Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The ovaries develop from a thickening of cells that form ridges medial to the müllerian and wolffian bodies. These germinal ridges appear at the sixth week. Primary oocytes, arising in the umbilical vesicle (yolk sac) and migrating along the mesentery of the hindgut, arrive in the embryonic gonads and are thought to provide the countless thousands of ova that crowd the ovary at birth.
By the third month of gestation, the ovaries descend toward the pelvis. The pull of the gubernaculum—an abdominal fold that grows more slowly than the rest of the fetus—exerts a downward traction on the gonadal ridges. Later, these folds fuse in their midportion with that part of each müllerian duct that develops into the uterine fundus. The lateral half and the medial portion of the folds become the round ligaments and the suspensory ligaments of the ovary, respectively.
The infant ovary is a sausage-shaped structure, with a pale and smooth surface. A gradual thickening and shortening occur throughout the first decade of life. The major gain in size and weight takes place after the menarche and during adolescence. Two layers, the germinal epithelium and the tunica albuginea, constitute the surface of the prepubertal ovary. They are crowded with primordial ova that are surrounded by darkstaining cells, the origin of the future granulosa cells. The granulosa cells are polygonal and rather uniform, round, with sharply outlined nuclei in a poorly stainable cytoplasm that contains, however, numerous granules, from which this layer derives its name.
As the primordial follicle develops, it sinks, with its single layer of epithelial cells, toward the center of the ovary. The attendant cells proliferate to form a many-layered coating of granulosa cells around the developing follicle. A crescentic cavity forms eccentrically, in which follicular fluid accumulates. From the surrounding ovarian stroma, a capsule of theca cells differentiates. The theca interna is rich in capillaries, upon which the avascular theca granulosa must depend for nourishment. Before menarche, while still little or no follicle-stimulating hormone is present, these follicles develop no further but degenerate and become atretic.
The mature gonad is an approximately almond-shaped structure, pitted and scarred by the stigmata of ovulation. Spiral arteries enter at the hilum and are involved in sequential changes during the cyclic ebb and sway of follicle growth and development of corpora lutea. In the hilum are also found cells with morphologic and histochemical properties, similar to the interstitial cells of the testis—vestiges from the fetal period, before sex differentiation took place. Proliferation of these cells or tumor formation may result in virilization.
In the ripening follicle, the oocyte is a spherical body composed of clear protoplasm. It contains a round, dark-staining nucleus, with a definite surrounding membrane and an eccentric nucleolus. A transparent membrane, the zona pellucida, encloses a fluid-filled perivitelline space in which the egg floats freely. A dense layer of granulosa cells, the cumulus oophorus, closely envelops the egg and attaches it to the follicular wall. The cumulus cells immediately next to the zona arrange themselves radially outward to form the corona radiata.
The two-layered theca envelope coats the follicle. The theca interna is composed of large epithelioid cells interspersed in connective tissue and rich in blood and lymph vessels. The theca externa is thick and dense, consisting of circularly arranged connective tissue fibers.
