Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
![]() Additional content is available online at Elsevier eBooks for Practicing Clinicians
Additional content is available online at Elsevier eBooks for Practicing Clinicians
It is estimated that almost a third of people will have a problematic tachyarrhythmia, most often atrial fibrillation (AF), at some point during a normal life span. Thus, most clinicians will need to manage their patients’ rhythm problems, or those treatments may impact or may be impacted by treatment of the patient’s other disorders. Treatment of patients with tachyarrhythmias has evolved dramatically over the last 40 years and has become more complex and specialized. A few, relatively ineffective antiarrhythmic drugs (AADs) were the only therapeutic option until the late 1960s, when surgical therapy to cure (not just suppress) tachyarrhythmias was developed. This mode in turn was replaced by catheter ablation for better control or even cure of many types of supraventricular tachycardias (SVTs) and ventricular tachycardias (VTs) in the absence of structural heart disease starting in the 1980s. The implantable cardioverter-defibrillator (ICD) was introduced in the early 1980s and has become standard therapy for patients with serious ventricular arrhythmias in the presence of structural heart disease. Some patients require a combination of treatments, such as an ICD and AADs or surgery and an ICD; drug therapy can also affect ICD function, positively or negatively. Drug therapy for arrhythmias, at one time the only option, has largely been replaced as the mainstay of therapy by ablation or implanted devices. In most patients, however, tachyarrhythmias are initially treated with AADs, and thus these agents continue to have a significant role in management of patients with a variety of arrhythmias.
The principles of clinical pharmacokinetics and pharmacodynamics are discussed in Chapter 9 .
Most of the AADs currently available can be classified according to whether they exert blocking actions predominantly on sodium (Na + ), potassium (K + ), or calcium (Ca 2+ ) channels and/or what receptors they block ( Table 64.1 ). The commonly used classification (Vaughan Williams) is still a useful framework for categorizing drug action but is limited because it is based on the electrophysiologic effects exerted by an arbitrary concentration of the drug, generally on a laboratory preparation of normal cardiac tissue.
| Drug | Channels | Receptors | Pumps | Predominant Clinical Effects | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Na ∗ | Ca | K r | K s | HCN | α | β | M 2 | P | Na-K ATPase | LV Function | Sinus Rate | Extracardiac | |||
| Fast | Med | Slow | |||||||||||||
| Quinidine | ●A | ◉ | — | ↑ | ◉ | ||||||||||
| Procainamide | ●I | ◉ | ↓ | — | ◉ | ||||||||||
| Disopyramide | ●A | ◉ | ↓ | var | ● | ||||||||||
| Ajmaline | ●A | — | — ↓ | ||||||||||||
| Lidocaine | — | — ↓ | |||||||||||||
| Mexiletine | — | — | |||||||||||||
| Phenytoin | — | — | ◉ | ||||||||||||
| Flecainide | ●A | ↓ | — | ||||||||||||
| Propafenone | ●A | ◉ | ↓ | ↓ | |||||||||||
| Propranolol | ● | ↓ | ↓ | ||||||||||||
| Nadolol | ● | ↓ | ↓ | ||||||||||||
| Amiodarone | ◉ | ● | ◉ | ◉ | ◉ | — | ↓ | ● | |||||||
| Dronedarone | ◉ | ● | ◉ | ◉ | ◉ | — | ↓ | ||||||||
| Sotalol | ● | ● | ↓ | ↓ | |||||||||||
| Ibutilide | activator | — | ↓ | ||||||||||||
| Dofetilide | ● | — | — | ||||||||||||
| Verapamil | ● | ◉ | ↓ | ↓ | |||||||||||
| Diltiazem | ◉ | ↓ | ↓ | ||||||||||||
| Adenosine | ▪ | — | ↓ | ◉ | |||||||||||
| Digoxin | ● | ↑ | ↓ | ◉ | |||||||||||
| Atropine | ● | — | ↑ | ◉ | |||||||||||
| Ranolazine | — | — | |||||||||||||
| Ivabradine | ● | ↓ | |||||||||||||
∗ Fast, med (medium), and slow refer to kinetics of recovery from sodium (Na) channel blockade.
In practice, the actions of these drugs are complex and depend on tissue type, degree of acute or chronic damage, heart rate, membrane potential, ionic composition of the extracellular milieu, autonomic influences (see Chapter 102 ), genetics (see Chapter 63 ), age ( Chapter 90 ), and other factors (see Table 64.1 ). Many drugs exert more than one type of electrophysiologic effect or operate indirectly, such as by altering hemodynamics, myocardial metabolism, or autonomic neural transmission. Therefore, it is more appropriate to think of classes of action rather than classes of drugs, although the major classification schemes categorize drugs by their predominant action. Some drugs have active metabolites that exert effects different from those of the parent compound. Not all drugs in the same class have identical effects (e.g., amiodarone, sotalol, and ibutilide). Whereas all class III agents are dramatically different, some drugs in different classes have overlapping actions (e.g., class IA and class IC drugs). Thus, in vitro studies on healthy myocardium usually establish the idealized properties of AADs rather than their actual antiarrhythmic properties in vivo. Since many AADs affect ventricular repolarization and thus have the potential for producing lethal ventricular arrhythmias, development and approval of new agents is uncommon (no new agents in the United States since dronedarone in 2009).
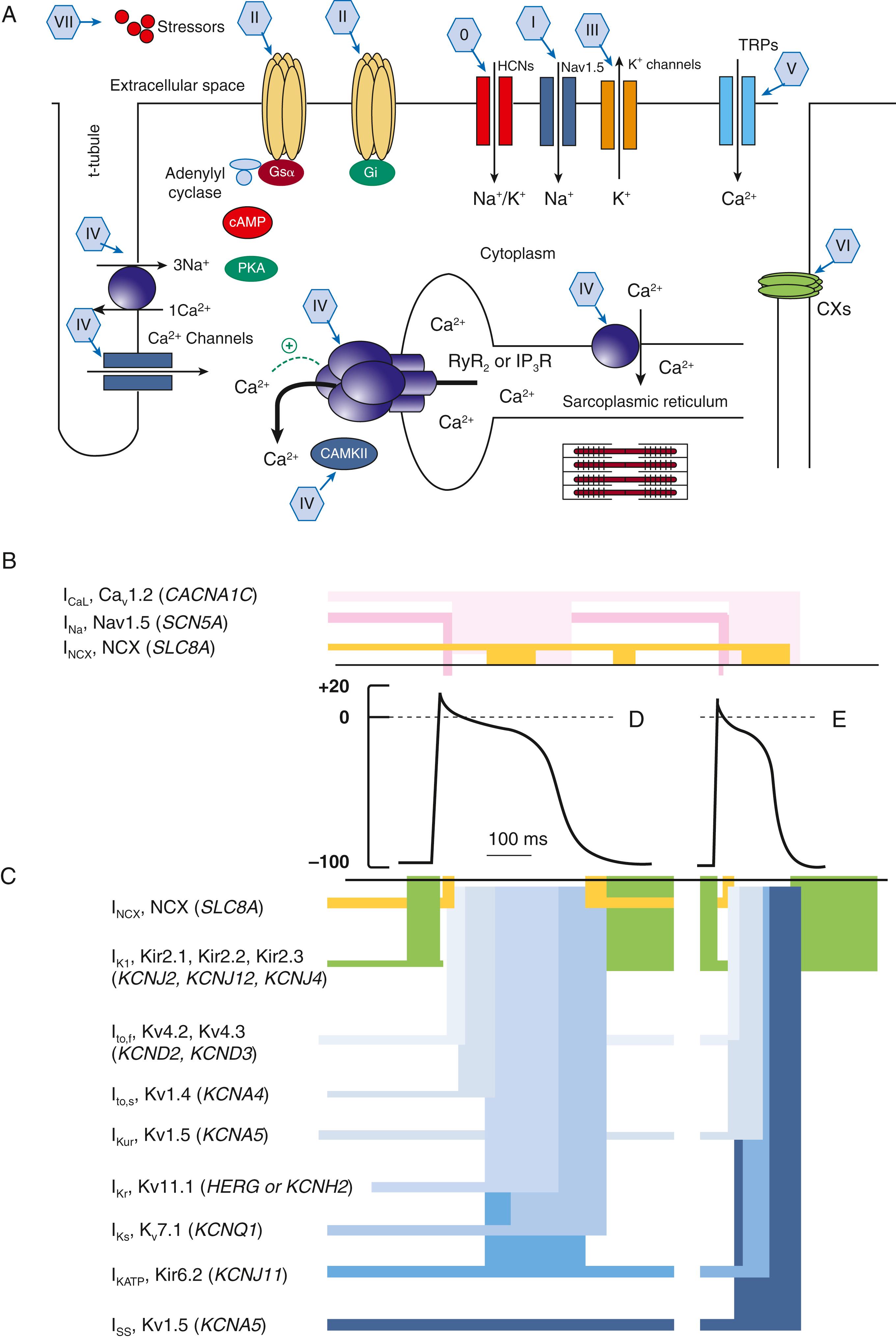
Despite its limitations, the Vaughan Williams classification is widely known and provides a useful communication shorthand, but the reader is cautioned that drug actions are more complex than those depicted by the classification. A more realistic but not widely used framework regarding AADs is provided by the “Sicilian Gambit.” This approach to drug classification is an attempt to identify the mechanisms of a particular arrhythmia, to determine the vulnerable parameter of the arrhythmia most susceptible to modification, to define the target most likely to affect the vulnerable parameter, and then to select a drug that will modify the target ( Table 64.2 ; also see Table 64.1 ). More recently, a modified Vaughan Williams classification has been proposed that takes into account additional drug targets, including effects on connexins, molecules underlying longer-term signaling processes, and mechanically sensitive ion channels ( Fig. 64.1 ). This revised classification, incorporating advances in both basic and clinical sciences, also emphasizes that most AADs affect multiple targets and can be used to facilitate decisions about drug choices in specific clinical settings. Classes 0-VII have been described; currently available agents are limited to Classes 0-IV and several unclassified drugs.
