Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Acknowledgment: We would like to acknowledge Robert M. Szabo, MD, and William W. Eversmann, Jr., MD, for their contributions to the chapter on this subject in previous editions of this textbook.
Compression neuropathies in the upper extremity are common. The rising prevalence of obesity in North America coupled with an aging population suggests that the problems of upper extremity compression neuropathy will likely increase in the next decade. The etiologic relationship between nerve compression disorders and occupation was controversial in the 1990s. , The existence of multiple levels of nerve compression is now generally recognized, although the contributions of work-related issues of these problems is still debated. , More recent evidence supports an increased risk of developing carpal tunnel syndrome with forceful grip/exertion, high hand/wrist repetition rates, and high body mass index. It is generally accepted that work is just one component of many factors that contribute to and aggravate compression neuropathy. , There is increased awareness that certain sleep positions will, over time, result in compression neuropathy.
Surgeons treating nerve compression in the upper extremity must be aware of other neurologic problems, such as brachial plexus neuritis, neuralgic amyotrophy (also known as Parsonage-Turner syndrome), mononeuritis, radiculopathy, and motor/sensory neuropathies that can mimic entrapment. Similarly, individuals who have hereditary neuropathy with liability to pressure palsies (HNPP) are genetically predisposed to the development of nerve compression. The prevalence of genetic predisposition is unknown but may help explain some clinical manifestations of entrapment neuropathy, as do systemic factors such as obesity, diabetes, and endocrine disease.
The clinical findings in patients with chronic nerve compression are variable and reflect the broad spectrum of histopathologic changes that occur in the nerve. Because biopsy of neural tissue related to chronic nerve compression is not performed, much of the information known about the histopathology of human nerve compression has been extrapolated from animal models. , Studies have suggested neural ischemia as contributing to compression neuropathies, , and animal models have been developed to induce chronic nerve compression. ,
The continuum of neural changes seen with compression neuropathy depends on the force and duration of the compression. The histopathologic changes that occur with chronic nerve compression begin with breakdown of the blood-nerve barrier, followed by endoneurial edema and, subsequently, perineurial thickening ( Fig. 28.1 ). , Increased endoneurial pressure will result in changes in the microneural circulation and render the nerve susceptible to dynamic ischemia. With increased compression, there will be localized demyelination, followed by more diffuse demyelination and finally axonal degeneration. Neural changes typically do not occur uniformly across the nerve and may vary depending on the distribution of compressive forces across the nerve. Fascicles susceptible to greater pressure will undergo changes sooner and may result in variable patient symptoms within a nerve’s distribution. For example, in early carpal tunnel syndrome, the superficial fascicles to the long finger and ring finger are usually affected before the fascicles to the thumb and radial side of the index finger. In cubital tunnel syndrome, the fascicles to the intrinsic muscles are located closer to the bony groove and are affected more than those to the flexor digitorum profundus and flexor carpi ulnaris, which are located farther from the bone.
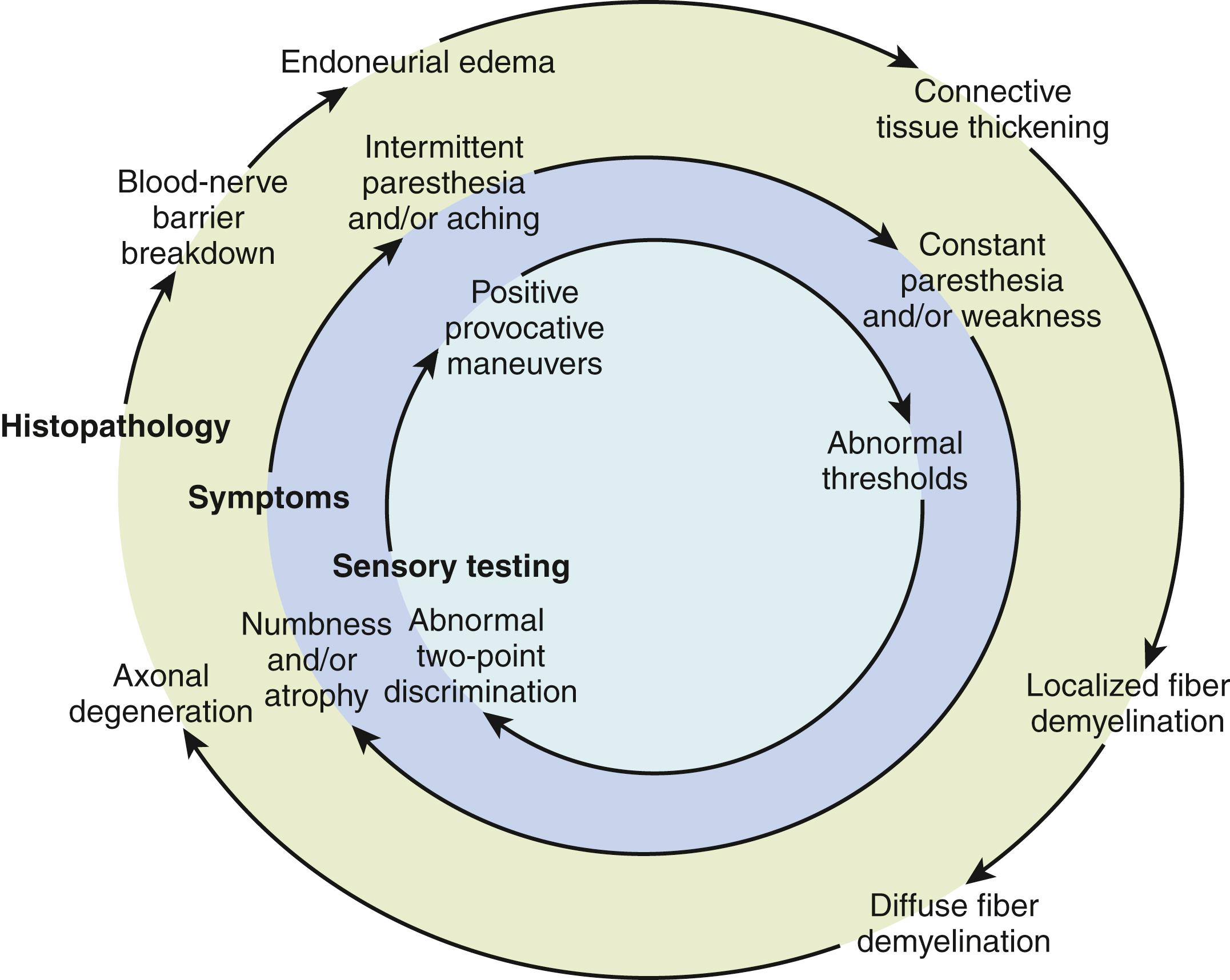
It is theorized that patient sensory complaints parallel histopathologic neural changes, progressing from intermittent paresthesia to persistent numbness (see Fig. 28.1 ). Sensory testing will also vary with the degree of nerve compression. Initially, patients will have altered threshold tests (vibration and Semmes-Weinstein monofilament testing), and with more severe nerve compression, they will progress to deficits in tactile discrimination testing (static and moving two-point discrimination).
First introduced by Upton and McComas, the double-crush mechanism is an important concept in nerve compression. In the clinical review by Upton and McComas of 115 patients with median or ulnar nerve compression, 81 patients had evidence of a cervical root lesion, and the authors concluded that compression of a nerve at one level makes the nerve more susceptible to damage at another level. The authors hypothesized that axoplasmic flow would be compromised by proximal compression on the nerve and then be further compromised by the distal compression site. The reverse double-crush syndrome was described by Lundborg. He hypothesized that a distal site of compression would decrease the flow of neurotrophic substances back to the neuron and thus decrease the production of substances to be transported distally. Other clinical studies have supported the double-crush mechanism with associations between the cervical spine, thoracic outlet, and distal sites of compression, including the carpal and cubital tunnels. Hurst and associates reported on 1000 cases of carpal tunnel syndrome in 888 patients and noted a significant relationship between bilateral carpal tunnel syndrome and patients with cervical arthritis and diabetes. In a case control study, Wessel and associates reported that patients with a history of cervical spine surgery had poorer outcomes compared with patients undergoing only distal nerve decompression. Other studies have reported similar findings with cervical radiculopathy, thoracic outlet syndrome, and distal nerve entrapment in the arm.
Animal models demonstrating the double-crush mechanism have been described , to support the double-crush and the reverse double-crush mechanisms of nerve compression. Although described in 1973, it is still controversial to some hand surgeons. The concept of a susceptibility to compression at known areas of nerve compression along the path of a single nerve is a powerful tool in the management of many patients with motor system impairment.
Other medical conditions and personal factors have been associated with carpal tunnel syndrome, including diabetes mellitus, acromegaly, hypothyroidism, excessive alcohol use, obesity, and tobacco use. Compared with a control group without symptoms of carpal tunnel syndrome, patients with carpal tunnel syndrome demonstrated a higher prevalence of hypothyroidism, diabetes, and obesity. Smoking history was not associated with an increased incidence of carpal tunnel syndrome.
Hereditary motor-sensory neuropathy consists of a group of inherited disorders that affect the motor and sensory nerves of the peripheral nervous system, including Charcot-Marie-Tooth disease and HNPP. HNPP is an autosomal dominant demyelinating motor-sensory neuropathy. It is characterized by underexpression of the peripheral myelin protein-22 gene on chromosome 17 and is manifested as recurrent, multifocal nerve compression. Patients may have rapid-onset neuropathies after minor trauma with decreased nerve conduction (motor and sensory) in the affected and unaffected nerves. In most cases, recovery is complete without surgery. In the upper extremity, the ulnar nerve is most commonly affected, and in the lower extremity, the peroneal nerve is affected. The presence of HNPP should be considered in patients with multiple recurrent neuropathies.
Longitudinal nerve mobility is important for normal nerve function. Although some longitudinal movement is permitted via the plexus formation within a nerve and via the loose attachment of the mesoneurium, damage may occur with excessive or prolonged traction. Venous obstruction has been shown with acute stretching of 8% of a nerve’s resting length, and ischemia occurred when it was stretched 15% of its resting length. Watanabe and colleagues investigated nerve strain in a rat model. The authors used the same amount of strain in the continuous and the repetitive strain groups and reported abnormalities in the repetitive strain group as determined by histologic, electrophysiologic, and functional evaluation. They concluded that a small amount of repetitive strain on neural tissue may result in neural dysfunction, particularly in cases with underlying subclinical nerve pathologic findings.
Studies have shown evidence of strain on the median and ulnar nerves during median and ulnar nerve tension tests and reported restriction of median nerve excursion in patients with carpal tunnel syndrome. Nerve compression may induce connective tissue and synovial thickening that may secondarily result in restriction of neural mobility with joint motion. ,
Much of the controversy associated with nerve compression is related to patients with diffuse symptoms and to the relationship of occupation to nerve compression disorders. Certain postures and positions may contribute to nerve compression. It is well accepted that wrist positions of moderate flexion and extension will increase pressure within the carpal canal, and this is hypothesized to contribute to carpal tunnel syndrome. , Similarly, elbow flexion will increase pressure within the cubital tunnel and thereby compromise the ulnar nerve. , However, these postures and positions will not only have an impact on the nerve but may also affect the surrounding musculature ( Fig. 28.2 ). We believe that prolonged or extreme postures or positions will affect the nerves and soft tissues in the upper extremity and cervicoscapular region and that multifocal nerve compression and muscle imbalance can contribute to diffuse upper extremity symptoms.
