Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
As the population ages and indications for implantation of cardiac implantable electronic devices (CIEDs) expand, increasing numbers of CIEDs are implanted with some data suggesting the rate of CIED infection is outpacing the rate of implant. CIED infection carries a significant risk for morbidity and mortality, and CIED-related endocarditis raises this risk even further. This chapter will review the pathogenesis, management, and prevention of CIED infections with special attention paid to lead and device extraction, emerging therapies, and recent data.
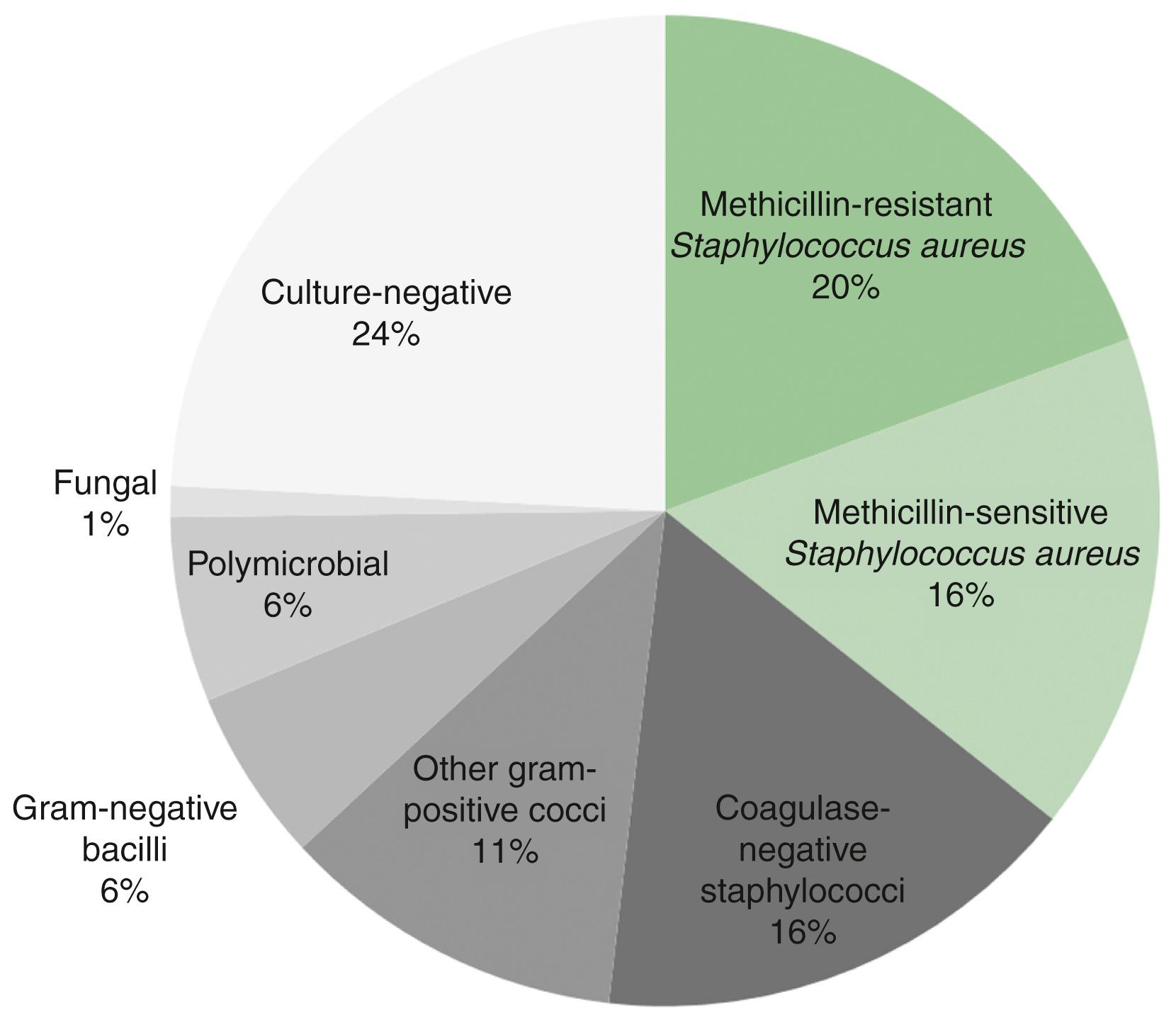
CIED infection can have many different sources, although most cases of pocket infection are likely acquired at the time of CIED implant or generator change. It can also arise because of hematogenous seeding of the CIED components from another source. Although bacteremia can be caused by a vast array of bacterial species, the majority of CIED infections are caused by Staphylococcus species ( Fig. 126.1 ). In contrast, gram-negative bacilli, such as Escherichia coli, are much less likely to lead to CIED infection (9% of all CIED infections), despite the fact that these pathogens are relatively frequent causes of human bacteremia in general. , The reason for this apparent disparity seems to be caused by the proclivity of Staphylococcus species to adhere to foreign materials and form biofilms, which protect from host defenses and antibiotics. , Polymicrobial infections and non- Staphylococcus gram-positive infections each constitute less than 10% of all CIED infections. Fungal species, most often Candida , constitute approximately 1% to 2% of CIED infections. , Although most patients never develop a CIED infection, microbiological studies have found bacterial DNA in noninfected device pockets or on noninfected CIED surfaces in 38.5% of 78 patients who underwent screening at the time of CIED replacement or upgrade. Although 30% of these were coagulase-negative Staphylococcus species, only one (3.3%) was S. aureus, and the remainder constituted a vast array of both gram-positive and gram-negative organisms. During follow-up, two patients (6.7%) became symptomatic with the same species of microorganism (including the one patient with S. aureus and one with coagulase-negative Staphylococcus ).
