Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Testing for the presence of drugs in the blood and other body fluids of patients has undergone a vast increase over the past 20 years.
Testing for the presence of drugs of abuse and/or poisons in patients has become mandatory both in the emergency department and in employment screening.
Most drugs for which monitoring is standard can be assayed using homogeneous immunologic techniques.
Gas and liquid chromatography–mass spectroscopy, which involve separation and identification of compounds in the gas phase from their mass/charge ratios and fragmentation patterns, are the gold standards for detection and quantitation of drugs in body fluids.
The mechanisms of action of many therapeutic drugs have been at least partially elucidated. Many of these, such as the antiinflammatory, antiasthmatic, and immunosuppressive drugs, block specific points in signal transduction pathways.
Ingestion of poisons causes life-threatening illness; poisons include cyanide, carbon monoxide, and a number of metals, such as lead, mercury, iron, and arsenic. If detected, the effects of these poisons can often be reversed.
Interpretation of results for drugs of abuse must be made in conjunction with other signs and symptoms for appropriate patient management.
Toxicology is the study of substances introduced exogenously into the body. Elsewhere in this textbook, the analytic methods presented are concerned with determining the presence and levels of natural substances involved in normal body function. In this chapter, we discuss the biological effects and methods for detection of exogenous chemical compounds that profoundly influence bodily functions, often in a deleterious way but also for therapeutic benefit.
Toxicology has become divided into four areas. The first two areas are the detection of drugs of abuse and the determination of levels of therapeutic drugs being administered to patients. Also, it has been recognized that certain environmental compounds that are mutagens and carcinogens, such as benzopyrene and acetylaminofluorene, cause mutations in critical sequences of human DNA, leading to the frank development of cancer. In the ongoing revolution in molecular biology, this field has now expanded into the detection of certain markers such as abnormal DNA sequences, the presence of mutated proteins, or the presence of carcinogens bound to DNA. This rapidly expanding field is the subject of discussion in Parts 8 and 9, especially in the chapters concerning serum and solid-tissue diagnostic markers for cancer and pharmacogenomics. Finally, there are numerous toxins to which individuals become exposed, such as carbon monoxide, cyanide, metals, and so forth, for which detection is vital if physicians are to be able to reverse the adverse acute physiologic effects. In this chapter, we discuss each of these divisions of toxicology with special reference to the detection of drugs and toxins in body fluids In the course of this chapter, drug dosages for some therapeutic drugs are noted. These dosages are approximate and are noted only as examples, i.e., typical dosages that have been found to give levels of the drug in serum or in other body fluids that are usually, but not always, therapeutic. These listed doses are not in any way to be taken as endorsements of these doses and are not recommended doses. They serve merely as examples.
The techniques involved in detecting the presence and/or the level of particular drugs, whether they are drugs of abuse or therapeutic drugs, are of two basic types: immunochemical and chromatographic.
Much drug testing today is performed using the so-called homogeneous immunoassay . The term homogeneous refers to the fact that these assays are all performed in solution, without any requirement for a phase separation that is traditionally used to separate bound from free ligand. This technology has revolutionized toxicology because it allows performance of rapid, stat analyses of blood and urine constituents. The technique is shown schematically in Figure 24.1 . Here, we show examples of two types of assays. In the first, the enzyme-mediated (or multiplied) immunologic technique (EMIT), the drug itself is covalently attached to an enzyme such as glucose-6-phosphate dehydrogenase ( Fig. 24.1A-1 ). When the drug-enzyme complex is incubated with an antibody to the drug, enzyme activity is markedly decreased as a result of blocking of the active site of the enzyme by the antibody. When, as in Figure 24.1A-2 , exogenous drug (such as in serum) is added to the immune complex, this exogenous drug competes with the drug-enzyme complex for the antibody. Liberated drug-enzyme results in increased enzymatic activity. Increasing concentrations of drug in serum result in increased observed enzymatic activity. This method was pioneered largely by the Syva Corporation (now part of Siemens, Palo Alto, CA) and has been applied both to therapeutic drug monitoring and to detection of drugs of abuse.
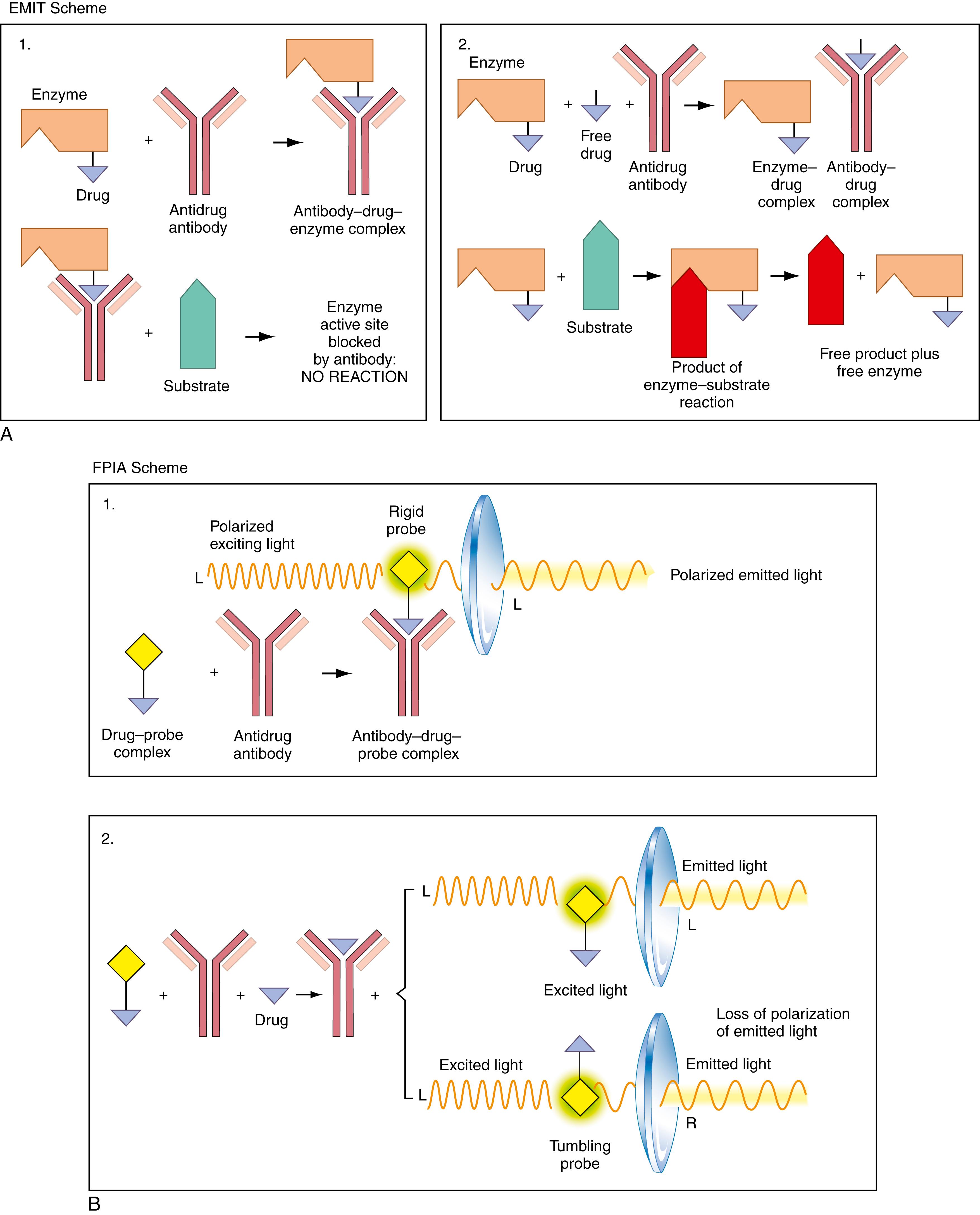
Fluorescence polarization immunoassay (FPIA) is the second type of homogeneous drug assay, as shown in Figure 24.1B . Rather than being linked to an enzyme, as in Figure 24.1A , the drug is covalently attached to a fluorescent probe molecule. If a fluorescent molecule is excited with polarized light and is stationary (i.e., it does not “tumble” in solution), it will emit polarized light as a fluorophore. This emitted light has the same polarization as the exciting light. Thus, for example, if the exciting light is polarized to the left, the emitted light will also be polarized to the left. If a fluorophore tumbles freely in solution, however, the polarization is lost; that is, the emitted light is now polarized equally to the left and right. However, if the fluorophore is bound to a macromolecule such as an antibody, the polarization is strong because attached to the nontumbling antibody it remains relatively stationary. In these assays, the probe-labeled drug is incubated with the antibody. The fluorescence polarization of the probe-labeled drug is, of course, high because the fluorescence probe is relatively immobilized when bound to the antibody directed against it ( Fig. 24.1B-1 ). Addition of exogenous drug, as in serum, to the incubated mixture results in displacement by the exogenous drug of some of the fluorescent probe-labeled drug molecules, as shown in Figure 24.1B-2 . These displaced molecules can now tumble freely in solution. The result is a decrease in fluorescence polarization. This decrease is directly related to the concentration of drug in serum.
This assay can detect drug levels in the nanomolar range and is both highly sensitive and specific. Both Abbott Laboratories (Chicago), currently on the Architect Analyzer and formerly on the TDX, IMX, and AXSYM analyzers, and Roche Diagnostic Laboratories (Nutley, NJ), on the COBAS and INTEGRA analyzers, have pioneered this effective technique in monitoring a wide variety of therapeutic drugs and drugs of abuse.
In both of the homogeneous methods discussed previously, a nonlinear relationship exists between the concentration of drug in serum and the response of the system—that is, the color that results from the enzymatic reaction (see Fig. 24.1A ) or the decrease in fluorescence polarization (see Fig. 24.1B ). This nonlinearity in response is due to the phenomenon of binding (i.e., the drug must bind to antibody before it is detected). This phenomenon may be expressed by the following equilibrium:
where D is the drug concentration in serum, D∗ is the “marker” drug (i.e., drug labeled with an enzyme or a fluorescent probe), and Ab is the antibody. The concentration of free D∗ is a measure of D–Ab, because both are equimolar. The concentration of D–Ab is, in turn, related to D; the more D is present, the more D–Ab is formed. However, because the concentration of Ab is fixed in a given experiment, at sufficiently high concentrations of D, all of the Ab will be saturated so that at higher concentrations of D, no further D–Ab can form. The relationship between D and D–Ab is given by the Langmuir expression:
where (Ab 0 ) is the total concentration of antibody, k is the equilibrium constant for formation of the D–Ab complex, n is the number of antibody-binding sites per molecule of antibody, and D is free drug concentration as defined earlier in the chapter. Equation 24.2 is very similar to the Michaelis-Menten equation discussed in Chapter 21 ( Eq. 21.10 ), except that there is no catalytic step here. This equation shows that the concentration of D–Ab is nonlinear in D, except where Kd ≪ 1. Where kD ≫ 1, saturation is achieved. This equation can be linearized in the form used for a Scatchard plot, that is,
where r/D is plotted versus r. Given the results for a set of experiments, a least-squares–best-fit line is drawn through the points. The slope of the line is –k, and the intercept is nk, so that n (the number of sites on the antibody) is equal to the intercept/slope. Thus, the values of n and k are readily determined. Once values for n and k are known, the value of D for any measured value of r can be directly computed from Equation 24.3 .
Two problems that arise in using Equation 24.3 are that often the antibodies are nonhomogeneous so that the Scatchard plot is nonlinear, and possible blockage of free antibody sites by drug molecules is seen in solid-phase immunoassays. The first problem was solved by , wherein the analysis mentioned previously was applied to multiple-binding equilibria. This analysis is used commonly in microprocessors that analyze the calibration curves in immunoassays. The second problem has been analyzed using a different theoretical approach ( ). Equation 24.3 illustrates the basic principle of how the results of the drug immunoassay on serum are converted into drug concentrations in serum.
