Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Blood group antigens play a variety of physiologic roles as membrane structures involved in maintaining erythrocyte cytoskeleton integrity, as well as in membrane transport, cell signaling, and immune complement regulation, and as receptors/modulators of disease.
The ABO histo-blood group antigens are widely expressed throughout the body and are the single most important blood group for selection and transfusion of blood products, as well as a major consideration in solid-organ and bone marrow transplantation.
The recipient immune response to exposure to foreign red cell antigens through transfusion or pregnancy may include antibody production and complement activation, resulting in hemolysis (e.g., transfusion reaction, hemolytic disease of the fetus and newborn).
Pretransfusion and perinatal blood testing is performed to prevent transfusion reactions and hemolytic disease of the fetus and newborn. This must include the key serologic evaluations of ABO and Rh antigen typing, antibody detection/identification, and crossmatching.
Antihuman globulin reagents, as used in direct or indirect testing, are integral to virtually all red cell antibody detection and identification techniques.
Patients with complex serologic problems, such as antibodies to high-frequency antigens and autoantibodies, may require utilization of a variety of special immunohematologic studies (enzymes, adsorption, elution) to identify compatible blood for transfusion.
The term immunohematology refers to the serologic, genetic, biochemical, and molecular study of antigens associated with membrane structures on the cellular constituents of blood as well as the immunologic properties and reactions of blood components and constituents. Fundamental discoveries in the area of immunohematology have played an integral role in the development of transfusion medicine, which includes the transfusion of blood, its components, and its derivatives (see Chapter 37 ). In this integrated relationship, immunohematologists perform and interpret a wide variety of serologic and molecular assays to aid in the diagnosis, prevention, and management of immunization associated with transfusion, pregnancy, and organ transplantation. Over the years, research in the field of immunohematology has contributed significantly to the fundamental understanding of human genetics and immunology, with broad applications to membrane physiology and function, epidemiology, anthropology, and forensic science.
The term blood group refers not only to genetically encoded erythrocyte antigens but also to the immunologic diversity expressed by other blood constituents, including leukocytes, platelets, and plasma. Most blood group genes, with few exceptions, are located on the autosomal chromosomes and are inherited following Mendelian rules of inheritance. A majority of blood group alleles demonstrate codominance as well, meaning that genetic heterozygotes at a particular locus will express both gene products.
Many membrane-associated structures on blood cells may be defined as antigens because they have the capability of reacting with a complementary antibody or cell receptor. A majority of these antigens are also immunogens in that they are able to elicit an antibody-mediated immunologic response in a responsive host. Each antigen may have a variety of different epitopes or specific antigenic determinants. Epitopes are discrete, immunologically active regions of the antigen whose molecular configuration can interact with specific lymphocyte membrane receptors or secreted complementary antibody. Clinically, about a dozen antigen systems are significant and are commonly encountered on the transfusion service. In general, these antigens demonstrate polymorphic epitopes with varied distribution in the population, often along racial or ethnic lines. Patients who lack certain antigens may form antibodies when exposed to them; these antibodies may be detected on routine testing in the blood bank.
The ability of an antigen to elicit an immune response is known as its immunogenicity . The immunogenicity of an antigen is determined not only by certain innate characteristics of the antigen itself but also by the host’s genetically determined immune responsiveness. Characteristics of antigens that determine their immunogenicity include degree of foreignness; molecular size and configuration, which may change with temperature, pH, and ionic environment; and antigenic complexity, as measured by the number of available epitopes or antigenic determinants.
Blood group antigens vary greatly in their ability to elicit an immune response. The A, B, and RhD antigens are certainly the most immunogenic. Thus, all blood transfused must be matched for these antigens between the blood donor and the recipient. Approximately 50% to 75% of D-negative individuals would produce anti-D if transfused with only one unit of D-positive blood. After the D antigen, K is the next most immunogenic, followed by Fy a and common Rh antigens, based on the frequency with which their corresponding antibodies are encountered. Using the same criteria, other common blood group antigens—such as Fy b , Jk a , Jk b , and s—are much less immunogenic. The relative immunogenicities of some clinically important red cell antigens are listed in Table 36.1 .
| Antigen | Relative Potency | Antigen | Relative Potency |
|---|---|---|---|
| D | 0.70 | K | 0.10 |
| C | 0.041 | E | 0.0338 |
| k | 0.030 | e | 0.0112 |
| Fy a | 0.0046 | C | 0.0022 |
| Jk a | 0.0014 | S | 0.0008 |
| Jk b | 0.0006 | s | 0.0006 |
∗ These figures represent the approximate percentage of persons negative for a specific antigen who, if transfused with one unit of corresponding antigen-positive blood, would develop antibodies to that specific antigen. When the relative potency (frequency) of K antigen is 0.1, as estimated by Kornstad and Heisto (Kornstad L, Heisto H: The frequency of formation of Kell antibodies in recipients of Kell-positive blood. In: Proceedings of the 6th Congress of the European Society of Haematology, Copenhagen, Denmark, August 1958, pp 754–758), the relative potency of other blood groups can be estimated as shown by Klein and Anstee (Klein HG, Anstee DJ: Mollison’s blood transfusion in clinical medicine , ed 11, Oxford, 2006, Blackwell Scientific Publications).
The chemical composition, complexity, and molecular size of an antigen determine most of its physical and biological properties, including immunogenicity. As a general rule, pure polysaccharides are not immunogenic except in certain species, such as humans and mice ( ). Pure lipid and nucleic acids are not immunogenic but can be antigenic because they can serve as haptens. Haptens are well-defined chemical groupings that are too small to be immunogenic by themselves but can induce an antibody response when attached to a carrier protein.
Although pure protein may be immunogenic, the most potent immunogens are usually complex macromolecular glycoproteins and lipoproteins. Thus, it is not surprising that red blood cell (RBC) antigens are glycoproteins, lipoproteins, and glycolipids. Experiments with peptide chain polymers have shown that aromatic amino acids, such as tyrosine and phenylalanine, can contribute significantly to overall immunogenicity ( ). In glycoproteins, immunogenicity may also be influenced by the extent of branching in the polysaccharide side chains. Whereas the immunogenicity of an antigen relates to the total complex molecular structure, the areas where antigen combines with specific antibody (i.e., the epitopes) are usually limited to one or a few simple structures (terminal sugars, amino acids) exposed on the exterior, mobile surface of the molecule. These are often referred to as immunodominant structures because they determine the specificity and optimal binding energy of antigen–antibody interactions.
The number of antigenic sites on a foreign substance, whether a complex molecule or a cell, will contribute to the strength of an immunologic response. Studies of blood group antigens have demonstrated that antigen density contributes to the efficiency of antibody binding and the extent of complement activation, thus determining the likelihood of RBC hemolysis.
Various techniques have been used over the years to determine the number of copies of specific blood group antigens on the RBC membrane. Historically, radioimmunoassay, enzyme-linked immunosorbent assay (ELISA), electron microscopy using ferritin-labeled anti-immunoglobulin, and flow cytometry have been used to indirectly calculate the number of antigen sites on RBC membranes. Table 36.2 lists the estimated densities of common RBC antigens.
| Antigen | Phenotype | Number of Antigenic Sites | Antigen | Phenotype | Number of Antigenic Sites |
|---|---|---|---|---|---|
| A | A 1 adult | 810–1170 × 10 3 | D | DCce | 9.9–14.6 × 10 3 |
| Newborn | 250–370 × 10 3 | Dce | 12–20 × 10 3 | ||
| A 2 adult | 240–290 × 10 3 | DcEe | 14–16.6 × 10 3 | ||
| Newborn | 140 × 10 3 | DCe | 14.5–19.3 × 10 3 | ||
| A 1 B adult | 460–850 × 10 3 | DcE | 15.5–33.3 × 10 3 | ||
| Newborn | 220 × 10 3 | DCcEe | 23–21 × 10 3 | ||
| A 2 B adult | 140 × 10 3 | D– – | 110–202 × 10 3 | ||
| B | B adult | 750 × 10 3 | Weak D (D u ) | 0.8–3 × 10 3 | |
| A 1 B adult | 430 × 10 3 | c | c+C– | 70–85 × 10 3 | |
| I | I+ | 500 × 10 3 | c+C+ | 37–53 × 10 3 | |
| K | K+k– | 6.1 × 10 3 | e | e+E– | 18.2–24.4 × 10 3 |
| K+k+ | 3.5 × 10 3 | e+E+ | 13.4–14.5 × 10 3 | ||
| E | e–E+ | 0.45-25.6 × 10 3 |
∗ Figures taken from Klein HG, Anstee DJ: Mollison’s Blood Transfusion in Clinical Medicine , ed 11, Oxford, 2006, Blackwell Scientific Publications.
A majority of clinically significant blood group antibodies are immunoglobulin G (IgG) or IgM, although occasionally an IgA antibody is encountered. Blood group antibodies are usually classified as (1) an alloantibody , which reacts with a foreign antigen not present on the patient’s own erythrocytes, or (2) an autoantibody , which reacts with an antigen on the patient’s own cells. RBC autoantibodies are discussed later in this chapter.
Some alloantibodies to erythrocyte antigens are called naturally occurring —that is, the antigenic stimulus is unknown. Naturally occurring antibodies may appear regularly in the serum of persons who lack the corresponding antigen, such as in the ABO blood group system. Other naturally occurring antibodies are produced only in a small subset of individuals.
Most blood group alloantibodies are produced as the result of immunization to foreign erythrocyte antigens by exposure through transfusion of blood components or through pregnancy. Alloantibodies to RBCs frequently require the selection of specific antigen-negative components for transfusion. Identification of alloantibodies and selection of compatible blood components remain the most important functions of a transfusion medicine service.
Complement plays a key role in the pathophysiology of hemolysis through its involvement in the sensitization and destruction of transfused RBCs by alloantibody or the destruction of autologous RBCs by autoantibody. Complement is also important in immunohematologic testing.
Antibody binding to RBC antigens is the most common reason for complement activation on the RBC membrane in vivo. Complement may also be activated on RBCs via a carrier–hapten antibody complex such as penicillin-coated RBCs and antipenicillin antibodies. Complement components may also be attached to the membrane via a nonspecific mechanism induced by certain drugs or when erythrocytes are innocent bystanders in another immune reaction.
RBC–antibody complexes usually activate complement by the classical pathway. However, the mode of destruction and the extent of RBC hemolysis depend primarily on the class of Ig involved and the activity of an individual’s reticuloendothelial (RE) system.
Intravascular RBC hemolysis is usually caused by antibodies directed against the ABO antigens. Rarely, other IgM blood group antibodies, as well as some complement-fixing IgG antibodies (e.g., anti-Kidd antibodies), can induce intravascular hemolysis. Intravascular lysis occurs when large amounts of complement are rapidly activated, resulting in complete activation of the complement cascade with assembly of the terminal membrane attack complex (C5b6789). This complex polymerizes to form pores in the RBC membrane so that extracellular fluid enters the cell, causing it to swell and burst by osmotic lysis.
IgG antibodies cause the majority of extravascular hemolysis via the RE system, which removes complement-coated RBCs. When IgG antibodies bind RBCs and activate complement, complement regulatory proteins generally stop the activation process at the C3/C4 level. RBC-bound C3b is degraded to iC3b, which is enzymatically inactive, by factor I and factor H. iC3b is further degraded to C3c and C3dg by factor I and CR1, a cofactor and C3b/C4b receptor ( ) ( Fig. 36.1 ). Decay accelerating factor (DAF) participates by inhibiting C3 convertase (C4b2b) formation and by promoting C3 convertase degradation. On RBCs, CR1 and DAF carry the Knops and Cromer blood group antigens, respectively.
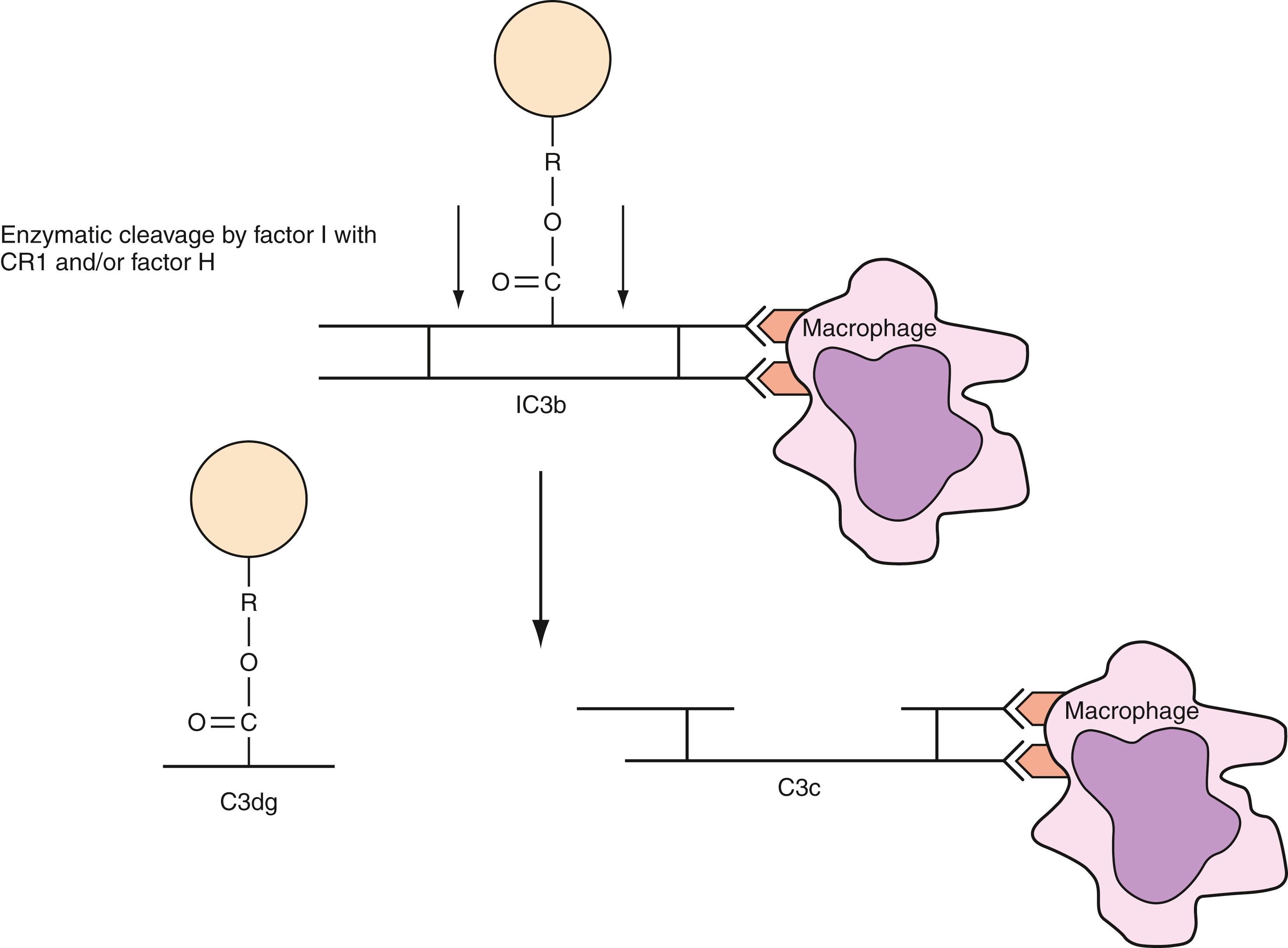
Initially, C3b/iC3b-coated RBCs are rapidly sequestered in the liver by monocytes and macrophages, which have receptors for C3b ( Table 36.3 ). Although phagocytic cells also have receptors for C4b, the role, if any, of C4b in immune hemolysis of erythrocytes is not defined ( ). A portion of the RBCs sequestered in the liver are immobilized and destroyed by phagocytosis with a half-life of about 2 minutes ( ). However, within 15 to 20 minutes, destruction slows and many of the cells escape extravascular destruction through the action of the complement regulatory protein, factor I, as previously described. C3dg, the iC3b fragment produced by factor I cleavage, remains attached to RBCs but has no enzymatic or opsonic properties. As a result, sequestered C3dg-coated RBCs are released back into the circulation and survive normally (see Fig. 36.1 ). In the circulation, C3dg is cleaved, leaving C3d attached to the RBC membrane.
In the absence of complement activation, IgG-coated RBCs are removed by phagocytic cells via Fcγ receptors. Although phagocytosis is not complement dependent, demonstrated that RBCs coated with both IgG and complement tend to show accelerated removal by the liver, whereas RBCs coated only with IgG tend to be destroyed more slowly in the spleen, displaying a linear pattern of removal with a minimum half-life of 20 minutes. Theoretically, RBCs coated only with IgG antibody could also be the targets of antibody-dependent cellular cytotoxicity mechanisms ( ) because natural killer (NK) cells possess Fcγ receptors.
More than 700 erythrocyte antigens have been reported in the literature and have been organized into 35 blood group systems by the International Society of Blood Transfusion (ISBT) ( Table 36.4 ). Many described erythrocyte antigens are high-frequency or public antigens expressed by most donors (>90%–99%), whereas others are extremely rare (private antigens). In the following section, we review the more common RBC antigens and antibodies encountered on the transfusion service. Table 36.5 summarizes several commonly encountered RBC alloantibodies according to their Ig class, serologic phase of detection, clinical significance, and statistics on finding compatible blood.
| TRADITIONAL NOMENCLATURE | ISBT NOMENCLATURE | ISGN NOMENCLATURE | ||||
|---|---|---|---|---|---|---|
| Name | Symbol | Symbol | Number | Gene | Chromosome | Gene Product Name |
| ABO | ABO | ABO | 001 | ABO | 9q34.1 | α1,3 N -acetyl-galactosaminyltransferase (A antigen) |
| α1,3-galactosyltransferase (B antigen) | ||||||
| MNS | MNS | MNS | 002 | GYPA | 4q28.2 | Glycophorin A (CD235 A) |
| GYPB | Glycophorin B (CD235B) | |||||
| GYPE | Glycophorin E (CD235E) | |||||
| P1 | P1 | P1PK | 003 | A4GALT1 | 22q13 | α1,4-galactosyltransferase |
| Rh | Rh | RHD | 004 | RHD | 1p36.1 | RhD protein (CD240) |
| RHCE | RHCE | RhCE protein | ||||
| Lutheran | Lu | LU | 005 | LU | 19q13.3 | Lutheran glycoprotein, B-CAM |
| Kell | K | KEL | 006 | KEL | 7q34 | Kell glycoprotein |
| Lewis | Le | LE | 007 | FUT3 | 19p13.3 | α-3/4-fucosyltransferase |
| Duffy | Fy | FY | 008 | DARC | 1q23 | Duffy-associated receptor cytokine glycoprotein |
| Kidd | Jk | JK | 009 | SLC14A1 | 18q12 | Urea transporter (HUT11) |
| Diego | Di | DI | 010 | SLC4A1 | 17q21.3 | Anion exchanger 1 (AE1, Band 3) |
| Yt | Yt | YT | 011 | ACHE | 7q22 | Acetylcholinesterase |
| Xg | Xg | XG | 012 | XG | Xp22.3 | Xg glycoprotein (CD99) |
| Scianna | Sc | SC | 013 | ERMAP | 1p34 | Human erythroid membrane–associated protein |
| Dombrock | Do | DO | 014 | ART4 | 12p13.2 | ADP-ribosyltransferase (CD297) |
| Colton | Co | CO | 015 | AQPI | 7p14 | Aquaporin-1 (CHIP) |
| Landsteiner-Wiener | LW | LW | 016 | LW | 19p13.3 | ICAM (CD242) |
| Chido/Rodgers | Ch/Rg | CH/RG | 017 | C4A, C4B | 6p21.3 | C4A, C4B complement glycoproteins |
| Hh | Hh | H | 018 | FUT1 | 19q13.3 | α1,2-fucosyltransferase |
| Kx | Kx | XK | 019 | XK | Xp21.1 | Kx glycoprotein |
| Gerbich | Ge | GE | 020 | GYPC | 2q14 | Glycophorin C and glycophorin D (CD236) |
| Cromer | Cromer | CROM | 021 | DAF | 1q32 | Decay-accelerating factor (CD55) |
| Knops | Kn | KN | 022 | CR1 | 1q32 | Complement receptor 1 (CD35) |
| Indian | In | IN | 023 | CD44 | 11p13 | CD44 |
| Ok | Ok | OK | 024 | CD147 | 19p13.3 | CD147Basigin |
| Raph | Raph | RAPH | 025 | CD151 | 11p15.5 | Tetraspanin (CD151) |
| John Milton Hagen | JMH | JMH | 026 | SEMA7A | 15q24.3 | Semaphorin (CD108) |
| I | I | I | 027 | GCNT2 | 6p24.2 | β1,6 N -acetylglucosaminyltransferase |
| Globoside | P(Gb4) | GLOB | 028 | B3GALNT1 | 3q26 | β1,3 N -acetylgalactosaminyltransferase |
| GIL | Gill | GIL | 029 | AQP3 | 9p13 | Aquaglyceroporin |
| RHAG | RHAg | RHAG | 030 | RhAG | 6p21-qter | Rh-associated glycoprotein (CD241) |
| Forssman | Fors | FORS | 031 | GBGT1 | 9q34.13 | α1,3 N -acetylgalactosaminyltransferase |
| Jr | Jr | JR | 032 | ABCG2 | 4q22 | ATP-binding cassette, G family |
| Lan | Lan | LAN | 033 | ABCB6 | 2q36 | ATP-binding cassette, B family |
| Vel | Vel | VEL | 034 | SMIM1 | 1p36.32 | Small integral membrane protein 1 |
| CD59 | CD59 | CD59 | 035 | CD59 | 11p13 | Membrane inhibitor of reactive lysis |
| Augustine | Ata | AUG | 036 | SLC29A1 | 6p21.1 | Nucleoside transporter ENT1 |
| KANNO | KANNO | 037 | PRNP | 20p13 | Prion protein | |
| SID | SID | 038 | B4GALNT2 | 17q21 | B1,4 N-acetylgalactosaminyltransferase | |
| Antibody | Usual Ig Class | Most Common Phase of Reactivity | Clinical Significance | Approximate % of Compatible Donors | ||||
|---|---|---|---|---|---|---|---|---|
| Sal | Alb | AGT | HTR | HDFN | White | Black | ||
| D | IgG | Few | X | X | Yes | Yes | 15 | 8 |
| C | IgG | X | X | Yes | Yes | 30 | 68 | |
| E | IgG | Few | X | X | Yes | Yes | 70 | 98 |
| c | IgG | X | X | Yes | Yes | 20 | 1 | |
| e | IgG | X | X | Yes | Yes | 2 | 2 | |
| Cw | IgG/IgM | Some | X | X | Yes | Yes | 98 | 100 |
| K | IgG | Rare | X | Yes | Yes | 91 | 97 | |
| k | IgGX | Yes | Yes | 0.2 | 0.1 | |||
| Kp a | IgG | Rare | X | Yes | Yes | 98 | 99.9 | |
| Kp b | IgG | X | Yes | Yes | <0.1 | 0.1 | ||
| Js a | IgG | X | Yes | Yes | >99.9 | 81 | ||
| Js b | IgG | X | Yes | Yes | <0.1 | 1 | ||
| Fy a | IgG | X | Yes | Yes | 34 | 90 | ||
| Fy b | IgG | X | Yes | Yes | 17 | 77 | ||
| Jk a | IgG | X | Yes | Yes | 23 | 9 | ||
| Jk b | IgG | X | Yes | Yes | 28 | 5 | ||
| M ∗ | IgM | X | Few | Yes | 22 | 30 | ||
| N | IgM | X | Rare | Rare | 28 | 26 | ||
| S | IgG/IgM | Some | X | Yes | Yes | 45 | 69 | |
| s | IgG | X | Yes | Yes | 11 | 3 | ||
| U | IgG | X | Yes | Yes | 0 | 1 | ||
| Lu a † | IgM | X | ? | Yes | 92 | 96 | ||
| Lu b † | IgG | X | Yes | Mild | <0.1 | <0.1 | ||
| P1 ‡ | IgM | X | Some | Rare | No | 21 | 6 | |
| P | IgM | X | Some | Some | Probable | Yes | <0.1 | 0.1 |
| PP 1 P k † | IgG/IgM | X | Some | Some | Probable | Yes | <0.1 | 0.1 |
| Le a ‡ | IgM | X | Some | Yes | No | 78 | 77 | |
| Le b ‡ | IgM | X | Yes | No | 28 | 45 | ||
| I | IgM | X | Few | Rare | No | <0.1 | <0.1 | |
| i | IgM | X | Few | ? | No | <0.1 | <0.1 | |
∗ Most examples of anti-M also have a small but significant IgG component.