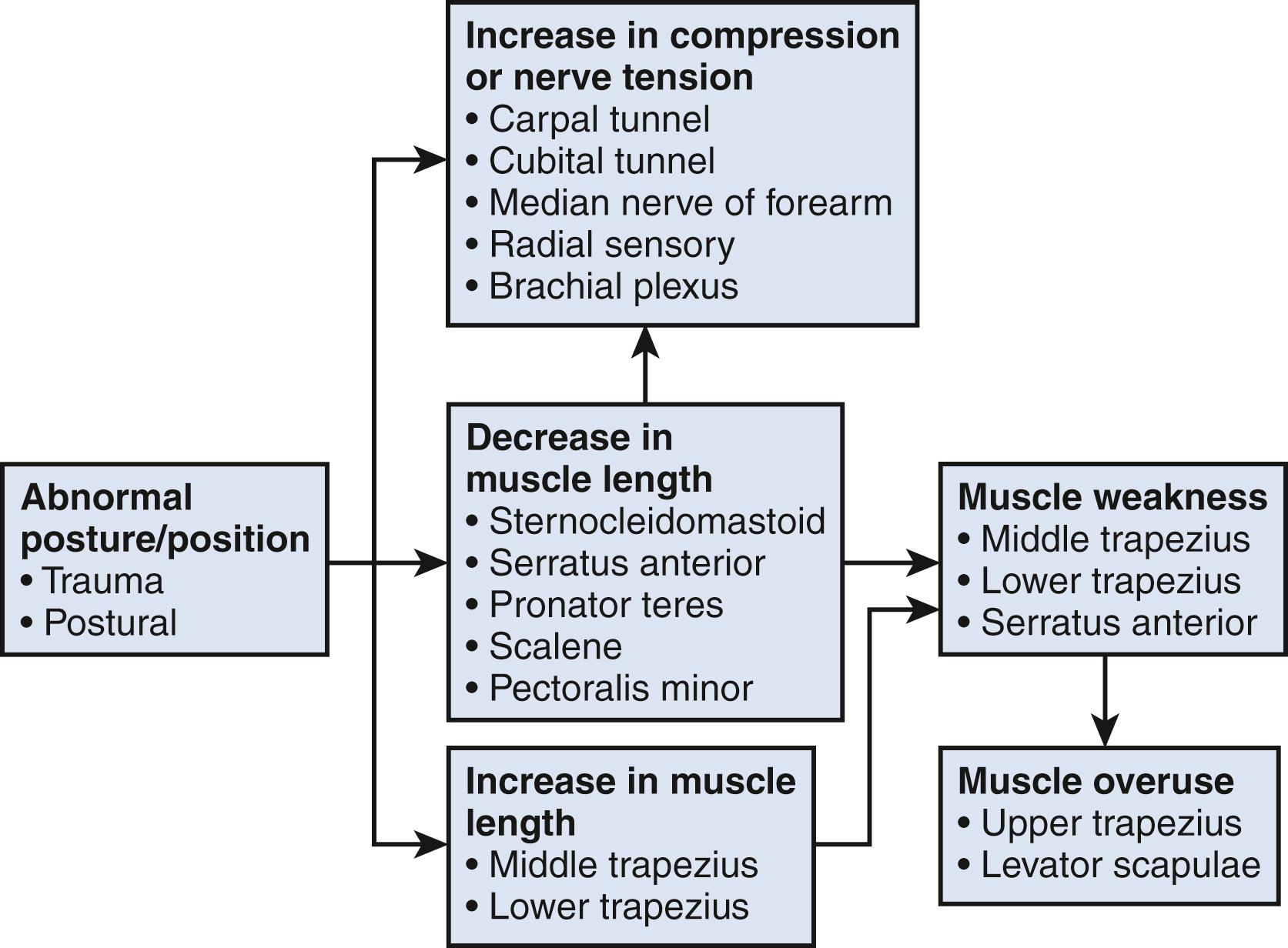
Associated postural related upper extremity issues and especially scapular dyskinesia should be considered in patients with substantial symptoms and relatively normal electrodiagnostic studies. Physicians performing electrodiagnostic studies on these patients should evaluate for subtle signs of cervical radiculopathy and thoracic outlet syndrome. Patients with persistent median nerve symptoms following carpal tunnel release or with relatively normal nerve conduction studies should be evaluated for median nerve compression in the forearm. Patients with cubital tunnel syndrome and normal nerve conduction studies should be evaluated for weak parascapular muscles resulting in forward flexed posture of the shoulder causing elongation and traction of the lower plexus and ulnar nerve.
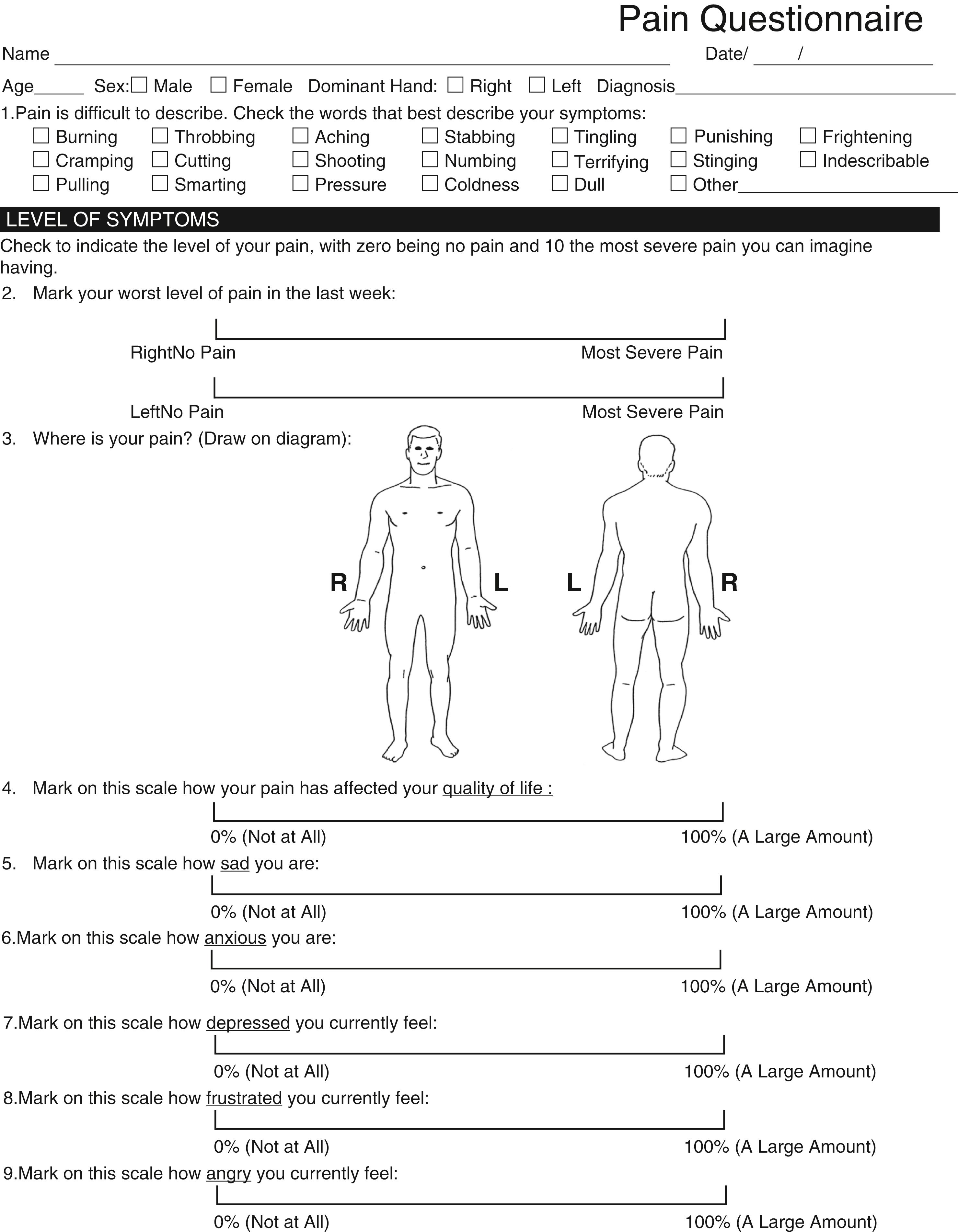
To develop an effective treatment plan, it is necessary to identify all sites of nerve compression and the musculoskeletal disorders that are contributing to a patient’s symptoms. Different diagnostic tests will yield important information at different stages of nerve compression (see Fig. 28.1 ). Not all tests will be positive at all stages; therefore it is important to use the assessment tools that will give the most accurate information for identifying a pathologic change or determining the grade severity. A complete sensory and motor evaluation, including electrodiagnostic studies, can determine the severity of nerve compression. Use of a subjective pain evaluation questionnaire is helpful to identify all symptomatic areas and other factors that may contribute to a patient’s symptoms. We have found the use of a pain evaluation questionnaire that includes a body diagram, a subjective questionnaire, and visual analog scales to be very useful in the evaluation of patients with nerve compression and/or diffuse symptoms ( Fig. 28.3 ).
Self-report questionnaires are useful to capture the patient’s perception of physical impairment, impact of the condition, and level of disability. A number of validated questionnaires to assess both global upper extremity and specific nerve-related symptoms and disability have been used in the assessment of nerve compression. The carpal tunnel syndrome questionnaire is a disease-specific measure that allows specific items related to carpal tunnel syndrome to be assessed from the patient’s perspective and has been shown to be reliable, valid, and sensitive to change. , The carpal tunnel syndrome questionnaire assesses symptom severity (11 items) and functional status (8 items). Each item is ranked on a Likert scale (1 is the low level and 5 is the high level of symptoms or difficulty), and the higher scores indicate decreased functional status. A modification of this questionnaire has been described for use with cubital tunnel syndrome. One of the most commonly used generic upper extremity measures for disability is the Disabilities of Arm, Shoulder, and Hand (DASH) questionnaires; using a standardized 30 items allows for comparison of upper extremity conditions. Upper extremity disability is assessed based upon physical symptoms and functional status; each item is ranked from 1 to 5. Scores are calculated, and higher scores indicate higher levels of disability as a single disability score. A factor analysis of patients with nerve injury revealed three factors within the DASH items representing different aspects of function and symptoms (light effort tasks, greater effort tasks, and work/social limitations and pain), which may provide more insight into the specific independent factors influencing outcomes. The Michigan Hand Outcomes Questionnaire (MHQ) is a longer questionnaire (67 items) and has the capability of addressing specific domains of overall hand function, physical function, cosmesis, and satisfaction. Similar to the DASH, each item is scored on a Likert scale (1 to 5), but in contrast to the DASH, the MHQ assesses function of each upper extremity (independent use, hand dominance, and bilateral involvement). A brief 12-item version of the MHQ has been introduced and correlates highly with the original MHQ. Computer adaptive testing of patient-reported outcomes has been described to increase the ease of application and scoring through electronic online applications. The Patient-Reported Outcomes Measurement Information System (PROMIS) was developed to provide an online platform with a variety of physical and psychosocial health domains, which are applicable to the assessment of nerve compression. Studies have utilized PROMIS instruments for comparisons with carpal tunnel–specific and generic upper extremity questionnaires and to report the minimal clinically important differences. , The carpal tunnel–specific questionnaire was most responsive, and the PROMIS instruments provided an assessment of overall health status.
