Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Wada testing was originally developed to anticipate postoperative surgical deficit. Test characteristics vary based on agent used, anatomic location of the AVM, and clinical situation.
The advent of microcatheter techniques has encouraged the use of superselective Wada testing. It may be less successful in predicting complication profiles with embolization than with surgery.
Intraoperative and postoperative digital subtraction angiography remain the mainstay gold standard for the assessment of successful resection of iAVMs.
Indocyanine green angiography is a useful adjunct to the microsurgeon in the operating room and can be helpful in determining initial approach and technical resection.
4D MRI, covered extensively in Chapter 4 by Hsiao and colleagues, has some applications in the operating room and for surgical planning when combined with these modalities and surgical navigation.
Preoperative planning and intraoperative imaging serve as useful tools to assist the neurosurgeon in operative resection of intracranial arteriovenous malformations (iAVMs). In this chapter, we discuss the use of preoperative Wada testing and 4D flow MRI to supplement standard digital subtraction angiography (DSA). This provides both functional and dynamic understanding of AVM anatomy and pathophysiology prior to planned resection. Intraoperative DSA and indocyanine green (ICG) are examined as real-time techniques to assess for extent of AVM resection. Postoperative angiography is performed to document complete resection and demonstrate an absence of early venous drainage. The cerebrovascular surgeon should be familiar with these imaging modalities, as their careful use and assessment can lead to improved patient outcomes.
Preoperative planning is critical for iAVM surgery. This includes a thorough understanding of location, 3D angioarchitecture, blood supply, and eloquence of adjacent tissue. Provocative pharmacologic assessment, or Wada testing, can be performed prior to iAVM surgery to help recognize and prevent potential functional deficits. The Wada test was initially developed by Dr. Juhn A. Wada in the late 1940s to establish cerebral language dominance. During the test, amobarbital (or another anesthetic agent, such as methohexital, propofol, etomidate, or lidocaine) is administered intra-arterially to transiently inactivate cortical regions supplied by the injected artery. Since its development, the Wada test has become widely used for functional localization in epilepsy patients who are surgical candidates.
The Wada test is classically performed on awake patients to assess neurological function by clinical examination. Functional testing can be supplemented with physiologic monitoring. Rauch et al. suggested that evaluation of both EEG and clinical neurological examination findings could increase testing sensitivity in iAVM patients. In this report, nearly half of all positive Wada tests occurred in patients who exhibited EEG changes without clinical symptoms. Limitations to awake testing include image degradation and potential complications secondary to patient movement. If multiple interventions are planned, the anesthetic dose may accumulate over the course of the procedure and induce drowsiness, thus decreasing patient cooperation. Alternatively, the Wada assessment can be performed with general anesthesia and simultaneous neuromonitoring (somatosensory evoked potentials [SSEPs], motor evoked potentials [MEPs], and electroencephalography [EEG]). Bican et al. performed Wada testing under general anesthesia in 28 patients (54 procedures), the majority of whom were being considered for AVM treatment. Transcranial MEPs were used to assess neural pathway integrity during injection. Embolization was performed after every negative test, and no neurological complications occurred. Although evoked potentials are able to assess motor and sensory function, complete evaluation of higher-level cortical functions, such as language or vision, typically requires awake testing.
An important consideration is the choice of anesthetic agent for Wada testing. Traditionally, amobarbital has been used, but recent supply has been limited. Amobarbital is a short-acting barbiturate that inhibits postsynaptic neurons in the deep and cortical gray matter without affecting white matter. In contrast, lidocaine affects both white and gray matter through the inhibition of voltage-gated sodium channels. Lidocaine is often used in Wada tests involving AVMs of the spinal cord, due to the abundance/importance of white matter tracts. A small clinical series compared the neurobehavioral differences of amobarbital and lidocaine in patients with iAVMs undergoing superselective Wada testing. The study showed that lidocaine detected clinically significant neurological deficits that amobarbital did not in all four patients. Embolization of one vessel was performed following a positive Wada test using lidocaine and a negative test using amobarbital. After embolization, the patient developed right-hand weakness, a deficit identical to that seen on Wada testing using lidocaine. The authors suggested that the administration of both lidocaine and amobarbital may improve the predictive value of the Wada test.