Chromatographic procedures (see , for an excellent brief history of liquid chromatography, and Chapter 4 ) have been applied mainly to the qualitative detection of drugs of abuse and toxins and less to the determination of levels of therapeutic drugs. The three major methods are thin-layer chromatography (TLC), high-performance liquid chromatography (HPLC), and gas chromatography–mass spectroscopy (GC-MS). Although GC-MS is considered the gold standard for detection and quantitation of volatile drugs and poisons, newer analytic techniques such as capillary electrophoresis (CE) and liquid chromatography–mass spectroscopy (LC-MS) and mass spectroscopy-mass spectroscopy (MS-MS) are available. These techniques are all discussed in turn.
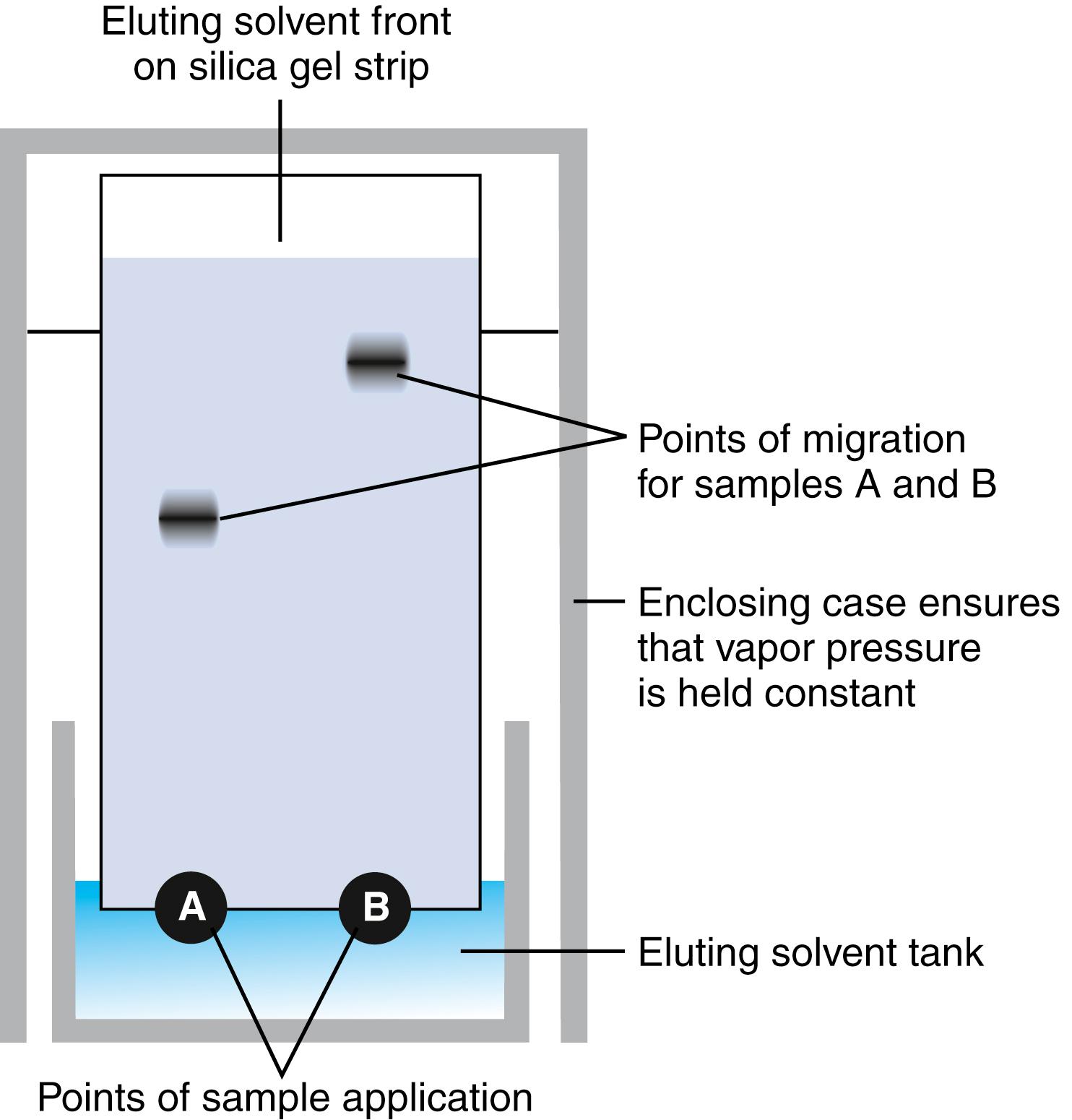
Many compounds can be separated from one another with this method based on their relative affinities for a polar solid stationary phase (usually a hydrated silicate) and a mobile liquid phase that is nonpolar (such as 10% methanol in chloroform). Depending on these affinities, different compounds adsorb to the hydrated silicate at different positions as the nonpolar solvent migrates up the stationary hydrated silicate. This principle is illustrated in Figure 24.2 . For a given solvent system, the ratio of the distance traversed by the compound to the distance traversed by the solvent front is a constant for the compound and can be used to identify the compound in a mixture. This ratio is called the r f . This technique is central to identifying different drugs of abuse, many of which can be separated from one another using TLC. The method has been packaged in the form of Toxi-Lab kits (Irvine, CA) in which the user is supplied with discrete strips of silicate, extraction solvents, and color-developing solutions.
The best specimen for drug detection by chromatography is urine because large quantities can be collected noninvasively. Once a urine specimen is collected, it is subjected to concentration and extraction procedures. In extraction procedures, acidic drugs are separated from basic ones. Almost all drugs of abuse are basic drugs, all of which are amine derivatives. The important acid drugs comprise almost exclusively the barbiturates. In aqueous solution, the basic drugs are charged because of the equilibrium in Equation 24.4A :
in which a primary amine (secondary and tertiary amines exhibit the same equilibrium) is represented by RNH 2 . The ammonium ion form (right side of Eq. 24.4A ) is soluble in water but not in nonpolar organic solvents. However, the amine-free base (left side of Eq. 24.4A ) is soluble in nonpolar organic solvents. Extraction procedures to isolate the basic drugs are aimed at treating the urine with base so that significant amounts of the basic drugs will be uncharged as the amine-free base ( Eq. 24.4B ). This form can then be extracted into a nonpolar organic phase and applied to the silicate strip. The reverse process is carried out for acidic drugs (i.e., these are treated with acid and are extracted into nonpolar solvents). In practice, a small paper disk is added to the organic extraction mixture; the solvent is then evaporated so that all basic drugs adsorb onto the paper disk. This disk is then applied to one end of the silicate strip, and the strip is placed in the migrating nonpolar solvent. A separate strip is used for each extraction; the A strip is used for basic drugs and the B strip for acidic drugs. The chief utility of the B strip is in identifying the barbiturates, as is discussed subsequently.
After separation of the drugs on the plate, it is necessary to identify them. This objective is achieved by subjecting the drugs to standard color reactions for each separate compound. In this procedure for basic drugs, the strip is simply dipped successively in three different solvents, which results in characteristic color patterns for each drug. The strip is also subjected to ultraviolet (UV) light, which excites fluorescence in selected compounds. Similar procedures are used for the acidic drugs extracted onto the B strip. As shown in Figure 24.3 , which is from the Toxi-Lab reference pattern book, each drug can be identified not only by its r f but also by its color and characteristic color change in different reagents. These patterns are reinforced by the fluorescence characteristics. As an example, notice in Figure 24.3A on the Toxi-Lab A worksheet that morphine, the main metabolite of heroin, has a characteristic r f of 0.14 and a characteristic dark-red or purple color in the first solvent that diminishes in intensity and changes color in the second solvent, water. It is nonfluorescent. If one or more of these characteristics differ(s) from this pattern, strong doubt about identification of the spot as morphine would exist. If all criteria are met, the sensitivity and specificity of the method are increased.
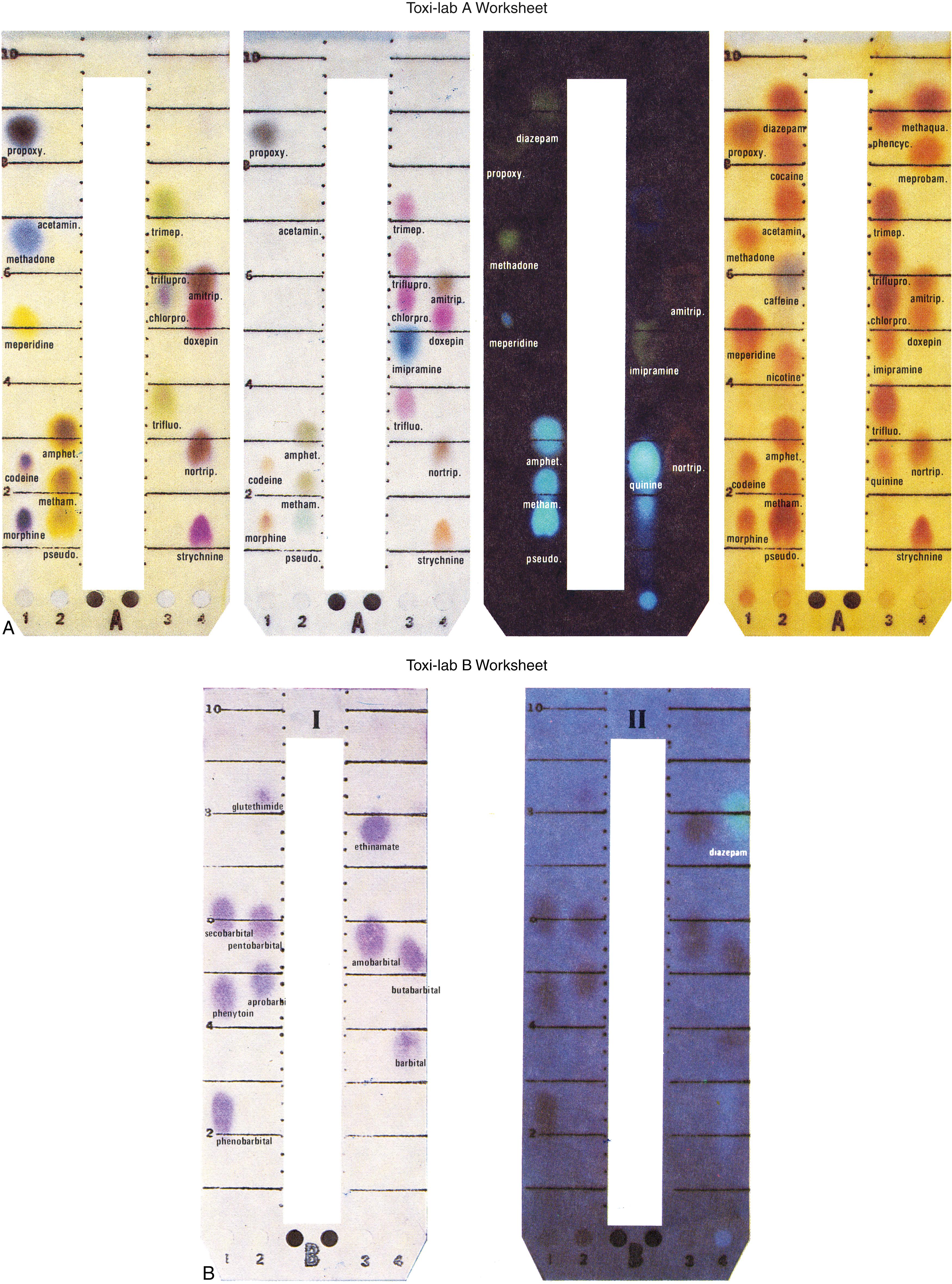
Because the identification of each drug depends on the use of qualitative color changes and/or the presence of fluorescence, the sensitivity of the method is limited by the ability of the naked eye to detect these changes. Practically, the level of detection is on the order of 1 μg/mL of compound present on the strip. The chief value of the chromatographic method is confirmatory (i.e., confirmation of a positive immunoassay test result). The two methods are often performed together on a single specimen.