† Exhibits characteristic mixed-field agglutination pattern.
Originally discovered in 1900, the ABO blood group system is the single most important blood group for the selection and transfusion of blood. As histo-blood group antigens, ABO epitopes are found on many tissues and body fluids, including RBCs, platelets, and endothelial cells ( ). Because they are so widely expressed, ABO antigens are a major consideration in solid organ and bone marrow transplantation ( ).
The ABO blood group system consists of two antigens—A and B—and four phenotypes—groups A, B, AB, and O. A and B are autosomal-codominant antigens (ISBT No. 001) and are expressed on group A, B, and AB red cells, respectively. In contrast, the group O phenotype is an autosomal-recessive phenotype, reflecting the absence of a functional ABO gene. Group O individuals express the H antigen (ISBT No. 018), the biosynthetic precursor of both A and B antigens ( Fig. 36.2 ). Group O is the most frequent ABO phenotype in most populations tested, particularly among Native Americans. Expression of ABO antigens on RBCs is usually accompanied by the presence of naturally occurring antibodies against the missing antithetical antigen(s). Table 36.6 shows the serologic reactions and frequencies of the four major ABO phenotypes.
| CELLS AGAINST KNOWN ANTISERA | SERUM AGAINST RED CELLS OF KNOWN PHENOTYPE | Interpretation | FREQUENCIES IN U.S. POPULATION, % | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Anti-A | Anti-B | A | B | O | White | Black | Native American | Asian | |
| – | – | + | + | – | O | 45 | 49 | 79 | 40 |
| + | – | – | + | – | A | 40 | 27 | 16 | 28 |
| – | + | + | – | – | B | 11 | 20 | 4 | 27 |
| + | + | – | – | – | AB | 4 | 4 | <1 | 5 |
| – | – | + | + | + | Bombay | rare | rare | rare | rare |
The ABO system also contains several phenotypes associated with weakened, anomalous, or complete absence of ABO antigen expression. The most common ABO subtypes encountered in the blood bank are A 1 and A 2 . A 1 red cells are distinguished from A 2 (and other weak A subtypes) by agglutination with the lectin Dolichos biflorus . Comparison of A 1 , A 2 , and other weak A subtypes indicates both quantitative and qualitative differences in A antigen expression ( ; ). A 2 is the most commonly encountered weak subtype in whites, accounting for nearly 20% of all group A donors. The ABO system contains additional weak A and weak B phenotypes, including several that can cause ABO discrepancies during routine testing ( ).
Anomalous ABO expression can be inherited ( cis -AB, B[A]) or acquired (acquired B). In the cis -AB phenotype, A and B antigens are synthesized by the same enzyme and are inherited as a single autosomal-dominant allele. Likewise, the B(A) phenotype, an autosomal-dominant phenotype characterized by trace A antigen expression on group B RBCs, is due to synthesis of A antigen by the B -gene enzyme. The acquired B phenotype, on the other hand, is an acquired enzymatic modification of group A 1 red cells in vivo. The acquired B phenotype usually occurs in the setting of bacterial infection or cancer and reflects enzymatic deacetylation of group A antigen to form a B-like antigen on RBCs. The cis -AB, B(A), and acquired B phenotypes are usually detected because of discrepancies in ABO typing ( ; ).
Bombay and para-Bombay are two rare null phenotypes characterized by an absence of all ABH antigens on RBCs. In the classic Bombay phenotype (O h ), neither AB nor H antigens are present on RBCs or in secretions. Para-Bombay also shows few or no ABH antigens on RBCs, sometimes accompanied by normal expression of ABH antigens in secretions and body fluids.
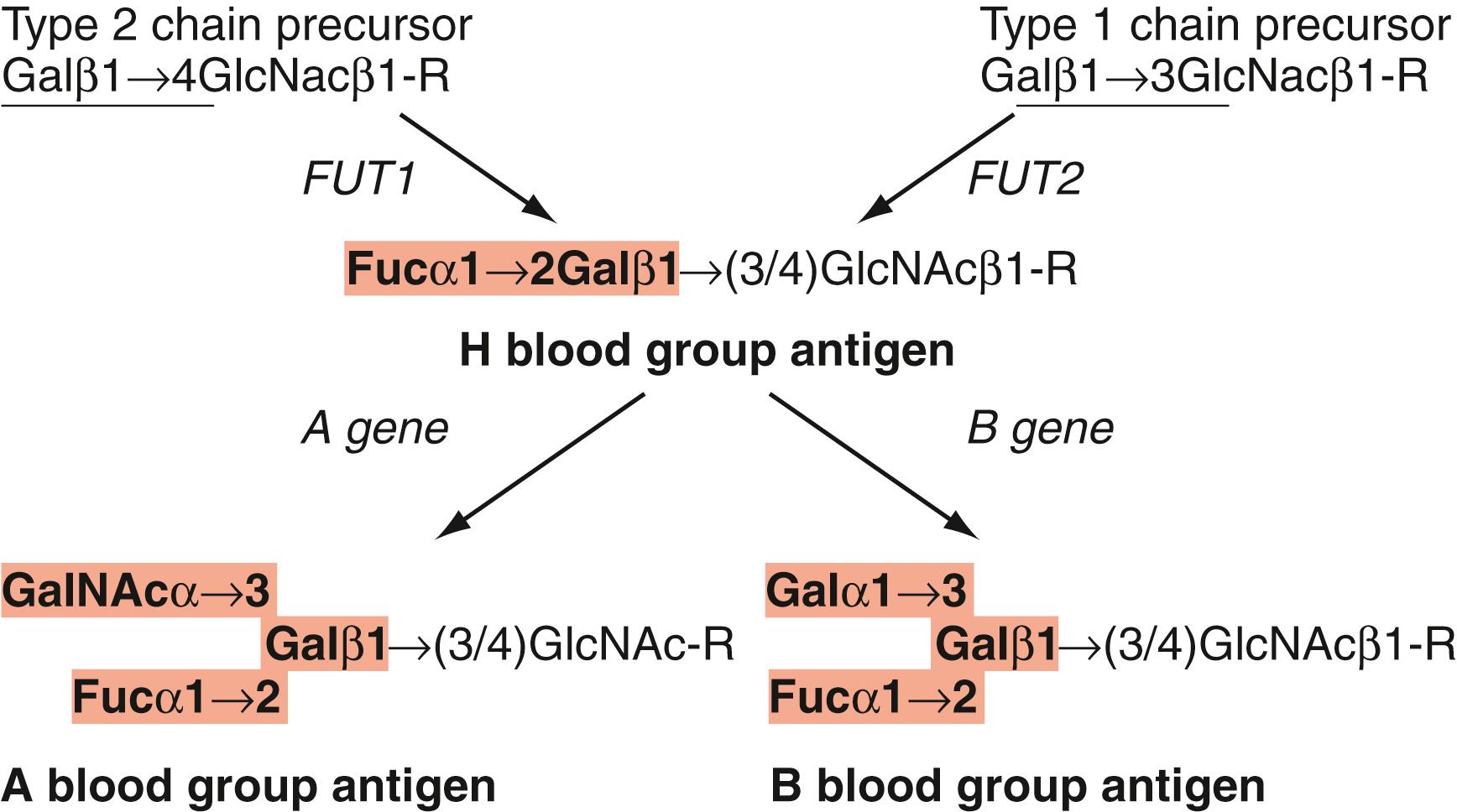
The ABO antigens are carbohydrate antigens and, therefore, represent a posttranslation modification of glycoproteins and glycolipids. On RBC glycoproteins and polylactosaminylceramides, ABO antigens are usually expressed on type 2 chain oligosaccharides, characterized by repeating lactosaminyl (Galβ1–4GlcNAcβ1–3) n motifs (see Fig. 36.2 ). On glycosphingolipids, ABO antigens can be expressed on multiple (types 1, 2, 3, and 4 chain) oligosaccharide precursors. ABH antigens expressed on RBC glycoproteins and most glycosphingolipids (type 2, 3, and 4 chain) are of RBC origin. In contrast, type 1 chain ABO antigens are synthesized by gastrointestinal mucosa, secreted into plasma, and passively adsorbed onto red cell membranes. Synthesis of type 1 chain ABO antigens is linked to the Lewis blood group system.
The first step in the synthesis of ABH antigens is the synthesis of the H or group O antigen, the immediate biosynthetic precursor of both A and B antigens. The H antigen is formed by the addition of fucose (Fuc), in an α1–2 linkage, to a terminal galactose. This reaction is catalyzed by two different enzymes, depending on whether the fucose is being added to a type 1 or type 2 chain oligosaccharide acceptor. Fucosyltransferase type 1 ( FUT1 ), the product of the H or FUT1 gene, catalyzes the formation of type 2 chain H antigen. In contrast, fucosyltransferase type 2 ( FUT2 ), the product of the Secretor gene, catalyzes the transfer of fucose to type 1 chain precursors to form type 1 chain H or Le d antigen ( ). Inactivating mutations in FUT1 are responsible for the Bombay and para-Bombay phenotypes ( ). Bombay and para-Bombay nonsecretors also have inactivating mutations in FUT2 ( ).
Once H antigen is formed, it can serve as a substrate for A gene and B gene glycosyltransferases. The A antigen is formed by A gene ( ABO∗A ) glycosyltransferase, which adds an N -acetylgalactosamine (GalNAc), in an α1–3 linkage, to the subterminal galactose of H antigen. Likewise, the B antigen is formed by the addition of an α1–3 galactose (Gal) to the same galactose by the B gene ( ABO∗B ) glycosyltransferase. Biochemically, the A and B antigens are very similar, differing only by the presence of an N -acetyl group. It is fascinating that such a minor chemical modification should have such profound immunologic consequences. Removal of the N -acetyl group on A antigen by circulating deacetylase enzymes is responsible for the acquired B phenotype ( ).
FUT1 ( H gene) and FUT2 ( Se gene) are located together on chromosome 19q13.3 and reflect a gene duplication ( ). FUT1 is a 365 amino acid, type II transmembrane glycoprotein, composed of a large, 240 amino acid, carboxy-terminal catalytic domain, which is anchored within the Golgi lumen by a short transmembrane and a cytosolic domain. FUT1 messenger ribonucleic acid (mRNA) is widely expressed in most tissues, with the exception of salivary and parotid glands ( ). The last is the basis for testing saliva to determine ABH secretor status. The ISBT currently lists more than 40 mutant FUT1 alleles, including 19 null alleles and 23 weak alleles ( ).
The ABO gene locus is located on chromosome 9q34 and encodes the A and B glycosyltransferases ( ). The gene is large, spanning 19.5 kb, and contains seven exons, although exons 6 and 7 encode the majority of the active enzyme (66%). The product of the ABO gene is a 41-kD, 354 amino acid type II transmembrane glycoprotein. Comparison of A and B enzymes shows nearly 98% identity, differing by four key amino acids at residues 176, 235, 266, and 268 ( Table 36.7 ). Amino acids 235, 266, and 268 are critical in determining A versus B enzyme activity: The polymorphism at 176 is not biologically active. Because both A and B recognize the type 2 chain acceptor, any differences in amino acids 235, 266, and 268 dictate whether the enzyme will recognize UDP-GalNAc or UDP-Gal nucleotide sugar donor. Crystallography data indicate that the amino acids associated with ABO∗A gene product are smaller and able to accommodate the larger UDP-GalNAc donor. To date, more than 200 ABO alleles have been identified.
| ABO Allele | AMINO ACID NUMBER OF A/B GLYCOSYLTRANSFERASE | Gene Type † | ||||
|---|---|---|---|---|---|---|
| 176 | 234 | 235 | 266 | 268 | ||
| ABO∗A.01 | Arg | Pro | Gly | Leu | Gly | AAAA |
| ABO∗B.01 | Gly | Pro | Ser | Met | Ala | BBBB |
| ABO∗cisAB.01 | Arg | Pro | Gly | Leu | Ala | AAAB |
| ABO∗cisAB.04 | Gly | Pro | Gly | Met | Gly | AABA |
| ABO∗cisAB.02 | Gly | Pro | Ser | Leu | Ala | BBAB |
| ABO∗BA.03 | Gly | Pro | Gly | Met | Ala | BABB |
| ABO∗BA.02 | Gly | Ala | Ser | Met | Ala | BBBB |
| ABO∗O.02 | Arg | Pro | Gly | Leu | Arg ‡ | AAAX |
∗ Data from Reid RE, Lomas-Francis C: The blood group antigen facts book , ed 3, San Diego, 2012, Academic Press; and Daniels G: Human blood groups , ed 3, Oxford, 2013, Blackwell Science. Amino acids that differ from the ABO∗A.01 (A 1 type) consensus allele are highlighted in bold.
† Gene type refers to amino acid positions 176, 235, 266, and 268. These four positions differ between A (AAAA) and B alleles. Amino acids at 235, 266, and 268 strongly influence substrate specificity. Hybrid glycosyltransferases have amino acids matching both A and B consensus alleles at these positions.
‡ ABO∗O.02 allele (historically O 2 , O03 ), associated with a group O phenotype, possesses an inactivating missense mutation at amino acid 268.
The cloning and sequencing of the ABO gene locus have also uncovered the molecular basis of group O and weak ABO subtypes. Group O is an autosomal-recessive phenotype due to homozygosity for amorph ABO alleles ( ABO∗O ). There are many ABO∗O alleles described to date; however, most ABO alleles can be classified as either O 1 or O 2 . Alleles belonging to the O 1 family ( ABO∗O1 ) share a nucleotide deletion and frameshift (G251D, fs88stop) and account for 95% of all ABO∗O alleles. O 2 alleles ( ABO∗O2 ; O03 ) carry a G802>A, leading to Gly268Arg in the translated protein (see Table 36.7 ). O 2 and related alleles (Aw08) have been linked to ABO typing discrepancies by an absence of anti-A and/or anti-B in these individuals ( ). It has been suggested that the absence of anti-A or anti-B is the result of weak residual enzyme activity ( ). However, this has not been confirmed ( ).
Weak ABO subtypes are the result of mutations at the ABO gene locus. Most mutations are located within exon 7, which encodes the catalytic domain of the molecule. The majority of described mutations are single-nucleotide polymorphisms (SNPs), although nonsense, frameshift, and translation-initiator mutations are known ( ; ; ). A2, the most common weak A subtype, is the result of a single-nucleotide deletion (nucleotide 1060) and frameshift, resulting in the loss of a stop codon and synthesis of a longer A enzyme with decreased enzyme activity. The cis -AB and B(A) phenotypes are the consequence of hybrid alleles, with characteristics of both A 1 and B gene consensus alleles. cis -AB and B(A) individuals can synthesize both A and B antigens (see Table 36.7 ) ( ; ).
Antibodies against ABO antigens are the most important antibodies in transfusion medicine. The practicing pathologist should note that the routine antibody screen does not test for ABO antibodies. All reagent cells in the antibody screen are group O. Patients demonstrate anti-A or anti-B on reverse typing (see later discussion). A valid ABO type requires agreement between the forward and reverse types.
In general, ABO antibodies are naturally occurring. It is believed that the immune stimulus for the formation of ABO antibodies may be exposure to ABH-like substances found in nature, particularly on the bacterial polysaccharides ( ). It is interesting that ABO titers have progressively decreased over the past 2 decades with increasing consumption of pasteurized, commercially packaged foods that are relatively sterile ( ). This trend may be reversed with increasing use of probiotic nutritional supplements that contain live bacteria. The latter have been shown to stimulate ABO antibodies, with marked increases in ABO titers within several months ( ).
ABO antibodies are weak or absent in the sera of newborns until 3 to 6 months of age. Adult levels of ABO antibodies are reached by 5 to 10 years of age and decrease only slightly with advancing age ( ). Anti-A,B is found exclusively in group O individuals and appears to recognize an epitope common to both A and B antigens. Before the development of anti-A and anti-B monoclonal antibody typing reagents, anti-A,B was useful in identifying weak and B subgroups. Anti-A,B is still used for typing donor units and cord samples (see ABO Grouping section).
In general, ABO antibodies are detected as room temperature, saline agglutinins with optimal reactivity at 4°C (see Table 36.5 ). Most naturally occurring ABO antibodies are of IgM isotype, although IgA and IgG antibodies with ABO specificity are also present ( ). ABO IgG antibodies, reactive at 37°C, can also occur following immune stimulation by transfusion or pregnancy. These antibodies generally are of higher titer and are less readily neutralized by soluble blood group substances. ABO antibodies can fix complement and can cause hemolysis in vivo and in vitro.
Clinically, ABO antibodies are a cause of hemolytic transfusion reactions (HTRs) and hemolytic disease of the fetus and newborn (HDFN). ABO antibodies are also a cause of acute rejection in solid-organ transplantation. As a result, solid-organ transplants should be ABO compatible with the recipient’s sera. Rare exceptions to the latter are heart transplantation in children younger than 6 to 8 months of age who have not yet developed ABO antibodies ( ) and transplantation of A 2 organs, which have very weak ABO expression on epithelium and vascular endothelium ( ). In ABO-incompatible bone marrow transplantation, ABO antibodies can result in hemolysis and a delay in erythroid and megakaryocyte engraftment ( ; ). For additional information on ABO-incompatible marrow and organ transplants, see the section on Antibody Titers.
Anti-A 1 is a naturally occurring antibody found in the sera of some A 2 , A 2 B, and other weak A subtypes. Anti-A 1 hemagglutinates A 1 RBCs but not A 2 and other weak A phenotypes. Although uncommon, anti-A 1 has been implicated in transfusion reactions and solid-organ rejection.
Anti-H is usually a benign, naturally occurring antibody in the sera of A 1 and A 1 B nonsecretors. Anti-H reacts most strongly with group O erythrocytes, followed by A 2 , B, A 2 B, A 1 , and A 1 B (see Table 36.41 ). Because H antigen is present to some degree on all RBCs, anti-H is an autoantibody in most individuals. In contrast, alloanti-H is a clinically significant alloantibody in Bombay (O h ) and para-Bombay individuals. These individuals require transfusion of rare O h red cells.
| Antibody Specificity | Clinical Significance | IAT Antibody Screen ∗ | Ig Class | RELATIVE REACTION STRENGTHS WITH SELECTED RED CELLS AT ROOM TEMPERATURE † | ||||
|---|---|---|---|---|---|---|---|---|
| O adult | O cord | A 1 adult | A 2 adult | Autologous | ||||
| I | Acute CAD associated with Mycoplasma pneumoniae with antibody titers >1000 at 4°C | Pos | IgM | 3+ | w+ | 3+ | 3+ | 3+ |
| i | Acute CAD associated with mononucleosis | Pos | IgM | w+ | 3+ | w+ | w+ | Weaker than O cord |
| Pr | Rare cause of CAD ‡ | Pos | Reported cases of IgM, IgA, IgG | 3+ | 3+ | 3+ | 3+ | 3+ |
| P | PCH associated with certain viral infections in children | Neg | IgG § | Negative in routine agglutination tests; autoanti-P is a biphasic hemolysin (Donath-Landsteiner antibody) | ||||
| H | Benign except as alloantibody in Bombay phenotype | Weak to neg | IgM | 3+ | 3+ | 1+ | 2+ | 0 to w+ |
| IH | Benign | Weak to neg | IgM | 3+ | 1+ | 1+ | 2+ | 0 to w+ |
∗ Antigen expression: O adult (I+i– H+s); O cord (I–i+ H+s); A 1 (I+i– H+w); A 2 (I+i– H+).
† Reagent cells showing agglutination in 37°C phase may be much weaker after conversion to indirect antiglobulin test (IAT).
‡ May be differentiated from anti-I by enzymes or increasing pH; anti-Pr reactivity is decreased by both techniques.
§ Autoanti-P is the only pathologic cold autoantibody known to be routinely of the IgG class to IAT.
The biological role of ABH antigens is still not known. Multiple studies have linked specific ABO types with a higher incidence of many diseases, including autoimmune, neoplastic, and infectious disorders. A and B antigen expression may stabilize the clustering and spatial organization of sialoglycoproteins ( ). Depression of A and B antigen expression can occur in malignancy and is often associated with increased metastatic potential. Groups A, B, and AB individuals also have a twofold higher risk of thrombotic events, including venous thrombosis and pulmonary embolism ( ). This may correlate to ABO differences in circulating von Willebrand factor (vWF), low-density lipoprotein (LDL), cholesterol, and P- and E-selectin. Plasmodium falciparum has been shown to bind A and B antigens with rosette formation, a possible risk factor in cerebral malaria ( ). Group O is a receptor for many gastrointestinal pathogens, including norovirus, rotavirus, Helicobacter pylori , Campylobacter jejuni , and Vibrio cholerae El Tor ( ). Group O may be protective against some infections due to anti-A and anti-B, including some enveloped viruses (human immunodeficiency virus [HIV], severe acute respiratory syndrome–associated coronavirus [SARS-CoV]), schistosomiasis, E. coli , and other gram-negative organisms ( ).
Discovered in 1927, the MNSs blood group was the second blood group system identified after ABO. Today, the MNSs blood group system consists of 49 antigens, although only four (M/N and S/s) are commonly encountered in the clinical setting ( ). As shown in Table 36.8 , the M and N antigens are fairly evenly distributed in both blacks and whites, with approximately 25% of donors homozygous for M or N antigen. In contrast, the S antigen is nearly twice as frequent in whites (57%) as in blacks (30%). In a minority (<1%) of blacks, an S–s– or null phenotype can be observed. As with Rh antigens, the MNSs blood group antigens are expressed only on RBCs. Approximately 1 million M/N and 170,000 to 250,000 S/s epitopes are present per RBC.
| GLYCOPHORIN A ANTIGENS | GLYCOPHORIN B ANTIGENS | Phenotype | PHENOTYPE FREQUENCIES, % | ||||||
|---|---|---|---|---|---|---|---|---|---|
| M | N | En (a) | “N” | S | s | U | Caucasian | Black | |
| + | 0 | + | M+N– | 28 | 26 | ||||
| + | + | + | M+N+ | 50 | 44 | ||||
| 0 | + | + | M–N+ | 22 | 30 | ||||
| + | + | 0 | + | S+s–U+ | 11 | 3 | |||
| + | + | + | + | S+s+U+ | 44 | 28 | |||
| + | 0 | + | + | S–s+U+ | 45 | 69 | |||
| Null Phenotypes | |||||||||
| 0 | 0 | 0 | + | +/0 | +/0 | + | En (a–) | Rare | Rare |
| 0 | 0 | 0 | 0 | 0 | 0 | 0 | M k M k | Rare | Rare |
| 0 | 0 | 0 | 0 | S–s–U– | Rare | <1 | |||
| 0 | 0 | 0 | wk+ | S–s–U var (23% Henshaw+) | Rare | <1 | |||
Three major null phenotypes are present in the MNSs system: U–, M k , and En (a–). The U– phenotype is the most common and is observed exclusively in blacks. In S–s–U– individuals, complete loss or a recombination of glycophorin B occurs, leading to altered expression of S/s and U antigens. Recombinant glycophorin B, such as the Henshaw phenotype, can react weakly with some examples of human anti-U and are known as U variants (S–s–U var ). The En (a–) phenotype is the result of recombination between glycophorin A and B genes to form a Lepore-type A-B hybrid (exons A1-B2-B5) lacking most of glycophorin A (GYPA). The M k M k phenotype lacks all MNSs antigens, including En(a), as the result of recombination and deletion of glycophorins A and B (GYPA and GYPB). Loss of GYPA can coincide with loss of Wr b expression, an antigen on Band 3. It is believed that Wr b requires an electrostatic interaction between a glutamic acid (Glu658) on Band 3 and the ENEP antigen on glycophorin A ( ).