In the follicles that do not mature but degenerate, the granulosa layer first becomes disorganized. The corona loses its radial arrangement. Thereafter, the follicular cavity shrinks, and soon the egg itself loses its characteristic features. Hyaline is deposited in a wavy, concentric band. Up to this point, the theca interna has continued to be a prominent layer of large, vesicular, nucleated cells. Degenerative changes rapidly progress until nothing is left except an amorphous hyaline scar.
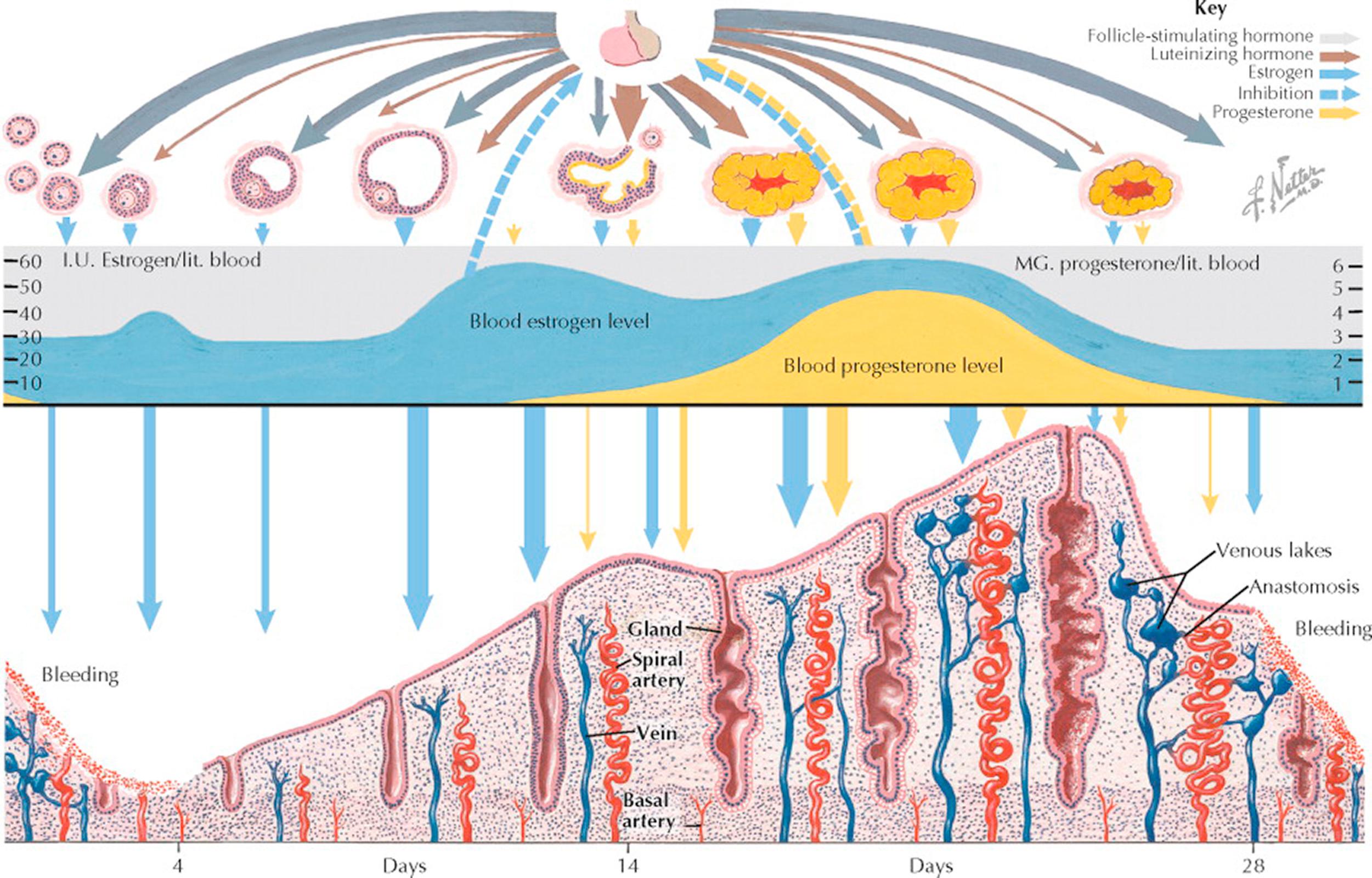
Menarche, or the first menstrual cycle, heralds the onset of adult reproductive function, which continues until menopause, when the major part of ovarian function ceases. Between these events, the hormonal ballet of cyclic menstruation takes place.
Two anterior pituitary gonadotropins and two ovarian steroids are primarily involved in this periodic occurrence of menstruation: the pituitary contributes follicle-stimulating hormone (FSH) and luteinizing hormone (LH), whereas the ovary secretes β-estradiol and progesterone.
Up to puberty—even during fetal life—ovarian follicles are constantly developing to a stage in which an antrum has formed, regressing then to become atretic. Eventually, one or more follicles produce enough estrogen to cause a proliferation of the endometrium. It is unlikely that any of these early follicles, though producing estrogen, achieve ovulation; more likely, atresia sets in and the endometrium breaks down, with resultant bleeding. It is probable that the mature cycle will not become established for several months after this, the first menstruation.
At the onset of menstrual flow (day 1), production of estrogen is low but FSH levels rise and initiate another round of folliculogenesis. A cohort consisting of a number of follicles begins the maturation process approximately 375 days prior to day 1 of the menstrual cycle. By the onset of menses, these have matured enough to respond with growth and an increase in estrogen. Most of the follicles, however, have a relatively short life. Their granulosa cells and ovum degenerate, leaving an atretic follicle. A few continue to enlarge, but in most cycles only one emerges as the mature graafian follicle that ruptures or ovulates about day 14.
Through about day 12 of the cycle, the secretion of FSH decreases with the increase of estrogen production. Although follicle growth beyond the stage of antrum formation must be initiated by the pituitary and continues to be dependent on this stimulus for the first week or so of the cycle, after about day 8 further development is autonomous. A surge of LH (and to a lesser extent FSH) at midcycle reflects this rising estrogenic tide and it provides the endocrine trigger for ovulation.
After ovulation the estrogen level drops slightly during a lag period between the functional peak of the mature follicle and that of the fully developed corpus luteum. Some uterine spotting or even bleeding for a day or two is not rare at this time (“midcycle bleeding”).
Within a few hours after ovulation, the empty cavity of the ruptured follicle becomes filled with blood clots, and a network of capillary fingers stretches tentatively inward along fibrin strands from the theca interna. Theca cells containing a yellow lipochrome, named lutein, proliferate centripetally at a rapid rate along with the capillary mesh. Progesterone production is quickly accelerated, and its effect can be detected by secretory changes in the endometrium within 48 hours after ovulation.
Stimulation of the thermal center in the brainstem, by progesterone, causes a rise in basal body temperature, which is sustained as long as the corpus luteum functions. Cervical mucus becomes scanty and viscid and, when rapidly dried on a slide, no longer crystallizes in a “fernlike” pattern. By day 20, the estrogen level is usually as high as that just before ovulation, and the corpus luteum has also reached a peak of production of progesterone.
