Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
![]() This chapter includes an accompanying lecture presentation that has been prepared by the authors: .
This chapter includes an accompanying lecture presentation that has been prepared by the authors: .
Major depressive disorder (MDD) is the most common psychiatric illness, with a lifetime prevalence of 10%–20%. Of those cases, approximately 20% will be refractory to conventional therapies, being termed treatment-resistant depression.
Recent advances in neuroscientific research have helped elucidate the neurocircuitry underlying depression. Dysfunctional activity within the limbic frontal-striatal-thalamic-frontal loops is thought to underlie the depressive phenotype. These loops include structures such as the cingulate cortex, orbitofrontal cortex, ventral striatum, and mediodorsal thalamus.
Ablative neurosurgery for depression has come a long way since the times of the prefrontal lobotomy. Cingulotomy and capsulotomy are the two most commonly performed ablative procedures for depression today. Both can be performed with open surgery using radiofrequency ablation, or with Gamma Knife radiosurgery. Magnetic resonance imaging–guided focused ultrasound is now a third option for performing capsulotomy.
Deep brain stimulation (DBS) for depression continues to evolve. The most common targets are the subcallosal cingulate cortex (SCC), the ventral capsule/ventral striatum, and the medial forebrain bundle (MFB). Diffusion tensor imaging is now being used extensively to optimize targeting, especially in the case of the SCC and the MFB targets.
Randomized control trial (RCT) results have suggested that studying DBS for depression requires nuanced, adaptive, patient-specific outcome measurement and optimization periods, as evidence by the RCT published by Bergfeld and colleagues (see ref. 95).
Major depressive disorder (MDD) is a common and challenging psychiatric disorder that is responsible for a significant proportion of global morbidity. Current estimates suggest that up to 12% of men and 20% of women will experience a major depressive episode (MDE) in their lifetime, with the total economic burden to society estimated at US $40 billion annually. The last 30 years have seen a dramatic improvement in our understanding of the neural underpinnings of MDD. Technical advances, such as improved neuroimaging, and advances in genetics and biology have now provided a clearer picture of mood circuitry, in both health and disease states, and are further fueling the development of more focused and effective antidepressant treatments.
MDD is a heterogeneous condition consisting of more than just a deficit state. For example, traditional downregulation symptoms such as sadness and psychomotor retardation often coexist with upregulation symptoms such as rumination, pathologic crying, and suicidal ideation. Diagnostic criteria for MDD have been formalized in the fifth edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-5) and are shown in Box 123.1 . An MDE is defined by distinct symptoms, as shown in Box 123.1 , existing continuously for a period of 2 weeks, and should be distinguished from those symptoms associated with medical or other conditions, the effects of substance use, and bereavement. As a result of the recognized diversity of depression types, a diagnosis by an expert psychiatrist is required prior to initiating treatment for an MDE. It is important to note that current expert opinion is moving away from rigid disease definitions to more transdiagnostic symptoms and circuit-based approaches. This is illustrated through the Research Domain Criteria, a framework put forward by the National Institute of Mental Health to guide neuroscience toward assessing domains and constructs of behavior that cut across typical disease boundaries. Future iterations of the DSM may focus on symptom clusters, as well as dysfunctional neural circuitry and biomarkers, as aids in diagnosis.
Five (or more) of the following symptoms have been present during the same 2-week period and represent a change from previous functioning; at least one of the symptoms is either (1) depressed mood or (2) loss of interest or pleasure.
Note: Do not include symptoms that are clearly attributable to another medical condition.
Depressed mood most of the day, nearly every day, as indicated by either subjective report (e.g., feels sad, empty, hopeless) or observation made by others (e.g., appears tearful). (Note: In children and adolescents, can be irritable mood.)
Markedly diminished interest or pleasure in all, or almost all, activities most of the day, nearly every day (as indicated by either subjective account or observation).
Significant weight loss when not dieting or weight gain (e.g., a change of more than 5% of body weight in a month), or decrease or increase in appetite nearly every day. (Note: In children, consider failure to make expected weight gain.)
Insomnia or hypersomnia nearly every day.
Psychomotor agitation or retardation nearly every day (observable by others, not merely subjective feelings of restlessness or being slowed down).
Fatigue or loss of energy nearly every day.
Feelings of worthlessness or excessive or inappropriate guilt (which may be delusional) nearly every day (not merely self-reproach or guilt about being sick).
Diminished ability to think or concentrate, or indecisiveness, nearly every day (either by subjective account or as observed by others).
Recurrent thoughts of death (not just fear of dying), recurrent suicidal ideation without a specific plan, or a suicide attempt or a specific plan for committing suicide.