The M/N antigens reside on GYPA (CD235A), a major RBC membrane glycoprotein. In the membrane, GYPA is present as a dimer, usually in association with Band 3, the erythrocyte anion exchanger ( ). Following cleavage of a 19 amino acid leader sequence, the mature GYPA is a 31-kD, 131 amino acid, type 1 glycoprotein composed of a large 72 amino acid extracellular domain, a transmembrane domain, and a short cytoplasmic tail ( Fig. 36.3 ). The molecule is heavily glycosylated, possessing 15 O -linked and one N -linked carbohydrate side chain. The O -linked glycans consist predominantly of a disialotetrasaccharide linked to a serine or threonine residue. Because of the large number of sialylated O -linked glycans on GYPA, nearly 60% and 50% of the total molecular weight is carbohydrate and sialic acid, respectively. Not surprisingly, GYPA is the major sialomucin on RBCs and contributes significantly to the overall negative charge or ζ potential ( ). The M and N antigens reside on the extreme amino-terminus of GYPA.
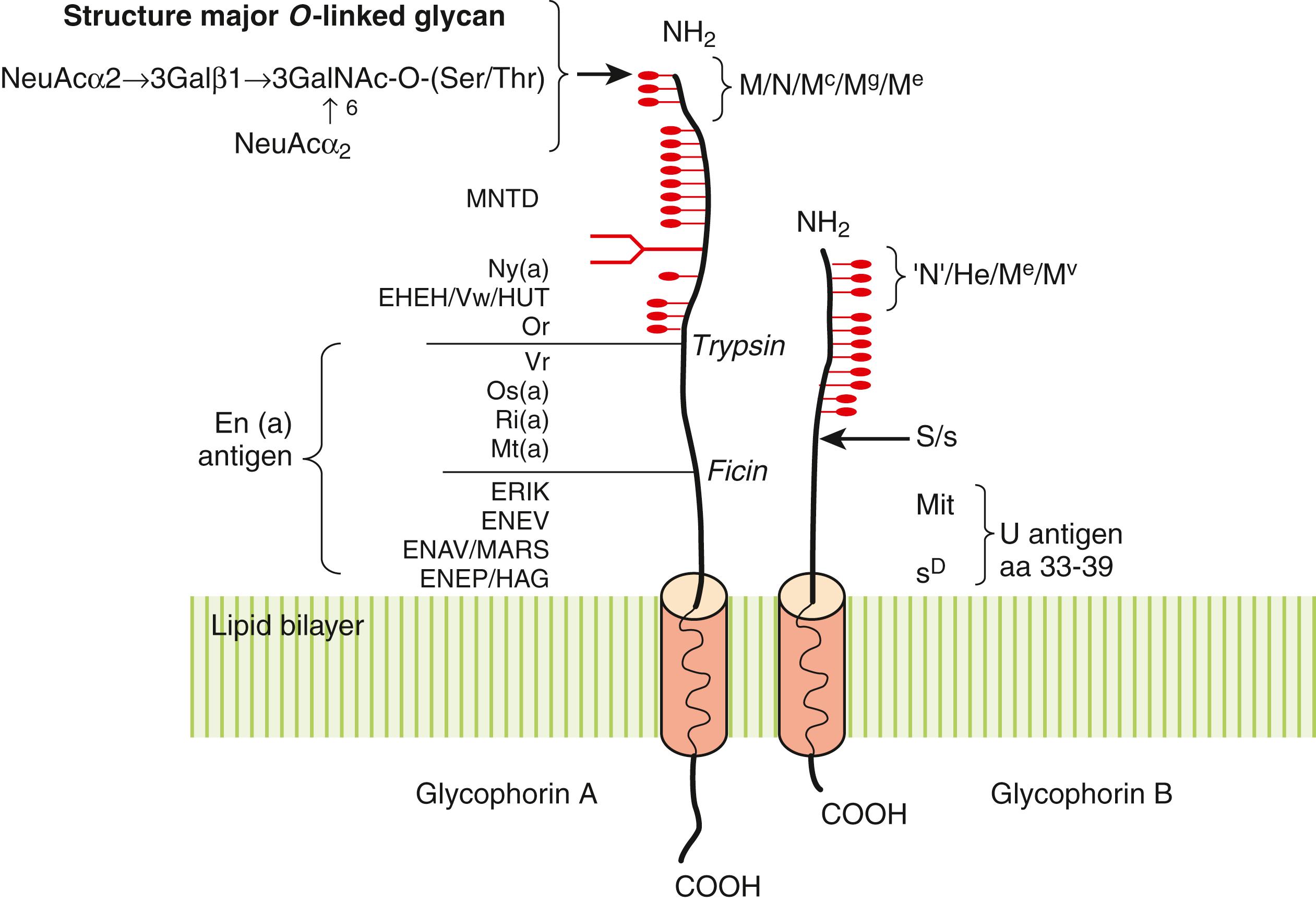
The S/s and U antigens reside on GYPB(CD235B), a related RBC glycoprotein (see Fig. 36.3 ). Like GYPA, GYPB undergoes posttranslational processing, resulting in a 20-kD, 72 amino acid glycoprotein composed of a large extracellular N -terminal domain containing 11 O -linked glycans. Although GYPB shares considerable homology with GYPA at the amino-terminus, GYPB is smaller, lacking both an N -glycan and a cytoplasmic tail. In the membrane, GYPB appears to be closely associated with Diego at the AE1-ankyrin and junctional complexes, often as a heterodimer with GYPA ( ). The S/s epitope (Met48Thr) is located at amino acid 29 in the mature protein ( ).
The genes for GYPA ( GYPA ) and GYPB ( GYPB ) reside on chromosome 4q28-q31 as part of a 330-kb gene cluster encoding GYPA , GYPB , and glycophorin E ( GYPE ) (5′-A-B-E-3′). Studies indicate that GYPB and GYPE arose from GYPA by gene duplication and nonhomologous recombination. Similar to many erythroid-specific genes, the promoter region contains consensus sequences for Sp1 and GATA-1, an erythroid transcription regulatory binding factor ( ). The greater stability of GYPA mRNA (>24 hours) over GYPB mRNA (<17 hours) may explain the four- to fivefold greater numbers of GYPA on RBCs ( ).
There are nearly 20 allelic GYPA variants reported to date that are responsible for several high- and low-incidence antigens. The biochemical nature of the MNSs antigens has long been known. The M and N antigens lie at the extreme amino-terminus of GYPA (amino acids 1–5) and include both protein and carbohydrate as part of the immune epitope. It is amino acid differences at positions 20 and 25 (1 and 5 in the mature protein), however, that define the M/N antigens ( Table 36.9 ). Further investigation of these amino acid differences revealed that the amino terminal amino acid (Ser for M and Leu for N) are the antigenic determinants ( ) It is not surprising that several low-incidence M and N antigen variants are the result of different amino acid substitutions (M g , M c ) and/or altered expression of O -linked glycans (M 1 , Tm, Can). In addition to GYPA, the N antigen is expressed on the extreme amino-terminus of GYPB. The latter is referred to as the “N” antigen to distinguish it from N antigen on GYPA ( ).
| ISBT No. | Antigen | Glycophorin | Allele | Amino Acid Change ∗ |
|---|---|---|---|---|
| MNS1 | M | GYPA | GYPA∗01 | Ser 20 -Ser-Thr-Thr-Gly 24 ∗ |
| MNS2 | N | GYPA | GYPA∗02 | Leu 20 -Ser-Thr-Thr-Glu 24 ∗ |
| MNS8 | Mc | GYPA | GYPA∗08 | Ser 20 -Ser-Thr-Thr-Glu 24 |
| MNS11 | Mg | GYPA | GYPA∗11 | Ser 20 -Ser-Thr-Asn 23 -Gly |
| MNS30 | “N” | GYPB | GYPB∗01 | Leu 20 -Ser-Thr-Thr-Glu 24 |
| MNS6 | He | GYPB | GYPB∗06 | Trp 20 -Ser-Thr-Ser 23 -Gly 24 |
| MNS3 | S | GYPB | GYPB∗03 | Met48∗ |
| MNS4 | s | GYPB | GYPB∗04 | Thr48∗ |
| Null phenotypes | ||||
| MNS: -1, -2 | M-N- | No GYPA | GYPA∗01N | Δ GYPA Exons 2–7 |
| En (a–) | Δ GYPB Exon 1 | |||
| MNS: -3, -4, -5 | S-s-U- | No GYPB | GYPB∗01N | Δ GYPB Exons 2–5 |
| MNS: -1, -2, -3, -4, -5 | M-N- | No GYPA | GYP∗01N | Δ GYPA Exons 2–7 |
| MkMk | S-s-U- | No GYPB | Δ GYPB Exons 1–5 | |
∗ The first 19 amino acids are cleaved, with the mature glycophorins A and B starting at amino acid 20. M/N antigens reside along the first 5 amino acids on the mature GYPA protein (aa1 to aa5). Likewise, the S/s polymorphism resides at amino acid 29 on the mature GYPB protein. Allele nomenclature per ISBT.
Twelve GYPB alleles are recognized, responsible for S/s, Mv+, Mit+, S D +, He, and U var phenotypes. Unlike the complexity of the M/N antigens, the S/s antigen is a single amino acid polymorphism on GYPB (Met29Thr on mature protein). The U antigen is a high-incidence antigen (amino acids 33–39 on mature GYPB). Loss of S, s, and U antigens can be observed with M k and some recombinant GYPB alleles such as Henshaw. It is estimated that 90% of Henshaw+ RBCs are U– or U var and account for 23% of all S–s–U– patients.
Over 20 deletion and recombinant GYPA and GYPB alleles are known. Recombination and gene conversion are responsible for several rare MNS antigens that were historically part of the Miltenberger system. These include misalignments with single crossovers leading to A-B Lepore-type (e.g., Hil) and B-A anti-Lepore (Dantu) variants as well as double crossover events, in which segments of one glycophorin are inserted into the other to form B-A-B and A-B-A hybrids ( ; ). Deletion mutants include En(a–), a GYPA null ( GYPA∗01N ) and GYPB (U-) null phenotypes ( GYPB∗01N ). The M k allele ( GYP∗01N ) is characterized by a large deletion of GYPA and GYPB due to recombination between GYPA and GYPE . Mutant GYP molecules are frequently accompanied by loss of high-frequency MNS antigens and can display unusual resistance or sensitivity to enzyme treatment ( ). GP.Mur, which is present in 2% to 10% of Southeast Asians, is associated with increased AE1/Band 3 (Diego) expression, decreased Rh, and the alloantibody anti-Mi a ( , ).
Antibodies against M and N antigens are naturally occurring antibodies of IgM isotype, usually detected as room temperature saline agglutinins (see Table 36.5 ). Anti-M and anti-N may show dosage, reacting more weakly with heterozygous (M/N) cells than with homozygous (M/M or N/N) cells. Because the M and N antigens reside on GYPA, the reactivity of anti-M and anti-N is destroyed by pretreatment of RBCs with proteolytic enzymes or neuraminidase. Some examples of anti-M and anti-N can be enhanced by acidification of serum to pH 6.5, use of an albumin diluent, or preincubation of RBCs in a glucose-containing solution.
Clinically, anti-M is a commonly encountered antibody in the blood bank. In contrast, anti-N is distinctly uncommon despite the fact that 25% of patients are negative for N antigen (M homozygous). The rarity of anti-N is due to the presence of “N” antigen on GYPB. When observed, anti-N is usually an autoantibody, reacting with both N and “N” antigens. An autoanti-N (anti-N f ) was reported in hemodialysis patients in the past, caused by the use of formaldehyde to sterilize membranes. Formaldehyde reacted with the terminal leucine on N and “N” antigens, creating a neoantigen ( ). In general, anti-M and anti-N are clinically insignificant antibodies and only rarely cause hemolytic transfusion reactions or HDFN. In contrast, potent hemolytic alloanti-N is observed in patients lacking GYPB (M+N–S–s– phenotype). In these patients, severe hemolytic transfusion reactions and HDFN can occur after transfusion of N+ RBCs.
Unlike anti-M and anti-N, antibodies against S, s, and U antigens are always clinically significant (see Table 36.5 ). All are antibodies of IgG isotype, reactive at 37°C, arising from immune stimulation. Some examples of anti-S and anti-s show dosage. Enzymatic modification of RBCs with proteases, but not neuraminidase, can decrease the reactivity of some anti-S and anti-s. The reactivity of anti-U is resistant to proteolytic digestion. Anti-S, -s, and -U are causes of hemolytic transfusion reactions and HDFN.
Despite the prevalence of GYPA and GYPB on RBCs, their biological role is still unknown—their absence is not associated with any known hematologic or pathologic sequelae. Because they are rich in O -glycans and sialic acid, GYPA and GYPB contribute significantly to the ζ potential of red cells, decreasing homotypic and heterotypic red cell adhesion. GYPA also facilitates transport and expression of Band 3 (AE1/Diego), a critical protein in gas exchange. Increased Band 3 expression and osmotic resistance can be observed with Miltenberger type III red cells, a GYP B-A-B hybrid ( ). Finally, GYPA and GYPB play a role in Plasmodium falciparum infections. P. falciparum can adhere to RBCs via sialic acid, which is highly expressed on glycophorins. Glycophorin-deficient phenotypes, such as En (a–) and U-, are relatively resistant to P. falciparum in vitro. Similar results can be obtained after neuraminidase treatment of RBCs ( ). Recently, P. falciparum P f EBA-175 erythrocyte binding protein was shown to recognize GYPA O-glycans at Ser 66, Ser69, and Thr72 ( ). Selective pressure by P. falciparum may account for some hybrid glycophorins such as GP.Dantu, which lacks critical O-glycans and is protective against severe malaria. In addition, the malarial invasin protein MSP also appears to bind GYPA, in association with Band 3, along the En(a) epitope (aa 31-72) ( ). GYPA is also a receptor for Babesia, another intraerythrocytic parasite ( ).
Historically, the P blood group system consisted of three antigens; P 1 , P k , and P. With the cloning of the glycosyltransferases necessary for their synthesis, the “P group” is now classified into three separate blood group systems containing six antigens. The antigens are assigned based on the last glycosyltransferase necessary for their synthesis. The P1PK system (ISBT 003) encompasses P k (P1PK3), P 1 (P1PK1), and NOR (P1PK4) antigens, which all share a terminal α1,4-Gal. GLOB (ISBT 028) contains P (GLOB1) and PX2 (GLOB4) antigens, which share a terminal β1,3-GalNAc. FORS (ISBT 031) contains a single antigen, Forssman, present on rare Apae RBCs. The LKE or Luke antigen is still classified under GLOB collection 209. Related antigens include globo-ABH (type 4 chain ABH) and galactosylgloboside (Gb5). Globo-A is a characteristic feature of A 1 RBCs and may account for antigenic differences between A 1 and A 2 RBCs.
Similar to the Lewis system (see later discussion), the P blood group antigens are glycosphingolipids, consisting of an antigenically active carbohydrate moiety covalently linked to a ceramide lipid tail. P k and P antigens are high-frequency antigens on most donor RBCs (>99.9%). RBCs are particularly rich in P antigen, which makes up nearly 6% of the total RBC lipid ( ). P k and P antigens are also expressed on nonerythroid cells, including lymphocytes, platelets, plasma, kidney, lung, heart, endothelium, placenta, uroepithelium, fibroblasts, and synovium. In contrast, the P 1 antigen is uniquely expressed on RBCs. Approximately 79% of white and 94% of black donors express P 1 on their RBCs ( Table 36.10 ). P 1 strength is variable between individuals and can be lost with in vitro storage.
| RBC Phenotype | RBC Antigens | Possible Antibodies | MOLECULAR BASIS | FREQUENCIES, % | ||
|---|---|---|---|---|---|---|
| A4GalT1 | B3GALNT1 | White | Black | |||
| P 1 | P k , P, P 1 | None | Normal | Normal | 79 | 94 |
| P 2 | P k , P | Anti-P 1 | Alternate start codon, intron 1 SNPs ∗ | Normal | 21 | 6 |
| Null Phenotypes | ||||||
| P 1 k | ↑P k , P 1 | Anti-P | Normal | Null allele † | Rare | Rare |
| P 2 k | ↑P k | Anti-P, anti-P 1 | Alternate start codon, intron 1 SNPs ∗ | Null allele † | Rare | Rare |
| p | None | Anti-P k PP 1 (Tj a ) | Null allele ‡ | Normal | Rare | Rare |
| Weak Phenotypes | ||||||
| Variant P k | ↑P k , ↓P | Anti-P | Unknown | Unknown | Rare | Rare |
| Weak P | ↓P k , ↓P | None | Unknown | Unknown | Rare | Rare |
∗ Recent studies suggest that P 1 is transcribed by an alternate A4GALT1 transcript and/or regulated by polymorphisms in intron 1 (Thuresson B, Westman JS, Olsson ML: Identification of a novel A4GALT1 exon reveals the genetic basis of the P1/P2 histo-blood groups, Blood 117:678–687, 2011; Lai YJ, Wu WY, Yang CM, et al.: A systematic study of single-nucleotide polymorphisms in the A4GALT1 gene suggests a molecular basis for the P 1 /P 2 blood groups, Transfusion 54:3222–3231, 2014).
† Multiple inactivating mutations have been identified in the B3GALNT1 open reading frame associated with the P k phenotype.
‡ Multiple inactivating mutations have been identified in the A4GALT1 open reading frame associated with the p phenotype (Steffensen R, Carlier K, Wiels J, et al.: Cloning and expression of the histo-blood group P k UDP-galactose: Galβ1-4Glcβ1-1Cer α1,4 galactosyltransferase, J Biol Chem 275:16723–16729, 2000).
Several P blood group phenotypes have been described (see Table 36.10 ). The P 1 and P 2 phenotypes account for more than 99% of donors. Both possess P k and P antigens and differ only in expression of the P 1 antigen. Three autosomal-recessive null phenotypes have been identified, as well as weak variants ( , ; ). The molecular basis for the null phenotypes has been elucidated ( ; ). An association between the P k variant and Luke (LKE)-negative phenotype has been noted in some donors ( ; ). Because they lack P antigen, p and P k individuals are resistant to parvovirus B19 ( ).
Synthesis of the P k , P, and P 1 antigens proceeds from the stepwise addition of sugars to lactosylceramide, a ceramide dihexose (CDH) ( Fig. 36.4 ). The first step is the synthesis of the P k antigen, the ultimate precursor of all globo-type glycosphingolipids. To make P k antigen, Gb 3 synthase (α4GalT1) adds a galactose, in an α1–4 linkage, to CDH. The P k antigen can then serve as a substrate for Gb 4 synthase (β3GalNAcT1). In some cells, including RBCs, the P antigen is further elongated to form additional globo-family antigens, such as Luke (LKE), Forssman, NOR, and type 4 chain ABH antigens (globo-ABH).
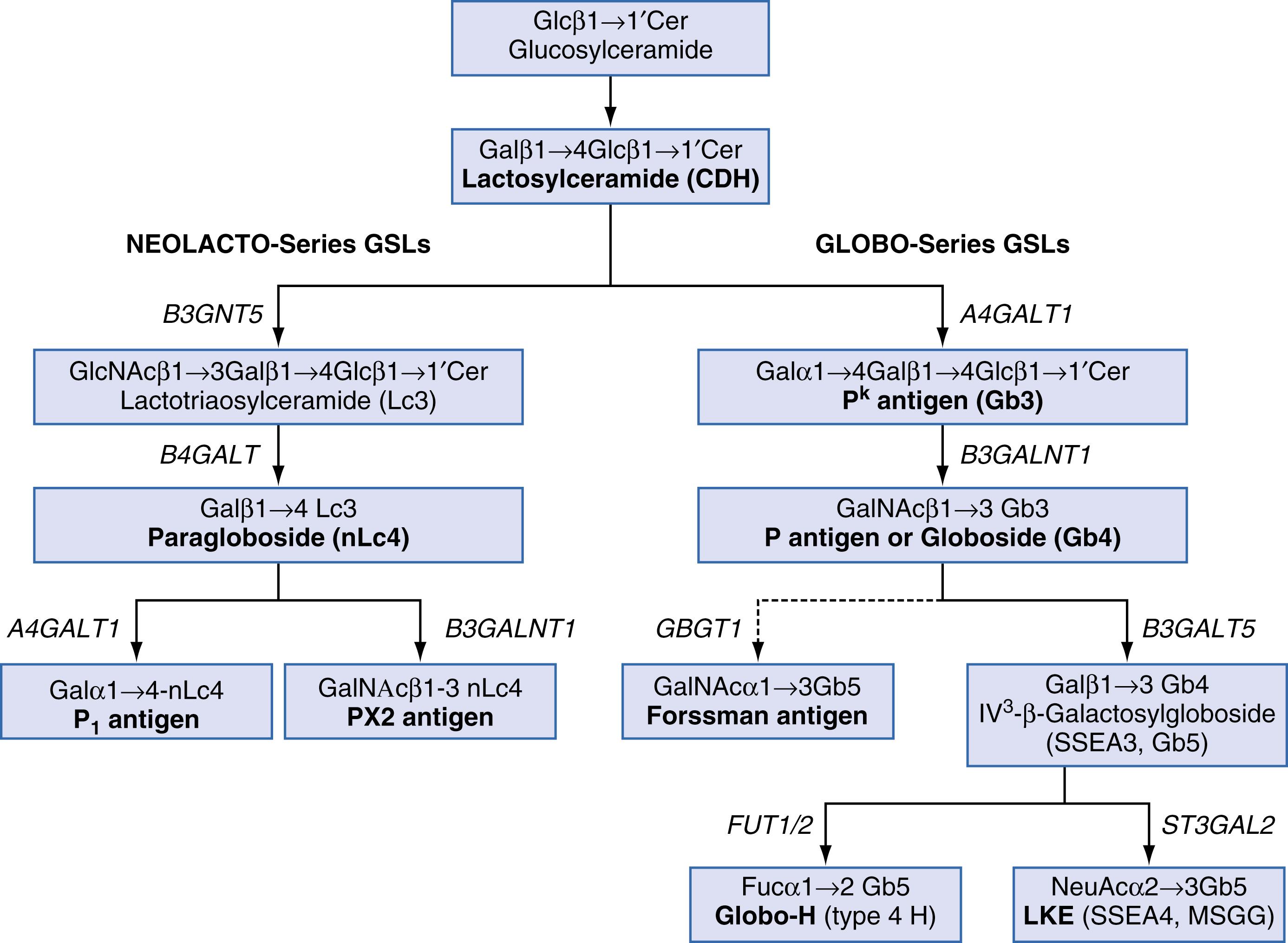
Unlike P k and P antigens, the P 1 antigen is not a globo-glycosphingolipid but is a member of the neolacto-family (type 2 chain glycosphingolipids). In P 1 individuals, a terminal α1,4 galactose is added to paragloboside to form P 1 . Although red cells are rich in type 2 chain glycoproteins, P 1 antigen is only weakly expressed on glycoproteins ( ). PX2 (GLOB4) is a related neolacto-GSL formed by the action of β3GalNAcT1. PX2 is expressed by rare p cells and can react with alloanti-P.