Under the influence of estrogen and progesterone, growth and the secretory activity of the endometrium progress continuously through day 25 or 26. Unless fertilization has occurred, degeneration of the corpus luteum is initiated. With the consequent decline of both estrogen and progesterone, changes occur in the endometrium that lead inevitably to slough and necrosis. By day 28, the pituitary, now released from the inhibitive levels of estrogen, starts again and rapidly reaches its peak of FSH output, which supports a new crop of primary follicles for the next cycle.
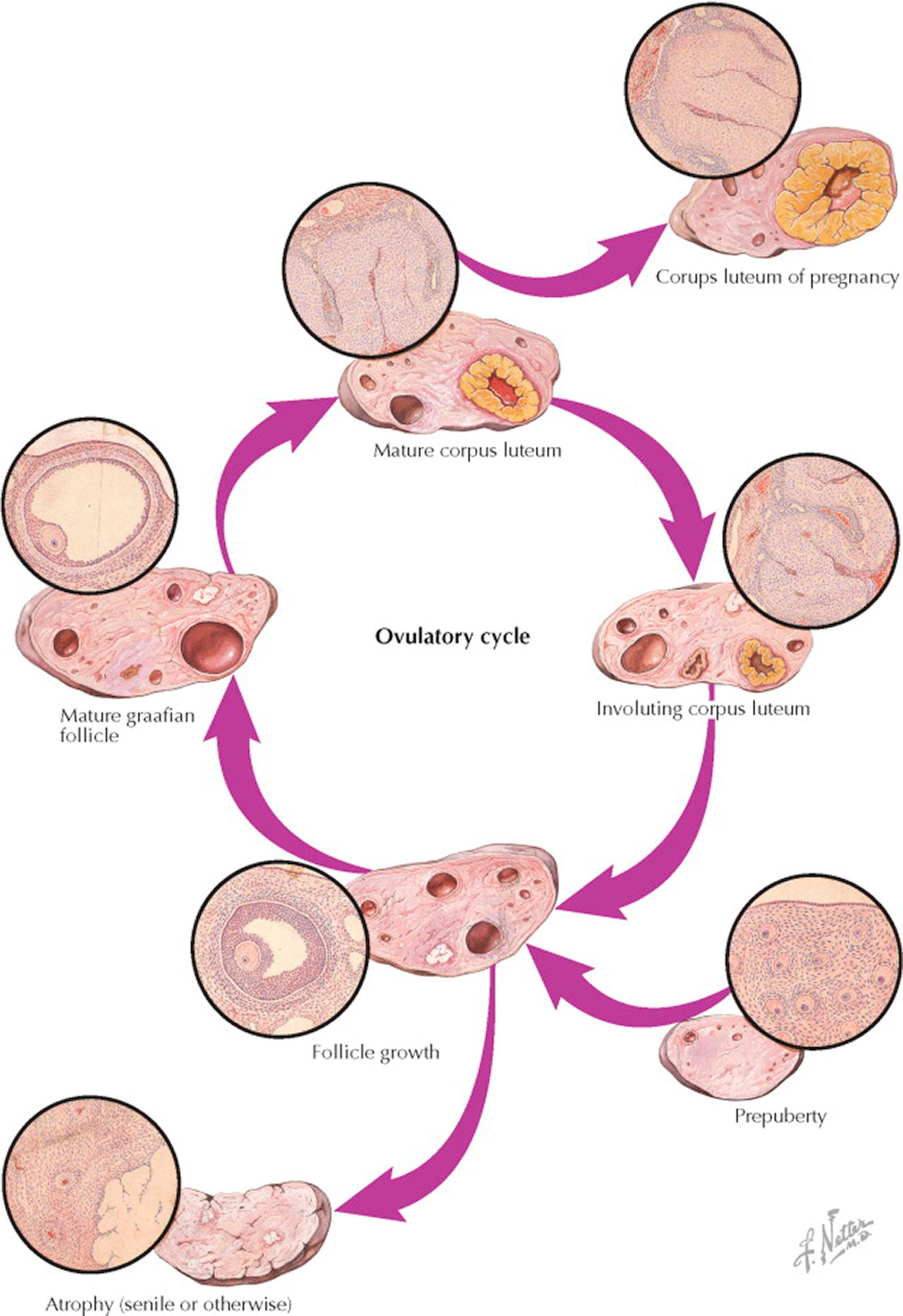
Although follicle growth is relatively even, it is slow for 10 to 12 days, during which time the granulosa layer thickens and the antrum becomes distended by increasing amounts of estrogen-rich liquor folliculi. After the follicles have reached a diameter of about 0.5 cm, they start their migration outward toward the surface, and about 12 days after the start of this cycle of growth, one follicle “gains ascendancy” in one of the ovaries, reaching a diameter of 1.5 to 2 cm in a short time. Simultaneously, it begins to protrude from the ovarian surface. This surge of development is accompanied by a similarly sudden wave of atretic processes, which wipes out all lesser developing follicles in both ovaries. Because the granulosa is the first layer to degenerate in this change leading to atresia, it is probable that the theca is responsible for transient continuing secretion.