Although standardized item questionnaires provide the opportunity to compare scores and answers between patients and conditions, these generic questionnaires may not reflect specific changes in each patient or the items that are most important to each patient. Questionnaires such as the Patient-Specific Functional Scale (PSFS) assess items that are identified by patients. In the PSFS, each patient identifies three activities or tasks that the patient finds difficult or impossible to perform. Each item is ranked on a scale from 0 to 10 and anchored based on perceived ability to perform these separate activities. Good validity, reliability, and responsiveness have been shown in patients with upper extremity nerve and musculoskeletal disorders. The minimally important differences have been described for different body regions, including the upper extremity, to better understand patient-perceived changes. Although the PSFS allows patients to select items that are specifically relevant, comparison between patients is more challenging. Combined use of generic questionnaires such as the DASH, MHQ, PROMIS, and our pain evaluation questionnaire in combination with the PSFS may provide a more comprehensive self-report evaluation. Changes in quality of life parallel improvement in pain scores after nerve surgeries. Surgeons who might be reluctant to measure pain response may be more receptive to assessing quality of life using a visual analog or numeric scale to better understand the impact of surgery on patient outcome.
For successful management, evaluation and treatment should be directed at all levels of nerve compression and any soft tissue disorders that are present.
Many signs and provocation tests have been described for clinical evaluation of peripheral compressive neuropathy. The concept of increasing tension or compression on a nerve to assess for nerve compression at the carpal tunnel may be extrapolated to other sites of nerve compression in the upper extremity. The concept of the double-crush mechanism is important in the assessment of patients with suspected upper extremity nerve compression. Because one site of nerve compression may affect other sites of nerve compression, all potential entrapment sites of nerve compression should be evaluated.
Provocation tests involving direct pressure or joint movement to increase compression on the nerve can be performed ( Table 28.1 ). Beginning at the more distal sites and advancing proximally, a Tinel sign or a nerve percussion test is performed by applying repeated digital percussion at each potential entrapment site (carpal tunnel, median nerve in the forearm, cubital tunnel, radial sensory nerve, spiral groove, brachial plexus). The test is considered positive with radiation of a tingling sensation into the affected nerve’s sensory neural distribution. All provocative tests are done in both upper extremities in all patients, even in those with symptoms in only one upper extremity. In patients who are hyperresponsive to stimuli or have positive nerve percussion test results at multiple entrapment sites, it is helpful to perform a nerve percussion test at points where nerves are not anatomically located as a negative control.
| Nerve | Entrapment Site | Provocative Test | Conservative Management |
|---|---|---|---|
| Median | Carpal tunnel | Pressure proximal to the carpal tunnel Phalen test Reverse Phalen test (hyperextension of the wrist) |
Splint the wrist in neutral position at night |
| Proximal forearm | Pressure over the proximal forearm in the region of the pronator teres with the forearm in supination Resisted elbow flexion, pronation, and finger flexion |
Use stretching exercises for the pronator teres | |
| Ulnar | Guyon canal | Pressure proximal to Guyon canal Reverse Phalen test |
Splint the wrist in neutral position at night |
| Cubital tunnel | Elbow flexion and pressure proximal to the cubital tunnel | Educate about the elbow pad, positioning in elbow extension, and decreasing direct pressure on the nerve Evaluate and treat for weak middle trapezius muscle |
|
| Radial (posterior interosseous) | Arcade of Fröhse | Pressure over the supinator Resisted supination Resisted long-finger and wrist extension |
Position in supination and avoid repetitive pronation and supination activities |
| Radial (sensory) | Forearm | Pressure over the junction of the brachioradialis/extensor carpi radialis tendon Forearm pronation with wrist ulnar flexion |
Avoid repetitive pronation and supination activities |
| Brachial plexus | Supraclavicular | Elevation of arms above the head Pressure over the brachial plexus in the interscalene region |
Avoid provocative positions Stretch shortened muscles and strengthen weakened scapular stabilizers |
The Phalen test uses wrist flexion to increase pressure on the median nerve and is a common provocation test for assessment of carpal tunnel syndrome. , Since the Phalen test was first described in 1966, a number of provocation tests using position or pressure, or both, to increase compression on the nerve have been reported. , , Provocation tests should be maintained for 1 minute and are considered positive when symptoms are reproduced in the appropriate neural distribution. To correctly identify a site of chronic nerve compression, it is important to compress only one nerve entrapment site with each test. Pressure provocation can be performed to assess the median nerve at the carpal tunnel by placing the wrist in either flexion or extension and then applying digital pressure just proximal to the carpal tunnel. In patients with restricted wrist movement, the pressure provocative test can be done with the wrist in a neutral position. Because the median nerve and the ulnar nerve can be compressed in this position, sensory alteration in the median and ulnar nerve distribution may be reported and should be documented. To evaluate the median nerve in the forearm, the patient’s forearm is placed in full supination with full elbow extension, and digital pressure is applied to the median nerve in the region of the pronator teres muscle. A positive response is noted with sensory alteration in the median nerve distribution. It is helpful to monitor the radial arterial pulse at the wrist during this maneuver, as inadvertent arterial compression in the forearm may cause a false positive compression test. To evaluate the cubital tunnel, the elbow is placed in full flexion with neutral forearm rotation and the wrist in neutral. Digital pressure is placed on the ulnar nerve just proximal to the cubital tunnel. The radial sensory nerve is evaluated by placing the wrist in flexion and ulnar deviation and the forearm in a pronated position. Compression of the brachial plexus at the thoracic outlet should also be screened by having the patient raise both arms overhead with the elbows extended and noting reproduction of the patient’s symptoms in the hands. Axillary nerve compression in the quadrangular space can be elicited by positioning the arm in shoulder abduction and elbow extension, and assessing for discomfort as the elbow is flexed and the long head of the triceps tightens. Pain is exacerbated as the teres minor is tightened with internal rotation of the shoulder.
Cervical nerve root impingement is assessed clinically with the Spurling test. This foraminal nerve root encroachment test is performed by placing the patient’s cervical spine in slight extension and lateral flexion. Axial compression is then applied to the patient’s head, and if a “spray” of symptoms into the arm is reported, it is considered positive for nerve root compression. The test is repeated with lateral flexion to the contralateral side. Tong and colleagues evaluated 255 patients and found the Spurling test to have a sensitivity of 30% and a specificity of 93%. This study would suggest that the test correctly identifies patients with no cervical radiculopathy but is not an effective screening test to identify patients with cervical radiculopathy. Therefore in a patient with symptoms suggesting involvement of the cervical spine and a positive response to the Spurling test, further cervical spine radiologic investigation may be indicated. Patients with cervical radiculopathy typically report less pain when they rest their hand on their head. This maneuver takes tension off the nerve roots and decreases the “drag” on the nerve roots from the unsupported arm. They also report an increase in pain when they tilt their heads toward the affected side (which closes off the space around the nerve roots) and decreased pain when they tilt their heads toward the opposite shoulder (opening up the space around the nerve roots).
The sensory collapse test (previously referred to as the scratch collapse test) is another clinical test that may be used to identify a site of nerve compression and has been shown to have good psychometric properties. In 2019 Abdo and associates described nociceptive Schwann cells supporting free nerve endings and responding to sensory stimuli with a withdrawal response. These findings prompted the change of the name from the scratch collapse test to the sensory collapse test. A positive response relies on the loss of muscle strength (shoulder external rotation) and therefore provides an outcome unrelated to the site of nerve compression. This test has been shown to have good positive and negative predictive value in the assessment of patients with carpal and cubital tunnel syndrome with abnormal nerve conduction studies. However, this test is particularly useful in patients with diffuse symptoms, multiple levels of nerve compression, and normal nerve conduction studies. , The sensory collapse test takes focused practice to learn to perform and accurately identify sites of nerve compression. Specifically, the patient and examiner calibrated “equal opposing energy” between their hands “palm to palm” to establish that the force exerted is “just a balance.” For examination with the sensory collapse test, the examiner is positioned in front of the patient with arms in neutral shoulder rotation, 90 degrees of elbow flexion, wrists in neutral position, and fingers extended. The examiner assesses isometric shoulder external rotation strength by applying a force to both forearms, and the patient applies an equal resisted force of external shoulder rotation to maintain the arms in a static position. The skin over the site of nerve compression to be assessed is lightly stimulated (with a scratch, touch, or air) and a similar force is applied to the forearms to resist isometric shoulder external rotation. A loss of shoulder external rotation strength will result in “collapse” of the arm toward the abdomen. The key point is to exert the equal force between patient and examiner after the sensory stimulus and then wait for a pause as the collapse occurs after a few seconds. Collapse of the arm is considered a positive response and indicative of nerve compression at the site of provocation. Each site of nerve compression may be assessed independently. Ethel chloride can be used to evaluate a hierarchy of neural compression muscle imbalance issues ( ![]() ).
).
Examination of the shoulder and evaluation of scapular movement patterns are important components of the physical examination of patients reporting upper extremity and cervicoscapular pain. , , Abnormal postures of the neck, shoulder, and scapular region are frequently associated with muscle imbalance, especially weakness of the serratus anterior and middle/lower trapezius muscles. To evaluate the strength of the serratus anterior muscles, the patient is asked to flex the shoulders forward while raising the arms above the head. Scapular position and movement are noted during movement and at end range of motion. As the arms are slowly lowered, the examiner observes for any scapular winging and to evaluate for serratus anterior strength. Similarly, to evaluate trapezius muscle strength, the patient is asked to abduct the shoulders and raise the arms above the head and then slowly lower the arms (in the coronal plane) as the examiner looks for winging of the scapula.
Compression of the mixed motor/sensory nerves in the upper extremity will produce symptoms of paresthesia and numbness. By contrast, aching pain and arm fatigue symptoms are associated more with muscle imbalance. Pure motor nerve compression (e.g., long thoracic, suprascapular, dorsal scapular, spinal accessory nerve) can present with the above symptoms and atrophy. In patients with these symptoms, if examination of the scapular position and movement indicates weakness of the serratus anterior and middle/lower trapezius muscles, this should be further evaluated and treated. Similarly, if the patient has associated rotator cuff tendinitis or shoulder impingement, the symptoms will also be reflected in upper extremity discomfort. Examination of the shoulder for these problems is likewise important.
Even though provocation of the nerve can help identify sites of nerve compression, it does not quantify sensory function. Many instruments and assessment devices have been described for evaluation of sensibility, but no single test has been accepted as the gold standard. This in part relates to the varying capacities of the sensory tests to evaluate different parameters of nerve function.
The four sensory receptors that are found in the glabrous skin of the hand are categorized by differing receptive field size and response qualities. The slowly adapting receptors (Merkel cell neurite complex and Ruffini end-organs) respond to static touch, whereas the quickly adapting receptors (Meissner and Pacinian corpuscles) respond to moving touch. The threshold and tactile discrimination of the quickly and slowly adapting receptors can be evaluated. Threshold is the minimum stimulus required to elicit a response and is assessed with vibration thresholds (quickly adapting receptors) and with cutaneous pressure thresholds (slowly adapting receptors). Tactile discrimination reflects the number of innervated sensory receptors and is assessed with moving and static two-point discrimination. Vibration and cutaneous pressure thresholds will permit quantification of the early changes that occur with chronic nerve compression. Decreases in the sensory receptor innervation density will occur in the later stages of chronic nerve compression, and therefore two-point discrimination measures will become abnormal only in the more severe stages of nerve compression.