The genes responsible for P k , P 1 , and P have been cloned. As noted, P k , P 1 , and NOR antigens are synthesized by α4GalT1, an α1,4 galactosyltransferase ( ; ). The α4GalT1 gene resides on chromosome 22q13 and is organized into three exons, of which only one (exon 3) encodes the enzyme. The α4GalT1 enzyme is a 353 amino acid type II glycoprotein containing two N -glycosylation sites and five cysteine residues. Similar to many metal-dependent galactosyltransferases, it possesses a DXD motif or a UDP-Gal binding site. A point mutation (C631>G), leading to a Q211E in the mature enzyme, is responsible for the NOR phenotype ( ). The p phenotype is the result of null A4GALT1 alleles (A4GALT∗01N ), leading to a loss of all globo-GSL synthesis. To date, 37 null alleles are reported due to either missense or frameshift mutations ( ).
The molecular basis for the P 1 and P 2 phenotypes remains under investigation. It was originally reported that P 2 was a consequence of a mutation in the A4GALT1 promoter leading to decreased transcription ( ); however, subsequent studies questioned these findings ( ). A later study identified a minor A4GALT1 mRNA arising from an alternate exon and transcription start site. The minor transcript included an additional 28 amino acid peptide and contained a single SNP linked to lower A4GALT1 transcription and P 2 phenotype ( ). This study has now been challenged by a third group. Lai and colleagues (2014) identified 11 SNPs within intron 1, including 8 that showed a linkage with the P 1 /P 2 phenotypes. One SNP (G3084>T; rs5751348) was associated with P 2 and lower A4GALT1 transcription. It was hypothesized that intron 1 contains transcription factor binding sites that regulate P k and P 1 synthesis. Transcription sites for both KLF1 and RUNX1 were identified within intron 1; however, only RUNX1 was shown to bind and effect A4GALT1 expression ( ). Likewise, no significant decrease in A4GALT1 mRNA was observed in a KLF1-null individual ( ).
This has been somewhat challenged by Kaczmarek and colleagues, who carefully examined P 1 expression by serology, flow cytometry, and genotype (Kaczmarek et al., 2018). They were able to show dosage effect in P 1 expression by genotype, with rs5751348 showing the strongest association. However, the amount of P 1 antigen on red cells genotyping as either P 1 P 1 or P 1 P 2 was highly variable between individuals, with significant overlap between both groups. As a consequence, no single SNP can “universally predict the [P 1 ] phenotype” since P 1 expression may be affected by other factors, including transcription factors, paragloboside synthesis, and competing glycolipid pathways (Kaczmarek et al., 2018).
Globoside or P antigen, a β1,3 N -acetylgalactosaminyltransferase, is the product of B3GALNT1 (β3GalT3 in original literature; ). The gene resides on chromosome 3q25 and contains six exons, although only exon 6 encodes the enzyme. A member of the β1,3 galactosyltransferase family, β3GalNAcT1 possesses seven conserved domains common to most β1,3 galactosyltransferases, as well as a DXD motif. The gene is ubiquitously expressed in most tissues. Thirteen null alleles have been reported associated with the P k phenotype. In mice, the absence of β3GALNT1 is lethal ( ).
Forssman antigen is the product of GBGT1 , an α1,3 galactosaminyltransferase related to the ABO gene ( ). Forssman antigen is expressed on many animal species but is typically absent from humans and old-world apes. In humans, GBGT1 is located on 9q24 as a pseudogene. GBGT1 mRNA can be identified in tissues; however, Forssman antigen is not expressed due to two inactivating mutations (Gly230Ser, Gln296Arg) in the translated enzyme. Forssman expression can be observed in the rare weak Apae phenotype. Apae individuals possess an Arg296Gln mutation that restores partial enzyme activity ( ). Forssman antigen can react with human polyclonal anti-A due to the terminal α1,3-GalNAc epitope.
Clinically, the most common antibody observed is anti-P 1 , which is detected in one-quarter to two-thirds of P 2 donors (Issitt & Anstee, 1998). Anti-P 1 is a naturally occurring antibody of IgM isotype and is often detected as a weak, room temperature agglutinin. Rare examples of anti-P 1 are reactive at 37°C or show in vitro hemolysis. Because P 1 expression varies in strength among individuals, anti-P 1 may not react with all P 1 -positive cells tested. Anti-P 1 can bind complement and may be detected in the indirect antiglobulin test (IAT) if polyspecific antihuman globulin (AHG) is used. Antibody reactivity can be eliminated by prewarming sera or by adding soluble P1 substance from hydatid cyst fluid, earthworms, and bird eggs. Anti-P 1 titers are often elevated in patients with hydatid cyst disease or fascioliasis (liver fluke) and in bird fanciers ( ; ). Some examples of anti-P 1 have I blood group specificity (anti-IP 1 ).
In general, anti-P 1 is not clinically significant, and its presence rarely requires transfusion of antigen-negative blood. The exception is seen in patients with an anti-P 1 showing in vitro hemolysis. Because of the risk of immediate and delayed hemolytic transfusion reactions, these patients should receive P 1 -negative (P 2 ), crossmatch-compatible units. Anti-P 1 is not a cause of HDFN.
Anti-PP 1 P k (historically known as anti-Tj a ) is a separable mixture of anti-P, anti-P 1 , and anti-P k in the sera of p individuals. These antibodies are naturally occurring and may be IgM only or IgM plus IgG (IgG3). Because anti-PP 1 P k antibodies are potent hemolysins, patients can be transfused only with p RBCs. In women, alloanti-PP 1 P k and alloanti-P are associated with HDFN and spontaneous abortion. Early and frequent plasmapheresis has been used with therapeutic success in alloimmunized pregnant women of the p and P k phenotypes ( ).
Anti-P is also a naturally occurring IgM alloantibody in the serum of P k (and p) individuals. It is a potent hemolysin and can cause in vivo hemolysis following transfusion of P-positive (P 1 and P 2 ) RBCs. Some alloanti-P can react with PX2 present on p red cells with hemolysis. Alloanti-P is a cause of HDFN and is associated with spontaneous abortions.
An autoantibody with anti-P specificity is seen in patients with paroxysmal cold hemoglobinuria (PCH), a clinical syndrome that may occur in children following viral infection ( ). In PCH, autoanti-P is an IgG, biphasic hemolysin capable of binding RBCs at colder temperatures, followed by intravascular hemolysis at body temperature. This characteristic can be demonstrated in vitro in the Donath-Landsteiner test. See a full description in later sections on immunohematologic methods.
Unlike many antigens, the physiologic role of the P blood group antigens is not known. As GSLs, they are frequently organized into glycolipid-enriched microdomain (GEM) or lipid rafts in the outer cell membrane. GEMs can function as a glycosynapse as well as an organizing platform for glycoproteins, cell adhesion, and cell signaling ( ). Not surprisingly, globo-GSLs demonstrate differential expression during embryogenesis, cellular differentiation, and neoplastic transformation ( ; ). In mice, P and extended globo-GSLs are necessary for embryonic development ( ). The P k antigen is a marker of apoptosis in germinal center B cells, Burkitt lymphoma, and lymphoblastic leukemia ( ). LKE is a marker of embryonic and mesenchymal stem cells ( ) and is implicated in adhesion, cell signaling, and metastasis in renal cell and breast carcinoma ( ; ).
Several P blood group antigens are receptors for microbial pathogens. The P blood group antigen is the receptor for parvovirus B19, a single-stranded deoxyribonucleic acid (DNA) virus associated with multiple clinical sequelae, including aplastic crises ( ; ). P k can bind HIV and may confer resistance to HIV infection ( ). P, P k , and LKE blood group antigens on uroepithelium are cell receptors for P-fimbriae, a bacterial adhesin and colonization factor expressed on uropathogenic Escherichia coli strains. The P antigen was recently found to bind the LPS receptor TLR4-MD2 complex, acting as an antagonist to LPS and blunting inflammation ( ).
The P 1 and P k antigens are receptors for Shiga toxins, produced by Shigella dysenteriae and enterohemorrhagic E. coli (EHEC) strains ( ). In addition to gastroenteritis, EHEC infection is the most common cause of community-acquired hemolytic-uremic syndrome, probably reflecting toxin binding to P k antigen on glomerular vascular endothelium and platelets ( ). The P k antigen also serves as a receptor for Streptococcus suis ( ).
The first and most clinically important characterization of the Rh system antigens came when published studies of animal experiments involving the immunization of guinea pigs and rabbits with rhesus monkey RBCs. The resulting antiserum agglutinated 85% of human RBCs, and the antigen defined was called the Rh (rhesus) factor . This anti-Rh was later reported to have the same specificity as antibodies studied earlier by that were responsible for HDFN. It is interesting to note that the anti-Rh developed by Landsteiner and Wiener was later shown to recognize a different blood group antigen, named LW for its discoverers.
Today, the Rh system is probably the most complex red cell antigen system in humans, encompassing over 62 antigens, many phenotypic variants, and complex serologic relationships. Hence, the following review is basic and highlights the most current information. For a detailed historical review of the Rh system, readers should consult and .
Using five basic antisera—anti-D, anti-C, anti-E, anti-c, and anti-e—Wiener identified five different factors or antigens ( Table 36.11 ) that, from population and family studies, appeared to be inherited as two complexes of up to three factors each. Eight possible combinations of three-factor complexes were identified if one included “d” as designating the lack of D, because no anti-d had ever been demonstrated. Wiener proposed a single-locus inheritance system with eight alternative common alleles coding for two Rh agglutinogens, capable of expressing up to three different antigenic determinants. Wiener’s nomenclature for the eight different genes and allelic frequencies is provided in Tables 36.12 and 36.13 .
| Wiener | Fisher-Race | Rosenfield |
|---|---|---|
| Rho | D | RH1 |
| rh′ | C | RH2 |
| rh″ | E | RH3 |
| hr′ | c | RH4 |
| hr″ | e | RH5 |
| Wiener | Fisher-Race † | FREQUENCIES IN U.S. POPULATION | |||
|---|---|---|---|---|---|
| White | Black | Native American | Asian | ||
| R0 | Dce | 0.04 | 0.44 | 0.02 | 0.03 |
| R 1 | DCe | 0.42 | 0.17 | 0.44 | 0.70 |
| R 2 | DcE | 0.14 | 0.11 | 0.34 | 0.21 |
| Rz | DCE | 0.00 | 0.00 | 0.06 | 0.01 |
| r | Ce | 0.37 | 0.26 | 0.11 | 0.03 |
| r′ | Ce | 0.02 | 0.02 | 0.02 | 0.02 |
| r″ | cE | 0.01 | 0.00 | 0.01 | 0.00 |
| r y | CE | 0.00 | 0.00 | 0.00 | 0.00 |
∗ Composite figures calculated from Mourant AE, Kopec AC, Domaniewska-Sobczak K: The distribution of the human blood groups and other biochemical polymorphisms , ed 2, Oxford, 1976, Oxford University Press.
† In historical Fisher-Race nomenclature, RhD negative was designated as “d.” At this time, RhD-negative phenotypes using Fisher-Race denote only the RHCE antigens present on red cells.
| REACTION WITH ANTI- † | PHENOTYPE | GENOTYPE | FREQUENCIES, n (%) ‡ | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| D | C | c | E | e | Rh | DCE | Rh | DCE | White | Black | Native American | Asian |
| + | + | + | + | + | Rh 1 Rh 2 | DCcEe | R 1 R 2 | DCe/DcE | 0.1176 (89) | 0.0374 (100) | 0.2992 (89) | 0.294 (97) |
| R 1 r″ | DCe/cE | 0.0084 (6) | 0.0088 (3) | |||||||||
| r′R 2 | Ce/DcE | 0.0056 (5) | 0.0135 (4) | 0.0084 (2.8) | ||||||||
| rR z | ce/DCE | 0.0132 (4) | 0.0006 (0.2) | |||||||||
| + | + | + | – | + | Rh 1 rh | DCce | R 1 R 0 | DCe/Dce | 0.0168 (5) | 0.1495 (63) | 0.0176 (15) | 0.042 (50) |
| R 1 r | DCe/ce | 0.3108 (95) | 0.0884 (37) | 0.0968 (85) | 0.042 (50) | |||||||
| + | – | + | + | + | Rh 2 rh | DcEe | R 2 R 0 | DcE/Dce | 0.0112 (10) | 0.0968 (63) | 0.0136 (15) | 0.0126 (50) |
| R 2 r | DcE/ce | 0.1035 (90) | 0.0572 (37) | 0.0748 (85) | 0.0126 (50) | |||||||
| + | + | – | – | + | Rh 1 Rh 1 | DCe | R 1 R 1 | DCe/DCe | 0.176 (91) | 0.029 (81) | 0.194 (92) | 0.490 (93) |
| R 1 r′ | DCe/Ce | 0.017 (9) | 0.007 (19) | 0.017 (8) | 0.028 (7) | |||||||
| + | + | – | + | + | Rh 1 Rhz | DCEe | R 1 R z | DCe/DCE | 0.053 (100) | |||
| + | – | + | + | – | Rh 2 Rh 2 | DcE | R 2 R 2 | DcE/DcE | 0.02 (88) | 0.012 (100) | 0.116 (94) | 0.044 (100) |
| R 2 r″ | DcE/cE | 0.003 (12) | 0.007 (6) | |||||||||
| + | + | + | + | – | Rh 2 Rhz | DCcE | R 2 R z | DcE/DCE | 0.041 (100) | |||
| + | – | + | – | + | Rh 0 Rh 0 | Dce | R 0 R 0 | Dce/Dce | 0.0016 (5) | 0.1936 (46) | 0.0004 (8) | 0.0009 (33) |
| R 0 r | Dce/ce | 0.0296 (95) | 0.2286 (54) | 0.0044 (92) | 0.0018 (67) | |||||||
| – | – | + | – | + | rhrh | ce | rr | ce/ce | 0.1369 (100) | 0.0676 (100) | 0.0121 (100) | 0.0009 (100) |
| – | + | + | – | + | rh′rh | Cce | rr′ | ce/Ce | 0.0055 (100) | 0.0014 (100) | 0.0044 (100) | 0.0012 (100) |
| – | – | + | + | + | rh″rh | cEe | rr″ | ce/cE | 0.0028 (100) | 0.0022 (100) | ||
∗ Estimated from haplotype frequencies (p, q from Table 36.12 ), using p2 for homozygotes and 2pq for heterozygotes.
Fisher and Race later proposed a different inheritance theory and nomenclature system based on genetic evidence of the antithetical or allelic nature of the C/c and E/e antigens ( ). These investigators proposed a system of three closely linked loci or subloci on each chromosome, which were inherited as a block of genes (haplotype). They also introduced the DCE nomenclature to name the alleles, including the use of “d” to designate the lack of D locus (see Table 36.11 ). Rosenfeld proposed a numeric system of naming the antigens in 1962, because the increasing number of Rh antigens rendered an alphabetic notation impractical ( Table 36.14 ). It was also appreciated that this nomenclature contained no inferences as to the genetic inheritance of the antigens. The Rh numbering system only weakly applies to naming Rh alleles, which assigns the allele based on gene ( RHD or RHCE ). For Rh antigens on RHCE protein, allelic variants are further classified based on expression of both C/c and E/e antigens: RHCE∗01 =ce; RHCE∗02 =Ce; RHCE∗03 =cE; RHCE∗04 =CE.
| ISBT | Name | Frequency, % | RH Protein (D or CE) | Molecular Basis (Protein or Gene Exon) | Comments |
|---|---|---|---|---|---|
| RH (ISBT 004) | |||||
| RH1 | D | 85–92 | D | RHD, loops 3, 4, 6 | |
| RH2 | C | 68% whites 27% blacks |
CE | Ser103 + Cys16 | Antithetical RH4 |
| RH3 | E | 22–29 | CE | Pro226 | Antithetical RH5 |
| RH4 | C | 80 | CE | Pro103 | Antithetical RH2 |
| RH5 | E | 80 | CE | Ala226 Dependence on Arg229 |
Antithetical RH3 |
| RH6 | F | 65 | CE | Pro103 + Ala226 Dependence on Arg229 |
Compound antigen |
| RH7 | Ce | 27–28 | CE | Ser103 + Ala226 | Compound antigen |
| RH8 | C w | 1–2 | CE | Gln41>Arg | Antithetical RH51 |
| RH9 | C x | <0.01 | CE | Ala36>Thr | Antithetical RH51 |
| RH10 | V | 30% blacks | CE | Leu235>Val, +Gly336 | Often with RH20 |
| RH11 | E w | <0.01 | CE | Met167>Lys | E variant type I |
| RH12 | G | 84–92 | D, CE | Ser103 | Anti-C+D |
| RH17 | Hr 0 | 100 | CE | RHCE loops 3,4,6 | |
| RH18 | Hr | 100 | CE | Met238 | |
| RH19 | hr S | 98 | CE | Ala226, Met238 | |
| RH20 | VS | 40% blacks | CE | Leu226>Val | Often with RH10 |
| RH21 | C G | 68 | CE | Ser103 | |
| RH22 | CE | 1 | CE | Ser103 + Pro226 | Compound antigen |
| RH23 | D w | <0.01 | Partial D | Gln233, RHD loop 3,6 | |
| RH26 | c-like | 80 | CE | Gly96 + Pro103 | Related RH55 |
| RH27 | cE | 22–28 | CE | Pro103 + Pro226 | Compound antigen |
| RH28 | hr H | <0.01 | ? | Unknown | |
| RH29 | Total Rh | 100 | CE + D | RHD + RHCE | Made by Rh null |
| RH30 | Go a | 2% blacks | Partial D | On DIV a | |
| RH31 | hr B | 98 | CE | Unknown | Missing on R2R2 |
| RH32 | RN | <1% blacks | Partial D | Exons D4–CE5 | Antithetical RH46 |
| RH33 | Har | <0.01 | Partial D | Exons CE4–D5 | R 0 Har |
| RH34 | Hr B | 100 | D + CE | Cys336 | |
| RH35 | 1114(CeMA) | <0.01 | CE | Unclear; CeMA is JAL+ | Weak C,e |
| RH36 | Be a | <0.1 | CE | Pro221>Arg | Weak c,e,f |
| RH37 | Evans | <0.01 | D–CE hybrid | Exons D6–CE7 | |
| RH39 | C-like | 100 | ? | Unknown | On C– and C+ RBCs |
| RH40 | Tar | <0.01 | D | Leu100>Pro | |
| RH41 | Ce-like | 70 | CE | Exon 2, Ala226 | |
| RH42 | Ce S | 2% blacks | Partial D | Leu245>Val | Associated dCce S |
| RH43 | Crawford | 0.7% D– blacks | CE | Gln223Glu, VS+ | ce S variant; VS+, V+ |
| RH44 | Nou | 100 | ? | Unknown | |
| RH45 | Riv | <0.01 | Partial D | On DIVa | |
| RH46 | Sec | 100 | CE | CE exon 4 | Antithetical RH32 |
| RH47 | Dav | 100 | CE | Exon 7 | |
| RH48 | JAL | <0.01 | CE | Arg114Trp or Glu | Antithetical RH57 |
| RH49 | STEM | 0.4% Indians | CE | W16C,M238V, L278V | |
| RH50 | FPTT | <0.01 | Partial D | Exons CE4–D5 | |
| RH51 | MAR | >99 | CE | Ala36, Gln41 | Antithetical RH8,9 |
| RH52 | BARC | <0.01 | Partial D | Exons CE6–D7 | |
| RH53 | JAHK | <0.01 | CE | Exon D2 (Ser103), no Cys16 | |
| RH54 | DAK | <0.01 | Partial D | Unknown | |
| RH55 | LOCR | <0.01 | CE | Gly95>Ser | Weak c, e, f; Rh26± |
| RH56 | CeNR | <0.01 | CE–D hybrid | Complex epitope | D– –; RH32+ |
| RH57 | CEST | >99 | CE | Arg114 | Antithetical RH48 (Jal) |
| RH58 | CELO | <0.01 | CE | Trp16,Gln233,Leu245 | Antithetical RH43 |
| RH59 | CEAG | <0.01 | CE | Ala85 | RH31(-), partial e |
| RH60 | PARG | <0.01 | CE | Met167Ile | Ce allele |
| RH61 | CEVF | >99% | CE | Trp16Cys,Val233Phe | Absent on ceMO |
| RH62 | CEWA | >99% | CE | Leu38Phe | |
| RHAG (ISBT 030) | |||||
| RHAG1 | Duclos | >99 | RhAg | Gln106>Glu | |
| RHAG2 | Ol a | <0.01 | RhAg | Ser227>Leu | Weak Rh expression |
| RHAG3 | DSLK | >99 | RhAg | Lys164>Gln | |
| RHAG4 | RHAG4 | <0.01 | RhAg | Val270Ile | |
Tremendous progress has been made in deciphering the biochemistry and molecular biology of the Rh blood group system. It is now clear that the Rh complex consists of three integral membrane proteins: RhD, RhCE, and Rh-associated glycoprotein (RhAg). RhD and RhCE are highly homologous proteins, differing by approximately 30 amino acids. Both are 30-kD, 417 amino acid multipass proteins containing 12 transmembrane domains, six extracellular loops, and a cytoplasmic amino- and carboxy-terminus ( Fig. 36.5 ). Both proteins possess two to three molecules of palmitate (C16 fatty acid) covalently linked to transmembrane cysteine residues. Palmitoylation of Rh proteins may help maintain the phospholipid asymmetry of the RBC membrane ( ).