| Mechanism | Arrhythmia | Vulnerable Parameter (Effect) | Drugs (Effect) |
|---|---|---|---|
| Automaticity | |||
| Enhanced normal | Inappropriate sinus tachycardia | Phase 4 β-adrenergic induced rate acceleration and I f block | β-Adrenergic blocking agents and I f blockers |
| Abnormal | Atrial tachycardia | Maximum diastolic potential (hyperpolarization) | M 2 agonist |
| Phase 4 depolarization (decrease) | Ca 2+ or Na + channel blocking agents M 2 agonist |
||
| Accelerated idioventricular rhythms | Phase 4 depolarization (decrease) | Ca 2+ or Na + channel blocking agents | |
| Triggered Activity | |||
| EAD | Torsades de pointes | Action potential duration (shorten) | β-adrenergic agonists; vagolytic agents (increase rate) |
| EAD (suppress) | Ca 2+ channel blocking agents; Mg 2+ ; β-adrenergic blocking agents; ranolazine | ||
| DAD | Digitalis-induced arrhythmias | Calcium overload (unload) | Ca 2+ channel blocking agents |
| DAD (suppress) | Na + channel blocking agents | ||
| RV outflow tract ventricular tachycardia | Calcium overload (unload) | β-adrenergic blocking agents | |
| DAD (suppress) | Ca 2+ channel blocking agents; adenosine | ||
| Reentry—Na + Channel Dependent | |||
| Long excitable gap | Typical atrial flutter | Conduction and excitability (depress) | Type IA, IC Na + channel blocking agents |
| Circus movement tachycardia in WPW | Conduction and excitability (depress) | Type IA, IC Na + channel blocking agents | |
| Sustained uniform ventricular tachycardia | Conduction and excitability (depress) | Na + channel blocking agents | |
| Short excitable gap | Atypical atrial flutter | Refractory period (prolong) | K + channel blocking agents |
| Atrial fibrillation | Refractory period (prolong) | K + channel blocking agents | |
| Circus movement tachycardia in WPW | Refractory period (prolong) | Amiodarone, sotalol | |
| Polymorphic and uniform ventricular tachycardia | Refractory period (prolong) | Type IA Na + channel blocking agents | |
| Bundle branch reentry | Refractory period (prolong) | Type IA Na + channel blocking agents; amiodarone | |
| Ventricular fibrillation | Refractory period (prolong) | ||
| Reentry—Ca 2+ Channel Dependent | |||
| AV nodal reentrant tachycardia | Conduction and excitability (depress) | Ca 2+ channel blocking agents | |
| Circus movement tachycardia in WPW | Conduction and excitability (depress) | Ca 2+ channel blocking agents | |
| Verapamil-sensitive ventricular tachycardia | Conduction and excitability (depress) | Ca 2+ channel blocking agents | |
This class includes drugs that block the HCN channel mediated pacemaker current (I f ). Inhibition of the I f channel (or “funny” current) reduces the depolarization rate of the sinus node pacemaker cells and reduces heart rate.
According to the Vaughan Williams classification, class I drugs predominantly block the voltage gated fast sodium channel (I Na ). These in turn are divided into four subgroups, classes IA, IB, IC and ID ( eTable 64.1 ). Some also block potassium channels at pharmacologically relevant concentrations.
| Drug | APD | dV/dt | MDP | ERP | CV | PF Phase 4 | SN Auto | Contr | SI Curr | Autonomic Nervous System |
|---|---|---|---|---|---|---|---|---|---|---|
| Quinidine | ↑ | ↓ | 0 | ↑ | ↓ | ↓ | 0 | 0 | 0 | Antivagal; alpha blocker |
| Procainamide | ↑ | ↓ | 0 | ↑ | ↓ | ↓ | 0 | 0 | 0 | Slight antivagal |
| Disopyramide | ↑ | ↓ | 0 | ↑ | ↓ | ↓ | ↓ 0 ↑ | ↓ | 0 | Central: antivagal, antisympathetic |
| Ajmaline | ↑ | ↓ | 0 | ↑ | ↓ | ↓ | ↓ 0 | ↓ | 0 | Antivagal |
| Lidocaine | ↓ | 0↓ | 0 | ↓ | 0↓ | ↓ | 0 | 0 | 0 | 0 |
| Mexiletine | ↓ | 0↓ | 0 | ↓ | ↓ | ↓ | 0 | ↓ | 0 | 0 |
| Phenytoin | ↓ | ↓ 0 ↑ | 0 | ↓ | 0 | ↓ | 0 | 0 | 0 | |
| Flecainide | 0↑ | ↓ | 0 | ↑ | ↓↓ | ↓ | 0 | ↓ | 0 | 0 |
| Propafenone | 0↑ | ↓ | 0 | ↑ | ↓↓ | ↓ | 0 | ↓ | 0↓ | Antisympathetic |
| Propranolol | 0↓ | 0↓ | 0 | ↓ | 0 | ↓∗ | ↓ | ↓ | 0↓ | Antisympathetic |
| Amiodarone | ↑ | 0↓ | 0 | ↑ | ↓ | ↓ | ↓ | 0↑ | 0 | Antisympathetic |
| Dronedarone | ↑ | 0↓ | 0 | ↑ | ↓ | ↓ | ↓ | 0↓ | 0 | Antisympathetic |
| Sotalol | ↑ | 0↓ | 0 | ↑ | 0 | 0↓ | ↓ | ↓ | 0↓ | Antisympathetic |
| Ibutilide | ↑ | 0 | 0 | ↑ | 0 | 0 | ↓ | 0 | 0 | 0 |
| Dofetilide | ↑ | 0 | 0 | ↑ | 0 | 0 | 0 | 0 | 0 | 0 |
| Verapamil | ↓ | 0 | 0 | 0 | 0 | ↓ ∗ | ↓ | ↓ | ↓↓ | ? Block alpha receptors; enhance vagal |
| Adenosine | ↑ | 0↓ | More (−) | ↑ | 0 | 0↓ | ↓ | 0 | ↓ | Vagomimetic |
| Ranolazine | ↑ | 0 | 0 | ↑ | 0 | 0 | 0 | 0 | 0 | 0 |
| Ivabradine | 0 | 0 | 0 | 0 | 0 | 0(↓) | ↓ | 0 | ↓ | 0 |
This class includes drugs that reduce V̇ max (rate of rise in action potential upstroke [phase 0]) and prolong the action potential duration (APD; see Chapter 62 )—quinidine, procainamide, and disopyramide. The kinetics of onset and offset of class IA drugs blocking the Na + chan nel is of intermediate rapidity (<5 seconds) when compared with class IB and class IC agents.
This class of drugs does not reduce V̇ max and shortens the APD—mexiletine, phenytoin, and lidocaine. The kinetics of onset and offset of these drugs in blocking the sodium channel is rapid (<500 milliseconds).
