Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Congenital aortic valve disease is one of the more commonly encountered congenital cardiac defects, occurring in 3% to 6% of children born with congenital heart disease. Congenital aortic valve disease primarily manifests as aortic stenosis and tends to be a progressive disease with morbidity and mortality resulting from the hemodynamic burden imposed on the left ventricle. Aortic stenosis is generally defined as a narrowing at the valvar, subvalvar, supravalvar, or multiple levels along the left ventricular outflow tract (LVOT). Although the same pathologic mechanisms are operational in both congenital and acquired aortic valve disease, the context differs dramatically. Congenital aortic valve pathology often manifests alongside multiple associated cardiovascular lesions, and multiple levels of the LVOT can be involved. There are multiple considerations when caring for these children regarding the timing of intervention and approach (catheter-based intervention versus surgical). Because of growth, behavior, and anticoagulation further surgical considerations often include single versus biventricular repair, valve repair versus replacement, and type of valve replacement. Thus, the evaluation and treatment of congenital aortic valve disease may present unique anatomic and physiologic considerations. Although aortic insufficiency can afflict congenitally abnormal valves later in life, it is an uncommon lesion during infancy and early childhood. When present in these age groups, it is usually an iatrogenic consequence of a procedure designed to relieve congenital aortic stenosis. This chapter will address these considerations and the related challenges encountered in the surgical treatment of congenital aortic valve disease.
The aortic valve and root span the transition from the left ventricular chamber and the systemic circulation, and they include the subaortic LVOT, the aortic valve, and the aortic wall up to the level of the sinotubular junction. Congenital heart disease can involve one or more levels of the aortoventricular complex. A thorough understanding and appreciation of these inconspicuous anatomic relationships form the basis of successful surgical treatment of congenital aortic valve disease, and these relationships will be referred to throughout this chapter.
The normal aortic valve sits wedged into the LVOT. In addition to supporting the coronary circulation, this central location places the aortic valve at the nexus of several critical intracardiac structures. Among these structures are the anterior leaflet of the mitral valve, the membranous interventricular septum, and the conduction apparatus. Because multiple levels of the aortoventricular complex can be involved in congenital heart disease, it is helpful to consider the normal anatomy to consist of subvalvular, valvular, and supravalvular components.
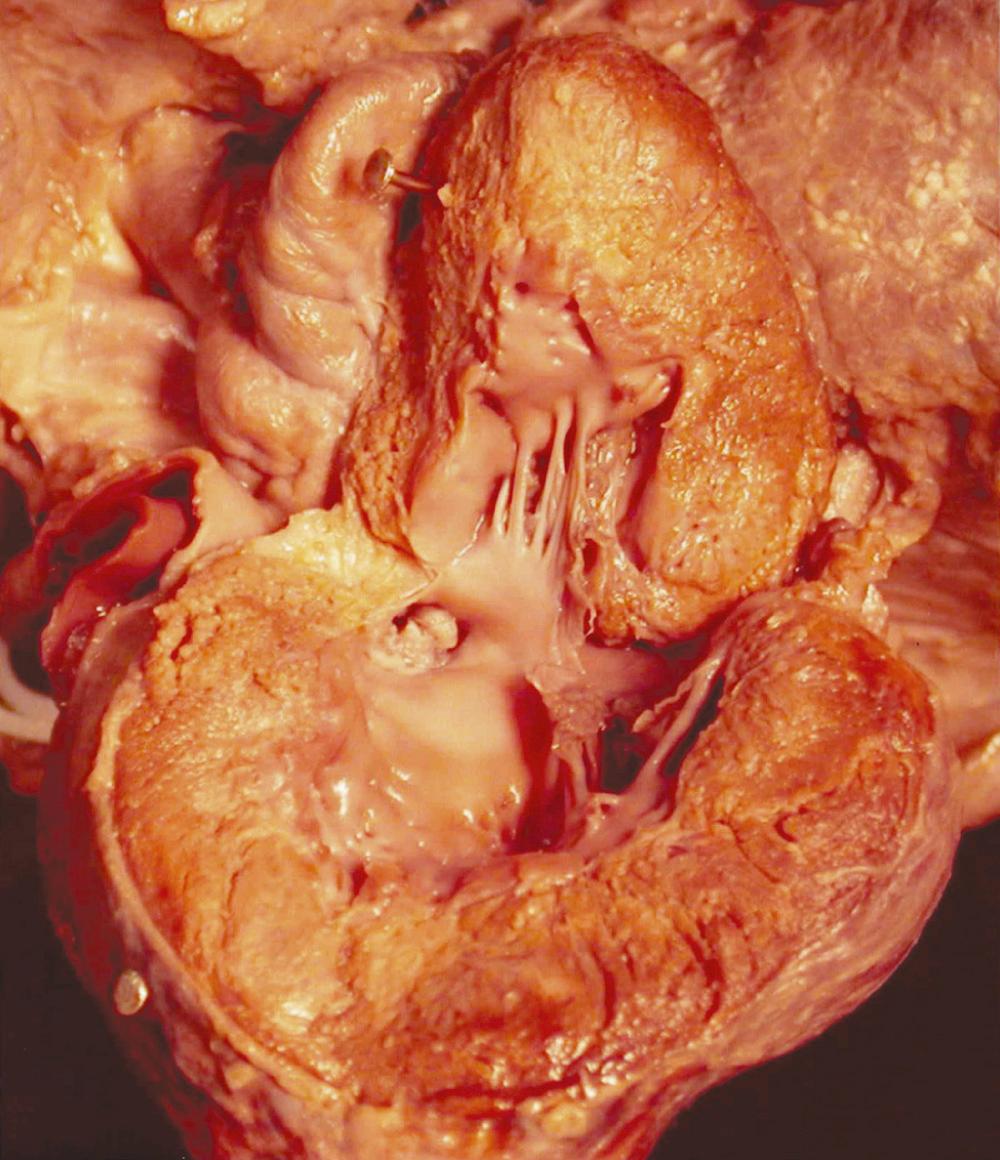