Quick screening of the large A-beta fibers can be performed with the ten test. Introduced by Strauch and coworkers, the ten test allows patients to rank their sensation of light moving touch on a scale from 0 to 10 in comparison with the normal contralateral digit or dermatome. Good reliability and validity were reported compared with Semmes-Weinstein monofilament testing. In patients with carpal tunnel syndrome, Patel and Bassini reported the ten test to be more sensitive than the Weinstein Enhanced Sensory Test and two-point discrimination. For assessment with the ten test, the examiner applies a light touch and moves the stimulus with a finger to an area of normal sensation on the contralateral digit. A similar stimulus with the examiner’s finger is applied simultaneously to the affected area, and the patient is asked to rank the sensation on a scale from 0 to 10, with 10 being perfect sensation and 0 being no sensation. Although this has proved to be an excellent screening test, it has limited use in patients with systemic sensory neuropathy and bilateral upper extremity nerve compression where there is no “normal” area to serve as a control.
Vibration can be used to assess the thresholds of quickly adapting receptors but is now rarely tested. Qualitatively, vibration thresholds may be evaluated with a tuning fork. A number of fixed-frequency and variable-frequency vibrometers have been described that permit quantification of vibration thresholds. Although good reliability has been reported with single-frequency vibrometers, the greatest limitation is that only one frequency is assessed. In a study of 130 factory workers, Werner and colleagues did not find single-frequency vibrometry useful as a screening tool for median nerve pathologic conditions. In those with nerve compression, it is hypothesized that perception of the higher frequencies is affected earlier; therefore evaluation at multiple frequencies may permit earlier identification of sensory loss. However, in a study of patients with brachial plexus nerve compression, vibration thresholds measured at multiple frequencies were not useful in identifying these patients.
Pressure thresholds of the slowly adapting receptors are commonly evaluated with Semmes-Weinstein monofilaments. These nylon monofilaments vary in diameter and thus differ in application force and produce different pressure thresholds. The set of Semmes-Weinstein monofilaments increases in diameter on a logarithmic scale (log 10 force of 0.1 mg). The examiner applies pressure with each successive nylon filament until the filament just begins to bend. The smallest monofilament that the patient can perceive is documented as the pressure threshold. Each monofilament size is associated with a grading of sensory impairment (e.g., normal, diminished light touch, diminished protective sensation). Consistency in monofilament diameter and the testing procedure is necessary to ensure reliability. , Pressure thresholds have been shown to be sensitive in testing for carpal tunnel syndrome.
Two-point discrimination reflects the number of innervated sensory receptors. A number of instruments with standardized distance between the probes, such as the Disk-Criminator, have been described for the measurement of static and moving two-point discrimination. Although good reliability has been shown with the Disk-Criminator, alterations in two-point discrimination occur only with advanced nerve compression. Therefore this test is not a very sensitive test for patients with mild chronic nerve compression and may be more appropriate for assessment of recovery after nerve repair. In our clinical experience, two-point discrimination of greater than 8 mm is essentially nonfunctional, and we therefore classify this as “no two-point discrimination.” Consequently, in the upper extremity, we have standardized our measurement of moving and static two-point discrimination to include two-point discrimination up to 8 mm.
Electrodiagnostic studies include electromyography and nerve conduction studies. The tests are not a substitute for a detailed clinical examination by an experienced clinician. Unfortunately, some physicians rely on these studies without recognizing the major limitations of electrodiagnostic examination. A well-performed electromyogram (EMG) and nerve conduction study can complement the clinical evaluation by helping to localize the level and severity of nerve compression.
A major limitation of the nerve conduction portion of the electrodiagnostic study is that it evaluates only the large myelinated fibers. This includes the motor and sensory axons relaying vibration and light touch but not the smaller axons conveying pain or temperature sensation. The determination of nerve latency reflects the conduction of the best myelinated fibers rather than the most severely affected axons, and normal latency may still be present even when many nerve fibers are affected. In electron microscopic studies, it was demonstrated that there were changes in the pathology of the unmyelinated nerve fibers prior to changes in the myelinated nerve fibers. These unmyelinated axons (C and A delta fibers) transmit pain. It is important to recognize that EMG study will not be able to assess these small unmyelinated fibers. This can account for the patients who present with pain and normal electrodiagnostic studies. Another limitation of nerve conduction studies relates to the location of the nerve injury in the extremity. Nerve problems occurring very distally or very proximally in the extremity are difficult to assess. Dynamic changes in blood flow that may produce intermittent alterations in peripheral nerve function may not be detected with electrodiagnostic studies. The timing of nerve conduction studies also influences their utility because even complete nerve transection will not become apparent for 2 to 6 weeks after injury. In situations with more than one level of injury or when a systemic polyneuropathy is present, nerve conduction studies may be less reliable. Although electrodiagnostic studies provide the surgeon with quantifiable values, they are extremely dependent on the expertise of the examiner who performs them.
For the EMG component of the study, needle recording electrodes are inserted into the muscles to evaluate spontaneous and volitional electrical activity. Muscle response with needle insertion at rest and during activation is noted. A normal muscle will respond with a brief burst of electrical activity on needle insertion (insertional activity). Abnormal insertional activity can include insertional positive sharp waves, as seen with early denervation, or electrical silence, which is associated with chronic muscle degeneration without reinnervation. In the rest phase there will normally be electrical silence, but after nerve injury, spontaneous activity with fibrillation potentials is noted. Fibrillation potentials are the earliest sign of muscle denervation and are generated by muscles after denervation of at least 2 weeks duration. A single neuron and motor axon innervates a variable number of muscle fibers, and this constitutes a motor unit. A motor unit can vary in size from a few muscle fibers in the ocular muscles to thousands of muscle fibers in the larger skeletal muscles; thus fibrillation potentials are the most sensitive indicator of motor axon loss and can be seen in nerve compression syndromes long before there is any clinical evidence of muscle weakness. During the muscle activation portion of the EMG, the patient is instructed to contract the muscle to elicit voluntary motor unit potentials (MUPs). In a normal muscle, because numerous MUPs are activated, individual MUPs cannot be seen, and a full interference pattern is noted. With nerve injury, incomplete MUP activation and reduced MUP recruitment are seen. Patients responding with pain or decreased effort can show incomplete MUP activation, but the rate will be slow to moderate. With reduced MUP recruitment because of a peripheral nerve physiologic problem, the firing rate will be faster, and the configuration will be of increased duration and amplitude. The degree of reduced recruitment can be graded from mild, moderate, to severe as a surrogate to the degree of axonal loss. The neurologist doing the EMG will be able to determine chronic neurogenic MUP changes from incomplete MUP activation as seen with upper motor neuron disease or decreased patient effort. MUPs associated with reinnervation will initially be of very low amplitude and extremely polyphasic, and they will fire at a slow to moderate rate. Eventually, they will remodel so that the duration and number of phases decrease and the amplitude increases.
Nerve conduction studies can be used to study motor, sensory, and mixed nerves. Surface electrodes are used to stimulate and record from the nerve. During motor nerve conduction studies, electrodes are placed over the muscle and the motor axons are stimulated proximal to the surface electrode. Motor axons are indirectly assessed by the size and configuration of the compound muscle action potentials (CMAPs) that are generated in response to nerve stimulation. Motor nerve conduction studies are characterized by a large-amplitude response, in contrast to sensory nerve conduction studies, in which the sensory axons themselves are directly assessed and the sensory nerve action potential (SNAP) is very small. Sensory nerve function can be assessed in antegrade (orthodromic) or retrograde (antidromic) fashion by placing the recording electrode either proximal or distal to the stimulating electrode, respectively. Because of their small size and low amplitude, SNAPs may be adversely affected by technical problems or by factors such as skin or room temperature. The parameters of nerve conduction studies include wave amplitude, duration, latency, the area of the recorded response, and conduction velocity. Laboratories have their own set of normal values based on age. It is customary for individuals with unilateral neurologic problems to have a drop in amplitude of 50% or more on the symptomatic side compared with the normal extremity.
Latency is an indirect measure of the speed of impulse conduction along the fastest conducting fibers and is expressed in milliseconds. Sensory nerves are stimulated at one point and recorded at a surface electrode, and the recorded latency is defined as the time elapsed until onset of the peak deflection. Motor latency is defined as the time elapsed between a supramaximal stimulus over the involved nerve and the onset of deflection at the motor point of the innervated muscle.
Amplitude is the height of the action potential expressed in millivolts for motor nerve conduction studies and in microvolts for sensory nerve conduction studies. The amplitude provides an assessment of the number of conducting axons. Amplitudes are more difficult to assess, but because they reflect the number of functioning axons, they give more information about muscle weakness and paresthesia than latency measurements, which reflect only the fastest conducting fibers. Conduction velocity is calculated by stimulating the nerve at two measured distances and determining the rate of conduction in meters per second between the two points. It is useful when comparing results between subjects with different limb lengths. Diffusely slowed conduction velocity across several nerves may also indicate a systemic peripheral neuropathy. However, conduction velocity reflects the speed of the fastest conducting fibers and, unlike amplitude, does not give any information regarding the number of conducting axons. Area beneath the curve is a function of amplitude and duration of response and more accurately reflects the number of axons, but, like amplitude, it is technically more difficult to assess.
Nerve conduction studies give information about axon and myelin pathologic conditions, and each component is important to properly identify such conditions of nerves. Latency and conduction velocity should not be viewed in isolation, because the speed of conduction relates only to the “healthiest surviving” myelinated nerve fibers. Similarly, even though amplitude is a reflection of the number of functioning axons, amplitude may also be reduced by other disorders, such as myopathy. In moderate cases of nerve compression, EMG studies may demonstrate scarce fibrillation potentials, and if the axonal problem is more severe, reduced MUP recruitment will be present. In rare cases, the examiner may identify fibrillation potentials on the EMG with maintenance of normal nerve latencies. With progression, the number of fibrillation potentials will increase and action potential amplitudes will decrease. SNAP amplitudes are affected before CMAP amplitudes. Eventually, MUP recruitment will decrease. With a complete injury, nerve conduction motor and sensory responses will be absent, fibrillation potentials will be marked, and no MUPs will be identified.
The early stage of nerve compression is associated with dynamic ischemic events involving the nerve and the results of electrodiagnostic studies are normal. As the nerve compression progresses, demyelination will occur, and conduction velocity will slow across the site of compression. Axonal loss does not usually take place until late in the course of the neuropathy. With carpal tunnel syndrome, nerve conduction study latencies may be prolonged, but the EMG is generally normal until late in the disease. Demyelination and focal conduction slowing are typical of most patients with carpal tunnel syndrome and at least half of those with cubital tunnel syndrome, with axonal changes being found much later in the course of the disease.
Electrodiagnostic studies are useful in ruling out other associated problems such as cervical disk disease, motor neuron problems, myopathy, or superimposed polyneuropathies. They are particularly useful in the investigation of symptoms related to the ulnar nerve, which can sometimes herald the presence of other, more sinister diagnoses. Patients with isolated motor deficits and spared sensory function must have a motor neuropathy ruled out before an entrapment neuropathy can be diagnosed. Electrodiagnostic studies can also help rule out problems that are “functional.” Muscles that are weak or atrophic because of disuse will show no EMG abnormalities other than poor voluntary MUP generation and reduced activation, in keeping with submaximal effort by the patient. If the muscle is weak or atrophic because of a peripheral nerve physiologic problem, the CMAP will be of low amplitude, MUP recruitment will be reduced, and fibrillation potentials will be noted, unless the process is extremely chronic, in which case fibrillations will no longer be present. Electrodiagnostic studies are not generally helpful in confirming a diagnosis of thoracic outlet syndrome or median nerve compression in the proximal forearm.
