Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Cardiac resynchronization therapy (CRT) is a device-based, electrical therapy for heart failure (HF) that has been available in clinical practice for the last quarter century. Delivery of CRT via biventricular (BiV) pacing has been validated in multiple large-scale randomized controlled trials (RCTs) and CRT is one of the most well-studied device therapies in modern medicine. The efficacy of CRT has primarily been shown in promoting ventricular remodeling, reducing HF hospitalization, and reducing all-cause mortality, particularly when combined with defibrillator therapy.
Despite the considerable success of CRT, a substantial minority of patients do not demonstrate measurable benefit, which has invigorated investigations into new approaches to optimize response, including algorithms to synchronize fusion of pacing wavefronts, procedural techniques to deliver leads outside the constraints of coronary venous anatomy, and alternative pacing targets within the conduction system. This chapter provides an overview into the history of BiV pacing for CRT including a review of landmark trials and discusses new approaches to addressing the known limitations and response to BiV pacing.
Fundamentally, the physiologic rationale for CRT is to correct or ameliorate mechanical dyssynchrony arising because of electrical delay. The concept that a wide QRS may be associated with electromechanical dysfunction is not self-evident, and it has remained disputed for much of the 20th century. Although Wiggers had observed a slower rise of intraventricular pressure from artificial ventricular pacing in animals in 1925, he incorrectly speculated that intraventricular conduction delay (IVCD) would not significantly reduce overall cardiac output, although this may be because his initial models did not investigate complete conduction block. , Later experimental investigation in canine models with complete transection of the bundle branches identified “ventricular asynchronism” resulting in alterations in the observed waveforms of arterial or venous pulsation, suggesting a change in stroke volume from left bundle branch block (LBBB). Indeed, it was even argued in 1939 that asynchrony assessed by optical recording of pulsations should be a fundamental criterion for complete LBBB as distinct from nonspecific delayed excitation leading to wide QRS.
Despite these observations, the topic of asynchrony-induced ventricular dysfunction continued to be debated, and it was only after the development of more contemporary techniques, including cine left ventriculography, that it was definitively shown that left ventricular (LV) mechanical activation was altered in LBBB. Depressed aortic and pulse pressures noted at catheterization, and the echocardiographic observation of paradoxical septal motion in the 1970s, led to greater awareness of the potential for hemodynamic dysfunction. The concept of improvement or restoration of electromechanical synchrony with pacing had not yet been conceived.
In 1983, de Teresa and colleagues were the first to report a pacing strategy tailored to treat wide QRS morphology. They studied patients with LBBB undergoing aortic valve surgery and demonstrated that timing LV free wall pacing to fuse with intrinsic activation was associated the best ventricular performance. During the same period, simulation of LBBB morphology with atrioventricular (AV) right ventricular (RV) pacing was demonstrated to be associated with significantly reduced peak systolic pressure compared with atrial pacing in patients with narrow QRS. Visionaries of the field, Michel Mirowski and Morton Mower began formulating the concept of CRT as early as 1974 while developing canine models that later led to the invention of the implantable cardioverter defibrillator (ICD). They first performed BiV pacing intraoperatively in 1986 and noted increased urine output in the first 30 minutes post pacing, although without changes in blood pressure.