The subvalvular anatomy is dominated by the anatomic relationships among the aortic valve, interventricular septum, membranous septum, mitral valve, and conduction apparatus ( Fig. 123-1 ). Parts of the aortic leaflets are in fibrous continuity with the anterior leaflet of the mitral valve as well as the tricuspid valve (via the membranous septum). These structures contribute to the central supporting structure of the heart, the fibrous skeleton. In addition to providing points of fixation for the atrioventricular valves, the fibrous skeleton also provides electrical insulation between the atria and the ventricles, restricting impulse conduction to the bundle of His. After arising from the atrioventricular node, the bundle of His penetrates the membranous septum, emerging on the surface of the left ventricular septum immediately below the aortic annulus. Looking through the aortic valve, the bundle will lie beneath the annulus, just below the commissure between the noncoronary and right coronary leaflet. From the surgeon's perspective, important radiations of the bundle reach to the nadir of the right coronary leaflet as they fall away, toward the apex of the heart.

Valvular anatomy is dominated by the semilunar leaflets, commissures, and sinuses of Valsalva. The leaflets and their sinuses are identified by the associated coronary artery. The right coronary artery ostium is found in the right coronary sinus, which is almost directly anterior as viewed by the surgeon. The left coronary artery emerges posteriorly from the left coronary sinus. The leaflets themselves are composed of a fibrous core lined by endothelium. The commissures, along with the free-edge coaptation provided by the leaflets themselves, provide the strength necessary to provide a competent valve.
The aortic sinuses are dilations of the aortic wall above distal to the insertion of the semilunar leaflets, and they are well suited to support the coronary ostia. While the leaflets retract during systole, eddy currents developing within the sinuses prevent occlusion of the coronary ostia by the retracted aortic leaflets. Their presence is probably important to the long-term function of the aortic valve leaflets.
Finally, the supravalvular area denotes the transition from the left ventricle–aorta complex to the aorta proper. For practical purposes, this area includes the sinotubular junction, the area just distal to the tips of the commissural posts, and the dilations of the aortic sinuses. Abnormalities at any level can be expected in congenital heart disease, and a discussion of the various forms of congenital aortic valve pathology will be categorized by its level: valvular, subvalvular, or supravalvular.
Congenital aortic stenosis has been reported to be present in between 3% and 6% of children with congenital heart disease. Males are affected more commonly than females with an incidence of 3 : 1.
The clinical presentation varies with the severity of the lesion and the age at presentation. In the neonate, critical aortic stenosis may present rapidly and dramatically, with abrupt hemodynamic deterioration, cardiovascular collapse, and shock. The cardiogram may reveal left ventricular hypertrophy with S-T and T wave abnormalities. On chest radiography, there may be cardiomegaly and pulmonary edema. Transthoracic echocardiogram will rapidly establish the diagnosis, and it is useful to determine the presence of associated abnormalities of the left ventricle, mitral valve, or aortic arch. Aggressive resuscitation with inotropes and prostaglandin is required to support the circulation.