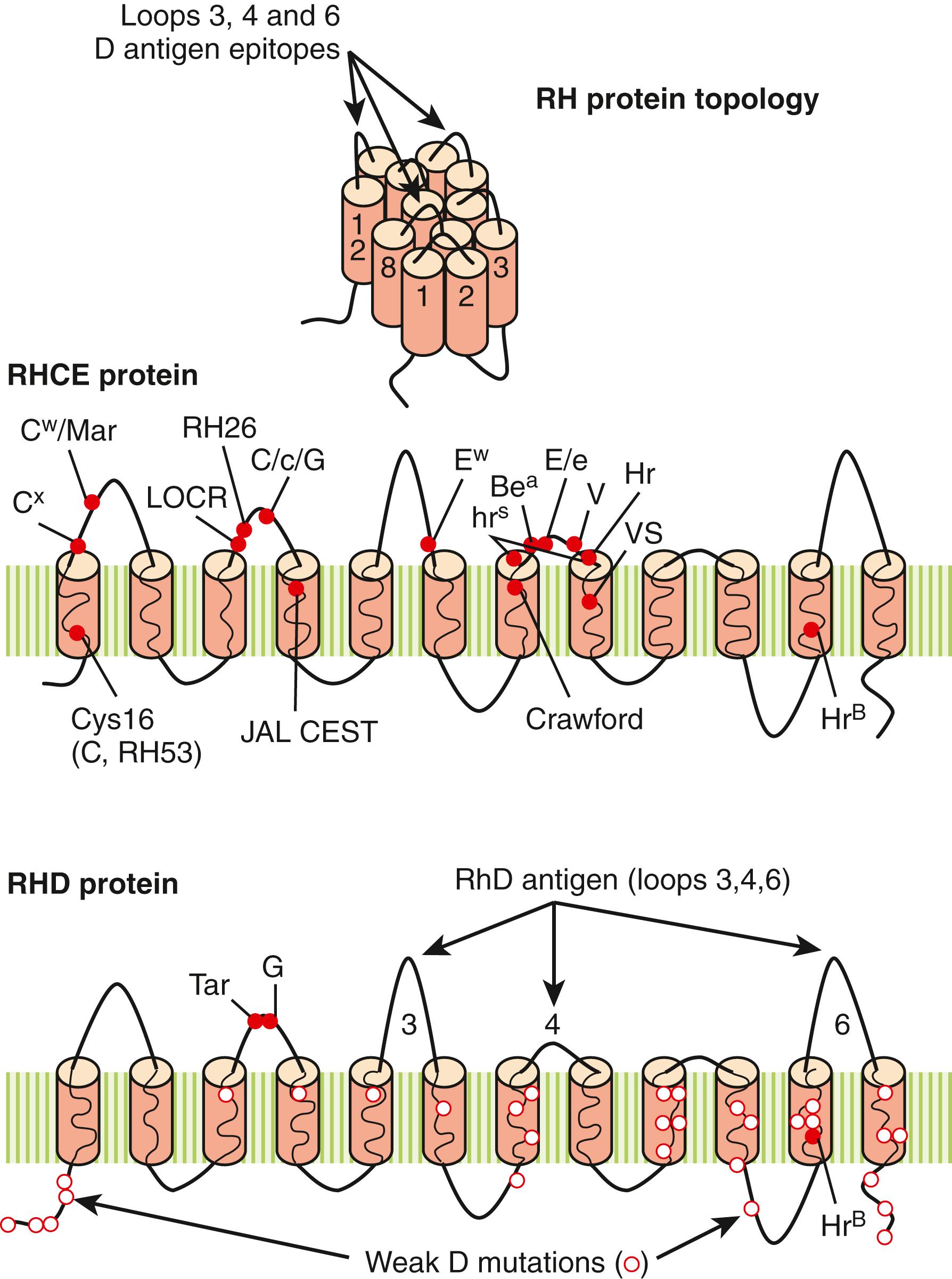
RhAg is a 45- to 70-kD multipass glycoprotein, evolutionarily related to RhD and RhCcEe glycoproteins. RhAg is a 409 amino acid glycoprotein with 12 transmembrane domains and a single large N -linked carbohydrate side chain on the first extracellular loop. Overall, approximately 170,000 molecules each of Rh and RhAg proteins are present per RBC. RhD, RhCE, and RhAg are erythroid-specific proteins ( ).
In the RBC membrane, RhD, RhCE, and RhAg proteins exist as a trimer, composed of three molecules of Rh (RhD and RhCE) and RhAg ( ). The importance of RhAg for the expression and correct assembly of Rh proteins cannot be understated. In the absence of functional RhAg protein, neither RhD nor RhCE proteins will be expressed (Rh null and Rh mod phenotypes). In addition to RhAg, Rh proteins may be topologically associated with CD47 (Lutheran), ICAM4 (LW), DARC (Duffy), Band 3 (Diego), and GYPB. At least two non-Rh antigens, Fy5 and U, may require noncovalent interactions between Rh, DARC glycoprotein (Fy5), and GYPB (U), respectively ( ). Rh is associated with the Band 3-ankyrin metabolon.
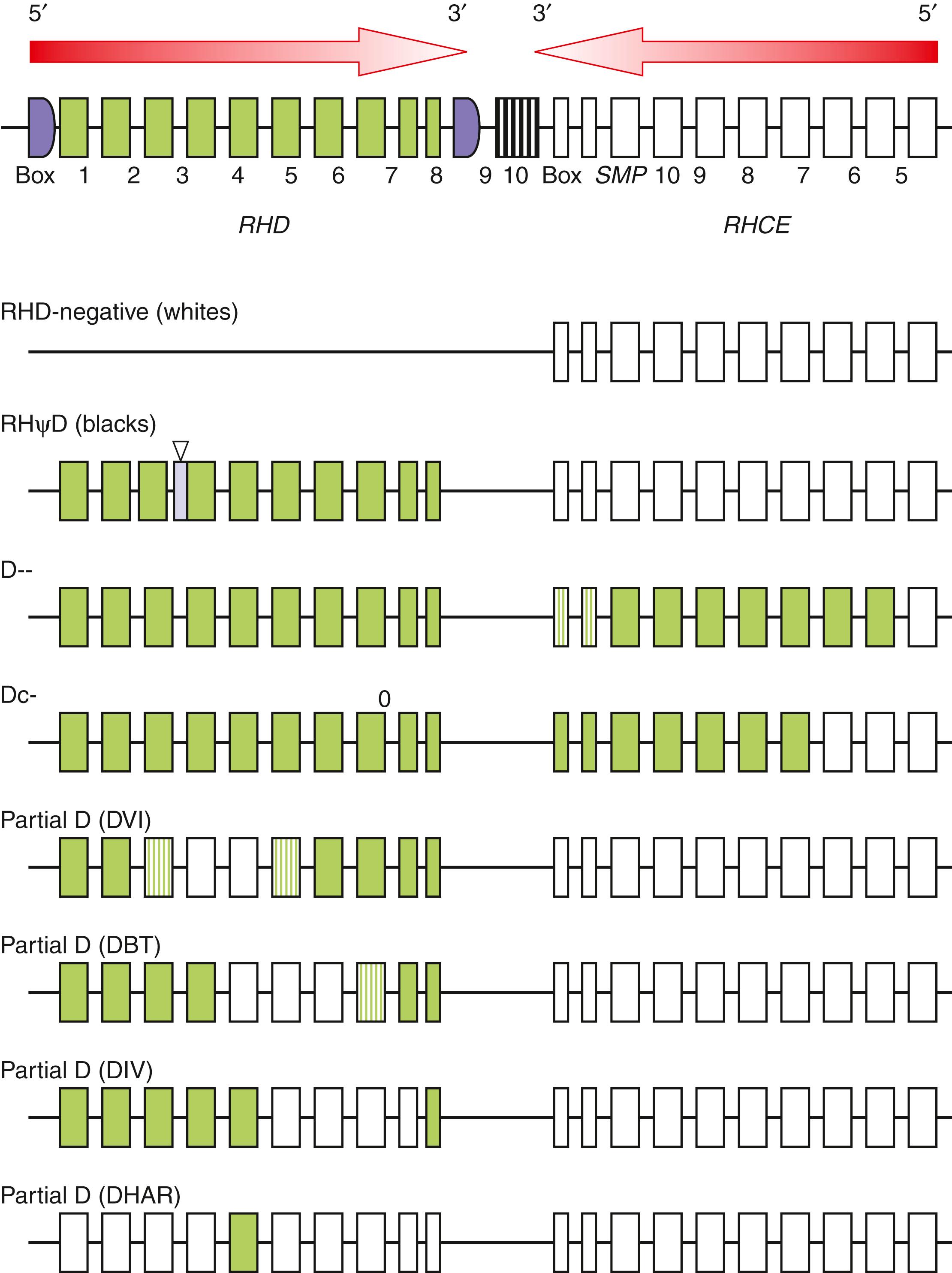
The genes for RhD ( RHD ) and RhCE ( RHCE ) proteins span 65 kb on chromosome 1p34-36.1 and share nearly 92% sequence identity. The two genes are separated by only 30 kb and have opposite orientations, facing each other at their 3′ ends ( Fig. 36.6 ). The RHD gene is also flanked by two homologous sequences known as rhesus boxes. The RHAG gene ( RHAG ) resides on chromosome 6p11.1 and shares 36% homology with the RHD and RHCE genes. All three genes possess 10 exons and at least one GATA-1 consensus sequence in the promoter region ( ; ). It is believed that RHAG and RH genes arose by gene duplication 250 to 350 million years ago. A second gene duplication 8 to 11 million years ago resulted in the ce and D alleles (cDe or R 0 haplotype). The remaining RHCe alleles are believed to be the product of point mutations, recombination, and gene conversion of the RHD and RHCE genes ( ).
The cloning of RHD and RHCE genes opened the door to understanding the complex immunology of many Rh antigens and phenotypes. The D antigen, the most immunogenic of all of the Rh antigens, resides on the RHD protein. Current evidence suggests that D is a highly complex antigen, depending on both specific amino acids and the tertiary structure of the RHD protein itself. At least nine “D-specific” amino acids (Met169, Met170, Ile172, Phe223, Ala226, Glu233, Asp350, Ala353, and Gly354) have been identified as functional D epitopes. The nine amino acids lie along the third, fourth, and sixth external loops of the RHD protein, creating six distinct D-epitope clusters or footprints (see Fig. 36.5 ). Expanded studies have identified 30 distinct D epitopes on RhD ( ).
Approximately 1% of D-positive individuals type as weak D (historically known as D u ), characterized by weak or absent RBC agglutination by anti-D at immediate spin during routine serologic testing. In weak D individuals, the D antigen usually requires enhancement with AHG owing to a quantitative decrease in RhD protein. In these individuals, the number of RhD molecules is decreased 40- to 100-fold, ranging from 66 to 5200 molecules per red cell ( ). A weak D phenotype can occur with many partial D phenotypes, Ce in trans with suppression of RHD , in the Rh mod phenotype, and via autosomal-recessive inheritance of two weak RHD alleles. To date, 147 weak D types are recognized. Some authors now refer to “weak D” as variant D alleles since the phenotype can arise from diverse genetic backgrounds ( ).
Many of the weak D alleles encode intact RhD with missense mutations along the transmembrane and cytoplasmic domains that may interfere with trafficking, assembly, or membrane stability (see Fig. 36.5 ) ( ). Weak D types 1, 2, and 3 belong to this category of weak D alleles and account for >90% of weak D alleles in whites. In general, these weak D genotypes are not at risk for making alloanti-D ( ; ). In contrast, the common weak D variant alleles observed in blacks (type 4, DAR) are at potential risk for making anti-D following transfusion of Rh-positive red cells. The most common weak D allele in Asian countries is DEL (D el ), which results in extremely weak D expression that can be detected only by adsorption and elution due to unstable splicesite variants ( ). As a result, D el individuals can type as Rh negative even in the presence of AHG enhancement. Despite extremely low aberrant D expression, D el individuals are not at risk for HDFN or anti-D if exposed to Rh-positive blood. D el red cells can induce anti-D in Rh-negative recipients ( ).
Partial D antigens are RHD proteins with missing D epitopes. Although they type as D-positive, persons with partial D antigens can make alloanti-D antibodies reactive with allogeneic, but not autologous, RBCs. The alloanti-D produced by these individuals recognizes D-specific epitopes missing on their own RBCs (see Fig. 36.6 ). Partial D can result from missense mutations (ex. DAR) or, more commonly, from genetic recombination of RHD and RHCE genes ( ). Partial D phenotypes arising from genetic recombination are frequently associated with the generation of new low-incidence antigens on both RhD and RhCE proteins (see Table 36.14 ). Studies of African Americans have shown that 29% carry altered RHD alleles and 6% possess partial D alleles ( ).
Rh-negative (D–) occurs in approximately 15% of white donors, almost always in association with a ce/ce or rr phenotype. In most whites, D– reflects a deletion of the entire RHD gene (see Fig. 36.6 ). In blacks, D– can result from gene deletion or from inheritance of an RHD pseudogene. Nearly 60% of D-negative black people inherit a mutant RHD allele (RHψD) containing a 37–base pair (bp) internal duplication, frameshift, and premature stop codon ( ). RHD genes containing nonsense mutations and nucleotide deletions have also been reported in some D-negative Japanese and white donors ( ; ).
In contrast to the complexity of the D antigen epitope, the C/c and E/e antigens are single–amino acid polymorphisms on the RhCE protein (see Fig. 36.5 ). Three additional amino acid polymorphisms (Cys16Trp, Ile60Leu, and Ser68Asn) may help stabilize the C and c antigens. Arg229 appears critical to e and f expression ( ). Because C/c and E/e epitopes are present on the same protein, alloantibodies dependent on the expression of both C/c and E/e antigens can occur. Four alloantibodies with “compound” Rh specificity have been described: anti-ce (f or RH6), anti-Ce (RH7), anti-cE (RH27), and anti-CE (RH22). These antibodies react only with RBCs carrying the appropriately paired antigens, in cis , on the same RhCE protein (see Table 36.14 ). The RHCE protein is home to several additional high- and low-incidence antigens.
The G antigen (RH12) is a high-frequency antigen present on virtually all D-positive and C-positive RBCs. G has been identified as Ser103, a C-type antigen, on RHD and RHCE proteins (see Fig. 36.5 ). It is not surprising that anti-G alloantibodies have both anti-C and anti-D specificity (anti-C + D) and are frequently accompanied by anti-C (anti-G + anti-C) ( ).
Similar to D, phenotypes with weakened or absent C/c and E/e are known. RHCE alleles with point mutations, deletions, and recombinations can lead to weakened CcEe expression. African ethnic groups, in particular, have a high incidence of altered RHCE alleles (53%) ( ). Studies have reported partial C phenotype in 21% to 25% of sickle cell patients ( , ). Likewise, point mutations and rearrangements resulting in weakened c and e expression are common among African Americans (18% to 19%) ( ; ; ). Like partial D, patients with altered RHCE alleles can make alloantibodies against C, c, and e antigens ( ; ). In one study, 23% of autoantibodies were actually Rh alloantibodies in patients homozygous for altered RHCE alleles ( ).
The deletion phenotypes D••, D–– (↑D+, C–c–E–c–), DC–, and Dc– are the result of genetic recombination between RHCE and RHD genes to yield RHCE-D-CE hybrids (see Fig. 36.6 ). As a consequence, total ablation of Ee and/or Cc epitopes occurs, with many recombinants expressing new low-frequency Rh antigens (see Table 36.14 ). Furthermore, many recombinants (e.g., D••, D––) demonstrate exalted D antigen expression on serologic testing owing to a double dose of some D epitopes on both RHD and mutant RHCE-D-CE proteins. For a detailed summary, the reader is referred to Reid and Lomas-Francis (2004).
RHAG is a separate blood group system with three antigens: Ol a , Duclos, and DSLK ( ; ). All three are the result of an amino acid polymorphism in the RhAg protein. Both Duclos and DSLK are associated with weak U expression, suggesting an interaction between RhAg and GYPB.
Rh null erythrocytes lack all Rh antigens as a result of an apparent absence of RhD and RhCE proteins. In addition, Rh null erythrocytes lack the high-frequency antigens Fy5 and LW and may have markedly decreased expression of S/s and U antigens. The absence of these non-Rh antigens reflects the complex topologic association of Duffy, LW, and GYPB proteins with Rh proteins on RBC membranes.
Extremely rare (<1 in 6 million), Rh null cells have abnormalities in RBC morphology (spherocytes, stomatocytes), water content, cell volume, cation fluxes, carbon dioxide (CO 2 ) permeability, and phospholipid asymmetry ( ; ). Rh null cells show increased osmotic fragility and a shortened circulating half-life, often accompanied by a mild hemolytic anemia (Rh deficiency syndrome). Because Rh null individuals can become sensitized to multiple Rh antigens, including high-frequency antigens, transfusion support can be quite difficult. Some alloimmunized Rh null individuals can produce anti-RH29, which reacts with all RBCs except Rh null .
The Rh null phenotype can arise from two distinct genetic backgrounds: regulator and amorph. The Rh null -amorph type is the result of nonsense mutations in the RHCE gene in D-negative people. Because of the absence of RhD and RhCE proteins, Rh null -amorph RBCs have reduced (but not absent) expression of RhAg protein ( ). The Rh null regulator type arises from mutations in RHAG ( ). To date, there are 17 RHAG alleles associated with Rh null -regulator phenotype ( RHAG∗01N ).
Mutations in RHAG are also observed in the Rh mod phenotype. Rh mod RBCs have markedly decreased Rh and RhAg expression, detectable only by careful adsorption and elution studies. Similar to Rh null individuals, persons with Rh mod may have laboratory evidence of Rh deficiency syndrome with a mild hemolytic anemia. Most (82%) Rh mod alleles ( RHAG∗01M ) possess missense mutations ( ).
Antibodies against Rh antigens are routinely encountered in the blood bank (see Table 36.5 ). D is the most immunogenic Rh antigen, followed by c, E, C, and e ( ). In general, antibodies against Rh antigens are the result of immune stimulation by transfusion or pregnancy. Exceptions include some examples of anti-C w and anti-E, which can be naturally occurring. Most antibodies against Rh antigens are of IgG isotype (IgG 1 and IgG 3 ), although rare examples of IgM and IgA are known. Anti-Rh antibodies are reactive at 37°C and are usually detected in the AHG phase of testing. The reactivity of anti-Rh antibodies can be enhanced with enzyme-treated RBCs. The gel method is extremely sensitive for the detection of Rh antibodies ( ).
Clinically, antibodies against Rh are associated with hemolytic transfusion reactions. However, because Rh antibodies do not fix complement, incompatible RBCs are almost always cleared through extravascular destruction. To prevent sensitization to the D antigen, Rh-negative patients should be transfused with Rh-negative RBCs. This is particularly true for young girls and women of childbearing age. For transfusion in alloimmunized patients, RBC units should be negative for the Rh antigen of interest and crossmatch compatible with the recipient’s serum through the AHG phase of testing.
Selection of units matched for D, C/c, and E/e antigens to prevent Rh alloimmunization is now standard practice for sickle cell anemia and other hemoglobinopathy patients. Historically, alloimmunization rates in sickle cell patients were as high as 50%, with most patients making at least one anti-Rh antibody ( ). Even with extended Rh matching, alloimmunization remains a significant issue in this population due to variant RHD and RHCE alleles, leading to efforts to provide genotyped units to at-risk patients ( ; ). Another example in which extended Rh matching is performed is R 1 R 1 (DCe/DCe) patients who have developed anti-E alloantibodies. Because these patients are at increased risk of delayed hemolytic transfusion reactions because of the subsequent development of anti-c, many blood bankers advocate transfusing only R 1 R 1 units to R 1 R 1 patients ( ).
Antibodies against Rh antigens are also a major cause of HDFN. All Rh-negative women should receive Rh immunoglobulin (IgG anti-D, RhIG) prophylactically in midpregnancy, following an invasive procedure (i.e., amniocentesis), and immediately after delivery to prevent alloimmunization. Rh immunoglobulin prophylaxis is also recommended in women with partial D and some weak D phenotypes because these women can be at risk for D alloimmunization ( ). Proposed algorithms have recommended RHD genotyping for women with discrepant or weak agglutination (1+–2+) by serology using commercial anti-D typing reagents. Women with weak D types 1, 2, or 3 are not at risk for alloimmunization and are not candidates for RhIG prophylaxis. Women with partial D or other weak D genotypes should receive RhIG ( ).
RhIG may also be given following transfusion of RhD+ platelet concentrates or after accidental transfusion of RhD+ RBCs. In the latter, RhIG is given after two-volume RBC exchange with RhD-negative RBCs ( ). Administration of one vial of RhIG is recommended for every 30 mL whole blood or 15 mL packed RBCs transfused ( ). RhIG should be given within 72 hours of exposure to prevent active immunization. RhIG is not given to Rh-negative women who are already immunized to D antigen (i.e., have anti-D).
It is also recommended to give RhIG to Rh-negative women with anti-G alloantibodies. As stated earlier, anti-G behaves as an anti-C + anti-D because of recognition of a Ser103 or C-type antigen on both RhD and RhCE proteins. In general, HDFN secondary to anti-G or anti-C + anti-G is mild when compared with HDFN due to anti-D. However, because these women may still become immunized to D-specific epitopes on the RhD protein, many blood bankers advocate giving RhIG to Rh-negative women with anti-G antibodies ( ). Separation of anti-G from a true anti-C + anti-D is very laborious, requiring sequential adsorption and elution ( ). One clue suggesting the presence of anti-G is an anti-C titer at least fourfold higher than anti-D ( ). It is not necessary, however, to separate anti-G from anti-C + anti-D for routine transfusion. With very rare exceptions, RBCs negative for D and C antigens are also negative for G antigen.
The specific physiologic function of the Rh proteins is still unclear; however, there is a strong belief that Rh proteins may constitute a membrane transporter. The Rh proteins are homologous with ammonium transporters of the Mep/Amt family. In addition, Rh-like homologues have been discovered in invertebrate life forms such as the nematode worm Caenorhabditis elegans and a marine sponge, Geodia cydonium ( ). Evidence suggests that RhAG is capable of facilitating NH 4 and CO 2 transport ( ).
The Lutheran (Lu) blood group system contains 22 antigens, including four pairs of allelic antigens and 14 high-incidence antigens ( ). Lutheran is a minor constituent of RBC membranes, averaging only 2000 to 5000 molecules per cell, and can vary in strength between donors ( ). Even within a single individual, considerable heterogeneity in Lutheran expression is seen, with only 40% to 50% of red cells positive for Lutheran antigens by flow cytometry ( ). Lutheran appears on red cells at the orthochromatic erythroblast stage, concurrent with binding of red cells to laminin ( ). Lutheran antigens are ubiquitously expressed on human tissues. In addition to RBCs, Lutheran glycoprotein is expressed by the colon, small intestine, ovary, testis, prostate, thymus, spleen, pancreas, kidney, skeletal muscle, liver, lung, placenta, brain, heart, and bone marrow ( ).
A Lu(a–b–) phenotype can occur in three settings with distinct patterns of inheritance: autosomal recessive, autosomal dominant ( In[Lu] ), and X-linked recessive ( Table 36.15 ). The autosomal-recessive phenotype is a true null phenotype, characterized by complete absence of all Lutheran antigens on RBCs. As a consequence, these individuals can make an alloantibody to Lutheran glycoprotein (anti-Lu3) that reacts with all Lu-positive RBCs. The Lu null phenotype is extremely rare and is not associated with altered expression of other red cell antigens.
| Reactions With Anti- | Phenotype | FREQUENCY IN U.S. POPULATION, % | Comments | ||||
|---|---|---|---|---|---|---|---|
| Lu a | Lu b | Lu-3 | White | Black | |||
| + | 0 | + | Lu (a+b–) | 0.1 | 0.1 | ||
| + | + | + | Lu (a+b+) | 7 | 5 | ||
| 0 | + | + | Lu (a–b+) | 93 | 95 | ||
| Null Phenotypes (Lu null ) | |||||||
| 0 | 0 | 0 | Lu (a–b–) | Very rare | Very rare | Autosomal recessive Normal CD44, i/I, CD75 Make an anti-Lu-3 |
|
| 0/W∗ | 0/w ∗ | w ∗ | Lu (a–b–) | Very rare | Very rare | In (Lu), autosomal dominant ↓CD44, I/i; ↑CD75 ELKF mutation |
|
| 0/W∗ | 0/w ∗ | w ∗ | Lu (a–b–) | Very rare | Very rare | X-linked recessive, GATA1 mutation nl CD44, i, P1 | |
∗ Weak Lu antigens detected only by adsorption and elution techniques.
