Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
![]() For videos accompanying this chapter see ExpertConsult.com . See inside cover for access details.
For videos accompanying this chapter see ExpertConsult.com . See inside cover for access details.
Nerve compression syndromes are a common clinical presentation in plastic surgery practice. The upper extremity is most commonly affected, with carpal tunnel syndrome being most frequent followed by cubital tunnel syndrome. The etiology of these disorders is usually multifactorial, with systemic disorders, prolonged postural changes, and repetitive activities contributing to their development. The increasing prevalence of obesity, metabolic disorders, and an aging population has resulted in increased numbers of patients seeking care for compression neuropathies. Surgeons treating these conditions must rule out neurological disorders that can mimic nerve compression, such as brachial neuritis, mononeuritis, and polyneuropathy. A subset of patients may have a genetic predisposition to multiple nerve compressions. Effective diagnosis and management require a detailed understanding of the anatomy and pathophysiology of nerve compression. A multidisciplinary approach to clinical evaluation, diagnosis, and management are key in attaining favorable outcomes.
The clinical presentation of patients with nerve compression is variable and dependent upon a spectrum of changes that occur within the nerve in response to a complex interplay of mechanical and ischemic forces applied over a period of time. This results in a classic constellation of symptoms, including pain, paresthesiae, and weakness ( Fig. 54.1 ). The histopathological response to chronic compression has been difficult to study in human patients for ethical reasons, and thus much of what we know originates from animal studies. As the nerve travels through a given compartment, the available volume is finite. Any reduction in compartment volume or increase in nerve size may result in compression of the nerve. External compression may occur from structures such as the transverse carpal ligament, bony prominences or the tendinous leading edge of muscles. Prolonged changes in limb position may reduce the overall size of the compartment and increase pressure on the nerve (e.g., prolonged wrist or elbow flexion). ,