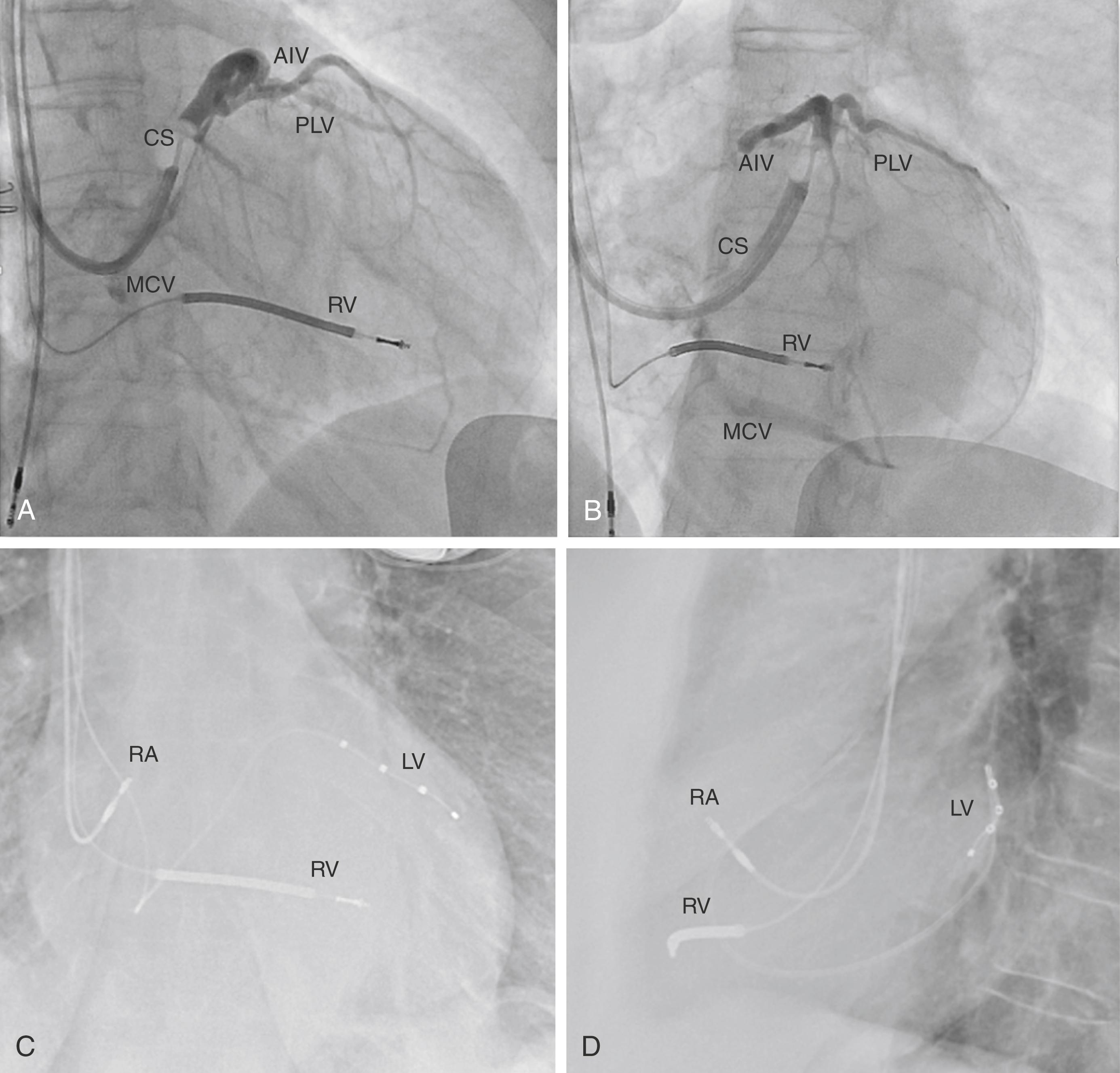
Morton Mower later filed a patent for a “method and apparatus for treating hemodynamic disfunction” in 1989. In it, he outlined a “pacer [which] is particularly suitable for treating bundle branch blocks or slow conduction in a portion of the ventricles” and formalized the concept for BiV pacing. Initial systems fashioned BiV systems using Y-adaptors connected to dual-chamber devices. Patricia Bakker, a cardiac surgeon, implanted the first permanent BiV system in 1993 and presented it as an abstract in 1994. Contemporaneously, Serge Cazeau, an electrophysiologist, published the first peer-reviewed case report in 1994 and provided the first systematic analysis of a series of patients in 1996, along with innovating on transvenous implantation techniques. Daubert reported coronary sinus (CS) lead implantation for resynchronization in 1998, and Auricchio described an initial over-the-wire technique, which later became the standard for BiV-CRT implants, and is still used in modern implants (see Fig. 125.1 for traditional intraprocedural venography and chest radiographs). , This period marked the beginning of an explosion of proof-in-concept and foundational research in the field of BiV pacing.
Early mechanistic work focused on evaluating the effect of BiV or left ventricle-only pacing on measures of cardiac output, reductions in pulmonary wedge pressure, improvement in maximal rate of LV pressure rise, and reductions in ventricular dyssynchrony. , Seminal work in Kass’ lab showed that LV or BiV pacing improved cardiac work at a diminished energy cost (i.e., reduction in MVO 2 ) in contrast to the effect of inotropes in HF. , These encouraging early physiologic proof-of-concept data paved the way for clinical trials of BiV in patients with HF.