Older children may be asymptomatic, and aortic stenosis may be suggested by physical examination results. Chest pain and exercise intolerance is possible but uncommon. Like the neonate, the diagnosis can be confirmed with echocardiography.
Physical examination results may be suggestive of the diagnosis of congenital aortic valve disease. There may be a harsh crescendo-decrescendo murmur heard best at the right second interspace, with transmission into the neck. A thrill will be present in the suprasternal notch, and over the right second intercostal spaces in severe aortic stenosis. Because ventricular systole is prolonged in the setting of aortic stenosis, the second heart sound may be prolonged, resulting in narrowly split second heart sound. An associated diastolic murmur would suggest an element of aortic insufficiency.
Electrocardiographic changes are consistent with ventricular hypertrophy, as evidenced by increased left-sided R wave voltage. Changes in S-T segment and T wave in the left precordial leads may denote LV strain. Except in the situation of obvious congestive heart failure, the chest radiograph is usually unremarkable.
Echocardiography is the mainstay for contemporary diagnosis of congenital aortic valve disease. This noninvasive test provides important anatomic and physiologic information, such as the number and anatomy of the aortic leaflets, the size of the aortic annulus and ascending aorta, the location of the coronary arteries, the adequacy of the subaortic LVOT, and the site of the hemodynamic stenosis. The peak instantaneous gradient can be estimated by measuring the velocity of blood flow across the stenosis, and using a modification of the Bernoulli equation (velocity [m/sec] 2 × 4).
Recent developments in imaging include the availability of three-dimensional echocardiographic technology. Ventricular mass and volume measurements obtained with three-dimensional echocardiography compare well with those obtained with cardiac magnetic resonance imaging, and their utility in determining the etiology of congenital aortic valvular disease is currently under investigation.
Stress testing may be helpful in older patients with mild to moderate aortic stenosis in whom symptoms may be vague or suspicious and are not clearly related to the aortic valve disease. Stress-related changes in the S-T segment or T wave morphology would suggest important stenosis with myocardium at jeopardy. In these instances, relief of the stenosis should be seriously considered, despite mild to moderate resting peak gradients.
Currently, the major roles of cardiac catheterization include diagnostic and therapeutic options. Diagnostically, catheterization may be helpful to assess left ventricular diastolic function, pulmonary artery pressure, and associated vascular lesions. Therapeutically, balloon valvotomy can be used in patients with isolated aortic valvular stenosis or occasionally in patients with complex multilevel LVOT obstruction.
Critical “valvular” aortic stenosis in the neonate often presents as an “emergent” situation, and without prompt treatment these children may succumb from cardiovascular collapse. By contrast, older children with aortic stenosis are often asymptomatic. Given the important considerations pertaining to pediatric aortic valve repair and replacement, the timing of the operation becomes an important consideration, and understanding the natural history and progression of asymptomatic children is useful.
Aortic valve disease in children tends to be a progressive disease. Valvar aortic stenosis can be classified as mild, moderate, or severe. Categorized by Hossack and colleagues, mild stenosis includes those patients with normal pulse volumes with a resting peak systolic gradient (measured at catheterization) between the left ventricle and aorta of less than 40 mm Hg. Patients are considered to have moderate aortic stenosis when they exhibit diminished pulse volumes by palpation and resting peak systolic gradients of 40 to 75 mm Hg at rest. Patients are considered to have severe stenosis when they present with abnormal pulse volumes and a resting peak systolic pressure gradient in excess of 75 mm Hg.
It should be noted that these gradient criteria were obtained at catheterization by direct pullback measurements. Currently, gradients across the LVOT are frequently obtained with echocardiography, and in most cases Doppler-derived peak instantaneous gradient correlates well with catheter-derived data; however, at lower gradients, Doppler echocardiography can result in an overestimation.
Because aortic valve disease in children is a progressive disease, the natural history of this progression is of some interest. Among children with nonobstructive aortic lesions, Mills and colleagues reported that 7% progressed to mild obstruction after 7 to 15 years. When mild stenosis was present upon the initial evaluation, progression was rapid. Twenty percent of patients developed moderate or severe stenosis within 10 years, with 45% progressing within 20 years. Finally, approximately 60% of patients presenting with moderate stenosis will progress to severe stenosis within 10 years.