Whether follicle migration is accomplished by hormonal or enzymatic reactions or merely by the physical forces involved in a rapidly expanding cystic structure enclosed in a tough, fibrous stroma by a relatively inelastic tunica is not known. During this development under the influence of the luteinizing hormone (LH) surge, the ovum undergoes the first maturation division and the first polar body is extruded, reducing the number of chromosomes from 46 to 23. The granulosa cells become widely separated by edema, thus loosening the egg and its surrounding cumulus for detachment from the inside of the follicle cavity. Finally, through compression of the capillary net at the weakest point of the follicle wall near the surface, a relatively avascular area is produced, and through this area a break occurs. Intrafollicular pressure forces the egg, cumulus, and some detached granulosa cells with the liquor folliculi out into the peritoneal cavity.
Bleeding to some degree from the break in the richly vascular theca interna always attends ovulation. Usually, the rupture point is rapidly sealed off, though in patients with compromised clotting ability, this can result in hemorrhage. The cavity of the follicle is filled with blood-containing fluid—the so-called corpus haemorrhagicum—and an immediate differentiation of cells sets in, which spreads inward from the granulosal remnants. These lipid- and pigment-containing cells—the lutein cells—grow on a network of capillaries from the theca interna. This process causes an appearance of infolding as the walls of the former follicle thicken and encroach more and more on the fibrin-containing, blood-filled cavity ultimately representing the mature corpus luteum. When pregnancy occurs, this development continues under the influence of human chorionic gonadotropin and increases until the bright-yellow body may make up as much as one half the total volume of the ovary—the corpus luteum of pregnancy. Some time after the second month of gestation, a slow process of regression of the corpus luteum occurs and is accompanied by the luteal–placental shift, where the placenta becomes the primary organ of hormonogenesis for the remainder of pregnancy.
If conception does not take place, the fate of the corpus luteum is involution. The crenated, yellow margin shrinks relatively rapidly, and the lutein cells degenerate into amorphous, hyaline masses held together by strands of connective tissue. The yellow color is in most part lost. The shrunken convolutions of hyalinized material are known as a corpus albicans. It is during this regressive phase that the decrease of estrogen and progesterone production prompts a new output of pituitary gonadotropins, producing stimulation of a new crop of ovarian follicles, and a new ovulatory growth cycle is once more initiated.
The senile ovary is a shrunken and puckered organ, containing few if any follicles, and made up for the most part of old corpora albicantia and corpora atretica. In the menopause, the senile ovary continues to produce hormones, albeit at lower levels and with a more androgenic profile.
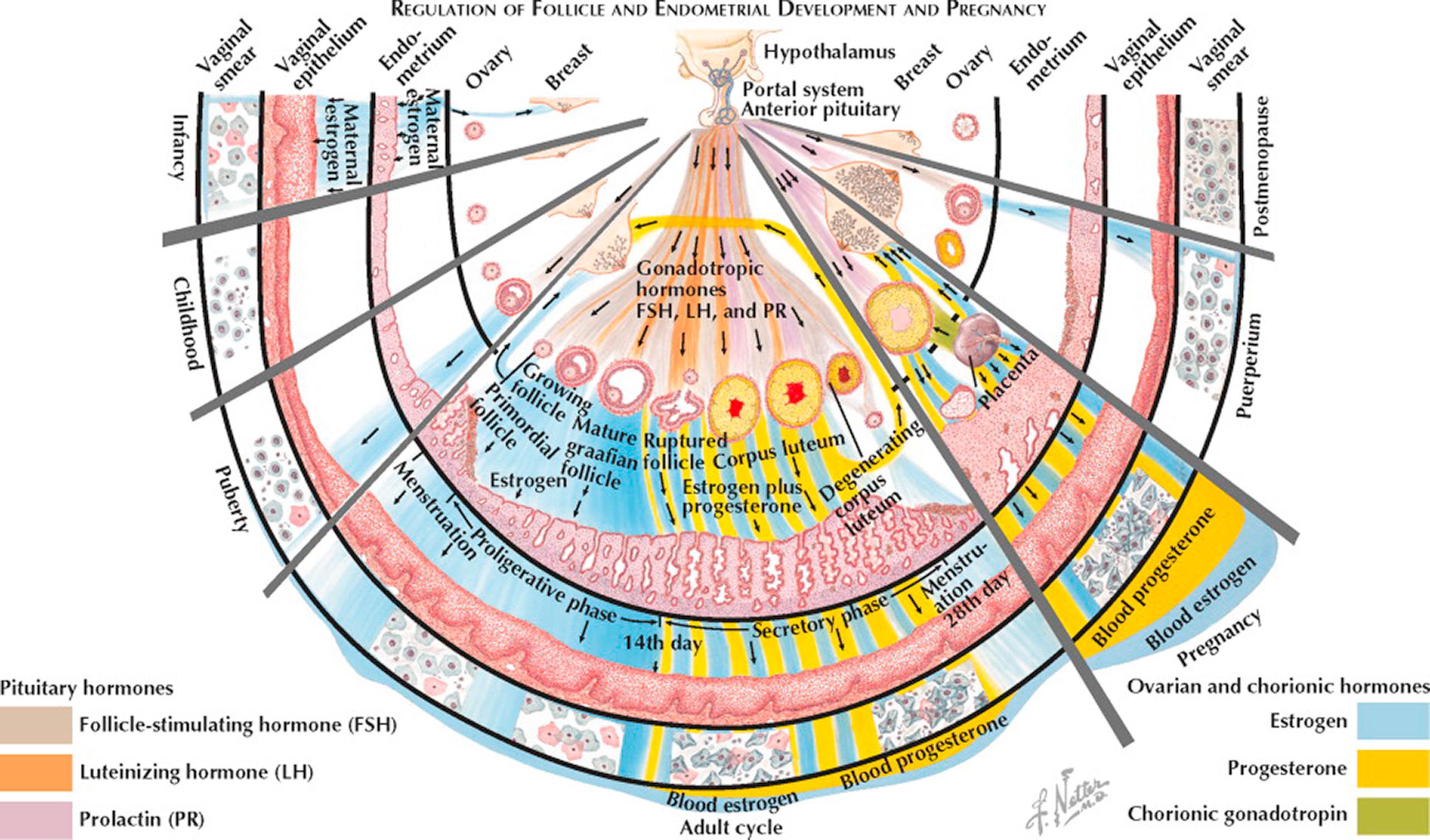
The placenta provides no barrier to the high concentration of maternal estrogens prior to parturition. As a result, the infant's breasts may show some enlargement, and milk can occasionally be expressed. The external genitalia are precociously developed, and the endometrium has been stimulated to proliferate. Within a week or so after birth, all the stigmata of estrogen stimulation recede.
From the postnatal recessional changes to the time of puberty, the ovaries gradually show a buildup of interstitial tissue from an accumulation of fibrous stroma, as a constant succession of primordial follicles degenerate in atresia.