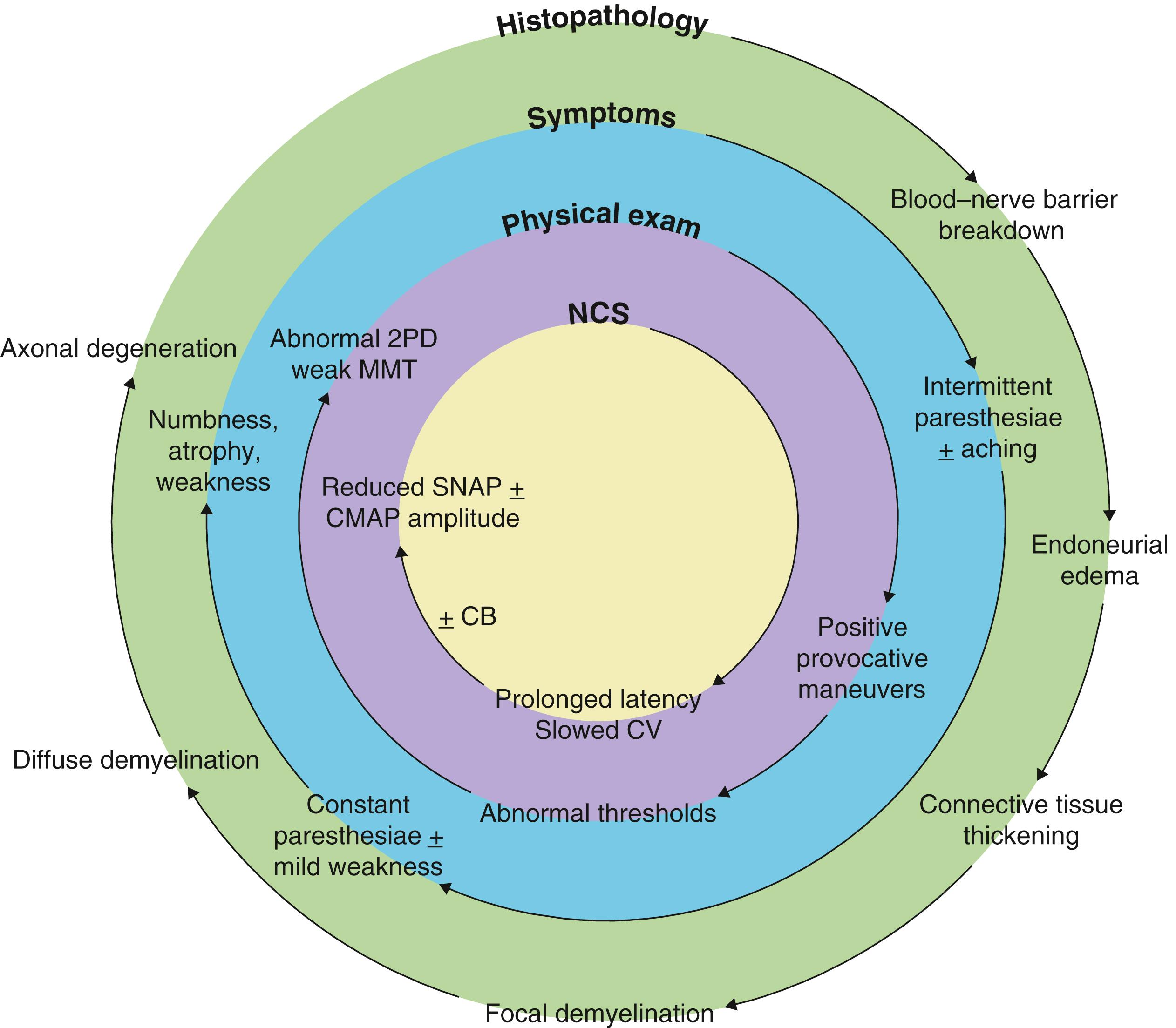
Chronic compression leads to breakdown of the blood–nerve barrier, followed by endoneurial edema and perineurial thickening and fibrosis. Increased endoneurial pressure may restrict blood flow to the nerve, making it susceptible to dynamic ischemia. If not corrected, areas of focal demyelination develop, followed by diffuse demyelination. This progresses to axonal injury and Wallerian degeneration in the absence of intervention. These changes may not be uniform across the nerve, and are related to the compressive forces applied to different areas of the nerve. For example, patients with early carpal tunnel syndrome often present with paresthesiae to the long and ring finger, owing to the superficial location of these fascicles relative to those of the thumb and index finger.
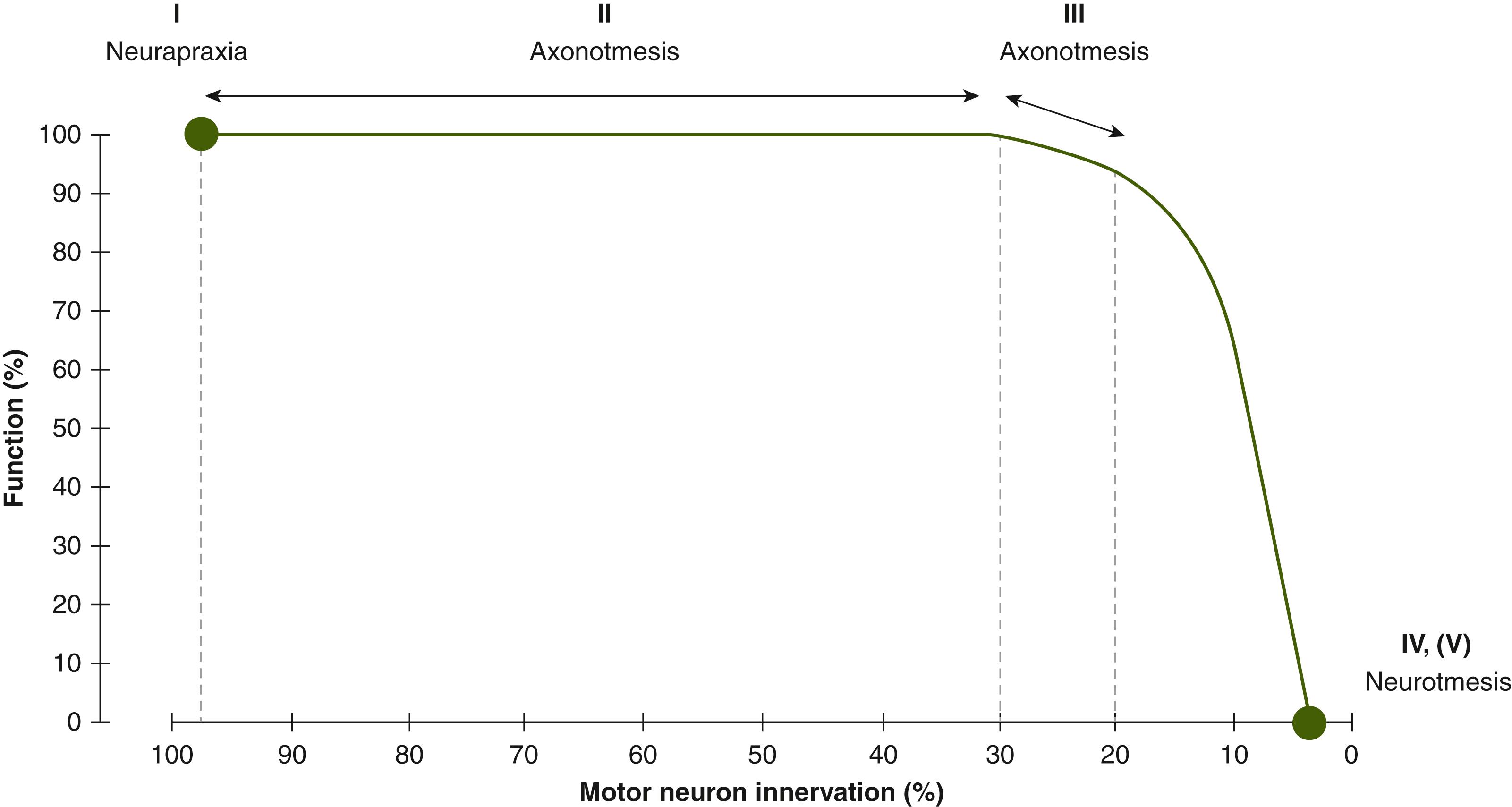
Accurate determination of the type of nerve injury is critical when approaching a patient with compression neuropathy as it guides management and helps to determine their prognosis. The Sunderland and Seddon classifications of nerve injury were originally described for acute nerve injuries, but can also be applied to compression neuropathy ( Table 54.1 ). A first-degree injury (neurapraxia) has areas of focal demyelination, with no axonal loss. Recovery is expected to be complete once the compressive insult is corrected. A second-degree injury (axonotmesis) has mild axonal loss and complete recovery is anticipated, whereas a third-degree injury (axonotmesis) has significant axonal loss with recovery that is variable and incomplete. Fourth- and fifth-degree injuries (neurotmesis) have no functional recovery because the axonal loss is complete. Fourth-degree injuries are uncommon in compression neuropathy but may occur with severe compression or iatrogenic injury. Fifth-degree injuries occur following traumatic or iatrogenic injury. When axonal injury occurs, any surrounding healthy axons collaterally sprout to innervate territories up to five times their original size. Therefore, weakness detected on clinical examination may not directly correspond with the degree of axonal loss. When linked to the classification of nerve injury, this concept can be used to better relate electrodiagnostic findings with the clinical examination ( Fig. 54.2 ).
