Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
© 2018 Elsevier Inc. All rights reserved. Please note that the copyright for the original figures submitted by the contributors is owned by Contributors.
Chordomas are rare primary bone tumors with an annual incidence of less than 0.1 per 100,000 individuals. They develop from the remnants of the primitive notochord and have a predilection for the axial skeleton, most commonly the sphenooccipital synchondrosis and sacrum. They grow slowly, rarely metastasize, and occur at any age, with a peak incidence in the fifth decade.
Virchow in 1857 initially described the unique intracellular “bubble-like” vacuoles of chordoma cells, which he termed physaliferous. Chordomas were initially thought to be derived from cartilage, but this hypothesis has since been abandoned as more contemporary evidence has confirmed Ribbert’s hypothesis from the 1890s that these tumors are derived from undifferentiated notochordal remnants within the vertebral bodies and throughout the axial skeleton. Studies of human embryos and fetuses and cell tracking experiments in mice have revealed that notochordal cells topographically distribute to sites where chordomas occur. Although direct evidence is still lacking that these cells transform to chordoma cells, the similarities of their distributions and their histological, molecular, and immunophenotypes suggest that these primitive cells are the substrate for transformation into chordoma tumor cells.
Perhaps the most compelling evidence in support of this notochordal hypothesis is the duplication of the T (brachyury) gene in familial chordoma. Brachyury is an important transcription factor in notochord development and is found to be expressed in rests of normal undifferentiated embryonic notochord in the axial skeleton. High-resolution array comparative genomic hybridization (CGH) of the chordoma genome has revealed unique duplications in the 6q27 region of familial chordomas. The duplicated region contains only the T (brachyury) gene, which is uniquely overexpressed in almost all sporadic chordomas compared with other bone or cartilaginous lesions. Brachyury regulates several stem cell genes and has recently been implicated in promoting epithelial–mesenchymal transition (EMT) in other human carcinomas. Although the specific pathogenetic role of brachyury of chordomas is undefined, T gene duplication and its overexpression suggest that brachyury is a critical molecular driver of tumor initiation and propagation.
Microscopically, chordomas display a lobular architecture in which fibrous bands encapsulate groups of vacuolated (physaliferous) atypical epithelioid neoplastic cells embedded in a myxoid stromal cellular matrix and classically display reactivity for cytokeratin, and a variable expression for S-100 and epithelial membrane antigen (EMA) on immunohistochemistry (IH) panel ( Fig. 3.1 ). Chordomas are classified into four different subtypes based on histological and IH findings: conventional (i.e., classical), representing the majority of cases; chondroid, containing a matrix with cartilaginous differentiation and carrying a better prognosis; dedifferentiated, with highly undifferentiated spindle cells, no immunoreactivity for cytokeratin, and worse prognosis; and sarcomatoid, similar to the dedifferentiated subtype but with cytokeratin reactivity.
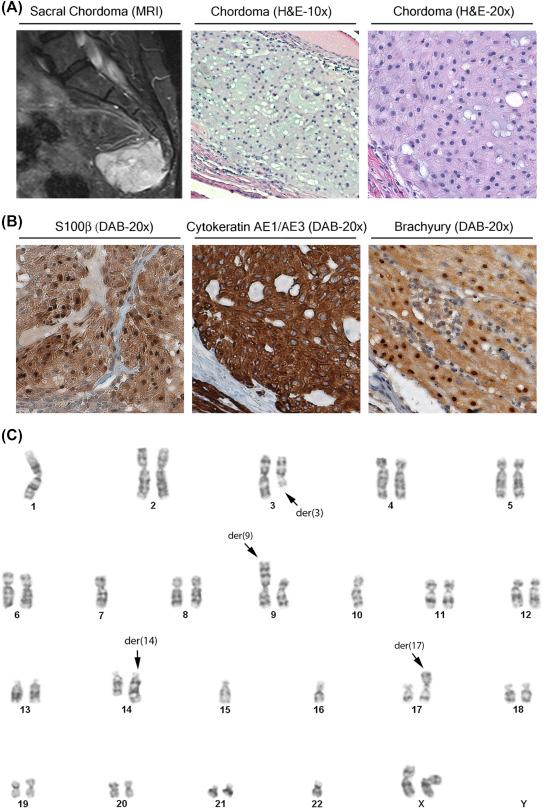
Classically, chordomas were pathologically identified by their physaliferous cells and immunoreactivity for S-100 and epithelial markers such as EMA and cytokeratins. Distinguishing between chondroid chordomas and chondrosarcomas was challenging because they share S-100 immunoreactivity and interpretation of cytokeratin expression on small biopsies is difficult until the discovery that the notochord developmental transcription factor, brachyury, is a discriminating novel biomarker for chordomas. A tissue microarray analysis of 103 skull base/head and neck chondroid tumors found 98% sensitivity and 100% specificity for brachyury and cytokeratin staining in detecting chordoma ( Fig. 3.1 ).
Chordomas may display different karyotypic and chromosomal anomalies depending on their stage (i.e., primary vs. recurrent), histopathology (i.e., classical vs. dedifferentiated), and anatomical site (skull base vs. spine).
Many chordomas have nonrandom multiple copy number alterations in which gains are less frequent than losses. Chromosome 7 polysomy and alterations were seen in 73% of chordomas, in 62% of primary tumors, and in all recurrent chordomas. Gains were most commonly seen for 7q and 20q. Gains of chromosome 7 occurred at loci 7q22, 7q33, 7q34, 7q36, 7p15, and 7p21–p22. These 7q gains correlated significantly with the expression of proto-oncogene tyrosine kinase, cMET. These findings suggest that alteration of chromosome 7 occurs early in chordoma development. Gene duplication of brachyury is the most common single gene anomaly in familial chordoma, and mutations have been found in four different oncogenes (ALK, CTNNB1, NRAS, PIK3CA). 22
Chromosomal losses in chordomas occur on 1p, 3p, 9p, 10q, and 13q ( Fig. 3.1C demonstrates the karyotype of a sacral chordoma; published data ). The loss of heterozygosity of tumor suppressor genes and overexpression and/or mutation of oncogenes may contribute to the genesis of chordomas. Mutations occur in tumor suppressor genes located in areas of frequent chromosomal loss and in oncogenes encoding molecular inhibitors that might be of therapeutic interest. Analysis of 865 hotspots for mutation in 111 oncogenes and selected tumor suppressor genes in chordomas identified mutations in ALK (A877S), CTNNB1 (T41A), NRAS (Q61R), PIK3CA (E545K), PTEN (R130), CDKN2A (R58∗), and SMARCB1 (R40∗). Of note, PTEN and CDKN2A lie in chromosomal regions found to have losses in CGH analysis (9p21 and 10q23, respectively), and 40% of mutations occurred in SMARCB1 and its companion chromatin regulatory genes.
SMARCB1 is part of the SWI/SNF chromatin remodeling complex, which controls chromatin compaction and gene expression. The absence of SMARCB1 expression, attributable to SMARCB1/INI1 gene deletions and a feature of rhabdoid tumors, is particularly characteristic of pediatric chordomas, which are poorly differentiated, highly aggressive malignancies with a poor prognosis.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here