Previous studies suggest a high negative predictive value for Wada testing using amobarbital and lidocaine. The sensitivity, specificity, and positive predictive value can often not be determined, as embolization/resection is generally not performed following a positive Wada test. Wada testing using lidocaine (amobarbital was not available) was performed prior to embolization of iAVMs in sensory-motor areas. A total of 58 Wada tests were performed and importantly no false negatives were observed. Nimi et al. conducted a retrospective study of 60 Wada tests using amobarbital and lidocaine prior to embolization of spinal cord AVMs. The negative predictive value of this Wada testing protocol was 97.6%.
Methohexital is an alternative to amobarbital and has a shorter duration of action. Repetitive Wada testing with methohexital can be completed without inducing incremental drowsiness. Studies have reported no methohexital-related side effects, such as postdelivery vasopasm, at doses up to 10 mg. Furthermore, no complications were reported following a 10- or 20-mg dose of methohexital in two patients. Etomidate and propofol have also been used as anesthetic options. While low-dose etomidate has been safely used for both intracarotid and superselective Wada testing, propofol seems to have more documented side effects. Seizures are one such side effect, but most reported instances occurred following injection of propofol directly into the internal carotid artery as opposed to superselective injection into distal branches. Studies using superselective Wada tests have reported no adverse effects of 7- or 20-mg doses of propofol.
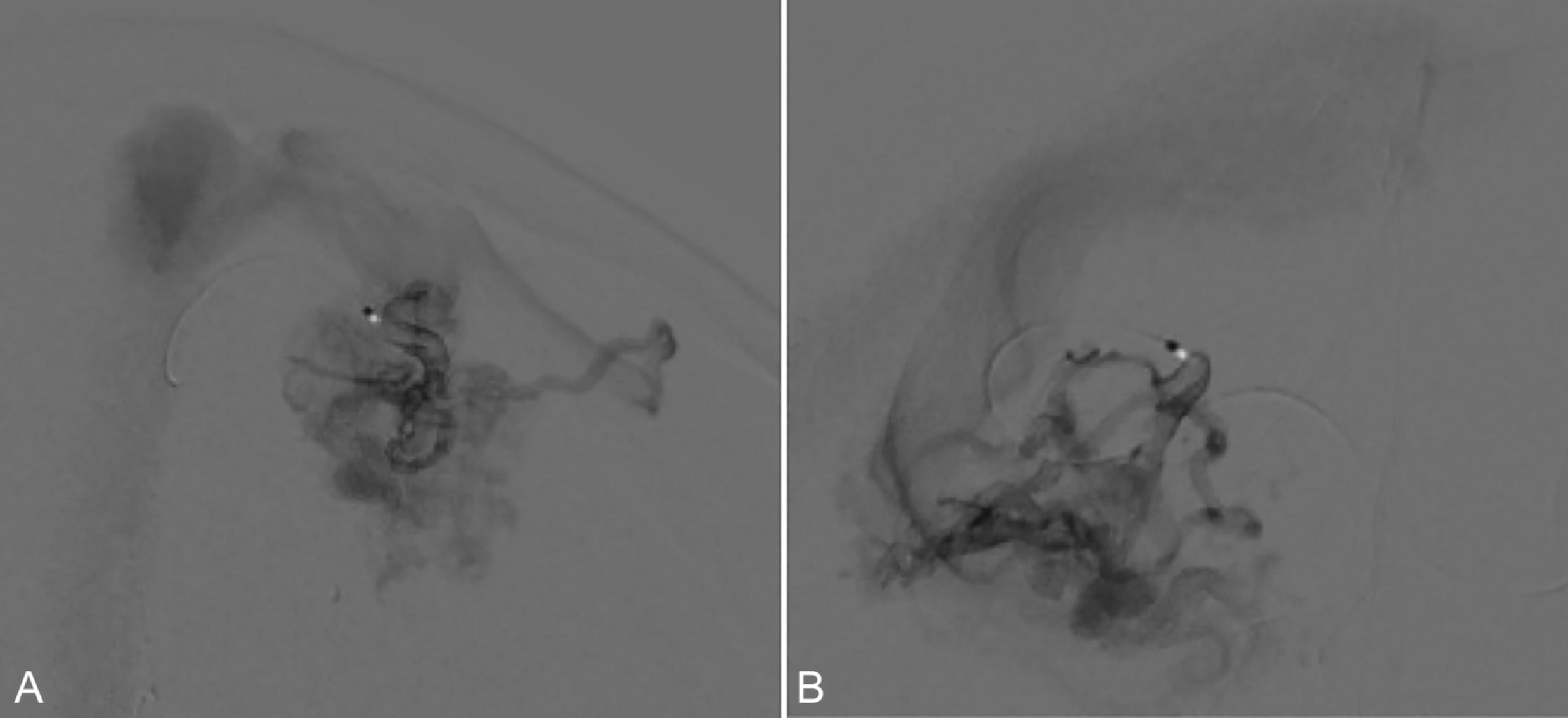
For iAVM evaluation, superselective Wada testing is preferred. In this technique, the anesthesic is administered to a targeted vessel through a microcatheter ( Fig. 29.1 ). A positive Wada test occurs when injection of the anesthetic results in a neurologic deficit. Superselective Wada testing pharmacologically simulates the proposed suspension of blood flow through the selected vessel. One of the earliest reports performed superselective Wada testing prior to resection of a posterior temporal AVM. Although the Wada test demonstrated no language deficits, the patient exhibited mild expressive aphasia postoperatively that resolved within 2 weeks. Although difficult to know with certainty, these transient postoperative changes could be due to surgical edema secondary to retraction or resection. Additional studies have since validated the efficacy of superselective Wada testing.
