Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Atrial fibrillation (AF) is the most common sustained cardiac arrhythmia, with an estimated prevalence of 2% among unselected adults, an increasing prevalence with each decade of life, and a twofold higher risk in men relative to women. , The three most common symptoms directly attributed to AF are impaired exercise tolerance caused by an inappropriate heart rate response during exertion, palpitations caused by an irregular or rapid pulse, and lightheadedness or presyncope often caused by post-AF conversion pauses. Restoring and maintaining sinus rhythm effectively addresses these symptoms.
Embolic strokes and heart failure are additionally known to complicate the clinical course of many patients with AF. Although still debated, a growing body of evidence suggests an association between AF burden and the cumulative incidence of these two major complications. , Accordingly, the natural progression of AF from paroxysmal to persistent forms is associated with increasing morbidity and mortality. , Achieving durable sinus rhythm can prevent this progression and therefore perhaps its associated risks.
Multicenter randomized trials have demonstrated the superiority of catheter ablation over antiarrhythmic drugs in two ways: (1) sinus rhythm maintenance, with recent data from continuous rhythm monitoring demonstrating a dramatic decrease in arrhythmia burden in patients with paroxysmal AF, and (2) reduction in cardiac hospitalization and likely mortality, with the latter outcome primarily shown in populations with heart failure, whereas it was only a secondary end point in less selected populations. ,
Current guidelines therefore recommend that catheter ablation be considered after failed antiarrhythmic drugs and that it is reasonable as a first-line treatment in symptomatic patients with or without heart failure. However, given the expected natural progression of AF to increasingly persistent forms, which are more difficult to successfully ablate and which have been associated with deleterious long-term outcomes, questions remain regarding the optimal management, particularly of asymptomatic patients with persistent AF.
During the second half of the 20th century, our understanding of AF mechanisms was dominated by the model of multiple reentrant wavelets described by Moe and coworkers, which was further supported by the experimental works of Allessie and associates, which introduced the leading circle theory: the wavelength (WL) is the shortest path length (PL) able to sustain reentry in a cardiac tissue with a given refractory period (RP) and conduction velocity (CV), according to the simple formula WL = RP × CV. , If the PL of the electrical reentry is shorter than the theoretical WL of