The symptoms cause clinically significant distress or impairment in social, occupational, or other important areas of functioning.
The episode is not attributable to the physiological effects of a substance or to another medical condition.
The occurrence of the major depressive episode is not better explained by schizoaffective disorder, schizophrenia, schizophreniform disorder, delusional disorder, or other specified and unspecified schizophrenia spectrum and other psychotic disorders.
There has never been a manic episode or a hypomanic episode.
The mainstay of MDD treatment is a combination of psychotherapy and pharmacotherapy. Cognitive behavior therapy is the most commonly used and studied psychosocial treatment, and involves the identification and subsequent correction of maladaptive cognitive and perceptual biases that influence mood and behavior. There are currently several classes of medications available for the treatment of depressed mood, each aiming to correct an underlying neurotransmitter deficit. Although some of these medications are selective for specific receptor types, such as serotonin or dopamine, their effect is widespread throughout the brain, often leading to side effects and poor tolerance. Nevertheless, current therapeutic regimens for MDD are effective in the majority of patients. However, up to 30%–40% of patients remain depressed despite optimal care, and are characterized as having treatment-resistant depression (TRD). , Patients with TRD are significantly impaired, are unable to work, and suffer from poor personal relationships and quality of life. Up to 15% of TRD patients commit suicide. ,
For patients who have failed conventional treatments, neuromodulation approaches may be appropriate. These include noninvasive approaches, such as electroconvulsive therapy and transcranial magnetic stimulation, as well as invasive surgical approaches, such as lesional and stimulation-based operations (for a review of the former, see Lipsman and associates ). Here we review the rationale and experience with neurosurgical approaches to MDD.
The generation and maintenance of mood is believed to be a consequence of cortical-subcortical circuits involved in affective and emotional processing. Top-down, largely cortical structures, such as the ventromedial prefrontal cortex, interact with bottom-up, largely subcortical structures, such as the amygdala and hippocampus, via key regulatory structures in the extended basal ganglia ( Fig. 123.1 ). Although such circuit models of mood and emotion have been recognized for some time, it is only through advances in neuroimaging that many of the details have now been provided.
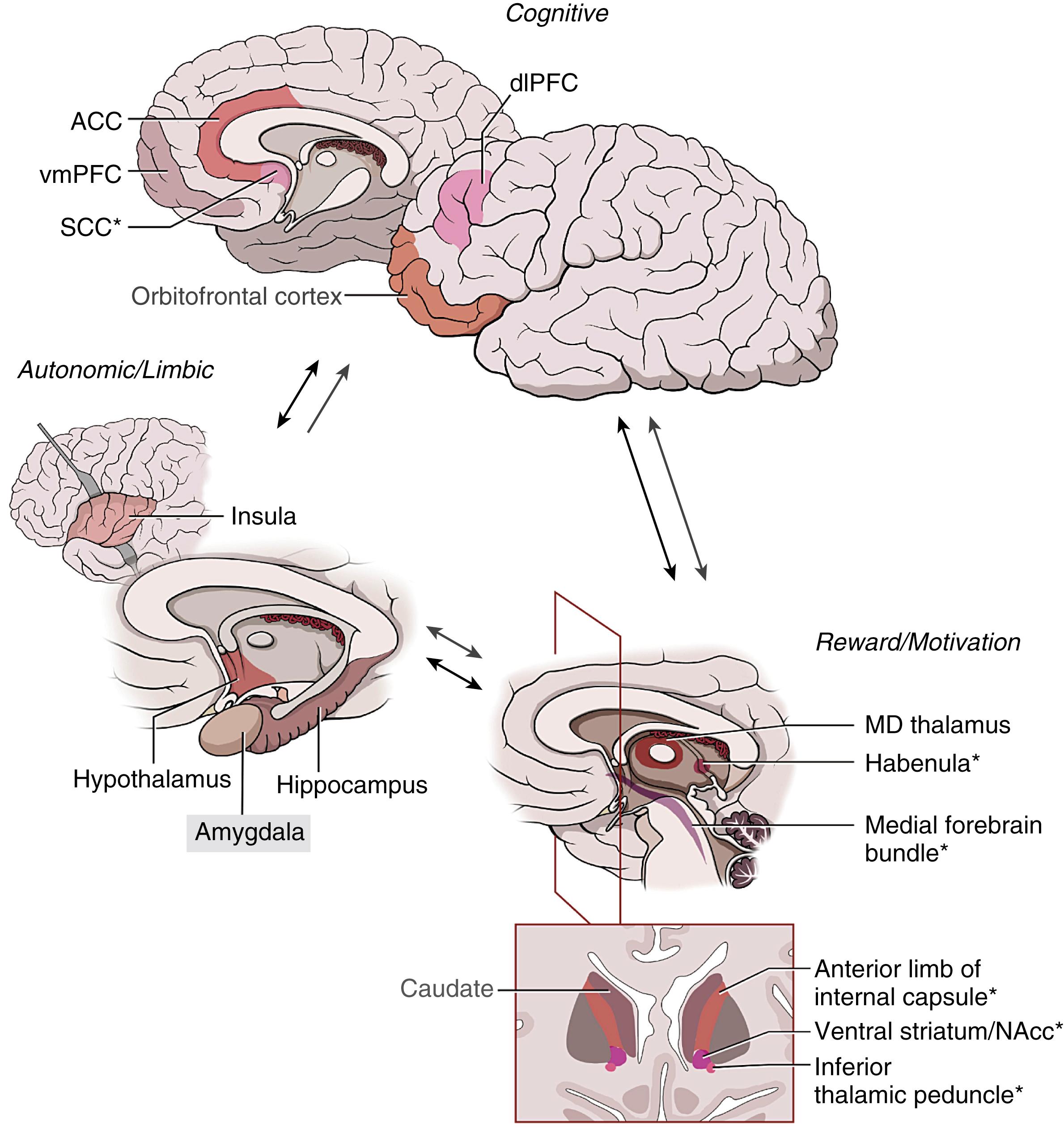
Individual brain structures integrally associated with depressive symptoms have been pinpointed through positron emission tomography (PET) and task-based functional magnetic resonance imaging studies, which identify regions with abnormal metabolism or blood flow in depressed patients, respectively. These structures include the nucleus accumbens (NAcc), the subcallosal cingulate cortex (SCC), and the dorsolateral prefrontal cortex (dlPFC). Not only do these regions display abnormalities when considered in isolation, they also play a key role in resting state networks, such as the default mode, reward, cognitive control, and affective networks, whose dysfunction may underlie not only the depressive phenotype but also subtypes within depression. The activity of these regions and the networks they participate in often normalize with successful treatment. ,