Tawk et al. evaluated the efficacy of Wada testing in patients with occipital AVMs. Visual field testing was performed before and after Wada testing to monitor for potential deficits. The group conducted 39 Wada tests on 13 patients. Of the 39 tests performed, 33 were negative, and embolization was performed. One patient subsequently developed a permanent visual deficit despite a negative Wada test. In four of the six patients with positive Wada tests, embolization was not performed. In the remaining two cases, the catheter was advanced distally until a negative Wada test occurred, and embolization was then performed. No further complications occurred. Rauch et al. investigated the utility of pre-embolization Wada testing in 30 awake patients with supratentorial AVMs. A total of 147 embolizations were completed. Five endovascular procedures were performed following a positive Wada test. Two of these five procedures were associated with subsequent neurological complications. None of the endovascular procedures that were performed immediately following a negative Wada test (82 total) resulted in neurological sequelae. A further report suggested a negative predictive value of 99.2% and a positive predictive value of 83.3% for the superselective Wada test.
For staged endovascular and operative interventions, it is important to repeat Wada tests before each treatment. High blood flow within an AVM nidus reduces flow to nearby vessels, termed the sump effect . Following each treatment, this effect may become less pronounced. Embolic agents introduced in subsequent endovascular procedures may reach and occlude new vessels due to blood flow changes. Rauch et al. suggested this potential in discussing 60 repeat embolization procedures during which repeat Wada testing was not performed. Six procedures (10%) resulted in neurological complications even though the patient had a negative Wada test during a prior procedure. The predictive application of Wada testing may therefore be less reliable when it is used as an antecedent to embolization than in anticipating postoperative surgical deficits with resection.
Intracranial AVMs exhibit complex angioarchitecture and flow dynamics. The presence of iAVMs alters regional cerebral perfusion and the distribution of flow within the extranidal vessels. DSA is the gold standard for iAVM characterization and guides staged treatments and operative approaches. DSA has high spatial and temporal resolution and provides detailed information on iAVM anatomy and hemodynamics, but it is an invasive modality and carries risks such as radiation exposure and potential for neurological complications. 4D flow MRI (time-resolved 3D phase-contrast MRI) can provide noninvasive in vivo hemodynamic assessment of cerebral vessels. (This topic is extensively covered in Chapter 4 by Hsiao and colleagues.) Studies can visualize and measure the temporal changes associated with blood flow within an acquired 3D volume. This hemodynamic AVM data may help inform treatment planning by identifying and targeting arteries with the highest flow. Quantitative blood flow measurements may be useful in assessing outcomes and therapeutic efficacy.