The major problems that occur with this method are that extraction procedures are occasionally inefficient so that insufficient amounts of drug are absorbed onto the disk. Also, the extraction and evaporation procedures are somewhat time-consuming, requiring approximately a half-hour for full processing. Furthermore, cocaine has a number of metabolites that are polar (e.g., ecgonine) and that barely migrate from the origin. Thus, it is sometimes difficult to detect the presence of this drug of abuse because it has been converted completely to the polar metabolites before excretion. Some difficulty may also be encountered in distinguishing among various opiates, such as between morphine and other opiates, because the r f values of these drugs can be close to one another (see Fig. 24.3 ). However, experienced personnel can make this distinction in most cases. Also, some nontoxic drugs may give characteristic color changes and r f values that are similar to those for the drug of abuse. A case in point is that certain antihistamines appear on the A strip to be very similar to amphetamines.
TLC allows direct qualitative detection of drugs in a panoramic way. HPLC allows quantitative detection of drugs and allows sharper separation of these same drugs. In HPLC, the stationary phase, which can be either polar (silicic acid) or nonpolar (such as the C-18 columns), in reverse-phase chromatography is composed of uniform, ultrafine particles that vastly increase its adsorptive surface area. This stationary phase is packed into a column. The resistance to flow in this column is high so that large pressures are required to deliver constant reasonable flow rates. In the Waters HPLC instrument (Waters Corporation, Milford, MA), a constant pressure head is delivered to the column by the use of two pumps that operate so that as one withdraws, the other pushes forward (i.e., the two operate 180 degrees out of phase). The eluate from the column is monitored by a variety of detectors, ranging from UV multiwavelength detectors to redox potential electrode detectors. It is the usual practice in performance of quantitative HPLC to use an internal standard—that is, a compound similar in structure to the drug(s) of interest, which is added to the specimen to be analyzed in a known concentration. By knowing how much of this marker compound or internal standard is placed on the column and how much is recovered from the column in the eluate, the percentage recovery from the column can be calculated for this compound and, by extrapolation, for all of the drugs of interest for which concentrations are being quantitated. Thus, losses due to the column (in addition to losses in extraction procedures) can be corrected for using this technique. Generally, HPLC has been used for the quantitation of specific therapeutic drugs but has found use in detection of cocaine and heroin in urine. The sensitivity of the method is in the nanomolar to micromolar range.
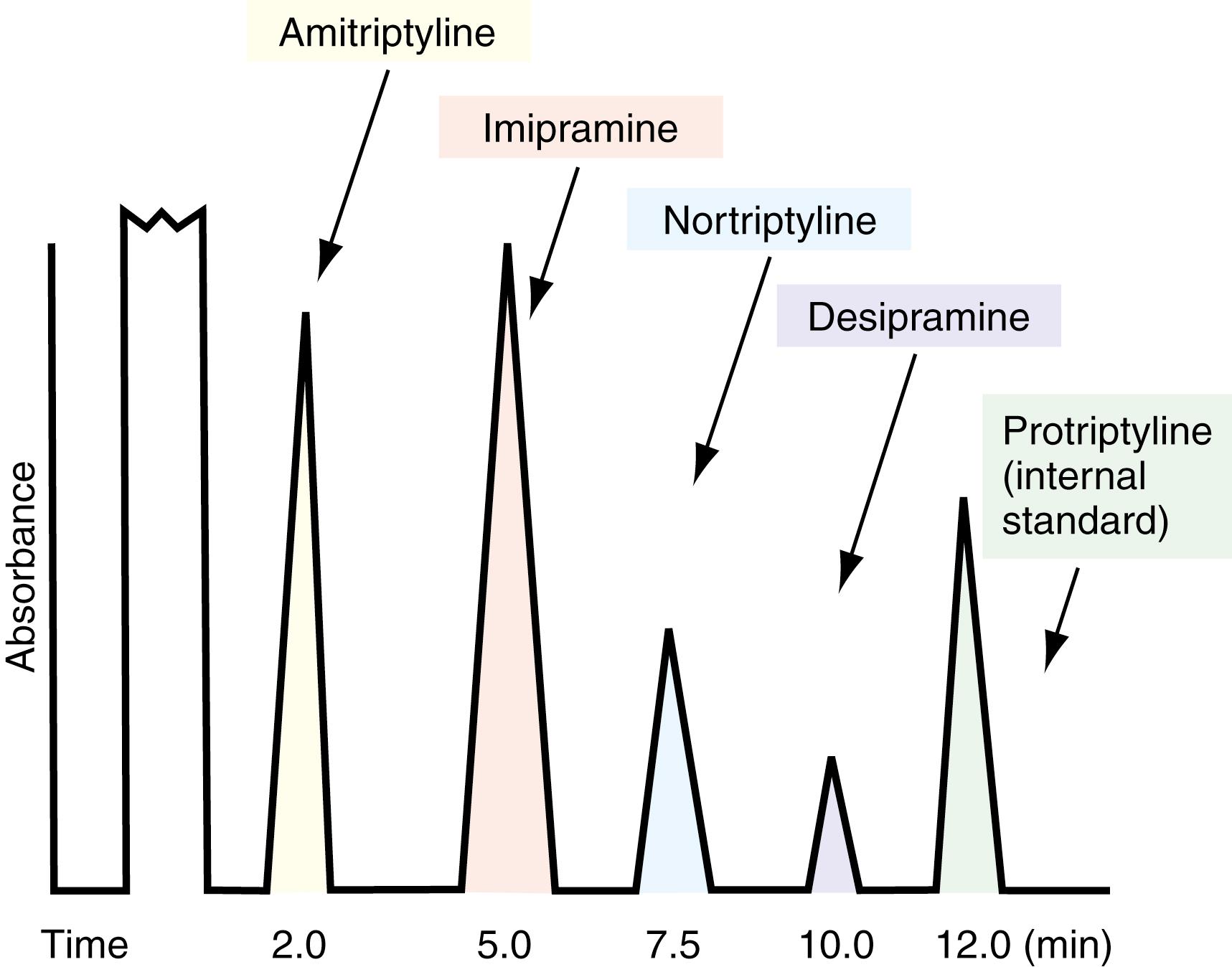
One of the greatest uses of HPLC is in the separation and quantitation of the tricyclic antidepressants and their metabolites. These are among the most commonly prescribed drugs and are also used in excess as drugs of abuse in suicide attempts. It is often necessary to determine the levels not only of the parent tricyclic antidepressant but also of its active and inactive metabolites reviewed subsequently. Figure 24.4 shows a typical separation on a silicate column. Protriptyline, a less inactive tricyclic compound, is the internal standard. It is clear that the separation among the metabolites and parent compounds is quite sharp. This separation is completely reproducible.
A variant of TLC that includes the advantages of HPLC is capillary electrophoresis (CE) ( ; ). In this method, a capillary tube lined with silicate, 10 to 100 μm in internal diameter and 100 to 1000 mm in length, is used as the solid support in an electrophoresis apparatus. Here, the driving force for separation is the voltage (on the order of 25 kV) rather than pressure, as in HPLC. Because of the vast surface area, separations are quite sharp. The system is highly versatile and can be used to separate serum proteins and small molecules. CE possesses a wide analytic spectrum (including biopolymers, pesticides, aromatic compounds, drugs, inorganic ions) because of high versatility in terms of separation modes. Different analyte selectivity is based on different physicochemical principles of separation without changes in instrumental hardware—a distinct advantage of this technique. Capillary zone electrophoresis, micellar electrokinetic capillary chromatography (MECK), capillary isotachophoresis, capillary isoelectric focusing, capillary electrochromatography, and capillary gel electrophoresis are different separation modes utilized in CE separations. For example, by adding a detergent to the buffer system, as with MECK, micelles will form. A neutral compound (such as a pesticide) will partition into the detergent, forming micelles, and the complex will separate out based on the mobility/charge of the micelle. In addition, new platforms such as chip-based CE and immunoaffinity CE are currently being developed. CE has not yet enjoyed as widespread use as the immunoassay techniques (discussed previously) in clinical toxicology but is commonly utilized in analytic forensic toxicologic studies as well as in molecular diagnostic studies and can easily be extended to other uses in clinical laboratory medicine ( ; ; ). Recent studies have compared CE to HPLC in the determination of asialo-transferrin (Tf), a crucial marker for the reliable interpretation of carbohydrate-deficient transferrin (CDT), to serve as biomarkers in the study of alcohol abuse disorders ( ). CE allowed the identification of asialo-Tf in 108 out of 165 CDT “positive” cases, based on disialo-Tf measurement (cutoff, 1.8%), whereas HPLC showed a detectable asialo-Tf peak in only 2 cases. In addition, in some cases of disputed CDT increases, the quasi-absence of this Tf component in front of an important increase of disialo-Tf allowed the ruling out of a diagnosis of alcohol abuse, in agreement with all other clinical and laboratory data. These studies showed a superior performance of CE versus HPLC for the determination of asialo-Tf and the importance of this CDT component to avoid misinterpretation of non-alcohol–related CDT increases.
Testing for drugs of abuse has become one of the most rapidly developing areas in the clinical laboratory in view of the widespread and ever-expanding use of these drugs among large segments of the working population. In view of the increasing requirements for routine drug screening, it is necessary to have gold standard techniques to confirm the results obtained using screening methods such as EMIT and TLC.
GC-MS has proved to be such a gold standard because of its great sensitivity and reliability. This method, as its name implies, involves two techniques: gas-liquid chromatography and mass spectroscopy. In the former, compounds are directly heated into the gas phase or are derivatized to make them labile to facilitate heating them into the gas phase. They are then passed over a column containing the stationary phase, which often consists of a liquid, usually a hydrocarbon or silicone oil, that coats a solid support in the column and offers a large surface area for adsorption. Separation is based, much as in TLC, on the ability of each compound to adsorb to the stationary phase, which partially depends on the relative solubilities of the compound in the gas versus liquid phase. Normally, the compounds eluting from the column could be detected by conventional techniques, as discussed previously, except that once the compounds are in the gas phase, where they are heated, advantage can be taken of another feature of the system—the ability of compounds that are heated to high temperatures to lose or gain electrons.
The technique of mass spectroscopy is discussed comprehensively in Chapter 5 . Here, we summarize briefly the salient features of this technique. At high temperatures, the highest energy electrons of a compound (i.e., the ones of lowest ionization potential) can be excited such that the molecule can lose electrons and become charged. This process may be aided by such techniques as electron bombardment in a specially designed chamber that directly creates molecule-ions. Most of these resulting molecule-ions are single cations. Different molecule-ions in general have different sizes and different molecular weights. These molecule-ions decompose into characteristic fragments whose ratios with respect to one another and whose positions of migration relative to one another are also constant. The molecule-ions are then passed through an electric field generated by four rods that are subjected to rapidly alternating currents, the so-called quadrupole detector . Depending on the frequency of the alternating current, certain molecule-ions with specific mass/charge ratios can pass through the field to a detector. Thus, the molecule-ions can be separated on the basis of molecular weight or, more exactly, on their mass/charge ratios. The overall design of GC-MS is shown in Figure 24.5 .