The autosomal-dominant and X-linked–recessive forms are characterized by very weak Lutheran expression, often detected only after adsorption and elution. In(Lu) is an autosomal-dominant Lu mod phenotype with an incidence of 0.02% to 0.12% ( ). In addition to Lutheran antigens, In(Lu) red cells can show weakened expression of P 1 , i, Indian/CD44, Knops/CD35, OK/CD147, LW, and Colton antigens and enhanced expression of CDw75 ( ; ; ). The X-linked Lu mod phenotype has weakened Lutheran; enhanced i and CDw75; and normal P 1 , i, and CD44 expression. Because both In(Lu) and X-linked–recessive Lu mod phenotypes express some Lutheran antigen on RBCs and other tissues, neither is associated with the development of anti-Lu3. In(Lu) RBCs can display subtle abnormalities, including increased poikilocytosis and increased hemolysis, during in vitro storage ( ). Cases of congenital dyserythropoietic anemia (CDA) have also been linked to the In(Lu) phenotype ( ; ).
The Lutheran antigens reside on two isomeric, type 1 glycoproteins: Lu-glycoprotein (85 kD) and epithelial cancer antigen (B-CAM, 78 kD) ( ). The 85-kD glycoprotein is the predominant isoform found on RBCs and normal tissues. Members of the Ig superfamily, the 78- and 85-kD glycoproteins share a common large (518 amino acid) extracellular domain with five Ig regions and five potential N -glycosylation sites ( Fig. 36.7 ). Three Ig domains are of the constant-region-2 (C2) type, whereas the remaining two are variable-region (V) domains. A laminin binding site resides in a flexible hinge region between C2 and V domains.
The 7-kD difference between the 78-kD and 85-kD isoforms reflects the length of the COOH-terminal cytoplasmic tail. In addition to its longer length, the cytoplasmic domain of the 85-kD isoform possesses an SH3 motif, a potential binding site for Src protein. The cytoplasmic domain also interacts with spectrin, a cytoskeletal protein ( ). Because it is a highly folded protein, stabilized by disulfide bonds, Lutheran is destroyed by sulfhydryl-reducing agents and many proteases.
The gene for Lutheran ( LU ) resides on chromosome 19q13.2-13.3. It is a 12.5-kb gene containing 15 exons: alternate splicing of exon 13 is responsible for the 78-kD minor isoform ( ; ). Although no TATA or CAAT boxes are present in the 5′ flanking or promoter region of the gene, it does possess consensus sequences for several cis -regulatory elements, including the ubiquitous Sp1, AP2, EGR-1, EKLF, Ets, and GATA-1, an erythroid transcription binding protein ( ; ).
The molecular basis for most Lutheran antigens is now known (see Fig. 36.7 ). Lu b is the consensus allele ( LU∗02 ). Most Lu antigens are the result of SNPs, including Lu a (His77, LU∗01 ) and Lu b (Arg77) ( ). The autosomal-recessive, true Lu null phenotype arises from inheritance of two silent amorph LU alleles due to nonsense mutations and deletions ( ; ). The molecular basis for the autosomal-dominant In(Lu) phenotype was finally identified in 2008. Most In(Lu) individuals are heterozygous for a mutant EKLF gene, an erythroid transcription factor active in late erythroid differentiation ( ). The In(Lu) phenotype, therefore, is the result of reduced transcriptional activation and expression of LU glycoprotein. Reduced EKLF activity likely accounts for decreased expression of other blood group antigens. KLF1 mutations are also linked to hereditary persistence of fetal hemoglobin and congenital dyserythropoietic anemia ( ). Over 60 KLF1 allelic variants have been described with variable effects on red cell phenotype ( ; ). The basis for the X-linked recessive In(Lu) phenotype has been identified as mutations in the transcription factor GATA1, which resides on the X chromosome.
In general, Lutheran antibodies are not clinically significant and are only rarely associated with HDFN and hemolytic transfusion reactions. Anti-Lu a is the most common Lutheran alloantibody encountered in the blood bank and is often an IgM, room temperature agglutinin. Because not all RBCs express detectable Lu antigens ( ), anti-Lu a can display mixed-field agglutination. Antibodies against Lu b and other Lutheran antigens are most often of IgG isotype, reacting best in the IAT. Reactivity of anti-Lu a , Lu b , and other Lutheran antibodies can be inhibited by pretreatment of RBCs with chymotrypsin, trypsin, 2-aminoethylisothiouronium bromide (AET), and dithiothreitol (DTT).
Biologically, Lutheran is a high-affinity receptor for laminin, a basement membrane protein involved in cell differentiation, adhesion, migration, and proliferation. Overexpression of LU glycoprotein in ovarian carcinoma and other cancers is hypothesized to facilitate tumor cell adhesion and metastasis ( ). In sickle cell patients, increased Lutheran expression on reticulocytes and sickle cells may contribute to the pathophysiology of vaso-occlusive crises. Patients with sickle cell disease have indirect evidence of chronic vascular injury with shedding of microvascular endothelial cells and exposure of vascular basement membrane ( ). In these patients, increased exposure of laminin on the thrombotic subendothelium, coupled with increased expression of laminin receptor on reticulocytes and sickle cells, may promote red cell adhesion and circulatory stasis ( ; ). Likewise, LU glycoprotein may contribute to thrombosis in polycythemia vera. The JAK2 mutation was shown to increase phosphorylation of LU glycoprotein and red cell adhesion ( ). In normal erythropoiesis, LU may play a role in migration of maturing erythroid cells out of the marrow ( ).
First identified in 1945, the Kell (KEL) blood group system currently consists of 39 high- and low-frequency antigens ( Tables 36.16 and 36.17 ). Sixteen Kell antigens belong to 8 sets of allelic antigens, whereas the remainder are predominantly high-incidence antigens (>99% of the population). Many low-incidence Kell antigens (K, Js a , Ul a , Kp a , Kp c ) show distinct racial differences. The Kell antigen is found on RBCs, erythroid and megakaryocyte progenitors, skeletal muscle, and testis. RBCs express approximately 2000 to 6000 copies of Kell protein per cell ( ).
| ANTIGEN (HIGH FREQUENCY) | Percent Donors | Amino Acid Change (High→Low Frequency) | Percent Donors | ANTIGEN (LOW FREQUENCY) | ||
|---|---|---|---|---|---|---|
| ISBT | Name | Name | ISBT | |||
| KEL2 | K | 99.8 | Thr193Met Thr193Ser |
9 Rare |
K | KEL1 |
| Ala423Val | Unknown | Weak KEL2 | ||||
| KEL4 | Kp b | 99.9 | Arg281Trp | 2 | Kp a | KEL3 |
| Arg281Gln | <0.1 | Kp c | KEL21 | |||
| KEL7 | Js b | >99.9 | Leu597Pro | <1% whites 19.5% blacks |
Js a | KEL6 |
| KEL5 | Ku | >99.9 | Heterogeneous | 0.8 | K 0 | K null |
| Heterogeneous | Rare | K mod | ||||
| KEL20 | Km | >99.9 | unknown | |||
| XK1 | Kx | >99.9 | Mutation XK protein | Rare | McLeod | |
∗ As referenced in Daniels G: Human blood groups , ed 3, Oxford, 2013, Blackwell Science; Daniels G, Flegel WA, Fletcher A, et al.: International Society of Blood Transfusion Committee on Terminology for Red Cell Surface Antigens: Cape Town report, Vox Sang 92:250–253, 2007; Daniels G, Fletcher A, Garratty G, et al.: Blood group terminology 2004: From the International Society of Blood Transfusion Committee on Terminology for Red Cell Surface Antigens, Vox Sang 87:304–316, 2004; and Reid RE, Lomas-Francis C: The blood group antigen facts book , ed 3, San Diego, 2012, Academic Press.
| REACTIONS WITH ANTI- | Phenotype | FREQUENCY IN U.S. POPULATION, % | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| K | k | Kp a | Kp b | Js a | Js b | Ku | Km | Kx | White | Black | |
| + | 0 | ++ | ++ | w | K+k– | 0.2 | Rare | ||||
| + | + | ++ | ++ | w | K+k+ | 8.8 | 2 | ||||
| 0 | + | ++ | ++ | w | K–k+ | 91 | 98 | ||||
| + | 0 | ++ | ++ | w | Kp(a+b–) | Rare | 0 | ||||
| + | + | ++ | ++ | w | Kp(a+b+) | 2.3 | Rare | ||||
| 0 | + | ++ | ++ | w | Kp(a–b+) | 97.7 | 100 | ||||
| + | 0 | ++ | ++ | w | Js(a+b–) | 0 | 1 | ||||
| + | + | ++ | ++ | w | Js(a+b+) | Rare | 19 | ||||
| 0 | + | ++ | ++ | w | Js(a–b+) | 100 | 80 | ||||
| Null Phenotypes | |||||||||||
| 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ++ | K 0 | Exceedingly rare | |
| 0/w | 0/w | 0/w | 0/w | 0/w | 0/w | w | w | ++ | K mod | Rare | |
| 0/w | 0/w | 0/w | 0/w | 0/w | 0/w | 0/w | 0 | 0 | McLeod | Rare | |
K 0 K 0 is an autosomal-recessive, null phenotype that completely lacks all Kell antigens (see Table 36.22 ). As a consequence, these individuals can make an alloantibody to the Kell glycoprotein (anti-Ku). Unlike McLeod RBCs (see later discussion), K 0 K 0 RBCs have enhanced expression of Kx antigen, present on the XK protein. Unlike Rh null red cells, K 0 K 0 red cells have no decrease in CO 2 permeability ( ). A K 0 K 0 RBC can be produced in the laboratory by treating Kell-positive RBCs with sulfhydryl-reducing agents.
| REACTIONS WITH ANTI-DOMBROCK ANTIBODIES | Phenotype | ISBT | Amino Acid Change | FREQUENCY (%) U.S. POPULATION | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Do a | Do b | Gy a | Jo a | Hy | D06 | D07 | White | Black | |||
| + | 0 | + | + | + | + | + | Do (a+b–) | DO1 | Asn265 | 18 | 11 |
| + | + | + | + | + | + | + | Do (a+b+) | 49 | 44 | ||
| 0 | + | + | + | + | + | + | Do (a–b+) | DO2 | Asp265 | 33 | 45 |
| Null/Weak Phenotypes | |||||||||||
| 0 | 0 | 0 | 0 | 0 | 0 | 0 | Gy(a–) or Do null † | DO3 | Loss exon 2 ∗ | Rare | Rare |
| 0 | w | w | 0/w | 0 | w | + | Hy(–) | DO4 | Gly108Val | Rare | Rare |
| w | 0/w | + | 0 | w | w | 0/w | Jo(a–) | DO5 | Thr117Ile | Rare | Rare |
| 0 | 0 | w | w | w | 0 | ? | DOYA(–) | DO6 | Tyr183Asp | Rare | Rare |
| 0 | + | 0 | w | w | ? | 0 | DOMR(–) | DO7 | Ala144Glu | Rare | Rare |
| + | 0 | + | + | + | ? | ? | DOLG(–) | DO8 | Leu225Gln | Rare | Rare |
| + | 0 | + | + | + | + | ? | DOLC(–) | DO9 | Thr189Met | Rare | Rare |
| + | 0 | + | + | + | + | + | DODE(–) | DO10 | Asp135Glu | Rare | Rare |
∗ Loss of exon 2 has been associated with splice-site, frameshift, and nonsense mutations (Reid RE, Lomas-Francis C: The Blood Group Antigen Facts Book , ed 3, San Diego, 2012, Academic Press).
† Also associated with PNH III red blood cells (RBCs), which lack all glycophosphoinositol (GPI)-linked glycoproteins, including Dombrock, Cartwright, Cromer, JMH, and CD59 antigens.
Kell antigens are significantly depressed/absent on McLeod RBCs, an X-linked recessive phenotype characterized by the absence of XK protein on RBCs (Kx antigen, XK1; ISBT 019), acanthocytes, and neuromuscular disorders. Because McLeod individuals lack XK and Kell proteins, these individuals can make alloantibodies directed against both proteins. As a consequence, McLeod individuals are incompatible with both Kell-positive and K 0 K 0 RBCs. Depressed Kell expression is also observed on K mod and Gerbich-negative RBCs, two autosomal-recessive phenotypes. As with the K 0 K 0 phenotype, some Kell mod individuals have increased Kx (KEL15) expression and can develop an anti-KEL5 (anti-Ku) following RBC transfusion. Transient depression and masking of Kell antigens have been reported in septic patients and in autoimmune hemolytic anemia due to anti-Kell autoantibodies ( ; ; ). Kell antigens are also depressed (up to 80%) in alleles bearing KEL3 in cis (e.g., KKp a , KEL∗1,3; kKp a , KEL∗2,3) ( ; ).
The Kell antigens (ISBT No. 006) reside on a 93-kD, 732 amino acid glycoprotein (CD238) possessing a short 47 amino acid amino-terminal cytosolic domain, a single transmembrane domain, and a large 665 amino acid extracellular domain that includes the carboxy-terminus and 4-5 N -linked glycans ( Fig. 36.8 ). In the KEL1 phenotype, a Thr183>Met substitution voids a glycosylation site, resulting in only four N -linked glycans ( ).
Overall, the molecule has a complex, folded tertiary structure due to the formation of multiple disulfide bonds by the 15 cysteine residues present in the extracellular domain of the molecule ( ). Because of its highly folded nature, the Kell glycoprotein is fairly resistant to proteolytic digestion but is exquisitely sensitive to sulfhydryl-reducing agents such as DTT, AET, 2-mercaptoethanol (2-ME), and ZZAP, a combination of DTT and cysteine-activated papain.
In the RBC membrane, the Kell glycoprotein is covalently linked to the XK protein (ISBT No. 019), a 444 amino acid, nonglycosylated, multipass protein containing 10 transmembrane domains. Encoded by a gene on chromosome Xp21, the XK protein is hypothesized to be a membrane transport protein. On RBCs, the XK protein is covalently linked to the Kell protein through a disulfide bond at C347 on the fifth extracellular loop of XK protein and C72 of Kell. In the absence of XK protein (McLeod phenotype), Kell expression is decreased on RBCs, suggesting that XK protein may help transport Kell. Transfection studies in COS cells, however, show normal transport and folding of the Kell protein in the absence of XK protein ( ). Nor is expression of XK dependent on Kell expression. The XK protein is expressed on several nonerythroid tissues, including liver, skeletal muscle, heart, brain, and pancreas ( ).
Cloned in 1991, the Kell gene ( KEL ) contains 19 exons spanning 21.5 kb on chromosome 7q34 near the cystic fibrosis gene. The promoter region and exon 1 contain several consensus-binding sequences for GATA-1, an erythroid transcription regulatory binding factor. Kell is a member of the M13 neprilysin family of zinc-neutral endopeptidases and shares homology with common acute lymphocytic leukemia antigen (CALLA, CD10), endothelin-converting enzyme-1 (ECE-1), neutral endopeptidase 24.11 (NEP-24.11), and PEX. The region of greatest homology lies in the carboxy-terminal half of the molecule (amino acids 550–652) and is sometimes referred to as the NECK domain (N-24.11, EC-1, K family). This relatively conserved region shares 34% to 36% homology with the carboxy-terminal catalytic domains of NEP-24.11 and ECE-1, as well as a pentameric zinc-binding motif (HELLH), a common feature of zinc-dependent metalloproteinases ( ).
With cloning of the Kell gene, the molecular and biochemical basis of several Kell antigens became possible. As shown in Table 36.16 , all Kell alloantigens identified and sequenced are single–amino acid polymorphisms in the translated Kell protein, generally in the amino half of the molecule ( ). The consensus allele KEL∗02 encodes 20 high-frequency antigens, including k, Kp b , and Js b ( ).
Several individuals with absent or depressed Kell expression have also been studied. K 0 K 0 phenotype arises from homozygosity for a silent KEL amorph gene. Thirty K 0 alleles ( KEL∗02N ) are recognized, including splice-site mutations and premature stop codons ( ). K mod is the result of missense mutations and may occur on either K1 or k background. Twelve K mod alleles ( KEL∗02M ) are recognized by the ISBT. Several mutations have been identified in the XK protein on Xp21, responsible for the McLeod phenotype ( ). At present, 39 mutant XK alleles ( XK∗01N ) have been described, including deletion mutants, splice site, and nonsense mutations. The molecular basis for decreased Kell antigen expression with Gerbich-negative phenotypes is unknown. It is speculated that Kell and glycophorin C (Gerbich) may be near neighbors on the RBC membrane.
Alloantibodies against antigens in the Kell blood group system are clinically significant (see Table 36.5 ). They can be associated with both immediate and delayed hemolytic transfusion reactions. Anti-Kell antibodies are also associated with HDFN. HDFN secondary to maternal anti-Kell antibodies is often characterized by reticulocytopenia, with little or no bilirubinemia. It is now known that maternal anti-Kell (anti-KEL1 or K1) directly suppresses erythroid progenitors, leading to a severe reticulocytopenic anemia in the fetus ( ) with up to 30% of affected infants presenting with fetal hydrops ( ). Anti-K1 is present in approximately 1% of pregnancies, with HDFN affecting 40% of K1-positive infants ( ). Several centers now routinely screen and refer prenatal patients with Kell antibodies, with improved perinatal outcomes ( ). Reports have also described neonatal thrombocytopenia due to suppression of marrow megakaryocytes ( ). In general, thrombocytopenia is mild, with only 2% of infants having platelet counts of less than 100 × 10 9 /L ( ). Similar to RHD, fetal genotyping can be performed on maternal plasma ( ).
The most commonly encountered antibody against the Kell blood group system is anti-K1, which is second only to RhD in immunogenicity. Antibodies against Kell antigens are of IgG isotype, arising from immune stimulation via transfusion or pregnancy. There are rare reports of naturally occurring anti-K1 antibodies following severe bacterial infections and may be the result of cross-reactive antibodies against bacterial neprilysin homologues ( ), although examples of naturally occurring anti-Kell alloantibodies are known ( ). Because Kell antigens are sensitive to sulfhydryl-reducing agents, the activity of anti-Kell antibodies can be eliminated by pretreatment of RBCs with AET, DTT, 2-ME, or ZZAP.
Kell cleaves endothelin-3, a vasoactive peptide that functions in endothelial cell migration, neovascularization, axonal growth, and neural crest development. In a murine Kell null model, absence of Kell led to only subtle effects, including decreased blood pressure, blunting endothelin-3 activation of Ca ++ -dependent K + channels, decreased microvascular formation, and mild changes in motor function ( ). Because endothelin-3 is also cleaved by ECE-1, a related endothelin-converting enzyme, Kell may have only a minor physiologic role in endothelin-3 homeostasis. The ability of anti-Kell to suppress fetal erythropoiesis also suggests a possible role for Kell during erythroid differentiation and maturation ( ). However, only minor differences in mean corpuscular volume, mean corpuscular hemoglobin, hemoglobin, and reticulocyte counts were observed in Kell null mice ( ).
In contrast to Kell, the absence of XK protein is strongly associated with several abnormal clinical and laboratory findings. McLeod RBCs have shortened survival, decreased permeability to water, and abnormal morphology (acanthocytes). The McLeod syndrome, characterized by both hematologic and neuromuscular abnormalities, typically presents with areflexia, dystonia, and choreiform movements late in life. Late-onset muscular dystrophy and cardiomyopathy can also be seen. Both hematologic and neuromuscular defects in McLeod patients are believed to result from the absence of XK protein on red cells, brain, heart, and skeletal muscle ( ). It is interesting that the Huntington disease gene, another neurodegenerative disorder, is located near the XK gene on the X chromosome ( ). In fact, modern historians have hypothesized that a K1+ McLeod phenotype might explain Henry VIII’s physical and psychological decline late in life, as well as his wives’ high miscarriage rate ( ).
The McLeod phenotype can also be associated with chronic granulomatous disease (CGD), a functional 0p defect resulting in severe, recurrent, life-threatening bacterial infections. In two thirds of patients, CGD results from a deletion or mutation of the cytochrome b gene (CYBB) on the X chromosome. Because of the proximity of the CYBB and XK genes on the X chromosome, approximately 7% of patients with X-linked CGD also express a McLeod phenotype ( ).
The Lewis blood group system is unusual in that the Lewis antigens are not of erythroid origin. It primarily consists of two antigens: Lewis a (Le a , LE1) and Lewis b (Le b , LE2). Four additional antigens (Le ab , Le bH , ALe b , BLe b ) reflect the influence of ABO on Lewis synthesis and antigenicity. On RBCs, the Lewis antigens reside on glycosphingolipids, composed of a Lewis-active carbohydrate head group linked to a ceramide ( N -acyl sphingosine) lipid tail. Lewis antigens are synthesized in the gastrointestinal tract and passively adsorbed onto RBCs from a soluble pool of secreted Lewis substance in plasma ( ). Tissues and fluids expressing Lewis include plasma, saliva, RBCs, platelets, lymphocytes, endothelium, uroepithelium, and bowel mucosa. In some tissues, Lewis antigens are expressed on glycosphingolipids, glycoproteins, and mucins ( ).
Three Lewis phenotypes are observed in adults: Le (a+b–), Le (a–b+), and Le (a–b–). The Le (a+b+) is only rarely observed, usually on RBCs of very young children and some individuals of Polynesian, Japanese, or Taiwanese ancestry ( ). As shown in Table 36.18 , the Le (a–b–) phenotype is five times more common in blacks than in whites. The Le (a–b–) phenotype is also increased in neonates owing to developmentally delayed expression of the Lewis and Secretor genes. By 5 years of age, most children will express adult levels of Lewis antigens on their RBCs. The amount of Lewis antigen on RBCs is also influenced by ABO type. Le (a–b+) RBCs from group O donors appear to have more Le b than A 1 and B cells. As will be discussed later, group A 1 and B donors can convert Le b to ALe b and BLe b , respectively ( ).
| FREQUENCIES IN U.S. ADULTS, % | POSSIBLE GENOTYPE | ABH AND LEWIS SUBSTANCES IN SALIVA AND PLASMA | |||||
|---|---|---|---|---|---|---|---|
| Phenotype | White | Black | Le Gene | Se Gene | Group O | Group A | Group B |
| Le (a+b–) | 22 | 23 | Le/Le or Le/le | se/se | Le a | Le a | Le a |
| Le (a–b+) | 72 | 55 | Le/Le or Le/le | Se/Se or Se/se | Type 1H | Type 1H, A | Type 1H, B |
| Le a , Le b | Le a , Le b , ALe b | Le a , Le b , BLe b | |||||
| Le (a–b–) | 6 | 22 | le/le | Se/se | Type 1H | Type 1H, A | Type 1H, B |
| le/le | se/se | Type 1 chain precursor (Le c ) | Type 1 chain precursor (Le c ) | Type 1 chain precursor (Le c ) | |||
Although biosynthetically related to each other, the Le a and Le b antigens are not allelic antigens. They represent the complex interaction of two distinct glycosyltransferases: fucosyltransferase type II (FUT2) and fucosyltransferase type III (FUT3). FUT3 is the product of the Lewis gene ( Le/FUT3 ) and is an α1–3/4 fucosyltransferase. FUT2, the product of the Secretor gene ( Se ), is an α1–2 fucosyltransferase related to the H gene, FUT1. How Le, Se, and ABO genes interact to yield the different combinations of RBC and plasma phenotypes is shown in Table 36.18 and Figure 36.9 .