This class of drugs, including flecainide and propafenone, can reduce V̇ max slow conduction velocity, and prolong refractoriness minimally. These drugs have slow onset and offset kinetics (10 to 20 seconds).
This class of drugs includes ranolazine, which preferentially inhibits the late Na + current affecting APD and recovery and increases refractoriness and repolarization reserve. Class ID drugs cause a reduction in early afterdepolarization-induced triggered activity.
These drugs block beta-adrenergic receptors and include propranolol, metoprolol, nadolol, carvedilol, nebivolol, and timolol.
This class of drugs predominantly blocks potassium channels (e.g., I Kr ) and prolongs repolarization. Included are sotalol, amiodarone, dronedarone, and ibutilide. Although these drugs are all classified as class III, they differ significantly in their effects on additional ion channels. For example, amiodarone is a nonselective K + channel blocker while the other agents primarily block I Kr .
This class of drugs predominantly blocks the L-type or slow calcium channel (I Ca.L )—verapamil, diltiazem, nifedipine, and others (felodipine blocks I Ca.T ).
Antiarrhythmic agents appear to cross the cell membrane and interact with receptors in the membrane channels when the channels are in the resting, activated, or inactivated state (see Table 64.1 and Chapter 62 ), and each of these interactions is characterized by different association and dissociation rate constants of a drug from its receptor. Such interactions depend on voltage and time. Transitions among resting, activated, and inactivated states are time- and voltage-dependent. When the drug is bound (associated) to a receptor site at or close to the channel pore (the drug may not actually “plug” the channel), the channel cannot conduct, even in the activated state.
Some drugs exert greater inhibitory effects on the upstroke of the action potential at more rapid rates of stimulation and after longer periods of stimulation, a characteristic called use dependence. Drugs with this property depress V̇ max to a greater extent after the channel has been “used” (i.e., after action potential depolarization rather than after a rest period). Agents with class IB action exhibit rapid binding and unbinding from their receptor site on the channel protein, or exhibit use-dependent block of the fast channel at fast rates. Class IC drugs have slow kinetics, and class IA drugs are intermediate. With increased time spent in diastole (slower rate), a greater proportion of receptors unbind drug, and the drug exerts less effect. The clinical consequence is that these drugs with slower kinetics have greater electrophysiologic effects at more rapid heart rates. For example, a class IC drug would cause more Na + channel blockade at more rapid heart rates, and this translates into greater QRS widening with faster heart rates. Unhealthy cells with reduced (i.e., abnormal) membrane potentials recover more slowly from drug actions than do healthier cells with more negative (i.e., normal) membrane potentials. This is referred to as voltage dependence of block.
Some drugs exert greater effects at slow rates than at fast rates, a property known as reverse use dependence. This is particularly true for drugs that lengthen repolarization; in the ventricle the QT interval becomes more prolonged at slow rather than at fast rates. This effect is not an ideal antiarrhythmic property, because prolongation of refractoriness should be increased at fast rates to interrupt or prevent a tachycardia and should be minimal at slow rates to avoid precipitation of torsades de pointes (TdP).
Given that enhanced automaticity, triggered activity, or reentry can cause cardiac arrhythmias (see Chapter 62 ), mechanisms by which AADs suppress arrhythmias in general can only be postulated (see Table 64.2 ) as some arrhythmias may encompass multiple mechanisms. AADs can slow the spontaneous discharge frequency of an automatic pacemaker by depressing the slope of diastolic depolarization, shifting the threshold voltage toward zero, or hyperpolarizing the resting membrane potential. In general, most AADs at therapeutic concentrations depress the automatic firing rate of spontaneously discharging ectopic sites while minimally affecting the discharge rate of the normal sinus node. Other agents act directly on the sinus node to slow heart rate, whereas drugs that exert vagolytic effects, such as disopyramide and quinidine, can increase the sinus rate. Drugs that suppress early (early afterdepolarization [EAD]) or delayed (delayed afterdepolarization [DAD]) afterdepolarizations can eliminate triggered arrhythmias based on these mechanisms.
Reentry depends critically on the interrelationships between refractoriness and conduction velocity, the presence of unidirectional block in one of the pathways, and other factors that influence refractoriness and conduction, such as excitability (see Chapter 62 ). An antiarrhythmic agent can stop ongoing reentry that is already present or can prevent it from starting if the drug depresses or, alternately, improves conduction. For example, improving conduction can (1) eliminate unidirectional block so that reentry cannot begin or (2) facilitate conduction in the reentrant loop so that the returning wavefront reenters too quickly, encounters cells that are still refractory, and is extinguished. A drug that depresses conduction can transform unidirectional block into bidirectional block and thus terminate reentry or prevent it from starting by creating an area of complete block in the reentrant pathway. Conversely, a drug that slows conduction without producing block or significantly lengthening refractoriness can actually promote reentry. Lastly, most AADs share the ability to prolong refractoriness relative to their effects on APD; that is, the ratio of the effective refractory period (ERP) to APD exceeds 1.0. If a drug prolongs the refractoriness of fibers in the reentrant pathway, the pathway may not recover excitability in time to be depolarized by the reentering impulse, and reentrant propagation ceases. Different types of reentry influence the effectiveness of a drug.
In considering the properties of a drug, it is important to define carefully the situation or model from which conclusions are drawn. Electrophysiologic, hemodynamic, autonomic, pharmacokinetic, and adverse effects can all differ in normal individuals compared with patients, in normal versus abnormal tissue, in cardiac muscle compared with specialized conduction fibers, and in atrial versus ventricular muscle ( eTable 64.2 ).
| Drug | Electrocardiographic Measurements | Electrophysiologic Measurements | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Sinus Rate | PR | QRS | QT | JT | ERP-AVN | ERP-HPS | ERP-A | ERP-V | AH | HV | |
| Quinidine | 0 ↑ | ↓ 0 ↑ | ↑ | ↑ | ↑ | 0 ↑ | ↑ | ↑ | ↑ | 0 ↓ | ↑ |
| Procainamide | 0 | 0 ↑ | ↑ | ↑ | ↑ | 0 ↑ | ↑ | ↑ | ↑ | 0 ↑ | ↑ |
| Disopyramide | ↓ 0 ↑ | ↓ 0 ↑ | ↑ | ↑ | ↑ | ↑ 0 | ↑ | ↑ | ↑ | ↓ 0 ↑ | ↑ |
| Ajmaline | 0 | 0 ↑ | ↑ | ↑ | ↑ | 0 | ↑ | ↑ | ↑ | ↓ 0 ↑ | ↑ |
| Lidocaine | 0 | 0 | 0 | 0 ↓ | ↓ | 0 ↓ | 0 ↑ | 0 | 0 | 0 ↓ | 0 ↑ |
| Mexiletine | 0 | 0 | 0 | 0 ↓ | ↓ | 0 ↑ | 0 ↑ | 0 | 0 | 0 ↑ | 0 ↑ |
| Phenytoin | 0 | 0 | 0 | 0 | 0 | 0 ↓ | ↓ | 0 | 0 | 0 ↑ | 0 |
| Flecainide | 0 ↓ | ↑ | ↑ | 0 ↑ | 0 | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ |
| Propafenone | 0 ↓ | ↑ | ↑ | 0 ↑ | 0 | 0 ↑ | 0 ↑ | 0 ↑ | ↑ | ↑ | ↑ |
| Propranolol | ↓ | 0 ↑ | 0 | 0 ↓ | 0 | ↑ | 0 | 0 | 0 | 0 | 0 |
| Amiodarone | ↓ | 0 ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ |
| Dronedarone | ↓ | 0 ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | 0 |
| Sotalol | ↓ | 0 ↑ | 0 | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | 0 |
| Ibutilide | ↓ | 0 ↓ | 0 | ↑ | ↑ | 0 | 0 | ↑ | ↑ | 0 | 0 |
| Dofetilide | 0 | 0 | 0 | ↑ | ↑ | 0 | 0 | ↑ | ↑ | 0 | 0 |
| Verapamil | 0 ↓ | ↑ | 0 | 0 | 0 | ↑ | 0 | 0 | 0 | ↑ | 0 |
| Adenosine | ↓ then ↑ | ↑ | 0 | 0 | 0 | ↑ | 0 | ↓ | 0 | ↑ | 0 |
| Digoxin | ↓ | ↑ | 0 | 0 | ↓ | ↑ | 0 | ↓ | 0 | ↑ | 0 |
| Ranolazine | 0 | 0 | 0 | ↑ | ↑ | 0 | 0 | ↑ | ↑ | 0 | 0 |
| Ivabradine | ↓ | 0 | 0 | 0 ∗ | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
∗ Rare reported cases of QT prolongation and torsades de pointes.
