Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Fibrous dysplasia is a benign proliferation of fibro-osseous tissue that may be diagnosed at any age; most manifest in the second to third decades of life ( Table 24.1 ). Males and females are affected equally, and it may occur in all racial groups. Most (70%–80%) are solitary (monostotic), but a minority of patients have multiple bone involvement (polyostotic). Although symptoms vary depending upon the anatomic site, most patients are asymptomatic and, consequently, fibrous dysplasia is typically an incidental finding. Patients with polyostotic disease usually develop symptoms in childhood, primarily due to developmental malformations leading to complaints of pain and/or gait disturbances. The polyostotic form can be associated with crippling deformities.
| Fibrous Dysplasia | Osteofibrous Dysplasia | |
|---|---|---|
| Peak Age | Second and third decades | First decade |
| Sex | Males = females | Males = females |
| Symptoms | Painless, soft tissue swelling, abnormal bone growth, Albright syndrome (polyostotic lesions, skin pigmentation, precocious puberty, Mazabraud syndrome (polyostotic lesions, soft tissue myxomas) | Painless, soft tissue swelling, bowing of the tibia/fibula. |
| Involved Bones (decreasing order of frequency) | Skull and craniofacial skeleton, femur, tibia and ribs | Tibia (almost exclusively) Fibula (rarely) |
| Radiographic Findings | Metaphyseal/diaphyseal, lytic, expansile, sharply marginated; ground glass appearance; may affect multiple bones (polyostotic) | Cortical diaphysis, lytic, expansile, multiloculated; no periosteal reaction |
| Osteoblastic Rimming | Usually absent; when present typically minimal and focal | Present, dominant and diffuse |