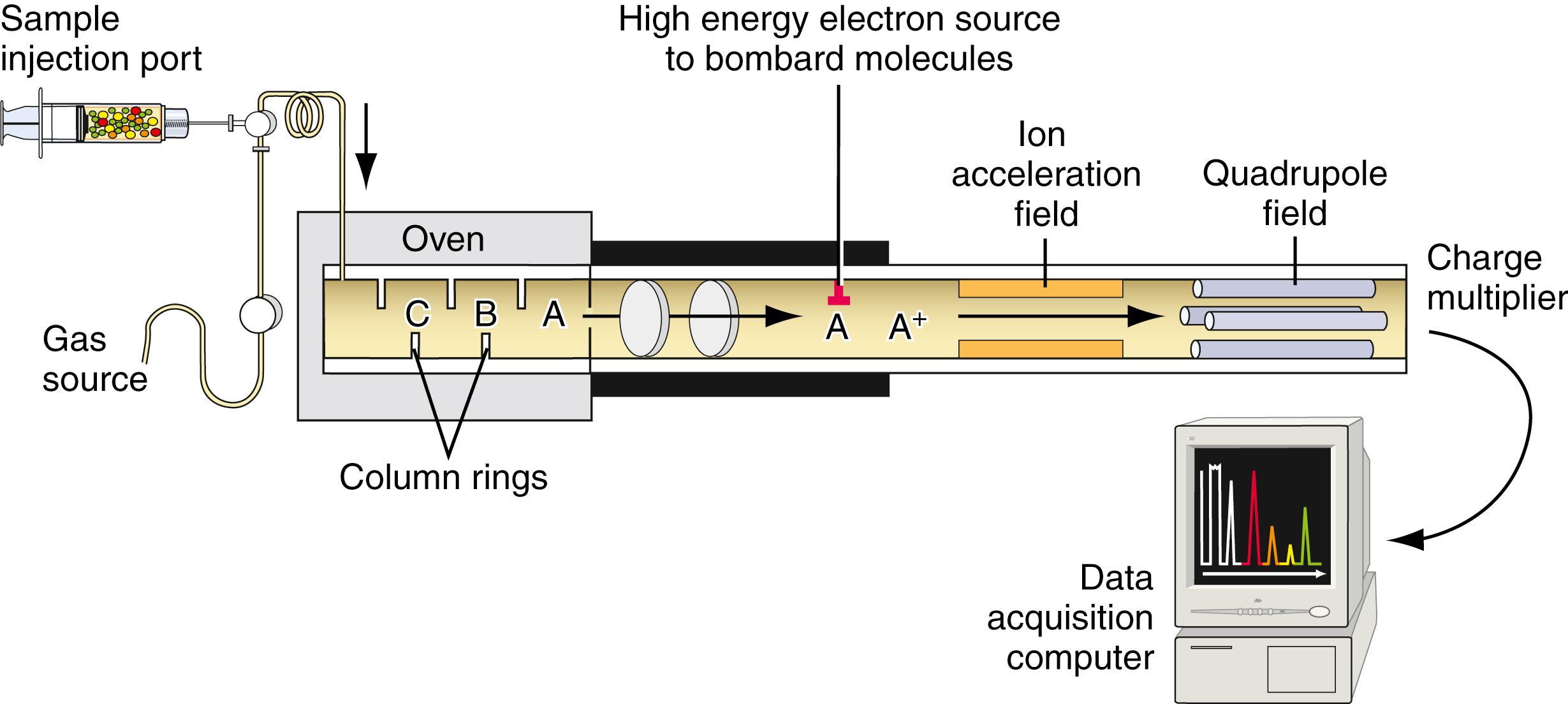
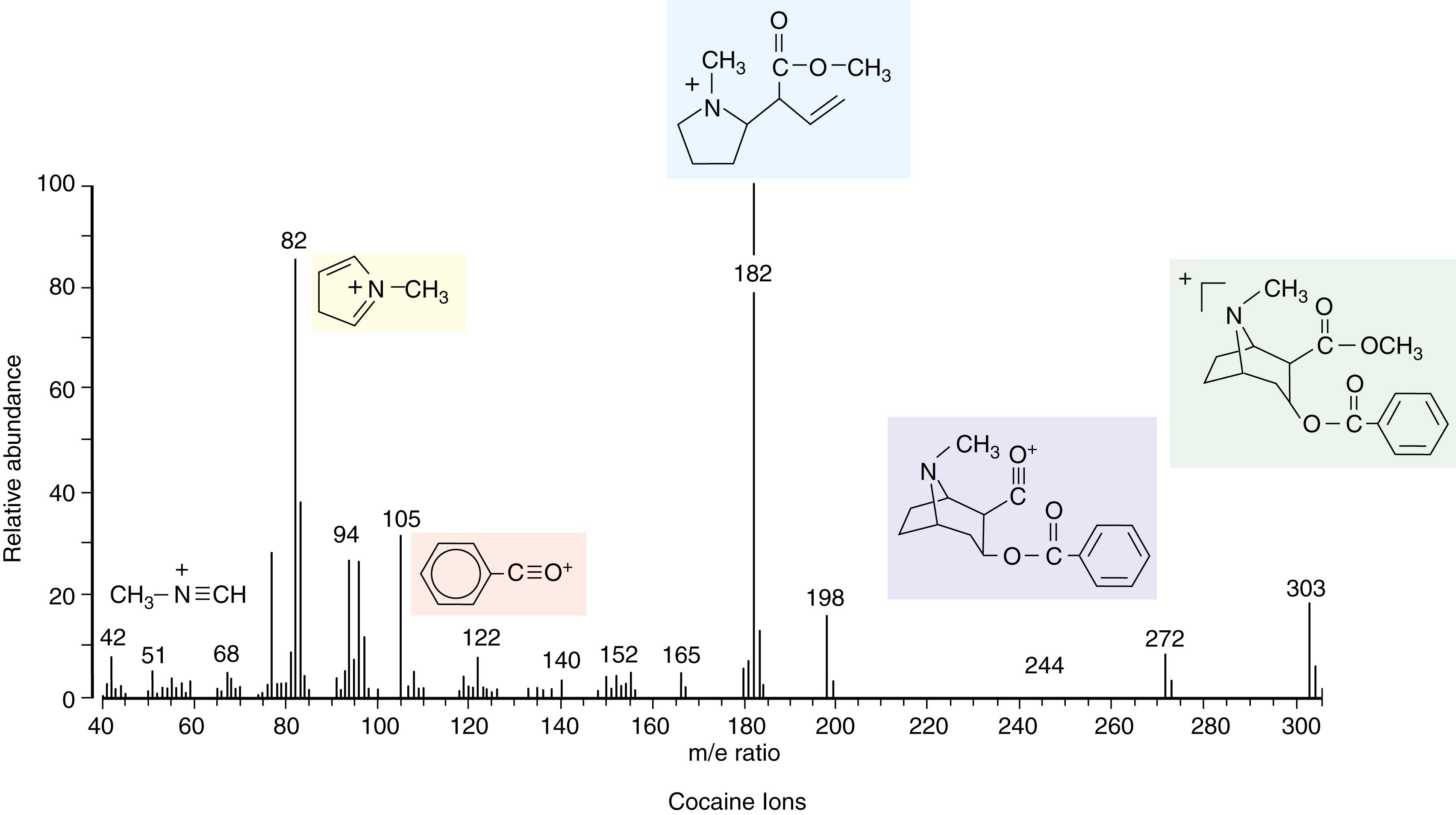
The presence of the molecule-ion on the plate is detected by a charge multiplier detector system. The technique of GC-MS has become highly refined. Each molecule-ion created in the gas phase can undergo further changes, such as elimination reactions and rearrangements, and further degradation to small fragments that, in turn, ionize and give characteristic decomposition patterns. The patterns of thousands of compounds have now been determined. The position of the parent molecule-ion of the compound and the decomposition fragments give rise to a fingerprint pattern unique to the compound. These patterns are stored in a computer so that when a pattern for an unknown compound or group of compounds is obtained, the pattern is compared with the stored patterns to identify the compound(s) of interest. The entire method has been highly successful in detecting even low levels of cocaine and/or its metabolites in body fluids. A typical cocaine pattern is shown in Figure 24.6 . Because single molecule-ion species give rise to significant currents in the detector, it is possible to detect very low levels of drugs, making this technique the ultimate reference method and the best confirmatory testing procedure available at present.
As discussed previously, GC-MS is the gold standard for the identification of volatile compounds. Nonvolatile compounds, however, can be detected utilizing LC-MS ( ; ; ; , ). Unlike GC-MS, however, the coupling of LC with MS requires sophisticated interfaces between the LC and MS components. The interface must volatilize nonvolatile compounds that have been separated on the LC; must remove the liquid solvent from the LC; and must correct flow-rate incompatibility between the LC and MS. Two interface methods, electrospray (ES) and atmospheric pressure chemical ionization (APIC), appear to have become the gold standards for LC-MS. Both interface methods are utilized with atmospheric pressure ionization devices to facilitate removal of the mobile phase. They differ primarily in the range of analytes that can be accommodated and in how the solvent phase is nebulized. Ionizable analytes of high molecular weight and/or high polarity can be accommodated with ES, and APCI can be used for analytes of less polarity and lower molecular weight. At present, ES appears to have more clinical and forensic applications than APCI in toxicology.
LC-MS can be used to both screen and confirm positive test results from a screening assay. It has been used for confirmation of drugs-of-abuse assays, poisoning detection in acute or chronic intoxication, therapeutic drug identification and quantitation, and pharmacokinetic and drug metabolism studies. Although LC-MS has limitations when compared with GC-MS, it has become a powerful “mature and validated” ( ) technique in analytic toxicology and a complementary method to GC-MS. To this end, LC-MS/MS offers significant advantages over other traditional testing, such as immunoassay and gas chromatography–mass spectrometry (GC/MS) methodologies. Major strengths of LC-MS/MS include improvement in specificity, flexibility and sample throughput when compared with other technologies ( ).
LC-MS/MS offers much better specificity for a target molecule because the quadrupole recognizes not only the original ionized molecule but also all the fragments derived from the original ionized molecule. Combined with the HPLC technique, the separation of the analytes is much easier and the retention time is another characteristic factor that enhances the selectivity of the mass spectrum.
LC-MS/MS exhibits flexibility and versatility for the clinical labs to develop and validate new assays in house within a short time. LC-MS/MS assays, as laboratory-developed tests, are highly attractive for target analytes where no commercial immunoassays are available. In contrast to GC/MS, which is limited to volatile molecules, LC-MS/MS has a much wider range of applications since most biologically active molecules are polar, thermolabile, and nonvolatile. In addition, LC-MS/MS sample preparation is simpler and does not require derivatization techniques. Although LC-MS/MS offers the flexibility of developing assays to meet the clinical needs, the shortcoming of the flexibility is that method development, validation, and quality control may vary among different laboratories because most of the LC-MS/MS methods used in the clinical labs are often laboratory developed. Accuracy experiments are a prerequisite step in method validation to ensure that the interlaboratory result variance is less than 20% ( ).
Infrared (IR), or Fourier-transformed infrared, spectroscopy (FTIR) utilizes light of high wavelength (low frequency), which excites vibronic states of molecules involved in bond stretching and bond angle bending. Every compound has a characteristic IR “fingerprint,” that is, IR absorption pattern, so that even closely related compounds can be distinguished readily from one another by gas-phase IR. Thus, the fractions of patients’ samples that elute from a GC or liquid chromatography column can be analyzed using this technique. As with mass spectroscopy, the FTIR spectra for different compounds can be stored in a computer attached to the GC-FTIR system. The spectra obtained from the eluates from the column are then analyzed for the presence of specific drugs of abuse. This method is particularly well suited for the detection of amphetamines ( ).