Drug metabolites can add to or alter the effects of the parent compound by exerting similar actions, competing with the parent compound, or mediating drug toxicity. Quinidine has at least four active metabolites, but none with a potency exceeding that of the parent drug, and none implicated in causing TdP. About 50% of procainamide is metabolized to N -acetylprocainamide (NAPA), which prolongs repolarization and is a less effective sodium channel blocker but competes with procainamide for renal excretion and can increase the parent drug’s elimination half-life. A lidocaine metabolite can compete with the parent drug for sodium channels and partially antagonize its blocking effect.
Genetically determined metabolic pathways account for many of the differences in patients’ responses to some drugs (see Chapter 9 ). The superfamily of cytochrome P-450 (CYP450) enzymes metabolize propafenone, hydroxylate several beta blockers, and biotransform flecainide. The CYP4502D6 exhibits extensive genetic and functional heterogeneity. Lack of this enzyme (in approximately 7% of white patients and 5% of Blacks) reduces metabolism of the parent compound and thereby leads to increased plasma concentrations of the parent drug and reduced concentrations of metabolites. Propafenone is metabolized by CYP450 to a compound with slightly less antiarrhythmic and beta-adrenergic blocking effects, as well as fewer central nervous system (CNS) side effects. Thus, poor metabolizers may experience more heart rate slowing and neurotoxicity than extensive metabolizers do.
Drugs such as rifampin, phenobarbital, and phenytoin induce the synthesis of larger amounts of various CYP450 isoforms, which leads to lower concentrations of the parent drugs because of extensive metabolism, whereas erythromycin, clarithromycin, fluoxetine, and grapefruit juice inhibit enzyme activity, which leads to accumulation of the parent compound. Therefore, clinicians caring for patients who take AADs should be sensitive to the effects of non-cardiac medications and supplements on AAD metabolism and elimination and drug-drug interactions. Over-the-counter (OTC) drugs such as proton pump inhibitors can promote hypokalemia and hypomagnesemia and interact with a simple antibiotic such as ceftriaxone to cause TdP. , Many astute clinicians use, and refer their patients to, websites such as Crediblemeds.org , where updated information on drug interactions of this type is available. A list of drugs that prolong the QT interval can also be found at www.sads.org .
In treating cardiac rhythm disorders, most drugs are given on a daily basis (in one to three doses) to prevent episodes from occurring or, in some cases of AF, to control the ventricular rate. Efficacy can be judged in various ways, depending on the clinical circumstances. Symptom reduction (in the case of benign arrhythmias, such as most premature ventricular complexes [PVCs]) and electrocardiographic monitoring (long-term or event; see Chapter 61 ) are useful; electrophysiologic study (EPS) has been used in the past, with suppression of electrical induction of arrhythmia being the goal, but is rarely used for this purpose currently. Interrogation of implanted devices can also provide an indicator of the success of drug therapy by providing PVC counts and burden of atrial arrhythmias.
In some patients, tachycardia episodes are infrequent enough (months between occurrences) and symptoms mild enough that reactive drug administration is more reasonable than chronic daily dosing. The patient takes a medication only after an episode has started, in the hope that the tachycardia can be terminated and a visit to a physician’s office or emergency department avoided. This “pill in the pocket” strategy has worked well for some patients with AF, who have been given one of various medications orally in a monitored setting to ensure safety as well as efficacy before allowing self-medication at home or elsewhere.
AADs produce one group of adverse effects related to excessive dosage and plasma concentrations that result in both non-cardiac (e.g., neurologic defects) and cardiac (e.g., heart failure, some arrhythmias) toxicity. Another group of side effects unrelated to plasma concentrations is termed idiosyncratic ; examples include amiodarone-induced pulmonary fibrosis and some arrhythmias, such as quinidine-induced TdP, which can occur in individuals with a forme fruste of long-QT syndrome (i.e., marked prolongation of normal QT interval in the presence of certain medications; see Chapter 63, Chapter 9 ). Genetic variants can underlie susceptibility to idiosyncratic reactions.
The U.S. Food and Drug Administration (FDA) has recently determined that the risk of adverse events during pregnancy and lactation (previously categorized as A, B, C, D, and X; see Table 64.3 ) should be modified (the Pregnancy and Lactation Labeling rule of 2015). The old classification scheme was confusing and did not accurately differentiate the risks to the fetus. This rule applies to all new prescription drugs after June 2015 and is being phased in gradually for drugs approved between 2001 and 2015. This process is underway but until completed, adverse event risk in this setting is characterized using the previous classification. We have attempted to summarize some information about AAD safety during pregnancy in Table 64.4 .
| Considered Safe |
| Adenosine (C) |
| Propranolol (C) |
| Metoprolol (C) |
| Lidocaine (B) |
| Digoxin ∗ (C) |
| Sotalol (B) |
| Verapamil (C) |
| Limited Data; Recommend Use With Caution |
| Adenosine (C) |
| Propranolol (C) |
| Metoprolol (C) |
| Carvedilol (C) |
| Propafenone (C) |
| Flecainide ∗ (C) |
| Propafenone (C) |
| Sotalol ∗ (B) |
| Diltiazem (C) |
| Verapamil (C) |
| Ivabradine (D) |
| Contraindicated |
| Amiodarone (D) |
| Dronedarone (X) |
∗ Digoxin, flecainide, and sotalol have been used to treat fetal arrhythmias during pregnancy.
| Drug | Usual Dosage Ranges | Time TO Peak Plasma Concentration (Oral) (HR) | Effective Serum or Plasma Concentration (mcg/mL) | Half-Life (hr) | Bioavailability (%) | Major Route of Elimination | Pregnancy Class | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Intravenous (mg) | Oral (mg) | |||||||||
| Loading | Maintenance | Loading | Maintenance | |||||||
| Quinidine | 6–10 mg/kg at 0.3–0.5 mg/kg/min | — | 800–1000 | 300–600 q6h | 1.5–3.0 | 3–6 | 5–9 | 60–80 | Liver > kidneys | C |
| Procainamide | 6–13 mg/kg at 0.2–0.5 mg/kg/min | 2–6 mg/min | 500–1000 | 250–1000 q4–6h | 1 | 4–10 | 3–5 | 70–85 | Kidneys > liver | C |
| Disopyramide | 1–2 mg/kg over 15–45 min ∗ | 1 mg/kg/hr ∗ | N/A | 100–300 q6-8h | 1–2 | 2–5 | 4–10 | 80–90 | Kidneys | C |
| Lidocaine | 1–2 mg/kg at 20–50 mg/min | 1–4 mg/min | N/A | N/A | N/A | 1–5 | 1–2 | N/A | Liver | B |
| Mexiletine | 500 mg ∗ | 0.5–1.0 g/24 hr ∗ | 400–600 | 150–300 q8-12h | 2–4 | 0.75–2.0 | 10–17 | 80–90 | Liver | C |
| Phenytoin | 100 mg q5min for ≤1000 mg | N/A | 1000 | 100–400 q12-24h | 8–12 | 10–20 | 18–36 | 50–70 | Liver | D |
| Flecainide | 2 mg/kg ∗ | 100–200 q12h ∗ | 50–200 q12h | 3–4 | 0.2–1.0 | 20 | 95 | Kidneys | C | |
| Propafenone | 1–2 mg/kg ∗ | N/A | 600–900 | 150–300 q8-12h | 1–3 | 0.5–2.0 | 2-10 (extensive metabolizers); 10-32 (poor metabolizers) | 3–25 | Liver | C |
| Propranolol | 0.25–0.5 mg q5min to ≤0.20 mg/kg | N/A | N/A | 10–200 q6–8h | 4 | 0.02–0.2 | 3–6 | 35–65 | Liver | C |
| Amiodarone | 15 mg/min for 10 min 1 mg/min for 6 hr 0.5 mg/min thereafter |
0.5 mg/min | 400 to 800 mg/day for 7–14 days | 200–600 qd | Variable | 0.5–1.5 | 56 days | 25 | Liver | D |
| Dronedarone | N/A | N/A | N/A | 400 mg q12h | 3–4 | 0.3–0.6 | 13–19 | 70–90 | Liver | X |
| Sotalol | 60–112.5 mg over 1 h | 75–112.5 mg over 5 h | N/A | 80–160 q12h | 2.5–4 | 2.5 | 10–20 | 90–100 | Kidneys | B |
| Ibutilide | 1 mg over 10 min | N/A | N/A | N/A | N/A | N/A | 2–12 | Kidneys | C | |
| Dofetilide | N/A | N/A | 0.125–0.5 q12h | 7–13 | 90 | Kidneys | C | |||
| Verapamil | 5–10 mg over 1–2 min | 0.005 mg/kg/min | N/A | 80–120 q6–8h | 1–2 | 0.10–0.15 | 3–8 | 20–35 | Liver, kidneys | C |
| Adenosine | 6–18 mg (rapidly) | N/A | N/A | N/A | N/A | N/A | Seconds | 100 | Blood cells | C |
| Digoxin | 0.5–1.0 mg | 0.125–0.25 qd | 0.5–1.0 | 0.125–0.25 qd | 2–6 | 0.0008–0.002 | 36–48 | 60–80 | Kidneys | C |
| Ranolazine | N/A | N/A | N/A | 500–1000 bid | 4–6 | N/A | 7 | 60–75 | Kidneys > liver | C |
| Ivabradine | N/A | N/A | N/A | 2.5–7.5 mg bid | 1 | N/A | 6 | 40% | Gut and liver | D |
| Pill in the Pocket Approach |
|
|
|
| OR |
| Propafenone as single dose of 450–600 mg; 450 mg if weight <70 kg |
| Recommended that the first dose of a class IC agent or first trial of pill in the pocket consideration should be given to have it administered under monitored therapy |
∗ Intravenous use investigational or unavailable in United States.