Beginning in the late 1990s and early 2000s, enrollment started in a series of pivotal RCTs that studied the use of BiV pacemakers and defibrillators in patients with HF and wide QRS and established the efficacy of CRT with BiV. Landmark trials and their findings are summarized in Table 125.1 . Early work focused on patients with moderate and severe HF with New York Heart Association (NYHA) class III or IV symptoms. The Multisite Stimulation in Cardiomyopathies (MUSTIC) investigators were among the first to report a controlled, single-blind, crossover study of 58 NYHA class III patients with QRS width of 150 ms or greater who underwent successful BiV pacemaker implant and were randomized to either inactive or active pacing over two successive 12-week periods. In MUSTIC, patients walked significantly further while receiving BiV CRT (399 ± 100 m vs. 326 ± 134 m, P < .001), demonstrated improved quality of life as assessed by the Minnesota Living with Heart Failure questionnaire (32% improvement, P < .001), and demonstrated higher peak oxygen uptake (8% increase, P < .03). Importantly, hospitalizations were also decreased by two-thirds ( P < .05), and 85% of patients preferred assignment during BiV pacing.
| Trial | Year | N∗ | Intervention | Population | ECG Pattern | Follow-up (mo) | Outcomes |
|---|---|---|---|---|---|---|---|
| MUSTIC | 2001 | 67 | Crossover BiV pacing | NYHA III, LVEF ≤35%, LVEDD ≥60 mm, SR with QRS ≥150 ms | 87% LBBB | 6 | ↑ 6 MWD, ↑ QOL, ↑ pVO 2 , ↓ hospitalization, ↑ preference for BiV |
| MIRACLE | 2002 | 453 | BiV pacing vs. OMT | NYHA III or IV, LVEF 35%, LVEDD 55 mm, QRS 130 ms, 6MWD 450 m | NA | 6 | ↑ 6MWD, ↓ NYHA, ↑ QOL, ↑ exercise time, ↓ hospitalization and diuretic use |
| MUSTIC-AF | 2002 | 59 | Crossover BiV pacing | NYHA III, LVEF 35%, LVEDD 60 mm, persistent AF, RV paced QRS duration >200 ms, 6MWD 450 m | 100% RV paced | 6 | ↑ 6MWD, ↑ pVO 2 , ↓ hospitalization, ↑ preference for BiV |
| PATH-CHF | 2002 | 41 | Crossover BiV pacing | NYHA III or IV, SR ≥55 bpm with QRS 120 ms, PR ≥150 ms | 87% LBBB, 13% RBBB | 12 | ↑ pVO 2 , ↑ 6MWD |
| MIRACLE ICD | 2003 | 369 | BiV-ICD on vs. off | NYHA III or IV, LVEF 35%, LVEDD 55 mm, QRS 130 ms, cardiac arrest caused by ventricular arrhythmia | 13% RBBB | 6 | ↑ QOL, ↓ NYHA, ↔ 6MWD, ↑ pVO 2 , ↑ exercise duration, ↔ LV size or function, survival, or hospitalization |
| VENTAK CHF/CONTAK CD | 2003 | 490 | BiV-ICD on vs. off | NYHA II–IV, LVEF 35%, QRS >120 ms, ICD indication | 54% LBBB, 14% RBBB, 32% IVCD | 6 | ↑ pVO 2 , ↑ 6MWD, ↔ QOL and NYHA, ↓ LV dimensions, ↑ LVEF |
| MIRACLE-ICD II | 2004 | 186 | BiV-ICD on vs. off | NYHA II, LVEF 35%, LVEDD 55 mm, QRS 130 ms, ICD indication | 17% RBBB | 6 | ↔ pVO 2 , ↑ VE/VCO 2 , ↓ LV dimensions, ↑ LVEF, ↑ clinical composite |
| COMPANION | 2004 | 1520 | BiV pacing vs. BiV-ICD vs. OMT | NYHA III–IV, LVEF 35%, QRS >120 ms, PR ≥150 ms, SR, no clinical indication for pacemaker or ICD | 70% LBBB, 9% RBBB | 12 | ↓ Primary end point (time to death or HF hospitalization), ↓ any cause death in BiV-ICD but not BiV-pacing ( P = .059) |
| CARE-HF | 2005 | 813 | BiV pacing vs. OMT | NYHA III–IV, LVEF 35%, LVEDVI ≥30, QRS >120 ms (QRS 120–149 with dyssynchrony), no clinical indication for pacemaker or ICD | ∼90% LBBB | 29.4 | ↓ Primary end point (time to death or unplanned hospitalization), ↓ death, ↓ mechanical delay, ↓ LV volumes, ↓ MR, ↑ LVEF, ↑ QOL |
