Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Areas of fibrosis that are common to many cardiac disease states predispose to reentry. Dense fibrosis can create areas of fixed conduction block that define parts of a reentry path (see Fig. 129.2A ). Alternatively, reentry circuits can be defined by functional conduction block or collision of wavefronts in regions where conduction is slowed (see Fig. 129.2B ). Both mechanisms of conduction block may occur within a single circuit. Separation of myocyte bundles by interstitial fibrosis causes circuitous propagation, resulting in slow conduction across an area, even though propagation velocity along an individual myocyte bundle may be relatively normal. , Asynchronous activation of myocyte bundles produces multicomponent electrograms (EGMs) described as split potentials or fractionated potentials (see Chapter 127 ). The geometry of reentry pathways also influences susceptibility to unidirectional conduction block, which enables initiation of reentry.
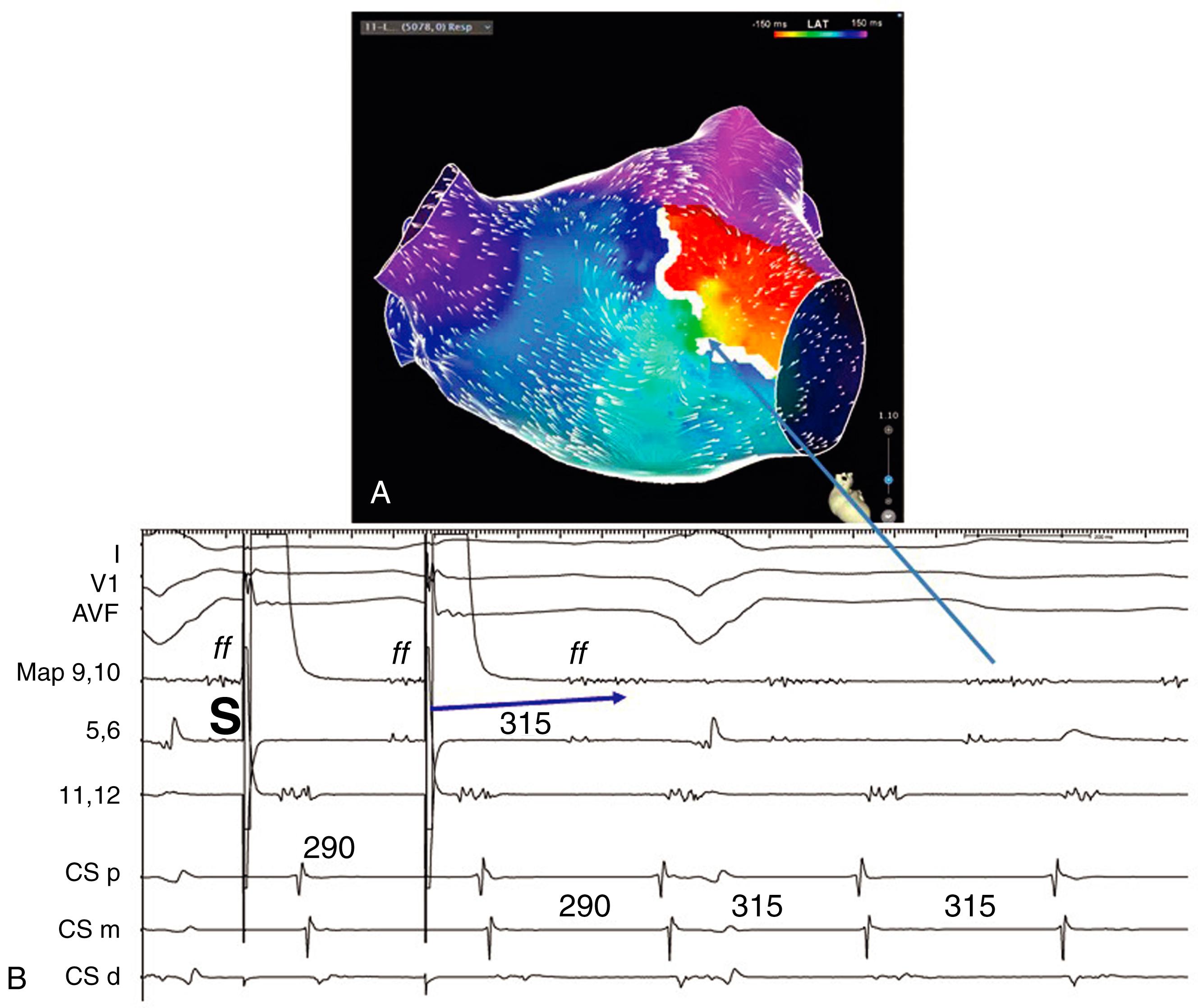
A variety of scar-related reentry circuit configurations have been described. Throughout the cardiac cycle, some part of the circuit is activated. Recording from a small circuit within the view of a single bipolar pair of electrodes would then be expected to define the entire circuit or show continuous activity, and such small circuits have been described in diseased atria ( Fig. 129.1 ). , , More commonly, long fractionated EGMs are due to slow conduction across adjacent fiber bundles in a region that can be in a reentry circuit or in a bystander area. Scar-related reentry circuits are often large, extending over several centimeters or completely around the circumference of a chamber, as with perimitral atrial flutter. A figure-of-eight type of circuit has been commonly described in experimental infarct models and is seen in humans ( Figs. 129.2 and 129.3 ). , This circuit has a common isthmus or channel. Propagation through the isthmus, which is within a region of scar, typically does not generate sufficient signal to contribute to the surface electrocardiogram (ECG). The QRS or P wave is inscribed when a larger mass of myocardium is activated as the wavefront emerges from the exit region and propagates away from the scar across the chamber. From the exit, the activation wave propagates through one or more loops to return to the channel. Outer loops are reentry paths along the border of the scar. Inner loops are reentry paths through the scar. All sites outside the reentry circuits are bystanders. Activation maps traditionally use the QRS onset or P wave (when definable) for a timing reference. If the exit is at the border of the scar, activation precedes the QRS (presystolic activation). Activation becomes progressively earlier at the central and proximal parts of the isthmus. The loops are typically activated during the QRS. Occasionally a reentry circuit is within a scar with the exit several centimeters from the scar border region, such that activation time at the exit is substantially earlier than the QRS onset. Multiloop and single loop types of reentry occur (see Figs. 129.1 to 129.3 ).