Drug-induced or drug-exacerbated cardiac arrhythmias (proarrhythmia) constitute a major clinical problem. , Proarrhythmia can manifest as an increase in frequency of a preexisting arrhythmia, sustaining of a previously non-sustained arrhythmia (even making it incessant), or development of arrhythmias that the patient has not previously experienced. Electrophysiologic mechanisms are probably related to prolongation of repolarization or an increase in transmural dispersion, development of EADs with resultant TdP, and alterations in reentry pathways to initiate or sustain tachyarrhythmias. Proarrhythmic events can occur in as many as 5% to 10% of patients receiving antiarrhythmic agents; heart failure increases this risk. Reduced left ventricular function, treatment with digitalis and diuretics, bradycardia and a longer pretreatment QT interval identify patients who experience AAD-induced ventricular fibrillation (VF). The more commonly known proarrhythmic events occur within several days of beginning drug therapy or changing dosage and are represented by such developments as incessant VT and long-QT–related TdP. In the Cardiac Arrhythmia Suppression Trial (CAST), however, researchers found that encainide and flecainide reduced spontaneous ventricular arrhythmias but were associated with a total mortality of 7.7%, versus 3.0% in the placebo group. Deaths were equally distributed throughout the treatment period, indicating that another type of proarrhythmic response can occur sometime after the beginning of drug therapy. Such late proarrhythmic effects may be related to drug-induced exacerbation of the regional myocardial conduction delay caused by ischemia and to heterogeneous drug concentrations that can promote reentry. In the future, a candidate antiarrhythmic compound’s potential for proarrhythmia may be modeled computationally and tested in stem cells.
The availability of catheter ablation (see later) and implantable devices (pacemakers and ICDs; see Chapter 69 ) to treat a wide variety of arrhythmias has largely relegated drug therapy to a secondary role in the treatment of serious arrhythmias. Drugs are still useful to prevent or to decrease the frequency of recurrences in patients who have relatively infrequent episodes of benign tachycardias, as well as in those who have had incomplete success with catheter ablation procedures and in patients with an ICD, to decrease the frequency of shocks because of supraventricular or ventricular arrhythmias.
Quinidine and quinine are isomeric alkaloids isolated from cinchona bark. Although quinidine shares the antimalarial, antipyretic, and vagolytic actions of quinine, only quinidine has direct cellular electrophysiologic effects. It blocks several channels, including the rapid inward sodium channel (I Na ), I Kr , I to , and to a lesser extent the slow inward calcium channel, I Ks , and the adenosine triphosphate (ATP)–sensitive potassium current (K ATP ). Quinidine causes alpha-adrenergic and cholinergic blockade. The ultimate biologic effect of the drug in a given patient depends on heart rate, drug concentration, and which channels are more prominently affected. Because of decreased demand for quinidine, manufacturing had ceased for a time, with little remaining supply in many countries, but recent renewed demand for its use in patients with Brugada syndrome and idiopathic VF have resulted in quinidine becoming more readily available.
Quinidine exerts little effect on automaticity of the normal sinus node but suppresses automaticity in normal Purkinje fibers (see eTable 64.2 ; see also Tables 64.1, 64.2 , and eTable 64.1 ). In patients with sinus node dysfunction, quinidine can further depress sinus node automaticity. Quinidine lengthens the QT interval in part via formation of EADs in experimental preparations and in humans, which appears to be responsible for TdP. Because of its significant anticholinergic effect and the reflex sympathetic stimulation resulting from alpha-adrenergic blockade, which causes peripheral vasodilation, quinidine can cause a reflex increase in the sinus node discharge rate and improve atrioventricular (AV) nodal conduction. Quinidine prolongs repolarization, an effect that is more prominent at slow heart rates (reverse use dependence) because of block of I Kr (as well as enhancing the late inward Na + current at low concentrations). Faster rates result in more block of sodium channels and less unblocking because a smaller percentage of time is spent in the rested state (use dependence). Isoproterenol can modulate the effects of quinidine on reentrant circuits in humans. Quinidine at higher doses inhibits the late inward Na + current. As noted, quinidine blocks the transient outward current I to , which probably explains its efficacy in suppressing ventricular arrhythmias in Brugada syndrome and patients with idiopathic VF (see Chapter 63 ).
Quinidine induces vasodilation by blocking alpha-adrenergic receptors and can cause significant hypotension. It does not cause significant direct myocardial depression.
Plasma quinidine concentrations peak at approximately 1.5 to 3 hours after an oral dose of a quinidine gluconate preparation (see Table 64.4 ). Approximately 80% of plasma quinidine is protein bound, especially to α 1 -acid glycoprotein. Both the liver and the kidneys remove quinidine; dose adjustments may be made to achieve appropriate serum concentrations. Its elimination half-life is 8 to 9 hours after oral administration.
The usual oral dose of quinidine sulfate for an adult is 300 to 600 mg four times daily, which results in a steady-state level within about 24 hours (see Table 64.4 ). A loading dose of 600 to 800 mg produces an earlier effective concentration. Oral doses of the gluconate are about 30% higher than those of the sulfate form. Important interactions with other drugs occur.
Quinidine is a versatile AAD that was used previously to treat premature supraventricular and ventricular complexes and sustained tachyarrhythmias. However, because of its side effect profile and potential for causing TdP, as well as its limited usefulness in preventing VT and VF in most applications, its use has decreased greatly. In recent years, however, interest has increased in quinidine for treating primary (idiopathic) VF, ventricular arrhythmias in patients with Brugada syndrome (see Chapter 63 ), and short-QT syndrome. Quinidine crosses the placenta so it can be used to treat fetal arrhythmias.
The most common adverse effects of chronic oral quinidine therapy are gastrointestinal (GI) and include nausea, vomiting, diarrhea, abdominal pain, and anorexia (milder with the gluconate form). CNS toxicity includes tinnitus, hearing loss, visual disturbances, confusion, delirium, and psychosis (cinchonism). Allergic reactions include rash, fever, immune-mediated thrombocytopenia, hemolytic anemia, and rarely, anaphylaxis. Side effects may preclude long-term administration of quinidine in 30% to 40% of patients.