| Nerve | Site of Compression | Test | Conservative Therapy |
|---|---|---|---|
| Median | Carpal tunnel | Carpal compression test Phalen test Reverse Phalen test |
Splint wrist in neutral at night |
| Proximal forearm | Pressure over pronator teres with the forearm supinated Resisted elbow flexion with the forearm supinated (lacertus fibrosus) Resisted forearm pronation (PT) Resisted long finger flexion (FDS) |
Stretching of pronator teres | |
| Ulnar | Cubital tunnel | Elbow flexion Pressure proximal to cubital tunnel |
Splint elbow in extension at night Elbow pad use |
| Guyon’s canal | Pressure proximal to Guyon’s canal Reverse Phalen test |
Splint wrist in neutral at night | |
| Radial | Proximal forearm (Radial tunnel/PIN) | Pressure over supinator (arcade of Fröhse) Resisted forearm supination Resisted long finger and wrist extension |
Favor positioning in supination Avoid repetitive forearm rotation |
| Distal forearm (Radial sensory) | Pressure over brachioradialis and extensor carpi radialis longus tendinous junction Forearm pronation with wrist flexion and ulnar deviation |
||
| Brachial plexus | Supraclavicular | Pressure over brachial plexus at the level of the scalenes Elevated arm stress test |
Stretching of shortened muscles Strengthening of weak scapula stabilizing muscles |
| Infraclavicular | Pressure over pectoralis minor |
A thorough history and physical examination is essential to identify all potential points of compression and musculoskeletal disorders contributing to patient symptomatology. The history should explore the nature and time course of symptoms, and address all relevant comorbidities, medications, and risk factors for nerve compression. Typical symptoms of compression neuropathy include numbness, paresthesiae, pain, and/or weakness in the distribution of the affected nerve(s). A pain questionnaire is a useful adjunct, especially in patients with diffuse symptomatology.