In addition to conventional blood and urine testing using fresh fluid samples such as blood and urine, dried blood spot (DBS) sampling is emerging as a minimally invasive microsampling technique. DBS testing provides advantages for analyte stability, transport, and ease of storage, making it an attractive matrix in forensic and clinical settings. Recent studies have demonstrated successful determination of various drugs, including illicits such as cocaine, amphetamine, methamphetamine, MDA, MDMA, morphine, codeine, 6-AM, cocaine, benzoylecgonine, methadone and EDDP, that were variably comparable to conventional testing ( ; ; ; ). These methods employ select aspects of DBS extraction systems coupled with standard LC-MS/MS configurations, high-resolution mass spectrometry (HRMS), and ultra-performance liquid chromatography (UPLC), among others. Concerns pertaining to DBS include hematocrit (Hct) levels, which can affect diffusion rates on the absorbent filter paper, matrix effects, and recovery bias that may impact on the accuracy of an analytic result, possibly leading to an under- or overestimation of the analyte being assessed ( ). Various strategies have been designed that maintain the benefits of DBS but eliminate the Hct-based area bias, such as heated flow-through desorption, fixed volume capillary dispensation (HemaPEN) to filter paper spots, and precision volumetric absorptive microsampling (Mitra), among others ( ; ).
In most states of the United States, two levels of testing for drugs of abuse have become recognized: emergency department/point-of-care (POC) testing and employment screening/forensic testing. The former involves rapid, stat screening methods, in particular, EMIT (or FPIA) and TLC. The purpose of this type of screening test is to detect the presence of a drug or several drugs of abuse in the patient’s urine. Rarely are the more sophisticated chromatographic procedures such as HPLC and GC-MS used for this purpose.
Forensic testing, on the other hand, requires not only a screen but also an independent confirmatory method, which is almost always chosen to be GC-MS or, less commonly, HPLC. It should be noted that, strictly by law, any confirmatory method is valid provided that it is a completely different method from the primary one. Thus, TLC can confirm EMIT, whereas FPIA cannot confirm it because both EMIT and FPIA are immunochemical methods. Another important legalistic consideration in forensic testing is the chain of custody ( ; ; ). This process is used in the collection of urine from the individual from whom the specimen is taken. It may begin by observation of specimen collection by one person. Then, that person or another specifically designated individual, usually a police officer (in the case of prisoners or suspects) or some other designated official, accompanies the messenger, who brings the specimen from the individual to the laboratory. This individual is a witness to the testing (and must sign a legal document to this effect) of the specific urine sample collected. More than one designated individual may be involved as the witness in this chain.
Specimens submitted for urine toxicology testing may be manipulated during the preanalytic phase of collection ( ). These can encompass metabolic “tricks” that a patient can incorporate prior to providing a urine sample as well as postvoiding to mask the presence of a substance that may cause clinical and/or legal issues if detected, including dilution, substitution, and adulteration.
The simplest of these interferences involves diluting one’s urine to the point that the concentration of the drug in question is below the detection limits of the test. The subject either adds liquid to the urine sample or drinks something that causes the urine to be very dilute. Though this is a very effective technique, it can be easily detected by the testing facility either by observing the collection process wherein any manipulation can be directly observed and/or by detection of abnormally low values for urine creatinine and specific gravity. A normal urine has a creatinine greater than 20 mg/dL, along with a specific gravity that is greater than 1.0030. If a urine specimen has both creatinine and specific gravity values below these cutoffs, the urine is considered to be dilute.
Subjects can attempt to substitute their urine with either someone else’s urine or with a urine-like liquid. This type of interference is detected with either an observed collection, recording the temperature of the urine immediately after collecting, and/or the testing facility testing the creatinine and specific gravity of the sample. A sample that is not between 90° F to 100° F is not humanly possible and should be questioned as to the source of the specimen (NRC Regulations Title 10, Code of Federal Regulations, Part § 26.111). Additionally, a creatinine that is less than 2 mg/dL with a specific gravity that is less than or equal to 1.0010 or greater than or equal to 1.0200 is not physiologically possible and is thus considered to be substituted.
Another way a sample can be tampered with is by the addition of a substance that interferes with screening test, causing the antibody to not bind to the drug in question. Specimen validity testing for oxidative substances and the pH of the sample usually detects these and other types of interference. A specimen will be reported as substituted when at least one of the following criteria is met:
pH < 3 or ≥ 11
Nitrite ≥ 500 μg/mL
Chromium (VI) is present
A halogen (e.g., bleach, iodine, fluoride) is present
Glutaraldehyde is present
Pyridine is present
A surfactant is present
There is variability among clinical collection sites with regard to adherence to ascertaining whether strict collection protocols are part of a standard operating procedure and enforced. Thus, careful assessment of collection processes in light of an inconsistent laboratory toxicology result can often shed light as to whether adulteration or specimen mishandling, including accidental patient specimen mix-up, may have taken place.
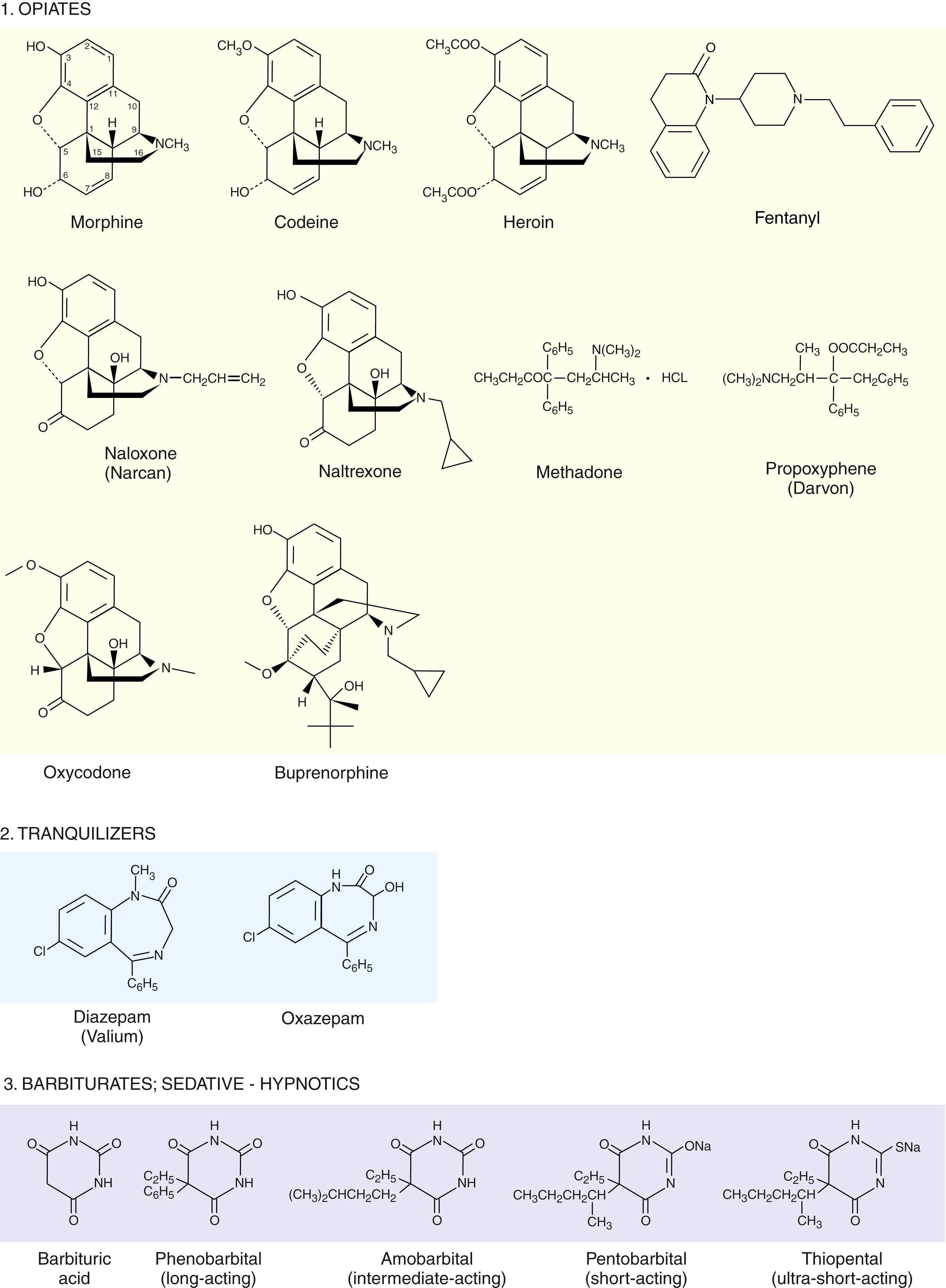
The major drugs of abuse ( ; ) are shown in Figure 24.7 ( ; ). As may be seen in this figure, these drugs, with the exception of the barbiturates and cannabinoids, are all basic amino group–containing compounds, most of which also contain benzene rings. The steric relationship of the amino group with respect to the aromatic benzene rings is rather similar, especially in cocaine, the opiates, and methadone. As might be expected, these compounds can cross-react, although with lower affinities, with each other’s target receptors. The primary physiologic mechanisms of action of these drugs are not well understood, but some rudimentary knowledge has been gained as to some of the main targets of these drugs. Many of these drugs act directly on dopaminergic and norepinephrinergic neurotransmitter systems, especially the limbic system (sometimes referred to as the smell brain ). This system is a more primitive one associated with pleasure seeking and is discussed further later.