the tissue, the activation front will return to its starting point while that point is still refractory, thus halting its propagation. This concept relied on two conditions: (1) the reentrant wavelets are functional and generated in the three dimensions of the atrial tissue, ignoring anatomic obstacles, , and (2) the critical number of reentrant wavelets necessary for AF perpetuation depends on atrial size and WL. In this framework, the number of wavelets needed for AF perpetuation requires that the atrial tissue should have a very short RP and a very fast CV, characteristics that are not observed at basal states.
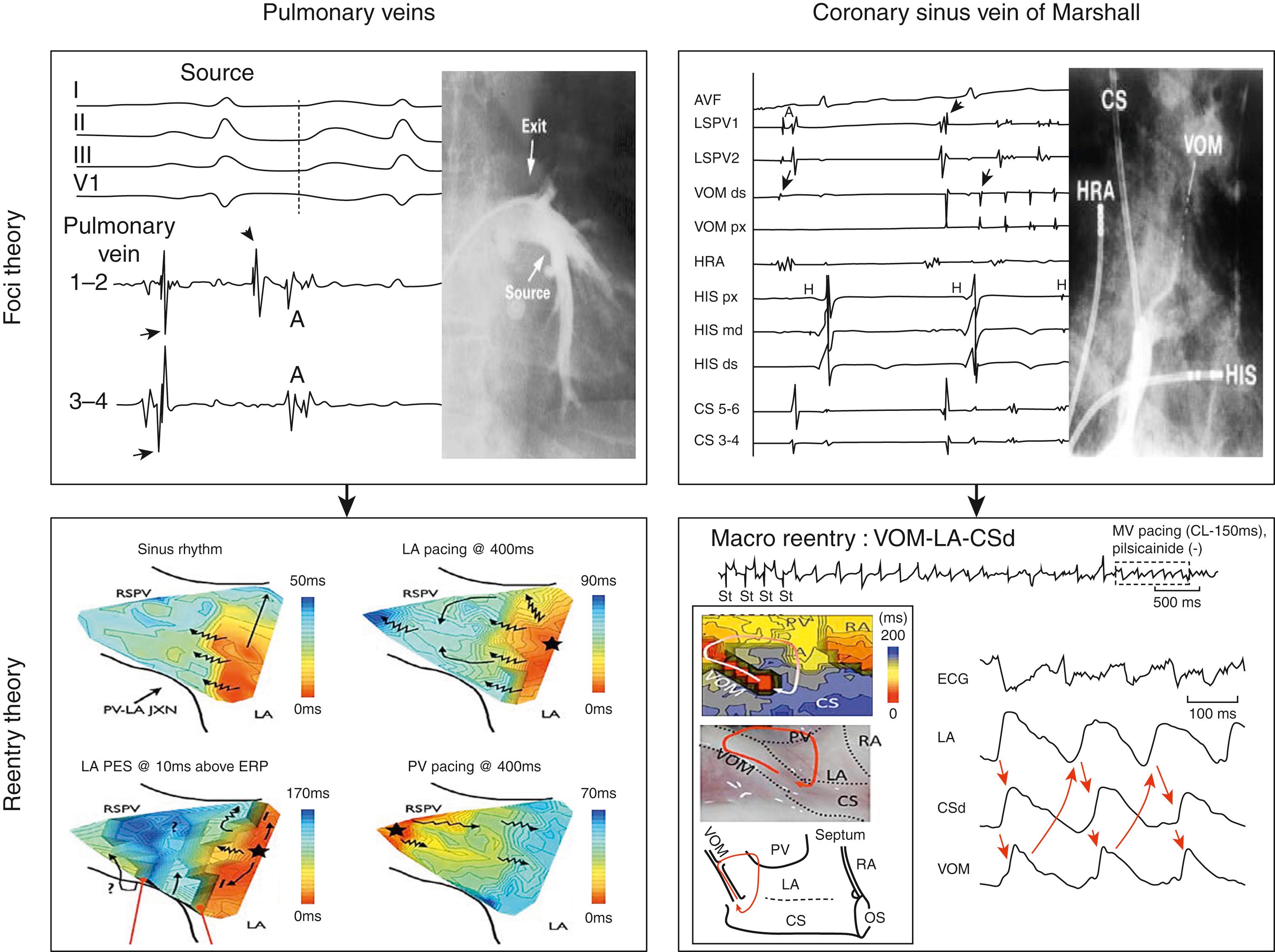
At the end of the 20th century, the focal ectopic sources theory emerged from clinical observations to explain AF mechanistic behavior: distinct structures of the atria can trigger the fibrillatory process by discharging rapid bursts of electrical impulses with a centrifugal activation pattern. Our institution has first identified the pulmonary veins (PVs) as critical structures harboring such focal sources, but other structures were similarly characterized including the vein of Marshall (VOM), the coronary sinus (CS), and the superior vena cava (SVC). These findings had two key implications: (1) there may exist a strong link between specific electrophysiologic mechanisms and distinct anatomic structures, and (2) the same mechanism may be supported by multiple anatomic structures. If correct, AF initiation would be difficult to fully eliminate because localizing triggering foci would be time consuming (if relying on spontaneous focal discharges) or potentially imprecise (if relying on pharmacologic provocation of focal discharges).
Over the past decade, experimental data from high-resolution tissue mapping have demonstrated the heterogeneous architecture of the two atria, with a complex arrangement of distinct muscular structures exhibiting distinct RPs and CVs. The musculature of the PVs, the VOM, the CS and Bachmann’s bundle have proved propitious for functional conduction block and activation shifts that favor anatomic reentry using these tracts. Such experimental findings might help reconcile the two aforementioned theories into one theory of the native atrial network for reentry: (1) reentry is the main mechanism underlying the fibrillatory process, and (2) atrial anatomy is a native network of muscular paths capable of supporting reentry. These anatomic reentries randomly running through native structures (PV bundles, CS-VOM bundles, anatomic isthmuses, etc., may constitute mechanistic primers that then promote functional reentries favoring AF perpetuation) ( Fig. 76.1 ). (See also Chapter 42, Chapter 43, Chapter 44, Chapter 45, Chapter 75 for further discussions of AF mechanisms.)