The NAcc is a gray matter structure located at the ventral interface of the caudate and putamen (i.e., ventral striatum), which is intimately involved with reward processing. When the individual is expecting or experiencing a reward, dopamine is released by neurons in the ventral tegmental area (VTA) projecting to the NAcc in the ventral striatum. Extracellular levels of dopamine rise in the NAcc, which in turn sends dopaminergic projections to the orbitofrontal cortex, the dlPFC, and other cortical areas. Drugs such as cocaine, methamphetamine, and caffeine, as well as natural rewards such as food and sex, all increase, either directly or indirectly, dopamine release in this mesocorticolimbic pathway. In the rat model, self-administration of cocaine is correlated with dopamine concentration released at the NAcc. When researchers administered haloperidol, a dopamine receptor antagonist, the rats self-administered less cocaine, suggesting an attenuation of their perception of reward. Furthermore, Doyon and colleagues trained rats to press a lever to receive sugar water combined with ethanol. Dopamine concentration was measured in the area around the NAcc, and researchers found that self-administration of the ethanol solution was significantly correlated with increased dopamine concentration in the NAcc area, released by VTA neurons. More recently, optogenetic studies have been used to precisely stimulate the VTA to release dopamine into the NAcc, recapitulating addiction behaviors in rodents. In humans, the perceived “rush” reward of cocaine use is associated with increased metabolic activity in the NAcc. These results implicate the NAcc as a brain structure intimately associated with the perception of reward.
The NAcc is also functionally related to the experience of anhedonia, which is the abject lack of pleasure in typically pleasurable activity. Anhedonia is a core feature of MDD, and work by several research groups has linked NAcc activity to anhedonia in MDD patients. , For example, MDD patients who exhibited increased pleasure response to dextroamphetamine, with concomitant increased NAcc activation, also exhibited greater severity of anhedonia.
The NAcc is a highly connected node within a well-defined cortico-striato-thalamo-cortical loop. , The NAcc receives afferents from the anterior cingulate cortex as well as the limbic temporal cortex, including the hippocampus, entorhinal cortex, and amygdala. The NAcc sends efferent projections to the ventral (limbic) portions of the globus pallidus externa and globus pallidus interna. The globus pallidus interna then projects to the mediodorsal nucleus of the thalamus, which in turn projects to the anterior cingulate, completing the circuit. Nestler and colleagues have suggested that this striatal circuit is responsible for the emotional memory associated with depression. This is congruent with findings of increased activity in both the rostral anterior cingulate and the amygdala in response to emotional memory related to depression. ,
The SCC (a.k.a. Cg25; Brodmann area [BA] 25) is a critical regulatory node in the medial ventral frontal lobe, immediately below the genu of the corpus callosum. Located at the interface of several key white matter pathways, the SCC governs affective regulation, emotional decision making, basal vegetative functions, and autonomic states.