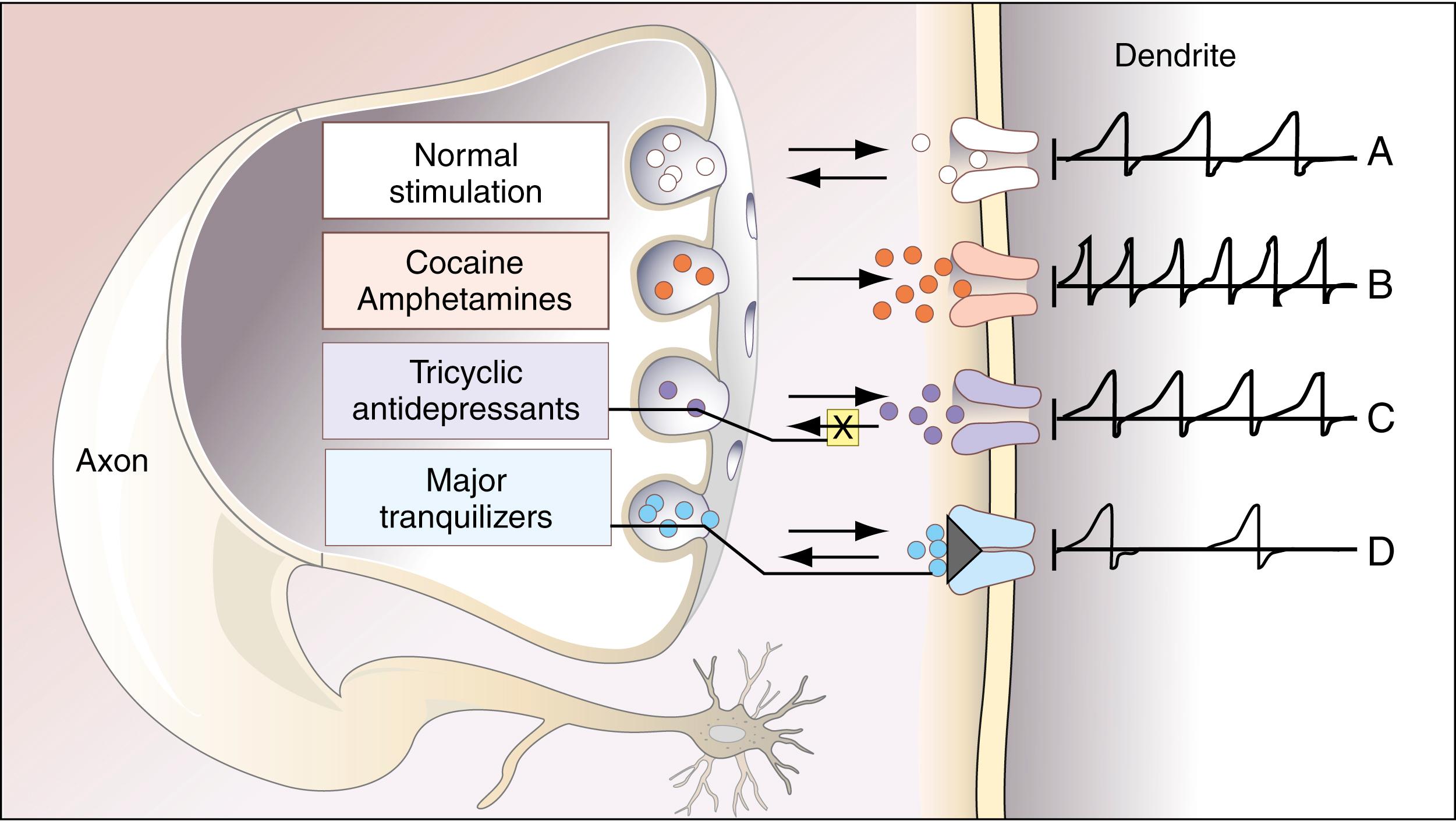
In Figure 24.8 , possible effects of several of the most important drugs on this system are shown. It appears that the amphetamines, closely related structurally to dopamine and the catecholamines, and cocaine cause release of dopamine from the vesicles at the axonal side of the synapse, which may partially be responsible for their producing a pleasant sensation (a so-called high ) in many individuals ( ). The tricyclic antidepressants stimulate pathways that utilize norepinephrine as the neurotransmitter. These pathways, like the dopaminergic pathways, are involved in arousal and pleasure seeking. In this case, the tricyclic antidepressants, rather than promoting release of the neurotransmitters, block the reuptake of norepinephrine into the vesicles on the axonal side of the synapse. They also may exert nonspecific reuptake blockade of dopamine in the dopaminergic pathways ( ). It is of great interest that, paradoxically, the tricyclic antidepressants such as imipramine (Tofranil) have been used successfully to treat the effects of cocaine, although, as described later, benzodiazepine tranquilizers are now preferred. The major tranquilizers, such as haloperidol (Haldol) and chlorpromazine, used to treat psychotic states such as schizophrenia, appear to block attachment of dopamine to the dendritic receptors in the synapse, thereby blocking the stimulatory effects of dopamine. Associated with many dopaminergic neurons are inhibitory neurons that use γ-aminobutyric acid (GABA) as their neurotransmitter. It appears that many benzodiazepine receptors exist on these neurons, causing potentiation of GABA at the synapses in this system, reducing the dopaminergic effects of the stimulatory pathways on the limbic system. Thus, some of the tranquilizing effects of diazepam (Valium) and other benzodiazepines can be explained.
Widely distributed throughout the central nervous system and periphery are a variety of opioid receptors classified mainly as μ-, δ-, κ- and ε-receptors ( ; ; ). The μ-receptors appear to be specific for morphine and heroin, both of which produce a general analgesic state. These opioid receptors are discussed further in the following section.
Many of the drugs of abuse also act on two other major pathways in the brain: those using serotonin (serotonergic) and those utilizing N -methyl- d -aspartate (NMDA) and L-glutamate as their neurotransmitters. Neurotransmission by serotonin occurs by its binding to the 5-hydroxytryptamine (5-HT) receptor on the dendritic side of the synapse. There is a rather wide range of 5-HT receptors, not all of which produce the same physiologic effects. The major ones appear to be 5-HT 1 and 5-HT 2 receptors. The serotonin pathways encompass a rather wide swath of the brain and even the spinal cord. This neurotransmitter is the principal one for the limbic system and, in addition, for the basal ganglia, especially the amygdala, which is involved in aggressive behavior. As mentioned earlier, the limbic system is involved in pleasure seeking and pleasure reinforcement. Serotonergic pathways also extend to the hippocampus and are involved in memory. As a neurotransmitter in the spinal cord, serotonin induces muscle contraction. NMDA pathways are more involved in nociceptive (pain) pathways and are involved in memory and neuronal plasticity ( ). They have been found to be involved in chronic pain reinforcement. Blockade of NMDA pathways by drugs of abuse can therefore remove this perceived undesirable effect.
Overall, in the U.S. population as of 2017, there has been a general increase in the use of all drugs of abuse in individuals 12 years of age and older, about 51.8 million individuals compared with 48.5 million in the previous year, representing an ≈6.6% increase ( ; www.cdcfoundation.org ). Although these data refer mostly to cannabinoid (marijuana) use (≈79%), compared with other illicit drugs, misuse/abuse of other prescribed pharmacopeia, such as psychotherapeutics, account for another substantial group (≈18 million). The highest number of drug abusers (≈75%) occurs in 15 to 39 year old individuals. Whites and low-income adults have shown increased odds of lifetime prescription opioid use disorder. Interestingly, individuals in the 40- to 59-year-old bracket comprise most of the remainder, numbering over 20% of the overall number of drug abusers. Persons older than 26 years have a 20% to 25% lifetime probability of using cocaine whether they are working full-time, part-time, or are unemployed. Health care costs were ≈$80 billion in 2013, including ≈$29 billion in health care and substance abuse treatment as well as ≈$20 billion in lost productivity costs. Overdose deaths were >80,000 Americans in 2018 compared with >33,000 Americans in 2015, an increase of 2.5 times and 4 times the number in 2002. It is interesting to note that 3.8 million Americans reported nonmedical use of prescription opioids (2015 data) and that nearly one in every two adolescents who reported the medical use of prescription opioids after initiating nonmedical use of prescription opiates had two or more substance use disorder symptoms at age 35 years (McCabe, 2019). It is also important to note that drug abuse does not spare any age, race, gender, or socioeconomic, employment, or educational status, demonstrating the scope of the issue.
Cocaine is derived from the coca plant and has enjoyed much popularity as an additive to certain foods. At the beginning of the 20th century, it was used in Coca-Cola, but owing to its addictive effects, this practice was discontinued. Cocaine is a derivative of the alkaloid ecgonine (i.e., the methyl ester of benzoylecgonine), as shown in Figure 24.7 (Group 4 drugs). The normal route of administration of cocaine is nasal (i.e., inhalation, called snorting ), such that the drug passes through the nasal membranes. A particularly potent form of cocaine, called crack, is the free-base form that passes rapidly across the nasal membranes such that, for a given dose, most or all of it enters the bloodstream rapidly. The half-life of cocaine is 1 to 2 hours; the parent compound and its metabolites are usually cleared from the body within 2 days.
It is estimated that as many as 25 million people in the United States have used cocaine at least once ( ); fortunately, most of these individuals do not continue. Fatalities from cocaine abuse are of two types: direct toxicity of the drug ( ) and crime related to the illicit acquisition of the drug. Up to 25% of myocardial infarctions in patients between 18 and 45 years of age have been attributed to cocaine abuse ( ).
Cocaine is used medically to induce local anesthesia during nasopharyngeal surgery. However, in large doses, it induces a euphoric state (the “high” experienced by the user) and may also induce hallucinatory states. It can also promote violent behavior ( ; ). Many of these results can be explained by cocaine’s dopaminergic effects. One study ( ) suggests that cocaine induces increased calcium ion influx in dopaminergic neurons. The increased intracellular calcium activates phospholipases that possibly act as second messengers in causing ultimate release of dopamine in synapses. Prolonged action of phospholipases, however, ultimately causes cell death. In the previously mentioned study, in fact, cocaine was found to be neurotoxic. It also has a general cytotoxic effect from formation of an N -oxide free-radical produced in the metabolism of this compound in the liver. It appears then that, over time, cocaine induces neuronal loss. In addition, binding of cocaine to cell receptors in the limbic system induces synthesis of cyclic adenosine monophosphate (cAMP) that appears to be critical in activating cell processes involved in dopamine release ( ). Cocaine may also block the reuptake of dopamine at the axonal side of the synapse. As if becoming toxic from cocaine abuse were not sufficient, many cocaine abusers consume this drug together with alcohol. Ethanol becomes esterified to cocaine in the liver to form cocaethylene, which blocks reuptake of dopamine in dopaminergic pathways more effectively than does cocaine and causes pronounced vasoconstriction of the coronary arteries, inducing increased myocardial oxygen demand. This cocaine derivative is deadlier than either cocaine or ethanol alone ( ).
Studies ( ; ; ; ) further indicate that prolonged use of cocaine results in cardiotoxicity—that is, cocaine can cause progressive atherosclerosis and causes constriction of the coronary arteries that can, in turn, induce myocardial ischemia and sometimes frank infarction. Cocaine has been found to induce sympathomimetic effects on the myocardium by increasing heart rate. At the same time, it induces increased vasoconstriction. The net effect is increased chronotropy and afterload, resulting in increased oxygen demand by the myocardium. At the same time, cocaine induces platelet aggregation and stimulates production of plasminogen activator inhibitor ( ). These events all predispose to development of myocardial infarction.
One highly disturbing aspect of cocaine abuse is the fact that cocaine passes readily across the placenta and into the lactating mammary gland, thus is readily passed from mothers to nursing infants. Often in the hospital setting, mothers receive the drug from dealers and breastfeed their newborn babies, who are therefore maintained on this drug. Cocaine causes mental retardation, delayed development, and strong drug dependence in newborns. It can also produce malformations in utero.
Cocaine has not been considered classically to be an addictive drug, as it does not cause the true physical dependence typical of abusers of barbiturates and opiates. However, the high produced by the drug is extraordinarily reinforcing so that the drug-seeking behavior of the cocaine and opiate abuser is similar. Evidence in experimental animals suggests that cocaine can induce the release of β-endorphins that bind to μ-receptors in the limbic system ( ). This induces a pleasant and positive feeling of reinforcement. Clinically, patients who are overdosed with cocaine may become violent and irrational, requiring sedation. The treatment of choice for patients in hyperexcitable states with cardiac symptoms such as palpitations is one of the benzodiazepines. Thus, it is not uncommon to find cocaine and Valium in the urine of cocaine addicts. Occasionally, overdosed patients will become obtunded or comatose. The treatment for these patients is usually supportive. As noted in the previous section, antidepressants, including the tricyclics, and selective serotonin reuptake inhibitors (SSRIs), such as fluoxetine (Prozac), have been found to inhibit some of the undesirable effects of cocaine and have been used in the treatment of cocaine abuse.
The half-life of cocaine, as stated previously, is approximately 1 to 2 hours. It is metabolized to more polar compounds that have significantly less potency than the parent compound. These metabolites have longer half-lives and, with techniques such as GC-MS, can be detected up to 48 hours after administration of the drug. The immunoassay methods can detect the drug for about 24 to 36 hours after administration. If a patient has inhaled cocaine free-base (“crack”), it is possible to detect the parent compound, cocaine, by TLC up to several hours after administration owing to the high doses of drug present.
