Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Autoimmune disorder
Autoinflammatory disorder
Adipose-derived stem cells
Autologous hematopoietic stem-cell transplantation
Chronic antibiotic-refractory pouchitis
Crohn's disease
Extraintestinal manifestation
The Food and Drug Administration
Familial Mediterranean fever
Fecal microbiota transplantation
Granulocyte colony-stimulating factor
Gastrointestinal
Graft-versus-host disease
Genome-wide association studies
Inflammatory bowel disease
Inflammatory bowel disease–unclassified
Inflammatory bowel disease–variant
Indeterminate colitis
Ileal pouch-anal anastomosis
Intestinal tuberculosis
Interleukin
Intravenous immunoglobulin
Mycobacterium avium paratuberculosis
NOD2-associated autoinflammatory disease
Nucleotide-binding oligomerization domain containing
Orthotopic liver transplantation
Primary sclerosing cholangitis
Tumor necrosis factor
Ulcerative colitis
Dr. Bo Shen is supported by the Ed and Joey Story Endowed Chair.
Classic inflammatory bowel disease (IBD) consists of Crohn's disease (CD) and ulcerative colitis (UC), which run chronic diseases with relapsing and remitting clinical courses. The diagnosis of IBD is made with histologic evidence of inflammatory and structural changes, with or without acute inflammation, combined with supporting clinical, endoscopic, and radiological features. The exact “triggers” for IBD are not entirely clear but the diseases are believed to be caused by a combination of genetic predisposition, abnormal immunity, and environmental exposures. Generally speaking, classic IBD has been considered to be idiopathic.
As it is apparent in clinical practice, the phenotype IBD is far from uniform. Whereas one patient may have severe Crohn's colitis with debilitating arthropathy, another patient may have mild UC with pyoderma gangrenosum (PG). The diagnosis may be unclear in 10%–15% of patients who will carry a disease entity of IBD-unclassified (IBD-U). To confuse the picture even further, infectious mimics to IBD such as intestinal tuberculosis (ITB) are frequently difficult to differentiate from CD.
Inflammatory bowel disease can be associated with traditionally defined extraintestinal manifestation (EIM) such as primary sclerosing cholangitis (PSC) and PG. However, various autoimmune and autoinflammatory diseases occur concomitantly with IBD but are not considered to be classic IBD-associated EIMs. For example, IBD patients may have concurrent psoriasis, autoimmune hepatitis, or celiac disease. Those immune-mediated diseases have normally viewed as separate, distinct entities from IBD. However, those immune-mediated disorders, including classic, idiopathic IBD, may share part(s) of common pathways.
Aside from the Montreal classification for CD and UC, multiple different classifications have been proposed. Vermeire et al. advocated for a molecular reclassification of IBD, noting that there is frequently a poor correlation between genetic-based subgroups and clinical phenotypes. Shen et al. proposed a systemic overlap syndrome of the gut (IBD) and liver. Cleynen et al. reported genetic evidence supporting a location-focused classification of IBD, for example, ileitis, colitis, ileocolitis, and proctitis. Levine et al. elaborated on the Montreal classification, advocating for pediatric IBD to be subdivided into diagnosis before or after age 10. We propose an adjustment from the traditional concept of IBD as a stand-alone field to IBD as a part of a spectrum of immune-mediated intestinal diseases and overlap syndromes. IBD can be further delineated into primary (“classic”) and secondary IBD. Variables such as the genome, exposome, microbiome, and immunome contribute to the variation in disease presentations, disease course, and outcomes.
The conventional theory in the pathogenesis of UC and CD begins with a dysbiotic shift followed by dysregulated innate and adaptive immunity in genetically susceptible hosts. Noticeable shifts in bacterial flora are apparent in IBD patients, with an overrepresentation of select bacterial taxa and decrease in overall diversity of the bacterial community. This dysbiosis leads to an inflammatory response. Genetic variants such as nucleotide-binding oligomerization domain containing 2 (NOD2), lead to alterations in host innate immunity such as defective sensing of bacteria and decreased production of antimicrobial peptides. Defects in innate immunity result in dysregulated T-cells of the adaptive immune system, chronic inflammation, and enterocyte apoptosis. The etiopathogenesis of UC and CD is also described in Chapter 1 . Genetic factors, such as interleukin-10 (IL-10)/IL-10R mutations may play a more important role in pathogenesis of infant or pediatric onset of IBD.
The definition of IBD has been traditionally limited to CD and UC. In some cases the diagnosis is unclear, despite clinical, endoscopic, radiographic, and gross pathologic evaluations. The label of IBD-indeterminate or indeterminate colitis (IC) is usually given in these cases. The Montreal classification is commonly used to categorize CD in terms of age, behavior (inflammatory, stricturing, penetrating), and location (ileal, colonic, ileocolonic, upper gastrointestinal (GI) tract, and perianal). In UC, patients are categorized based on the extent of disease (proctitis, left-sided colitis, and extensive colitis) and disease severity.