Quinidine can slow cardiac conduction, sometimes to the point of block, which is manifested as prolongation of the QRS duration or as sinoatrial (SA) or AV nodal conduction disturbances. Quinidine can produce syncope in 0.5% to 2.0% of patients, most often the result of a self-terminating episode of TdP. Quinidine prolongs the QT interval in most patients (not dose related), regardless of whether ventricular arrhythmias occur, but significant QT prolongation (QT interval of 500 to 600 milliseconds) is often a characteristic of patients with quinidine-related syncope, who may have a genetic predisposition underlying such a response (see Chapter 9 ). Many of these patients are also receiving digitalis or diuretics or have hypokalemia; women are more susceptible than men. Importantly, syncope is unrelated to plasma concentrations of quinidine or the duration of therapy, although most episodes occur within the first 2 to 4 days of therapy initiation, often after conversion of AF to sinus rhythm. This proarrhythmic effect during initiation of treatment is reproducible and because of this, the drug should not be taken on an intermittent basis. Therapy of proarrhythmia requires immediate discontinuation of use of the drug; magnesium given intravenously (2 g over 1 to 2 minutes, followed by an infusion of 3 to 20 mg/min) is the initial drug treatment of choice. Atrial or ventricular pacing can be used to suppress the ventricular tachyarrhythmia, perhaps by suppressing EADs. When pacing is not available, isoproterenol can be given with caution. The arrhythmia gradually dissipates as quinidine is cleared and the QT interval returns to baseline. Affected patients should not use quinidine thereafter and also should avoid other drugs that prolong the QT interval (see Crediblemeds.org ).
Drugs that induce hepatic enzyme production, such as phenobarbital and phenytoin, can shorten the duration of action of quinidine by increasing its rate of elimination. Quinidine can increase plasma concentrations of flecainide by inhibiting the CYP450 enzyme system. Quinidine can elevate serum digoxin concentrations by decreasing its clearance and volume of distribution and the affinity of tissue receptors.
The cardiac actions of procainamide on automaticity, conduction, excitability, and membrane responsiveness resemble those of quinidine (see Tables 64.1, 64.2 , and eTables 64.1, 64.2 ). Procainamide predominantly blocks the inactivated state of I Na . It also blocks I Kr , I K1 , and I K.ATP . Like quinidine, procainamide usually prolongs the ERP more than it prolongs the APD and thus may prevent reentry. Procainamide exerts the least anticholinergic effects among type IA drugs. High levels of NAPA, such as in patients with renal disease, can produce EADs, triggered activity, and TdP. Because of decreased demand, availability of intravenous (IV) and oral procainamide is greatly limited.
Procainamide can depress myocardial contractility in high concentrations. It does not produce alpha blockade but can result in peripheral vasodilation, possibly through antisympathetic effects on the brain or spinal cord, which can impair cardiovascular reflexes (e.g., provoking orthostatic symptoms).
Oral administration produces a peak plasma concentration in approximately 1 hour. Approximately 80% of oral procainamide is bioavailable; the overall elimination half-life of procainamide is 3 to 5 hours, with 50% to 60% of the drug eliminated by the kidneys and 10% to 30% metabolized by the liver (see Table 64.4 ). The drug is acetylated to NAPA, which is excreted almost exclusively by the kidneys. As renal function decreases and in patients with heart failure, NAPA levels increase and, because of the risk for serious cardiotoxicity, need to be carefully monitored in these situations. NAPA has an elimination half-life of 7 to 8 hours, but the half-life exceeds 10 hours if high doses of procainamide are used. Increased age, congestive heart failure, and reduced creatinine clearance lower the clearance of procainamide and necessitate a reduced dosage.
Procainamide can be given by the oral, IV, or intramuscular (IM) route to achieve plasma concentrations in the range of 4 to 10 μg/mL and produce an antiarrhythmic effect (see Table 64.4 ). Several IV regimens have been used to administer procainamide, but usually doses of 10 to 15 mg/kg are used at a rate of up to 50 mg/min until the arrhythmia has been controlled, hypotension results, or the QRS complex is prolonged more than 50%. With this method, the plasma concentration falls rapidly during the first 15 minutes after the loading dose, with parallel effects on refractoriness and conduction. A constant-rate IV infusion of procainamide can be given at a dosage of 2 to 6 mg/min, depending on the patient’s response.
Oral administration of procainamide requires a 3- to 4-hour dosing interval at a total daily dose of 2 to 6 g, with a steady-state concentration being reached within 1 day. When a loading dose is used, it should be twice the maintenance dose. Frequent dosing is required because of its short elimination half-life in normal persons. For the extended-release forms of procainamide, dosing is at 6- to 12-hour intervals. Procainamide by IM injection is almost 100% bioavailable.
Procainamide is used to treat both supraventricular and ventricular arrhythmias in a manner comparable to that of quinidine. Although both drugs have similar electrophysiologic actions, either drug can effectively suppress a supraventricular or ventricular arrhythmia that is resistant to the other drug. Procainamide can be used to convert recent-onset AF to sinus rhythm. As with quinidine, prior treatment with beta or calcium channel blockers is recommended to prevent acceleration of the ventricular response during atrial flutter or fibrillation after procainamide therapy. Procainamide can block conduction in the accessory pathway of patients with Wolff-Parkinson-White (WPW) syndrome and can be used in patients with AF and a rapid ventricular response related to conduction over the accessory pathway. It can produce His-Purkinje block and is sometimes administered during an EPS to stress the His-Purkinje system and evaluate the need for a pacemaker (see Fig. 61.15). However, it should be used with caution in patients with evidence of His-Purkinje disease (bundle branch block) for whom a ventricular pacemaker is not readily available.
Procainamide is more effective than lidocaine in acutely terminating sustained VT. IV procainamide is recommended along with IV amiodarone and IV sotalol in the Advanced Cardiac Life Support (ACLS) Guidelines for the treatment of stable wide QRS tachycardia presumed to be VT. Most consistently, procainamide slows the VT rate, a change correlated with the increase in QRS duration. The drug also has diagnostic applications when given intravenously (10 mg/kg over 5 to 10 minutes). In patients with suspected Brugada syndrome who have a normal resting electrocardiogram (ECG), drug infusion can result in the characteristic “Brugada sign,” whereas in patients with WPW syndrome, the drug can cause sudden loss of preexcitation.
Noncardiac adverse effects from administration of procainamide include rash, myalgia, digital vasculitis, and Raynaud phenomenon. Fever and agranulocytosis may be the result of hypersensitivity reactions, and a complete blood count should be assessed at regular intervals. GI side effects are less frequent than with quinidine, and adverse CNS side effects are less frequent than with lidocaine. Toxic concentrations of procainamide can diminish myocardial performance and promote hypotension. Various conduction disturbances or ventricular tachyarrhythmias can occur, similar to those produced by quinidine. NAPA can cause QT prolongation and TdP. In the absence of sinus node disease, procainamide does not adversely affect sinus node function. In patients with sinus node dysfunction, however, procainamide can prolong sinus node recovery time and worsen symptoms in some patients with bradycardia-tachycardia syndrome.
Arthralgia, fever, pleuropericarditis, hepatomegaly, and hemorrhagic pericardial effusion with tamponade have been described in a systemic lupus erythematosus (SLE)-like syndrome related to procainamide administration. The syndrome occurs more frequently and earlier in patients who are slow acetylators of procainamide and is genetically influenced (see Chapter 9 ). Acetylation of procainamide to form NAPA appears to block the SLE-inducing effect. In 60% to 70% of patients receiving long-term procainamide therapy, anti-nuclear antibodies (ANAs) develop, with clinical symptoms occurring in 20% to 30%; this is reversible when procainamide is stopped. Positive serologic test results are not necessarily a reason to discontinue drug therapy; however, the development of symptoms with a positive anti-DNA antibody generally indicates that drug therapy should be discontinued. Corticosteroid administration in these patients may eliminate the symptoms. In this syndrome, in contrast to naturally occurring SLE, the brain and kidneys are typically spared, and there is no predilection for women.
Disopyramide has been approved in the United States for oral administration to treat patients with ventricular and supraventricular arrhythmias.
Disopyramide produces electrophysiologic effects similar to those of quinidine and procainamide, causing use-dependent block of I Na and non–use-dependent block of I Kr (see Tables 64.1, 64.2 , and eTables 64.1, 64.2 ).
Disopyramide is a muscarinic blocker and can increase the sinus node discharge rate and shorten AV nodal conduction time and refractoriness when the nodes are under cholinergic (vagal) influence. It exerts greater anticholinergic effects than quinidine and does not appear to affect alpha or beta adrenoceptors. The drug prolongs atrial and ventricular refractory periods, but its effect on AV nodal conduction and refractoriness is not consistent. Disopyramide prolongs His-Purkinje conduction time, but infra-His block rarely occurs. It can be administered safely to patients who have first-degree AV delay and narrow QRS complexes.