The primary medicinal use of opiates such as codeine and morphine is to diminish or eliminate pain in a patient. As noted earlier, there are several classes of opiate receptors that are involved in the modulation of pain. These receptors are classified as μ, κ, δ, and ε. The endogenous ligands for each of these receptors are the antinociceptive peptides: endomorphin (Tyr-Pro-Trp-Phe-NH 2 and Tyr-Pro-Phe-Phe-NH 2 ) for μ-receptors, dynorphins A (Tyr-Gly-Gly-Phe-Leu-Arg-Arg-Ile-Arg-Pro-Lys-Leu-Lys) and B (Tyr-Gly-Gly-Phe-Leu-Arg-Arg-Gln-Phe-Lys-Val-Val-Thr) for κ-receptors, Met- and Leu-enkephalin (Tyr-Gly-Gly-Phe-Met and Tyr-Gly-Gly-Phe-Leu) for ε-receptors, and deltorphin (Tyr-D-Met-Phe-His-Leu-Met-Asp-NH 2 ) and Met and Leu-enkephalin for δ-receptors. Note that the first five amino acids of dynorphin are identical to those of Leu-enkephalin, suggesting a possible reason for the binding of enkephalin to κ-receptors. The exogenous opiates (e.g., morphine, codeine, fentanyl) whose structures are shown in Group 1 of Figure 24.7 , are known to be agonists, primarily, for μ-receptors. As can be seen in this figure, morphine, codeine, heroin, oxycodone, and buprenorphine have quite similar structures. In fact, morphine is a metabolite of heroin, the diacetyl precursor of morphine.
A major target for each of the endogenous opiate peptides and for the exogenous agents shown in Figure 24.7 is the main pain pathway (i.e., the spinothalamic tract). This neural pathway carries nerve impulses from peripheral pain receptors to the peripheral nerves that innervate them to the posterior horn of the spinal cord where the nerves synapse to ascending fibers in the spinothalamic pathway. These nerves travel to the next spinal level where they cross the midline and then travel to the medulla as the lateral lemniscus that synapses in the thalamus (mainly in the ventroposterior nucleus [VPN]) and then projects to the cortex where pain perception takes place. Activation of the opioid receptors that occurs at these synapses results in hyperpolarization of the dendritic side of the synapses, blocking nerve conduction, thereby diminishing the sensation of pain.
In addition, it appears that the opioid receptors also play another major role in pain modulation via the activation of descending tracts that emanate from the midbrain in the periaqueductal gray area and travel to nuclei in the median raphe of the medulla. After synapsing with these nuclei, these tracts synapse at interneurons in the posterior horn of the spinal cord where they activate the release of GABA, resulting in inhibition of nerve conduction in the spinothalamic tract. Normally, these pathways are quiescent, but release of any of the endogenous antinociceptive peptides or introduction of exogenous drugs (e.g., morphine or codeine) results in removal of inhibition of these pathways, again resulting in inhibition of nerve conduction in the spinothalamic pathway with diminished perception of pain.
Morphine, a μ-receptor agonist, in addition to acting on pain pathways as described earlier, further acts by binding to μ-receptors in the limbic system (central nervous system [CNS]), mainly in the nucleus accumbens and the ventral tegmental area, resulting in an analgesic state. Binding of morphine and the other opiates to the μ-receptor inhibits the release of GABA from the nerve terminal, reducing the inhibitory effect of GABA on dopaminergic neurons. The resulting increased activation of dopaminergic neurons results in sustained activation of the postsynaptic membrane, causing a sense of euphoria. On the molecular level, binding of morphine to these receptors activates a cell-signaling cascade via G-protein activation that results in elevated expression of many transcriptionally active proteins such as ERK, jun, and fos, and superactivation of adenyl cyclase, resulting in high intracellular levels of cAMP ( ). Besides being used as a major analgesic, morphine (Dilaudid) is important in treating acute congestive heart failure by lowering venous return to the heart (i.e., it is a powerful preload reducer by causing increased splanchnic pooling of blood).
Heroin induces a pleasant, euphoric state and is highly addictive both physically and psychologically. As can be seen in Figure 24.7 , heroin is a diacetyl form of morphine. This characteristic facilitates heroin’s crossing the blood-brain barrier, allowing it to reach higher concentrations in the CNS. Withdrawal from this drug is exceedingly difficult, with a myriad of symptoms such as hypothermia, palpitations, cold sweats, and nightmares. This is a true physical dependence, the molecular basis for which is not fully understood. It appears that the dependence is strongly linked to the number of cell surface μ-receptors ( ).
This class of compounds exhibits certain important paradoxical effects on the parasympathetic nervous system. These drugs exert a procholinergic effect on the eyes and on blood vessels in the periphery (i.e., they cause constriction of pupils [ pin-point pupils ] and peripheral vasodilatation). In contrast, in the gut they lower gastrointestinal (GI) motility (i.e., they exhibit anticholinergic effects in the GI tract). This fact enables rapid diagnosis of heroin or, in general, opiate abuse in a patient brought to the emergency department in an obtunded or comatose state. These patients typically have severe miosis (pupillary constriction). Although the sign is not useful in acute diagnosis, constipation commonly occurs.
Administration of heroin occurs via the intravenous route. Addicts are readily recognized by the presence of needle tracks on their arms and hands and by extensive thrombosis of their peripheral veins. The half-life of heroin via the intravenous route is about 3 minutes, and the effects of the drug last approximately 3 hours. The major metabolites are N -acetylmorphine and morphine. The half-life of morphine is about 3 hours. Overdoses of heroin are extremely dangerous and can cause severe obtundation, coma, respiratory arrest, hypotension (secondary to histamine release), and cardiac arrhythmias. One of the most common acute therapeutic modalities for heroin overdose is intravenous treatment with naloxone (Narcan; see Fig. 24.7 ), a strong competitive antagonist to the action of heroin. Heroin addiction, as a chronic problem, is treated pharmacologically with a partial agonist of heroin—methadone (shown in the Group 1 structures in Fig. 24.7 ), which is discussed later.
Another opiate antagonist is naltrexone, whose structure is shown in the Group 1 structures in Figure 24.7 . The primary effect of this drug is to lower the euphoria experienced by opiate abusers. However, it has no effect on opiate craving by drug abusers. On the other hand, it has been found to be effective in reducing the physical dependence of patients treated with the drug. However, to achieve abstinence, psychosocial support of the patient is required. Surprisingly, naltrexone has been found to be effective in the treatment of alcohol dependence; in particular, it has been found to reduce relapse rates after abstinence and to reduce heavy drinking to lower levels of consumption. The half-life of naltrexone is about 4 hours. Treatment of opiate abuse requires daily administration of one 50-mg tablet per day (often missed by opiate drug abusers). Excretion is via urine.
The structure of codeine is similar to that of morphine and heroin (Group 1 structures in Fig. 24.7 ). Codeine acts in a manner similar to that of morphine and is used as a milder analgesic and as an antitussive. The codeine analog, dextromethorphan ( d -3-methoxy- N -methylmorphine), is an antitussive agent that is a component of cough-suppressive medications.
Dextromethorphan, an analog of codeine, is the active component of cough syrups because of its antitussive effects. Recently, there has been a “run” on cough medicines by addicts, who can obtain them legally and then consume quantities sufficient to reach their desired euphoric state. Unlike codeine, dextromethorphan is believed generally not to be addictive, although cases of drug dependency have been documented. For therapeutic use, the recommended dose of dextromethorphan is 15 to 30 mg given three to four times per day. Moderate intoxication is achieved at about 100 to 200 mg, and heavy intoxication is reached at around 1500 mg ( ). It is surprising to note that dextromethorphan does not have analgesic properties because of its lack of affinity for μ-, κ-, and δ-receptors. It has been found to induce the release and to block the reuptake of serotonin. Similar to the action of phencyclidine (PCP), discussed later, dextromethorphan has also been found to block NMDA receptors that are critical for neuronal plasticity and memory and are involved in central pain pathways in the brain, as discussed earlier ( ). Dextromethorphan is readily absorbed from the GI tract and, in about 85% of individuals, is rapidly metabolized to dextrophan, an active metabolite, and d -hydroxymorphinane via the 2D6 cytochrome P450 isozyme ( ). It is dextrophan that has a high affinity for NMDA receptors, so that most individuals experience PCP-like effects (i.e., euphoria; tactile, auditory, and visual hallucinations; paranoia; altered time perception; and general disorientation). For the 15% of individuals who are slow metabolizers of dextromethorphan, these effects are much less pronounced and are replaced by sedation and dysphoria ( ). Overdoses of dextromethorphan can result in mainly neurologic effects, such as lethargy, or, conversely, hyperexcitability; ataxia; slurred speech; tremors and fasciculations; hypertonia and hyperreflexia; and nystagmus, as well as either pupillodilation or pupilloconstriction. Diaphoresis may also occur. In addition, cardiovascular sequelae include tachycardia and hypertension. Unfortunately, a number of antitussives contain, in addition to dextromethorphan, anticholinergic agents such as chlorpheniramine. Thus, abuse of antitussive medication can give rise to such symptoms as tachycardia, mydriasis, flushed skin, urinary retention, and constipation.
This drug is effective in reducing pain, especially pain associated with malignancy. Its structure is shown in the Group 1 compounds in Figure 24.7 , where it can be seen to be similar in structure to codeine, with the difference that there is a keto rather than a hydroxyl group at the 6– position (lower cyclohexone ring in Fig. 24.7 ) and a hydroxyl group rather than a hydrogen atom at the carbon between the two bridgehead carbons. On an empty stomach, pain diminution commences within about 15 minutes after oral drug administration. Peak serum levels are achieved in about 1 hour. The slow release form of oxycodone is Oxycontin, which achieves peak serum levels in about 3 hours. Although there is some controversy about the site of action of oxycodone, i.e., primary action on κ-receptors rather than μ-receptors ( ), oxycodone does bind to μ-receptors and one of its metabolites, oxymorphone, is known to have a high affinity for μ-receptors ( ). The half-life for oxycodone is about 3.2 hours and for Oxycontin is 4.5 hours. Metabolites of oxycodone are α- and β-oxycodol, oxymorphone, α- and β-oxymorphol and noroxymorphone, noroxycodone, and α- and β-noroxycodol and noroxymorphone (N-desmethyloxycodone). Most of the parent compound and its metabolites are excreted in the urine.
As with morphine and codeine, oxycodone, especially at high doses, can induce euphoria and a sense of well-being. Like the other opiates, it also induces a true physical dependence. At high doses, side effects are particularly pronounced, which include fatigue, dizziness, constipation, vomiting, anxiety, shallow breathing and apnea, hypotension, meiosis, circulatory collapse, and death. Withdrawal from oxycodone includes such symptoms as myositis, anxiety, nausea, insomnia, fever, hypogonadism, and hormonal imbalance. Given the side effects and the physical consequences of withdrawal, it is difficult to fathom the appeal of abuse of this drug. Nonetheless, abuse of oxycodone has grown to the point where it has now been added to the roster of drugs of abuse that are routinely assayed for mainly in urine.