| REVERSE | 2008 | 610 | BiV ± ICD on vs. off | NYHA I–II, LVEF 40%, LVEDD ≥55, QRS >120 ms, no AF within 30 days | 60% LBBB, 40% non-LBBB | 12 | ↔ Primary end point (HF clinical composite worsened), ↓ hospitalization, ↓ LV volumes |
| MADIT-CRT | 2009 | 1820 | BiV-ICD vs. ICD only | NYHA I–II, LVEF 30%, QRS 130 ms | 70% LBBB, 13% RBBB, 17% IVCD | 28.8 | ↓ Primary end point (time to death or HF event), ↔ death, ↓ LV volumes, ↑ LVEF |
| RAFT | 2010 | 1798 | BiV-ICD vs. ICD only | NYHA II or III, LVEF 30%, QRS 120 ms, or paced QRS of 200 ms | 71% LBBB, 9% RBBB, 12% IVCD, 8% paced | 40 | ↓ Primary end point (time to death or HF hospitalization), ↓ death, ↑ adverse events |
| BLOCK-HF | 2013 | 691 | BiV ± ICD vs. ICD or pacing only | NYHA I–III, LVEF 50%, standard class I or IIa indication for pacing caused by atrioventricular block | 33% LBBB, 21% RBBB | 37 | ↓ Primary end point (time to death, IV diuresis for HF, or 15%↑ LVESVi) and clinical components of primary outcome |
| EchoCRT | 2013 | 809 | BiV-ICD on vs. off | NYHA III or IV, LVEF 35, QRS <130 ms with echo evidence of LV dyssynchrony, LVEDD 55 mm | NA | 19.4 | ↓ Primary end point in BiV-on (death or HF hospitalization), ↑ death |
The Multicenter InSync Randomized Clinical Evaluation (MIRACLE) trial was the first large RCT of BiV for CRT in NYHA class III and IV patients with QRS of 130 ms or greater. A total of 453 patients underwent device implant, 228 randomized to BiV pacing and 228 assigned to no pacing. MIRACLE confirmed and amplified the results of MUSTIC; patients demonstrated significant improvements in the 6-minute walking test, functional class, quality of life, time on treadmill during exercise testing, and improved peak oxygen consumption. Echocardiography demonstrated an increase in LV ejection fraction (LVEF) (4.6% vs. −0.2% in controls, P < .001), and favorable ventricular remodeling including greater reductions in LV end-diastolic dimension with reduction in mitral regurgitant jet area in those randomized to BiV-CRT. HF hospitalization was reduced (8% vs. 15%) and use of intravenous therapy for exacerbation (7% vs. 15%) ( P < .05 for both comparisons).
A number of other clinical studies reported consistent findings of improved functional class and exercise capacity after BiV-CRT, and reporting measures of improved ventricular remodeling (e.g., MUSTIC-AF, MIRACLE ICD, VENTAK/CONTAK-CD, PATH-CHF, PATH-CHF II, and MIRACLE ICD II ). Notably, however, the effect of BiV-CRT on hard outcomes such as HF hospitalization and mortality were either not measured, not the primary end point, or did not reach statistical significance in these studies.
The Comparison of Medical therapy, Pacing, and Defibrillation in Heart Failure (COMPANION) study was the largest comparison of BiV with or without ICD and had a primary composite end point of time to death or hospitalization for any cause. In COMPANION, 1520 patients were randomly assigned, in a 1:2:2 ratio, to receive either optimal pharmacologic therapy (OPT) and a BiV-pacemaker device, or OPT and a combination BIV and defibrillator device. COMPANION demonstrated that BIV-CRT decreased the primary end point of time to any-cause hospitalization or death in both patients assigned to BiV pacing only and BiV defibrillation-capable devices. There were reductions in mortality in those assigned to BiV that approached significance for those with BiV-pacemakers (24% reduced all-cause death, P = .059) and achieved significance in patients receiving BiV-ICDs (36% reduction in all-cause mortality, P = .003).
