Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Cartilage is an extracellular matrix rich in aggrecan, a proteoglycan that retains a large amount of water. The matrix consists of approximately 65% water, 15% proteoglycan, 15% collagen (predominantly type II), and less than 5% cells. Cartilage is unique in that it receives nutrients and oxygen by simple diffusion rather than a capillary network, thus limiting the extent of growth of native cartilaginous structures to, at most, 1 to 2 cm. The assessment of the presence of cartilage matrix is best made on hematoxylin and eosin (H&E)-stained sections where hyaline cartilage has a smooth, basophilic or amphophilic quality with clearing around individual chondocytes ( Fig. 17.1 ). Elastic cartilage has a similar appearance, with the addition of elastic fibers within the matrix that provide increased tissue elasticity ( Fig. 17.2 ). Fibrocartilage tends to be richer in collagen, imparting a pinker fibrillary quality on H&E staining ( Fig. 17.3 ).
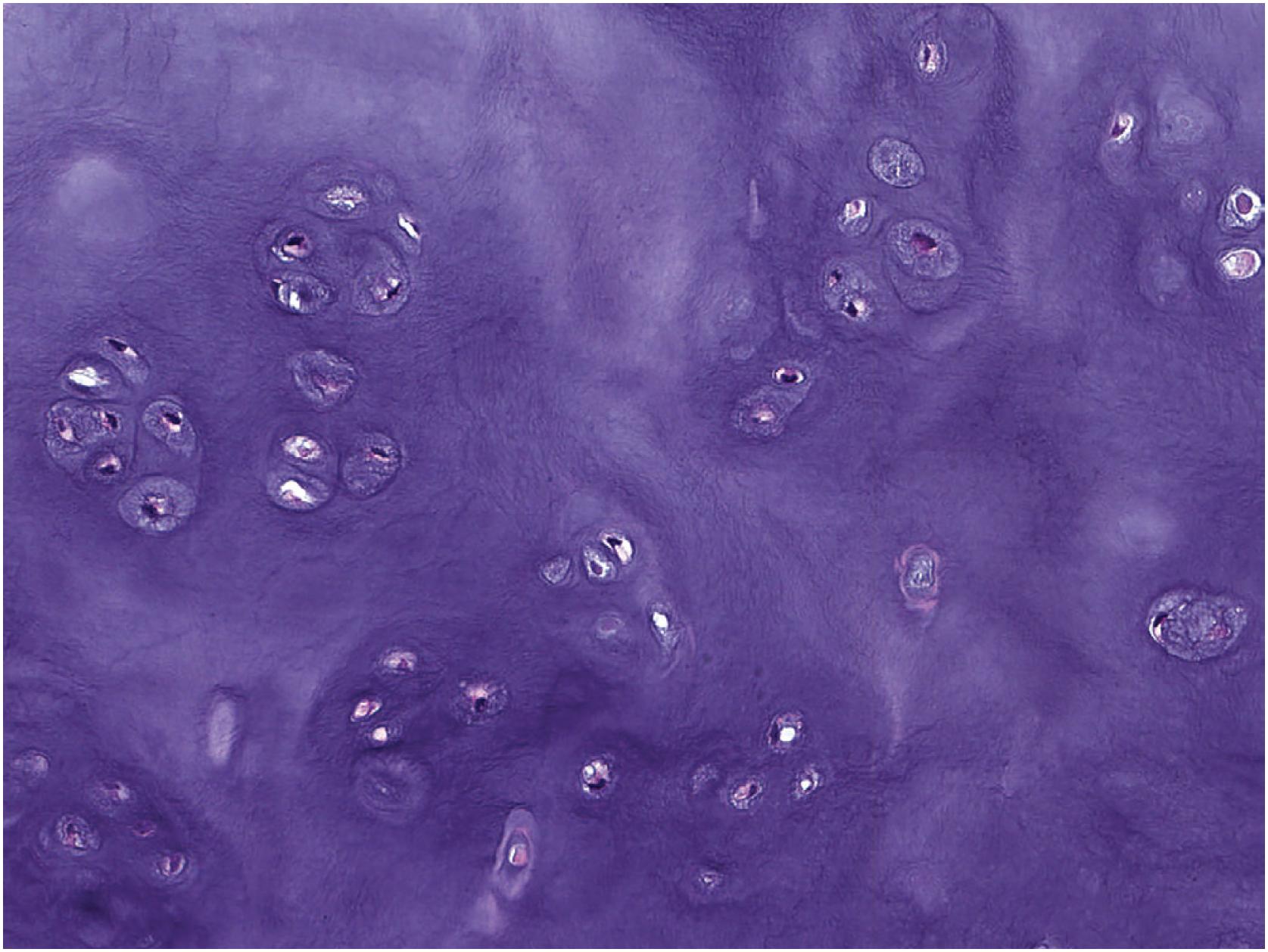
Chondrocytes are round with indistinct cytoplasmic borders and small, dark nuclei with perinuclear clearing. Benign chondrocytes typically demonstrate regular, albeit darkly staining, nuclei that are too small to make out nuclear detail of at intermediate (200X) magnification ( Fig. 17.4 ). Nuclear atypia in chondrocytes is generally defined as the ability to detect nuclear detail, vesicular chromatin or macronucleoli, especially at intermediate magnification. Although cellularity and cytologic atypia are not typical of benign cartilage tumors, enchondromas located in the small bones of the hands and feet can show increased cellularity and slight atypia. Periosteal chondromas and enchondromas in patients with enchondromatosis also characteristically show more cytologic atypia and hypercellularity when compared with other benign cartilage tumors and may demonstrate myxoid change. Although binucleation of chondrocytes has historically been correlated with cytologic atypia, this finding is nonspecific. Many benign tumors (e.g., synovial chondromatosis, periosteal chondroma, enchondroma) demonstrate binucleated chondrocytes. Mature chondrocytes have little to no proliferative capacity, but it is thought that undifferentiated mesenchymal stem cells can undergo chondrocyte differentiation.
As for all bone tumors, evaluation of a cartilage lesion requires adequate correlation with radiographic findings. Frequently plain films (two views) of the lesion are sufficient to guide the diagnosis. Computed tomography (CT) and magnetic resonance imaging (MRI) are useful adjuncts to further evaluate the pattern of mineralization, cortical involvement, and presence or absence of soft tissue involvement. Positron emission tomography (PET) may also have a role as benign cartilaginous tumors have lower maximum standardized uptake values (SUVs) than chondrosarcomas.
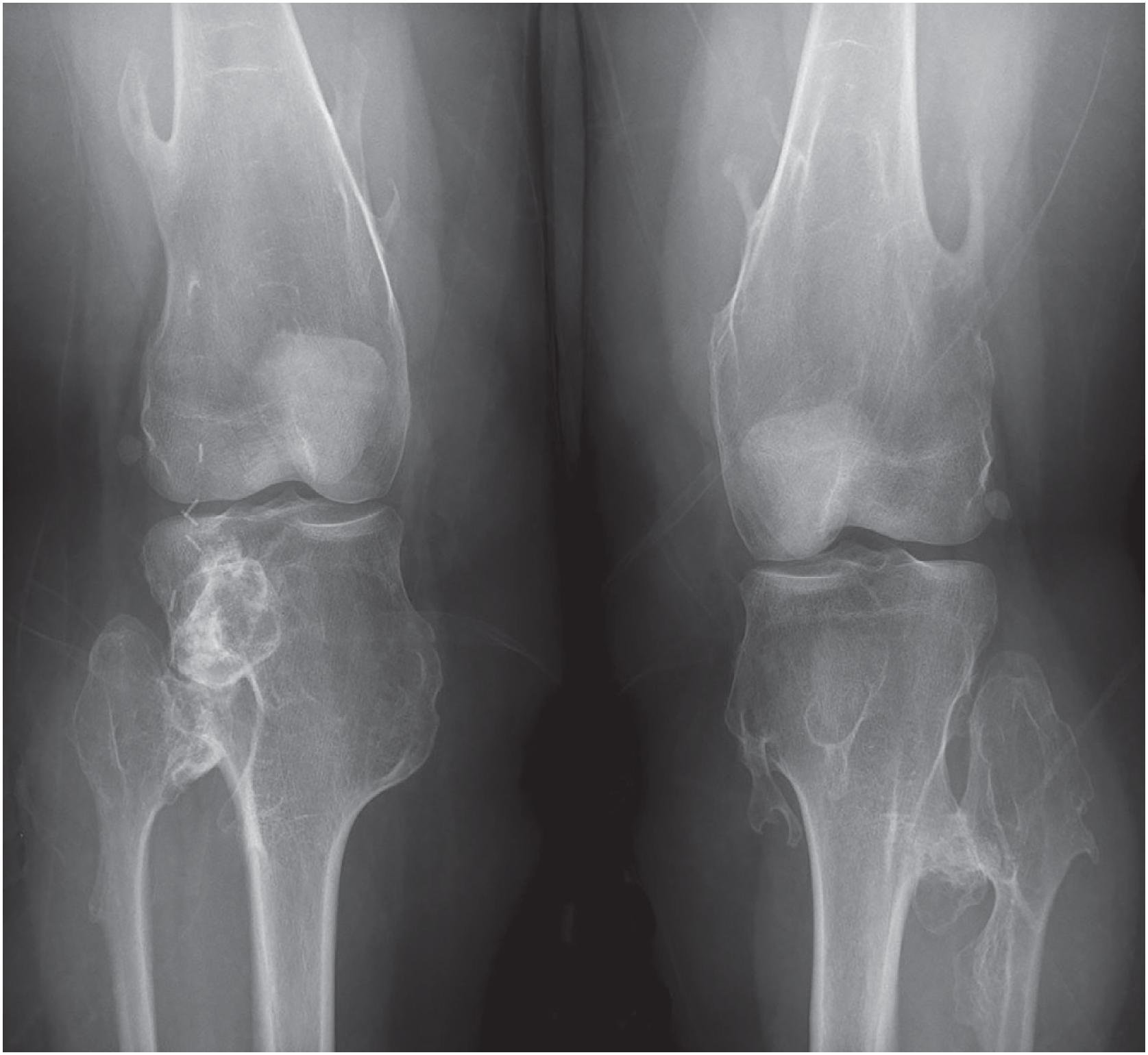
Osteochondroma is a cartilaginous neoplasm composed of a bony projection covered by a cartilaginous cap arising on the surface of bone, containing a marrow cavity and cortices that are continuous with that of the underlying bone. It most commonly arises in the distal femoral, proximal tibial, or proximal humeral metaphysis of adolescents and young adults (peak incidence in the second decade of life). It is more frequently seen in men than in women (2:1). Osteochondromas may be an incidental finding on imaging or present as a symptomatic, slow-growing mass of long duration. It may cause pain due to compression of nearby structures, associated bursitis or, less commonly, from fracture of the stalk. A recent and rapid growth of a preexisting osteochondroma, especially in a proximal skeletal location, raises the suspicion for malignant transformation.
Most patients have a solitary osteochondroma. Less commonly, it occurs as multiple lesions in patients with the autosomal dominant disorder, multiple osteochondromas (also known as hereditary multiple exostoses or multiple hereditary osteochondromatosis). The rate of malignant transformation for solitary osteochondromas is approximately 0.4%–2%. This is in contrast with patients with multiple osteochondromas who have higher rates of malignant transformation (0.2%–5%). Rates as high as 25% have been reported in multiple osteochondromas; however, these data are from large referral centers and likely overestimate the true incidence secondary to selection bias.