CIED infection seems to be increasing over time, and the proportion of lead extractions performed for infection has increased as well. It has been postulated that this may be caused by expanding indications into older and sicker patient populations. However, it may also be possible that as CIED extraction procedures and advanced imaging become more commonplace, the likelihood that a given episode of bacteremia is treated as a CIED infection increases.
The risk of CIED infection within 1 year of a de novo pacemaker implant is estimated at 0.5% to 1.0% with most infections occurring within the first 60 days. Infection rates after generator change seem to be significantly greater, estimated at 1% to 7% with rates varying across studies and predicted by several risk factors. , Using data from the recent Prevention of Arrhythmia Device Infection Trial (PADIT), five independent predictors of device infection were retrospectively identified: number of prior procedures, younger age, renal dysfunction, immunocompromised state, and procedure type (increased risk with implantable cardioverter-defibrillator [ICD] vs. pacemaker, cardiac resynchronization therapy [CRT], and revision/upgrade). The resultant PADIT score stratified patients into three CIED infection risk groups: low (0.51%), moderate (1.42%), and high (3.41%). Additional studies have found that hematoma, early reintervention, heart failure, temporary pacing, preoperative infection, central venous catheters, and chronic obstructive pulmonary disease also predict postoperative CIED infection. , ,
Expedient recognition of CIED infection can be essential to providing the correct treatment and to avoid delaying definitive therapy. CIED infection can have a highly variable presentation depending on the location of the infection, the virulence of the pathogen, and the host defenses. The 2017 Heart Rhythm Society Consensus Statement for Lead Management and Extraction describes 10 definitions of CIED-related infection based on the presence of pocket infection, bacteremia, and CIED/valvular involvement ( Table 126.1 ).