The unipolar EGM morphology can be particularly helpful for focal tachycardias in relatively normal myocardium, provided that high-pass filtering is kept to less than 5 Hz , (see Chapter 127 ). At a focal site of early activation, a monophasic S wave is expected. These recordings have a wide field of view, with substantial contribution of depolarization remote from the recording site. In areas of scar, the signal from depolarization of small bands of myocardium can be obscured by the far-field signal. Bipolar recordings essentially subtract the signal occurring at the same time on each electrode, removing much of the far-field component and making detection of low-amplitude signals in regions of scar easier. The signal of interest, however, may emerge from beneath either of the electrodes. The field of view of bipolar recordings is determined by electrode size and spacing. Reducing electrode size and spacing reduces the field of view (see Chapter 127 ).
Activation sequence mapping requires careful attention to defining the mapping window and annotation of activation (see Chapter 128 ). Difficulty identifying local activation from fractionated multicomponent EGMs is a common source of error. High-density mapping systems incorporate methods to synthesize and interpret large amounts of rapidly acquired data that have improved activation sequence mapping (see Chapters 127 and 128 ). For complex signals, surrounding activation can be considered to help define activation time. , , Alternatively, the entire time away from baseline for each site can be displayed, creating a visual display of activation without attempting to select a specific activation time. ,
Activation can suggest the components of the reentry circuit, particularly if an entire reentry circuit can be defined ( Fig 129.4 ; see also Figs. 129.1 to 129.3 ). , Commonly, however, only parts of the circuit are detectable. Intramural portions of the circuits may escape detection, or the signals from tiny fibers may be less than the noise level of the recording system. The activation time alone for a single site is not a reliable indication of whether a site is in a reentry circuit. Bystanders in the scar may have fractionated EGMs and be activated during electrical diastole, mimicking reentry circuit sites. In diseased atria, focal tachycardias or small reentry circuits arising adjacent to a region of block can mimic a macroreentry sequence (see Fig. 129.1 ). , In some tachycardias, apparent macroreentry loops are, in fact, bystanders (see Fig. 129.1 ). In some scarred atria, the conduction time through the atria exceeds the tachycardia cycle length (TCL), making interpretation of the activation sequence difficult. , Combining activation mapping with pacing maneuvers can be particularly useful when dealing with complexities (see Fig. 129.1 ).
Pacing to entrain tachycardia can distinguish reentry circuit sites and bystanders and is complementary to activation sequence mapping. There are several limitations. Tachycardia must have a stable cycle length and persist sufficiently long to allow pacing. When ventricular tachycardia (VT) is not tolerated, extensive entrainment mapping is not possible, but selective interrogation of one or a few sites can be helpful, particularly when a substrate-guided ablation approach fails or the ablation target is in a high-risk area, such as adjacent to the conduction system or a coronary artery. Undesired termination or change in the tachycardia can occur, although this can be minimized by paying attention to pacing initiation synchronization and cycle length. ,
Entrainment is continuous resetting of a reentry circuit. The mechanism is shown in Fig. 129.5 . During pacing a stimulated wavefront propagates to the reentry circuit and splits into two components. An antidromic component propagates in the reverse direction in the circuit and collides with a returning orthodromic wavefront. The stimulated orthodromic wavefront propagates through the circuit in the same direction as the tachycardia wavefronts and resets the tachycardia. After a few beats the location of collision of stimulated antidromic and orthodromic wavefronts stabilize, producing constant fusion, with part of the chamber activated by the antidromic wavefront and part activated by the orthodromic wavefront. A constant degree of fusion is a hallmark of entrainment, as defined by Waldo et al. During pacing, all EGMs and QRS complexes or P waves are accelerated to the pacing cycle length, followed by resumption of tachycardia on termination of pacing. The presence of constant fusion establishes the presence of entrainment rather than overdrive suppression of an automatic focus (see Fig. 129.5 ). Evidence of fusion can also be assessed from EGMs, which is particularly useful for atrial arrhythmias ( Fig. 129.6 ). During entrainment, sites that are activated by the orthodromic wavefront have the same EGM morphology during pacing as during tachycardia and typically follow the pacing stimulus with a relatively long interval consistent with propagation of the stimulated wavefront through the reentry circuit (see Fig. 129.6 ). Sites activated by antidromic wavefronts have a different morphology during pacing than during tachycardia. Antidromic and orthodromic activation is evidence of fusion.