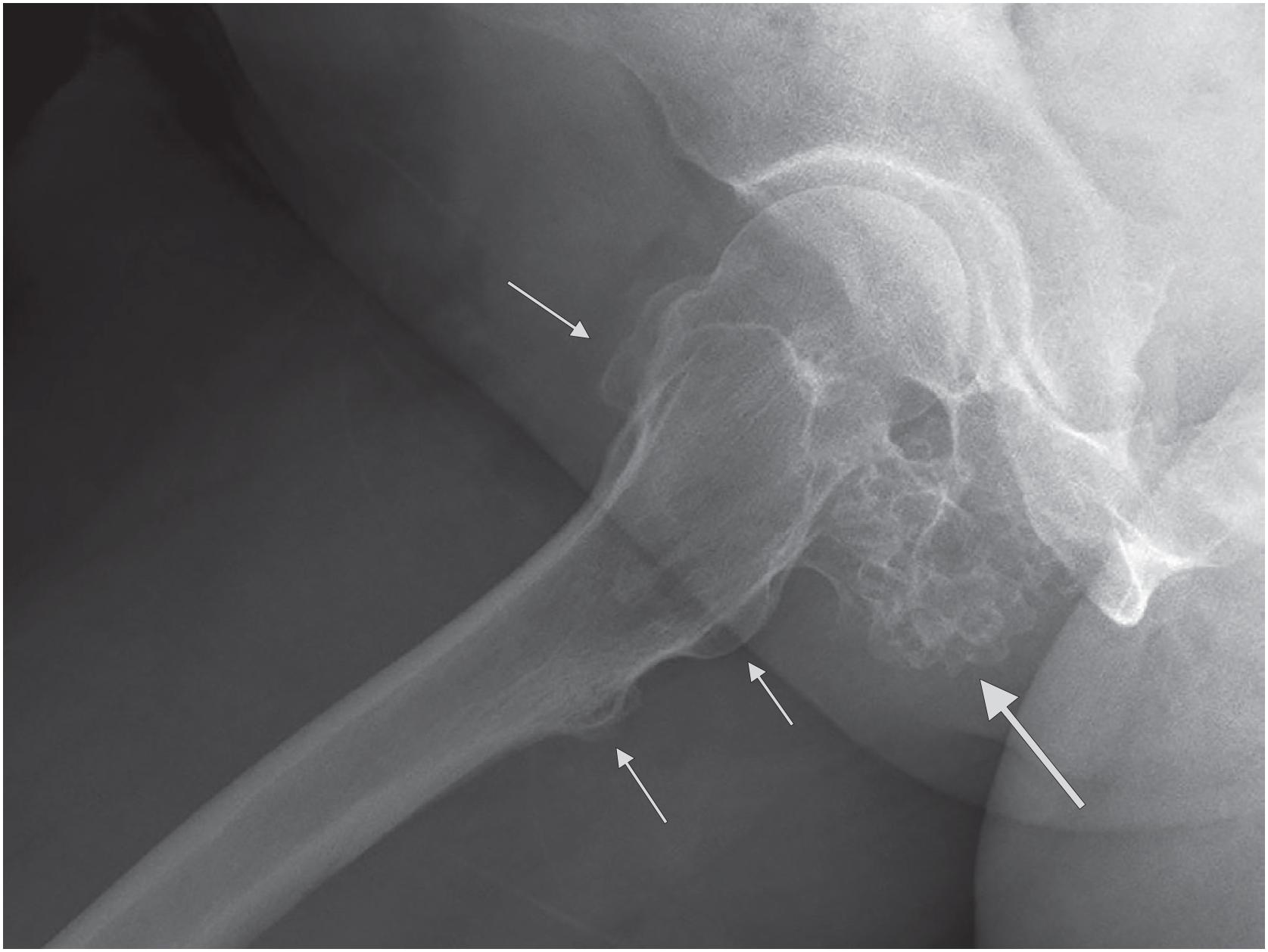
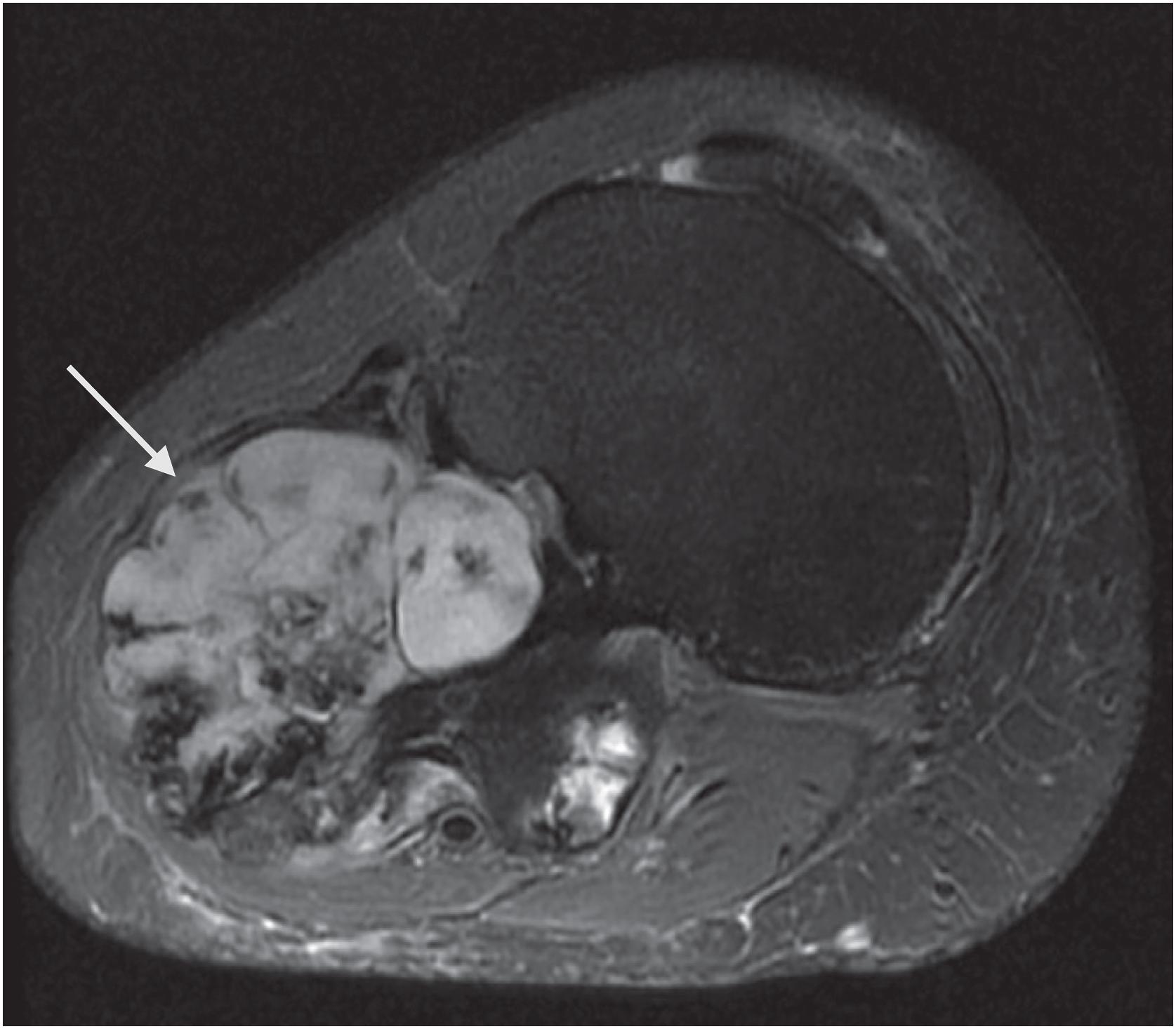
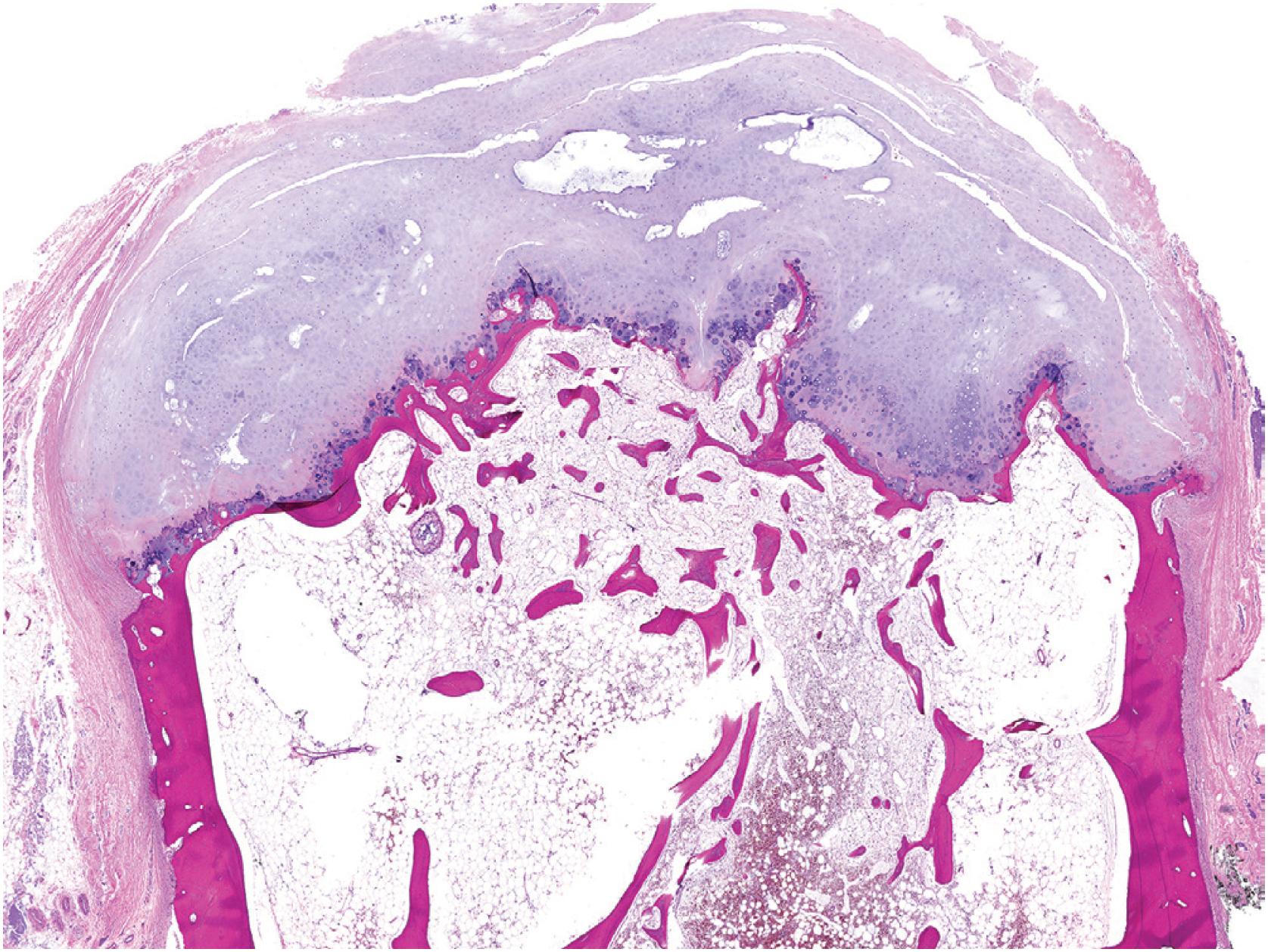
An osteochondroma presents as an exophytic, sessile, or pedunculated projection of bone capped by cartilage ( Fig. 17.5 ). It demonstrates both medullary and cortical components contiguous with the medulla and cortex of the underlying bone. These features are seen in each exostosis in patients with multiple osteochondromas ( Figs. 17.6 and 17.7 ). Features that have been associated with malignant transformation include irregularity of the margin, inhomogeneous mineralization ( Fig. 17.8 ), an associated soft tissue mass, and a thick cartilaginous cap ( Fig. 17.9 ).
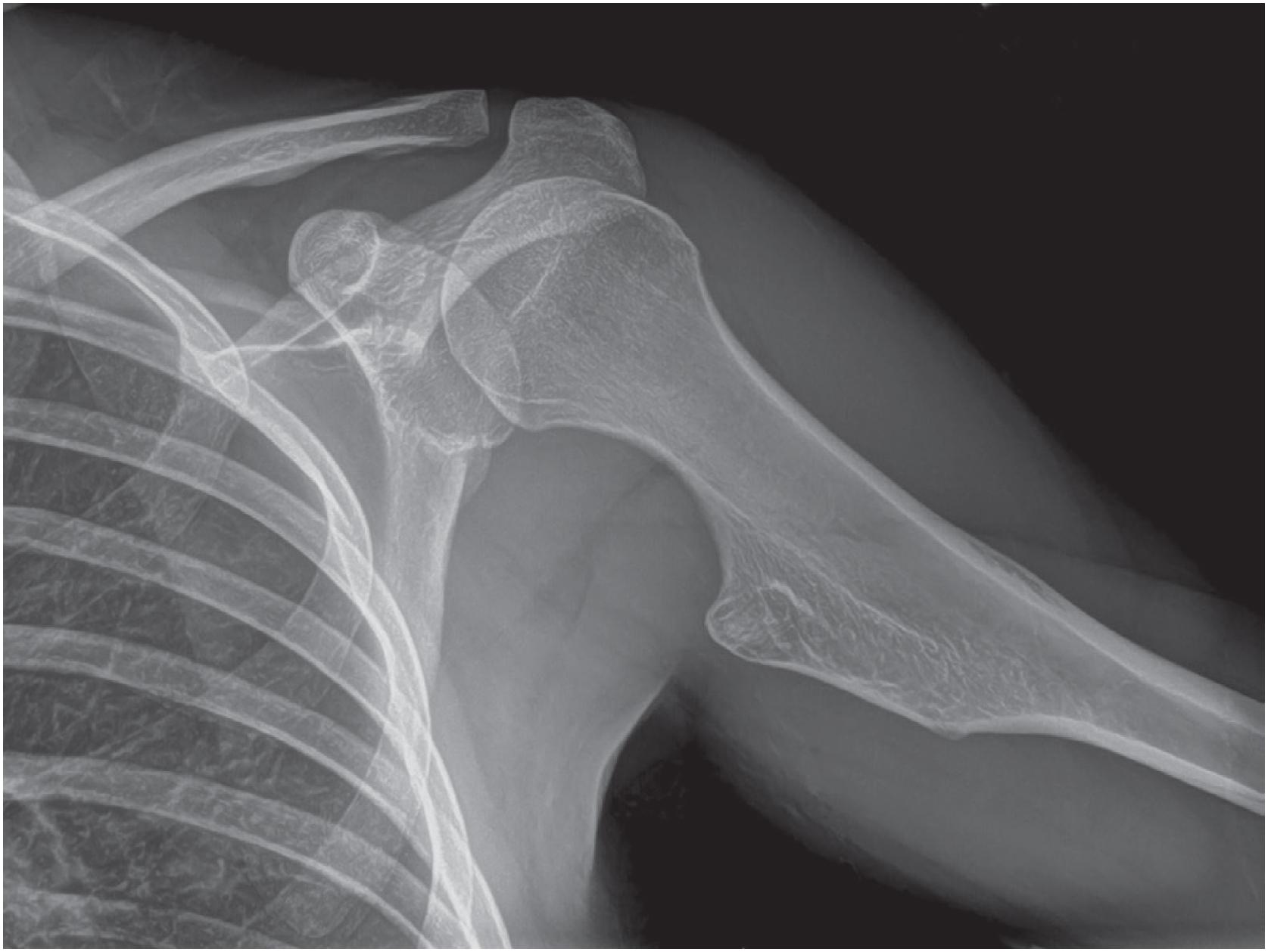
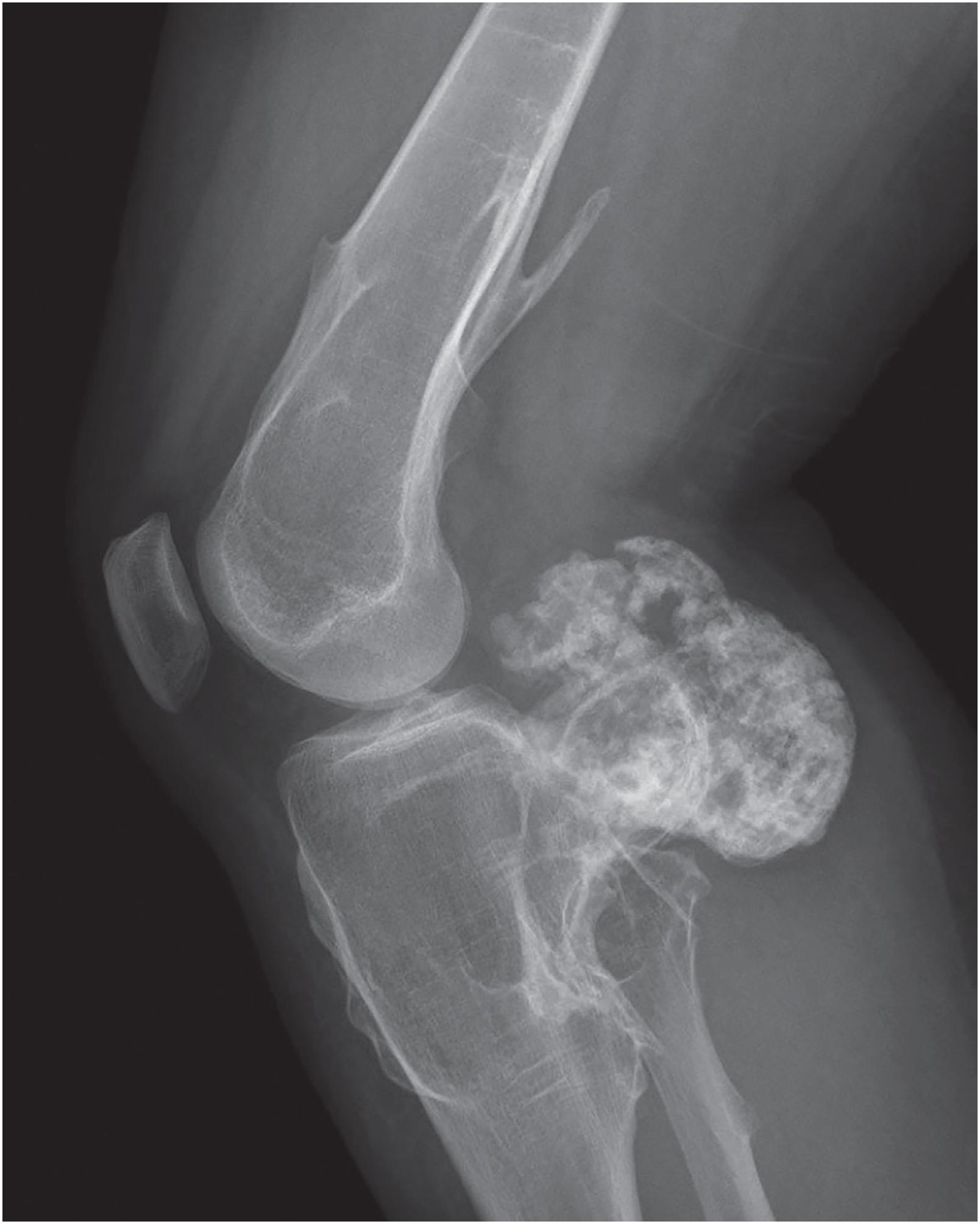
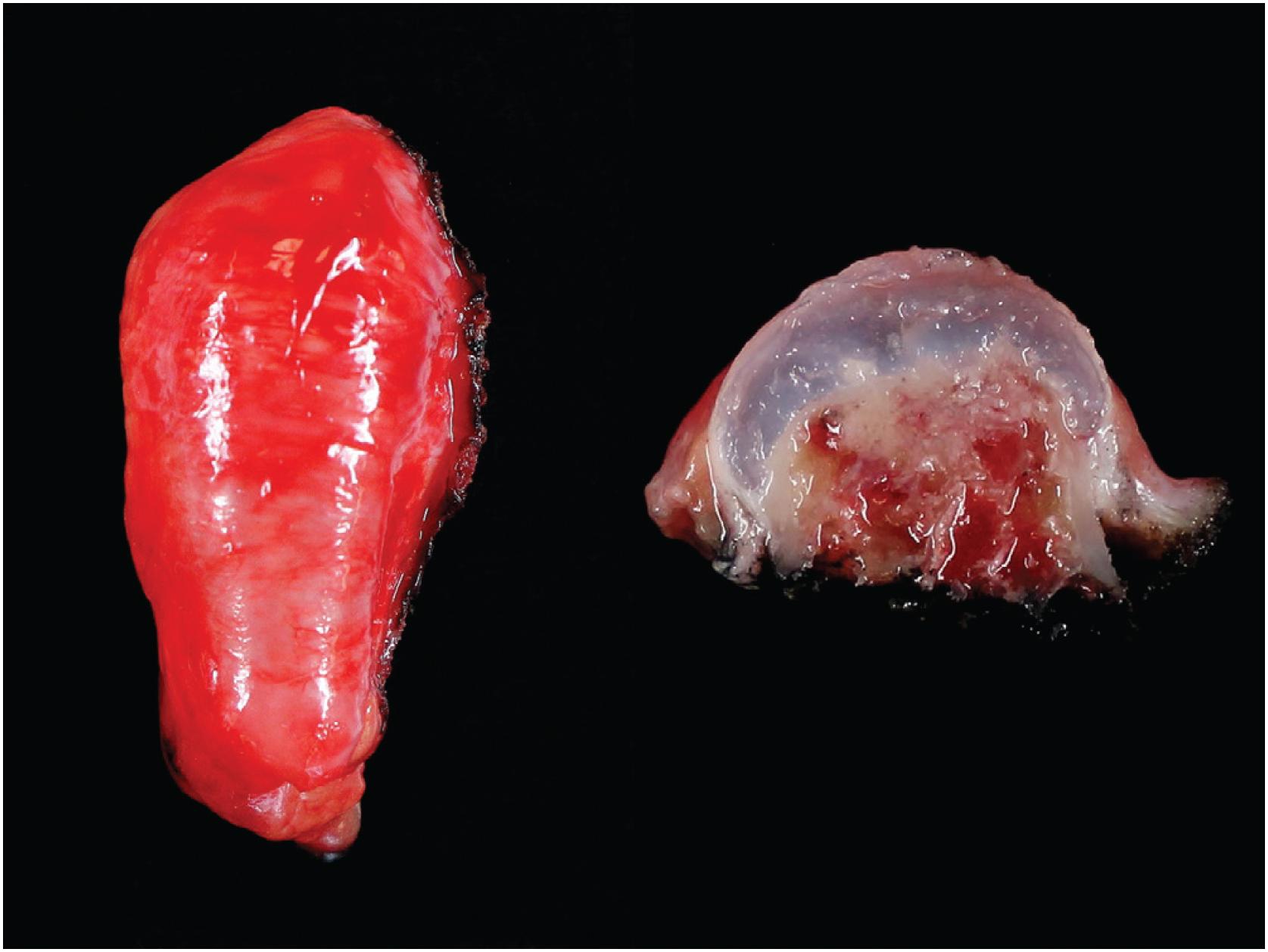
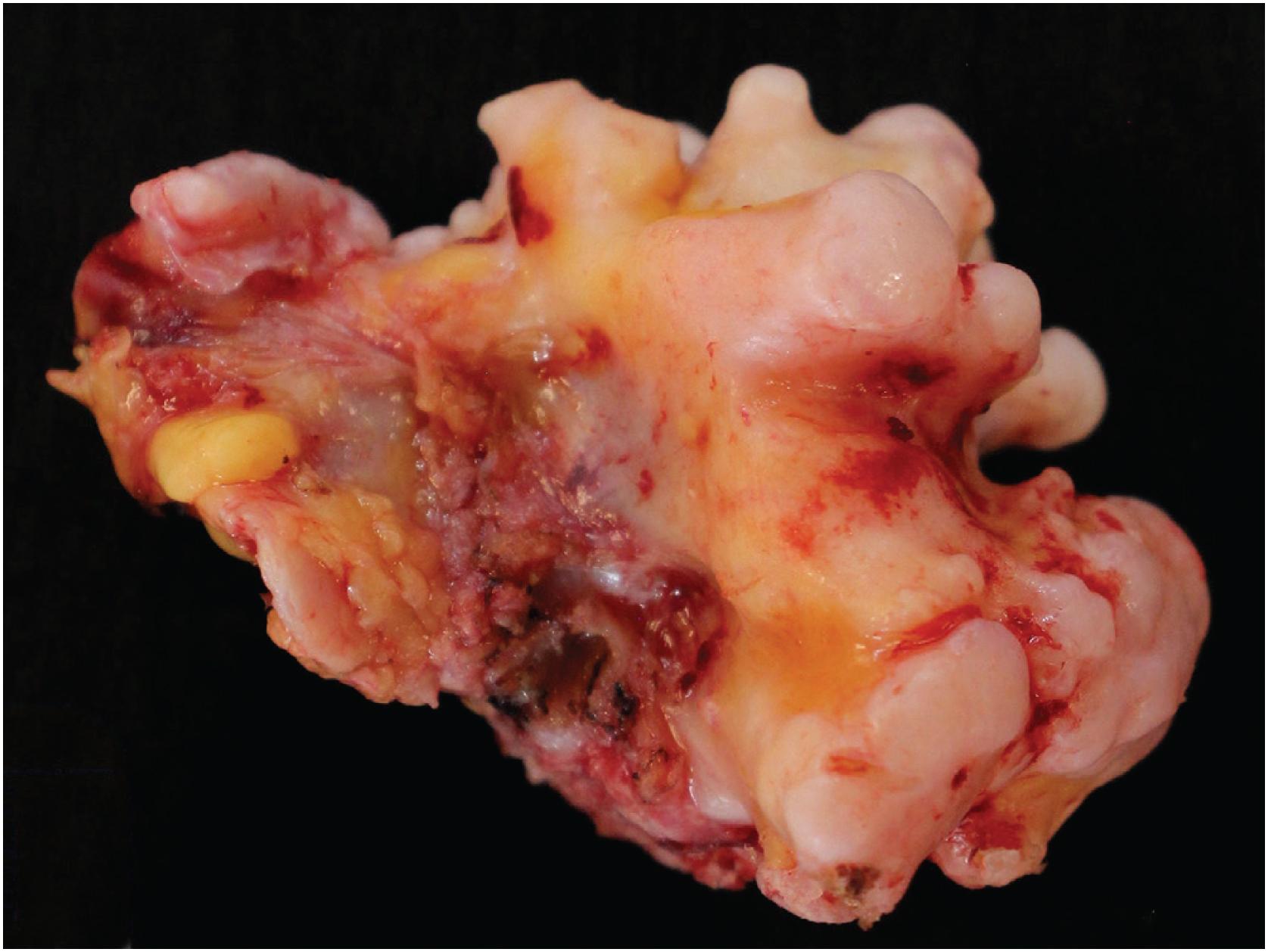
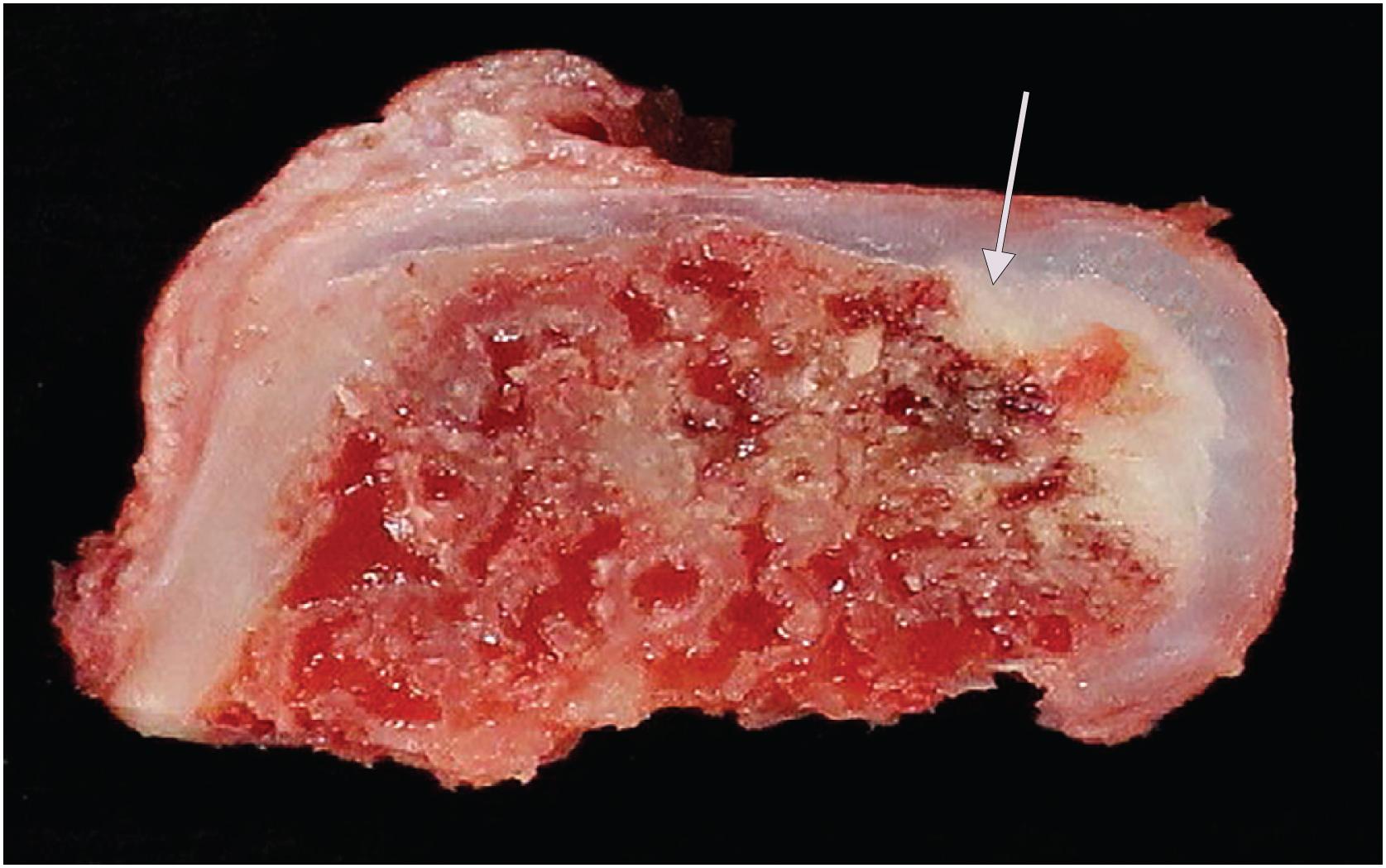
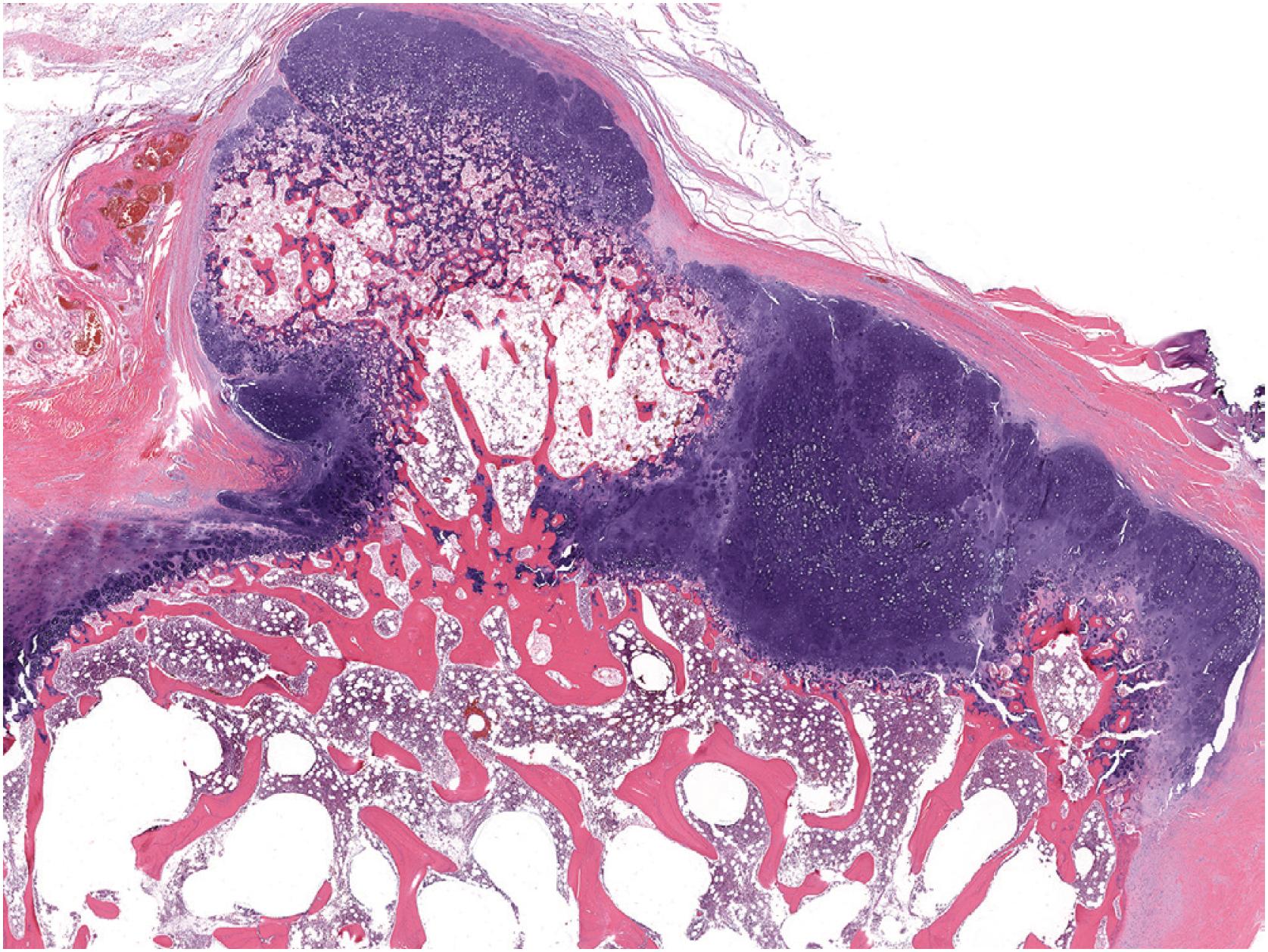
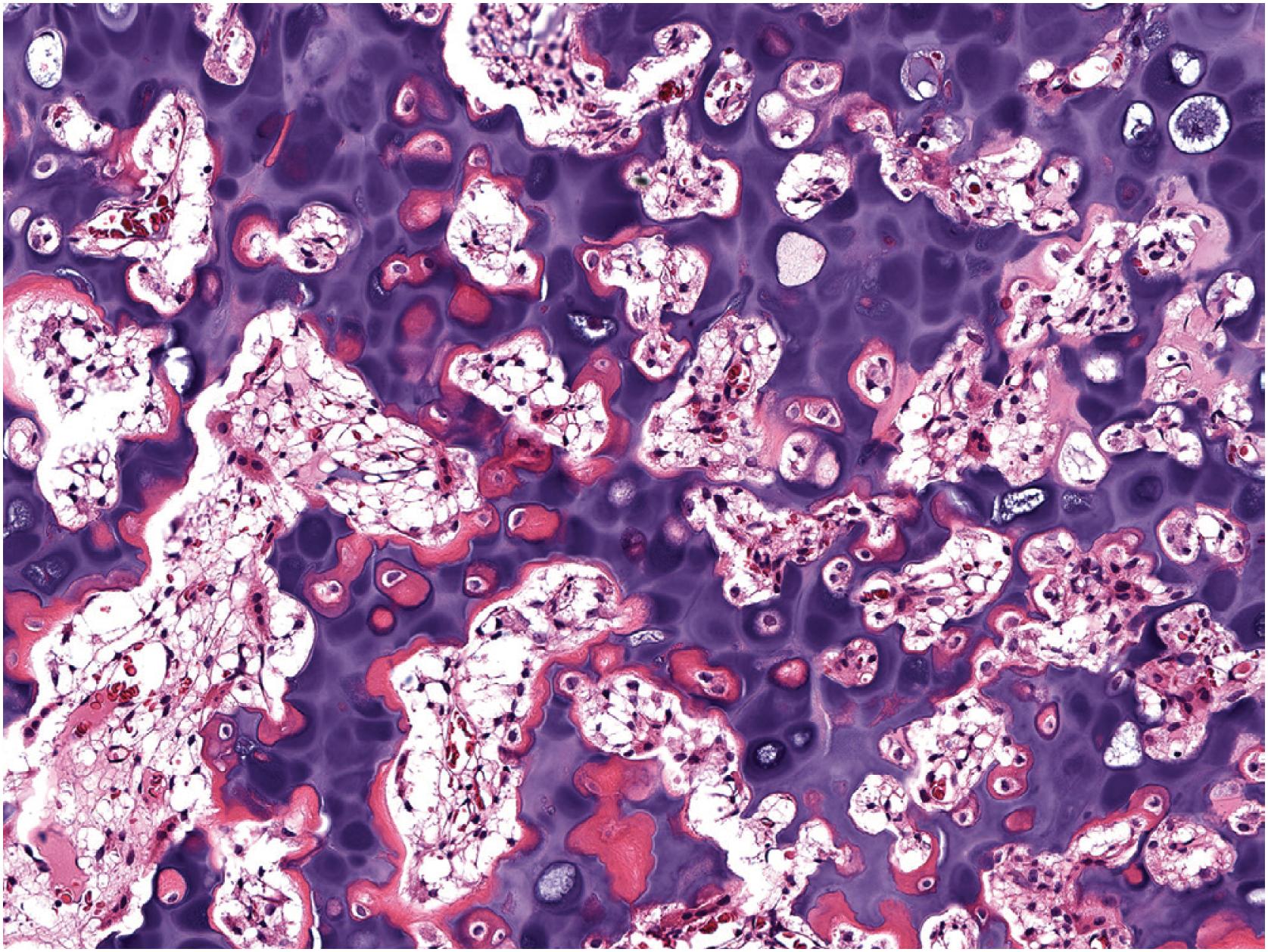
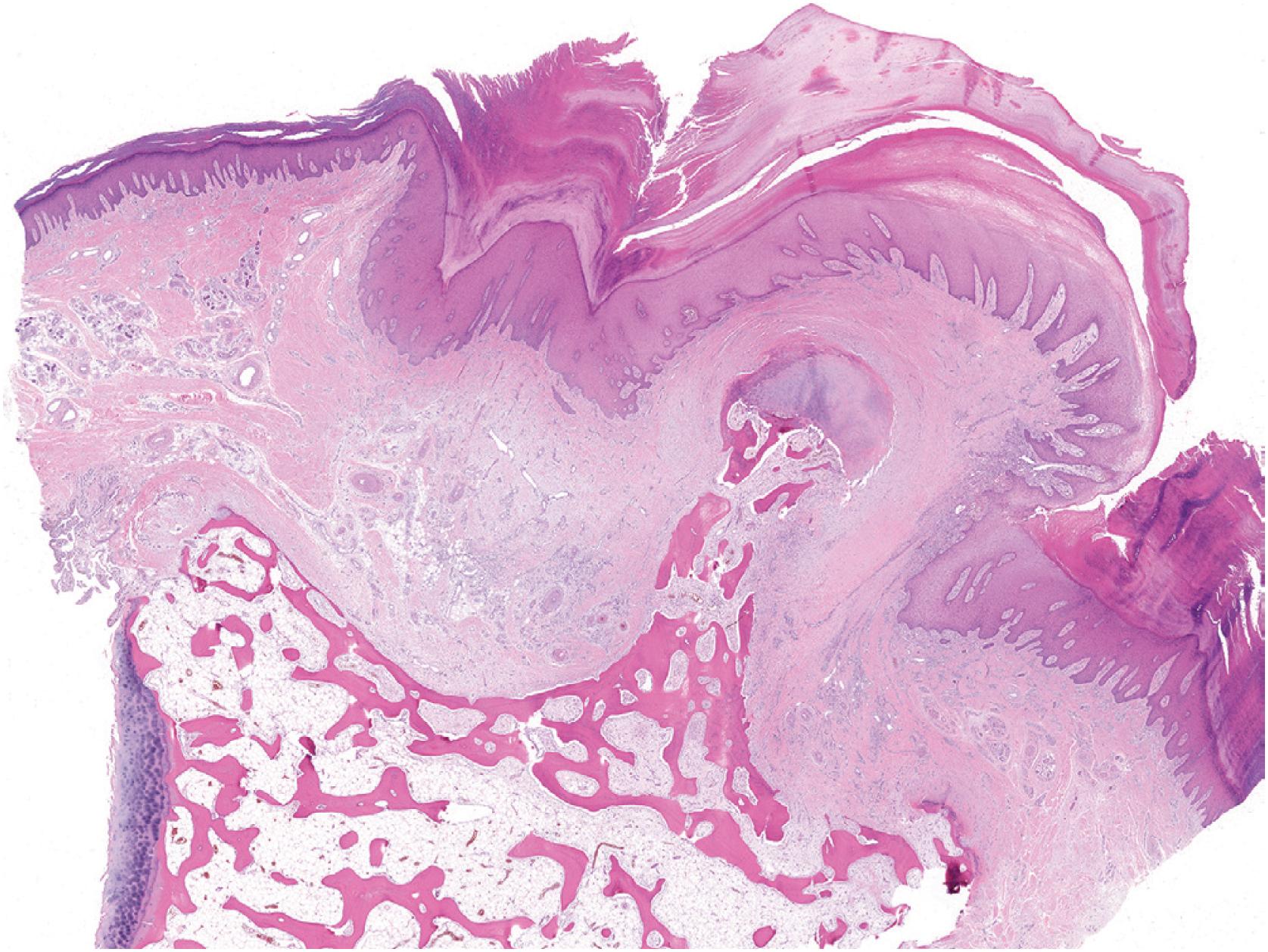
Grossly osteochondroma shows a thin (1 to 2 cm), pale blue, smooth cartilage cap overlying cortical and medullary bone ( Fig. 17.10 ). The thickness of the cartilaginous cap decreases with age. It may be a single dome-shaped cap with a mushroom-like configuration or be composed of multiple excrescences, imparting a bosselated appearance ( Fig. 17.11 ). A pedunculated osteochondroma demonstrates a prominent stalk, whereas the sessile form shows a broad attachment to the underlying bone. Lesions that have ceased active growth will show increasing amounts of endochondral ossification ( Fig. 17.12 ) and may be composed principally of bone.
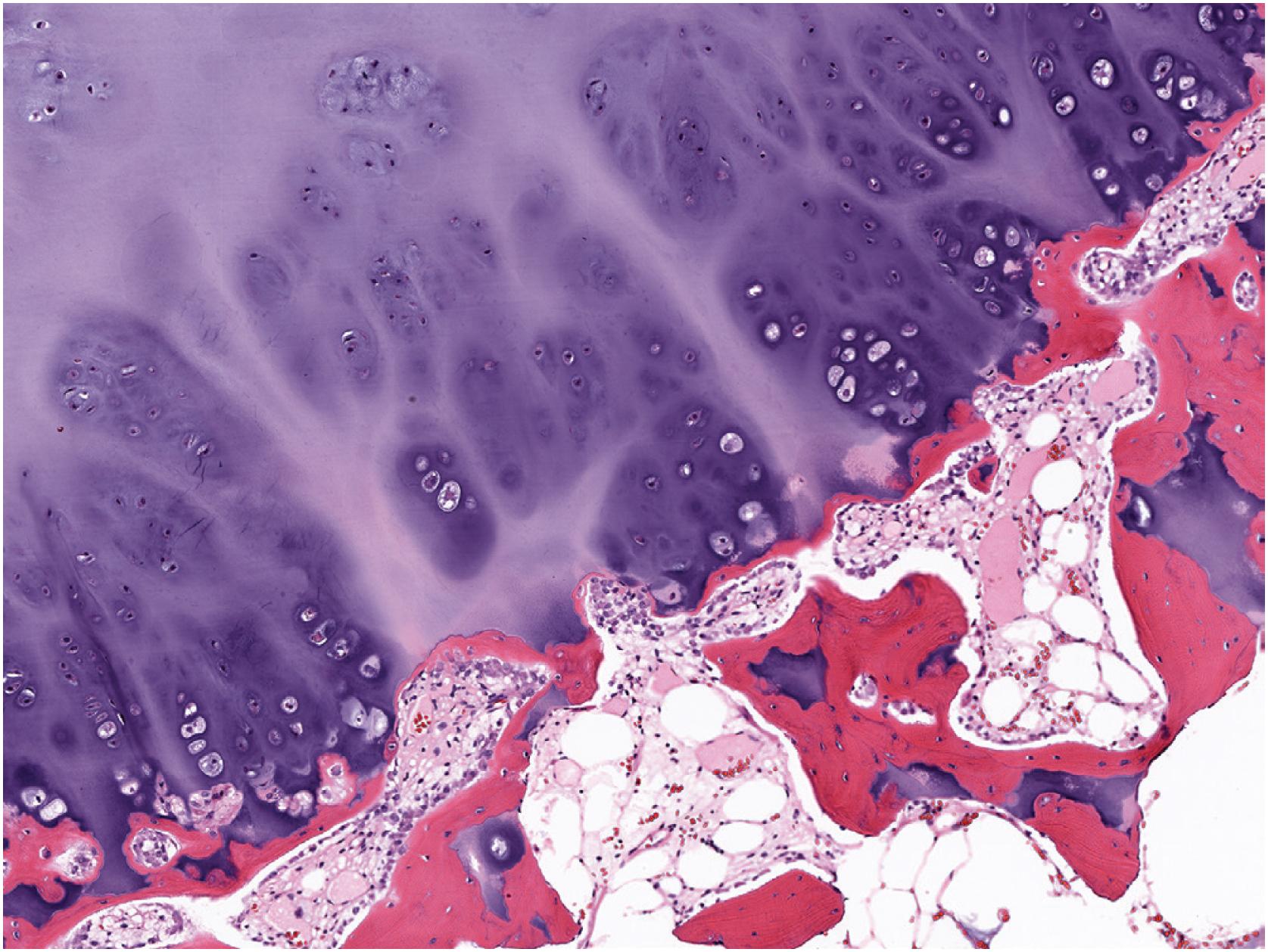
In general, osteochondroma mimics an epiphyseal growth plate beginning with a thin (usually <1 cm) cartilaginous cap superficially, a zone of endochondral ossification followed by lamellar trabecular bone, and fatty or hematopoietic marrow ( Fig. 17.13 ). The chondrocytes may be somewhat haphazard superficially but are arranged in orderly columnar growth at the ossification zone ( Fig. 17.14 ). Scattered zones of residual cartilage may be seen among the trabeculae. The chondrocytes lack nuclear atypia ( Fig. 17.15 ). Occasionally, degenerative change and calcification are seen in the cartilage cap. In the growing skeleton, osteochondromas have conspicuous endochondral ossification ( Fig. 17.16 ) with prominent regions of primary spongiosa-like tissue ( Fig. 17.17 ).
Immunohistochemical studies are not useful in the diagnosis of osteochondroma.