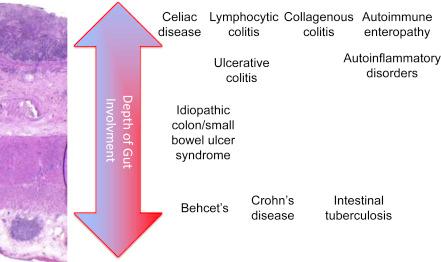
The conventional discriminating features have been used to differentiate UC from CD for decades. CD may involve any part(s) of the GI tract, whereas UC is confined to the colon, rectum, and in some cases, distal ileum (backwash ileitis). Inflammation in CD (with or without granulomas) may extend transmurally, leading to a diverse array of complications including stricture, fistula, and abscess, whereas inflammation in UC is generally limited to the mucosa, muscularis, and up to the superficial submucosa ( Fig. 2.1 ). In addition, a segmental distribution with skip lesions and rectal sparing are characteristics of CD, whereas the rectum is always affected in UC at presentation. Of interest, the disease location and extent of CD remain relatively stable, even after ileocolonic resection, whereas the disease extent of UC often migrates proximately. These phenomena suggest that etiopathogenetic pathways of CD and UC do not completely overlap.
The treatment of IBD involves use of antiinflammatory agents such as mesalamines, corticosteroids, immunomodulators (e.g., thiopurines and methotrexate), anti-tumor necrosis factor α (TNFα; e.g., infliximab, adalimumab, golimumab, and certolizumab pegol), anti-integrins (e.g., vedolizumab and natalizumab), anti-ILs (e.g., ustekinumab), and pathway-targeted small molecules (e.g., ozanimod). The newer agents have been developed to target various etiopathogenetic pathways involved in IBD.
Inflammatory bowel disease has traditionally been classified into UC and CD. The term of IC has been used by the GI pathologist to describe transmural inflammation in severely inflamed colon, which precludes the diagnosis of UC or CD. Patients with clinical, endoscopic, and histologic features, which do not completely fit the diagnosis of UC or CD, may be labeled as IBD-U.
Both UC and CD are then further subclassified based on the age of onset, disease location/extent, and disease phenotype and severity. The most commonly used is the Montreal classification (see Chapter 1 ).
However, the current classification systems are not able to cover the spectrum of immune-mediated IBD and IBD-like conditions, with a wide range of etiopathogenesis, disease course, phenotypes, and histopathologic features.
The classic boundary between UC and CD is frequently obscured. Up to 9% of patients diagnosed with either UC or CD may have their diagnosis changed within the first 2 years after diagnosis. Approximately 23%–35% of patients with CD have disease limited to the large bowel, that is, Crohn's colitis. The distinction between Crohn's colitis and UC can be challenging, especially if the colon has severe inflammation, leading to a histopathologic diagnosis of IC ( Fig. 2.2 ). It has also been reported that some patients with UC may have duodenal involvement, especially in those with concurrent PSC. These ill-defined “gray zones” have posed a great challenge for clinicians in the diagnosis and management of IBD.

Attempts have been made to further define these “gray zones” based on genetics. It is known that of the 163 confirmed IBD susceptibility loci, many are dually associated with both UC and CD, making the majority of IBD polygenic. Moreover, a recent genetic-phenotype profiling study redefined IBD into three groups, ileal CD, colonic CD, and UC, noting that disease location has a strong association with genetics. In contrast, traditional distinctions (UC and CD) or disease behavior (penetrating, stricturing, inflammatory) do not match up well with predictive models of genetic risk.
Single-gene mutations, rather than gene profiling, can also define phenotype in pediatric IBD. Monogenic mutations in IL-10RA and IL-10RB determine phenotype in a very early-onset IBD. In very early-onset IBD, infants develop symptoms of perianal fistulas, diarrhea, oral ulcers, and folliculitis within the first year of life. The defect in IL-10 signaling in this special form of IBD has a Mendelian inheritance pattern with complete penetrance. Owing to the unique genetic defect leading to disease, patients with very early-onset IBD do not usually respond to conventional IBD therapy and require alternative treatments such as allogeneic stem-cell transplants. Thus in many forms of IBD, there are genetically driven (monogenic and polygenic) disease phenotypes.
Inflammatory Bowel Disease frequently presents with EIMs involving the skin, eyes, joints, liver, lungs, or pancreas. The classic EIMs include erythema nodosum, PG, uveitis, episcleritis, iritis, ankylosing spondylitis, sacroiliitis, and PSC. The gut disease activity of IBD may or may not be associated with the presence of and severity of those EIMs. The treatment of the underlying IBD is a key to controlling many of these EIMs. In contrast the severity of some EIMs is not driven by underlying intestinal inflammation. For example, the disease courses PG, ankylosing spondylitis, and PSC are independent of bowel inflammation. This is particularly apparent in the disease course of PSC in postcolectomy UC patients.