Most ablation strategies for AF have thus far relied on one of two opposing global approaches: (1) targeted elimination of clear culprits, resulting in a tailored lesion set that varies from one patient to another, or (2) global and empiric treatment of a general mechanism resulting in a lesion set systematically applied to all patients. Although simplistic, such a classification works well for PV isolation and atrial flutter ablation. PV ectopy treatment was initially tailored, electively ablating only the sources of focal discharges within the culprit PV. , The advent of tools such as cryoballoon ablation and three-dimensional (3D) electroanatomic navigation facilitated a shift to antral electrical isolation of the four PVs, which drastically reduced the risk of severe stenosis of the ablated PVs and protected the left atrium (LA) from ectopy that could subsequently arise in the nonablated PVs. This systematic approach is now universally recommended. , Conversely, the treatment of perimitral or roof-dependent flutters was based on systematically completing a lateral mitral or roof line, respectively, which targeted classic anatomic isthmuses. , The advent of high-density mapping has since revealed narrow areas of slow conduction within these circuits, allowing for more targeted and time-sparing ablation of these critical isthmuses. However, because these isthmuses can be situated on the anterior and septal walls, adopting a tailored approach to their ablation poses the risk of creating areas of conduction block at crucial sites of interatrial conduction. ,
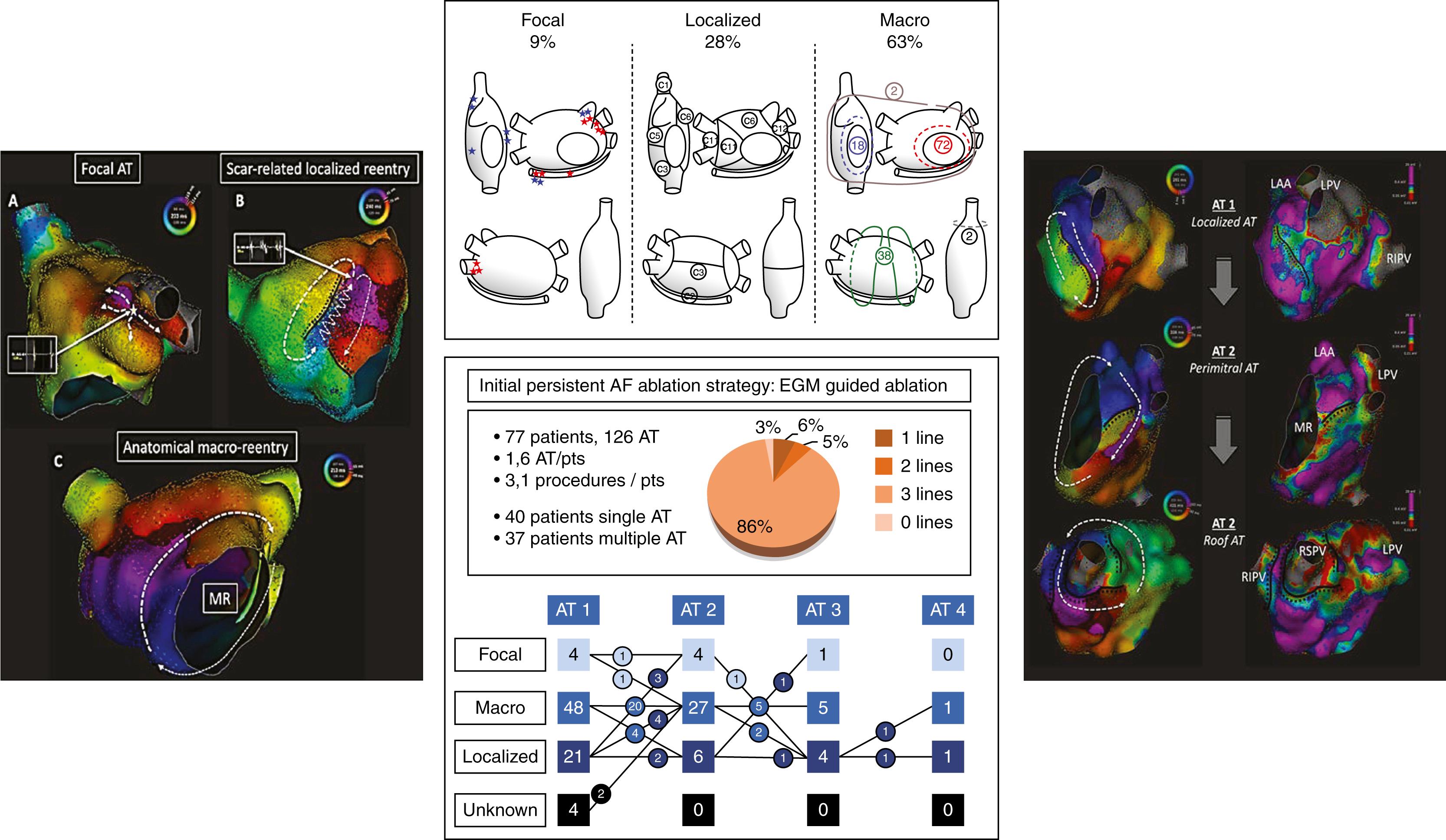
This simplified classification similarly describes the ablation strategies targeting the reentrant mechanisms for AF. At one end of the spectrum, electrical substrate ablation usually results in a patchy lesion set tailored to exhaustively target, wherever they are located, areas exhibiting the electrical signature of functional reentry maintaining AF, often with termination as an end point. At the other end of the spectrum, anatomic substrate ablation generally consists of a linear lesion set that systematically targets specific structures to block potential native paths for anatomic reentries priming AF, often