| Hyaline Cartilage | May be present; rarely can predominate | Absent |
In the more common monostotic form, fibrous dysplasia most commonly arises in bones of the craniofacial skeleton, followed by the long bones of the lower extremities, primarily the proximal femur and ribs. In addition to the craniofacial region, polyostotic fibrous dysplasia has a predilection for the pelvis and long bones of the lower extremities. Albright syndrome is polyostotic fibrous dysplasia associated with various stigmata, including pigmented skin lesions (café-au-lait pigmented lesions that have irregular borders) and precocious puberty. Mazabraud syndrome is the combination of polyostotic fibrous dysplasia and intramuscular soft tissue myxomas. The monostotic form does not evolve into the polyostotic form. Many tumors that were previously classified as liposclerosing myxofibrous tumor (LSMFT), most often involving the proximal femur, are now considered a form of fibrous dysplasia.
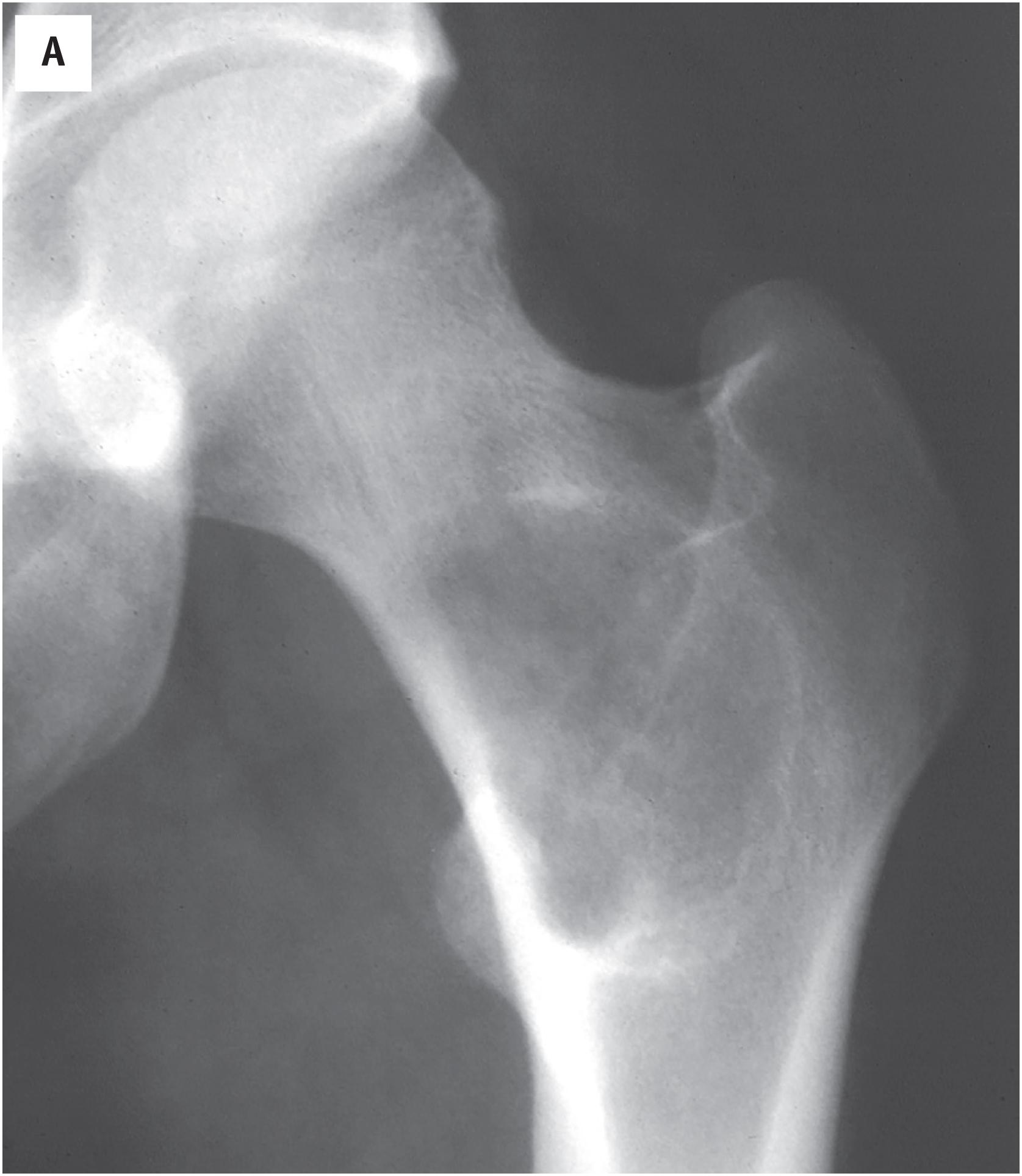
The radiologic appearance of fibrous dysplasia is variable, but most examples show an expansile, non-aggressive, sharply marginated, variably ossified lesion ( Fig. 24.1 A–D ). A sclerotic rim is often observed. With rib involvement, a marked expansion of the affected bone is typically seen. Intralesional densities may impart a “ground glass” appearance. Rarely, fibrous dysplasia may erode the overlying cortex or protrude beyond the contours of the bone (fibrous dysplasia protuberans).
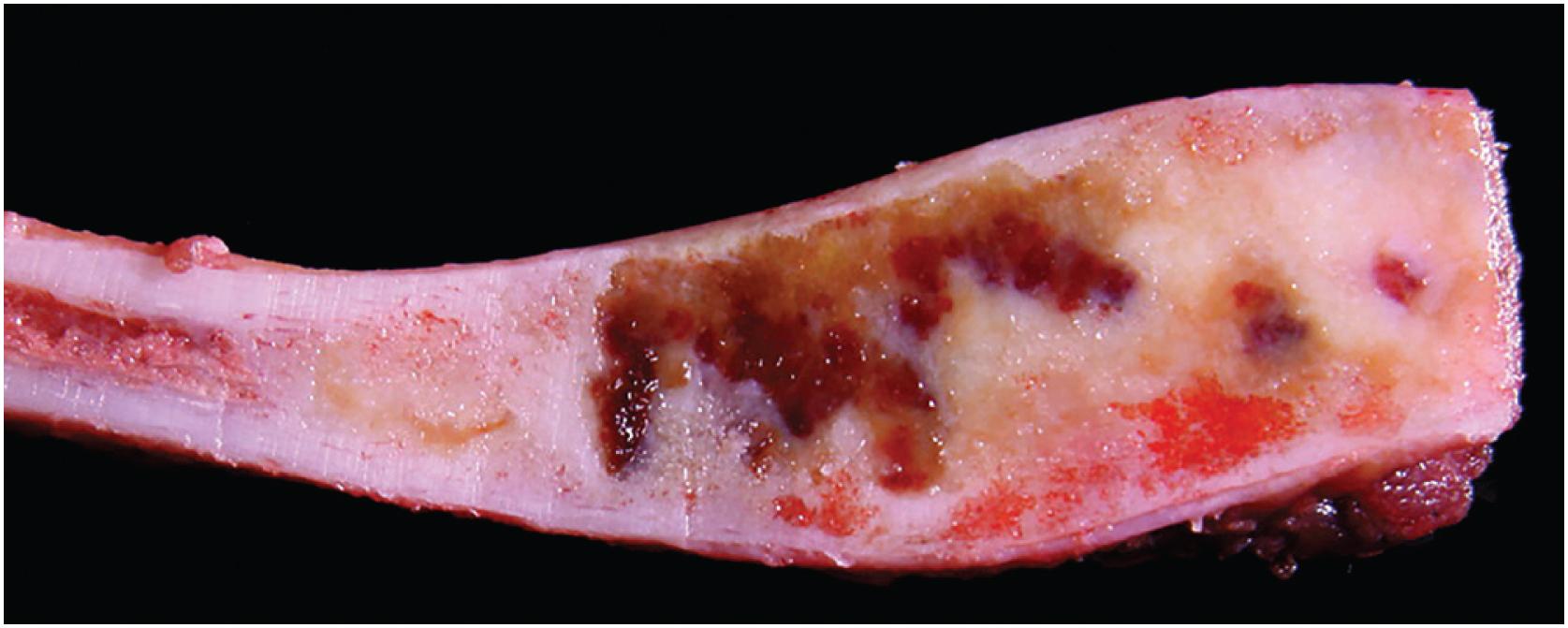
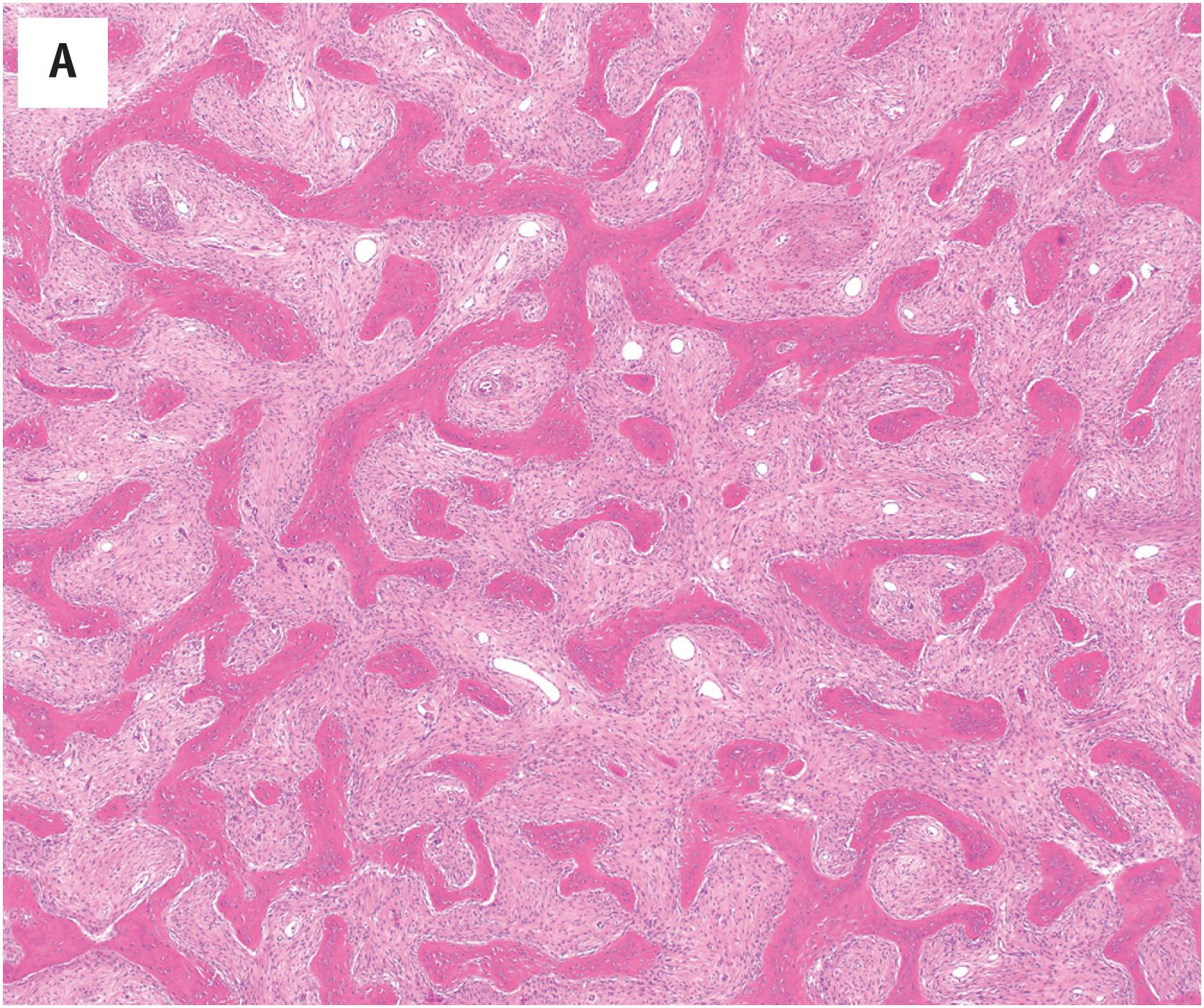
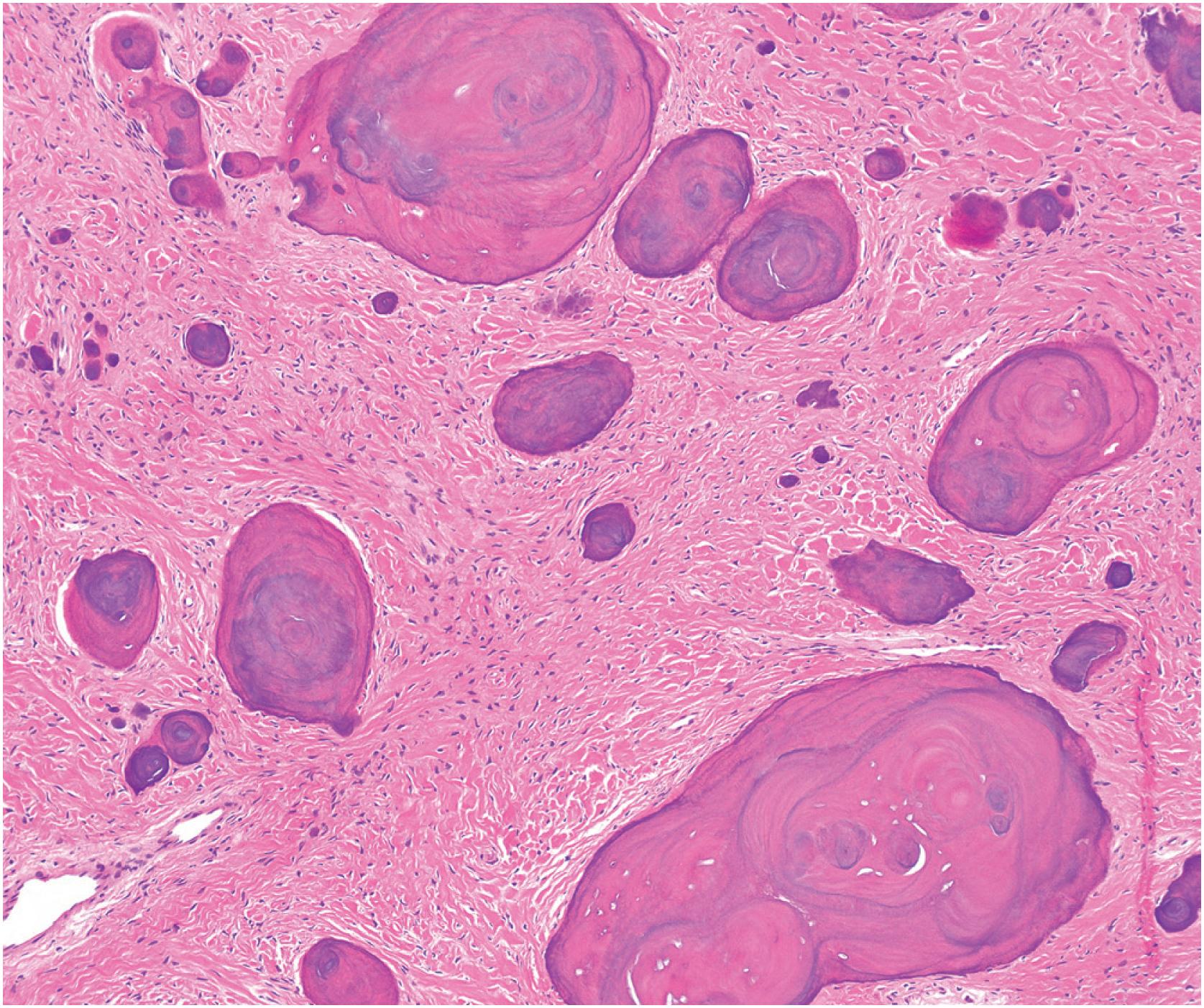
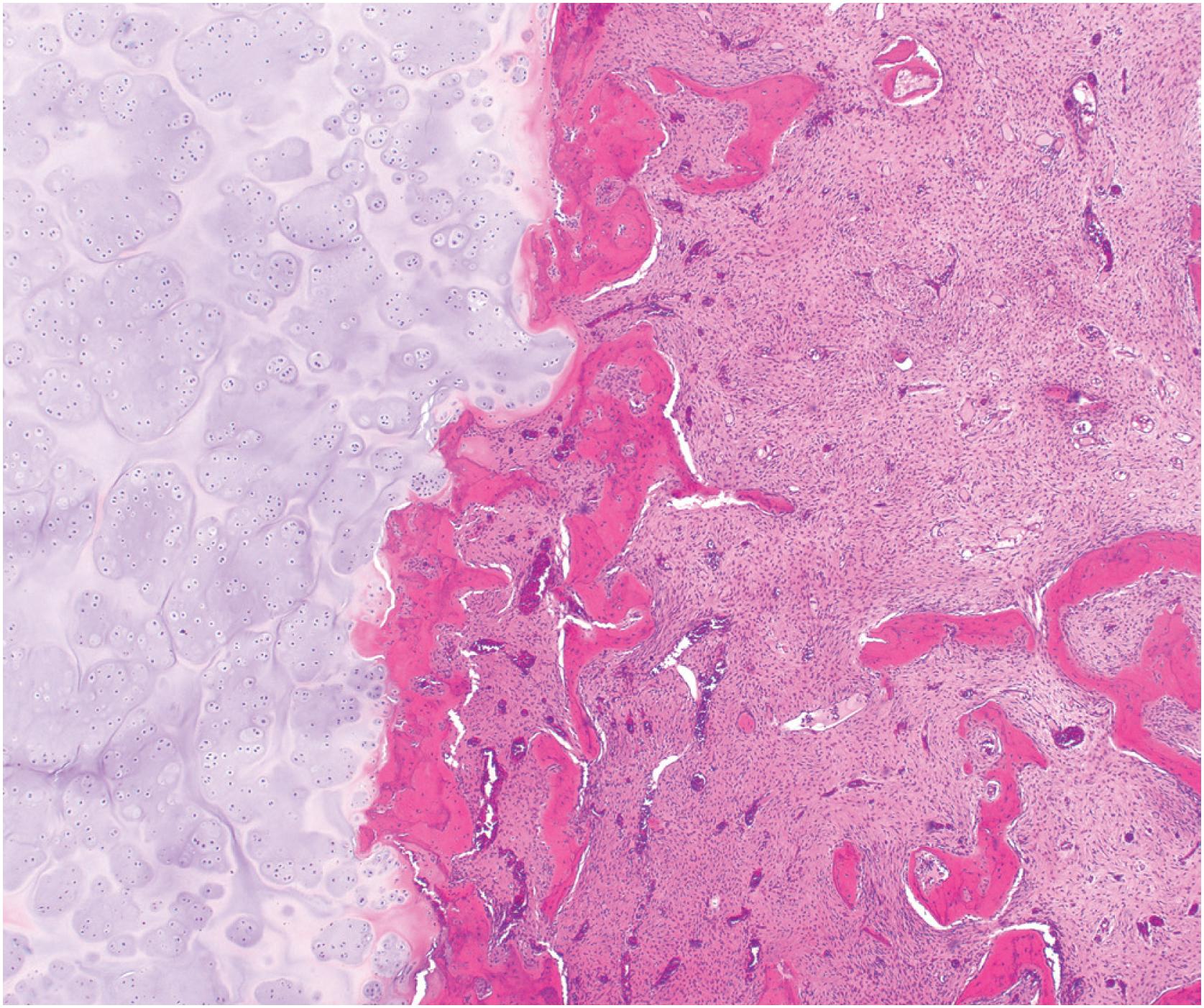
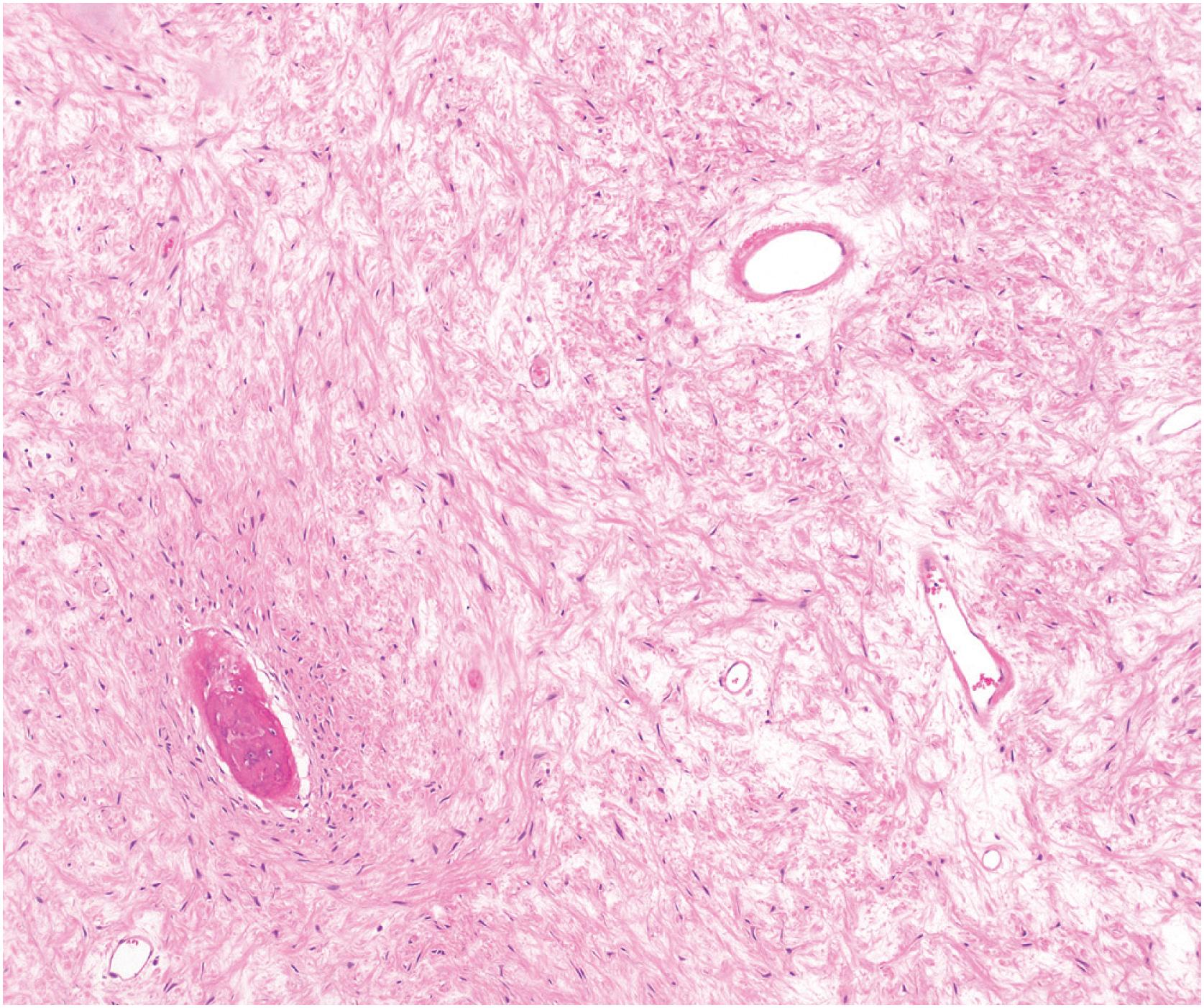
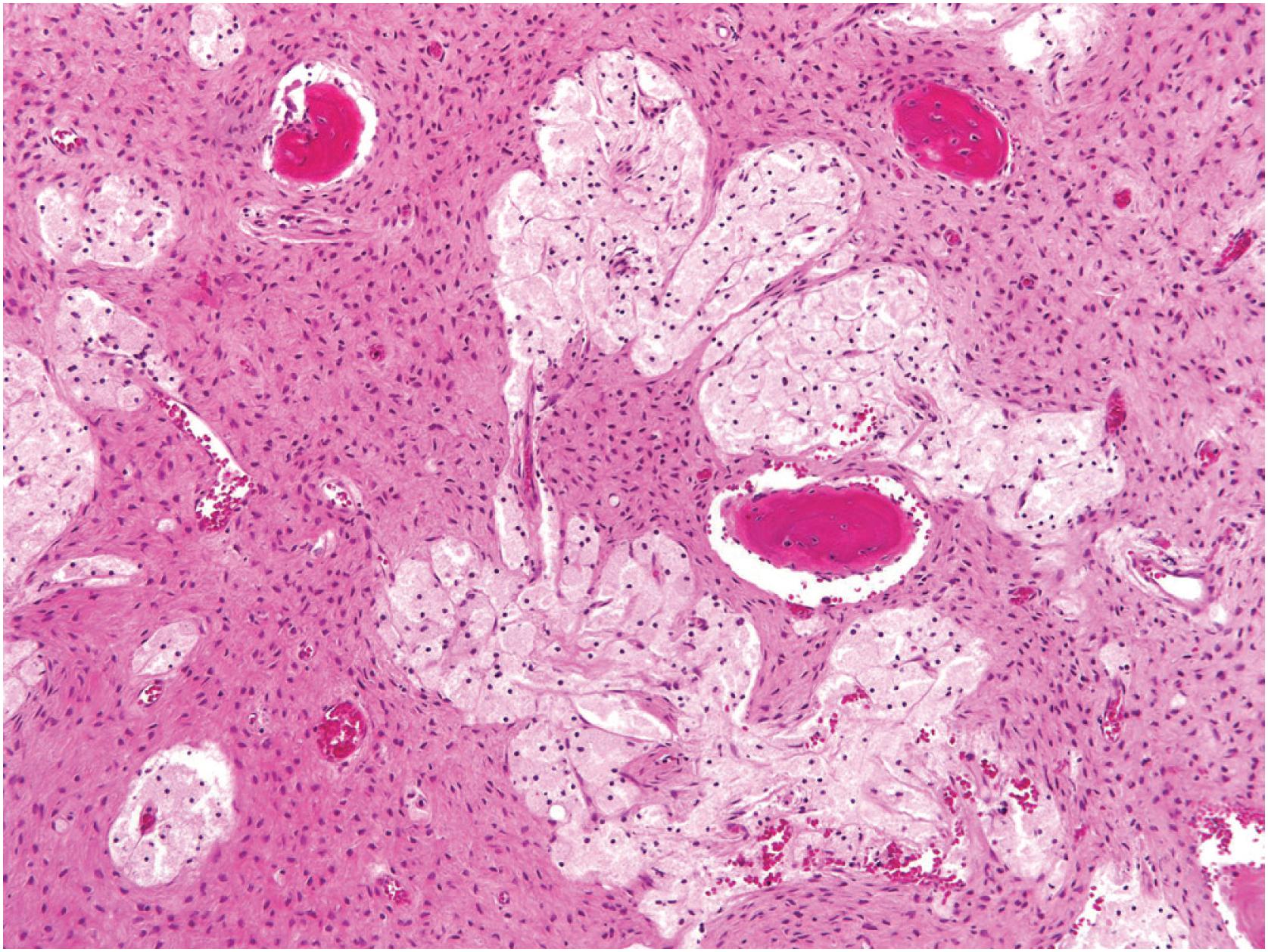