Carpal tunnel syndrome with compression of the median nerve at the wrist is the most commonly diagnosed site of nerve compression in the upper extremity. Symptoms include paresthesia or numbness (or both) in the median nerve distribution (thumb, index finger, middle finger, and radial side of the ring finger). Nocturnal paresthesias in the radial three digits of the hand is nearly pathognomonic for carpal tunnel syndrome. Paresthesias also occur characteristically in “fixed wrist activities” such as reading a book or a newspaper, driving, or use of a computer keyboard or mouse. Patients rarely describe aching in the thenar eminence and, with advanced nerve compression, weakness and atrophy of the abductor pollicis brevis and opponens pollicis muscles. Because of the slow onset of thenar weakness, patients typically adapt to this loss without functional impairments. Carpal tunnel syndrome is a clinical diagnosis based on a combination of symptoms and characteristic physical findings; its presence may be subsequently confirmed with electrodiagnostic studies. , , Electrodiagnostic studies are useful to stage the degree of nerve compression and assist the surgeon and patient in anticipating the time needed for recovery of nerve function. Patients with long-standing symptoms, severe atrophy of the thenar musculature, and dense sensory loss should be cautioned that release may not lead to complete recovery of sensation or thenar strength.
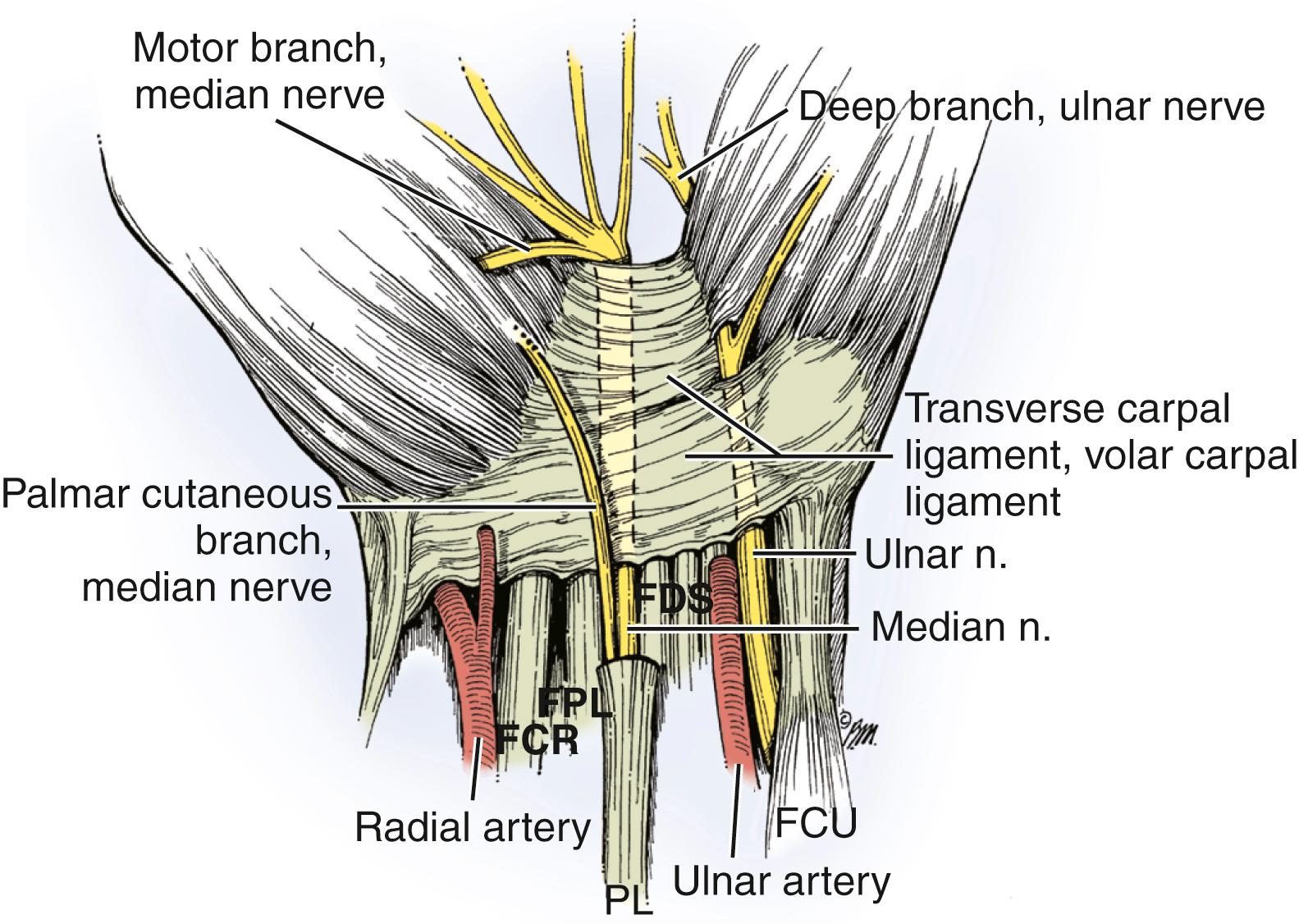
The roof of the carpal canal is the flexor retinaculum, which extends from the hamate and triquetrum on the ulnar side to the scaphoid and trapezium on the radial side. The median nerve and flexor tendons (flexor pollicis longus tendon, four flexor digitorum superficialis tendons, and four flexor digitorum profundus tendons) pass through this tunnel. Although the carpal tunnel is open at its proximal and distal ends, it maintains distinct tissue fluid pressure levels. The diameter of the carpal tunnel is narrowest at a point approximately 2 cm distal to its leading edge ( Fig. 28.4 ), and this corresponds to the site of morphologic changes in the nerve in patients with carpal tunnel syndrome. The median nerve lies just beneath the flexor retinaculum. At the distal end of the flexor retinaculum, the median nerve gives off the recurrent motor branch to innervate the abductor pollicis brevis muscle, superficial head of the flexor pollicis brevis muscle, and opponens pollicis muscles and then divides into the digital nerves that provide sensation to the thumb and index finger, middle finger, and radial half of the ring finger.
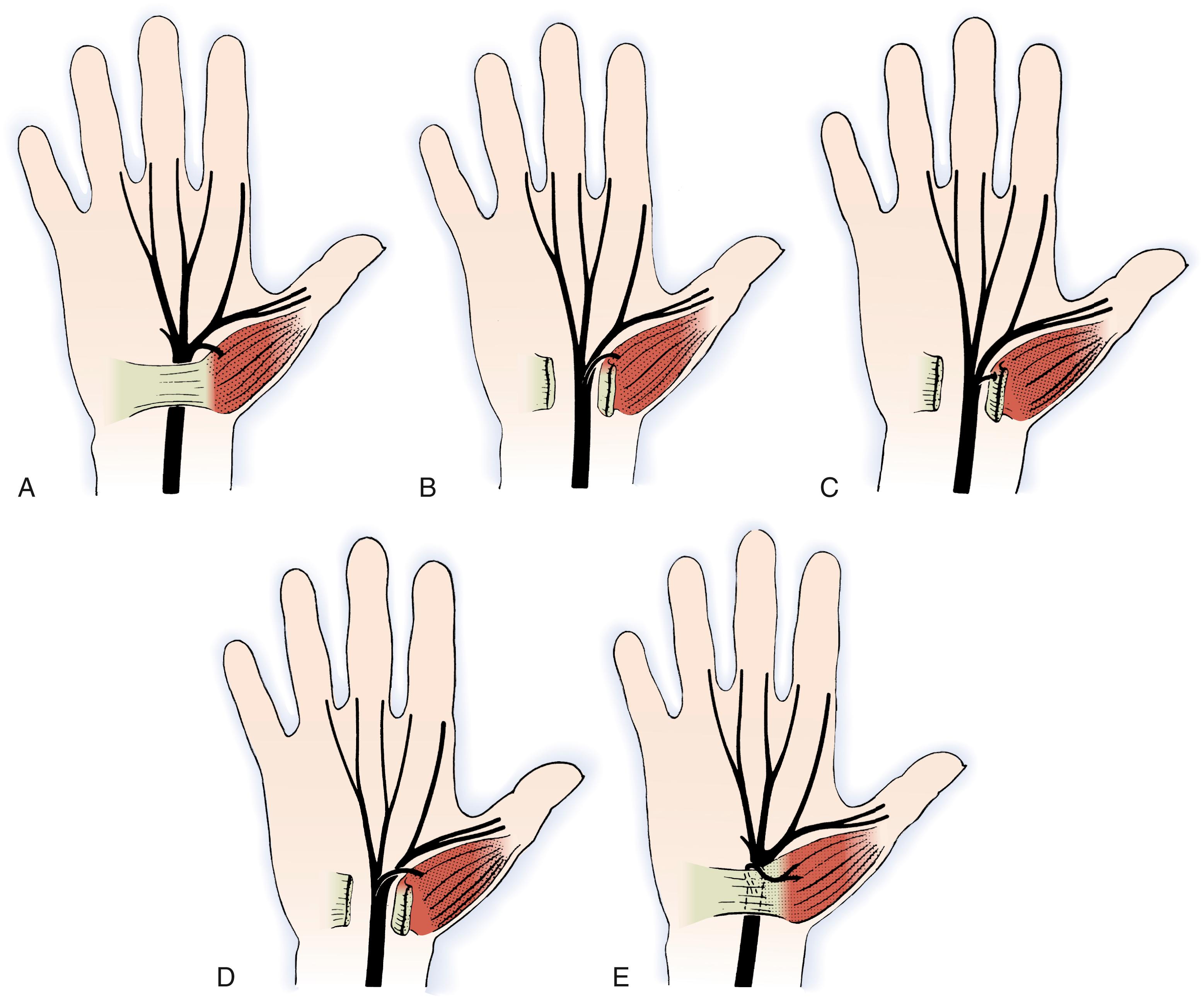
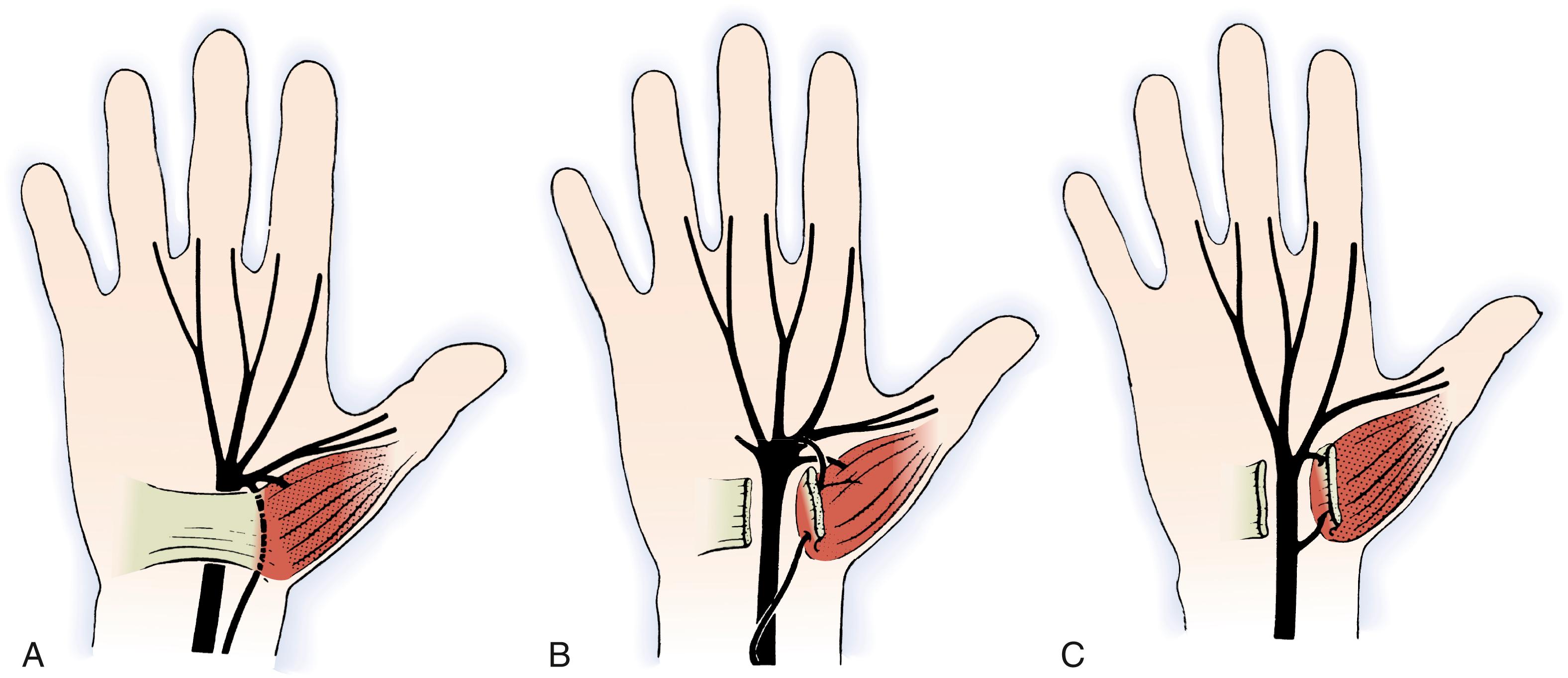
Recognition of variations in the branching pattern of the median nerve is important, particularly during surgical decompression. Lanz classified variations of the recurrent motor branch into four subgroups ( Figs. 28.5 to 28.7 ). In most cases, the motor branch divides from the median nerve distal to the flexor retinaculum in an extraligamentous pattern (46% to 90%). Less common variations include the subligamentous pattern (31%) and transligamentous pattern (23%). There have been reports of the recurrent motor branch dividing from the medial side of the median nerve and other unusual variations in which a separate compartment within the carpal tunnel contained half of a bifid median nerve. The recurrent motor branch can have a separate fascial tunnel that, if identified and released in patients with thenar atrophy, can improve thenar muscle recovery. Variation in the branching pattern of the palmar cutaneous branch of the median nerve has also been described. The palmar cutaneous nerve has been reported to branch through the palmaris tendon proximal to the palmar fascia and also through the antebrachial fascia proximal to the wrist crease ( Fig. 28.8 ). More proximally in the forearm, the palmar cutaneous branch of the median nerve has been found within the flexor carpi radialis tendon sheath in up to 6% of patients. , To avoid injury to these anomalous branching patterns during the surgical approach to the median nerve, Taleisnik recommended an incision ulnar to the axis of the flexed ring finger. Although considerable variation in the length and location of the surgical incision used for carpal tunnel release exists among hand surgeons, , consideration of the anatomy of the palmar cutaneous and recurrent motor nerves is essential to avoid inadvertent nerve injury.