Investigations have demonstrated the feasibility of 4D flow MRI for assessing hemodynamic and physiologic parameters in iAVMs, such as velocity, flow volume, wall shear stress, and pressure gradients. 4D flow MRI can identify and quantify flow in feeding arteries and draining veins and becomes particularly useful for evaluating AVM hemodynamic changes between staged interventions, helping to avoid overaggressive treatment and the associated risk of hemorrhage. After staged treatment, 4D flow MRI can quantify the redistribution of cerebral blood flow and elucidate changes in venous drainage that may lead to increased rupture risk. For example, venous stenosis is associated with an increased risk of AVM rupture, and 4D flow MRI can be used to localize venous stenoses by identifying regions with accelerated venous blood flow.
Results conflict with respect to whether iAVM flow parameters consistently correlate with clinical presentation and/or AVM rupture risk. A 4D flow MRI study found that macrovascular flow was inversely associated with perinidal perfusion. Both cerebral blood flow and cerebral blood volume were decreased in the region surrounding the nidus. However, these AVM flow parameters were not associated with clinical presentation or AVM risk factors. In contrast, Chang et al. noted increased wall shear stress in feeding vessels compared to contralateral vessels in symptomatic iAVM patients but did not find any significant difference in the corresponding comparison in asymptomatic individuals with iAVMs. Further studies are needed to assess the clinical utility of these hemodynamic parameters. There are technical limitations that restrict the widespread adaptation of 4D flow MRI for diagnostic and therapeutic iAVM applications. These constraints include relatively long scan times, limited velocity ranges, and restricted spatial resolutions.
Imaging iAVMs requires broad cerebral coverage. 4D flow studies must have sufficient spatial and temporal resolution to allow accurate assessment of vessel morphology and flow dynamics. These factors increase total scan time to durations that can be clinically prohibitive. However, acceleration methods, such as parallel imaging, can decrease scan times to under 20 minutes. GRAPPA and SENSE acceleration methods have conventionally been used to decrease total scan time by an acceleration factor of up to R = 3. Advanced spatiotemporal parallel imaging methods, such as k-tGRAPPA and k-tSENSE, have been developed to further reduce total 4D flow scan time.
To assess the hemodynamics of both arteries and veins in AVMs, imaging must be performed over a dynamic velocity range. However, most 4D flow MRI protocols measure flow with one velocity sensitivity (venc), limiting the ability to assess both higher arterial flow and lower venous flow. A high venc is used to measure arterial flow, which can hinder evaluation of slower venous flow in images with decreased signal-to-noise ratios (SNRs). Dual-velocity encoding (dual-venc) can be used to address this limitation. Dual-venc improves the quantification of both high- and low-flow vessels. Two vencs are used in the scan, which improves vessel characterization across a velocity range.
A significant limitation of 4D flow MRI is restricted spatial resolution, which often precludes the hemodynamic assessment of small vessels. Blood flow may be overestimated at low spatial resolution due to partial volume effects. Schnell et al. suggest that isotropic spatial resolution should be 0.5–1.2 to accurately measure flow in 2- to 5-mm–diameter cerebral vessels. Ultra-high-field MRI can be used to increase spatial resolution without sacrificing SNR. Increasing total scan time or decreasing imaging coverage can further improve resolution without decreasing SNR. 4D flow data can be co-registered with other imaging modalities with high spatial resolution, such as time of flight imaging, to better analyze small cerebral vessels. Postprocessing algorithms, such as HYPR (highly constrained projection reconstruction) or relative blood flow within vascular territories (rBFR), may be used to further improve spatial resolution ( Fig. 29.2 ). To increase imaging coverage without sacrificing resolution, radial and spiral undersampling methods can be employed.