One year later, the Cardiac Resynchronization–Heart Failure (CARE-HF) trial was the first and only randomized trial to demonstrate improved survival with BiV-CRT without a defibrillator. The landmark study enrolled 813 patients with NYHA class III and IV HF symptoms and QRS of 120 ms or greater, with longer median QRS complexes of 160 ms (interquartile range [IQR], 152–180 ms). BiV patients had a significant reduction in death from any cause or unplanned cardiovascular hospitalization (hazard ratio [HR], 0.63; 95% confidence interval [CI], 0.51–0.77; P < .001) and a significant reduction in all-cause mortality (HR, 0.64; 95% CI, 0.48–0.85; P < .002). BiV-CRT was associated with significant improvement in measures of echocardiographic remodeling, including reduction in LV end-systolic volume index (LVESVI), increases in LVEF and systolic blood pressure, with significant reduction in N-terminal pro brain natriuretic peptide (NT-proBNP) and reduction in mitral regurgitant area.
As studies began addressing whether BiV pacing might alter the natural history of HF in early-stage disease, the MIRACLE ICD II trial was the first large double-blind, parallel-controlled RCT to investigate the effect of BiV-CRT in patients with NYHA class II symptoms; 101 patients were randomized to CRT-off and 85 to CRT-on. Despite no significant improvement in peak VO 2 at 6 months, significant improvement in LV volumes and LVEF were observed.
The Resynchronization Reverses Remodeling In Systolic Left Ventricular Dysfunction (REVERSE) study group was the first study to explore outcomes after BiV-CRT in asymptomatic (NYHA class I) and mildly symptomatic (NYHA class II) patients with QRS of 120 ms or greater in a large RCT. A total of 610 patients underwent device placement and were randomized in a 2:1 fashion to receive active BiV-CRT (i.e., CRT-on). The primary end point was an HF clinical composite response. Because NYHA class I patients were included in the study and their functional status could not improve, the study was designed to compare the efficacy of therapy by testing the difference in percentage of patients who worsened while BiV-CRT was on, and this was not significantly different between the two groups (16% worsened with CRT-on vs. 21% with CRT-off, P = .10). Importantly, however, time to first HF hospitalization was significantly delayed in CRT-on (HR, 0.47; P = .03) patients. Echocardiographic remodeling, including significant reductions in LVESVI, reduction in LV end-diastolic volume index (LVEDVI), and increase in LVEF were noted in the CRT-on group.
The Multicenter Automatic Defibrillator Implantation Trial with Cardiac Resynchronization Therapy (MADIT-CRT) trial was the largest study to explore the role of BiV-CRT in NYHA Class I and II patients with QRS of 130 ms or greater, enrolling 1820 patients in a 3:2 ratio to combined CRT-ICD (i.e., CRT-D) or ICD-only systems. The trial was stopped early because of the significant superiority of CRT-D therapy. Although the primary end point was a composite of death from any cause and nonfatal HF events, overall mortality was low (3% annual mortality rate over an average follow-up of 2.4 years). The benefit conferred from BiV-CRT in this group was driven by a 41% reduction in HF events among patients assigned to CRT-D. Similar to REVERSE, significant reductions in LVESV and LVEDV and rise in LVEF were noted in the BiV-CRT group.
The most recent large-scale randomized trial to evaluate the effect of CRT-D versus ICD only was the Resynchronization-Defibrillation for Ambulatory Heart Failure Trial (RAFT), which evaluated NYHA class II or III HF with QRS of 120 ms or greater. The primary end point was death from any cause or HF hospitalization, which was significantly reduced in patients receiving BiV-CRT (HR, 0.75; 95% CI, 0.64–0.87; P < .001). Unlike REVERSE and MADIT-CRT, all-cause mortality was significantly reduced in RAFT (HR, 0.75; 95% CI, 0.62–0.91; P = .003). Importantly, however, the authors noted a significantly increased risk of adverse events, particularly lead dislodgement, in patients receiving CRT-D. Together with the findings of REVERSE and MADIT-CRT, however, RAFT solidified the role of BiV-CRT in patients with even mildly symptomatic HF and wide QRS. RAFT and other landmark trials summarized in Table 125.1 , led to the updated guidelines for CRT that are noted in Table 125.2 .