Osteochondromas are caused by biallelic loss of the tumor-suppressor genes EXT1 or EXT2 . In the setting of multiple osteochondromas, somatic loss of the wild-type allele occurs in the background of a germline alteration in EXT1 or EXT2 . In the sporadic setting, homozygous EXT1 deletions are present in a subset of chondrocytes within the cartilage cap of an osteochondroma. Mutations in IDH1 and IDH2 are absent.
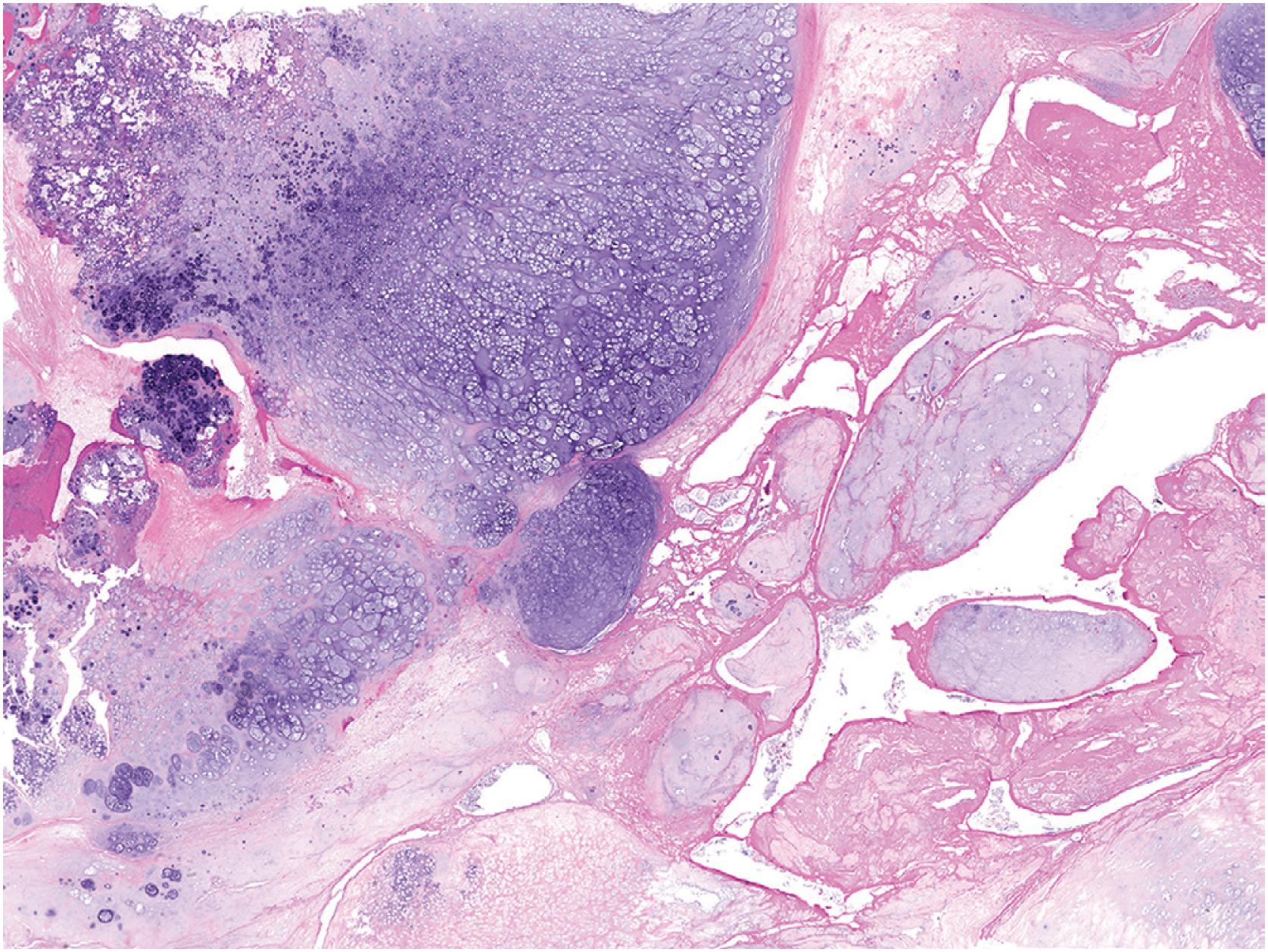
Rarely, secondary chondrosarcoma can arise from an osteochondroma. The distinction between these entities requires clinical, radiographic, and pathologic correlation. Clinical or radiographic evidence of recent growth, pain, the involvement of the proximal skeleton, irregular mineralization, or soft tissue destructive growth are concerning features. The thickness of the cartilage cap (usually <1 cm) has long been reported as an indicator of potential malignancy with malignancy suspected in cases with a cartilage cap of >2 cm in thickness. The best feature to confirm a diagnosis of malignancy is histologic evidence of permeative growth of bone or adjacent soft tissue. Other helpful microscopic features that suggest malignant change include prominent myxoid cystic change in the matrix, wide fibrous bands, increased cellularity, and loss of the normal columnar arrangement of chondrocytes at the ossification zone ( Fig. 17.18 ) along with mitotic activity and nuclear pleomorphism, if present. The diagnosis is difficult to make on biopsy specimens.