with the deliberate goal of preserving atrial conduction and physiology. The “stepwise approach” used in our institution for over a decade was a unique combination of these two opposing strategies. Lessons learned from this experience ultimately indicated a marked advantage of systematic anatomic substrate ablation over tailored electrical substrate ablation in terms of atrial physiology, end point reproducibility, and arrhythmogenicity.
First, methodically identifying and ablating electrical substrate risks neglecting the effect of ablation lesion location or extent on atrial conduction and mechanics. Considering the interplay between atrial anatomy and physiology may create opportunities to ablate in a manner that preserves intrinsic wavefront propagation and spares functional myocardium. Furthermore, the “signature” of functional reentries used to identify and target the electrical substrate is poorly and inconsistently defined. In contrast, a purely anatomic approach consists of successive objective end points that are much easier to reproduce by different teams. Finally, the remarkable AF termination rate seen with electrical substrate ablation is offset by a high recurrence rate of stable tachycardias, mostly of classic macroreentry with complex circuits caused by iatrogenic scars ( Fig. 76.2 ). In addition to avoiding patchy scars that predispose to reentry, systematically blocking the cavotricuspid, lateral mitral, and posterior wall (PW) isthmuses anticipates the risk of these anatomic macroreentries (i.e., cavotricuspid-dependent, perimitral and roof-dependent flutters).
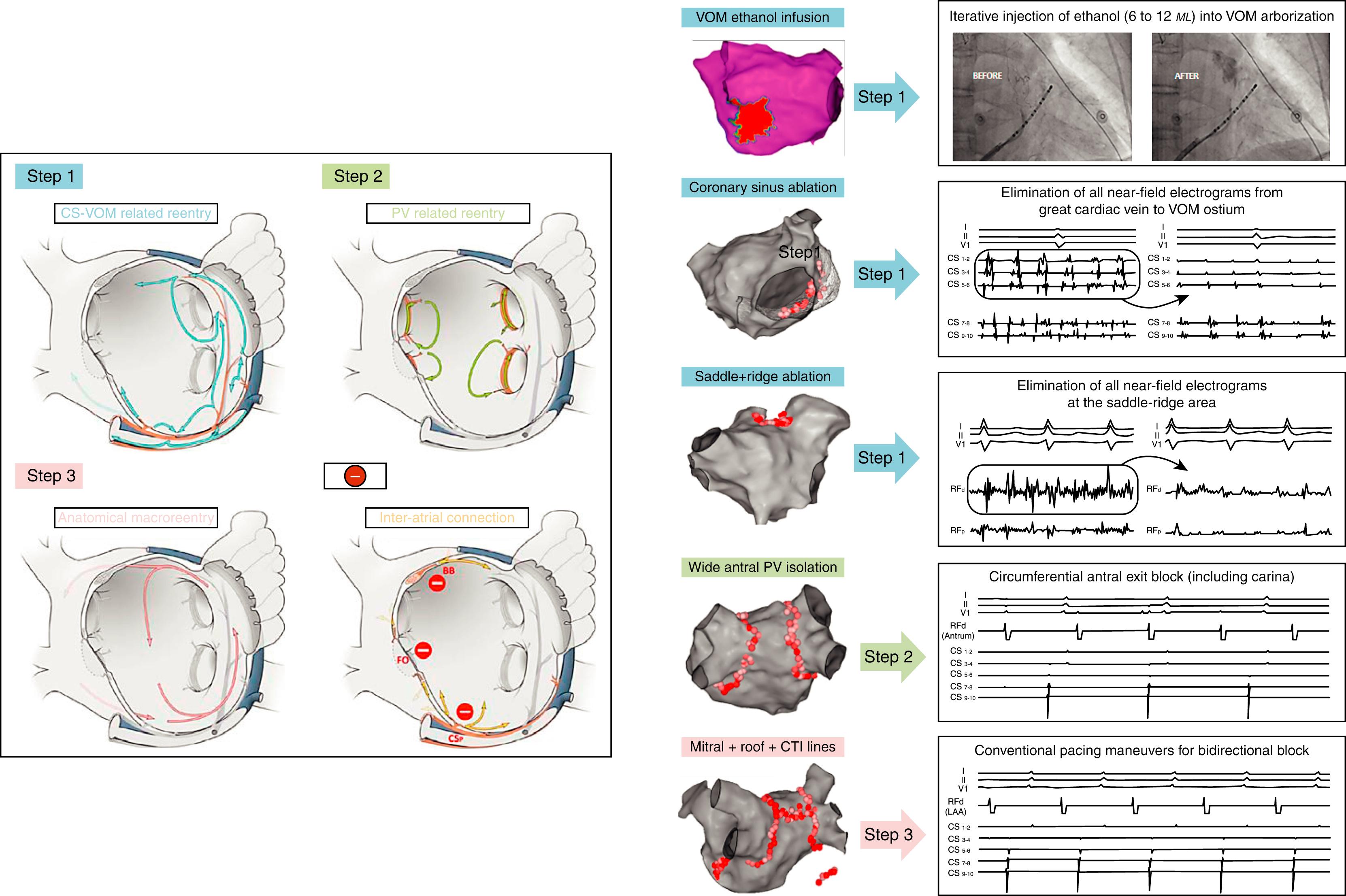
To date, the lesion set that best meets the previously mentioned objectives is the third “cut-and-sew” iteration of the Cox maze procedure ( Chapter 135 ), with excellent results in terms of preserved atrial physiology, end point reproducibility, and sinus rhythm maintenance. , With advances in ablation techniques and technologies, our ability to create durable lines of block continues to improve in the electrophysiology (EP) laboratory, raising the prospect of replicating the results seen with effective anatomic approaches for treating AF in the operatory room. The PV muscle bundles, VOM