Disopyramide suppresses ventricular systolic performance and is a mild arterial vasodilator. The drug should generally be avoided in patients with reduced left ventricular systolic function because they tolerate its negative inotropic effects poorly.
Oral disopyramide is 80% to 90% absorbed, with a mean elimination half-life of 8 to 9 hours in healthy volunteers but almost 10 hours in patients with heart failure (see Table 64.4 ). Renal insufficiency prolongs its elimination time. Thus, in patients with renal, hepatic, or cardiac insufficiency, loading and maintenance doses need to be reduced. Peak blood levels after oral administration occur in 1 to 2 hours. Approximately 50% of an oral dose is excreted unchanged in urine, with 30% occurring as the mono- N -dealkylated metabolite. The metabolites appear to exert less effect than the parent compound. As with other class IA antiarrhythmic drugs, macrolide antibiotics inhibit its metabolism.
Doses are generally 100 to 300 mg orally every 6 hours, with a range of 400 to 1200 mg/day (see Table 64.4 ). A controlled-release preparation can be given as 200 to 300 mg every 12 hours.
Disopyramide appears to be comparable to quinidine and procainamide in reducing the frequency of PVCs and effectively preventing recurrence of VT in selected patients. Disopyramide has been combined with other drugs, such as mexiletine, to treat patients who do not respond or respond only partially to one drug.
Although rarely used for this, disopyramide helps prevent recurrence of AF after successful cardioversion as effectively as quinidine and may terminate atrial flutter. In treating patients with AF, particularly atrial flutter, the ventricular rate must be controlled before disopyramide is administered, or the combination of a decrease in atrial rate with vagolytic effects on the AV node can result in 1:1 AV conduction during atrial flutter (see Chapter 66 ). It has been used in patients with hypertrophic cardiomyopathy for both AF therapy and its negative inotropic effect.
Three types of adverse effects follow disopyramide administration. The most common effects are related to the drug’s potent parasympatholytic properties and include urinary hesitancy or retention, constipation, blurred vision, closed-angle glaucoma, and dry mouth. Symptoms are less with the sustained-release form. Second, disopyramide can produce ventricular tachyarrhythmias frequently associated with QT prolongation and TdP. Cross-sensitization to both quinidine and disopyramide occurs in some patients, and TdP can develop while receiving either drug. When drug-induced torsades de pointes (DI-TdP) occurs, agents that prolong the QT interval should be used cautiously or not at all. Lastly, disopyramide can reduce contractility of the normal ventricle, but the depression of ventricular function is much more pronounced in patients with preexisting ventricular failure. Rarely, cardiovascular collapse can result.
Ajmaline, a rauwolfia derivative, has been used extensively to treat patients with ventricular and supraventricular arrhythmias in Europe and Asia but is not available in the United States.
As with other class IA drugs, ajmaline produces use-dependent block of INa; it also weakly blocks IKr. The drug has mild anticholinergic activity (see Tables 64.1, 64.2 , and eTables 64.1, 64.2 ).
Ajmaline mildly suppresses ventricular systolic performance but does not affect peripheral resistance. It also inhibits platelet activity more potently than aspirin does.
Ajmaline is well absorbed with a mean elimination half-life of 13 minutes in most patients, thus making it poorly suited to long-term oral use. The dose for termination of acute arrhythmia is generally 50 mg intravenously infused over 1 to 2 minutes.
Although it is useful for terminating SVTs by IV infusion, other medications have largely supplanted ajmaline for this purpose and its use has evolved to that of a diagnostic tool. When administered intravenously at doses of 50 mg over a 3-minute period, or 10 mg/min, to a total dose of 1 mg/kg, ajmaline can have the following effects: (1) delta wave disappearance in patients with WPW syndrome (indicating an accessory pathway anterograde ERP longer than 250 milliseconds); (2) ST-T abnormalities and interventricular conduction block in patients with occult Chagasic cardiomyopathy; (3) heart block in patients with bundle branch block and syncope, but in whom no rhythm disturbance had been discovered; and (4) right precordial ST elevation in patients with suspected Brugada syndrome in whom findings on the resting ECG are normal. It is in this last setting that ajmaline is used most frequently.
Ajmaline can produce mild anticholinergic side effects, as well as mild depression of left ventricular systolic function, and can worsen AV conduction in patients with His-Purkinje disease. Rare occurrences of TdP have been reported. Ajmaline can increase the defibrillation threshold.
Lidocaine blocks I Na , predominantly in the open or inactivated state. It has rapid onset and offset kinetics and does not affect normal sinus node automaticity in usual doses but does depress other normal and abnormal forms of automaticity, as well as EADs and DADs in Purkinje fibers in vitro (see Tables 64.1, 64.2 , and eTables 64.1, 64.2 ). Lidocaine has only a modest depressant effect on V̇ max ; however, faster rates of stimulation, acidosis, increased extracellular K + concentration, and reduced membrane potential (changes that can result from ischemia) increase the ability of lidocaine to block I Na . Lidocaine can convert areas of unidirectional block into bidirectional block during ischemia and inhibit the development of VF by preventing fragmentation of organized large wavefronts into heterogeneous wavelets.
Except in very high concentrations, lidocaine does not affect slow-Ca channel–dependent action potentials despite its moderate suppression of the Ca current. Lidocaine has minimal effect on atrial fibers and does not affect conduction in accessory pathways. Depressed automaticity or conduction can develop in patients with preexisting sinus node dysfunction, abnormal His-Purkinje conduction, or junctional or ventricular escape rhythms. Part of lidocaine’s effects may involve inhibition of cardiac sympathetic nerve activity.
Clinically significant adverse hemodynamic effects are rarely noted with lidocaine at the usual drug concentrations unless left ventricular function is severely impaired.
Lidocaine is used only parenterally because oral administration results in extensive first-pass hepatic metabolism and unpredictably low plasma levels, as well as excessive metabolites that can produce toxicity (see Table 64.4 ). Hepatic metabolism of lidocaine depends on hepatic blood flow; severe hepatic disease or reduced hepatic blood flow, as in heart failure or shock, can greatly decrease the rate of lidocaine metabolism. Beta-adrenoceptor blockers can decrease hepatic blood flow and increase the serum concentration of lidocaine. Prolonged infusion can reduce lidocaine clearance. Its elimination half-life averages 1 to 2 hours in normal individuals, longer than 4 hours in patients after uncomplicated myocardial infarction (MI), longer than 10 hours in patients after MI complicated by heart failure, and even longer in the presence of cardiogenic shock. Maintenance doses should be reduced by one third to one half in patients with low cardiac output.
Although lidocaine can be given intramuscularly, the IV route is most often used, with an initial bolus of 1 to 2 mg/kg body weight at a rate of 20 to 50 mg/min and a second injection of half the initial dose 20 to 40 minutes later to maintain the therapeutic concentration (see Table 64.4 ).
If the initial bolus of lidocaine is ineffective, up to two more boluses of 1 mg/kg may be administered at 5-minute intervals. Patients who require more than one bolus to achieve a therapeutic effect generally need a higher maintenance dose to sustain these higher concentrations, with infusion rates in the range of 1 to 4 mg/min to produce steady-state plasma levels of 1 to 5 mg/mL in patients with uncomplicated MI. These rates must be reduced during heart failure or shock because of the concomitant reduced hepatic blood flow. Higher doses and concentrations are unlikely to provide additional benefit but do increase the risk for toxicity.
Lidocaine has moderate efficacy against ventricular arrhythmias of diverse causes; it is generally ineffective against supraventricular arrhythmias and rarely terminates monomorphic VT. Although once used in an attempt to prevent VF in the first 2 days after acute MI, its efficacy was marginal, and because it can produce side effects such use is now not recommended.
The most frequently reported adverse effects of lidocaine are dose-related manifestations of CNS toxicity: dizziness, paresthesias, confusion, delirium, stupor, coma, and seizures. Occasional sinus node depression and His-Purkinje block have been reported. Rarely, lidocaine can cause malignant hyperthermia.
Mexiletine, a local anesthetic congener of lidocaine with anticonvulsant properties, is used for the oral treatment of patients with symptomatic ventricular arrhythmias. It is rarely used as a single agent for the treatment of ventricular arrhythmias.
Mexiletine is similar to lidocaine in many of its electrophysiologic actions. In vitro, mexiletine shortens the APD and ERP of Purkinje fibers and, to a lesser extent, ventricular muscle. It depresses the V̇ max of phase 0 by blocking I Na , especially at faster rates, and depresses the automaticity of Purkinje fibers but not of the normal sinus node. Its onset and offset kinetics are rapid. Hypoxia or ischemia can increase its effects (see Tables 64.1, 64.2 , and eTables 64.1, 64.2 ).