The physical examination should consist of a complete sensory and motor evaluation of both upper extremities, to localize the site of compression and determine the severity of neuropathy. All potential entrapment sites should be evaluated, owing to the double crush mechanism, wherein compression of a nerve at one site renders it more susceptible to compression at a second site. Failure to identify and address all involved compression points can lead to ongoing or residual symptoms following treatment.
Numerous methods are available to evaluate sensation and there is no accepted gold standard. These include assessment of light touch (e.g., the Ten Test ), vibration, pressure detection thresholds (e.g., Semmes–Weinstein monofilament testing), and two-point discrimination (static and dynamic). Abnormal sensory thresholds are an early finding in compression neuropathy, whereas abnormal two-point discrimination is typically found later.
Provocative tests using percussion (i.e., Tinel’s test), direct pressure, or joint movement can elicit patient symptoms by increasing compression at known nerve entrapment sites (see Table 54.1 ). The scratch collapse test (SCT) is a useful tool to identify areas of compression, and can be performed in hierarchical fashion to identify multilevel nerve compression.
Motor testing should include inspection of both upper extremities for atrophy and abnormal posture, as well as quantification of muscle strength using the Medical Research Council grading system, dynanometers (e.g., key pinch and grip strength), and special tests specific to each nerve. Weakness is usually a late finding in compression neuropathy.
Further details regarding symptomatology and exam findings unique to each compression neuropathy can be found in their respective sections.
Electrodiagnostic studies help to accurately localize the site of compression, assess the severity and progression of neuropathy, and exclude alternate diagnoses (e.g., cervical radiculopathy, systemic polyneuropathy, or disuse atrophy). However, they should be interpreted with an awareness of their limitations. Nerve conduction studies (NCS) evaluate the large, myelinated fibres and not smaller unmyelinated pain fibres. Since the latter are affected first in compression neuropathy, NCS may be normal early in the disease process. Similarly, dynamic ischemia resulting from compression cannot be detected on electrophysiological studies. History and physical examination therefore remain a critical component in the diagnosis of compression neuropathy.
Initial changes seen on NCS relate to focal demyelination and/or conduction block and include prolonged latency and slowing of conduction velocity. Axonal loss occurs in later stages of compression and results in decreased compound muscle action potential (CMAP) and sensory nerve action potential (SNAP) amplitudes (see Fig. 54.1 ). Electromyography (EMG) provides a qualitative assessment of muscle function and is a sensitive indicator of axonal injury. Increased insertional activity, the presence of spontaneous activity (e.g., fibrillation potentials, positive sharp waves), and changes in the morphology and recruitment of motor unit potentials (MUPs) signify axonal injury. In the setting of compression neuropathy, the presence of spontaneous activity provides evidence that denervated muscle is capable of receiving reinnervation. In very late stages of compression neuropathy, the sensory and motor NCS may have absent responses and EMG may demonstrate no MUPs.
Imaging is used as an adjunct to clinical examination and electrodiagnostic testing and generally does not establish the diagnosis of compression neuropathy on its own. It is most useful for identifying pathology contributing to the development of nerve compression at a specific location. Plain radiographs and/or computed tomography are useful for any compression neuropathy where trauma or bony abnormalities (i.e., a supracondylar process in pronator syndrome) are suspected. Ultrasonography and magnetic resonance imaging (MRI) can determine if cysts or other space-occupying lesions (e.g., ganglion or peripheral nerve sheath tumor) are present. Both ultrasound and MRI have also been used for the diagnosis of cubital tunnel syndrome with reasonable sensitivity and specificity: ultrasound evaluates the cross-sectional area of the ulnar nerve at the elbow, whereas MRI also looks at ulnar nerve hyperintensity.
The median nerve is formed by the medial (motor) and lateral (sensory) cords of the brachial plexus, then crosses superficial to the brachial artery and courses distally along its medial aspect. In the upper arm, the median nerve lies between the medial intermuscular septum and brachialis and then runs distally through the antecubital fossa deep to the lacertus fibrosis. It then passes between the superficial and deep heads of the pronator teres (PT). Less commonly, the median nerve will pass deep to both heads of the PT or through the substance of the superficial head.