bundle, distal CS bundle, mitral isthmus, PW isthmus, cavotricuspid isthmus, Bachman’s bundle, fossa ovalis, and proximal CS bundle are all distinct muscular components of the native atrial network that constitute critical paths for pathologic reentry or physiologic conduction. A sensible anatomic ablation strategy for AF would distinguish between them, and target the former, while respecting the latter ( Fig. 76.3 ).
Prior to ablation, patients must be carefully evaluated for comorbidities such as heart failure, cardiac ischemia, hypertension, sleep apnea, and obesity, which should be optimized whenever possible. Patients with a high CHA 2 DS 2 -VASc score, hypertrophic cardiomyopathy, or valvular heart disease should benefit from long-term anticoagulation. Otherwise, the expert consensus is that anticoagulation should be administered for 1 month before AF ablation and 2 months afterward. The use of uninterrupted direct oral anticoagulants (DOACs), when feasible, appears to be safer than uninterrupted vitamin K antagonists with a lower risk of minor bleeding events.
Transthoracic echocardiography should be performed prior to ablation to assess LA size and exclude significant structural abnormalities. Transesophageal echocardiography can be used to screen for LA thrombus and the presence of a patent foramen ovale. Cardiac computed tomography (CT) with 3D reconstruction of the two atria is a powerful tool for ablation planning and guidance by providing key information regarding (1) the presence of PV anatomic variants (e.g., early branching, common trunks, extra PVs), (2) left atrial appendage (LAA) anatomy, and (3) the position of the esophagus relative to LA structures to potentially reduce the risk of thermal injury. The data obtained from the CT scan can be directly imported into 3D mapping systems or presented as an overlaid and linked image on fluoroscopic imaging to assist the operator during the procedure. A variety of 3D navigational tools ( Chapter 128 ), such as CARTO (Biosense-Webster), Rhythmia (Boston Scientific), and EnSite Velocity (St. Jude Medical), can integrate CT images and assist during ablation of AF or stable atrial tachycardia (AT).
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here