| Infection Type | Description |
|---|---|
| Isolated generator pocket infection | Erythema, swelling, pain, tenderness, warmth, or drainage occurring at the pocket with negative blood cultures |
| Isolated pocket erosion | Protrusion of the generator or leads through the skin |
| Bacteremia | Positive blood cultures, regardless of signs of systemic infection |
| Pocket site infection with bacteremia | Signs of generator pocket infection accompanied by bacteremia |
| Lead infection | Lead vegetation with bacteremia |
| Pocket site infection with lead/valvular endocarditis | Signs of generator pocket infection accompanied by bacteremia and lead or valvular vegetation |
| CIED endocarditis without pocket infection | Bacteremia and lead or valvular vegetation without signs of generator pocket infection |
| Occult bacteremia with probable CIED infection | Bacteremia that resolves after CIED extraction with no other source present |
| Situations in which CIED infection is not certain | Impending or threatened device erosion, left-sided valvular endocarditis |
| Superficial incisional infection | Infection of the skin and subcutaneous tissue but not the deep soft tissues |
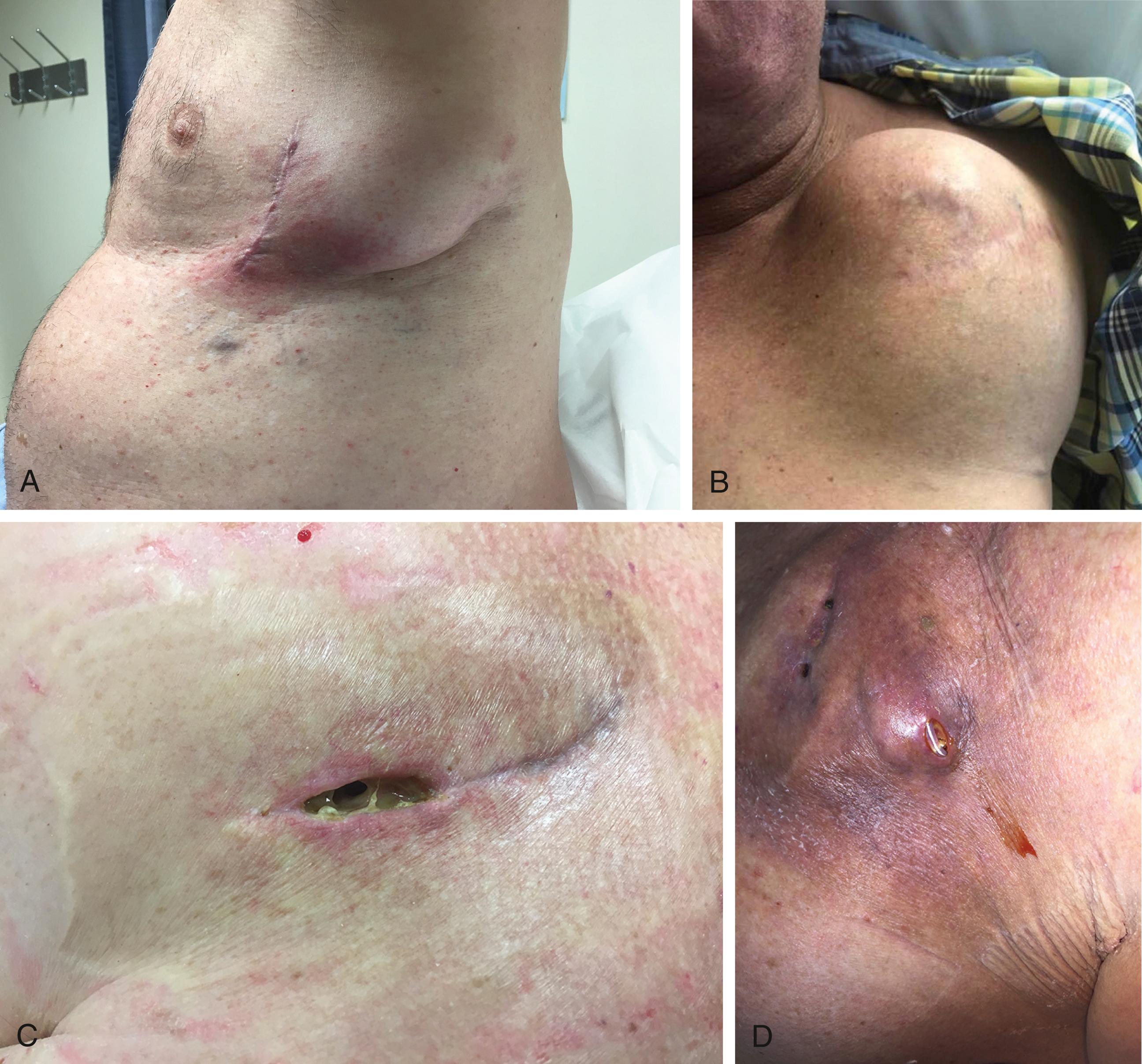
Localized pocket infection can present in a variety of ways, and diagnosis is more complex in the setting of recent pocket instrumentation. Clinical signs of pocket site infection include erythema (41%), swelling (38%), drainage (38%), pain/tenderness (28%), device exposure (21%), and warmth (18%) ( Fig. 126.2 ). Postoperative hematoma in the absence of infection may be associated with swelling, pain, and occasionally bloody drainage, although these signs and symptoms typically improve steadily in the absence of infection. Thinning of tissue caused by fat necrosis and skin adherence to the device suggest infection and portend impending erosion. , If left untreated, CIED components will often erode and protrude out through the skin. Most CIED erosions are caused by infection, and regardless, once a CIED is exposed to the external environment and cutaneous bacteria, the device should be considered infected even if the erosion was from another cause (inadequate suture closure, trauma, etc.).