The median nerve passes under the fibrous edge of the flexor digitorum superficialis (FDS), traveling in the forearm between the FDS and flexor digitorum profundus (FDP). It gives off four groups of branches in the proximal forearm: branches to PT (proximal, superficial); branches to flexor carpi radialis (FCR) and palmaris longus (PL) (deep, ulnar); branches to FDS (deep, ulnar, more distal); and, the anterior interosseous nerve (AIN), which arises from the radial aspect of the median nerve, approximately 3 cm distal to the intercondylar line, and innervates the FDP to the index and long fingers, the flexor pollicis longus (FPL), and the pronator quadratus.
In the distal forearm, the median nerve becomes more superficial and courses just deep to the FCR and PL tendons. The palmar cutaneous branch (PCM) arises from the volar–radial aspect of the median nerve 5 cm proximal to the wrist flexion crease and may course distally up to 6 mm ulnar to the thenar crease. The PCM usually crosses the wrist as a single branch and then arborizes in the palm superficial to the palmar fascia.
At the wrist the median nerve enters the carpal tunnel along with nine tendons (FDS ×4, FDP ×4, FPL). The carpal tunnel is bordered dorsally by the carpal bones, ulnarly by the hamate and triquetrum, and radially by the scaphoid, trapezium, and FCR sheath. The volar roof of the carpal tunnel is formed by the transverse carpal ligament (TCL), with contributions from the antebrachial fascia proximally and the thenar/hypothenar aponeuroses distally.
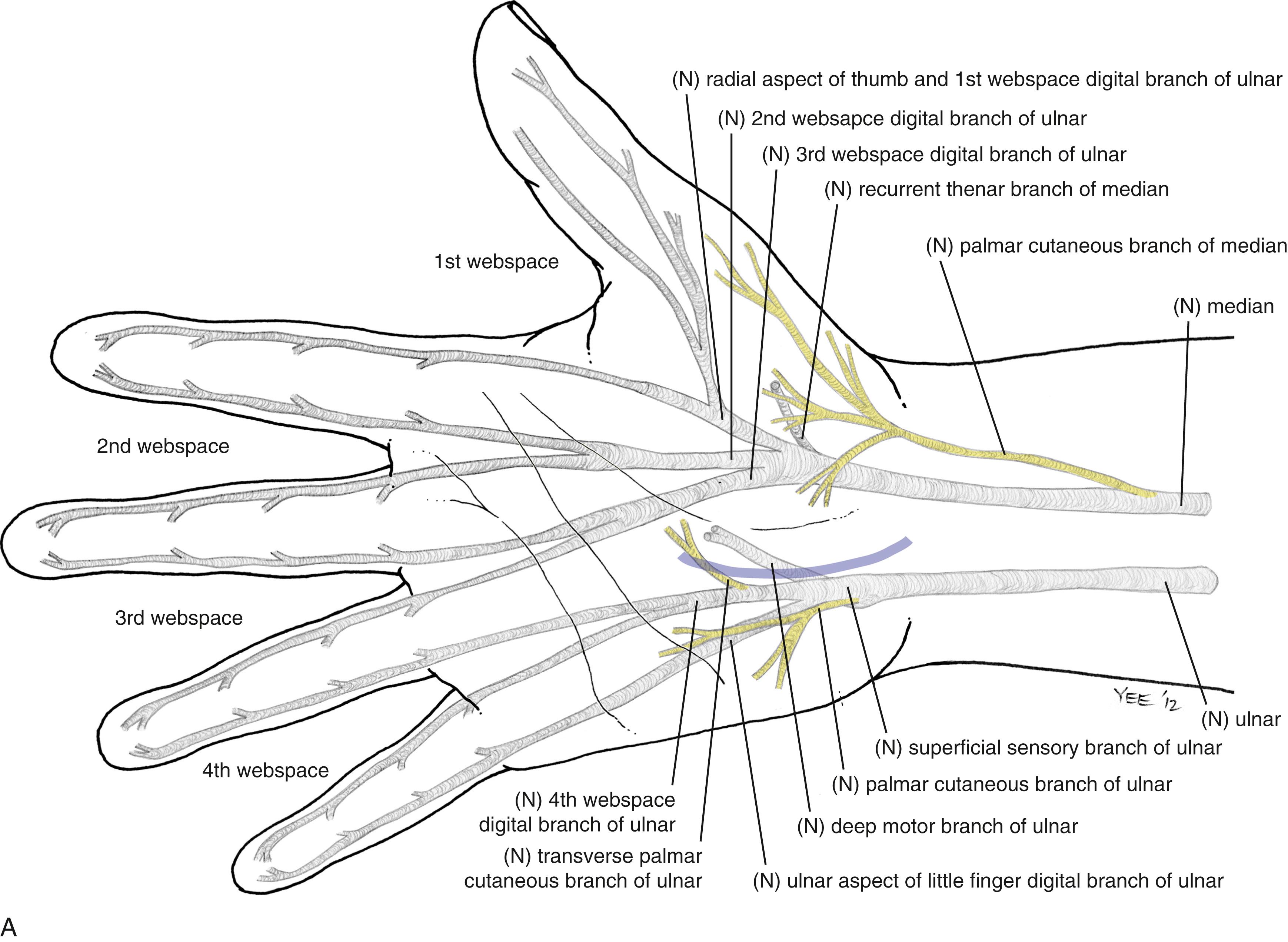
At the distal aspect of the carpal tunnel, the median nerve divides into the common digital nerves to the thumb and radial border of the index finger, and the common digital nerves to the second and third webspaces. The recurrent motor branch typically arises from the radial border of the median nerve just distal to the flexor retinaculum (extraligamentous course), turning back to innervate the abductor pollicis brevis (APB), opponens pollicis, and superficial head of the flexor pollicis brevis. The recurrent branch may also take a subligamentous (31%) or transligamentous course (23%). Other anatomic variations include an accessory median nerve branch that can arise distal or proximal to the carpal tunnel, and a bifid or duplicated median nerve.
The Riche–Cannieu anastomosis is an anomalous motor connection from the ulnar to median nerve in the palm that contributes an average 28% of the innervation to APB. When present, thenar function may be preserved in the setting of severe carpal tunnel syndrome.