Benign, exophytic tumor of cartilage and bone that recapitulates a growth plate
Syndromic form (multiple osteochondromas) estimated at 1 in 50,000
Sporadic form (solitary) six times more common than those in the setting of multiple osteochondromas
Most common in metaphysis of long bones, especially distal femur, proximal tibia, and proximal humerus
Pelvic bones and scapula are uncommon sites
More common in male than female sex (2:1); peak in second decade of life
Asymptomatic or painless mass of long duration
May be found incidentally on imaging for unrelated complaints
Pain may be present due to local effects or fracture of lesion; rarely pain signals malignant transformation
Asymptomatic lesions can be observed
Surgical excision is curative for symptomatic lesions
Malignant transformation < 1% (sporadic form) and ∼5% (multiple osteochondromas syndrome)
Thin (< 2 cm), pale blue, firm cartilage cap overlying a stalk composed of hard bone
Cut surface reveals medullary and cortical bone contiguous with underlying bone
Cartilage cap undergoing endochondral ossification with orderly arrangement of chondrocytes
Higher cellularity of cartilage cap in skeletally immature individuals
Endochondral ossification increases with time; lesions of longstanding duration show prominent mineralization
Cancellous bone beneath the cartilage cap
Intertrabecular spaces filled by fat and hematopoietic marrow
Occasionally, degenerative changes in cartilage cap
Chondrosarcoma arising in osteochondroma
Parosteal osteosarcoma
Bizarre parosteal osteochondromatous proliferation (Nora lesion)
Periosteal chondroma
Parosteal osteosarcoma may also demonstrate a cap of cartilage with underlying bone. However, radiographically and grossly, the bone is not contiguous with the medullary cavity of the native bone. Most importantly, the intertrabecular space within parosteal osteosarcoma is occupied by a spindle cell proliferation with nuclear atypia rather than fat and hematopoietic marrow, as is typically seen in osteochondroma.
Bizarre parosteal osteochondromatous proliferation (Nora lesion) differs clinically in the age of presentation (adults) and location (hands and feet). Although the lesion is composed of a mixture of bone and cartilage, it is not contiguous with the underlying medullary cavity, nor does it show the organization of a growth plate.
Periosteal (surface) chondroma is composed exclusively of lobules of cartilage and lacks endochondral ossification and growth plate-like architecture.
Surgical excision should be curative treatment for osteochondromas, though recurrence is possible if a portion of the cartilage cap or perichondrium is left behind.
Bizarre parosteal osteochondromatous proliferation (BPOP) or Nora lesion is an uncommon osteochondromatous proliferation involving the surface of the bone, in particular the small bones of the hands and feet (75% of cases). Approximately 25% of cases involve the long bones. BPOP tends to affect adult patients in the third to fourth decades of life. There is no sex predilection, and the typical presentation is one of a localized swelling, with or without pain. Some examples demonstrate rapid growth.
Subungual exostosis is a distinct lesion with similar morphologic features but with different genetic abnormalities.
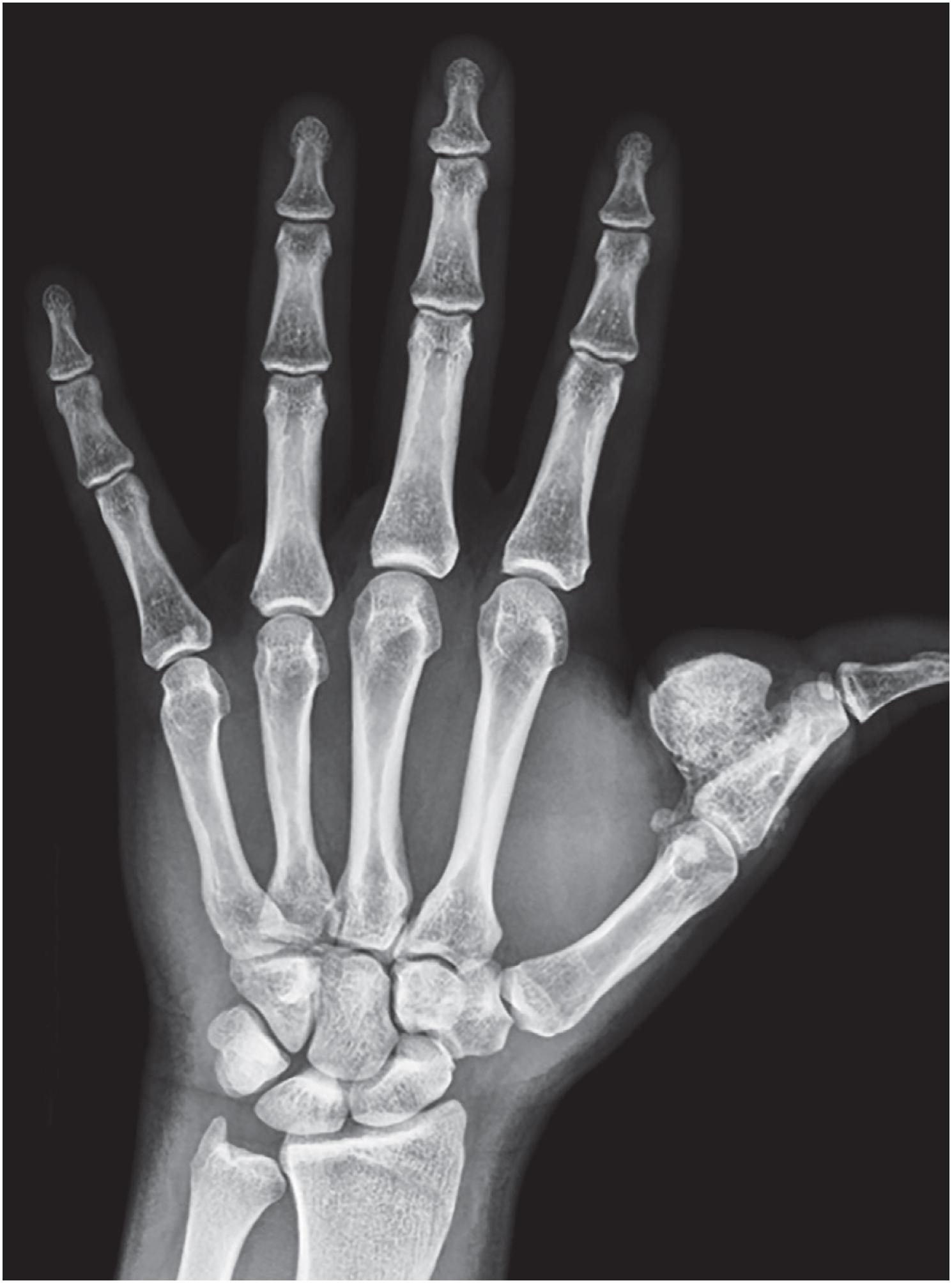
Radiographic studies show a well-marginated, mineralized mass attached to the surface of the affected bone with an intact underlying cortex ( Fig. 17.19 ).
Macroscopic exam shows a lobulated cartilage cap with attached bony stalk. Lesions range from 0.5 to 3 cm.
BPOP demonstrates a disorganized proliferation of fibrous tissue, cartilage, and bone. The surface is composed of fibrous tissue overlying a cartilaginous component that undergoes endochondral ossification. The cartilage may be hypercellular with enlarged and binucleated chondrocytes (atypical or “bizarre”) and undergoes endochondral ossification manifested by distinctive basophilic mineralization (so-called “blue bone”), which can remain after decalcification ( Fig. 17.20 ). Marked nuclear hyperchromasia and atypical mitotic figures are absent. Similar features are seen in subungual exostosis ( Fig. 17.21 ). The fibrous tissue ( Fig. 17.22 ) ranges in cellularity and may be hypercellular.
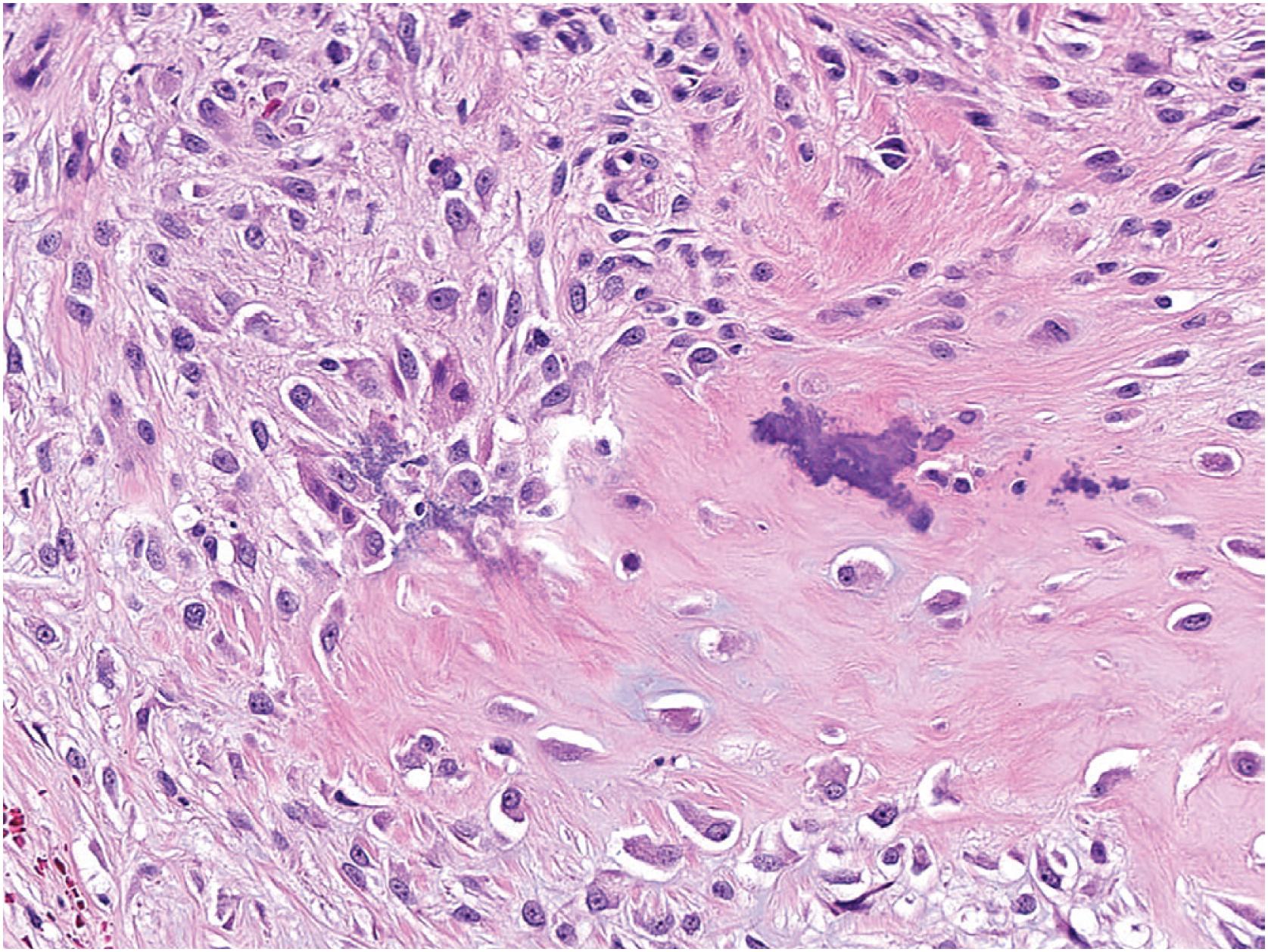
Several reports have documented the expression of a range of different growth factors in BPOP; however, immunohistochemical stains are generally not useful in diagnosing BPOP.
Most cases demonstrate a t(1;17) or variant thereof. A subset of cases demonstrates inv(7) or inv(6).
Osteochondroma demonstrates cortical and medullary continuity with the involved bone, whereas the cortex is intact in BPOP and related lesions; radiographic features are helpful to distinguish these entities. Histologically, in osteochondroma, the cartilage demonstrates growth plate-like architecture, and the lesion lacks a fibrous tissue component.
A subungual exostosis is a morphologically similar process that generally lacks “blue bone.” It exclusively affects distal phalangeal bones, typically the great toe, and genetically harbors a distinct translocation, t(X;6).
Parosteal osteosarcoma is very rare in the small bones of the hands and feet. The spindle cells in parosteal osteosarcoma show cytologic atypia and are oriented around trabeculae of tumor bone. The amplification of MDM2 seen in parosteal osteosarcoma is absent in BPOP.
Benign chondro-osseous matrix-forming tumor that arises on the surface of bone beneath periosteum
Rare; exact incidence unknown
Most common in small bones of hands and feet (75%)
Long bones of upper and lower extremities (25%); rarely in craniofacial skeleton
Diaphysis or metaphysis
Equal sex distribution; wide age range, predominantly third to fourth decades of life
Small, rapidly growing mass on the surface of tubular bones
May mimic a range of other neoplasms
Asymptomatic lesions can be observed
Frequently treated by simple excision; 50% recur
Cartilage cap with bony stalk, similar to osteochondroma; lesions range from 0.5 to 3 cm
Cortex and medulla of underlying bone are not contiguous with those of the lesion
Composed of disorganized fibrous tissue, cartilage, and bone
Hyaline cartilage is hypercellular with enlarged chondrocytes and frequent binucleation
Cartilage undergoes endochondral ossification (with a “blue bone” pattern) leaving bony trabeculae lined by plump osteoblasts
Fibrous tissue can be hypercellular or occasionally absent
Mitotic activity may be present without atypical forms
Osteochondroma
Subungual exostosis
Parosteal osteosarcoma
Asymptomatic lesions can be observed. Most cases are treated by simple surgical excision. Approximately 50% of cases recur, likely due to incomplete removal. Multiple, non-destructive recurrences are possible. Metastasis does not occur.