Because most CIEDs include transvenous components, the presence of bacteremia (defined as positive blood cultures not caused by a contaminant) is an important consideration in CIED infection. Bacteremia can exist without CIED involvement, although, as mentioned earlier, certain bacteria, when found in the bloodstream are more likely to lead to, or arise from, CIED infection. , CIED lead infection is defined as a lead vegetation, typically visualized using echocardiography, in the setting of bacteremia. However, not all material adherent to CIED leads is infectious, as discussed later, so the same finding with no clinical signs of infection usually does not constitute a lead infection. , CIED endocarditis is defined more broadly as a lead or valvular vegetation with bacteremia in the presence of a CIED. Pocket infection can occur with or without bacteremia and with or without CIED endocarditis. Similarly, CIED endocarditis can occur with or without a concomitant pocket infection.
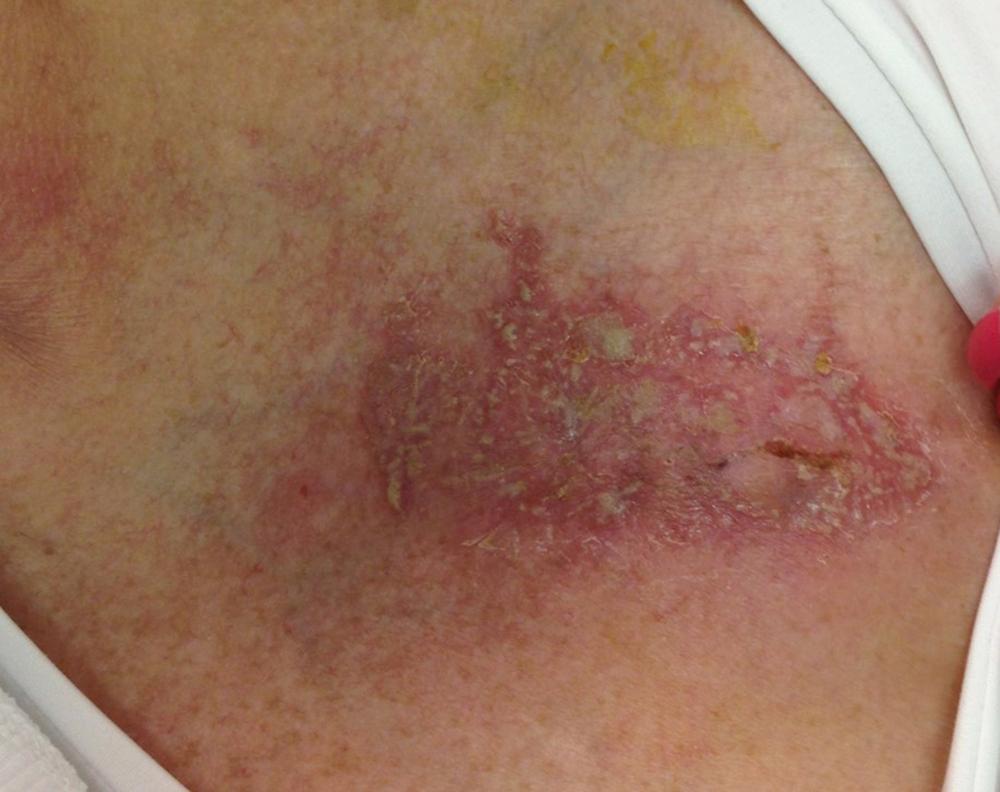
The diagnosis of pocket infection is often made based on history and physical exam. Superficial erythema at the skin site immediately after implant is commonly seen because of intradermal hemorrhage, local inflammation, or occasionally, skin allergy ( Fig. 126.3 ). Additionally, superficial stitch abscesses may arise days to weeks after implant and typically do not progress to pocket infection. , However, if increasing swelling, purulent drainage, wound dehiscence, or frank or threatened erosion is present, pocket infection is diagnosed. Aspiration of the CIED pocket should not be performed to assist in the diagnosis of pocket infection because of the risk of introducing microorganisms into the pocket. Once pocket infection has been diagnosed and a decision to extract the system has been made, it may be reasonable to aspirate the pocket prior to the initiation of antibiotics to obtain culture and sensitivity, assuming CIED extraction is planned in the near term. However, diagnostic yield tends to be low. Any time there is