| Society | Criteria | COR/LOE |
|---|---|---|
| 2012 ACC/AHA/HRS Focused Update of the 2008 Guidelines for Device-Based Therapy of Cardiac Rhythm Abnormalities | LVEF ≤35%, NSR, LBBB, QRS ≥150 ms, NYHA III/IV on GDMT | I A |
| LVEF ≤35%, NSR, LBBB, QRS ≥150 ms, NYHA II on GDMT | I B | |
| LVEF ≤35%, NSR, LBBB, QRS 120–149 ms, NYHA II–IV on GDMT | IIa B | |
| LVEF ≤35%, NSR, non-LBBB, QRS ≥150 ms, NYHA III/IV on GDMT | IIa A | |
| LVEF ≤35% and AF, if it requires ventricular pacing and otherwise meets CRT criteria or AV node ablation or pharmacologic rate control will allow near 100% ventricular pacing | IIa B | |
| LVEF ≤35% and undergoing device new implant or replacement with anticipated >40% ventricular pacing | IIa C | |
| LVEF ≤35%, ICM, NSR, LBBB, QRS ≥150 ms, NYHA I on GDMT | IIb C | |
| LVEF ≤35%, NSR, non-LBBB, QRS 120–149 ms, NYHA III/IV on GDMT | IIb B | |
| LVEF ≤35%, NSR, non-LBBB, QRS ≥150 ms, NYHA II on GDMT | IIb B | |
| 2013 ESC/EHRA Guidelines on cardiac pacing and cardiac resynchronization therapy | LVEF ≤35%, NSR, LBBB, QRS >150 ms, NYHA II–IV | I A |
| LVEF ≤35%, NSR, LBBB, QRS 120–150 ms, NYHA II–IV | I B | |
| LVEF ≤35%, NSR, non-LBBB, QRS >150 ms, NYHA II–IV | IIa B | |
| LVEF ≤35%, NSR, non-LBBB, QRS 120–150 ms, NYHA II–IV | IIb B | |
| LVEF ≤35%, permanent AF, QRS ≥120, NYHA III–IV, if close to 100% BiV pacing can be achieved or AV junction ablation performed for either incomplete pacing or uncontrolled heart rates | IIa B | |
| 2018 ACC/AHA/HRS Guideline on the Evaluation and Management of Patients with Bradycardia and Cardiac Conduction Delay | LVEF 36%–50%, AV block with indication for permanent pacing who are expected to require >40% ventricular pacing, it is reasonable to consider CRT or HBP | IIa B SR |
| LVEF 36%–50%, LBBB, QRS ≥150 ms | IIb C | |
| 2019 ESC/AEPC Guidelines for the management of patients with supraventricular tachycardia | AV nodal ablation with subsequent pacing (“ablate and pace”), either BiV or HBP if tachycardia responsible for TCM cannot be ablated or controlled by drugs | I C |
| AV nodal ablation followed by pacing (preferably biventricular or HBP) for LV dysfunction caused by recurrent multifocal AT refractory to drug therapy | IIa C | |
| AV nodal ablation with subsequent pacing, either BiV or HBP if catheter ablation or drug therapy fails and patient has symptomatic persistent macroreentrant atrial arrhythmias with fast ventricular rates | IIa C |
The premise of BiV for CRT was established as a means to correct intraventricular conduction disturbance causing delayed or dyssynchronous electrical and mechanical activation of the left ventricle. From first principles, BiV pacing is not expected to lead to hemodynamic or clinical improvements in patients without mechanical dyssynchrony or left-sided delay at baseline. The proxy for left-sided delay in early trials was primarily QRS width, and although the inclusion criterion for initial RCTs of BiV-CRT was QRS duration of 120 ms or greater in most studies, the mean QRS of enrolled patients was substantially longer (between 155 and 209 ms). Indeed, in early BiV studies the majority of patients demonstrated LBBB or wide IVCD with LBBB-like features. , Patients with right bundle branch block (RBBB) were typically excluded.
In 2001, the Bordeaux group were the first to specifically report on the use of BiV-CRT in 12 subjects with wide RBBB (mean 189 ± 26 ms) in whom it was suspected that RBBB might be masking concomitant left-sided delay, which was first reported in 1954. Decades later, in an elegant electroanatomic mapping study reported in 2005, Fantoni and colleagues clearly demonstrated that some subtypes of RBBB, particularly when present with broad or notched R waves in leads I and aVL, demonstrated left-sided electrophysiologic delay similar to patients with LBBB. Importantly, other patients with “isolated” RBBB did not show any significant left-sided delay, , and it would be unlikely that BiV pacing would be helpful for this group. The translation of QRS morphology to guidelines for BiV-CRT changed slowly with increasing data from large RCTs. Subgroup analysis of MIRACLE was the first RCT assessment of the effect of BiV among patients with RBBB and IVCD. Results were mixed from this initial study: Improvement in NYHA functional class was noted in RBBB but not IVCD, but improvements in survey-assessed quality of life were noted in IVCD but not RBBB patients. Changes in the 6-minute walk test, exercise time, and measured oxygen consumption numerically trended toward benefit for both groups relative to control, but it did not reach significance. NYHA functional class and quality of life improvements were not significantly different in LBBB versus RBBB in MIRACLE-ICD, although subsets were not separately reported.