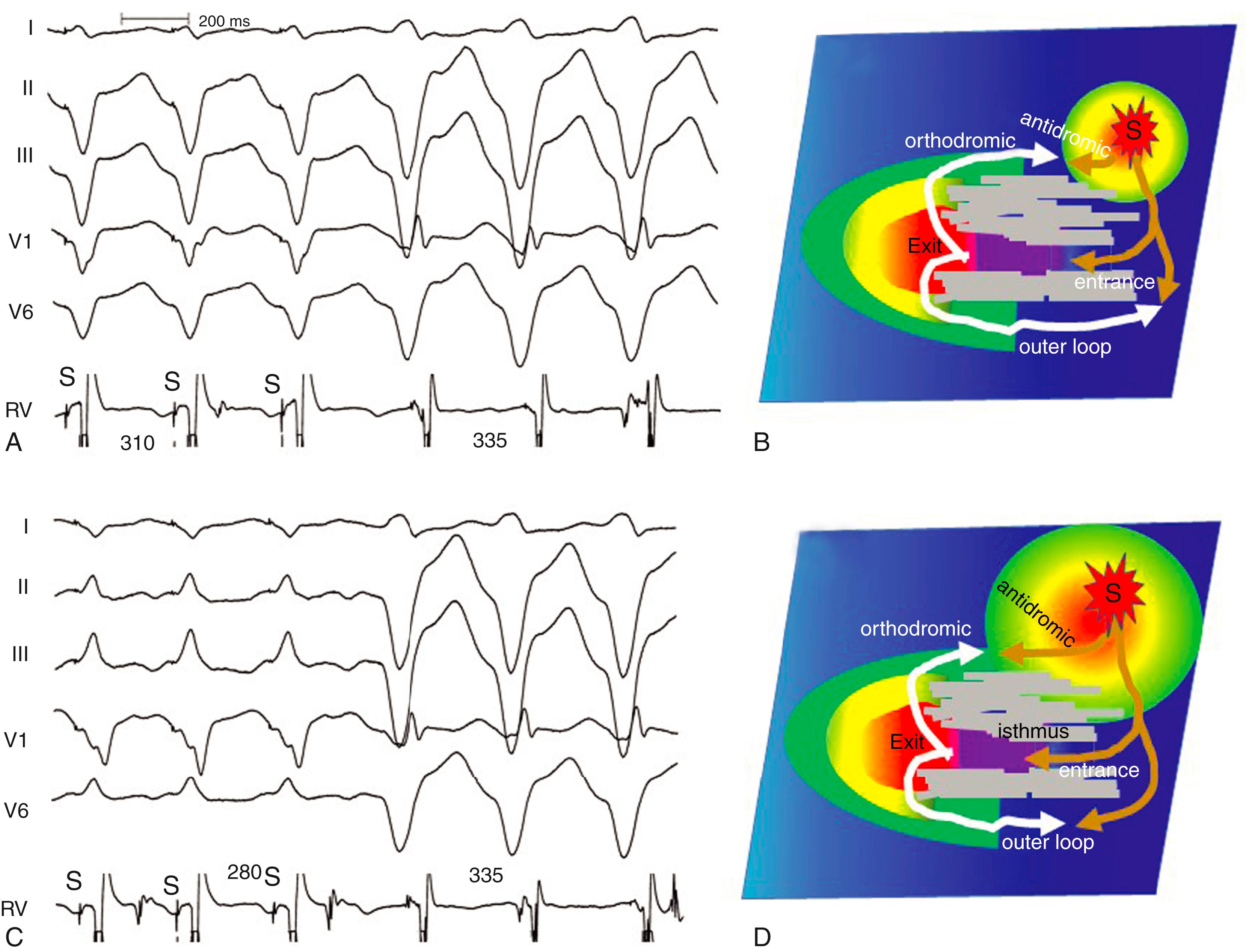
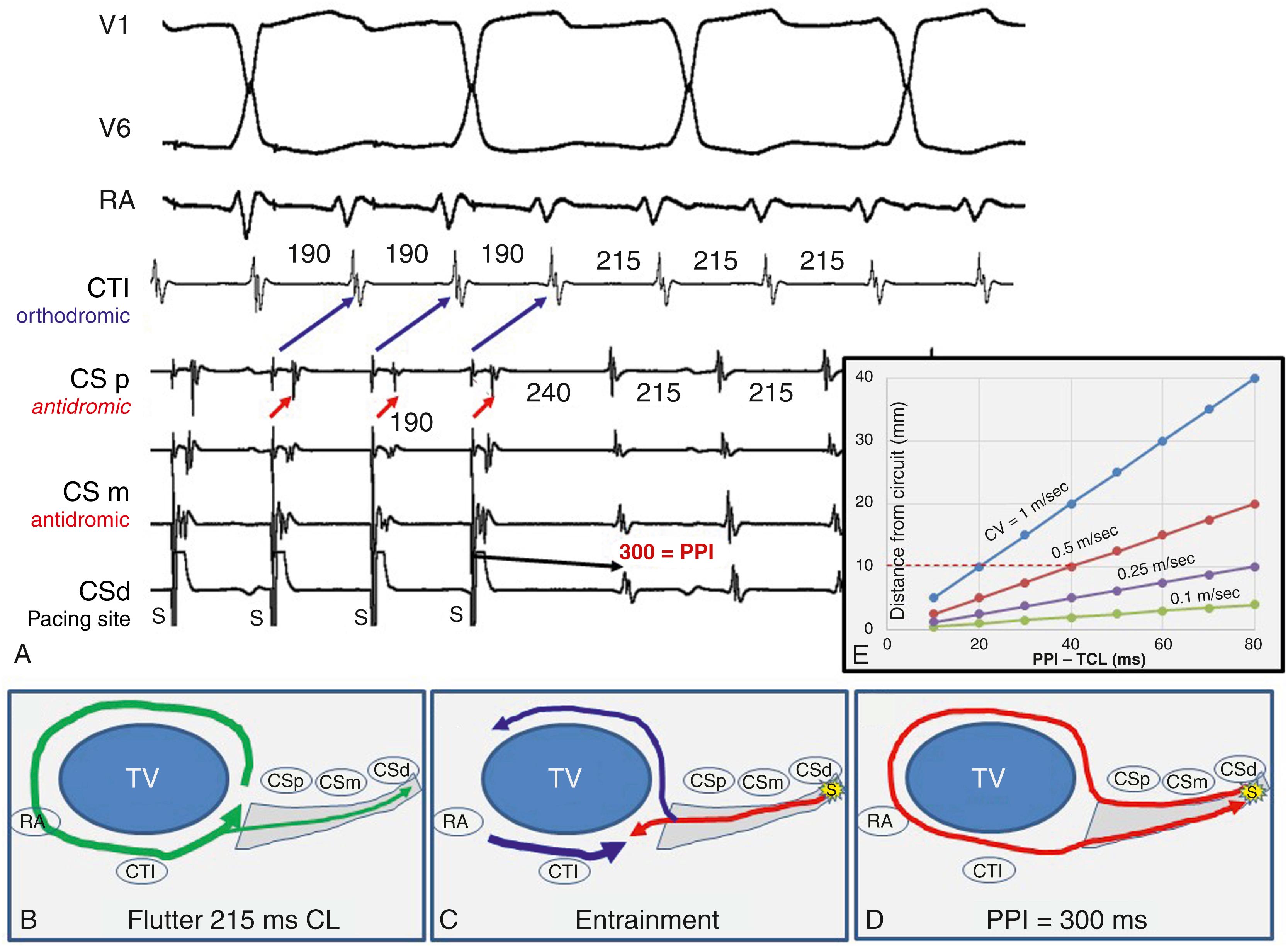
The postpacing interval (PPI) is an indicator of the conduction time between the pacing site and the reentry circuit. It is defined as the time from the last stimulus that entrains tachycardia to the next activation at the pacing site. At reentry circuit sites it approximates the VT cycle length and lengthens as the conduction time between the pacing site and reentry circuit increases ( Fig. 129.7 ; see also Fig. 129.6 ). In Fig. 129.4 , pacing outside the reentry circuit, the wavefront from the last paced stimulus propagates to the reentry circuit and its orthodromic component travels through the circuit and back to the pacing site. The revolution time through the circuit is the TCL, and hence the rest of the PPI is because of the conduction time from the pacing site to the reentry circuit and then from the circuit back to the pacing site. The relation between the PPI and distance between the pacing site and reentry circuit is also related to the conduction velocity between the pacing site and the circuit. If the conduction velocity is rapid, the distance between the pacing site and circuit can be relatively large for a small PPI-TCL difference, compared with when conduction velocity is slower (see Fig. 129.6E ). For scar-related VT, a PPI-TCL difference of 30 ms or less was associated with termination of VT by radiofrequency (RF) at that site.