concern for CIED infection, at least two sets of blood cultures should be drawn prior to the initiation of antibiotics. At the time of CIED removal, device pocket swabs and tissue should be sent for cultures and Gram stain; tissue is higher yield than pocket swab. It is recommended that the lead tip also be sent for culture, keeping in mind that the lead could be contaminated when extracted through the pocket.
CIED endocarditis in the absence of pocket infection may be more easily missed because of the nonspecific symptoms of malaise, fever, chills, weight loss, and other infectious manifestations. In addition, even if bacteremia is diagnosed, CIED infection is not always present. The likelihood that any given episode of bacteremia is associated with CIED infection is related to, among other things, the pathogen and duration of bacteremia. As discussed previously, Staphylococcus species cause most CIED endocarditis, and the presence of Staphylococcus bacteremia in a patient with a CIED often indicates CIED involvement. However, if Staphylococcus bacteremia is of short duration and echocardiography does not reveal a vegetation, many patients do not recur if treated with antibiotics alone. In bacteremia with many other bacterial pathogens, both gram negative and gram positive, antibiotics alone are often curative if no evidence of CIED infection is present. ,
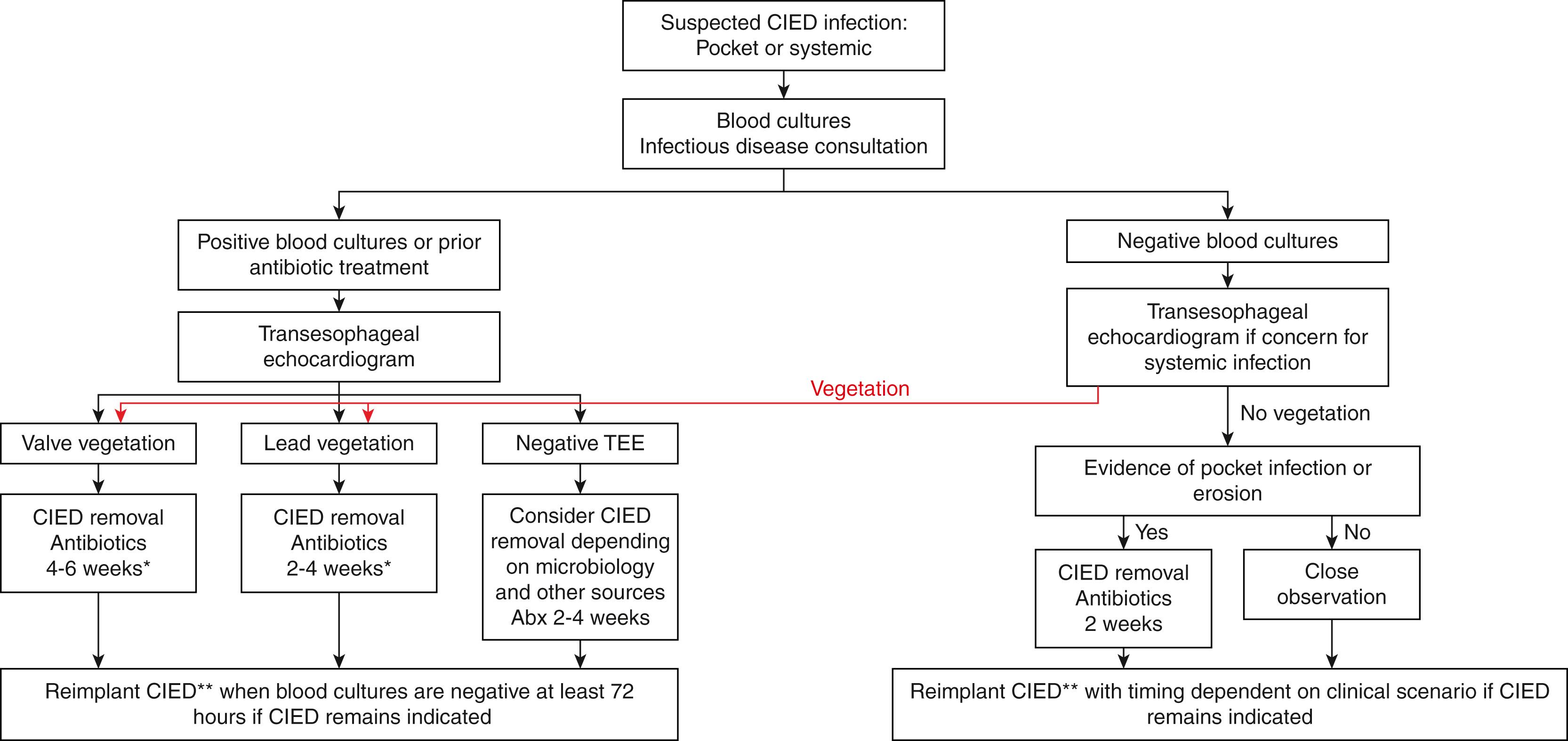
A diagnostic algorithm for suspected CIED infection was outlined in the 2010 American Heart Association (AHA) Scientific Statement on CIED infections ( Fig. 126.4 ). The focus of the algorithm is primarily centered on the results of blood cultures (in all patients) and transesophageal echocardiography (TEE; in any patient with positive blood cultures or with concern for systemic infection). Although a vegetation seen on transthoracic echocardiography (TTE) is a specific finding for CIED endocarditis, the sensitivity of TTE remains low (32% compared with TEE in one study). Therefore TTE is not helpful to rule out the possibility of CIED endocarditis. This