Periosteal chondroma (surface chondroma or juxtacortical chondroma) is a relatively uncommon benign cartilage tumor that arises on the surface of bone beneath the periosteum, frequently eroding the underlying cortex. It most commonly involves the small bones of the hands and feet or the long bones of the appendicular skeleton, in particular the proximal humerus. Periosteal chondroma affects all age groups with a peak in the second and third decades of life, demonstrates a male predilection (1.5:1), and is usually asymptomatic, solitary and found incidentally on radiographs obtained for other reasons. Patients can also present with a painful, palpable mass.
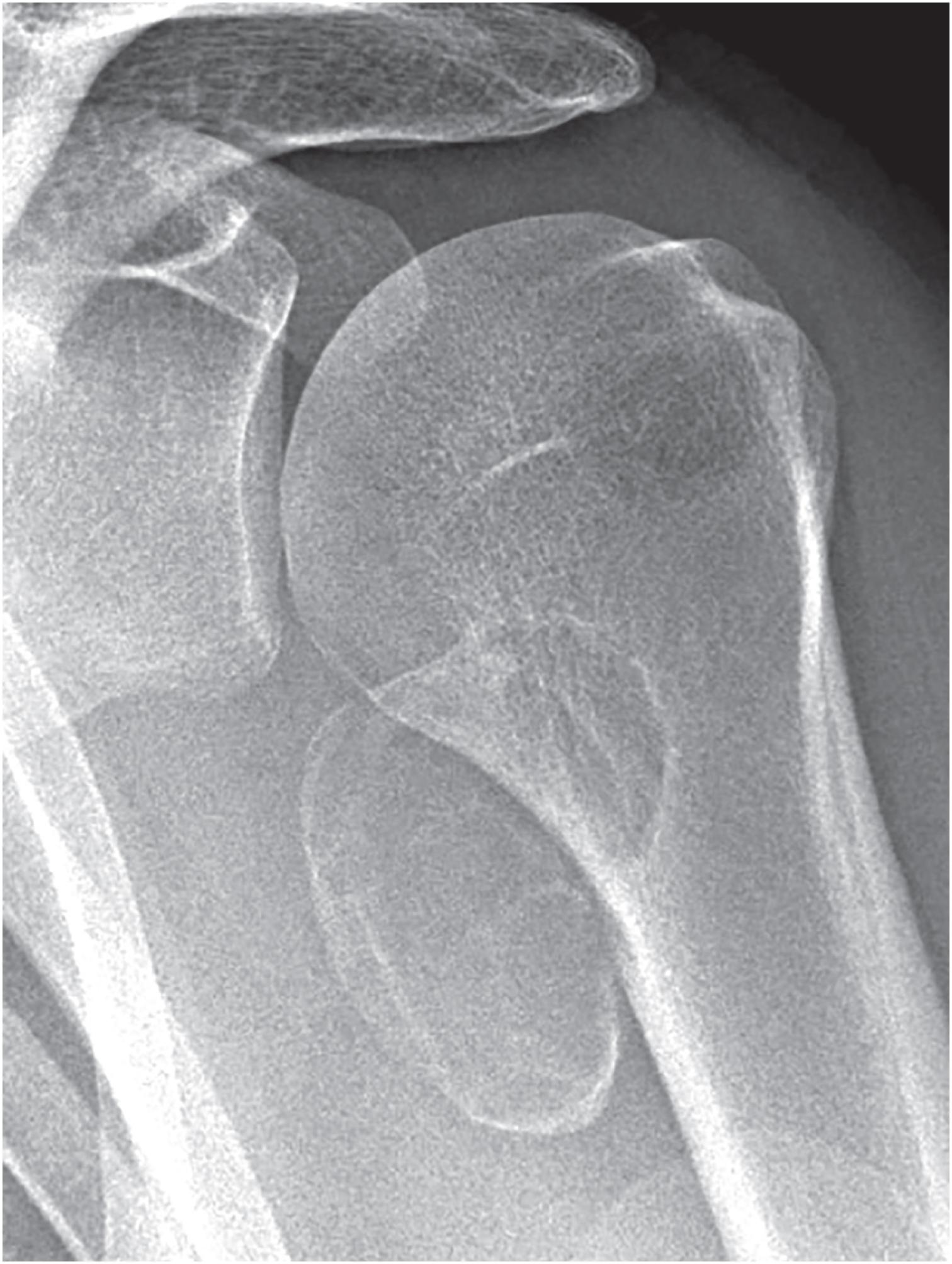
Periosteal chondromas are small (1 to 3 cm), radiolucent masses attached to the cortex with cortical erosion (saucerization) and underlying cortical sclerosis ( Fig. 17.23 ). Variable degrees of internal mineralization may be noted ( Fig. 17.24 ). Periosteal reaction with peripheral buttressing may be seen. MRI findings show a surface tumor that is bright on T2-weighted sequences ( Fig. 17.25 ), similar to other benign cartilage tumors.