There are four main compression syndromes associated with the median nerve: carpal tunnel syndrome (CTS), pronator syndrome, AIN syndrome, and PCM compression.
CTS is the most common compression neuropathy in the upper extremity, with an annual incidence of 0.5–5.1 per 1000 people. The etiology of CTS is thought to relate to compression of the median nerve within the carpal tunnel resulting in impaired microcirculation, subperineurial edema, and eventually fibrosis. Any condition that reduces the volume of the carpal canal or increases the volume of its contents can initiate this process. Several risk factors for the development of CTS have been proposed including: female gender, advanced age, diabetes, thyroid disease, pregnancy, rheumatoid arthritis, obesity, and smoking. Positioning the wrist in flexion or extension during sleep may exacerbate many of these risk factors. Occupational factors such as repetitive hand use and prolonged vibration exposure have also been associated with CTS. Anatomical causes are identified in a minority of cases. Most commonly, this involves a space occupying lesion within the carpal tunnel, but lumbrical incursion into the carpal tunnel can also occur.
CTS classically presents with pain and paresthesiae in the volar aspect of the thumb, index, long, and radial border of the ring fingers. This may progress to constant numbness over time. Thenar sensation is generally preserved since the PCM courses outside the carpal tunnel. Symptoms are often exacerbated by gripping and wrist flexion and are usually worse at night. With severe or long-standing compression, weakness and atrophy of the thenar muscles may occur.
Provocative tests for CTS include the SCT, Tinel’s test, Phalen test (wrist flexion to 90 degrees for up to 60 seconds), the carpal compression test, and the fist test for lumbrical incursion (gentle flexion of the fingers into the palm) (see Table 54.1 ). Clinical tests for CTS tend to have high specificity and moderate sensitivity. ,
Patients often have normal electrodiagnostic studies early in the disease, with symptoms attributed to dynamic ischemia of the median nerve. As the disease progresses, demyelination occurs first in a focal pattern, followed by diffuse demyelination. The majority of patients with carpal tunnel syndrome present with demyelinating disease and respond well to surgical treatment. However, axonal loss may be seen in longstanding disease.