![Fig. 28.6, Variations in median nerve anatomy in the carpal tunnel. Group III variations include high divisions of the median nerve (A) that may be separated by a persistent median artery (B) or an aberrant muscle (C) . (From Lanz U. Anatomic variations of the median nerve in the carpal tunnel. J Hand Surg Am. 1977;2[1]:44–53.) Fig. 28.6, Variations in median nerve anatomy in the carpal tunnel. Group III variations include high divisions of the median nerve (A) that may be separated by a persistent median artery (B) or an aberrant muscle (C) . (From Lanz U. Anatomic variations of the median nerve in the carpal tunnel. J Hand Surg Am. 1977;2[1]:44–53.)](https://storage.googleapis.com/dl.dentistrykey.com/clinical/CompressionNeuropathies/5_3s20B9780323697934000286.jpg)
A number of nonoperative treatments of carpal tunnel syndrome have been described that vary from wrist splinting to corticosteroid injection.
Wrist splinting is frequently recommended for nonoperative treatment. , Prefabricated wrist splints are often used and can present a unique problem if they are placed in a functional position of 30 degrees of extension. Carpal canal pressures have been shown to be elevated in patients with carpal tunnel syndrome, and pressure is further elevated when the wrist is in a position of extension. To control symptoms related to nerve compression at the wrist, wrist splints are most effective in a neutral position. Splinting the wrist in neutral will restrict function due to restricted motion in a nonoptimal position, and for this reason we do not recommend wrist splints during normal daily activity. We recommend splinting only at night to maintain the wrist in a neutral position. A subset of patients will report sleeping with their hands in a “fisted” position, and these patients should be provided with a custom nighttime splint to block both wrist flexion and proximal interphalangeal (PIP) flexion to prevent lumbrical excursion into the canal.
Oral antiinflammatory medications and corticosteroid injections have been used for the treatment of carpal tunnel syndrome with varying reports of success. , , , Using a randomized placebo-controlled trial to investigate the efficacy of steroid injection, Atroshi and coauthors reported good temporary symptom improvement but little difference in likelihood of surgery within 1 year. Celiker and coauthors reported a randomized controlled trial conducted to evaluate the efficacy of corticosteroid injection versus nonsteroidal antiinflammatory drugs and splinting. They reported significant improvement with both methods of treatment; however, the brief follow-up time of just 8 weeks limited the applicability of the study. Green reported that temporary relief after carpal tunnel injection was an excellent prognostic factor for successful carpal tunnel surgery. Complications with steroid injection have been reported, including nerve injection injury to the median nerve. In a study evaluating the effects of steroid nerve injection in a rodent model, dexamethasone was found to have no deleterious effects on the nerve, even when injected directly into the nerve. All other corticosteroids caused varying degrees of nerve injury when directly injected into the rodent sciatic nerve. However, given its temporary relief of symptoms and finite risk for nerve injury, in our practice corticosteroid injection is not routinely indicated.
Nerve gliding exercises have been described to alleviate symptoms of nerve compression. Rozmaryn and associates used nerve and tendon gliding exercises to treat a group of patients with carpal tunnel syndrome and noted less surgical intervention in this group than in a historical control group. Seradge and coauthors reported good symptomatic relief and avoidance of surgery in up to 80% of patients with mild or moderate compression treated with nerve gliding and stretching exercises who were monitored for 18 months.
When a regimen of nonoperative treatment fails to relieve the patient’s symptoms, surgical decompression of the median nerve is usually recommended. The first carpal tunnel release was reportedly performed by Herbert Galloway in 1924. Numerous approaches for carpal tunnel release have been described that range from an open technique to a small incision to endoscopic release. The decision regarding whether to perform open versus endoscopic carpal tunnel release is largely dependent on surgeon preference and patient selection. A metaanalysis was published in 2008 to evaluate outcomes related to endoscopic and open carpal tunnel release. The authors concluded that the data related to symptom relief and return to work are inconclusive and there is increased likelihood of transient nerve injury with endoscopic release. Although release of the median nerve is the goal of treatment, injury to even a few fascicles of the nerve can have devastating consequences for the patient. Therefore to avoid nerve injury, the surgeon should choose the surgical method that offers the best visualization of the median nerve.
Minimal incision and endoscopic techniques were introduced to decrease the length of the incision and thus potentially decrease postoperative incisional discomfort. A number of endoscopic systems have been described, but the risk for complications, including iatrogenic nerve injury, poor visualization, inability to identify anatomic variations, incomplete release, and apparent beneficial cost savings, is still debated. Those who support endoscopic carpal tunnel release also advocate specialized training in cadaveric courses before performance of endoscopic carpal tunnel release. Others have introduced surgical releases with smaller incisions termed “minimally invasive” to decrease postoperative incisional pain and thus minimize postoperative morbidity. In this chapter, we have reviewed the original endoscopic techniques as introduced by Chow and Agee, but others have also introduced variations of endoscopic approaches.
Though not indicated in all patients, a tenosynovectomy for biopsy should be performed during carpal tunnel decompression in patients in whom there is concern for amyloidosis or inflammatory arthritis. Carpal tunnel syndrome may be the presenting sign in patients with amyloidosis, especially in men >50 years of age and women >60 years of age with bilateral carpal tunnel syndrome. In this subset of patients, 10.2% were found to have a positive biopsy for amyloid. Early diagnosis of amyloidosis is beneficial as current treatments are better at preventing progression than reversing existing disease. ,
A two-portal endoscopic technique was introduced by Chow. Local anesthesia with 1% lidocaine without epinephrine is used, with or without intravenous sedation. The entry portal is located by drawing a line 1 to 1.5 cm radially from the proximal pole of the pisiform and a second line 0.5 cm proximally from the first line. A 1-cm transverse incision is made through the skin radially from the end of the second line. A longitudinal incision is made through the fascia. A curved dissector-obturator slotted cannula assembly is inserted through the distal edge of the entry portal incision along the long axis of the forearm. It is used to free synovial tissue from the inferior surface of the flexor retinaculum. The assembly tip touches the hook of the hamate, the hand is lifted off the table, and the wrist and fingers are hyperextended. At 1 cm proximal to a line drawn that bisects the angle formed by the distal border of the fully abducted thumb and the third web space, a 0.5-cm exit portal incision is made. The slotted cannula assembly is advanced distally, and the tip palpated at the exit portal in the palm. The assembly is passed through, and the hand is stabilized in a custom hand-holding device. The endoscope is inserted into the proximal tube opening, and a probe is inserted distally to identify the distal edge of the flexor retinaculum. The probe knife is used to release the distal edge of the flexor retinaculum in a distal to proximal direction. The triangle knife is then used to release the midsection of the flexor retinaculum, and the retrograde knife is positioned in the second cut and drawn distally to join the first cut to release the distal part of the flexor retinaculum. The endoscope is withdrawn and replaced in the distal tube opening, and the probe knife is used to release the proximal edge of the flexor retinaculum. The retrograde knife is inserted into the midsection and drawn proximally to complete release of the flexor retinaculum. The trocar is reinserted and the cannula removed. The antebrachial fascia is released through the proximal incision with tenotomy scissors, and the skin is sutured.
A single-incision technique was described by Agee and associates. A tourniquet is used for this procedure. The authors recommend that initially a general or regional anesthetic be used, and with more surgeon experience, they recommend local anesthesia. A 2- to 3-cm transverse skin incision is made in the distal wrist flexion crease between the flexor carpi radialis and flexor carpi ulnaris tendons. With the use of spreading longitudinal dissection, the cutaneous nerves are protected, and the forearm fascia is identified. A “U”-shaped, distally based flap of forearm fascia is incised and elevated in a palmar direction. A probe is passed down the ulnar side of the carpal tunnel, radial to the hook of the hamate. The wrist is placed in slight extension, and the blade assembly is inserted with the viewing window toward the inner aspect of the flexor retinaculum. The tip of the blade assembly is palpated with the surgeon’s contralateral hand. The distal edge of the flexor retinaculum is located by video, ballottement, and light touch through the skin. When the correct position is ensured, the blade is deployed upward from within the device, the device is withdrawn, and the distal aspect of the flexor retinaculum is incised. The blade is retracted, the assembly is reinserted, the flexor retinaculum division is inspected, and additional cuts are made to release the remaining flexor retinaculum. Using tenotomy scissors, the antebrachial forearm fascia is released proximal to the skin incision. The skin incision is closed, and splinting is performed at the discretion of the surgeon. Agee and colleagues strongly recommend that if complete visualization is not obtained, the endoscopic technique should be abandoned, and the carpal tunnel released with an open technique.