Grossly, the tumor is centered in the medullary cavity and has a tan cut surface that may be cystic ( Fig. 24.2 ). Microscopically, fibrous dysplasia is a fibro-osseous lesion, consisting of a variable mixture of haphazardly arranged bony trabeculae often in alphabet-like shapes, embedded within a slightly to moderately cellular fibrous stroma ( Fig. 24.3A–B ). Less commonly, the stroma is more cellular and accompanied by numerous osteoclast-type giant cells, resembling an aneurysmal bone cyst ( Fig. 24.3C ). In most cases, the bony trabeculae represent woven bone, but more mature lamellar bone is usually at least focally present and may predominate, especially in the craniofacial region. The presence of reversal cement lines mimicking Paget disease is frequently observed. Calcified spherules (cementicles) are sometimes seen, again, commonly in the craniofacial region but also in the extremities ( Fig. 24.4 ). Their presence in various anatomic sites has led some to postulate that cemento-ossifying fibroma and fibrous dysplasia represent a spectrum of similar disease and not specific clinicopathologic entities. Although virtually never conspicuous, osteoblastic rimming may be focally seen and does not preclude the diagnosis of fibrous dysplasia in the appropriate clinicoradiologic setting. Occasional examples of fibrous dysplasia contain aggregates of hyaline cartilage, which rarely may be prominent, raising the differential diagnosis of an enchondroma ( Fig. 24.5 ). Such lesions are sometimes referred to as fibrocartilaginous dysplasia. Between the bony trabeculae, the fibrous stroma ranges from slightly cellular with abundant collagenization to highly cellular with minimal collagen deposition. Marked stromal myxoid and/or cystic changes may potentially confound the diagnosis ( Fig. 24.6 ). Cytologically, the individual fibroblasts are small and uniformly round to ovoid with plump to spindled, hyperchromatic nuclei. As with virtually all bone tumors, randomly distributed osteoclast-type giant cells are not uncommon. Mitotic activity is scant and never atypical. Xanthomatous changes are frequently present and, similar to hyaline cartilage, may obscure the more characteristic diagnostic features ( Fig. 24.7 ). Meticulous sampling and attentively seeking more typical areas representative of fibrous dysplasia will lead to the correct diagnosis. It is likely that many examples of the so-called “xanthoma of bone” represent forms of fibrous dysplasia.
SATB2, a marker of osteoblastic differentiation, is positive in virtually all cases of fibrous dysplasia. However, MDM2 and CDK4, typically associated with low-grade central osteosarcomas, are not expressed in fibrous dysplasia, providing an excellent diagnostic tool for distinguishing these morphologically similar lesions when necessary.
GNAS (guanine nucleotide-binding protein/alpha-subunit) activating mutations appear to be directly involved in the pathogenesis of fibrous dysplasia. Regardless of histologic features (e.g., conventional, aneurysmal bone cyst-like, or osteocartilaginous), these mutations are detected in approximately 50% of cases. Nevertheless, they appear relatively specific and are not seen in other similar fibro-osseous lesions, including osteofibrous dysplasia and low-grade central osteosarcoma. Karyotyping in a subset of cases has revealed a variety of numerical and structural changes, with the most common being trisomy 2. These findings suggest that fibrous dysplasia is a neoplastic process.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here