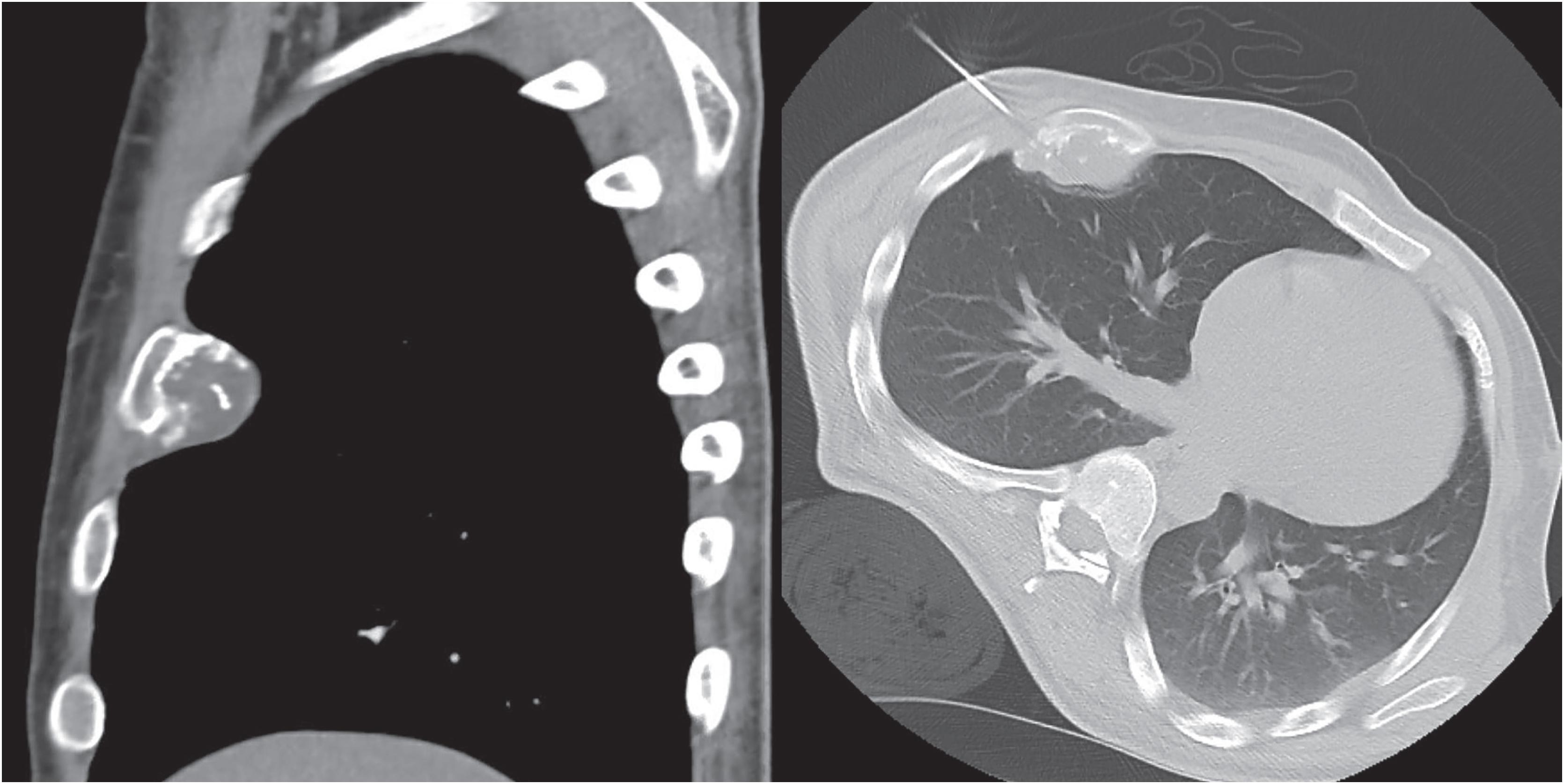
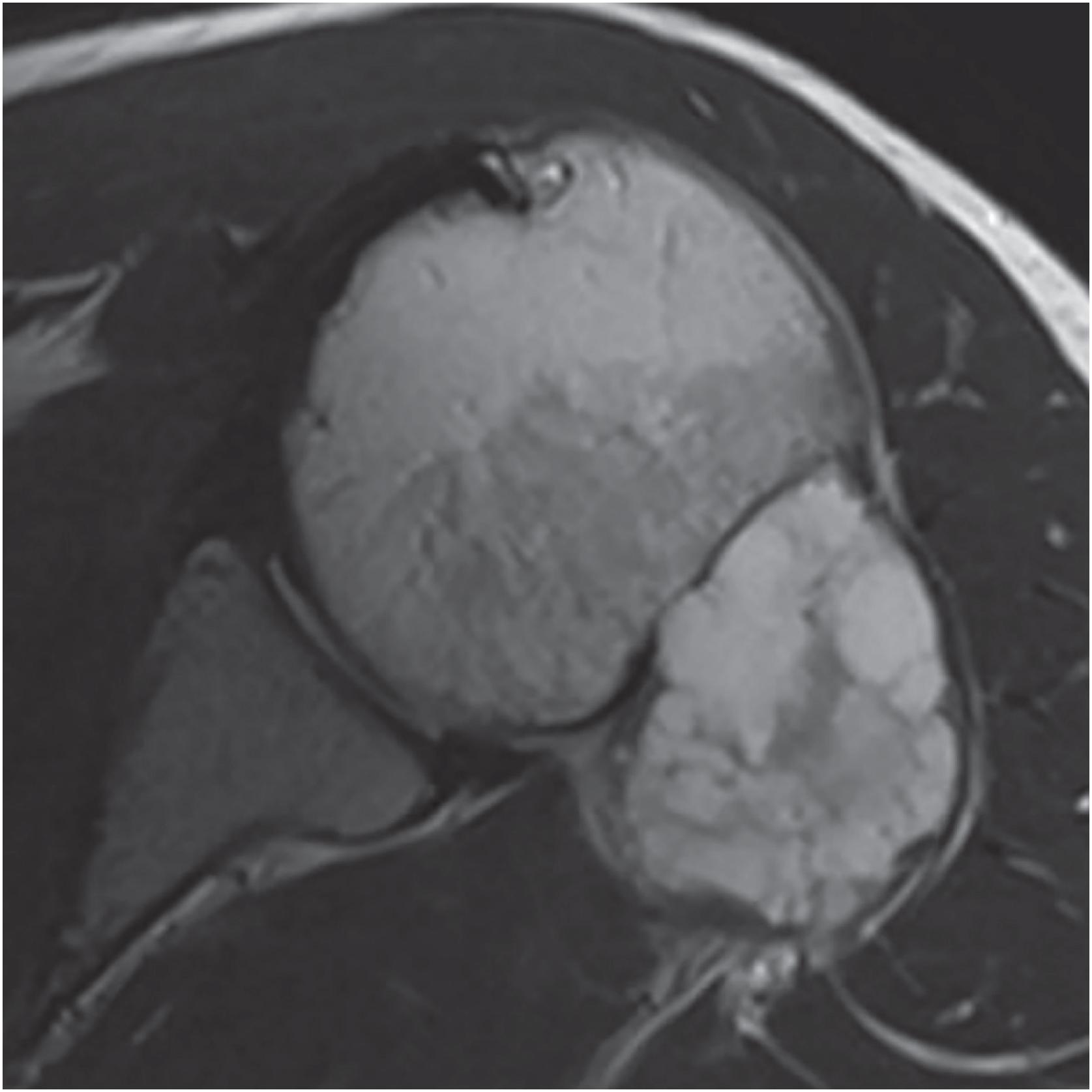
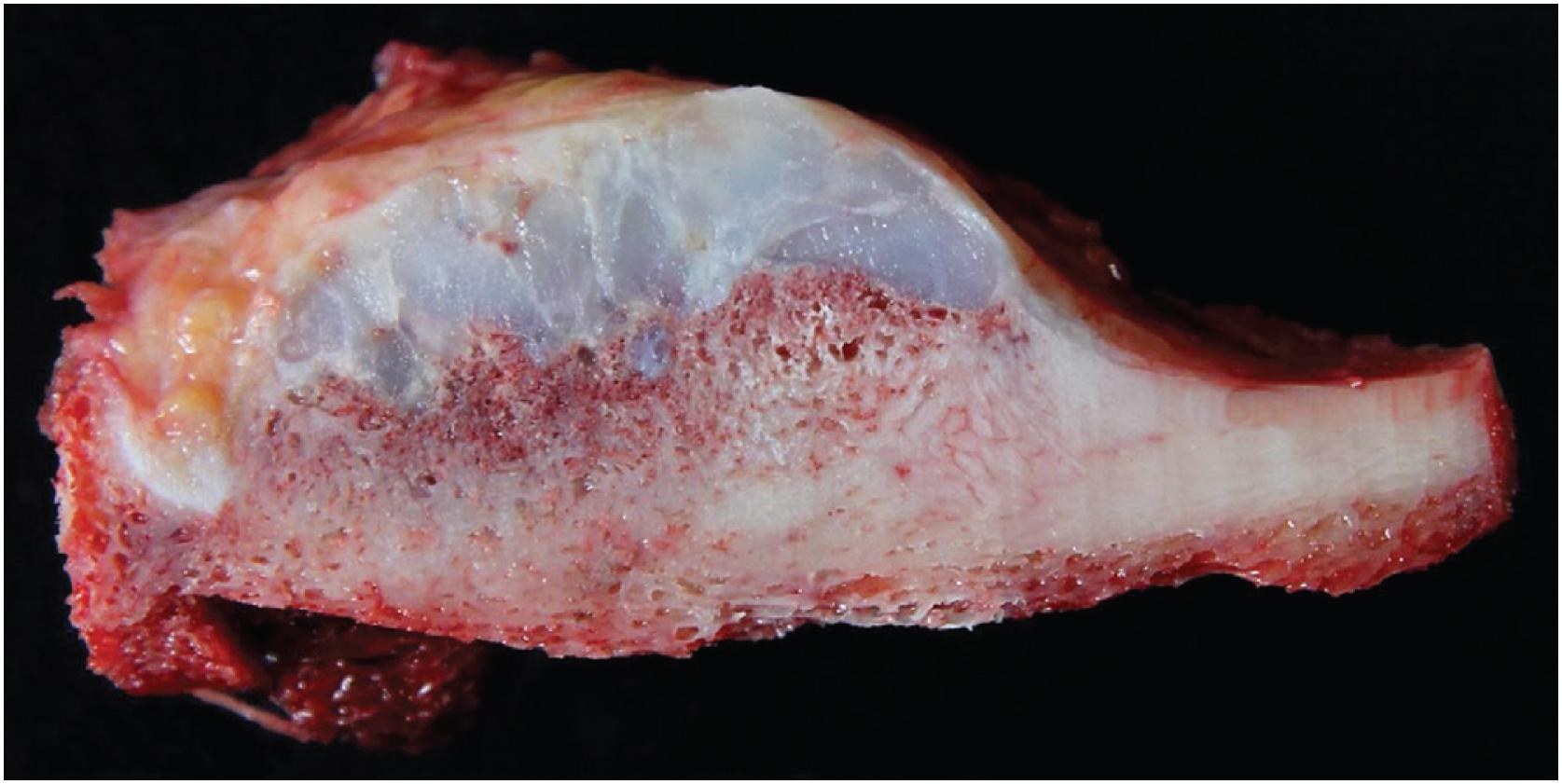
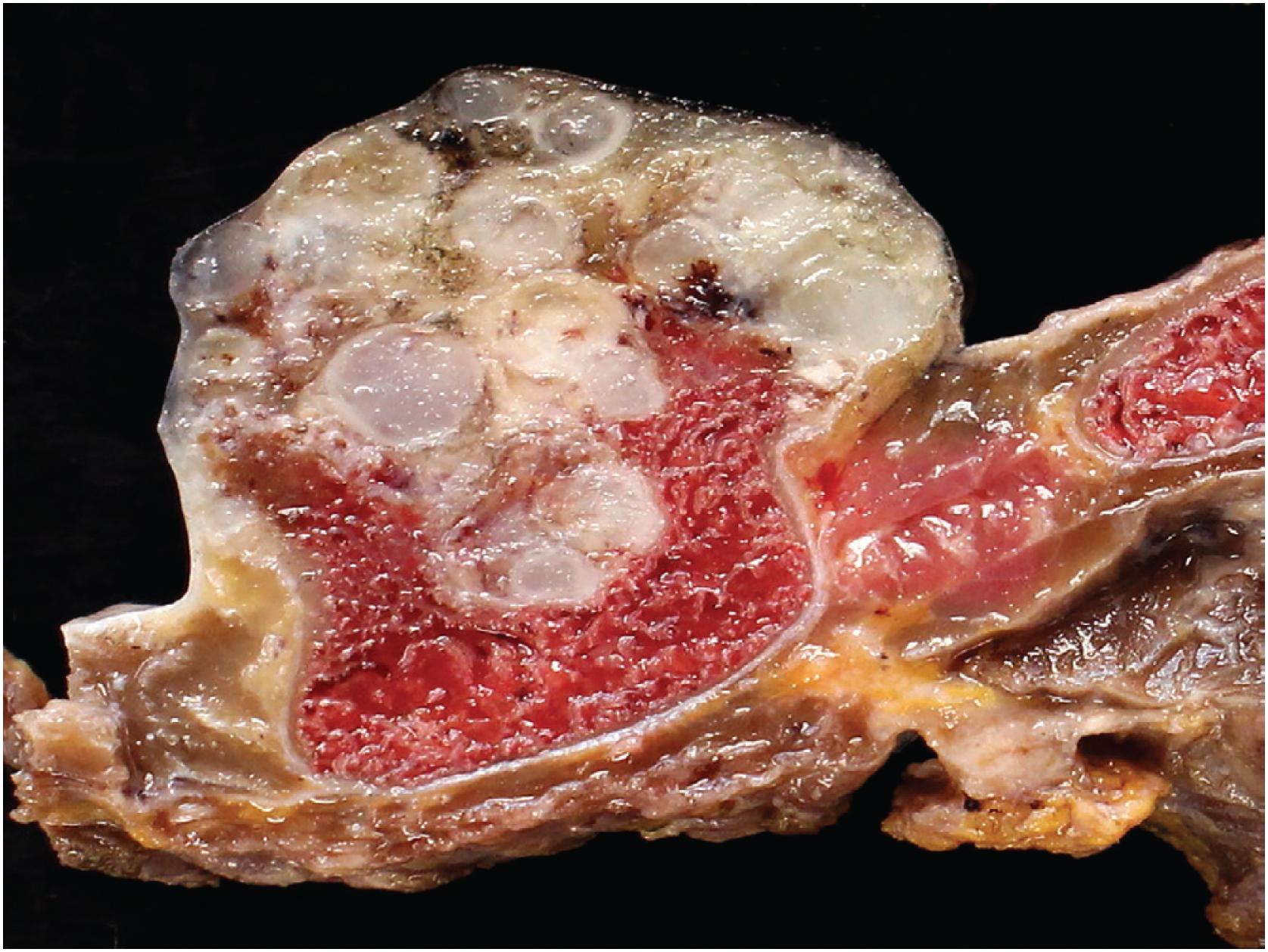
The mass is firm, translucent, and pale blue, indicative of hyaline cartilage. The interface between the tumor and the underlying cortex is sharp with a zone of sclerotic bone ( Figs. 17.26 and 17.27 ). The medullary cavity may be impinged upon my reactive endosteal bone but is not invaded.
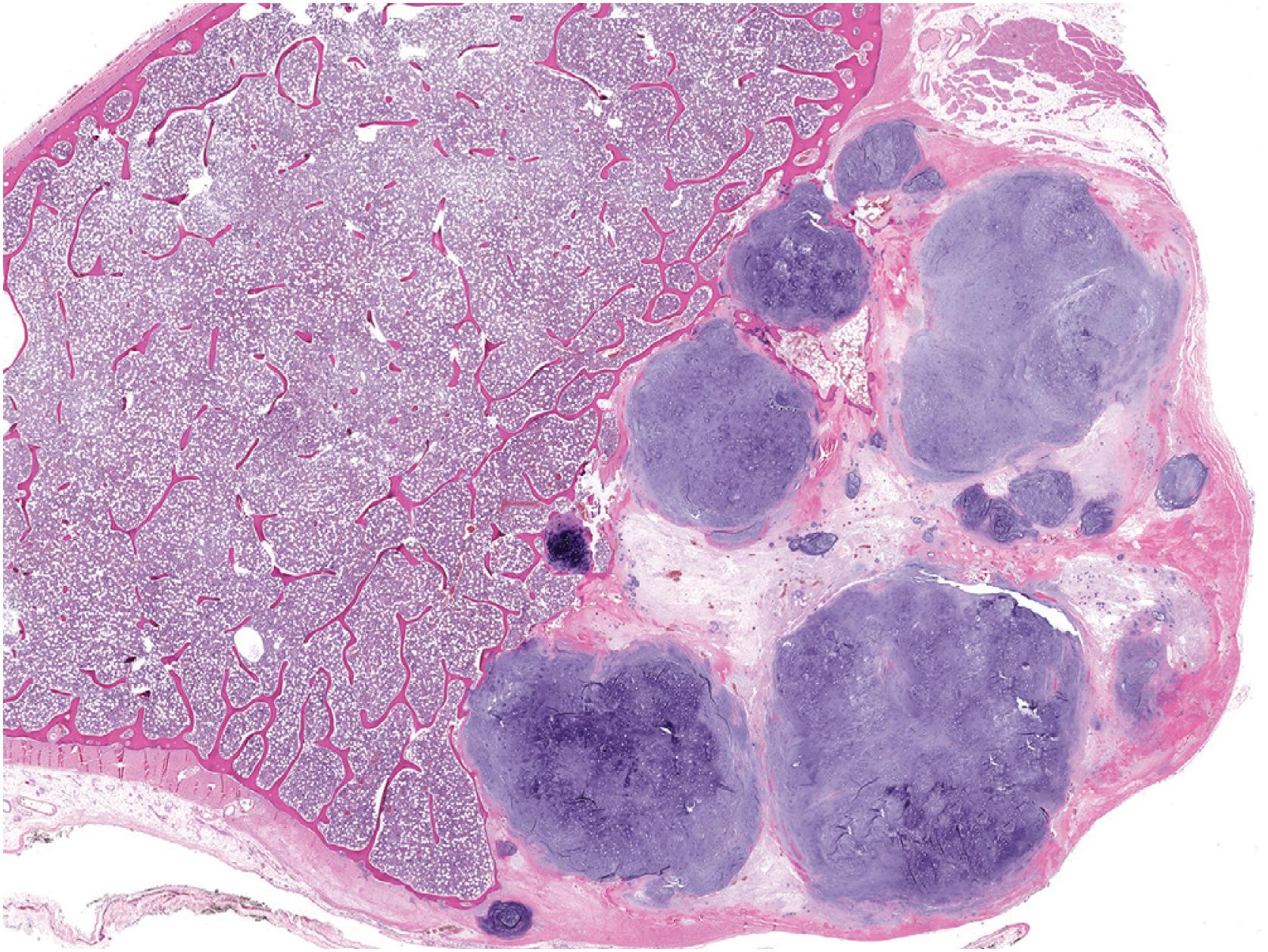
The tumor consists of lobules of hyaline cartilage of low-to-moderate cellularity ( Fig. 17.28 ). Focal myxoid matrix can be present. The tumor has a sharp interface with the underlying bone ( Fig. 17.29 ) and does not permeate Haversian canals or the medulla. Chondrocytes are generally bland but mild to moderate atypia, binucleated chondrocytes, and chondrocyte drop out ( Fig. 17.30 ) may be present. Rare examples can be hypercellular in comparison to enchondroma ( Fig. 17.31 ).

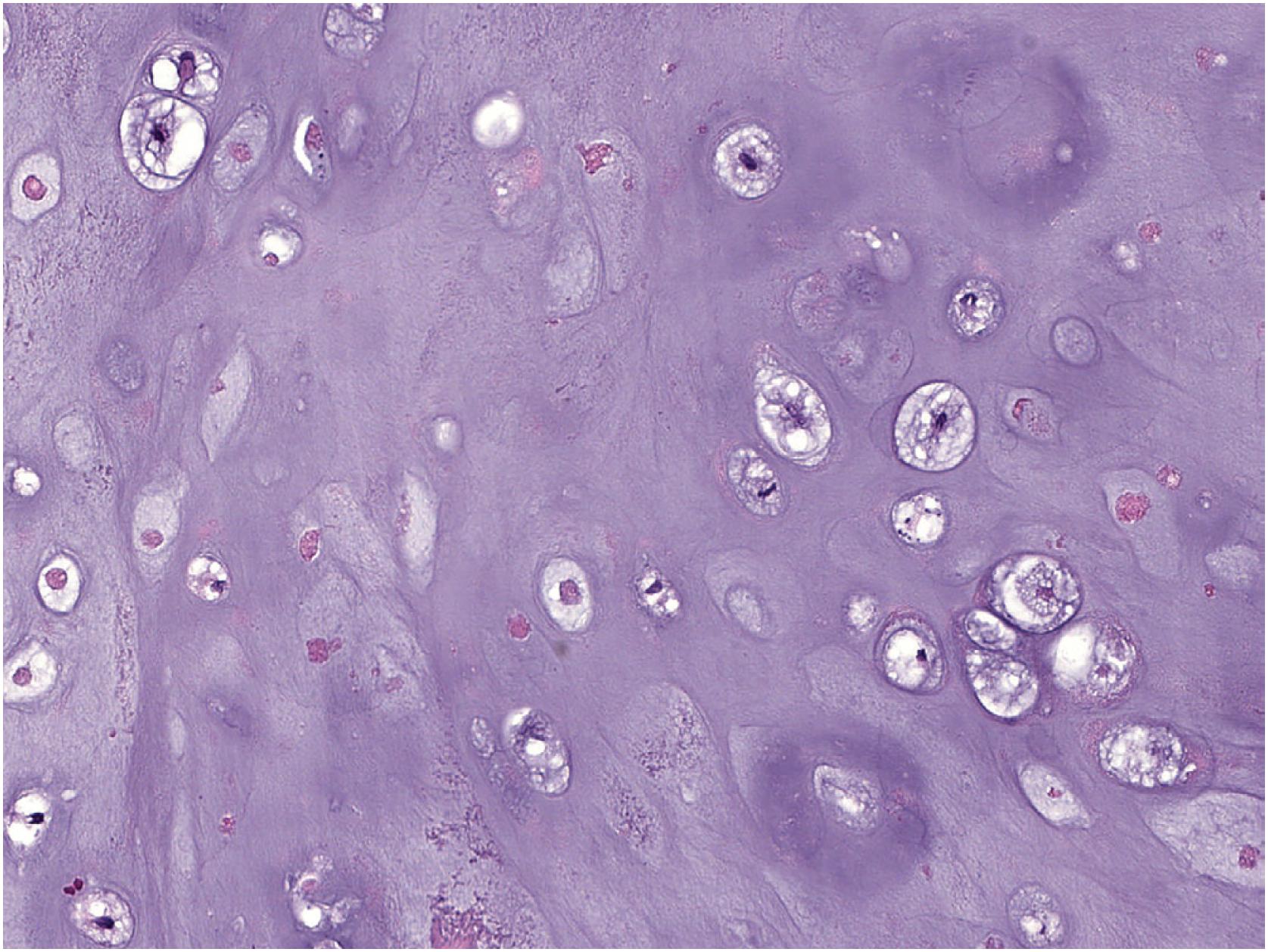
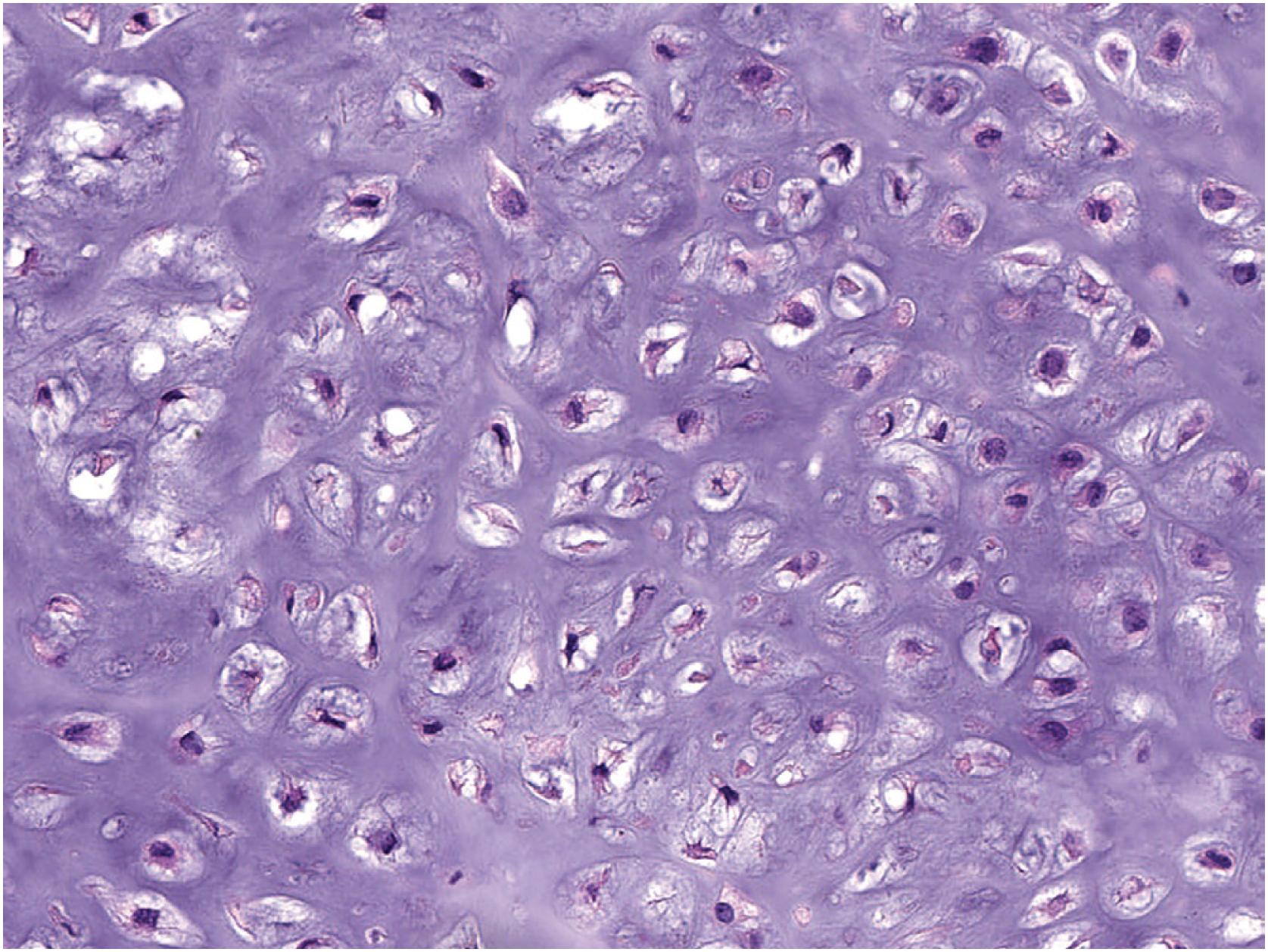
Immunohistochemistry is not helpful in the diagnosis.