Aortic stenosis presenting in the neonatal period can be a severe hemodynamic lesion that is life threatening ( Fig. 123-2 ). These children often present in shock as a consequence of ductal closure in the setting of left heart structures that are inadequate to support the systemic circulation; their systemic circulation is “ductal dependent.” These children require urgent medical stabilization, including assisted ventilation and inotropic support. Prostaglandin E1 is administered to reopen the duct and to maintain ductal patency. Once stabilized, a thorough echocardiographic examination of the left-sided structures is required to determine their suitability to support the systemic circulation.
When approaching critical aortic stenosis in neonates, the single most important decision regarding treatment is to decide which patients will benefit from biventricular repair, and which are better suited to a single ventricle approach. The importance of proper treatment selection is reflected by the high mortality in older, unstratified series of neonates undergoing valvotomy for critical aortic stenosis. An accurate determination of which patients will benefit from a single ventricle treatment pathway (Stage I/Norwood operation) is critical, and it has been the impetus for the study sponsored by the Congenital Heart Surgeons Society (CHSS) and designed to accomplish this. An estimate of survival benefit for specific left heart morphology can be obtained at the CHSS website ( www.chssdc.org ). The management of these patients has been detailed elsewhere. This discussion will focus on patients with aortic stenosis as the dominant lesion in the setting of two adequate ventricles.
For patients with anatomy deemed suitable to support a two-ventricle circulation, aortic valvotomy provides effective relief of aortic stenosis. Two techniques of aortic valvotomy have been developed: balloon dilation of the stenotic valve and surgical valvotomy (open and closed). While balloon valvotomy is the favored technique at many institutions, surgical valvotomy remains preferred by some. Proponents of balloon valvotomy cite avoidance of potential surgical morbidity, while advocates of surgical valvotomy maintain that a more accurate valvotomy is possible under direct vision.
Although there are no prospective randomized studies directly comparing these two techniques, some data are available. McCrindle and colleagues reported a CHSS-sponsored multi-institutional review of 110 neonates undergoing either surgical (28) or balloon (82) valvotomy for critical aortic valve stenosis in the neonatal period. Survival was similar between the two procedures (82% at 1 month, 72% at 5 years). Balloon valvotomy was more effective at relieving stenosis (mean residual gradient 20 vs. 36 mm Hg), but it was accomplished at the expense of a higher incidence of important aortic insufficiency (18% after balloon valvotomy versus 3% after surgical valvotomy). Despite these differences, the outcome data between the two techniques is comparable. Overall freedom from reintervention was similar for both groups (91% at 1 month, 48% at 5 years). The need for subsequent procedures, regardless of technique, emphasizes the palliative nature of valvotomy for critical aortic stenosis.
McElhinney and colleagues reported medium- and long-term follow-up of 113 patients (age ≤ 60 days) from Children's Hospital Boston performed between 1985 and 2002. They reported a normalization of aortic annular and left ventricular end-diastolic dimensions within 1 to 2 years. Freedom from moderate or severe aortic regurgitation was 65%, with a reintervention free survival of 48% at 5 years. These data suggest that early relief of the LVOT obstruction allows for catch-up growth without neonatal surgery.
Although balloon valvotomy has assumed a prominent role in the treatment of congenital aortic stenosis in many institutions, its role vis-à-vis surgical valvotomy remains controversial. Hawkins and colleagues have estimated the incidence of aortic valve operation after balloon valvotomy to be 5% to 7% per year. Others have reported the risk of surgery to be lower, but the incidence of reintervention, including subsequent balloon dilation, remains high (60% at 8 years). It may be, however, that specific aortic valvular substrates lend themselves to one treatment or the other. In a group of 54 infants (57% neonates) undergoing surgical aortic valvotomy, Bhabra and colleagues reported significant differences in the long-term outcomes based on leaflet morphology. When valvotomy resulted in a trileaflet structure, patients did significantly better than when only a bileaflet valve was achieved. At 10 years, the actuarial freedom from reintervention was 92% among trileaflet valves, but only 33% among bileaflet valves ( P = 0.01). Similar differences were reported for freedom from aortic valve reoperation ( P = 0.04). Freedom from aortic valve replacement (AVR) was 100% in trileaflet valves and 57% in bileaflet valves. However, by echocardiogram, the authors were only able to retrospectively identify 14 of 28 bileaflet valves, whereas 7 of 8 valves with trileaflet potential could be identified (88% sensitivity, 50% specificity). These results have yet to be confirmed, but closer examination of the anatomic subtypes of aortic valve stenosis may be justified before selecting the appropriate technique.