It is unclear why certain diseases have been labeled as EIMs of IBD, whereas other commonly IBD-associated disorders are referred to as separate disease entities. For instance, ankylosing spondylitis is considered an EIM of IBD, whereas rheumatoid arthritis is regarded as a concurrent autoimmune disorder (AimD) ( Table 2.1 ). In fact, patients with IBD were shown to have comparably high odds ratios of having ankylosing spondylitis (odds ratio = 5.1) or rheumatoid arthritis (odds ratio = 3.5). As another example, unlike erythema nodosum or PG, psoriasis is not considered a dermatologic EIM of IBD, despite the known association between psoriasis and IBD. In fact, ustekinumab, an agent for psoriasis, was recently approved for the treatment of CD in the United States. Other immune-mediated diseases, such as autoimmune thyroiditis and autoimmune hepatitis, which occur concomitantly with IBD, are considered as concurrent AimDs but as classic EIMs of IBD. Up to now, we have taken this traditional classification system at face value, which has created confusion in clinical practice. This is now leading to our proposal for reclassification of IBD and its associated disorders ( Table 2.2 ).
| Classic Extraintestinal Manifestations of Inflammatory Bowel Disease | Examples of “Concurrent” Autoimmune Disorders of Inflammatory Bowel Disease | |
|---|---|---|
| Skin | Pyoderma gangrenosum, erythema nodosum | Psoriasis, Hashimoto thyroiditis, celiac disease |
| Liver | Primary sclerosing cholangitis | Primary biliary cirrhosis, autoimmune hepatitis |
| Joint | Ankylosing spondylitis | Rheumatoid arthritis |
| Vascular | Thromboembolism | Autoimmune vasculitis |
| Criteria | Class | Description | Examples | |
|---|---|---|---|---|
| Disease location, extent and depth +/− granulomas | Ulcerative colitis | Classic ulcerative colitis | ||
| Crohn's disease | Classic Crohn's disease | |||
| Indeterminate colitis | ||||
| Age of onset | Very early onset | Age 0 | IL-10/ILR mutations | |
| Early onset | Age 0–10 years | |||
| Age 10–17 years | ||||
| Regular onset | Age 17–40 years | |||
| Late onset | Age >50 years | |||
| Phenotype | Inflammatory | Inflammatory Crohn's disease; classic ulcerative colitis | ||
| Stricturing | Stricturing Crohn's disease; ulcerative colitis with stricture | |||
| Penetrating | Fistulizing Crohn's disease | |||
| Locations | Oral | |||
| Upper gastrointestinal | ||||
| Jejunum | ||||
| Ileum | ||||
| Colon | ||||
| Rectum | ||||
| Perianal | ||||
| Extraintestinal | Metastatic Crohn's disease of the skin, lung, liver | |||
| Concurrent or immune-mediated disorders | IBD | Isolated ulcerative colitis or Crohn's disease of the gut | ||
| IBD-variant | IBD + | IBD + classic extraintestinal manifestations | Ulcerative colitis with concurrent primary sclerosing cholangitis | |
| IBD ++ | IBD + autoimmune and/or autoinflammatory disorders ± classic extraintestinal manifestations | IBD with concurrent microscopic colitis, celiac disease, hidradenitis suppurativa | ||
| IBD +/− | Diseases sharing clinical features and possible etiopathogenetic pathways with classic IBD ± classic extraintestinal manifestations of IBD, autoimmune disorders or autoinflammatory disorders | Lymphocytic colitis, collagenous colitis; Behcet's disease, cryptogenic multifocal ulcerous stenosing enteritis, ulcerative jejunitis | ||
| Etiology of IBD | Primary or idiopathic | Monogenic | IL-10, IL-10RA, IL-10RB mutations Very early–onset IBD |
|
| Polygenic | Classic ulcerative colitis; classic Crohn's disease | |||
| Secondary | Identifiable pathogens | Mycobacterium avium paratuberculosis | ||
| Medication-induced | Mycophenolate-associated colitis; Ipilimumab-associated colitis | |||
| Organ transplantation-induced | Post-solid organ transplant IBD-like conditions, cord colitis syndrome; | |||
| Surgery-induced | Pouchitis, Crohn's disease-like conditions of the pouch, postcolectomy enteritis, bariatric surgery-associated IBD | |||
| Genetic etiology | Monogenic | IL-10/IL-R mutations, familial Mediterranean fever | ||
| Polygenic | Classic Crohn's disease and classic ulcerative colitis | |||
| Disease spread process | Intrinsic (“inside-out”) | Starting from the lymphatic system or mesentery, spreading to gut mucosa | Subset of obese Crohn's disease patients; subset of sclerosing mesenteritis or lymphangitis | |
| Extrinsic (“outside-in”) | External trigger (e.g. bacteria) leading to mucosal inflammation | Fulminant ulcerative colitis: from mucosal disease to transmural inflammation | ||
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here