Pituitary maturation and consequent secretion of gonadotropins results in the initiation of enhanced ovarian steroid production at the time of puberty. The uterus is the first to respond and the endometrium proliferates with the development of straight, tubular glands. Next, the vagina thickens and becomes stratified, with cornified superficial estrogenic cells appearing. In the ovary, primordial follicles progress beyond the stage of a one- or two-layer granulosa with a tiny antrum and exhibit identifiable several-thickness granulosa and theca interna layers. In the breast, the areolae show pigmentation along with a domelike change, becoming elevated as a conical protuberance. Fat is deposited about the shoulder girdle, hips, and buttocks, and the adult pelvic and, later, the axillary hair patterns typical of the female begin to develop.
In the decade of adolescence, the skeletal system reacts to estrogen, first, by an accelerated growth rate of the long bones, and, second, by a hastening of epiphyseal closure, the balance affecting final height.
In the mature cycle, the endometrium undergoes cyclic changes (described elsewhere) under the stimulus of estrogen secreted by ovarian follicles in response to follicle-stimulating hormone (FSH). By day 12 in a typical 28-day cycle, one follicle attains dominance and exhibits a rapid growth toward maturity. The release of luteinizing hormone (LH) at midcycle on day 14 induces ovulation of the mature follicle and initiates progesterone excretion from the rapidly forming corpus luteum. Endometrial glands become saw-toothed and secretory. If fertilization and implantation do not occur, the corpus luteum degenerates on about day 26, and in consequence with the rapid withdrawal of its estrogen and progesterone secretion the endometrium shrinks, undergoes autolysis, and breaks away with bleeding on day 28.
When conception occurs, the early excretion of chorionic gonadotropin maintains the corpus luteum. In pregnancy, the peak production of chorionic hormone is seen by about day 90 after the last menstrual period, declining thereafter to a plateau. The corpus luteum is responsible for increasing progesterone and estrogens throughout the first 3 months, after which the placenta takes over until the end of the pregnancy. The augmentation of both estrogen and progesterone is approximately linear throughout the 9 months of gestation. The breasts react to the increasing steroid stimulation with an extension of both ductile and alveolar growth, and there is congestion without actual lactation.
The withdrawal of estrogen and progesterone after placental delivery combined with the psychoneural mechanisms initiated by the suckling reflex bring about the release of prolactin. Breast tissues, already conditioned by growth, respond with milk production. Ovarian activity is often held in abeyance for approximately 6 months in women who are fully breastfeeding; it may well occur sooner in women who are partially breastfeeding. Reestablishment of the pituitary–ovarian cycle can, and often does, take place before weaning, so that another conception can occur before the advent of a menstrual flow.
In the United States, menopause occurs late in the fourth or early in the fifth decade (mean age 51 ± 2). The ovaries no longer contain any follicles capable of responding to pituitary stimulation, and increasing amounts of FSH are released in response to lower estrogen and inhibin levels. Estrogen deficiency is reflected by senile changes in the breasts, uterus, and vagina, and also in the skin, bony skeleton, and vascular system.