There are three major assumptions to consider when interpreting the PPI. The first assumption is that pacing captures reliably and is entraining the tachycardia. The second is that the TCL is stable and conduction through the circuit does not change during pacing. This second assumption is often violated. In 18% of common cavotricuspid isthmus (CTI)-dependent flutters, the PPI in the CTI exceeds the tachycardia cycle length by more than 30 ms because of an increase in conduction time with pacing, which could be because of conduction slowing or an increase in the reentry path if an area of function block occurs during pacing. An increase in conduction time should be suspected if the TCL oscillates or is unstable after pacing and is consistent with decremental conduction properties in the circuit, often associated with antiarrhythmic medications.
The third assumption is that EGM selected for measurement is because of activation at the pacing site. At multicomponent EGM sites, some far-field EGMs can be recognized because they remain visible during pacing ( Figs. 129.8 and 129.9 ). These can be excluded from PPI measurement. It may still not be possible to identify which of the remaining signals reflect local activation. In some cases the pacing stimulus artifact obscures EGMs. An EGM visible on the proximal electrode recordings that matches the distal electrode recordings can be used. If the pacing artifact decays quickly, two TCLs can be used for measurement. The S – N+1 method can also be used in which the stimulus to QRS or EGM of the second beat after the last stimulus is measured. It is possible that there could be some noncaptured far-field potentials in close proximity to the pacing site that are not captured because they are refractory or that could be captured at greater stimulus strength, but this issue has not been well studied.
