Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
This work was supported in part by an Agence Nationale de la Recherche (ANR) grant (10-IAHU04-LIRYC). F.D.R. was supported by a Canadian Institutes of Health Research (CIHR) Banting Postdoctoral Fellowship and a Royal College of Physicians and Surgeons of Canada Detweiler Traveling Fellowship. We thank Mr. Jean-Rodolphe Roux (Biosense-Webster) for his assistance with Fig. 111.2 .
Heart failure (HF) is considered a global pandemic, with a prevalence of approximately 1% to 2% in adults in developed countries and at least 10% among those older than 70 years. In the United States alone, more than 6 million individuals at least 20 years or older are estimated to have HF, and it is predicted that more than 8 million individuals aged at least 18 years or older will have HF by 2030, accounting for nearly 3% of the population. Remarkable advances in our understanding of the condition and in available therapeutic options have dramatically improved survival and quality of life of patients with left ventricular (LV) systolic dysfunction. Among available therapies, durable ventricular assist devices (VADs) have emerged as a viable option for those with severe and refractory HF, with more than 25,000 devices implanted thus far in the United States alone. VADs are usually implanted in patients with advanced HF but without acute hemodynamic instability or significant organ damage. They can support the LV (LVADs), right ventricle (RVADs), or both (BiVADs), but LVADs are the most widely used and best studied. For the purposes of this chapter, VADs will be considered distinct from other forms of mechanical circulatory support (MCS), although several from the latter group could be considered temporary and/or percutaneous VADs.
VADs were initially intended to be used as temporary forms of MCS to allow patients to survive to heart transplantation (HT). Their effectiveness soon created a different problem, however: expanding waiting lists and lengthening waiting times for HT because of a rising number of patients with VADs but stagnant or declining numbers of HT being performed. , It is estimated that currently only 10% of patients with an implanted MCS device as a bridge to HT (BTT) will receive an organ within 1 year of being listed. The concept of VADs as a permanent substitute rather than a bridge to HT (VADs as “destination therapy” [DT]) therefore largely arose from necessity rather than design.
As our experience with VADs has increased and the technology has been incrementally refined with a focus on durability and safety, patient outcomes have improved and indications for VAD use have evolved. Based on the most recent data available from the Society of Thoracic Surgeons Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS), between 2012 and 2017, 48% of VADs were implanted as DT (with an additional 2.5% that were implanted in patients who were deemed unlikely to be listed for HT), 25% were implanted as BTT, 25% were implanted in patients who were not listed for HT but were believed to be likely or moderately likely to be listed for HT in the future (bridge to candidacy), and less than 1% were implanted as a bridge to cardiac recovery. These values are comparable to those recently reported by the International Society for Heart and Lung Transplantation Mechanical Assisted Circulatory Support (IMACS) Registry. VADs are therefore often used as chronic or lifelong therapies in modern practice, a reality that speaks to the important gains made in the treatment of patients with advanced HF, but one that is also creating new challenges.
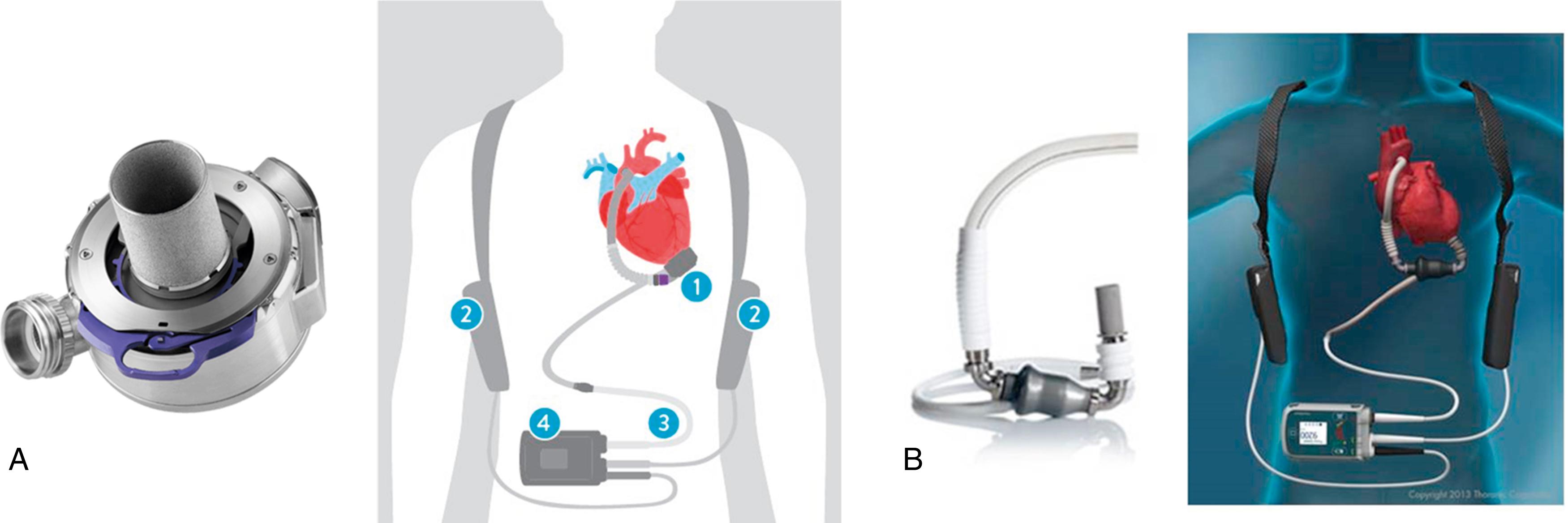
Early iterations of VADs were characterized by pulsatile flow and high rates of complications and device failure. Improved safety profiles and durability were seen with a transition to continuous-flow VADs (CF-VADs). Currently, there are two CF-VADs approved by the US Food and Drug Administration (FDA) for BTT and DT indications: HeartMate II and HeartMate 3 (Abbott). The HeartMate II is an axial flow device with an Archimedes screw–like pump supported by a single ruby bearing, whereas HeartMate 3 generates centrifugal flow using a magnetically levitated rotor ( Fig. 111.1 ). These CF-VADs can achieve cardiac outputs of up to 10 L/min by drawing blood from the LV via an inflow cannula and pumping it into the ascending aorta via an outflow cannula, bypassing the aortic valve. External power sources drive the pump via a driveline that is tunneled subcutaneously and externalized at the patient’s abdomen.