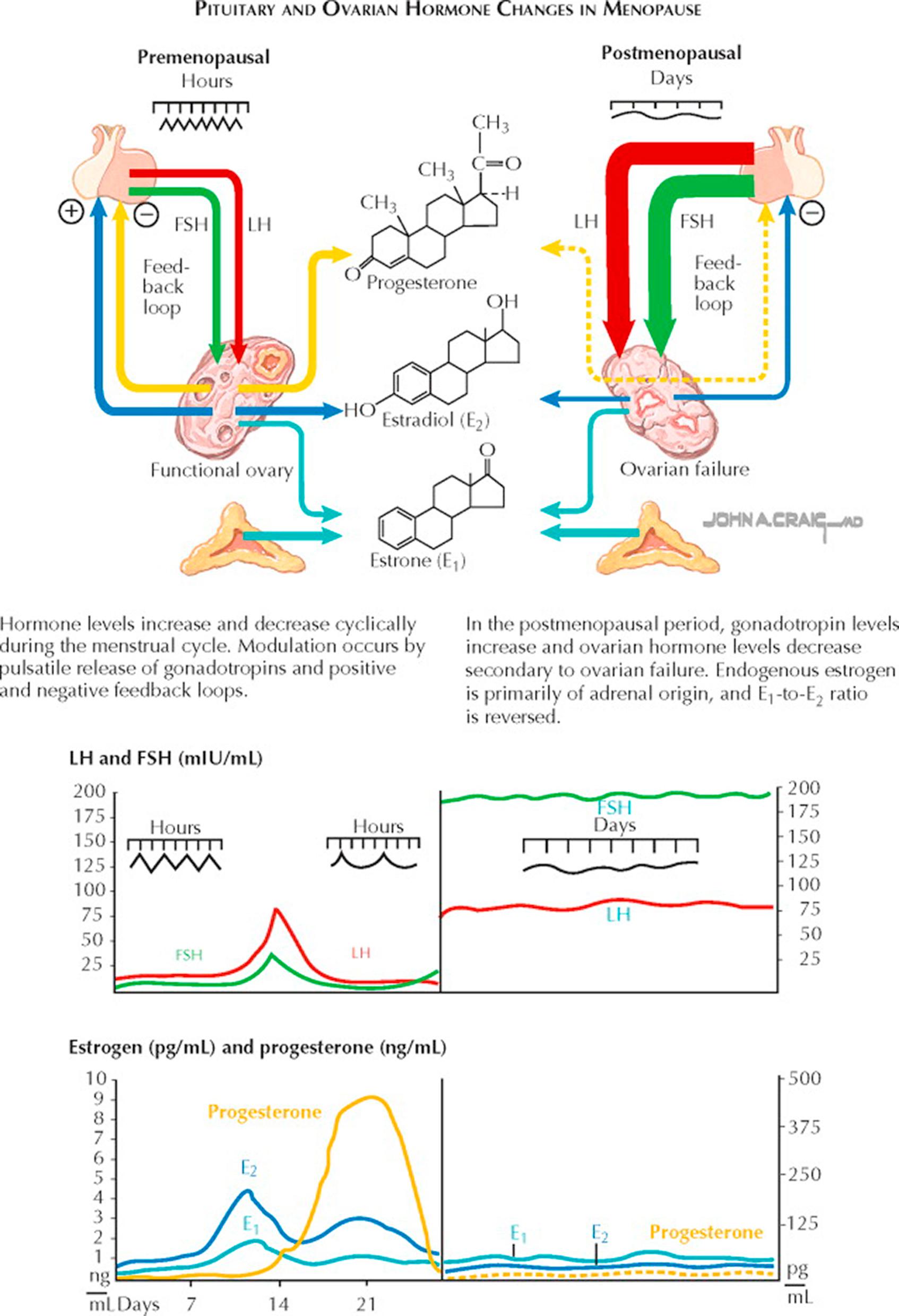
Menopause is the loss of normal ovarian steroidogenesis because of age, chemotherapy (alkylating agents), radiation, or surgical therapy. (Menopause may be viewed as an endocrinopathy: the loss of an endocrine function with adverse health consequences.) Menopause naturally occurs at a median age of 51.5, with 95% of women going through this transition between ages 44 and 55. Menopause may occur at a younger age in smokers, those with poor nutrition or chronic illness, or those who have a loss of genetic material from the long arm of the X chromosome.
When ovarian steroidogenesis is lost, menstrual function (if the uterus is present) ceases. Up to 85% of women will also experience hot flashes, flushes, and night sweats, with the most severe symptoms associated with the steepest or most abrupt declines in hormone levels (e.g., surgical menopause). Vaginal atrophy, vulvodynia, dysuria, urinary urgency, and urgency incontinence, urinary frequency, nocturia, and an increased incidence of stress urinary incontinence are also common with the loss of estrogen. For many women, there is a decrease in libido, independent of that caused by vaginal dryness and the attendant dyspareunia. Around the time of menopause, there is an estrogen-dependent accelerated loss of bone mass. There is a suggestion of an increased risk of cardiovascular disease associated with natural menopause and strong evidence of this in premature surgical menopause.