Currently there appears to be little if any role for surgical transventricular (closed) aortic valvotomy.
A few studies have compared balloon aortic valvotomy to surgical valvotomy in older children. McCrindle and colleagues reported their analysis of 630 balloon valvotomies on 606 patients from 23 institutions, with a median age of 6.8 years (range, 1 day to 18 years). The procedure was abandoned 4.1% of the time because of technical issues, and procedural mortality was 1.9%. A suboptimal result (including failure to complete procedure, residual gradient >60 mm Hg, left ventricle aortic pressure ≥1.6) or major morbidity or mortality were reported in 17% of patients. Independent risk factors for poor outcomes were age less than 3 months, earlier procedure date, higher preoperative gradient, unrepaired aortic coarctation, and the use of undersized balloons.
Other groups have reported similar results with balloon valvotomy in non-neonates. Moore and colleagues reported successful dilation in 87% of patients (129/148), with a very low procedural mortality (0.7%) and good long-term survival (95% at 8 years). Freedom from reintervention at 8 years was 50%, a figure that is consistent with other studies.
In these patients, the risk of repeated intervention was related to the degree of regurgitation and to residual gradients after initial balloon valvotomy. Reminiscent of the report by Bhabra and colleagues, they reported differential results based on angiographic morphology of the stenotic aortic valve; however, as in neonates, it is difficult to demonstrate clearly the superiority of balloon or surgical aortic valvotomy. Chartrand and colleagues reported their experience with 67 children (age > 6 months) undergoing surgical valvotomy during 1960 to 1992. There was no operative mortality, and the 20-year freedom from death, reoperation, and AVR was 94%, 63%, and 73%, respectively. The authors concluded that surgical valvuloplasty is a safe and effective procedure with durable results.
In summary, as in neonates, aortic valve morphology appears to influence the response to intervention in infants, and further characterization may be justified. Currently there appear to be institutional preferences for balloon or surgical valvotomy that can be defended on the basis of experience.
In contrast to clinical presentation of the neonate with critical aortic stenosis, older children are commonly asymptomatic. For them, durable preservation of left ventricular function becomes the primary goal of treatment. The etiology of aortic stenosis in the older child (>1 year of age) is most commonly due to valvular aortic stenosis (79%), followed by subvalvular aortic stenosis (7%) and supravalvular stenosis (6%) being much less common. Aortic regurgitation is often associated with congenital aortic stenosis and may be the result of previous interventions for the relief of stenosis.
Older children with aortic stenosis generally enjoy normal growth and development. When present, symptoms such as chest pain, exercise intolerance, or syncope constitute clear surgical indications. For asymptomatic patients, the indications for intervention are subtler. For patients with severe stenosis in whom the left ventricle to aortic (LV-Ao) gradient is greater than 75 mm Hg, operation is recommended. For children thought to have moderate stenosis (40-75 mm Hg LV-Ao gradient), more information may be needed before a recommendation can be made. In this setting, electrocardiographic changes (ST-T wave changes consistent with left ventricular strain, left ventricular hypertrophy) or a positive stress test result would be indications for operation. For these asymptomatic patients, somatic growth, surgical options, and timing of intervention become important concerns.
Because of the progressive nature of the disease, older children thought to have mild aortic stenosis (gradient < 40 mm Hg) should be followed with periodic examinations and echocardiograms. It should be remembered that the gradient depends on the cardiac output and in a severely dysfunctional left ventricle the gradient may be unimpressive.
The management options for older children depend on the context of their disease. Older children newly diagnosed with important aortic stenosis with minimal insufficiency may be well served by balloon valvotomy. The more common scenario is several years of excellent palliation following an initial valvotomy, during which time the child grows and develops normally. However, with time or repeated interventions, or both, many patients will develop important aortic insufficiency (see Aortic Regurgitation section). Any residual valvular stenosis can be magnified by the resulting volume load, and the combinations of these lesions conspire to threaten left ventricular function. However, by virtue of their older age and larger size, these children are better candidates for durable surgical palliation, usually including valve replacement.