The Berlin Heart EXCOR Pediatric VAD (Berlin Heart) is a pneumatically driven extracorporeal pulsatile VAD with various pump and cannula sizes, rendering it a feasible option in all pediatric age groups as BTT. ,
Other VADs have not been approved by the FDA but have received CE (Conformité Européenne) Mark certification. For instance, the Jarvik 2000 (Jarvik Heart) is a small axial-flow pump that offers a power supply connection site behind the ear, which may reduce the risk of driveline-associated infections. Several other devices are currently undergoing clinical testing.
Current 1-year survival rates for patients with contemporary LVADs exceed 80%; however, overall 5-year survival rates remain below 50%, underscoring how “sick” this patient population is and the high risk for adverse events associated with these devices. Nearly half of patients with LVADs are rehospitalized within 3 months of device implantation. By 12 months, approximately 80% will have been rehospitalized, 25% to 28% will have a pump-related infection, 20% to 25% will experience gastrointestinal (GI) bleeding, and 13% to 20% will have a stroke or transient ischemic attack. Additional complications include aortic insufficiency (30% incidence at 2 years), right heart failure (15%–25%), and pump thrombosis (1%–12%). Ventricular arrhythmias (VAs) occur in approximately 20% to 50% of patients with VADs, most often within the first month after surgery, , but are often well-tolerated over the short term because of the hemodynamic support provided by the device.
Total artificial hearts (TAHs) are not true VADs because they replace the ventricles completely. A temporary TAH (TAH-t, SynCardia Systems) consisting of a pneumatic pulsatile pump with an external portable drive unit has been approved by the FDA as a BTT. It is predominantly considered in select patients with severe biventricular failure. Other models of artificial hearts, such as Carmat and AbioCor (Abiomed), have also been developed.
Various short-term alternatives to VADs exist and are often used to rescue patients with acute cardiac decompensation and cardiogenic shock or to support patients during procedures that carry a high risk for hemodynamic instability, , settings in which durable VAD implantation are either impractical or not desired given expected prompt clinical recovery.
Intraaortic balloon pumps (IABPs) are among the simplest devices available for temporary MCS. IABPs consist of a balloon that is advanced to the descending aorta via the femoral artery where it inflates and deflates synchronously with the cardiac cycle, augmenting diastolic blood pressure when inflated (during diastole) and reducing afterload as it deflates (during systole). Nevertheless, they provide only modest increases in cardiac output and in a randomized trial of 600 patients with acute myocardial infarction (MI) complicated by cardiogenic shock, there was no evidence of benefit with IABP (vs. no IABP) on all-cause mortality or secondary end points at 30 days, 12 months, or 6 years. Although limited data exist on its use during catheter ablation of unstable ventricular tachycardias (VTs), fewer patients could undergo VT mapping, fewer VTs could be mapped and ablated per patient, fewer VTs were terminated by ablation, and more VTs were terminated by rescue shocks with IABPs compared with alternative options (see later).
The Impella device (Abiomed) is similarly typically introduced via the femoral artery but is advanced across the aortic valve into the LV. The Impella 5.0, which can deliver up to 5 L of blood per minute, is considered the Impella device most capable of supporting the LV in cardiogenic shock. The Impella RP is designed for right ventricle (RV) support, pumping blood from the inferior vena cava to the pulmonary artery. Given limited options for temporary MCS in pediatric patients, off-label use of Impella devices (especially the Impella 2.5 and Impella CP) in this population has grown, suggesting it to be a technically feasible strategy with acceptable safety profile in select cases. The TandemHeart (CardiacAssist) consists of an extracorporeal pump that draws blood from the left atrium through a cannula that is advanced through a femoral vein across the interatrial septum and propels it to one or both femoral arteries. Relative to the IABP, both the Impella and TandemHeart can substantially increase the cardiac index in patients with acute MI complicated by cardiogenic shock , and may facilitate mapping and ablation of unstable VTs. The TandemHeart has been associated with more bleeding and vascular complications than the IABP, likely because of the large size of the venous and arterial cannulae used with the former. Furthermore, TandemHeart’s large transseptal cannula impedes transseptal access for LV mapping and ablating during electrophysiologic procedures.