We prefer the classic open carpal tunnel release with intravenous regional anesthesia (Bier block) ( ![]() , ). General anesthesia is rarely indicated; more recently there has been a trend toward increased use of local anesthesia. Many surgeons use local anesthesia, but we believe that patients are more comfortable with some sedation and tolerate the tourniquet better with an intravenous regional block (250 mm Hg). In the rare cases in which use of a tourniquet is contraindicated (such as patients with an arteriovenous fistula undergoing dialysis), use of local anesthetic with or without epinephrine can provide adequate visualization. As a preemptive action against the sympathetic nervous system, dexmedetomidine hydrochloride is included with the intravenous regional anesthetic in patients with substantial preoperative pain. A double tourniquet on the upper part of the arm is used, and the tourniquets are changed at 10 minutes so that patients do not experience tourniquet pain. A forearm tourniquet is used for obese patients because the upper arm tourniquet can sometimes be less reliable and result in a venous tourniquet.
, ). General anesthesia is rarely indicated; more recently there has been a trend toward increased use of local anesthesia. Many surgeons use local anesthesia, but we believe that patients are more comfortable with some sedation and tolerate the tourniquet better with an intravenous regional block (250 mm Hg). In the rare cases in which use of a tourniquet is contraindicated (such as patients with an arteriovenous fistula undergoing dialysis), use of local anesthetic with or without epinephrine can provide adequate visualization. As a preemptive action against the sympathetic nervous system, dexmedetomidine hydrochloride is included with the intravenous regional anesthetic in patients with substantial preoperative pain. A double tourniquet on the upper part of the arm is used, and the tourniquets are changed at 10 minutes so that patients do not experience tourniquet pain. A forearm tourniquet is used for obese patients because the upper arm tourniquet can sometimes be less reliable and result in a venous tourniquet.
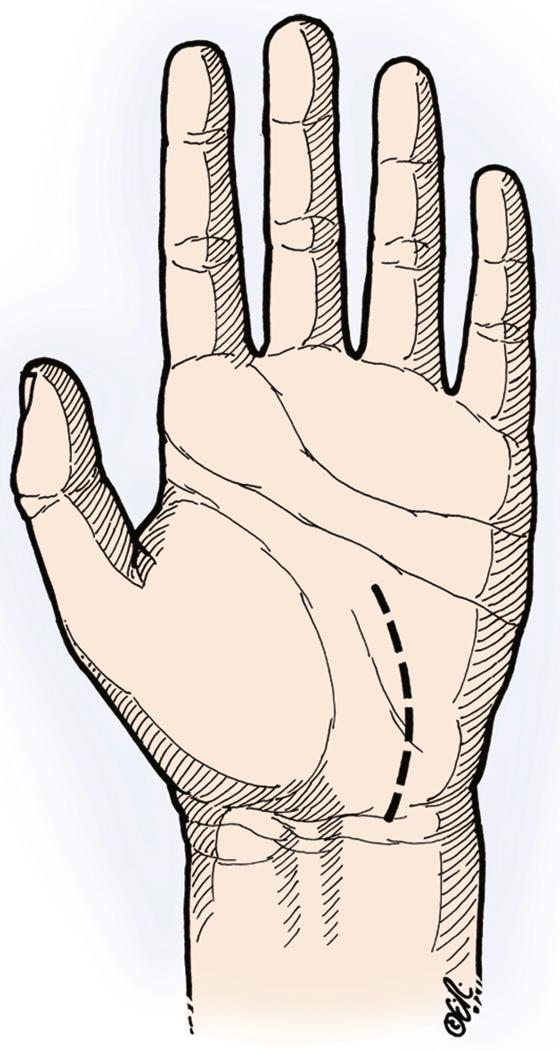
The incision is marked about 6 mm ulnar to the thenar crease to ensure that any scarring is away from the median nerve and that the incision is well ulnar to the palmar cutaneous branch of the median nerve, which is located deep to the thenar crease and radial to the palmaris longus ( Fig. 28.9 ). A longitudinal or curved incision is made that parallels the thenar crease, 2 cm in length, and ends 1 cm distal to the transverse wrist crease. If more exposure is necessary to release the antebrachial fascia, the incision is extended proximally in a zigzag fashion across the wrist in an ulnar direction so as not to injure the palmar cutaneous branch of the median nerve. With the increased prevalence of obesity, use of a longer incision should be considered in these patients to provide adequate visualization. Two sharp Senn retractors are used for retraction, and dissection is carried out through the soft tissue. We typically open the Guyon canal for exposure of the “V” between the hypothenar and thenar muscles to allow release of the transverse carpal ligament along its ulnar border. About 15% of the time, a crossing cutaneous branch from the ulnar nerve will be identified in the distal portion of the incision and protected. Senn retractors are used radially and ulnarly and a looped retractor is placed proximally and then distally to retract the fatty tissue to expose the palmar fascia, which is divided longitudinally. This will reveal the underlying transverse fibers of the transverse carpal ligament. A No. 15 blade is used very carefully, slowly, and with control to incise the transverse carpal ligament over a short segment to gain entry into the carpal tunnel. A different color of the darker synovium around the flexor tendons will be seen once the ligament has been cut. The blunt end of a Freer retractor can be placed gently into the carpal canal if desired to delineate the direction of release. Distal release is performed with tenotomy scissors or a knife, releasing the transverse carpal ligament along its ulnar border to the level of the superficial palmar arch to the point of the confluence of the “V” of the hypothenar and thenar muscles. In obese individuals or people with very large hands, the incision may need to be extended more distally to ensure that the entire ligament has been released and to identify the fatty tissue signaling the end of the ligament. Proximally, using the down-curved portion of two Senn retractors placed radially and ulnarly and a looped retractor proximally, the fatty tissue is retracted off the antebrachial fascia. The surgeon is positioned at the end of the hand table to ensure excellent visualization. With slight volar wrist flexion, the median nerve will drop away from the antebrachial fascia. Under direct vision, the antebrachial fascia is released with long tenotomy scissors. If difficulty releasing this antebrachial fascia under direct vision is encountered, the incision must be extended proximally above the wrist to ensure that the antebrachial fascia has been adequately released and that there is no injury to the median nerve. The rake end of the Senn retractor is used to retract the radial side of the divided flexor retinaculum up or anteriorly. The median nerve is visualized. If preoperatively, patients describe a deep, aching thenar pain or present with significant thenar wasting with minimal sensory complaints, specific exploration of the recurrent motor branch of the median nerve is warranted. The rake end of the Senn retractor is used to pull up on the radial side of the divided ligament, and the median nerve is carefully retracted toward the surgeon. This will cause the recurrent motor branch of the median nerve to tent up as it enters the thenar muscle mass and allow identification of the recurrent motor branch as it curves toward the thenar muscle. In patients with deep, aching thenar pain or thenar muscle wasting (or both), the recurrent motor branch will often enter the thenar musculature through its own tendinous tunnel. (Similarly, in these patients, the thenar motor branch may come off of the ulnar side of the median nerve, exposing it to more compression as it travels across the top of the nerve and directly under the ligament.) This maneuver of pulling up on the flexor retinaculum and pulling the median nerve ulnarly toward the surgeon will help with easy identification of the recurrent motor branch. The floor of the carpal canal can be visualized by retracting the flexor tendons to evaluate for a soft tissue mass such as a ganglion. We believe that neurolysis of the median nerve during primary carpal tunnel release is not indicated. Studies have also reported no benefit with epineurotomy. Similarly, synovectomy is not indicated during primary carpal tunnel decompression, unless a biopsy would be helpful to establish a histologic diagnosis of inflammatory arthritis or amyloidosis. The tourniquet is deflated. Bupivacaine is injected into the incision under careful direct vision for postoperative comfort. Microbipolar cautery is used for hemostasis. The incision is closed with interrupted 4-0 nylon suture, and a bulky dressing is applied while keeping the wrist in neutral. A sling may be used for the early postoperative period, but the patient is cautioned against unnecessary elbow flexion, which may result in flare-up of an associated cubital tunnel syndrome, and against shoulder immobilization, which may result in shoulder stiffness.
In cases of severe carpal tunnel syndrome with thenar muscle atrophy, opponensplasty may be considered. In this patient population, a palmaris longus (Camitz) transfer is the ideal transfer for improved thenar abduction because of its minimal donor deficit and immediate availability in the same surgical incision. In the authors’ opinion, it is rarely required, as patients have adapted to the slow progressive loss associated with the chronic neuropathy. Because this is an in-continuity nerve lesion, there is also the potential for muscle reinnervation and motor recovery, as is the case with sensory recovery. Finally, the older population that presents with severe thenar atrophy may not desire or tolerate the cast immobilization required after tendon transfer.
Timing of recovery is related to preoperative electrodiagnostic studies. Reinnervation in patients with decreased conduction velocity and increased latency will take just a few months, but axonal regeneration (decreased CMAP) will take much longer. Following carpal tunnel decompression for mild or moderate nerve compression, there will be return of “painless” sensation to the digits innervated by the median nerve. Reinnervation of the thenar muscles will also occur, although it takes longer and return of motor function may not be complete in patients with severe carpal tunnel syndrome. Patients can expect restoration of full range of motion at the wrist. Rarely, patients with severe carpal tunnel syndrome and thenar atrophy have been described to experience a postoperative flare with complaints of pain, stiffness, and swelling likely caused by a reinnervation hypersensitivity. These patients are treated with hand therapy and occasionally a short burst of oral steroids. Patients can also experience pillar pain after carpal tunnel release, which is likely microneuroma related. The risk of pillar pain can be minimized by placing the incision in the watershed area between the median and ulnar palmar cutaneous nerves, approximately 6 mm ulnar to the thenar crease. If pillar pain develops, the patients are referred to hand therapy for scar massage and desensitization.
Immobilization after decompression of the carpal tunnel was historically used to protect the wound and immobilize the wrist to prevent the flexor tendons from bowstringing. However, because of advancing knowledge in the importance of postoperative tendon and nerve gliding, such postoperative immobilization has been dramatically decreased. , , After carpal tunnel decompression, a bulky dressing is used to restrict wrist range of motion during the first 2 postoperative days for patient comfort and is then removed. The patient is instructed in range-of-motion exercises for the fingers, wrist, and arm. A splint in a wrist neutral position is used at night for 2 weeks for patient comfort or until the patient has regained comfortable full range of motion of the wrist. The sutures are removed 12 to 14 days postoperatively. At 1 month after surgery, patients are allowed to return to work with a 2-pound weight restriction, and at 6 to 8 weeks after surgery, they are allowed full activity without restrictions.
Failed night splinting with the wrist in neutral position
Electrodiagnostic studies to “stage” the degree of nerve compression for helping to predict the anticipated rate and degree of recovery
Document preoperative pain distribution.
Document preoperative examination of the entire upper extremity.
Use dexmedetomidine hydrochloride in a Bier block for patients with pain complaints.
Postoperatively, begin early movement.
Make an incision ulnar to the thenar crease and extend it proximally in obese patients.
Avoid the ulnar cutaneous branch (15%) in the distal portion of the incision.
Release the flexor retinaculum in a proximal to distal direction to the “V” between the thenar and hypothenar muscles.
Release the ligament distally to the fat surrounding the superficial palmar arch.
Release the antebrachial fascia proximally under direct vision.
Provide hemostasis after release of the tourniquet.
Use bupivacaine in the incision.
In obese patients, use a forearm tourniquet to avoid a “venous” tourniquet.
Failure to adequately release the distal ligament or the proximal antebrachial fascia may occur.
Remove the dressing on postoperative day 2 or 3.