Many periosteal chondromas harbor mutations in IDH1 . Chromosomal aberrations in 12q13-15 may also represent a recurring molecular event.
Periosteal (juxtacortical) chondrosarcoma demonstrates some clinical and morphologic overlap. Like periosteal chondroma, periosteal chondrosarcomas arise from the surface of the bone and may erode the cortex but usually does not involve the medulla. However, these tumors are typically large (>5 cm) with poorly defined margins and demonstrate greater cellularity and cytologic atypia. Infiltration of underlying bone is diagnostic of periosteal chondrosarcoma.
Periosteal osteosarcoma is a surface lesion that contains cartilage. However, it also contains neoplastic bone or osteoid production, typically in the center of the cartilage lobules, and surrounding spindle cells with significant cytologic atypia.
Parosteal osteosarcoma may contain a cartilaginous cap that is disorganized and may undergo endochondral ossification. Like periosteal osteosarcoma, it additionally contains malignant spindle cells and neoplastic bone.
Surgical removal is the treatment of choice by curettage or simple excision. Local recurrence is uncommon. Malignant transformation is exceedingly rare.
Benign tumor of cartilage that involves the surface of the bone
Most common in small bones of hands and feet and long bones, especially femur and humerus
More common in male than female sex (1.5:1); all ages involved with peak in second and third decades of life
Painless or painful mass present for years
Mass may be present for years
May be found incidentally on imaging for unrelated complaints
Asymptomatic lesions can be observed
Surgical excision or curettage is curative for symptomatic lesions
Small (1 to 3 cm), pale blue, firm cartilage attached to cortical surface
Sclerotic interface with cortex, no medullary involvement
Lobular cartilage of moderate cellularity, variable nuclear atypia
Periosteal chondrosarcoma
Periosteal osteosarcoma
Parosteal osteosarcoma
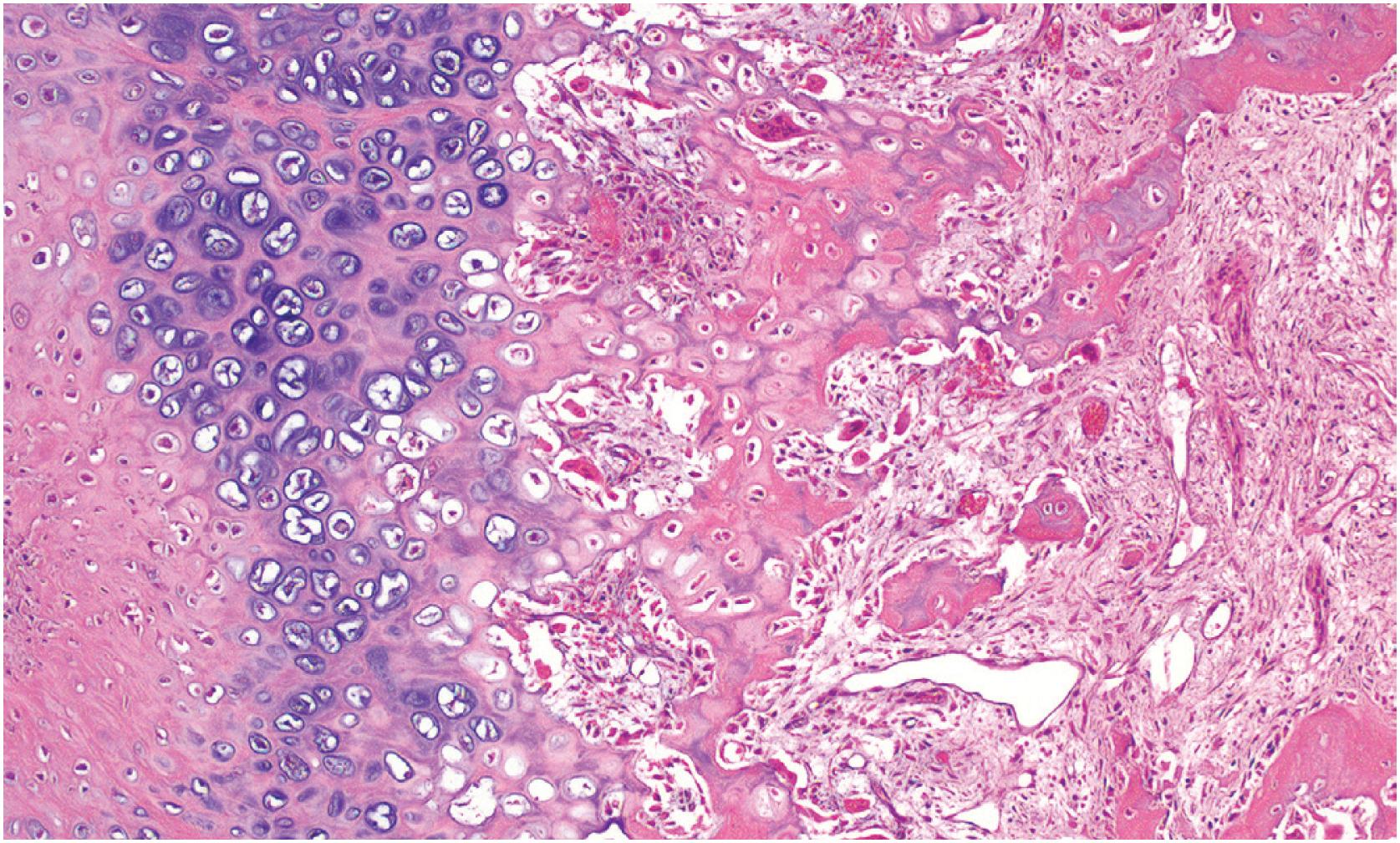
Chondroblastoma is a rare (<1% of primary bone tumors) benign chondroid matrix-producing tumor that affects the epiphyses (or apophyses) of skeletally immature individuals, adolescents, and young adults with a slight male predominance (1.5:1). It may occasionally present in older individuals. The most common complaint is several months to years history of pain involving a joint that may be accompanied by gait abnormality, swelling or decreased mobility. Although most chondroblastomas occur in the epiphyses of long bones, the small bones of the hands and feet are involved in approximately 10% of cases. A range of other sites, including temporal bone, ribs, the base of the skull, iliac crest, acetabulum, patella, and calcaneus, have also been reported. Almost all chondroblastomas are unifocal.
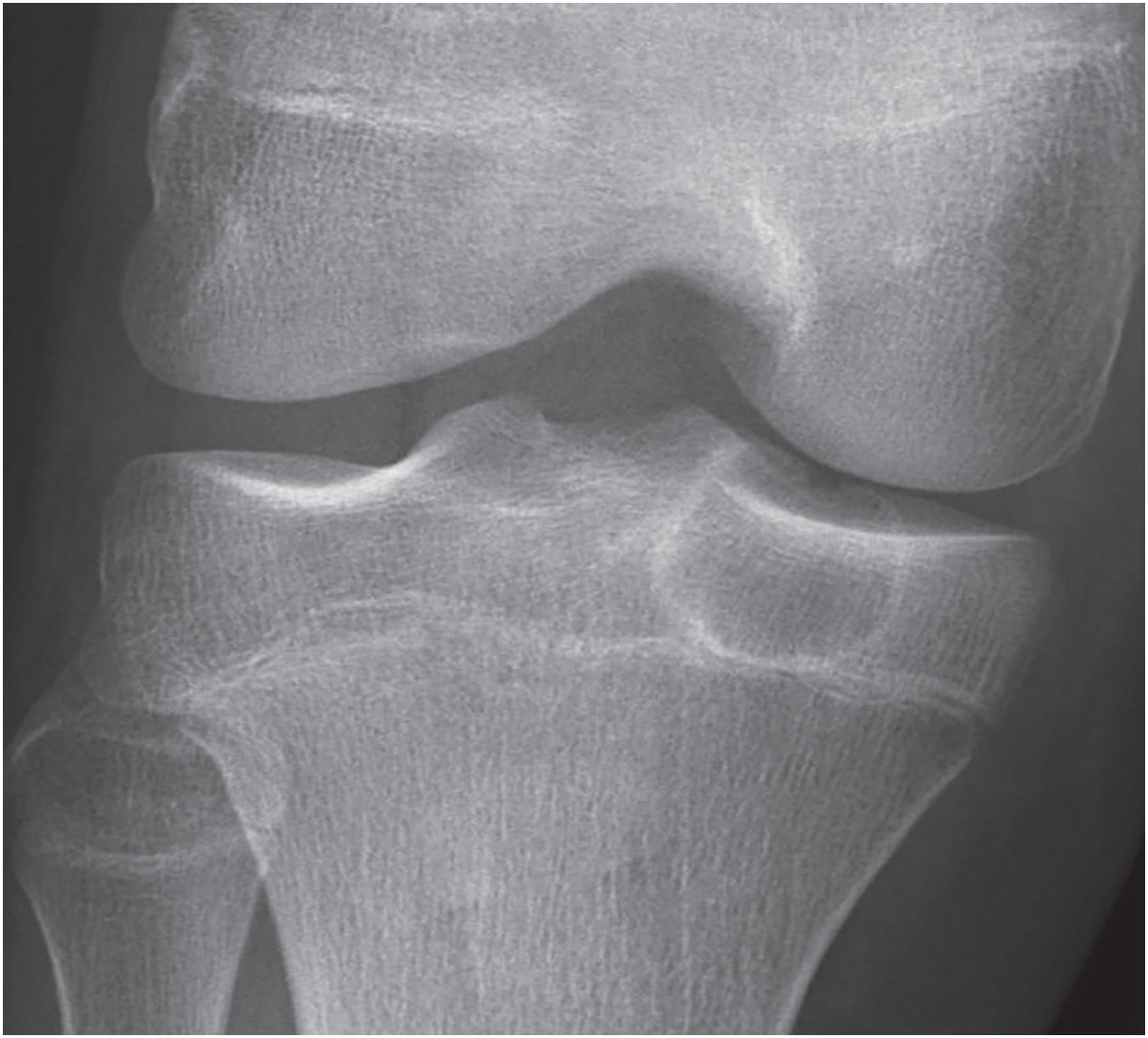
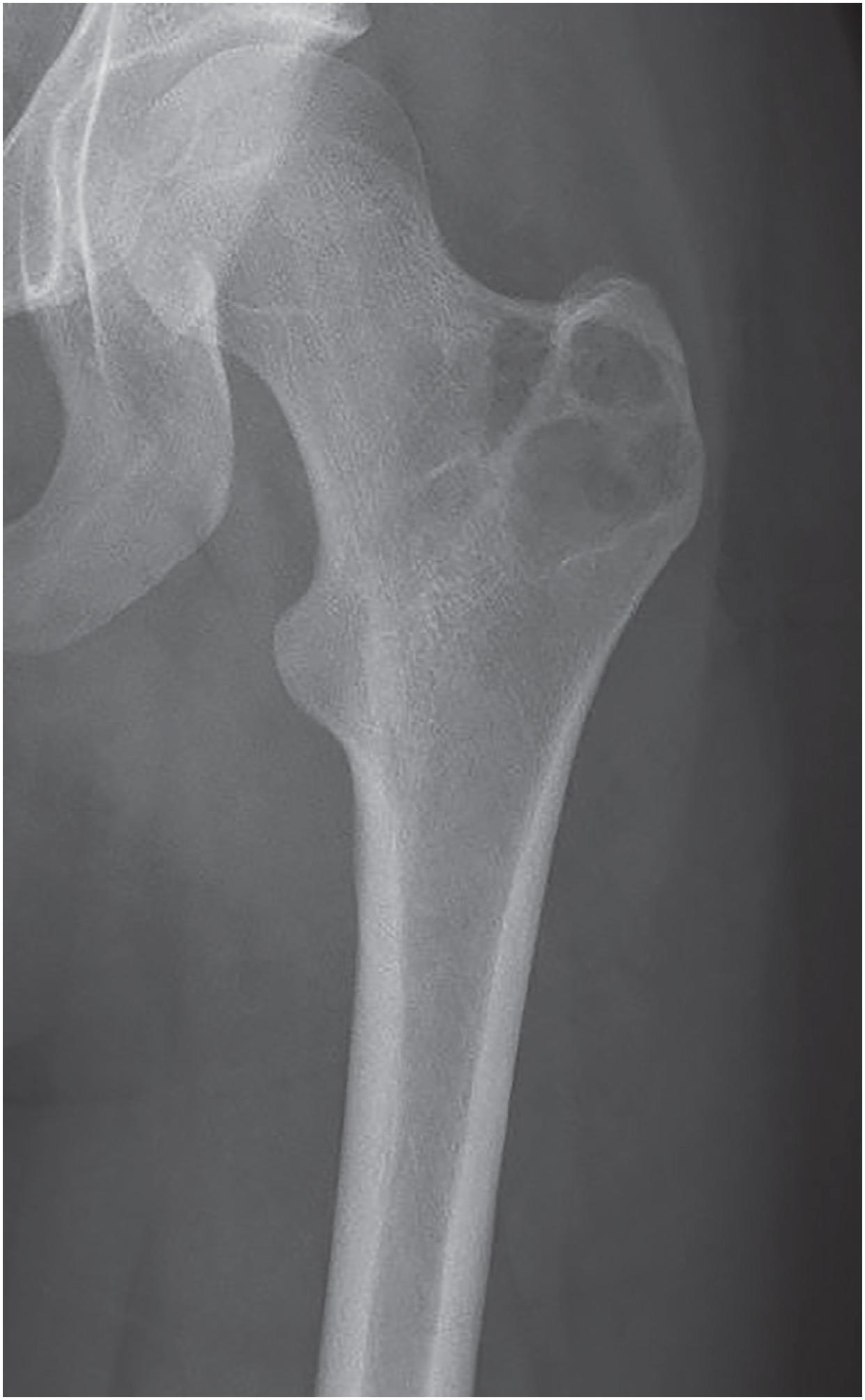
The lesion is sharply marginated, predominantly lytic, and rimmed by sclerotic bone ( Fig. 17.32 ). In long bones, it involves the epiphysis but may cross the physis into the metaphysis. Approximately 25% of cases are apophyseal ( Fig. 17.33 ). Periosteal reaction is uncommon. Matrix calcifications within the tumor are seen in 25% of cases. MRI often demonstrates tumor-associated reactive changes.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here