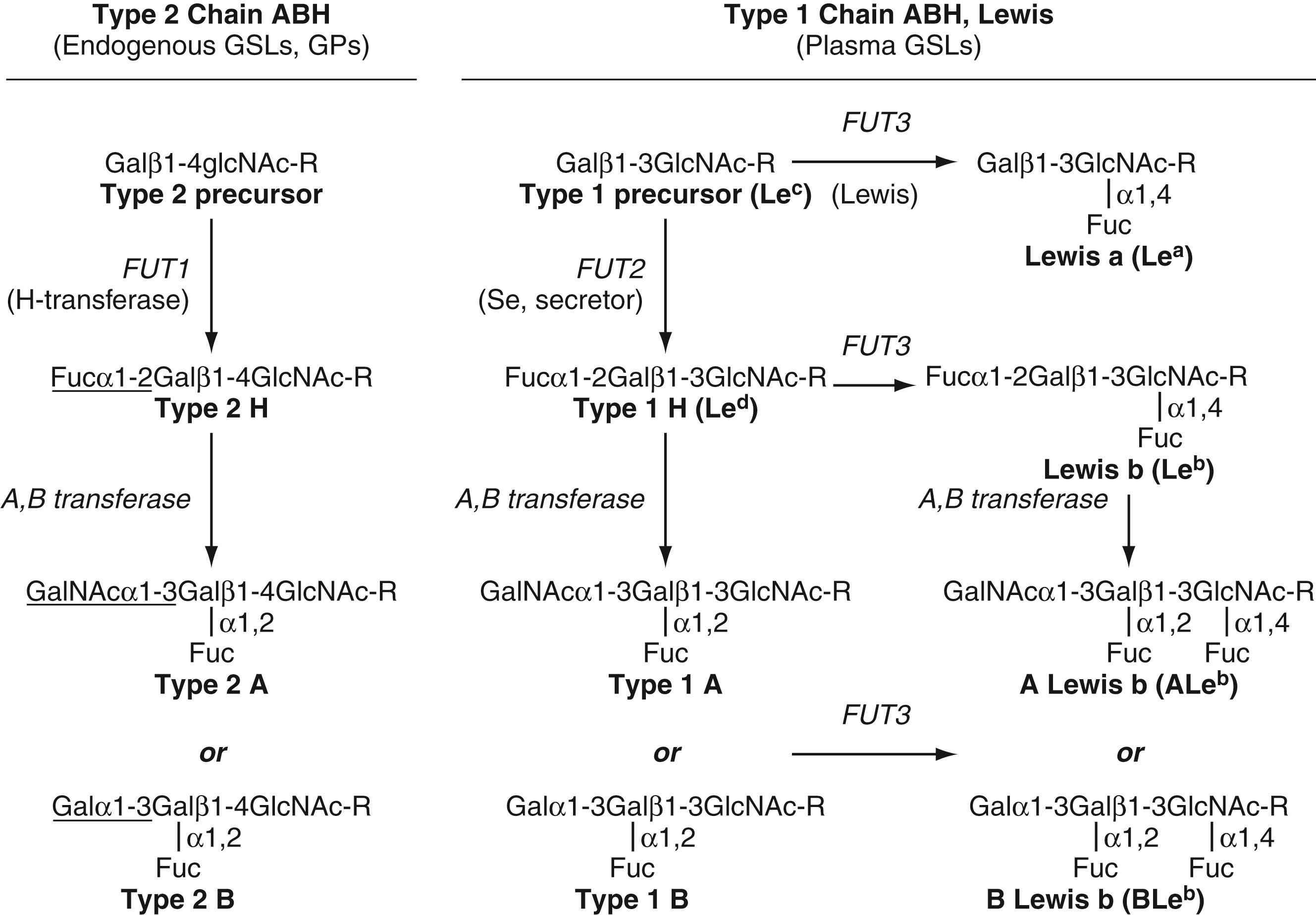
The precursor molecule for Lewis and related antigens is a type 1 chain precursor, historically known as the Le c antigen. In Le (a+b–) donors, FUT3 or Lewis adds an α1–4 linked fucose to Le c to form the Le a antigen. Because these donors lack the Se/FUT2 gene ( se/se ), no type 1 chain H (Le d ) is made. As a result, only Le a antigen is present in plasma, saliva, and RBCs. The Le (a–b+) red cell phenotype results from the inheritance of both Le and Se genes. FUT3 still converts a small amount of type 1 chain precursor to Le a antigen. However, most Le c substance is converted to type 1 chain H or Le d by FUT2 (see Fig. 36.9 ). FUT3 subsequently adds a second fucose to form Le b antigen ( ). In group A and B persons, type 1 chain H is further modified by the A/B glycosyltransferase to form type 1 chain A and B antigens. Similar to type 1 chain H and Le c antigens, these molecules can serve as substrates for FUT3 to form ALe b and BLe b (see Fig. 36.9 ). In group A 1 donors, ALe b is the major Le b antigen found in plasma ( ).
The Le (a–b–) red cell phenotype occurs in individuals who lack the Le/FUT3 gene. Individuals who are negative for both Le and Se alleles ( le / le , se / se ) are unable to synthesize Le a antigen or type 1 chain H antigens. As a result, only type 1 chain precursor or Le c antigen is present in secretions and plasma. In contrast, individuals who inherit at least one Se allele can express type 1 chain glycolipids with ABH activity.
The FUT3 or Lewis gene resides on chromosome 19p13.3 near two other α1–3 fucosyltransferase genes: FUT5 and FUT6 . All three α1–3 fucosyltransferases are highly homologous, consistent with gene duplication ( ). The gene encodes a 361 amino acid type II glycoprotein with both α1→4 (Le a ) and α1→3 (LeX) fucosyltransferase activity. FUT3 is highly expressed in colon, stomach, small intestine, lung, and kidney, with weaker expression in salivary gland, bladder, uterus, and liver. FUT6 is coexpressed with FUT3 in most tissues ( ).
The Le (a–b–) or null phenotype arises from inactivating mutations in FUT3 . More than 37 FUT3 null alleles have been reported, with most (89%) possessing at least two mutations ( ). Many alleles show distinct geographic and ethnic distributions. In whites, Le (a–b–) is most commonly associated with alleles containing mutations at Trp68Arg and Thr105Met ( le 202,314 ). In contrast, FUT3 null alleles common in Asia include le 59 , le 59,508 , and le 59,1067 ( ). The Lewis weak (Le w ) phenotype, often characterized by a Le (a–b–) RBC phenotype and the presence of Lewis-active substance in saliva, is due to a single missense mutation in the transmembrane domain (T59>G). The latter has decreased Golgi retention and reduced activity with glycolipid substrates, leading to discrepant Lewis expression on red cells, saliva, and gastrointestinal tissues ( ; ). Le w and a Le (a–b–) RBC phenotype can also be a consequence of gene dosage: Le/le heterozygous individuals show 50% decreased FUT3 activity in saliva and tissues ( ).
The FUT2 ( Se ) gene resides on chromosome 19q13.3 as part of a 100-kb gene cluster that includes the H gene ( FUT1 ) and Sec1 , an inactive FUT2 -like pseudogene ( ). Multiple null alleles have been reported, with distinct geographic and ethnic distributions. Most null alleles are the result of nonsense mutations ( ). There are also examples of recombination among FUT1 , FUT2 , and Sec1 ( ; ). Weak FUT2 alleles are known and common in Asia ( ; ). The most common partial secretor phenotype has been linked to an Ile129>Phe mutation and is associated with a Le (a+b+) phenotype ( ).
Similar to ABO, antibodies against Le a and Le b antigens are naturally occurring IgM antibodies (see Table 36.5 ), but unlike ABO antibodies, anti-Lewis antibodies are seldom clinically significant. Most examples are detected as room temperature agglutinins; however, some examples are reactive in the IAT. Although uncommon, some examples demonstrate in vitro hemolysis. Because Le a and Le b are glycosphingolipids, antibody reactivity can be enhanced by pretreatment of RBCs with enzymes. Antibody reactivity is neutralized by the addition of commercially available soluble Lewis substance or plasma containing the soluble Lewis antigen of interest. Anti-Le b can be observed in individuals of Le (a+b–) or Le (a–b–) phenotype, whereas anti-Le a is observed only in Le (a–b–) individuals. Anti-Le a is not observed in the Le (a–b+) phenotype because these individuals synthesize a small amount of Le a . It is interesting to note that some Le (a–b+) women can transiently become phenotypically Le (b–), with the development of anti-Le b , during pregnancy. Some examples of anti-Le b can demonstrate ABH specificity (anti-LebH, anti-ALe b , anti-BLe b ), reacting more strongly with Le b -positive RBCs of specific ABO types.
They are not associated with HDFN and are only rarely associated with hemolytic transfusion reactions. It is speculated that they could play a role in renal graft rejection in black Le (a–b–) individuals ( ). For transfusion, patients with anti-Lewis antibodies reactive only at room temperature may be safely transfused with crossmatch–compatible RBCs. In contrast, rare examples of anti-Le a or anti-Le b that are hemolytic in vitro should receive antigen-negative, crossmatch-compatible RBCs. If antigen-negative blood is not available, infusion of plasma containing the soluble Lewis antigen of interest may be helpful in neutralizing or inhibiting circulating antibody before RBC transfusion ( ).
The Lewis blood group antigens play an important role in disease. Le (a–b–) is linked to a twofold increased risk of atherosclerotic disease and coronary death, with the strongest association observed in Le (a–b–) individuals carrying the le 59 allele ( ; ). Aberrant expression of sialyl-Le a occurs in many gastrointestinal and uroepithelial cell cancers and may contribute to tumor metastasis. Sialyl-Le a is a ligand for the endothelial adhesion molecule E-selectin and may mediate tumor cell–endothelium interactions ( ). Sialyl-Le a is also the epitope for the tumor marker CA 19-9, a useful serologic marker for monitoring patients with gastrointestinal and other malignancies.
Secretor status is a host susceptibility factor in several infectious diseases. Helicobacter pylori , a causative agent of gastritis and ulcers, binds H, Le b , and Le y antigens via BabA recognition of a terminal Fucα1–2Gal epitope ( ; ). The latter might explain the increased incidence of ulcers and stomach cancer among blood group O secretors ( ). A Lewis null and/or nonsecretor phenotype has also been linked with a higher incidence of recurrent Candida vaginitis and urinary tract infection ( ). Le b and type 1 H antigen are also receptors for most norovirus and rotavirus strains. Several epidemiologic studies have shown that nonsecretors have inherent resistance against infection ( ). In contrast, nonsecretors were recently identified as a risk factor for necrotizing enterocolitis and sepsis in premature infants ( ).
The Duffy (FY) blood group system was discovered in 1951 and contains five antigens: Fy a , Fy b , Fy3, Fy5, and Fy6. Fy a and Fy b are autosomal-codominant antigens, whereas Fy3, Fy5, and Fy6 are high-incidence antigens present on all RBCs except the Duffy null phenotype. The Fy4 antigen, originally described on Fy (a–b–) RBCs, is now thought to be a distinct, unrelated antigen and is no longer included in the FY system.
Fy a and Fy b antigens are common in whites, whereas the Duffy null or Fy (a–b–) phenotype is the predominant phenotype in blacks ( Table 36.19 ). Fy x is generally characterized by extremely weak Fy b expression. In addition to RBCs, the Duffy antigens are expressed on cerebellar Purkinje cells and postcapillary venule endothelial cells. Duffy antigens have also been reported on endothelial cells of renal glomeruli, vasa recta, thyroid, and pulmonary capillaries, as well as on alveolar type 1 squamous cells and epithelial cells of renal collecting tubules ( ; ). RBCs possess approximately 12,000 to 14,000 copies of the Duffy glycoprotein per cell ( ).
| REACTIONS WITH ANTI- | RBC Phenotype | WHITES | BLACKS | ||||
|---|---|---|---|---|---|---|---|
| Fy a | Fy b | Fy3 | Frequency ∗ | Genotypes † | Frequency ∗ | Genotypes † | |
| + | – | + | Fy (a+b–) | 17 | FY∗A/FY∗A | 9 | FY∗A/FY∗A FY∗A/FY∗B ES |
| + | + | + | Fy (a+b+) | 49 | FY∗A/FY∗B | 1 | FY∗A/FY∗B |
| – | + | + | Fy (a–b+) | 34 | FY∗B/FY∗B | 22 | FY∗B/FY∗B FY∗B/FY∗B ES |
| – | – | – | Fy (a–b–) | Very rare | FY∗amorph | 68 | FY∗B ES /FY∗B ES |
| +/– | w | w | Fy (a+b w ) Fy (a–b w ) |
<0.1% | FY∗A/FY∗X FY∗X/FY∗X |
Very rare | FY∗A/FY∗X FY∗X/FY∗B ES |
∗ Frequency (%) U.S. population.
† FY∗A ( FY∗01 ), Fy a allele; FY∗B ( FY∗02 ), Fy b allele; FY∗B ES , FY∗B allele carrying a GATA-1 promoter mutation; FY∗X, FY∗B gene containing missense with weak Fy b expression; FY∗amorph ( FY∗N ), silent FY gene containing disruptive mutations (deletion, frameshift, nonsense). See accompanying text.
The Duffy glycoprotein was recently renamed ACKR1 (atypical chemokine receptor 1) from its previous designation as DARC (Duffy antigen receptor for chemokines) ( Fig. 36.10 ). ACKR1 is a 336 to 338 amino acid, integral membrane glycoprotein, containing a 62 amino acid extracellular amino-terminus, seven transmembrane domains, and an intracellular carboxy-terminus rich in serine and threonine residues. The amino-terminal domain possesses three N -glycans ( ) and is linked to the third extracellular loop by a disulfide bond to form a hepatohelical structure. A second disulfide bond exists between the first and second extracellular loops. DARC exists as a homodimer or as a hetero-oligomer with CCR5, a β-chemokine receptor that plays a role in HIV internalization ( ).
The Fy a , Fy b , and Fy6 antigens reside on the amino-terminal domain of ACKR1 and are sensitive to proteolytic cleavage. The negatively charged amino-terminal domain is the binding site for P. vivax PvDBP ligand. Site-directed mutagenesis has confirmed that amino acids 19 to 26, the Fy6 epitope, are critical for P. vivax binding ( ). The high-incidence antigen Fy3 is believed to reside on the third extracellular loop ( ; ).
Located on chromosome 1q22-23, the Duffy gene ( ACKR1, DARC, FY ) is a 1014-bp gene containing two exons, a small upstream exon encoding the first seven amino acids, and a second exon encoding the rest of the molecule ( ). There are two known ACKR1 mRNA transcripts, which give rise to a 336 (isoform B, predominant protein) and 338 amino-acid protein (isoform A, minor), respectively ( ). The promoter region for the major protein contains consensus sequences for multiple cis -regulatory elements, including AP-1, Sp1, and GATA-1, an erythroid transcription activator-binding protein that controls transcription of DARC in erythroid cells ( ). Phylogenetic studies indicate that FY∗B ( FY∗02 ) is the ancestral gene of human and nonhuman primates ( ). Genetic studies have identified four predominant Duffy alleles— FY ∗ A (FY∗01) , FY ∗ B (FY∗02) , FY ∗ B ES ( FY∗02N.01 ), and FY ∗ X (FY∗02W) —responsible for the Fy a , Fy b , Fy null , and Fy x phenotypes, respectively (see Table 36.19 ). The codominant alleles, FY ∗ A and FY ∗ B , differ by a single amino acid (Gly42Asp). The Fy x phenotype (FY ∗ X), characterized by extremely weak Fy b expression (10% normal), arises from an R89C substitution in the first cytoplasmic loop, leading to protein instability ( ; ). Although rare, FY∗A alleles bearing the R89C and weak Fy a expression have been identified. To date, 7 ACKR1 alleles with weak Fy a or Fy b expression have been identified, with the highest frequency among European whites ( ).
Three distinct mechanisms are responsible for the Fy null or Fy (a–b–) serologic phenotype. In whites, the FY gene is disrupted ( FY ∗ amorph, FY∗02N.02 ), leading to complete absence of DARC on all tissues ( ). As a result, these individuals can make alloantibodies to all Duffy antigens, including high-incidence antigens (Fy3, Fy5). In contrast, black Fy (a–b–) individuals are homozygous for FY ∗ B ES ( FY∗02N.01 ), an FY ∗ B variant that possesses a point mutation in the FY gene promoter (–67T>C) that abrogates the consensus binding site for GATA-1. As a result, there is a selective loss of FY transcription in RBCs; however, FY transcription is retained in endothelial and epithelial cells, which utilize other promoter enhancer elements. Because Fy b is expressed on nonerythroid cells, black Fy (a–b–) individuals do not make anti-Fy b and only rarely make anti-Fy3. In Asian and Oceania populations, GATA mutations can occur on an FY∗A background ( FY∗01N ) with selective loss of Fy a on red cells ( ; ). Finally, there is increasing recognition that some individuals, particularly donors, who historically typed as Fy (a–b–) by serology are actually Fy (a–b +w ) on genotyping ( FY∗B ES /FY∗X ).
Antibodies against Fy a , Fy b , and other Duffy antigens are clinically significant. They are associated with HDFN and both immediate and delayed hemolytic transfusion reactions. They are usually of IgG isotype, reactive at 37° C, and are detected only in the IAT. Antibodies against Fy a , Fy b , and Fy6 antigens, which reside on the long amino-terminal domain of DARC, can be inhibited by prior protease digestion of RBCs. In contrast, Fy5 and Fy3 antigens are relatively resistant to protease digestion. Antibodies against Duffy antigens can demonstrate dosage.
Clinically, anti-Fy a is the most common alloantibody encountered and can be observed in Fy (a–) individuals of all races. Anti-Fy b is relatively uncommon and is observed primarily in nonblacks. Alloantibodies against the high-incidence antigens Fy3 and Fy5 are relatively rare, occurring predominantly in white, Fy null individuals. Anti-Fy3 behaves like an anti-Fy a+b , reacting with all Duffy-positive RBCs. Occasionally, potent anti-Fy3 can be produced by sickle cell, Fy (a–b–) patients despite the expression of Fy b on nonerythroid tissues ( ). Anti-Fy5 also reacts like an anti-Fy a+b but requires the presence of Rh antigens for reactivity. Anti-Fy6 has not been observed clinically but is the epitope for an anti-Duffy monoclonal antibody that blocks P. vivax binding ( ).
ACKR1 is a chemokine-binding protein, capable of binding several inflammatory chemokines from the C-X-C and C-C families ( ), including interleukin-8, RANTES (regulated upon activation, normal T cell–expressed and presumably secreted), and monocyte chemotactic protein-1 (see Fig. 36.10 ). Because ACKR1 lacks a DRY motif, it does not transmit an intracellular signal upon binding chemokines. Atypical chemokine receptors are considered negative chemokine regulators, acting as chemokine scavengers and transporters. ACKR1 on red cells likely functions as a “chemokine sink” to control blood chemokine concentrations whereas ACKR1 on endothelial cells helps facilitate leukocyte recruitment and neutrophil diapedesis ( ).
In humans, the Fy null phenotype ( FY∗02N ) is associated with benign ethnic neutropenia, characterized by low neutrophil counts in people of African ancestry ( ). In both humans and mice, the absence of ACKR1 on nucleated marrow red cells alters hematopoietic stem cells, leading to generation of a distinct neutrophil population with increased CD16, CD45 and CCR2 ( ). As a result, neutrophils are primed to exit the vascular system into tissues, leading to neutropenia. It is hypothesized that the enhanced migration of neutrophils into tissues could represent a selective advantage against bacterial infection. Interestingly, studies in chemotherapy patients have shown that Fy a -positive individuals were at increased risk for hospitalization secondary to febrile neutropenia ( ).
The Fy null phenotype has also been linked to renal disease, reduced graft survival following renal transplantation, and insulin resistance. In patients with sickle cell disease, the Fy null phenotype is associated with increased chronic organ damage and proteinuria ( ). In ACKR1-null mice, there is a rise in inflammatory cytokines and insulin resistance, suggesting an increased risk for obesity-related illnesses ( ). The role of ACKR1 in renal transplantation is mixed, with some studies showing an increase in rejection and chronic lesions ( ; ).
ACKR1 is also the receptor for P. vivax , which binds ACKR1 via PvDBP protein at the Fy6 epitope. Fy null individuals are resistant to most P. vivax strains, providing a selective advantage to populations living in malaria-endemic areas ( ). Fy a may also be protective against P. vivax and may account for the high incidence of Fy a in Asia. Fy a is a less efficient receptor, binding 40% to 50% less PvDBP than Fy b . In the Amazon, the Fy(a+b–) phenotype is associated with a 250% lower risk of P. vivax infection ( ). Finally, the Fy null phenotype ( FY∗02N/FY∗02N ) may play a role in HIV susceptibility. However, there are conflicting reports regarding the latter ( ).
The Kidd (JK) blood group system is strongly associated with delayed hemolytic transfusion reactions and with intravascular hemolysis (recall that most hemolysis with non-ABO antibodies is extravascular). It consists primarily of two allelic antigens: Jk a ( JK∗01 ) and Jk b ( JK∗02 ). Inheritance is autosomal codominant with three predominant phenotypes ( Table 36.20 ). A fourth phenotype, Jk null or Jk (a–b–), is very rare, except among Polynesians (≤1%) and Finns. Weak or altered Jk expression due to novel JK alleles is also known ( ). In addition to RBCs, Kidd antigens are expressed along descending vasa recta endothelial cells of the renal medulla. Evidence suggests low-level, constitutive expression of Kidd on heart, skeletal muscle, colon, small intestine, thymus, brain, pancreas, spleen, prostate, bladder, and liver ( ). More than 14,000 Kidd epitopes are present per human RBC ( ).
| REACTIONS WITH ANTI- | Phenotype | FREQUENCY (%) U.S. POPULATION | Comments | |||
|---|---|---|---|---|---|---|
| Jk a | Jk b | Jk3 | White | Black | ||
| + | 0 | + | Jk (a+b–) | 28 | 57 | Autosomal codominant |
| + | + | + | Jk (a+b+) | 49 | 34 | Sensitive 2M urea |
| 0 | + | + | Jk (a–b+) | 23 | 9 | |
| Null Phenotypes (Jk null ) | ||||||
| 0 | 0 | 0 | Jk (a–b–) | Very rare | Very rare | Autosomal recessive Resistant 2M urea |
| 0/w ∗ | 0/w ∗ | w ∗ | Jk (a–b–) | — | — | In (Jk) , autosomal dominant Rare, Japanese only Partially resistant 2M urea |
∗ Weak Jk antigens detected only by adsorption and elution techniques.
Jk null is autosomal recessive, reflecting homozygosity for amorph JK null alleles (see Table 36.20 ). In(Jk) is a second Jk (a–b–) phenotype that is characterized by very weak Kidd expression. Similar to In(Lu) , the In(Jk) phenotype is autosomal dominant owing to a suppressor gene at a distant, unrelated locus. It is interesting to note that Jk null RBCs are resistant to lysis by 2M urea, a lytic agent used by some automated hematology analyzers. In(Jk) RBCs have intermediate resistance to urea ( ). No change in gas permeability is seen with Jk null cells ( ).
Apparent resistance to urea by Jk null RBCs became clear with cloning and isolation of the human erythroid urea transporter (UT-B). The latter is a 43- to 45-kD multipass glycoprotein, which shares 60% homology with the vasopressin-sensitive urea transporter of rabbits (UT2) and humans (HUT2, UT-A) ( ). The molecule is a 391 amino acid protein with 10 transmembrane domains and a cytosolic amino- and carboxy-terminus ( Fig. 36.11 ). A single N -glycan is present on the third extracellular loop (Asn222) and expresses ABO antigens ( ). The Jk a /Jk b antigens reside on the fourth extracellular loop at amino acid 280. The third extracellular loop may play a key role in urea transport.