Use night splinting in a wrist neutral position for 2 weeks for patient comfort.
Begin range-of-movement exercises on day 2 or 3.
Avoid unnecessary elbow flexion because it may result in irritation of the ulnar nerve.
Begin strengthening exercises after 4 weeks.
There are no restrictions after the second month.
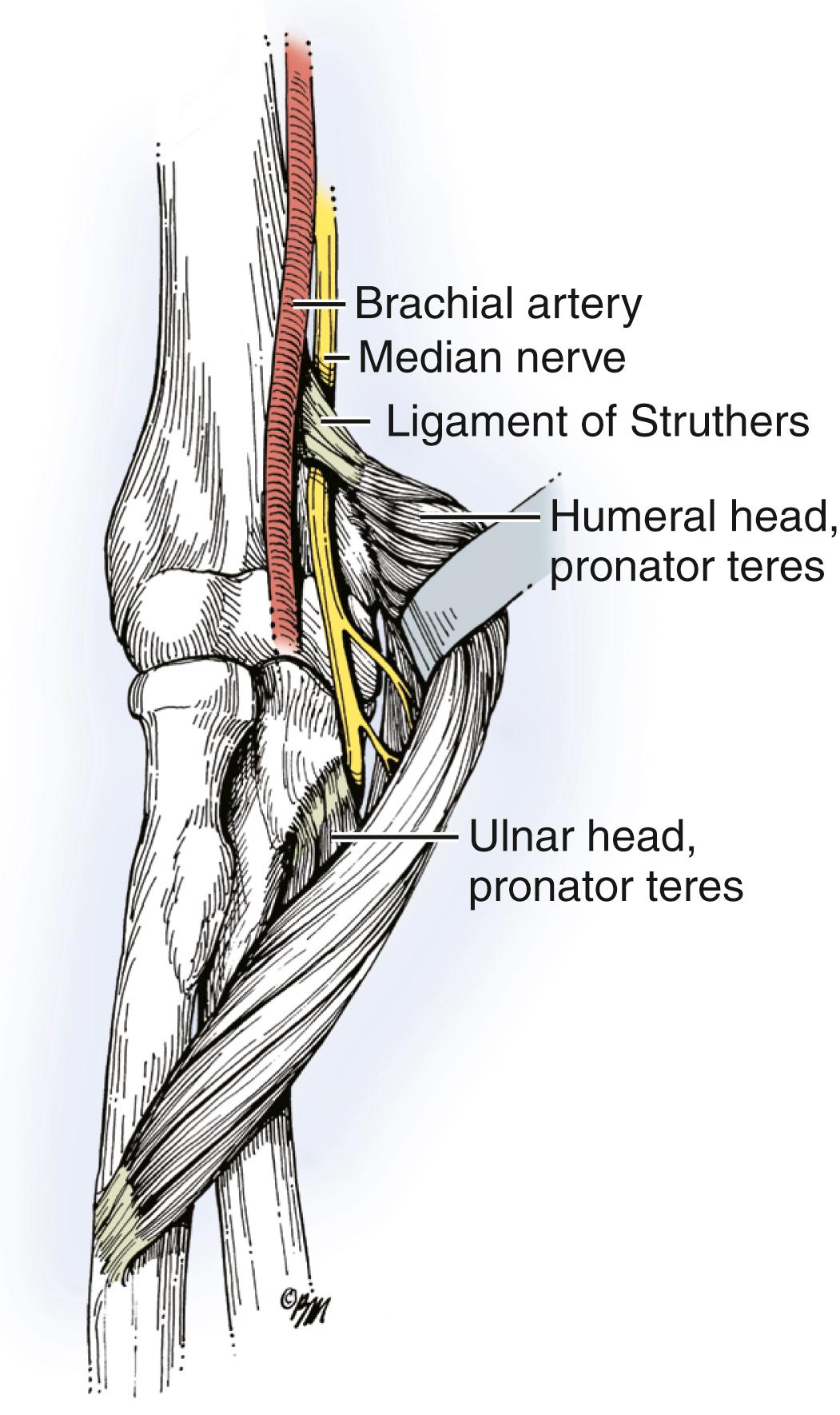
Proximal compression of the median nerve is much less common than carpal tunnel syndrome. The median nerve may be compressed proximal to the elbow under the ligament of Struthers in patients who have a supracondylar process ( Fig. 28.10 ). The most common site of proximal median nerve compression is in the forearm at the level of the pronator. The intersection of the deep and superficial heads of the pronator teres are frequently cited as the cause of compression in pronator syndrome. Compression may also occur from the lacertus fibrosis and the tendinous leading edge of the flexor digitorum superficialis arch. Other accessory and anomalous muscles, including the accessory head of the flexor pollicis longus (Gantzer muscle), the palmaris profundus, and the flexor carpi radialis brevis, have also been identified as potential compressive structures. The entrapment point of the pronator teres has been found to be located 3 to 7.5 cm distal to the humeral epicondylar line and the fibrous arch of the flexor digitorum superficialis to be 6.5 cm distal to the humeral epicondylar line in its most proximal position.
Compression in the forearm can produce sensory disturbance in the median nerve distribution or motor dysfunction of the muscles innervated by the anterior interosseous nerve and median nerve, or both sensory and motor dysfunction. Parsonage and Turner described spontaneous onset of periscapular pain, followed by flaccid paralysis of one or more muscles of the shoulder girdle or upper extremity, which they termed “neuralgic amyotrophy.” , Four of their 136 cases were localized to the anterior interosseous nerve. The pathophysiology of this motor axonopathy is largely unknown, and the leading theory is an immune-mediated process. Anterior interosseous nerve syndrome is associated with a shoulder or arm pain prodrome in nearly all cases and presents as an acute flaccid paralysis of the flexor pollicis longus with or without palsy of the flexor digitorum profundus-index and/or the pronator quadratus. It is generally preceded by a “trigger” such as a viral illness, trauma, or surgical procedure in the ipsilateral upper extremity. Spontaneous recovery to some extent is documented in most individuals; however, complete functional recovery cannot be assured. In one study, over 60% of a 246-patient cohort had residual weakness or sensory symptoms after 3 or more years. Recent literature suggests the pathophysiology of anterior interosseous syndrome may include intrinsic constrictions of the anterior interosseous nerve fascicle at or above the elbow.
The median nerve is formed from branches of the medial and lateral cords of the brachial plexus. It receives its sensory contribution predominantly from the lateral cord and its motor fibers predominantly from the medial cord. The median nerve crosses over the brachial artery to lie on the medial side in contact with the brachialis muscle. It continues distally between the brachialis muscle and the medial intermuscular septum and then passes through the antecubital fossa and under the lacertus fibrosus (bicipital aponeurosis). The nerve travels between the deep and superficial heads of the pronator teres and in some cases posterior to the pronator teres. The first branch from the main median nerve goes to the pronator teres, which branches from the ulnar side just above the elbow. A second pronator teres branch courses on the most anterior aspect of the median nerve. The next branches from the nerve are in order and on the ulnar side of the median nerve: the branches to the palmaris longus, flexor carpi radialis, and two branches to the flexor digitorum superficialis. The anterior interosseous nerve is the only branch to divide on the radial side of the median nerve. In a cadaveric anatomic study by Tung and Mackinnon, the pronator teres muscle received more than one branch in 73% of cases and the flexor digitorum superficialis received two or more branches in 94% of cases. After exiting the pronator teres, the median nerve courses deep to the fibrous arch of the flexor digitorum superficialis and becomes more superficial in the distal part of the forearm.
The anterior interosseous nerve provides innervation to the flexor digitorum profundus of the index and middle fingers, the flexor pollicis longus, and the pronator quadratus. The nerve provides sensory fibers to the radiocarpal, intercarpal, carpometacarpal, and radioulnar joints. The anterior interosseous nerve is relatively tethered as it separates from the main median nerve, which may predispose it to traction injuries. A Martin-Gruber anastomosis (connection between the median and ulnar nerves in the forearm) is found in 15% of the population, and in half of the cases the communicating branch originates from the anterior interosseous nerve.
The palmar cutaneous nerve branches from the median nerve approximately 5 cm proximal to the proximal wrist crease. It separates from the median nerve and enters a tunnel immediately medial to the flexor carpi radialis tendon and then innervates the skin of the thenar eminence. Variations in the branching pattern of the palmar cutaneous branch of the median nerve have been reported. It has been found to have an aberrant branching pattern in relation to the flexor carpi radialis tendon in 18.8% of patients including traveling within the tendon sheath in between 5% and 6% of patients. , Evaluation of thenar sensation is helpful in differentiating median nerve compression in the carpal canal from more proximal sites of compression.
Median nerve compression in the proximal forearm (previously referred to as pronator syndrome) is defined as nerve compression in the forearm that results in isolated sensory alteration and pain in the median nerve distribution in the digits and thenar eminence. This syndrome is much less common than carpal tunnel syndrome and is a clinical diagnosis; electrodiagnostic studies are usually normal. Patients report pain in the forearm and sensory abnormalities in the median nerve distribution distally to include the palmar cutaneous branch of the median nerve distribution. Provocation tests should reproduce symptoms in affected patients. These tests include pronator compression, resisted forearm pronation/supination, resisted flexion of the long finger flexor digitorum superficialis, and the sensory collapse test. Radiographs of the distal end of the humerus can identify the rare supracondylar process and alert the physician to the probability of the presence of a ligament of Struthers. Median nerve compression in the forearm should be considered in patients who have persistent symptoms after carpal tunnel release, or preoperatively in patients who have concomitant sensory findings in the palmar cutaneous distribution.
Anterior interosseous nerve syndrome is manifested as motor loss of the flexor pollicis longus, with or without involvement of the flexor digitorum profundus to the index finger, the pronator quadratus, or, occasionally, the flexor digitorum profundus to the middle finger. In a true anterior interosseous nerve syndrome, the loss of motor function occurs acutely and spontaneously. Patients may describe clumsiness with fine motor skills, such as writing and pinching. Because the anterior interosseous nerve does not innervate the skin, the syndrome is not associated with sensory loss.
Neuralgic amyotrophy (Parsonage-Turner syndrome) leads the differential diagnosis in patients with an anterior interosseous nerve palsy. Traumatic neuropathy, compressive neuropathy, and impingement by a forearm tumor are all decidedly rare. These patients typically have a history of severe periscapular or shoulder pain for several days or weeks. The pain has been reported to follow a viral illness, vaccination, trauma, surgery, pregnancy, or stress. High doses of corticosteroids are recommended if the neuritis is diagnosed early (within 72 hours). In a neuritis involving the anterior interosseous nerve, some degree of spontaneous recovery usually occurs, but full recovery cannot be assured. In patients with a spontaneous anterior interosseous nerve palsy and no electrodiagnostic evidence of reinnervation, consideration should be given to nerve exploration or nerve transfer (or both) at 7 to 10 months. Recent studies evaluating patients with anterior interosseous nerve syndrome have found that intrinsic fascicular constrictions of the anterior interosseous nerve are present, suggesting that anterior interosseous nerve syndrome represents a noncompressive neuropathy. These fascicular constrictions can be identified on magnetic resonance neurography or ultrasound and have been found at or proximal to the elbow in up to 99% of patients ( Fig. 28.11A ). When treating these patients surgically, it is essential to explore the median nerve proximal to the elbow. Under high-power microsurgical inspection, the constrictions are identified, and with microneurolysis and excision of the contracted perineurium, the fascicles can be unwrapped (see Fig. 28.11B ). The fascicles will appear very compressed, but excision and grafting are not recommended.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here