Extracorporeal life support (ECLS), also known as extracorporeal membrane oxygenation (ECMO), provides temporary respiratory and mechanical circulatory support. Different venoarterial and venovenous modes are available. The Extracorporeal Life Support Organization (ELSO) registry reported acute survival and survival to discharge rates of 56% and 41%, respectively, for adult patients treated with ECLS for cardiac indications. These rates decreased to 39% and 29%, respectively, when ECLS was initiated to support cardiopulmonary resuscitation. If sufficient myocardial function recovery does not occur when on ECLS, bridging to long-term VADs may be considered in some cases. Although this modality provides the best temporary hemodynamic support, there are considerable risks associated with its use, and its placement and management usually requires the expertise of a larger multidisciplinary team.
Sustained VAs are common in patients with VADs, particularly within the first month after implantation ( Table 111.1 ). , , Recurrent VAs that met the criteria for electrical storm (defined as ≥3 separate episodes of sustained VAs within a 24-hour period) occur in 6% of patients with LVADs within the first month postimplantation and in 9% by 9 months in multicenter observational studies, , although estimates as high as 28% have been reported in single-center studies. VAs in patients with VADs are often relatively well tolerated, particularly in those with CF-VADs and with VAs that are sustained for relatively short periods, because of the hemodynamic support provided by the device. In fact, cases of patients with VADs tolerating hours to months of sustained VF with minimal symptoms have been reported. Nevertheless, VAs after VAD implantation have also been associated with substantial morbidity and can increase hospitalizations, and the clinical presentation of VAs in this population can range from a lack of discernable symptoms to hemodynamic instability warranting immediate intervention. Furthermore, even in the absence of acute decompensation, VAs can precipitate RV failure in patients with LVADs because the RV is unsupported. RV failure can, in turn, lead to decreases in LV preload and LVAD flow. Underlying RV function, the degree of pulmonary hypertension, and the tachycardia cycle length may therefore influence the clinical presentation of sustained VAs. Many patients with VADs also have implantable cardioverter-defibrillators (ICDs) and thus can experience shocks while fully conscious. If sustained VAs are left untreated, thromboembolic risks may arise as well because of relatively stagnant RV blood flow.
| Study | n | % Patients With VAs After VAD Implantation | Follow-up (Days) a | % CF-VAD |
|---|---|---|---|---|
| Harding (2005) | 17 | 59 | 14 | 0 |
| Ziv (2005) | 91 | 35 | NS b | 0 |
| Bedi (2007) | 111 | 22 | 98 | 0 |
| Miller (2007) | 133 | 24 | 126 | 100 |
| Andersen (2009) | 23 | 52 | 341 | 100 |
| Ambardekar (2010) | 33 | 24 | 238 | 53 |
| Cantillon (2010) | 478 | 29 | 84 | 8 |
| Kuhne (2010) | 76 | 29 | 156 | 30 |
| Oswald (2010) | 61 | 34 | 365 | 100 |
| Brenyo (2012) | 61 | 31 | 662 | 100 |
| Raasch (2012) | 61 | 43 | NS | 100 |
| Refaat (2012) | 144 | 20 | 119 | 51 |
| Enriquez (2013) | 106 | 35 | 217 | 100 |
| Garan (2015) | 162 | 23 | 30 | 100 |
| Yoruk (2016) | 149 | 28 | 767 | 100 |
| Yap (2016) | 204 | 30 | 519 | 100 |
| Greet (2018) | 517 | 26 | 465 | 100 |
Despite the aforementioned plausible mechanisms for adverse events, the association of VAs with mortality in patients with LVADs remains unclear. Numerous observational studies have reported this association, , but not all, , , , raising the question of whether VAs cause or contribute to mortality as opposed to simply being a marker of a sick patient.
VAs pre-VAD implantation are the strongest predictor of VAs post-VAD implantation, especially early postsurgery (within the first 30 days). , Late VAs (those occurring >30 days after device implantation) are less frequent. The VT-LVAD score may help stratify patients with LVADs into low, intermediate, high, and very high risk categories for late VAs ( Table 111.2 ).
| VT-LVAD | Variables | Score |
|---|---|---|
| V | V As before LVAD implantation | 2 points |
| T | T herapy: no ACE inhibitor post-LVAD | 2 points |
| L | Fai L ure duration (>12 months) | 2 points |
| V | V As post-LVAD implantation (<30 days) | 2 points |
| A | A trial fibrillation before LVAD | 1 point |
| D | Idiopathic D ilated cardiomyopathy | 1 point |
| Maximum score | 10 points |
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here