COMPANION explored the effect of BiV-CRT on hard clinical outcomes in subgroups of patients with non-LBBB pattern at baseline receiving BiV or BiV-ICD systems, demonstrating numerical reductions in time to first hospitalization and death from any cause that did not reach statistical significance. CARE-HF enrolled a relatively low proportion of RBBB (∼5% of patients), and they were more likely to demonstrate HF hospitalization or death. The findings that patients with non-LBBB patterns were less likely to benefit were consistent across REVERSE, MADIT-CRT, and RAFT. , Reduction in LV volumes and improvement in LVEF were observed in LBBB but not in RBBB or IVCD patients in REVERSE. A trend toward reduction in hospitalization and death was noted in non-LBBB patients, but it did not reach significance.
In contrast, secondary analyses of MADIT-CRT suggested more starkly that there was no clinical benefit derived from BiV in patients with RBBB and IVCD, and observed a nonsignificant increased risk of the primary end point of HF event and death in patients with non-LBBB, particularly driven by the IVCD group. In longer-term follow-up, a trend toward harm was noted in non-LBBB patients (adjusted HR for death, 1.57; 95% CI, 1.03–2.39; P = .04). However, patients with non-LBBB in MADIT-CRT had significantly narrower QRS at baseline than patients with LBBB (163 ± 19 ms LBBB, 153 ± 15 ms RBBB, 142 ± 14 ms IVCD; P < .01 for comparisons vs. LBBB), and the authors did not report on the outcomes of non-LBBB stratified by QRS width. Investigators from RAFT similarly found that patients with non-LBBB (specifically RBBB and IVCD) were less likely to benefit from CRT, but they further noted that patients with non-LBBB who demonstrated a QRS of 160 ms or greater demonstrated a reduction in the risk of the primary end point of HF hospitalization or death (HR, 0.52; 95% CI, 0.29–0.96; P = .033). Contemporaneous with the publication of subgroup analyses from large CRT and meta-analysis showing lack of benefit in non-LBBB, data from large registries of Medicare patients showed similar findings (compiling data from 14,946 and 24,169 patients, respectively) of increased risk of HF hospitalization and all-cause death in patients with IVCD, RBBB, and non-LBBB. ,
In light of the data from RCTs and emerging real-world data, the American College of Cardiology Foundation (ACCF), American Heart Association (AHA), and the Heart Rhythm Society (HRS) issued a guidelines update in 2012 that downgraded BiV-CRT in patients with non-LBBB to a class IIa indication (Level of Evidence A) for patients with QRS width of 150 ms or greater, sinus rhythm, and NYHA class III–ambulatory IV symptoms. BiV-CRT in non-LBBB patients with NYHA class II symptoms and QRS width of 150 ms or greater or patients with NYHA class III–ambulatory IV symptoms and QRS width 120 to 149 ms were downgraded to a class IIb indication (Level of Evidence B).
More recently, ENHANCE-CRT was the first double-blind trial of CRT enrolling exclusively non-LBBB patients. The study evaluated conventional LV lead placement versus that targeted by electrical delay (i.e., based on the QRS to sensed LV time [QLV]). Responder rate by a clinical composite score was observed in 67.2% in the QLV arm and 73% in the control arm, without any differences with targeted guidance by QLV. Although these outcomes in non-LBBB patients cannot be directly compared with patients with LBBB (that comparator was not enrolled), the overall outcomes suggest favorable response rates in non-LBBB patients.
Recent large registry data also confirm that patients with non-LBBB (i.e., nonspecific IVCD and RBBB) who receive BiV devices have improved clinical outcomes relative to those receiving ICD only therapy. However, recent consensus remains cautious on the use of BiV-CRT in non-LBBB patients, and if pursued, with close attention to clinical performance and optimization in follow-up. The role of optimization strategies as a means to further augment response overall in BiV-CRT is discussed later in this chapter.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here