The Kidd glycoprotein is encoded by a 30-kb gene ( JK , SLC14A1 , UT-B ) on chromosome 18q12-21. This is the same location as the human HUT2/UT-A urea transporter (61% homology), suggesting that the two genes arose by gene duplication. The JK gene is organized over 11 exons, although only exons 4 to 11 encode the mature protein ( ). Similar to many blood group genes, the promoter region contains a GATA-1 consensus sequence, as well as other cis -regulatory elements (AP-2, AP-3, NF-ATp, Sp1, Ets-1). A single-bp transition (G823A) is the molecular basis of the Jk a /Jk b polymorphism, resulting in an Asp280 (Jk a , JK∗01 ) or Asn280 (Jk b , JK∗02 ) ( ). Seven alleles containing missense mutations are responsible for weak Jk a ( JK∗01W ) and Jk b ( JK∗02W ) expression and can lead to discrepancies in Jk phenotyping by serologic testing. One Jk a weak allele ( JK∗01W.01 ; Glu44Lys) is extremely common among some Asian populations (38%–40%) ( ). To date, 24 Jk null alleles ( JK∗0N ) are recognized by ISBT. The etiology of the In(Jk) phenotype is unknown.
Clinically, anti-Jk or Kidd antibodies are a common cause of hemolytic transfusion reactions, accounting for nearly one-quarter of all delayed hemolytic transfusion reactions and 75% of those with true hemolytic sequelae ( ). They are usually of IgG1 or IgG3 isotype and are capable of activating complement (see Table 36.5 ). Antibody reactivity can be enhanced with enzyme-treated RBCs and by the presence of complement. Anti-Jk is not enhanced by polybrene. An anti-Jk3, which reacts with all RBCs except Jk null , is observed in Jk null individuals (see Table 36.20 ). Anti-Jk3 is reported to react with some Jk(a+ w ) phenotypes ( ). Jk(a+ w ) and Jk(b+ w ) can also give discordant typing with commercial typing reagents ( ; ).
Anti-Jk antibodies can be difficult to detect or identify in the blood bank. Anti-Jk antibodies are often of low titer with weak avidity and can display dosage in vitro. Furthermore, anti-Jk antibodies are frequently transient, disappearing rapidly after immune stimulation. As a consequence, patients previously sensitized to Kidd antigens may be negative for anti-Jk antibodies in later blood samples. Following transfusion of crossmatch-compatible, Jk-positive RBCs, sensitized patients can mount a brisk anamnestic antibody response with rapid and extensive in vivo hemolysis as RBCs are cleared by extravascular and intravascular hemolysis. Although uncommon, anti-Jk can cause mild HDFN.
Biologically, JK/UT-B functions in the facilitated transport of urea. In the kidney, transport of urea by JK/UT-B on vasa recta endothelial cells is thought to help stabilize osmotic gradients in the renal medulla during the concentration of urine. On RBCs, JK/UT-B may help preserve the osmotic stability of RBCs as they pass through the kidney. Although Jk null individuals exhibit a slightly decreased capacity to concentrate urine, the absence of JK/UT-B is not associated with a clinical syndrome. It is likely that other mechanisms exist to compensate or reduplicate the function of JK/UT-B on tissues ( ). Because Jk antigens are strongly expressed by the kidney, there is some suggestion that they could act as alloantigens in renal transplant patients ( ). Four case reports describe the concurrent allograft rejection with new Jk alloantibodies several years after transplantation and transfusion exposure ( ).
The Diego blood group system (DI) consists of 22 antigens, including five sets of allelic antigens: Di a /Di b (DI1/DI2), Wr a /Wr b (DI3/DI4), Hg(a+)/Mo(a+) (DI12/DI11), Wu/DISK (DI9/DI22), and Sw(a)/SW1 (DI14/DI22). Most antigens (20/22) are rare, low-incidence antigens present on less than 1% of donors. In general, there are no apparent racial differences except Di a , which is rare in all populations except those of Mongolian ancestry. This includes Asian and Northern India populations due to Mongolian military expansion in ancient times ( ). Likewise, Asian populations of Mongolian descent who migrated to the Americas approximately 15,000 years ago are responsible for the high prevalence of Di a among several native South American Indian peoples, where the frequency of Di a can reach 50% ( ; ). Interestingly, Di a is not present in circumpolar North American native populations (Eskimo-Aleut), who arose from later Asian migrations ( ). In addition to RBCs, Diego antigens are expressed by the human kidney along the collecting ducts ( ). One million copies of Diego glycoprotein (AE1) are present per RBC.
The Diego blood group system resides on Band 3, also known as AE1. AE1 is a 100-kD, 991 amino acid glycoprotein containing two functionally distinct domains: a large 40-kD amino-terminal cytoplasmic domain and a transmembrane domain comprising the carboxy-terminal half of the molecule ( Fig. 36.12 ). On RBCs, AE1 is an oligomer, usually existing as a dimer or tetramer through the formation of intramolecular disulfide bonds. The carboxy-terminal is associated with carbonic anhydrase, whereas the amino-terminal cytoplasmic domain binds several key cytoskeletal proteins (Band 4.1, Band 4.2, and ankyrin) and glycolytic enzymes: glutaraldehyde-3-phosphate dehydrogenase, aldolase, and phosphofructokinase. Binding of hemochromes or denatured hemoglobin to the extreme amino-terminus of AE1 is believed to play a role in Heinz body formation. AE1 is a critical element in both ankyrin and junctional complexes that provide vertical and lateral stability to the membrane, respectively ( ).
The remainder of AE1 is membrane associated, containing 12 to 14 transmembrane domains, which constitute the carboxy-terminal half of the molecule. Whereas the amino-terminal domain is involved in membrane stability, the carboxy-terminal domain functions as an anion transporter, facilitating movement of Cl − and HCO 3 − anions across the cell membrane ( ). Crystal structures of AE1 dimers show that the transmembrane domain is arranged into two distinct subdomains responsible for anion transport and dimerization, respectively ( ). It is believed that anions diffuse along a pathway between the two subdomains, followed by anion binding to the transport domain, which transports the anion across the membrane in an “elevator-like” process.
AE1 is closely associated with several membrane proteins important to red cell function that is often referred to as a Band 3 metabolon or macrocomplex ( ). Other proteins physically associated with AE1 include Rh complex, glycophorins, aquaporin, glucose transporter, CD47, and carbonic anhydrase. The extracellular domain is also the site of all the Diego antigens described to date. There is a single N -glycosylation site, which displays a massive, highly branched, polylactosaminoglycan with both ABO and I blood group activity ( ). It is estimated that nearly half of all ABO epitopes on RBCs are associated with AE1.
AE1 is the product of a 20-exon, 17-kb gene ( SLC4A1 ) on chromosome 17q21-q22. Di(b+) is considered the reference allele (DI∗02). Every Diego antigen identified to date is the result of an amino acid polymorphism in the translated protein (see Table 36.26 and Fig. 36.12 ). The Diego system also contains two examples of allelic, low-incidence antigens: Mo a /Hg a and Jn a /KREP. In each case, two distinct amino acid substitutions at the same amino acid residue lead to different alloantigens. A similar example exists in the Kell system (Kp a , Kp b , and Kp c ).
| HIGH FREQUENCY | Frequency | Amino Acid Change (High→Low) | Frequency | LOW FREQUENCY | ||
|---|---|---|---|---|---|---|
| ISBT | Name | Name | ISBT | |||
| KN1 | Kn a | 99% | Val1561Met | 4% whites | Kn b | KN2 |
| KN3 | Mc a | 99% | Lys1590Glu | 45% blacks | Mc b | KN6 |
| KN4 | Sl1 (Sla) | 98% whites 52% blacks |
Arg1601Gly | Blacks only | Sl2 (Vil) | KN7 |
| Sl4 ∗ | 100% blacks 96% whites |
Ser1610Thr | 4% whites | Sl5 ∗ | ||
| KN8 | Sl3 † | Arg1601+Ser1610 | ||||
| KN9 | KCAM | 80% to 98% whites | Ile1615Val | 70% to 80% West Africans, Afro-Brazilians | KDAS | KN10 ‡ |
| KN5 | Yk a | 92% whites 98% blacks |
Unknown | |||
∗ Sl4 and Sl5 are theoretical antigens not formally added to the Knops family at this time.
† Sl3 is a conformational antigen that requires the presence of both Sl1 (Arg1601) and “Sl4” (Ser1610).
‡ KDAS, the allelic antigen to KCAM, is still pending final ISBT designation.
The most interesting Diego antigen, however, is Wr b . As stated earlier, AE1 is topically associated with GYPA in the RBC membrane. RBCs lacking GYPA (En [a–] phenotype) are phenotypically Wr (a–b–), implying that Wr b is on GYPA. Available evidence now suggests that Wr b is formed by an electrostatic interaction between GYPA and the Wr b antigen (Glu658) ( ). Which specific amino acid on GYPA is critical to Wr b formation is still a matter of debate. However, clearly, part of the Wr b epitope involves amino acids on GYPA.
Antibodies against Diego blood group antigens can be immune stimulated or naturally occurring. Antibodies against Di a , Di b , Wr b , and ELO are usually immune stimulated. These antibodies are of IgG isotype and are detected in the AHG phase of testing. Anti-Di a , Di b , and Wr b can be associated with decreased red cell survival, hemolytic transfusion reactions, and HDFN. Anti-Wr b is also associated with autoimmune hemolytic anemia ( ).
In contrast, antibodies against the majority of other Diego antigens are usually naturally occurring, room temperature saline agglutinins. Anti-Wr a is particularly common, occurring in 1 in 100 donors. Antibodies against Wd a and WARR are also fairly common, with anti-WARR reported in 13% to 18% of donors ( ).
Functionally, AE1 plays a critical role in gas transport and acid-base equilibrium. To facilitate the removal of CO 2 , RBCs hydrate CO 2 via carbonic anhydrase to form bicarbonate ion (HCO 3 – ), which is readily soluble in plasma. As the level of intracellular HCO 3 − rises, RBCs exchange HCO 3 − ions for Cl − in plasma (the Cl − shift, or Hamburger shift). This process is mediated by AE1 as RBCs pass through capillaries and small capillary venules. Because of the high copy number of AE1 on the RBC membrane, anion exchange is 90% complete within 0.4 to 0.5 second. An increase in HCO 3 − capacity and osmotic resistance is observed with Miltenberger III red cells owing to significantly higher AE1 expression ( ). In the kidney, AE1 is expressed along the basolateral membrane of type A intercalated cells of renal collecting ducts, where it functions in acid secretion and bicarbonate readsorption ( ). Given the physiologic importance of AE1, it is not surprising that Diego null phenotype is considered embryonic lethal.
AE1 also plays a role in the RBC cytoskeleton, impacting membrane stability and red cell circulation (see Fig. 36.12 ). Not surprisingly, mutations in AE1 are associated with red cell abnormalities. Southeast Asia ovalocytosis (SAO) is due to a 27 bp deletion (SLC4A1Δ27) in the amino-cytoplasmic domain and is common in malaria-endemic areas of the Pacific. Affected individuals are heterozygous for the mutation and have an apparent increased resistance to severe malaria ( ). The mutation affects AE1 oligomerization, membrane diffusion, and rigidity, suggesting that SAO red cells may be more susceptible to splenic clearance. AE1 is a target of senescent autoantibodies ( ). In addition, red cell damage is often accompanied by AE1 clustering, facilitating removal of red cells damaged by malaria, cold storage, or other insults ( ; ; ).
Finally, AE1 was recently identified as a receptor for Bartonella , the cause of Oroya fever, trench fever, bacillary angiomatosis and “cat scratch” disease ( ). Unlike malaria, Bartonella does not impact red cell survival or function, leading to prolonged and chronic bacteremia. There is no correlation between Bartonella and Di a phenotype, despite the high incidence of both in some areas of South America.
The YT blood group, historically known as Cartwright, was discovered in 1956 and consists of five antigens, including two autosomal-codominant antigens (Yt a , Yt b ). Yt a (Yt1) is a high-incidence antigen expressed by 99.8% of white donors. The incidence of Yt b (Yt2) varies by race, ranging from 0% in Japanese to 24% to 26% in the Middle East ( ). Three additional high incidence antigens have been identified: YTEG (Yt3), YTLI (Yt4), and YT0T (Yt5) ( ). Yt antigens are missing on PNH III RBCs, which are devoid of all glycosyl-phosphatidylinositol (GPI)-linked glycoproteins. In addition to RBCs, Cartwright antigens are expressed on neural synapses and neuromuscular junctions ( ). A total of 7000 to 10,000 molecules of Yt glycoprotein are present per RBC.
The Cartwright antigens are located on acetylcholinesterase (AChE), a β-carboxyesterase responsible for degradation of the neurotransmitter acetylcholine. Several different molecular forms of AChE have been isolated, including large heteromeric forms composed of several catalytic subunits covalently linked to collagen. On RBCs, AChE is expressed as a palmitoylated, GPI-linked glycoprotein ( Fig. 36.13 ) ( ). Human erythrocyte AChE is unique in the palmitoylation or covalent linkage of a palmitic fatty acid to the inositol ring, which renders the molecule resistant to phospholipase C. In RBC membranes, AChE usually exists as a 160-kD dimer and possesses both N - and O -linked glycans.
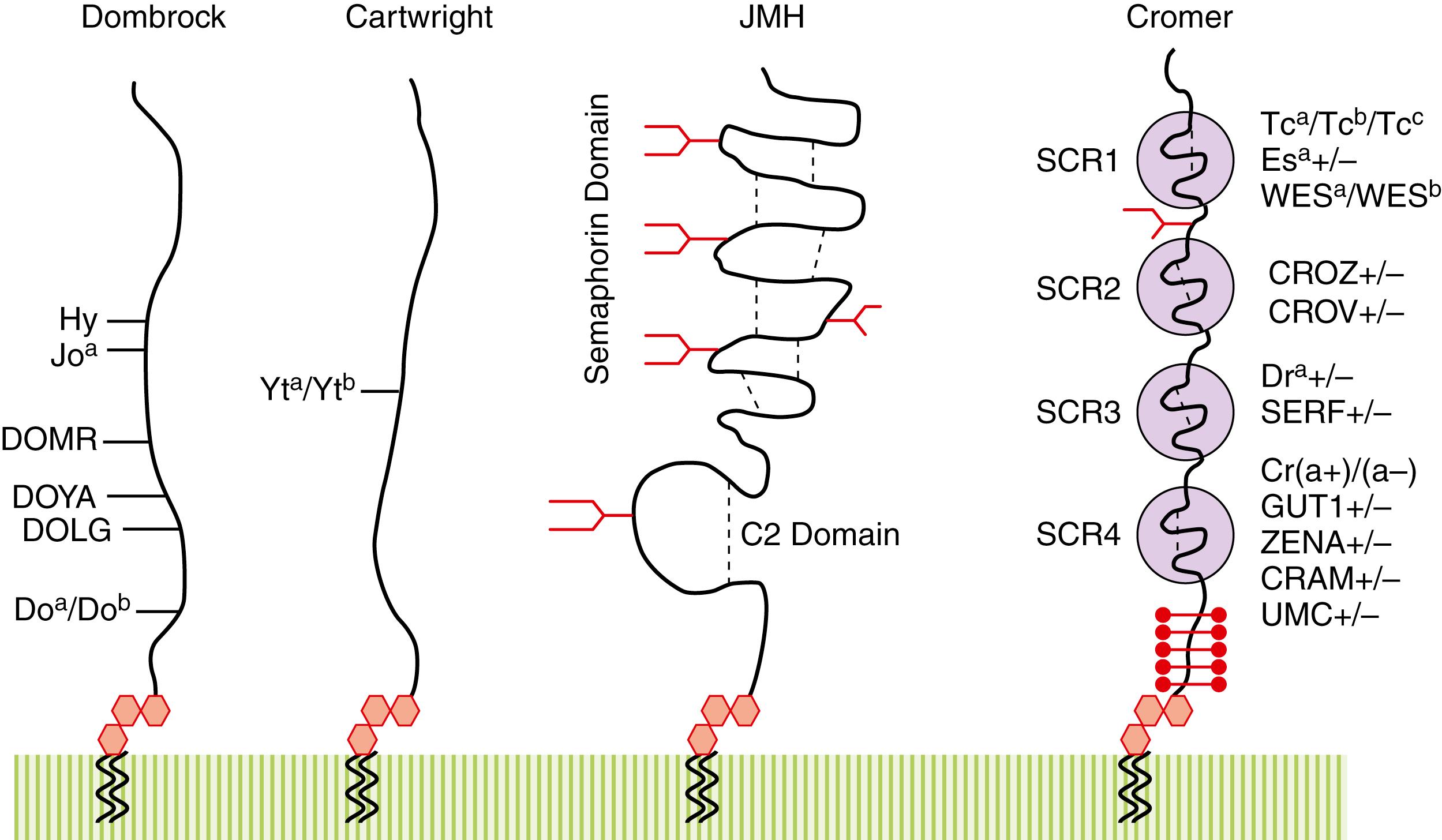
The gene for AChE possesses four exons, spanning 4.5 to 4.7 kb, on chromosome 7q22. Alternate splicing of exon 3 is responsible for the GPI-linked form of AChE observed in RBCs ( ). The Yt a and Yt b antigens represent a single–amino acid polymorphism at residue 322, where His322 is Yt a ( YT∗01 ) and Asn322 is Yt b ( YT∗02 ) ( ). The Yt a /Yt b polymorphism is not located near the catalytic site of the enzyme and has no effect on enzyme activity ( ). Missense mutations are responsible for the loss of high-incidence antigens YTEG ( YT∗03 , Gly89Glu), YTLI ( YT∗04 , Gly57Arg) and YTOT ( YT∗05 , Arg34Gln) ( ).
In general, anti-Yt a and anti-Yt b are usually clinically benign, although shortened red cell survival and even delayed hemolytic transfusion reactions have been reported. Neither antibody is associated with HDFN ( ). Anti-Yt a and anti-Yt b are of IgG isotype, arising from immune stimulation, and are usually detected in the IAT. Despite the high incidence of Yt a in the general population, anti-Yt a is more common than anti-Yt b , suggesting that Yt a is the more immunogenic antigen. Anti-Yt a is also more likely to cause a positive monocyte-monolayer assay with decreased red cell survival ( ). Antibodies against Yt can be inhibited by soluble recombinant AChE protein ( ).
AChE is a critical enzyme required for the rapid degradation of acetylcholine on postsynaptic membranes of nerves and muscles. The role of AChE in RBCs is unknown.
The Xg blood group system contains a single antigen, Xg a . As a result, only two phenotypes are known: Xg a -positive and Xg a -negative. Because the Xg a antigen is encoded by a gene on the X chromosome, the incidence of the Xg a -positive phenotype is higher among women. Among whites, approximately 89% of women and 66% of men are Xg a positive. Similar results have been found in other ethnic populations ( ). Xg appears to be specific for RBCs. Approximately 9000 molecules of Xg a are present per RBC ( ).
The Xg a antigen is located on a 24- to 29-kD, 180 amino acid glycoprotein named XG protein. A type 1 glycoprotein, XG protein possesses a large 117 amino acid, amino-terminal extracellular domain; a single transmembrane domain; and a short, carboxy-terminal cytoplasmic tail. Of 11 potential O -glycosylation sites, only 3 appear to be glycosylated. The protein is encoded by a 2.4-kb gene ( XG ) located on the X chromosome at the pseudoautosomal boundary/X-specific boundary (pAB1X). A mutation located 3.7 kb upstream of the XG transcription start site was identified as the basis for Xg(a–) phenotype in both men and women. The mutation (rs31103G>C) disrupted binding of the erythroid transcription factor GATA1 ( ).
In the RBC membrane, the XG protein is associated with a second 32 kD sialoglycoprotein, MIC2 or CD99, possibly as a heterodimer. Evidence suggesting a near-neighbor relationship of the XG and MIC2 proteins includes MIC2 inhibition of anti-Xg a antibodies and coimmunoprecipitation of MIC2 by anti-Xg a . In women, Xg(a–) is always associated with low CD99 expression. Conversely, CD99 is elevated in Xg(a–) men ( ).
Anti-Xg a is not associated with hemolytic transfusion reactions or HDFN ( ). Anti-Xg a may be immune stimulated or naturally occurring. Most examples are of IgG isotype, including some capable of activating complement. Because of differences in Xg a -positive phenotype between men and women, most examples (>85%) of anti-Xg a are observed in men.
The Scianna (Sc) blood group system contains seven antigens. Sc1 and Sc2 are antithetical antigens, with most individuals typing as Sc:1,–2. Sc2 and Sc4/Rd are low-incidence antigens, whereas Sc3, Sc5, Sc6, and Sc7 are high-incidence antigens ( Table 36.21 ) ( ). Sc3 is a high-incidence antigen present on all RBCs except Sc null (Sc: –1, –2, –3). Sc–1,2 is more common among oceanic populations of the Pacific Ocean, whereas Sc4 is found most often in Slavic and Ashkenazi Jews. Scianna appears to be specific for RBCs and erythropoietic tissues.
| Antigen (High Frequency) | Percent Donors | Amino Acid Change (High→Low Frequency) | Percent Donors | Antigen (Low Frequency) |
|---|---|---|---|---|
| Sc1 | 99 | Gly57Arg | 0.7 | Sc2 |
| Sc3 | 100 | 307delGA Arg332stop |
Rare | SC null |
| Pro60Ala | Rare | (Rd) Sc4 | ||
| Sc5 (STAR) | 100 | Glu47Lys | ||
| Sc6 (SCER) | 100 | Arg81Gln | ||
| Sc7 (SCAN) | 100 | Gly35Ser |
Scianna antigens reside on er ythrocyte m embrane- a ssociated p rotein (ERMAP), a 60- to 68-kD, 446 amino acid glycoprotein. Similar to Lutheran, Ok, and LW proteins, ERMAP is a member of the Ig superfamily. The molecule is a type 1 single-pass transmembrane protein possessing a single IgV domain and a large cytoplasmic domain that is likely involved in signal transduction. The latter possesses both SH3 and B30.2 domains as well as multiple phosphorylation sequences for protein kinase C, tyrosine kinase, and casein kinase II. The molecule possesses 11 cysteine residues and is sensitive to sulfhydryl-reducing agents.
The ERMAP gene resides on chromosome 1p34 and consists of 11 exons spanning 19 kb. Like many blood group antigens, ERMAP is regulated by the transcription factor KLF1 ( ). Most Sc antigens are the result of an SNP and amino acid polymorphism (see Table 36.21 ). Four Sc-null alleles ( SC∗O1N ) are known, all containing the same nonsense mutation (R332Stop) ( ). An additional amino acid polymorphism (His26>Tyr) has been identified in the leader sequence, which is not expressed on the mature red cell protein ( ). Approximately 25% of whites are positive for the His26>Tyr polymorphism.
Anti-Scianna antibodies are rare and generally benign. They are usually of IgG isotype, with some examples binding complement. Most examples are immune stimulated; however, naturally occurring anti-Sc2 antibodies are known. The antigens are relatively resistant to enzymes but can be weakened with DTT and AET. In general, they are not a cause of transfusion reactions although one case of AHTR due to anti-Sc2 was recently reported ( ). Both anti-Sc2 and anti-Sc4 (Rd), have been associated with HDFN. Autoantibodies against Sc1 and Sc3 antigens have been associated with warm autoimmune hemolytic anemia ( ). Anti-Scianna antibodies can be neutralized with soluble recombinant ERMAP protein ( ).
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here