is particularly important for any patient with S. aureus bacteremia, which has a very high pretest probability for CIED endocarditis. Patients with apparent isolated pocket infection should also be considered for TEE, as some patients will be found to have CIED endocarditis. TEE may inform the treatment plan by uncovering lead involvement, valvular involvement or dysfunction, or perivalvular abscess, which can inform not only the operative plan but also duration of antibiotic therapy and timing of reimplantation. , , Furthermore, characterizing not only the presence but also the size and shape of vegetations is important for procedural planning, as large (≥2.0 cm) vegetations with a globular shape are associated with increased mortality with transvenous extraction compared with more linear vegetations.
Although the finding of material adherent to a CIED lead in the setting of bacteremia often indicates lead infection, there is evidence that many noninfected leads have adherent material. In one study of 108 noninfected patients undergoing TEE prior to lead extraction, fibrinous material was detected on CIED leads in 18.5% of patients (all <2 cm). An earlier study of standard TEE examinations (not necessarily focused on the leads) found 5% of leads to have incidental masses. Intracardiac echocardiography (ICE) evaluation of CIED leads at the time of ablation in noninfected patients has revealed a 30% incidence of fibrinous material. In patients with “definite,” “possible,” or “rejected” diagnoses of endocarditis who were evaluated with both TEE and ICE, ICE seems to have a greater sensitivity for the detection of intracardiac masses. Together, although material on CIED leads seen by TTE, TEE, or ICE in the setting of bacteremia is usually best assumed to be a sign of lead infection, it is not proof, particularly when less than 2 cm. Additionally, adherent lead material noted incidentally in patients not previously suspected of having an infection is typically not infectious in nature.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here