Congenital subvalvular aortic stenosis is an obstruction below the aortic valve secondary to a discrete or short, localized fibrous or fibromuscular ridge or a longer diffuse fibromuscular tunnel ( Fig. 123-3 ). It is important to note that there is a spectrum between discrete and tunnel-like subaortic stenosis, which has contributed the variable difference in the reported prevalence. In addition, it is often difficult to distinguish hypertrophic obstructive cardiomyopathy from tunnel-like subaortic stenosis (see Hypertrophic Obstructive Cardiomyopathy ).
Subaortic membrane is found in association with other congenital lesions in 60% to 70% of cases, with ventral septal defect being the most common (35%). Membranous subaortic stenosis results from the proliferation of fibrous tissue just beneath the leaflets of the aortic valve. This tissue may be very thin and tough, and is often circumferential, involving the underside of the anterior leaflet of the mitral valve. In fact, careful review of the echocardiogram may suggest a hinge point representing tethering on the leaflet by the membrane. Although the etiology of this form of aortic pathology is not known, studies have implicated shear stress, precipitated by abnormal angles between the ventricular septum and the aortic barrel, as playing an important role. The addition of a ventral septal defect adds to the generation of shear stress in this setting.
In general, the surgical indications for membranous subaortic stenosis adhere to the general recommendations for aortic stenosis. However, citing a lower incidence of recurrence, some centers advocate earlier surgical resection for subaortic stenosis. Brauner and colleagues reported that the recurrence rate of subaortic stenosis (predominately composed of membranous, but with a few tunnel-like stenoses) was related to a preoperative gradient of 40 mm Hg or greater and suggested that surgical resection at lower gradients was justified. Others have advocated repair at the time of diagnosis, irrespective of the gradient. However, the advantages of early intervention have not been confirmed, as other investigators have reported no benefit of early surgery on recurrence.
Because the timing of surgery remains controversial, it is helpful to examine the natural history of membranous subaortic stenosis. There are data suggesting that some children with mild subaortic stenosis (peak systolic gradient < 40 mm Hg) might not require surgery for several years. A large representative study of the rate of progression of subvalvular aortic stenosis was reported by Rohlicek and colleagues studying children from several centers in Eastern Canada. To document the natural history and surgical outcomes, they followed 92 children from the time of diagnosis. There were slightly more males (1.6 : 1), and the mean age at diagnosis was 5.3 years. Thirteen patients had bicuspid aortic valves. At a mean follow-up of four years, 42 of these children ultimately came to surgery an average of 2.2 ± 0.4 years after diagnosis; 44 were followed medically and never came to operation. Children ultimately requiring surgery presented with higher initial gradients (40 ± 5 mm Hg vs. 21 ± 2 mm Hg) and were more likely to present with aortic insufficiency at diagnosis (35% vs. 13%). Analysis showed the echo gradient at diagnosis to be predictive of subsequent gradient progression as well as the appearance of aortic insufficiency. Eight children undergoing surgery required reoperation for recurrent subaortic stenosis at an average of 4.9 ± 0.9 years after initial resection. These patients initially presented with significantly higher gradients (66 ± 10 mm Hg).
In contrast to a more aggressive approach of aggressive surgical resection of membranous subaortic stenosis at time of diagnosis, these data suggest that a significant proportion of these patients will have stable or at least slowly progressive gradients. The management approach to patients remains variable, but it seems reasonable to pursue surgical resection for peak systolic gradients of 40 mm Hg or greater (obtained by echocardiography). The new onset of aortic insufficiency should be considered an important indication for surgery, regardless of the gradient. While these authors did not discern any improvement in aortic insufficiency following operation, it has been the experience of others that careful débridement of fibrous tissue encroaching on the aortic leaflets often results in significant improvement in aortic insufficiency.
The surgical approach to this lesion requires cardiopulmonary bypass. A single right atrial venous cannula is usually adequate. The aortic valve is exposed through a transverse aortotomy, which can be carried down into the noncoronary sinus if needed. Careful retraction of the aortic leaflets will reveal the subaortic membrane. The distance between the aortic valve and the membrane may vary slightly, but can usually be well visualized. The membrane is incised in the safe zone of the ventricular septum, just leftward of the nadir of the right coronary sinus. In many instances, the membrane can be peeled or endarterectomized from the endocardium anteriorly and rightward, and from the anterior leaflet of the mitral valve posteriorly. In severe cases, this membrane may encroach upon and even involve the belly of the aortic valve leaflets. In this instance, the leaflets require careful débridement of the thick, fibrous tissue.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here