The SCC has long been postulated as a central node in circuits mediating emotion. Early models of emotional circuits by Papez suggested the cingulate gyrus as the “seat of dynamic vigilance,” and an essential component for emotional processing. More recently, Seminowicz and colleagues proposed a model of depression circuitry based on fluorodeoxyglucose–PET across three cohorts of patients with MDD. In this model, the SCC receives afferent projections from the hippocampus and sends efferent projections to the lateral prefrontal cortex (BA 9). It also shares bilateral connectivity with both the rostral cingulate (Cg24a) and the orbitofrontal cortex (BA 11). This is particularly noteworthy because the rostral cingulate is in turn highly connected to both the dorsal and ventral compartments in the Mayberg model of depression. Additionally, the orbitofrontal cortex has been implicated in decision making and emotional processing. Hence, the SCC is well situated to affect, via retrograde and anterograde mechanisms, structures in both the dorsal compartment (BA 9 and BA 11) and ventral compartment (hippocampus) in order to modulate the depression circuit. , , In particular, SCC connections to cortical structures implicated in depression suggest that altering Cg25 activity may treat the cognitive aspects of depression: guilt, hopelessness, and suicidal ideation. ,
Activity of the SCC has been widely implicated in depressive disorders and depressive states in general. , Much of this work has been driven by functional neuroimaging, and by metabolic imaging with PET in particular. For example, when healthy individuals are induced to feel sad, blood flow and glucose utilization in the SCC is substantially increased. Furthermore, this hypermetabolism is attenuated in remitted MDD patients, following both pharmacotherapeutic and psychotherapeutic treatments. These decreases in metabolism in the ventral compartment, including the SCC, were further correlated with resolution of depressed mood. This reversal is also seen following deep brain stimulation (DBS) treatment in patients with TRD, as well as in patients with other affective regulatory conditions, such as anorexia nervosa. , Neurophysiological studies of SCC activity in depressed patients have also linked neural activity in the SCC to the experience of sad events. SCC single neurons appear to fire preferentially when viewing sad versus happy or neutral images, and populations of neurons, measured with local field potentials, are preferentially involved in the judgment of sad versus happy stimuli. ,
Functional connectivity between the SCC and the dlPFC is being increasingly considered as a diagnostic, therapeutic, and predictive tool in MDD. In healthy nondepressed individuals, the SCC and the dlPFC are anticorrelated (their spontaneous functional magnetic resonance imaging signals are 180 degrees out of sync). Depressed patients, on the other hand, tend to have more positively correlated activity linking the SCC and dlPFC. In the transcranial magnetic stimulation literature, precisely choosing a target within the dlPFC with maximal anticorrelation to the SCC leads to improved outcomes. , The degree of dlPFC-to-SCC positive correlation predicts the likelihood of response to pharmacotherapy for MDD. This work further strengthens the SCC’s place as a pivotal hub in MDD neurocircuitry since the early PET studies.
The ALIC is a white matter pathway connecting frontal cortical structures with subcortical, thalamic, and other basal ganglia structures. As a key limbic pathway, the ALIC has been postulated to be a critical connection between top-down and bottom-up regulatory structures important for mood and emotional processing as well as decision making. Imaging and anatomic tracer studies have shown that the ALIC connects the anterior cingulate and parts of the prefrontal cortex with lower limbic structures such as the hippocampus, amygdala, and mediodorsal nucleus of the thalamus. , In this way, the ALIC comprises the fibers that complete the “anterior cingulate” or limbic cortico-striato-thalamo-cortical circuit. This includes the projection fibers connecting limbic cortical structures to the ventral striatum, as well as the thalamocortical and striatal fibers that connect the thalamus to the anterior cingulate, closing the anatomic circuit.
Functional evidence directly linking the ALIC to MDD is scarce. Given its role in emotional decision making, lesioning of the ALIC (discussed later), known as capsulotomy, has been proposed in patients with treatment-refractory obsessive-compulsive disorder (OCD), as well as MDD. Leveraging the early experience with capsulotomy, DBS of the ALIC was used to treat patients with treatment-refractory OCD. , From these early studies, it was found that DBS was effective at treating not only obsessions and compulsions, but also comorbid depressive symptoms. As a result, Malone and colleagues have performed ALIC DBS on patients specifically with TRD, initially as an open-label trial and later as a randomized controlled trial (RCT). , The ALIC has also been targeted in combination with the bed nucleus of the stria terminalis. Although results have been variable to date, as discussed in more detail later, this experience underscores the place of the ALIC in conceptual models of both mood and depression.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here