Although, like oxycodone, this prescription drug has become a drug of abuse, buprenorphine is a mixed agonist-antagonist on opiate receptors. It has some opiate activity on μ-receptors, but it is purely an antagonist on κ- and δ-receptors. Its major use is the same as for methadone, as discussed later, in treating addiction to opiates, but it is also used in the treatment of pain. Since it has partial agonist activity, it does not cause life-threatening respiratory depression, as is true of agonists such as morphine. It undergoes extensive metabolism in the liver; therefore, excretion is via the hepatobiliary system in contrast to oxycodone, whose excretion is almost completely via the urinary tract. Thus, renal failure does not result in accumulation of buprenorphine in serum while it does cause increased levels of oxycodone. Treatment of patients with buprenorphine for addiction is carried out in programs in which the patient has access to private and group counseling during and after the treatment period. Suboxone is a combination of buprenorphine and naloxone (see Fig. 24.7 ). As described earlier, naloxone is an antagonist at μ-receptors and is administered with buprenorphine to block its toxic effects in patients who try to inject suboxone intravenously as a drug of abuse. The antagonism is limited due to the fact that the affinity of buprenorphine for the μ-receptor is about five times that of naloxone. Buprenorphine is also available as a transdermal patch (Butrans) that is used to treat chronic, rather than acute, pain and has been found to be effective for this purpose. However, the transdermal patch route has posed some challenges with regard to urine and oral drug testing as it does not require the same first-pass metabolism through the liver as with sublingual (Subutex, Suboxone) ingestion and releases microdoses compared with the pill form ( ). Buprenorphine is metabolized via CYP3A4 enzyme to norbuprenorphine via the liver; both analytes are often included in a standard urine drug test to demonstrate ingestion of the parent drug. The presence of both buprenorphine and its metabolite norbuprenorphine in the urine can help obviate suspicion of diversion in a patient suspected of adding straight buprenorphine directly to the urine, which would not yield norbuprenorphine in such a case. Buprenorphine has been found to be more effective than methadone in treating patients with depressive traits ( ), a phenomenon that is thought to be associated with its pure antagonistic effects on κ-receptors. Since it, like oxycodone, has become popular as a drug of abuse, screening for its presence in urine has become common. Toxic effects of this drug include nausea, vomiting, drowsiness, dizziness, headaches, memory loss, perspiration, dry mouth, miosis, orthostatic hypotension, impotence, decreased libido, and urinary retention. Constipation can occur but is less frequent than with morphine. Hepatic necrosis and hepatitis with jaundice, as with the major tranquilizers/neuroleptic drugs, have further been observed in patients with high levels of buprenorphine. The half-life of buprenorphine is 23 to 42 hours, while that of naloxone is 2 to 12 hours. This difference between the two drugs again limits the antagonistic effect of naloxone on buprenorphine.
This opiate analgesic (Group 1 drugs in Fig. 24.7 ) is about 80 times more potent than morphine in blocking pain. It can be taken orally as so-called fentanyl lollipops , smoked, inhaled, or administered by transdermal fentanyl patches ( ). Overdose effects of this drug are the usual ones seen for opiate abuse and include respiratory depression and miosis. Treatment may involve irrigation of the bowel, and anti-opiates such as naloxone may be administered. Hypotension is less common than with other opiates, such as morphine, because of the lack of histamine release.
Generally used in veterinary medicine to tranquilize large animals (e.g., rhinoceros), carfentanil has made its way into street drug applications and has been responsible for dozens of deaths ( ). Carfentanil is metabolized in hepatocytes via N -dealkylation and monohydroxylation of the piperidine ring. Carfentanil is 4-anilidopiperidine, structurally similar to fentanyl, and acts primarily on the μ opioid receptors as an agonist. Carfentanil is purported to have a clinical potency up to 10,000 times that of morphine and 100 times that of fentanyl, such that it is anticipated that 1 mg of carfentanil is approximately equivalent to 8 to 10 g of morphine. Carfentanil induces similar effects to other opioids; however, due to its potency, it also induces strong side effects such as sedation, respiratory depression, rise in systemic blood pressure and temperature, and decrease in heart and respiratory rates. Naltrexone is the antidote of carfentanil, eliminating its effects.
This interesting compound, whose structure is shown in the Group 1 drugs in Figure 24.7 , is a nonbicyclic drug that binds competitively with morphine to μ-receptors in the brain. However, although it can become addictive, the addictive effects are less than those of equivalent concentrations of heroin, possibly because its binding affinity is lower, so that it induces less of an effect than heroin. Thus, administration of methadone to heroin addicts allows them to experience the effects of heroin but in a modulated manner. By gradually lowering the methadone dose, physical dependence becomes reduced, and it appears that a trough serum methadone level greater than 100 ng/mL is adequate for effective methadone maintenance ( ). However, it should be noted that addiction to methadone can also occur. In toxicology laboratories, the most common request received for methadone screens comes from methadone clinics to test whether a patient is administering methadone or has relapsed into taking heroin. As can be seen in Figure 24.3 , it is a simple matter to distinguish methadone from the opiates by TLC. Similarly, EMIT or FPIA detects each drug with high specificity. Confirmation via GC/LC-MS is also utilized and can detect methadone in urine approximately 3 to 5 days postingestion. Methadone is further metabolized to 2-ethylidene-1,5-dimethyl-3,3-diphenylpyrrolidine (EDDP). Recent studies have reported the utility of methadone-to-EDDP ratios to monitor for patient compliance ( ).
These compounds, as can be seen in Figure 24.7 (Group 4 drugs), bear a close resemblance to the adrenergic amines such as epinephrine and norepinephrine and may be expected to exert sympathomimetic effects. They also resemble dopamine and may be expected to have effects on dopaminergic pathways. The amphetamines cause euphoria and increased mental alertness that may be attributed to their effects on these pathways. This group of drugs, however, also exerts pronounced stimulatory effects on γ- and β-receptors in the cardiovascular system and in the kidney to cause pronounced adrenergic effects such as increased heart rate, increased blood pressure, palpitations, bronchodilation, anxiety, pallor, and tremulousness. Studies indicate that amphetamines are also competitive inhibitors of the enzyme monoamine oxidase, which inactivates adrenergic neurotransmitters by oxidatively removing their amino groups. Blockage of this enzyme prolongs the effects of epinephrine and norepinephrine, with the attendant neurologic and cardiovascular sequelae. One particular amphetamine, 3,4-methylenedioxymethamphetamine (MDMA or ecstasy ), a derivative of methamphetamine (see Group 4A drugs in Fig. 24.7 ), has become popular as a recreational drug of abuse because it has euphoric and psychedelic effects but minimal hallucinogenic effects ( ; ).
The pharmacologic action of amphetamines includes CNS and respiratory stimulation and sympathomimetic activity (e.g., bronchodilation, pressor response, mydriasis). Loss of weight may also occur as the result of an anorectic effect. Psychic stimulation and excitability, leading to a temporary increase in mental and physical activity, can occur. Anxiety and nervousness can also be produced.
Initial manifestations of an overdose may be cardiovascular in nature. Symptoms may include flushing or pallor, tachypnea, palpitation, tremor, labile pulse rate and blood pressure (hypertension and hypotension), cardiac arrhythmia, heart block, circulatory collapse, and angina. Mental disturbances such as delirium, confusion, delusions, disorientation, and hallucinations may occur. Acute psychotic syndromes may be characterized by vivid auditory and visual hallucinations, restlessness, homicidal or suicidal tendencies, panic state, paranoid ideation, loosening of associations, combativeness, and changes in affect. A frequent and potential sign of acute intoxication is hyperpyrexia; rhabdomyolysis has also been associated with acute amphetamine overdose. Cardiovascular collapse is the usual cause of death.
Tolerance may be produced within a few weeks; physical or psychic dependence may occur with prolonged usage. Symptoms of chronic abuse include emotional lability, somnolence, loss of appetite, occupational deterioration, mental impairment, and social withdrawal. Trauma and ulcer of the tongue and lip may occur as a result of continuing chewing or teeth-grinding movements. A syndrome with the characteristics of paranoid schizophrenia can occur with prolonged high-dosage use. Aplastic anemia and fatal pancytopenia are rare complications.
No specific antidote for amphetamine overdose is known. Treatment of overdose is symptomatic, with general physiologic supportive measures immediately implemented. When cardiovascular symptoms are noted, propranolol (Inderal), discussed subsequently under therapeutic drugs, can be used as an antidote.
Both Toxi-Lab and EMIT (Syva, San Jose, CA) procedures are effective in detecting these drugs of abuse. Occasionally, on the Toxi-Lab A strip, amphetamines may be confused with antihistamines such as diphenhydramine.
The quest for euphoria-producing drugs has resulted in the advent of synthetic phenylethylamines, so-called designer drugs , such as MDMA, several further examples of which are shown in Category 4A of Figure 24.7 . With all of these drugs, the consequences of the sought-after effects of euphoria and hallucinations are headaches, nausea, vomiting, anxiety, agitation, violent behavior, tachycardia, hypertension, respiratory depression, and seizures, as discussed previously in the case of standard amphetamines.
Other phenylethylamine derivatives shown in Figure 24.7 , especially 2C-T-7 and 2CB, bind to 5-HT 2 receptors and induce hallucinogenic effects ( ). These drugs have been taken orally or have been insufflated, smoked, administered intravenously, and even taken rectally. Death from overdose of these designer drugs has been reported but is uncommon. Unfortunately, thus far, no specific assays are available for most of these drugs in urine. Their presence must be ascertained by history and/or symptoms reported in the absence of positive urine tests for standard amphetamines. On occasion, GC-MS is used to detect their presence.
These drugs are derivatives of serotonin, whose structure is shown in the Group 6 drugs in Figure 24.7 . These tryptamines, some of which occur in plants, are relatively simple to obtain and were not proscribed until relatively recently. An example is N,N -dimethyltryptamine (DMT), which has strong hallucinogenic properties. Smoking DMT results in the rapid onset of hallucinogenic effects that are short-lived, giving rise to the term businessman’s lunch . Other tryptamines contain modifications of the indole ring, as shown in the Group 6 drugs in Figure 24.7 . These also allows them to interact with 5-HT receptors ( ); this interaction is thought to result in their hallucinogenic effects. However, the mechanism of action of this class of drugs is not well understood. Psilocin shown in this figure is a component of the so-called Psilocybe , called magic mushrooms because of their hallucinogenic effects. The hallucinogenic effects of these drugs are enhanced by the presence of monoamine oxidase inhibitors such as β-carbolines ( ). The mixture of these two compounds is present in a South American tea called ayahuasca , which combines two plants—one containing DMT and the other carbolines, which themselves can induce nausea and vomiting. Like the amphetamines and other phenylethylamine derivatives, the tryptamines cause, in addition to the desired effects of euphoria and empathy, auditory and visual hallucinations, nausea, vomiting, diarrhea, and emotional distress. Symptoms further include agitation, tachycardia, hypertension, diaphoresis, salivation, dystonia, mydriasis, tremors, confusion, seizures, and, in a few cases, rhabdomyolysis and paralysis. Currently, no routine assays are available for these compounds. As with the amphetamines, many of the psychogenic and physiologic effects of the tryptamines can be countered with supportive therapy and the administration of benzodiazepines.
The structure of the parent compound, piperazine, is shown in Group 7 of Figure 24.7 . Several of the derivatives of piperazine are also shown in this figure. Many of these piperazines were used as antihelminthics during the 1950s but were subsequently discontinued. However, their euphoria-producing effects were discovered, leading to a “legal” way of obtaining drugs of abuse. Two classes of piperazine derivatives have been identified: N -benzylpiperazines, the parent compound of which is N -benzylpiperazine (BZP) and phenylpiperazines. The former group includes 1-(3,4-methylenedioxybenzyl)piperazine. The latter group includes 1-(3-chlorophenyl)piperazine, 1-(4-methoxyphenyl)piperazine, and 1-(3-trifluoromethylphenyl)piperazine (TFMPP). BZP (known as A2 ) and TFMPP (known as Molly ) are among the most popular piperazines. Studies have shown that these piperazines produced effects that were similar to those of the amphetamines, suggesting that the target receptors of these drugs are the same. Both classes of piperazines have been found to increase dopamine and serotonin levels. TFMPP has been found to act as a partial agonist at 5-HT 2A receptors and is a full agonist at other 5-HT receptors. Although TFMPP is three times less potent than MDMA, it produces a full MDMA effect when combined with BZP, with which it is synergistic ( ). The TFMPP-BZP combination at low doses induces euphoria with decreased motor action, making the euphoric experience more pleasurable. The acute undesirable effects of piperazines are similar to those of the amphetamines and MDMA (i.e., hallucinations, psychomotor agitation, increased heart rate and blood pressure, and increased body temperature). Two deaths from BZP have been reported when combined with another drug such as MDMA ( ). Both TFMPP and BZP have skin-irritant properties, causing sore nasal passages and throats. As with the tryptamines and designer amphetamines, no standard assay method detects these drugs in urine at this time. Treatment is supportive, as described in the preceding section.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here