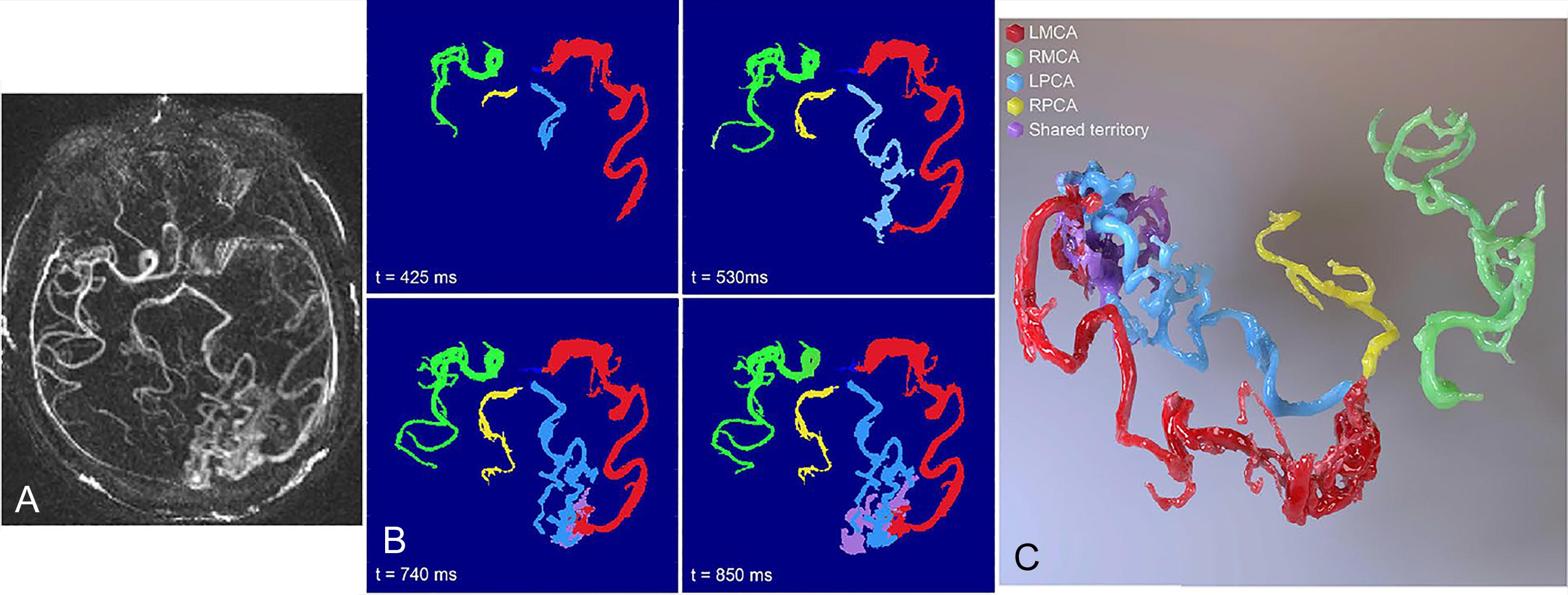
An in vitro 4D flow study assessed the association between spatial resolution and flow measurement accuracy. The study suggested that at least five voxels across a target vessel will yield accurate flow measurement within 10%–15%. However, four voxels across a cerebral vessel resulted in a flow conservation error less than 15% with high flow and peak velocity reproducibility. Four voxels can be attained in vivo with 0.8-mm isotropic resolution. This investigation found that the principal intracranial vessels could be imaged at high resolution within 20 minutes using dual-venc 4D flow MRI at 3T with the PEAK-GRAPPA acceleration method.
Ultimately, the technical temporospatial limitations of 4D flow coupled with the detailed overview provided in Chapter 4 offer a broad context for the clinical applications of these techniques to inform surgical treatment, baseline risk, and varying treatment effects. Their combined diagnostic use, similar to the complementary profiles of treatment strategies, will provide the appropriate context for the treating surgeon.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here