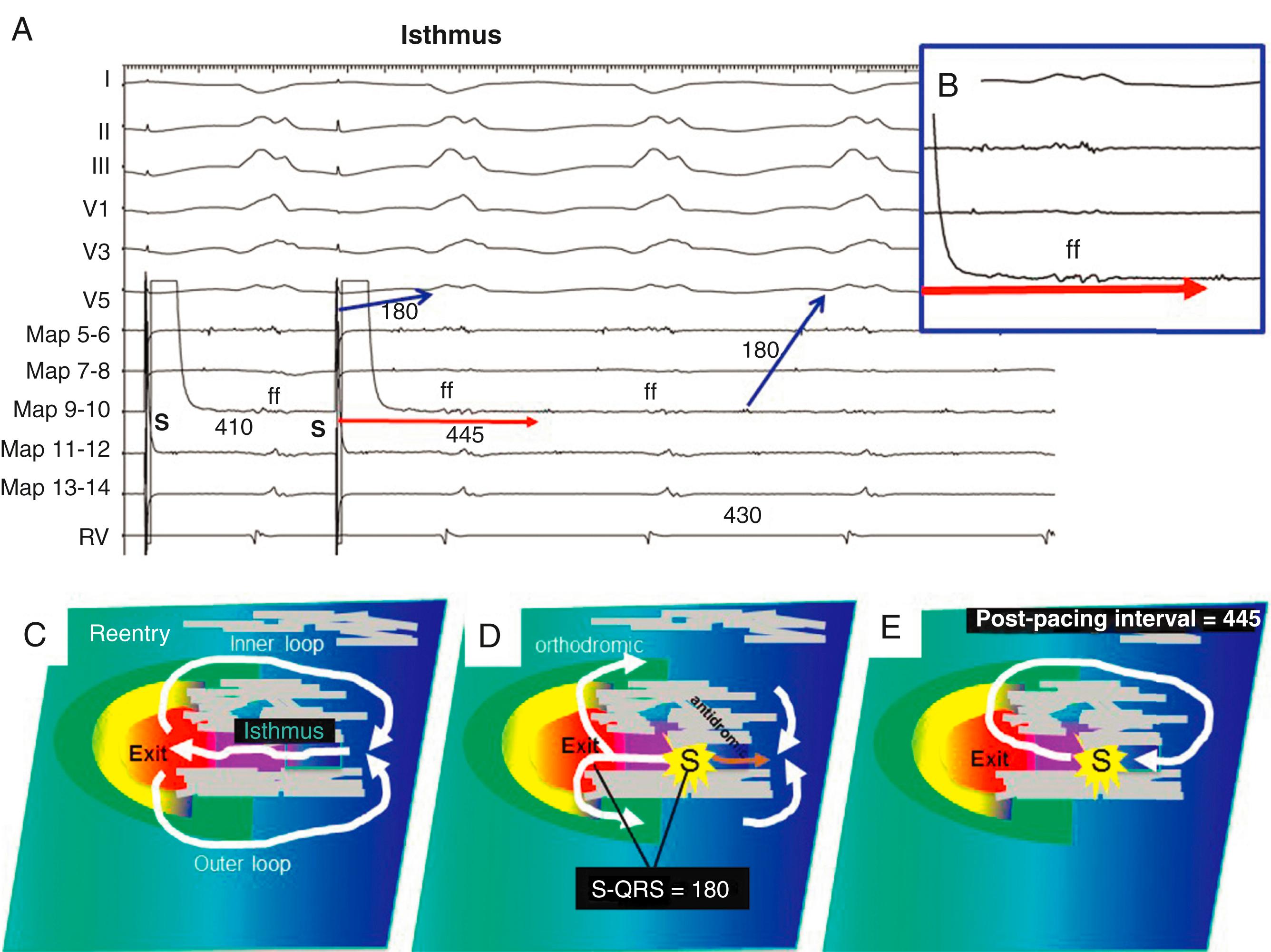
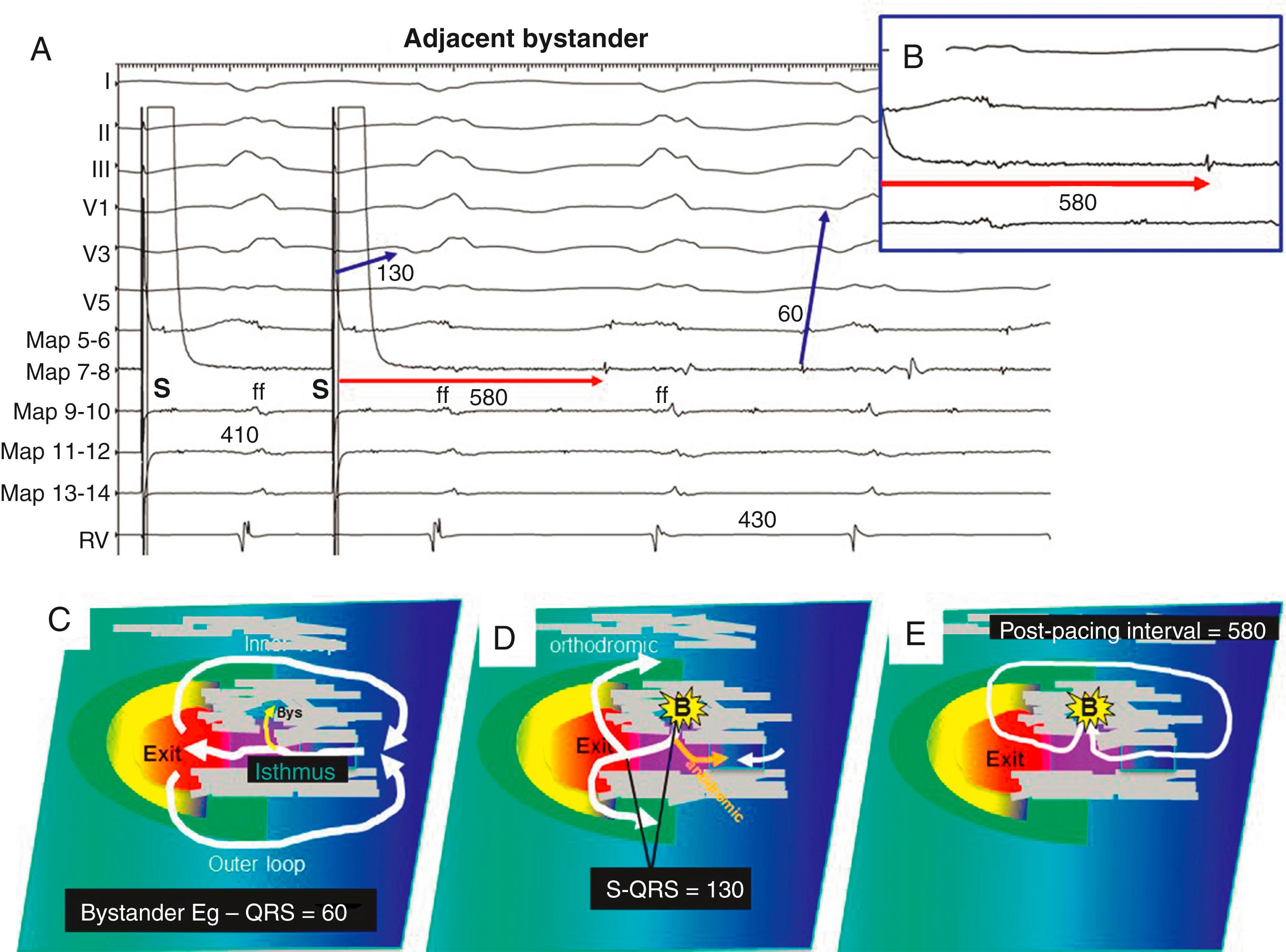
A PPI shorter than the TCL is usually because of measurement to a far-field EGM. High output pacing that captures tissue at a distance from the pacing site can also falsely shorten the PPI.
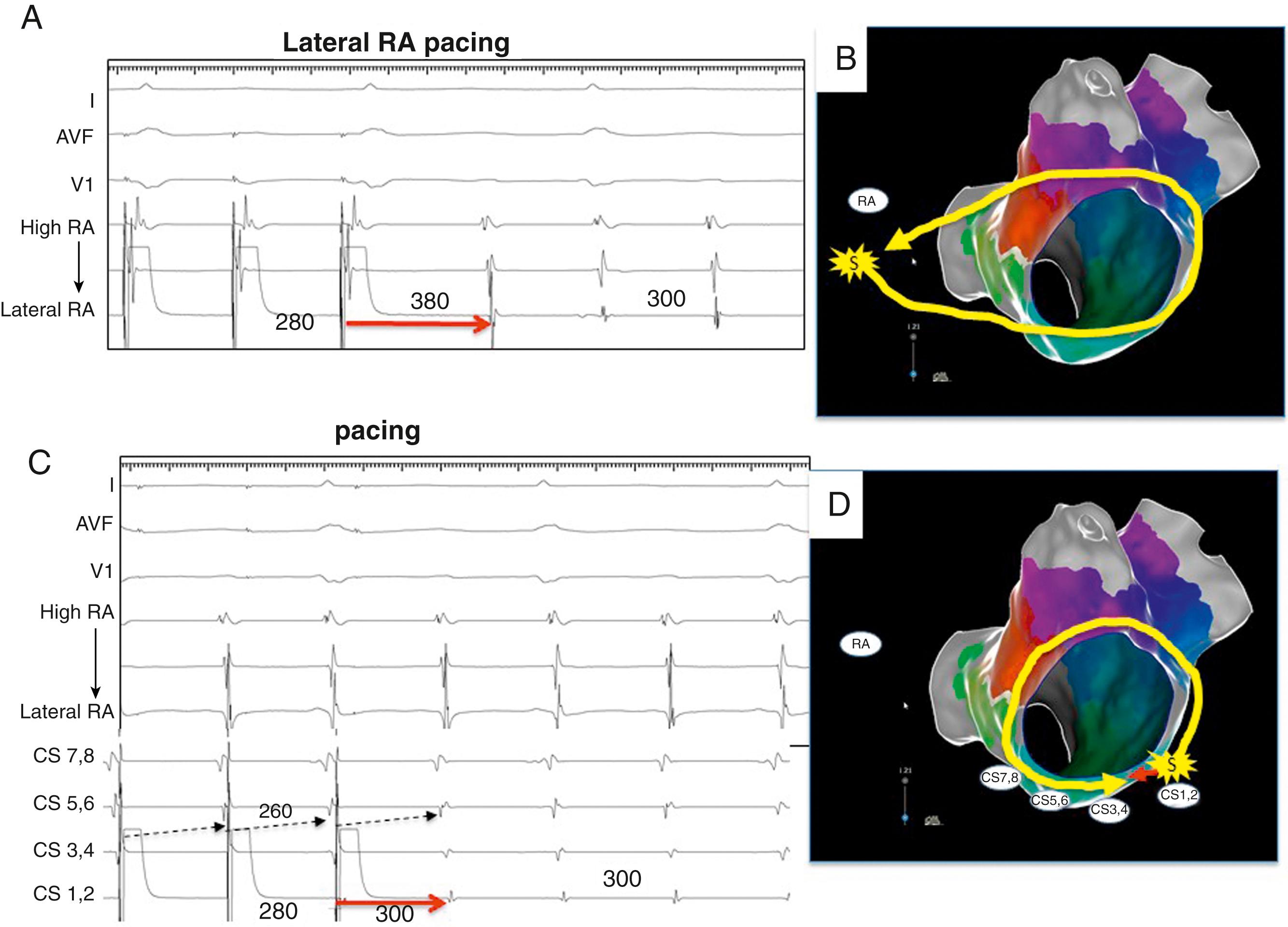
The PPI approximates the TCL at reentry circuit loops and at isthmus sites ( Fig. 129.10 ; see also Fig. 129.2 ). To detect an isthmus from entrainment requires analysis of fusion ( Fig. 129.11 ; see also Figs. 129.8 to 129.10 ). When pacing from a reentry