Mexiletine can result in severe bradycardia and abnormal sinus node recovery time in patients with sinus node disease, but not in those with a normal sinus node. It does not affect AV nodal conduction and can depress His-Purkinje conduction, but not greatly, unless conduction was abnormal initially. Mexiletine does not appear to affect human atrial muscle. It does not affect the QT interval. It has been used in treating a variety of other disorders, including erythromelalgia (red, painful extremities) in children and myotonia.
Mexiletine exerts no major hemodynamic effects on ventricular contractile performance or peripheral resistance.
Mexiletine is rapidly and almost completely absorbed after oral ingestion by volunteers, with peak plasma concentrations being attained in 2 to 4 hours (see Table 64.4 ). Its elimination half-life is approximately 10 hours in healthy individuals but 17 hours in post-MI patients. Therapeutic plasma levels of 0.5 to 2 mcg/mL are maintained by oral doses of 200 to 300 mg every 6 to 8 hours. Absorption with less than a 10% first-pass hepatic effect occurs in the upper part of the small intestine and is delayed and incomplete in patients receiving narcotics or antacids. Approximately 70% of the drug is protein bound; the apparent volume of distribution is large because of extensive tissue uptake. Normally, mexiletine is eliminated metabolically by the liver, with less than 10% being excreted unchanged in urine. Doses should be reduced in patients with cirrhosis or left ventricular failure. Renal clearance of mexiletine decreases as urinary pH increases. Its known metabolites exert no electrophysiologic effects. Metabolism can be increased by phenytoin, phenobarbital, and rifampin and can be reduced by cimetidine.
The recommended starting dose is 200 mg orally every 8 hours when rapid arrhythmia control is not essential (see Table 64.4 ). Doses may be increased or decreased by 50 to 100 mg every 2 to 3 days and are better tolerated when given with food. The total daily dose should generally not exceed 1200 mg. In some patients, administration every 12 hours can be effective.
Mexiletine is a moderately effective antiarrhythmic agent for the treatment of acute and chronic ventricular tachyarrhythmias, but not SVTs. Success rates vary from 6% to 60% and can be increased in some patients if mexiletine is combined with other drugs such as procainamide, beta blockers, quinidine, disopyramide, propafenone, or amiodarone. Most studies show no clear superiority of mexiletine over other class I agents. Mexiletine may be very useful in children with congenital heart disease and serious ventricular arrhythmias. In treating patients with a long QT interval, mexiletine may be safer than drugs that increase the QT interval further, such as quinidine. Limited experience in treating subsets of patients with long-QT syndrome (LQT3, which is related to the SCN5A gene for the cardiac sodium channel) suggests a beneficial role (see Chapter 63 ). Mexiletine has also been used to treat myotonia in patients with neuromuscular diseases such as myotonic dystrophy. Involvement of the conduction system in these diseases can predispose these patients to advanced life-threatening heart rate slowing, mandating mexiletine be used with caution or backup pacing in this situation ( Chapter 100 ).
Up to 40% of patients may require a change in dose or discontinuation of mexiletine therapy as a result of adverse effects, including tremor, dysarthria, dizziness, paresthesia, diplopia, nystagmus, confusion, nausea, vomiting, and dyspepsia. Cardiovascular side effects are rare but include hypotension, bradycardia, and exacerbation of arrhythmia. The adverse effects of mexiletine appear to be dose related, and toxic effects can occur at plasma concentrations that are sub-therapeutic or only slightly higher than therapeutic levels. Therefore, its effective use requires careful titration of dose and monitoring for adverse effects and possibly plasma concentration. Lidocaine use as an AAD should be avoided in patients receiving mexiletine.
Phenytoin was used originally to treat seizure disorders. Its value as an AAD is limited to rare cases of digitalis-toxic atrial and ventricular tachyarrhythmias (for which more rapid and effective control can be achieved with digitalis-specific antibodies) and occasional cases of ventricular arrhythmias when used in combination with other agents (see Tables 64.1, 64.2, 64.4 and eTables 64.1, 64.2 ).
Flecainide is approved by the FDA to treat patients with life-threatening ventricular arrhythmias, as well as various supraventricular arrhythmias.
Flecainide exhibits marked use-dependent depressant effects on the rapid sodium channel by decreasing V̇ max and has slow onset and offset kinetics (see Tables 64.1, 64.2 , and eTables 64.1, 64.2 ). Drug dissociation from the sodium channel is slow, with time constants of 10 to 30 seconds (versus 4 to 8 seconds for quinidine and <1 second for lidocaine). Thus, marked drug effects can occur at physiologic heart rates. Flecainide shortens the duration of the Purkinje fiber action potential but prolongs it in ventricular muscle, actions that, depending on the circumstances, could enhance or reduce electrical heterogeneity and create or suppress arrhythmias. Flecainide profoundly slows conduction in all cardiac fibers and, in high concentrations, inhibits the slow Ca 2+ channel (see Chapter 62 ). Conduction time in the atria, ventricles, AV node, and His-Purkinje system is prolonged. Minimal increases in atrial or ventricular refractoriness or in the QT interval result. Anterograde and retrograde refractoriness in accessory pathways can increase significantly in a use-dependent manner. Sinus node function remains unchanged in normal individuals but may be depressed in patients with sinus node dysfunction. Flecainide can facilitate or inhibit reentry and may transform AF to flutter. Pacing and defibrillation thresholds are characteristically slightly to significantly increased.
Flecainide depresses cardiac performance, particularly in patients with compromised ventricular systolic function, and should be used cautiously or not at all in those with moderate or severe ventricular systolic dysfunction.
Flecainide is at least 90% absorbed, with peak plasma concentrations achieved in 3 to 4 hours. Its elimination half-life in patients with ventricular arrhythmias is 20 hours, with 85% of the drug excreted unchanged or as an inactive metabolite in urine (see Table 64.4 ). Its two major metabolites have less potency than the parent drug. Elimination is slower in patients with renal disease and heart failure, and doses should be reduced in these situations. Therapeutic plasma concentrations range from 0.2 to 1.0 mcg/mL. Approximately 40% of the drug is protein bound. Increases in serum concentrations of digoxin (15% to 25%) and propranolol (30%) result during co-administration with flecainide. Propranolol, quinidine, and amiodarone may increase flecainide serum concentrations. Five to 7 days of dosing may be required to reach a steady-state concentration in some patients.
The starting dose is 100 mg every 12 hours, increased in increments of 50 mg twice daily, no sooner than every 3 to 4 days, until efficacy is achieved or an adverse effect is noted, or to a maximum of 400 mg/day (see Table 64.4 ). Cardiac rhythm and QRS duration should be monitored after changes in dose.
Flecainide is indicated for the treatment of life-threatening ventricular tachyarrhythmias, SVTs, and paroxysmal AF. Encouraging experimental and early clinical data support its use for catecholaminergic polymorphic VT (see Chapter 63 ). The dosage is adjusted to achieve the desired effect, but the serum concentration should not exceed 1.0 μg/mL. Flecainide is particularly effective in suppressing PVCs and short runs of non-sustained VT. As with other class I AADs, no data from controlled studies indicate that the drug favorably affects survival or sudden cardiac death, and data from CAST indicate increased mortality in patients with coronary artery disease (CAD). Flecainide produces a use-dependent prolongation of VT cycle length, which can improve hemodynamic tolerance. Flecainide is also useful for various SVTs, such as atrial tachycardia (AT), atrial flutter, and AF (including oral loading to terminate episodes acutely). Flecainide and propafenone may both be used in combination with beta or calcium channel blockers as a “pill in the pocket” approach to terminate AF as an outpatient ( Table 64.4 ). When flecainide toxicity occurs, isoproterenol can reverse some of its electrophysiologic effects. It is important to slow the ventricular rate before treatment of AF with flecainide (e.g., with beta blockers or verapamil or diltiazem) to avoid the 1:1 AV conduction of slowed atrial flutter that may result from the effect of flecainide on fibrillation. Flecainide has been used to treat fetal arrhythmias and arrhythmias in children. Flecainide administration can produce ST elevation in lead V 1 , characteristic of Brugada syndrome, in susceptible patients (see Chapter 63 ) and has been used as a diagnostic tool in persons suspected of having this disorder.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here