During the natural transition from ovulatory function to postmenopause ovarian activity (the “climacteric period”), many women will experience irregular vaginal bleeding and may experience the beginnings of hot flashes or flushes. Following menopause, the ovary is not truly quiescent: luteinizing hormone (LH) stimulation of theca cell islands in the ovarian stroma results in testosterone and androstenedione production. Although these are produced at a much lower level than before menopause, androgens become the primary hormonal product of the ovaries.
Although the timing and symptoms of menopause are sufficiently characteristic to allow a diagnosis to be made by history and physical findings alone, when the symptoms are atypical or the timing other than expected, alternative causes for the symptoms such as pregnancy, hypothyroidism, polycystic ovary syndrome (PCOS), a prolactin-secreting tumor, or hypothalamic dysfunction should all be considered. When the diagnosis of ovarian failure must be confirmed, measurement of serum follicle-stimulating hormone (FSH) can help support the diagnosis. FSH levels higher than 100 mIU/mL are diagnostic. Although lower levels (40 to 50 mIU/mL) may be sufficient to establish a diagnosis when symptoms are also present, a single measure of an elevation at these lower levels is not a reliable indicator of menopause. Serum estradiol levels may be determined (generally less than 15 pg/mL) but are less reliable as a marker of ovarian failure. A pregnancy test is always indicated in sexually active perimenopausal women who are not using contraception. A vaginal maturation index may be obtained but is generally not required for diagnosis. Bone densitometry may be indicated for those at special risk for bone loss. When noncyclic bleeding occurs in these patients, pelvic examination, Pap smear, and endometrial biopsy should be strongly considered. Women who have ovarian failure below age 30 should have a karyotype performed.
The management of menopause and its symptoms has become controversial in recent years. Estrogen replacement therapy is still indicated when symptoms such as vasomotor or urogenital symptoms warrant, but most suggest that this therapy should be time-limited. Hormone replacement therapy targeted primarily toward the prevention of bone loss or to reduce the risk of heart disease has generally been replaced by more specific osteoporosis therapies and cardiac risk–reduction strategies. When estrogens are used, progestins are usually added to the regimen if the patient has a uterus present to reduce the risk of endometrial hyperplasia or cancer. (Continuous estrogen exposure without periodic or concomitant progestins increases the risk of endometrial carcinoma six- to eightfold.)
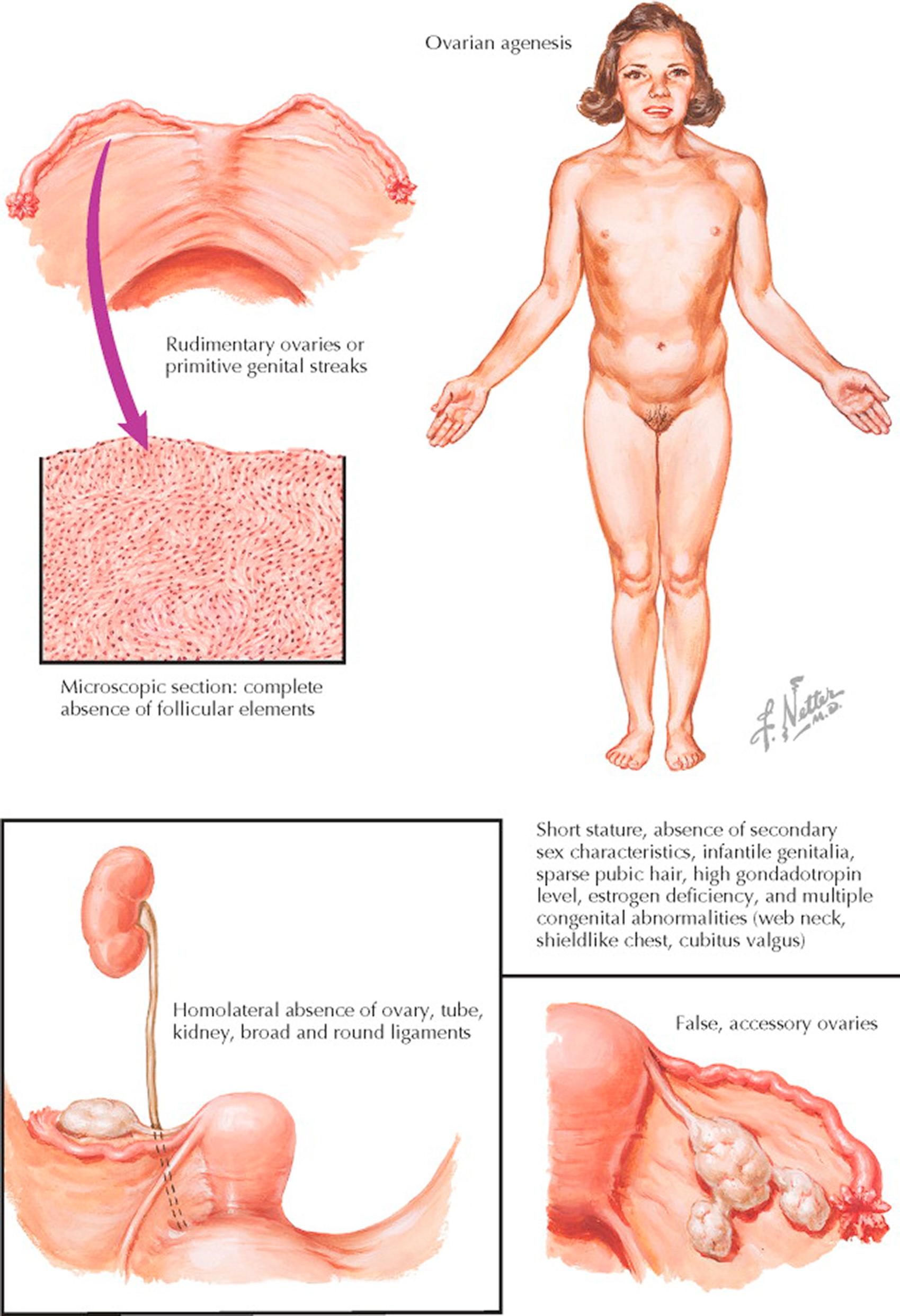
Ovarian failure is one of the hallmarks of Turner syndrome. The typical picture is produced not only by ovarian agenesis but also by coexisting congenital abnormalities of the skeletal, cardiovascular, and nervous systems. This entity is characterized by short stature, primary amenorrhea, sexual infantilism, high gonadotropin level, and multiple congenital abnormalities.
Following midgestation, there is a rapid increase in oocyte atresia as compared to normal ovaries, often resulting in early ovarian failure. The rate of complete depletion varies; some have primary amenorrhea with no secondary sexual characteristics, whereas others have varying degrees of pubertal development. The depleted ovaries are usually represented by thin, elongated, firm, whitish thickenings on the posterior surface of each broad ligament. On section, they are usually composed of spindlelike cells, arranged in whorls, without evidence of germ cells or follicles. The internal genitalia are markedly hypoplastic, being smaller than those seen in the newborn infant.