Initial management of mild-to-moderate CTS should be nonoperative and include activity modification, wrist-neutral splints, oral antiinflammatory agents, and/or corticosteroid injection. Wrist-neutral nighttime splinting can significantly improve symptoms, and there is no advantage to wearing splints full time. Corticosteroid injection into the carpal tunnel is effective at relieving CTS symptoms and may also be used for diagnosis and prognosis. The results tend to be short-lived and injection is most useful for patients requiring temporary relief (e.g., pregnancy-related CTS). A Cochrane review of other nonoperative modalities in CTS showed no benefit.
Surgery is indicated when conservative measures fail, and in severe CTS with thenar involvement. Patients with significant weakness and/or dense numbness should be advised that surgical intervention will prevent progression but is unlikely to yield complete recovery. An opponensplasty may be needed to restore thumb opposition in severe cases.
Carpal tunnel release (CTR) can be performed via endoscopic or open techniques. Multiple randomized controlled trials have been performed comparing open versus endoscopic CTR, none of which has demonstrated significant differences in outcomes or complications. The potential advantages of endoscopic CTR include less postoperative pain, which may allow earlier return to work. However, there is a learning curve to the endoscopic approach during which time there is increased risk of injury to the median nerve and a longer operative time. Limited-incision open techniques also carry an increased risk of nerve injury owing to the reduced visualization afforded by the small incision, and have no proven advantage over full-length open CTR. Since even a small injury to the median nerve can be life-altering for the patient, the senior author’s preferred approach remains open CTR with a full-length incision.
The procedure is performed under tourniquet control, with a Bier block or local anesthesia plus sedation. A 3-cm incision is made in the palm, with the proximal extent abutting the wrist crease. In obese patients, the incision is extended proximal to the wrist crease in a zig-zag fashion to allow complete visualization. The incision is placed 5 mm ulnar to the interthenar depression to avoid injury to the PCM and to avoid dividing the TCL directly over the median nerve. The skin is incised, and dissection is carried through the subcutaneous tissue to the palmar aponeurosis, which is divided longitudinally. Care is taken to identify and protect any crossing cutaneous nerve branches, as these may be a source of prolonged “pillar” pain postoperatively. The TCL is identified and sharply divided along its ulnar border. The distal extent of the release is the “V” junction between the hypothenar and thenar muscles. The proximal aspect of the palmar fat pad is also visible at this level. Proximally, the antebrachial fascia is divided under direct vision. Neurolysis of the median nerve, epineurotomy, and tenolysis is not routinely performed. Hemostasis is achieved with bipolar cautery, and the skin closed with nonabsorbable interrupted sutures. A well-padded wrist splint is applied for comfort and removed after 2–3 days. Immediate range of motion (ROM) of the digits and light hand use are encouraged. The wrist is splinted at night for 2–3 weeks postoperatively to prevent bowstringing of the flexor tendons. Sutures are removed at 14 days and a gradual return to full activity is permitted after 5–6 weeks.
Pronator syndrome is a sensory neuropathy resulting from compression of the median nerve in the proximal forearm and is much less common than CTS. Risk factors include female gender, advanced age, and repetitive pronation activities (which can result in a short, tight PT).
There are several potential compression points along the median nerve that can contribute to pronator syndrome. Proximally, there may be a supracondylar process (present in <5%, located 3–6 cm above the medial epicondyle) and ligament of Struthers, a fibrous band between the medial epicondyle and the supracondylar process. When present, the median nerve separates from the brachial vessels and passes posterior to these structures, where it can be compressed. Distally, the lacertus fibrosis, fibrous bands between the superficial and deep heads of the PT, a tendinous deep head of the PT, and the fibrous leading edge of the FDS can also compress the median nerve. Less common causes of compression include an anomalous PT, snapping brachialis, accessory FPL (Gantzer’s muscle), palmaris profundus, and FCR brevis.
Unlike CTS, sensory abnormalities in pronator syndrome include the PCM distribution and the radial three and a half digits. Patients frequently describe pain in the volar forearm, exacerbated by direct pressure and resisted pronation. Less commonly, patients report weakness or difficulty with small object manipulation.
On examination, pain is reproduced by passive supination with direct pressure over the median nerve at the leading edge of PT. The PT may be tender and occasionally a ligament of Struthers may be palpated. A positive Tinel’s sign and SCT may be present. Success in eliciting a positive SCT at this site requires firm pressure over the median nerve, owing to its deep location. Additional provocative maneuvers for pronator syndrome include resisted forearm pronation (PT), resisted elbow flexion with the forearm supinated (lacertus fibrosis), and resisted long finger PIP flexion (FDS).
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here