circuit isthmus, the antidromic stimulated wavefronts are contained in or near the reentry circuit by lines of block or collision with returning orthodromic wavefronts (see Fig. 129.8 ). The stimulated orthodromic wavefront emerges from the reentry circuit following the same path as the tachycardia wavefronts. Thus the QRS or P wave is identical during pacing to that during tachycardia. In outer loops, the PPI indicates that the pacing site is in the circuit, but pacing produces QRS fusion because of propagation of the antidromic wavefront away from the pacing site (see Fig. 129.10A ). This typically occurs with a short or minimal S-QRS interval because these sites are usually in the border of the scar. In outer loops near the exit region, QRS fusion may be minimal. Increasing the pacing rate can increase the degree of fusion, making it more obvious.
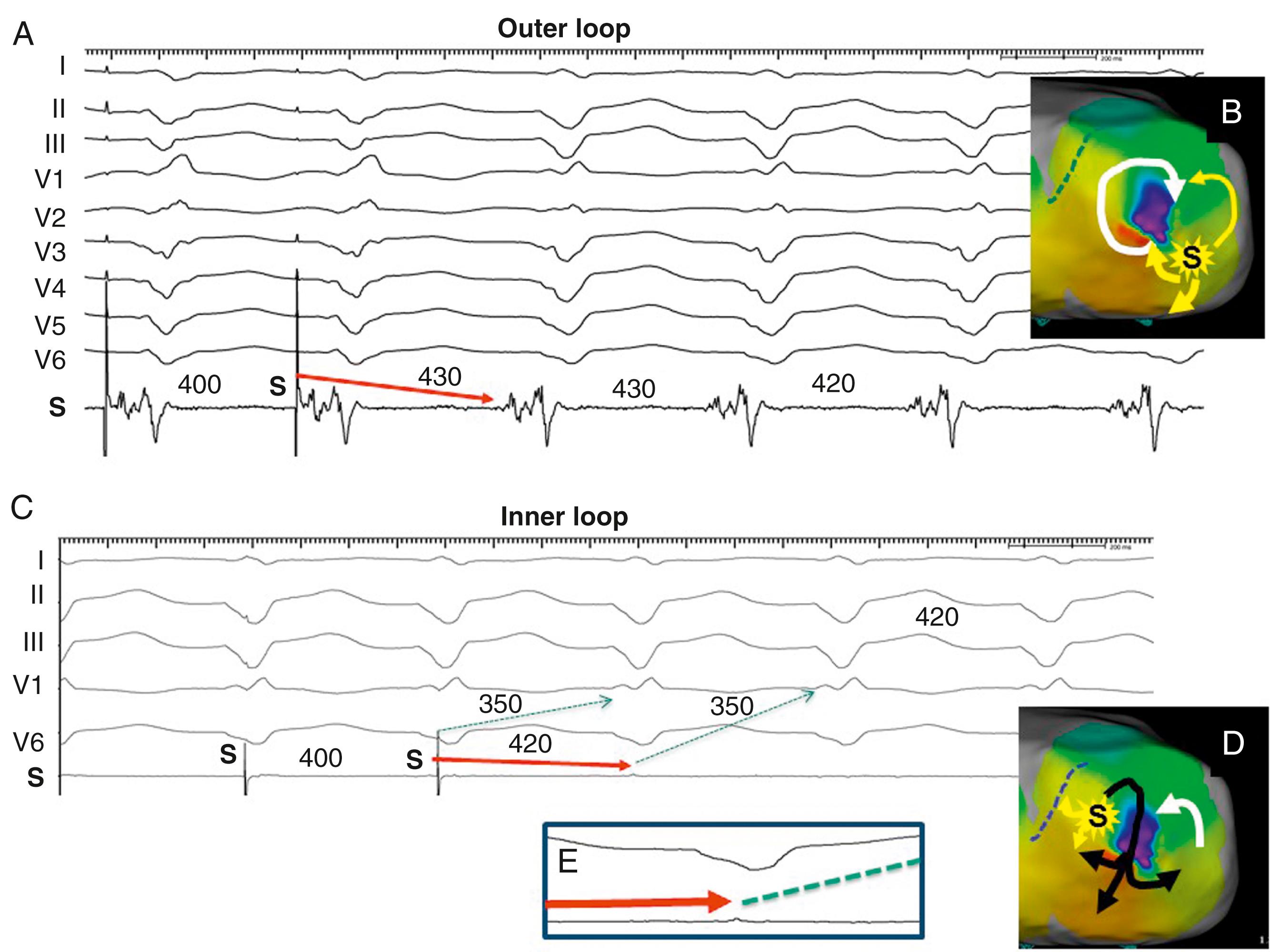
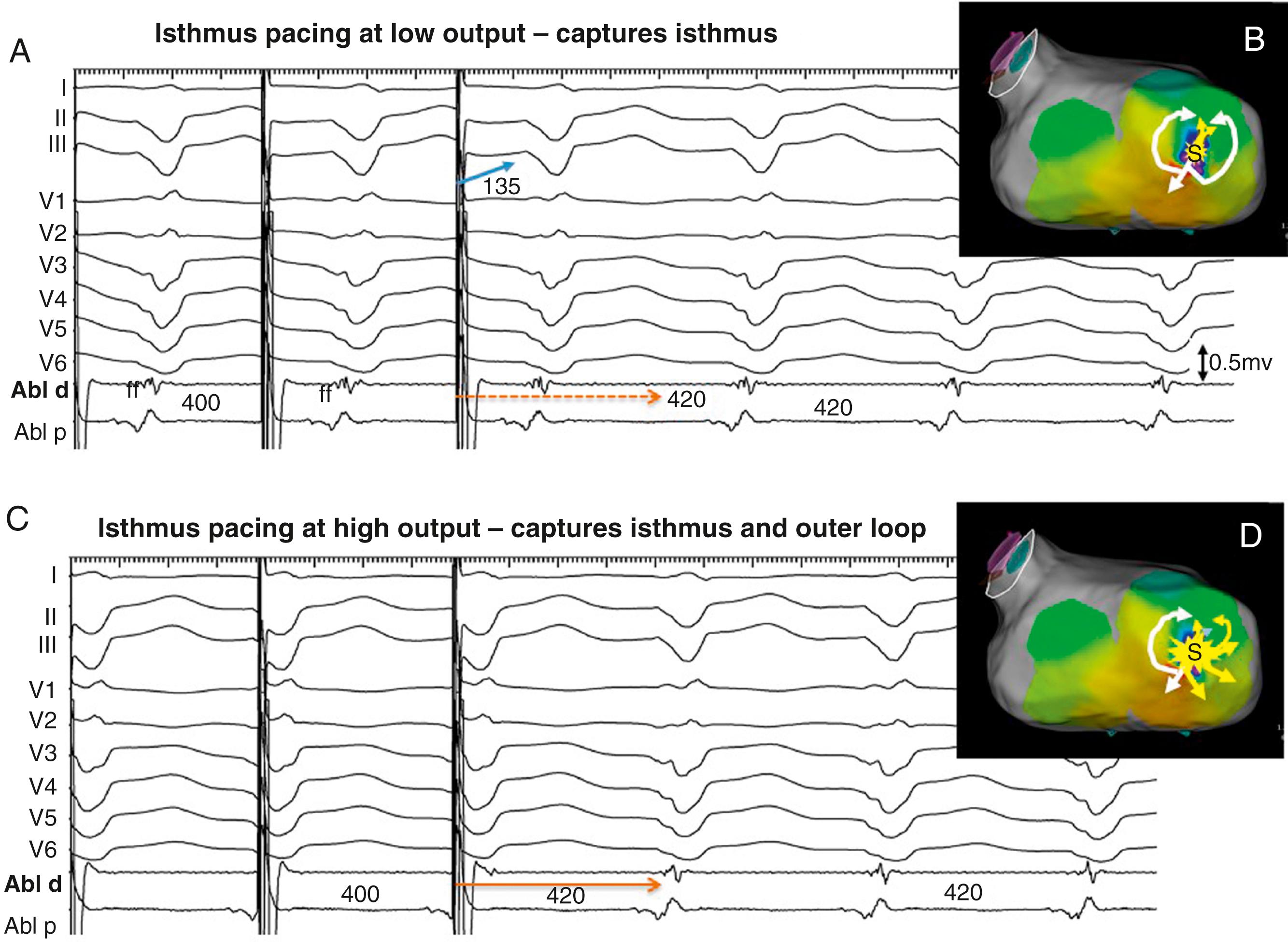
During entrainment with concealed fusion, the S-QRS interval indicates the conduction time from the pacing site to the reentry circuit exit (see Fig. 129.8 ). Although the relation of the S-QRS to the anatomic location in the circuit is not certain, a classification for VT based on likely VT termination by ablation has been suggested (see Fig. 129.2 ). At sites near the exit, the S-QRS is relatively short (<30% of the VT cycle length). Reentry circuit sites with S-QRS intervals between 30% and 70% of the VT cycle length are defined as central or proximal isthmus sites. Sites with very long S-QRS intervals (>70%) of the VT cycle length are likely in an inner loop (see Fig. 129.10C ), outside the isthmus.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here