The diagnosis is usually made after puberty, when a primary amenorrhea and the absence of secondary sex characteristics are noted in conjunction with other congenital defects. The estrogen deficiency is manifested by undeveloped genitalia and breasts, sparse pubic and axillary hair, delayed epiphyseal union, osteoporosis, and fine wrinkling of the skin (precocious senility).
The patients are short, averaging 52 in. and rarely attaining a height of more than 58 in. A variety of congenital anomalies have been associated with this syndrome, including cubitus valgus (increased carrying angle), webbing of the neck (symmetric winglike folds of skin extending from the base of the skull to the supraclavicular spaces), and a shield-like chest (broad, deep, stocky chest). Other abnormalities include spina bifida; syndactylism; malformation of the ribs, wrists, or toes; Klippel-Feil syndrome; coarctation of the aorta; deafness; mental deficiency; hypertension; and ocular disorders.
Laboratory abnormalities include a marked increase in gonadotropin levels, approximating titers found in castrated or postmenopausal women, and 17-ketosteroids that are only slightly reduced. This minimal decrease in adrenocortical function is insufficient to prevent the growth of sparse pubic and axillary hair. Karyotyping will unequivocally establish the diagnosis.
Therapy is substitutional, since the ovaries are incapable of stimulation. Estrogens may be given daily for 2 to 6 months to start sexual development and then changed to cyclical administration. After 4 to 6 months of estrogen-only therapy, progesterone is usually added in a cyclical fashion to achieve a more natural endometrial shedding, reducing the risk of iatrogenic hyperplasia. Under this regimen the breasts develop, the axillary and pubic hair increase, the external and internal genitalia mature, and the vagina becomes more capacious.
Developmental anomalies of the ovary other than ovarian aplasia are rare. They include absence of one ovary, ectopic ovary, third ovary, accessory ovaries, and congenital displacements. The absence of one ovary is almost invariably associated with a failure in development of the corresponding tube, half the uterus, a kidney, and the ureter. An ectopic ovary, usually with only the osteal end of the tube attached, may be present in the retroperitoneal lumbar region or inguinal area. A third ovary is exceedingly rare. It may conceivably arise from a duplication of the gonadal site. This diagnosis is unquestionable if an associated third fallopian tube is present. Such a true, supernumerary ovary may be intra- or extraperitoneal and is prone to the development of neoplastic cysts, teratomas, or sarcomas. False, accessory ovaries are separate segments of ovarian tissue, attached to a normally situated ovary by intervening bands of fibrous or attenuated ovarian tissue. The term bipartite or succenturiate ovary is sometimes applied to this splitting or partitioning effect.
Congenital displacements include herniation of the ovary within a peritoneal sac in the inguinal, femoral, sciatic, obturator, or perineal regions. Excessive degrees of prolapse into the cul-de-sac of Douglas may occur, sometimes leading to a true vaginal herniation of the ovary.
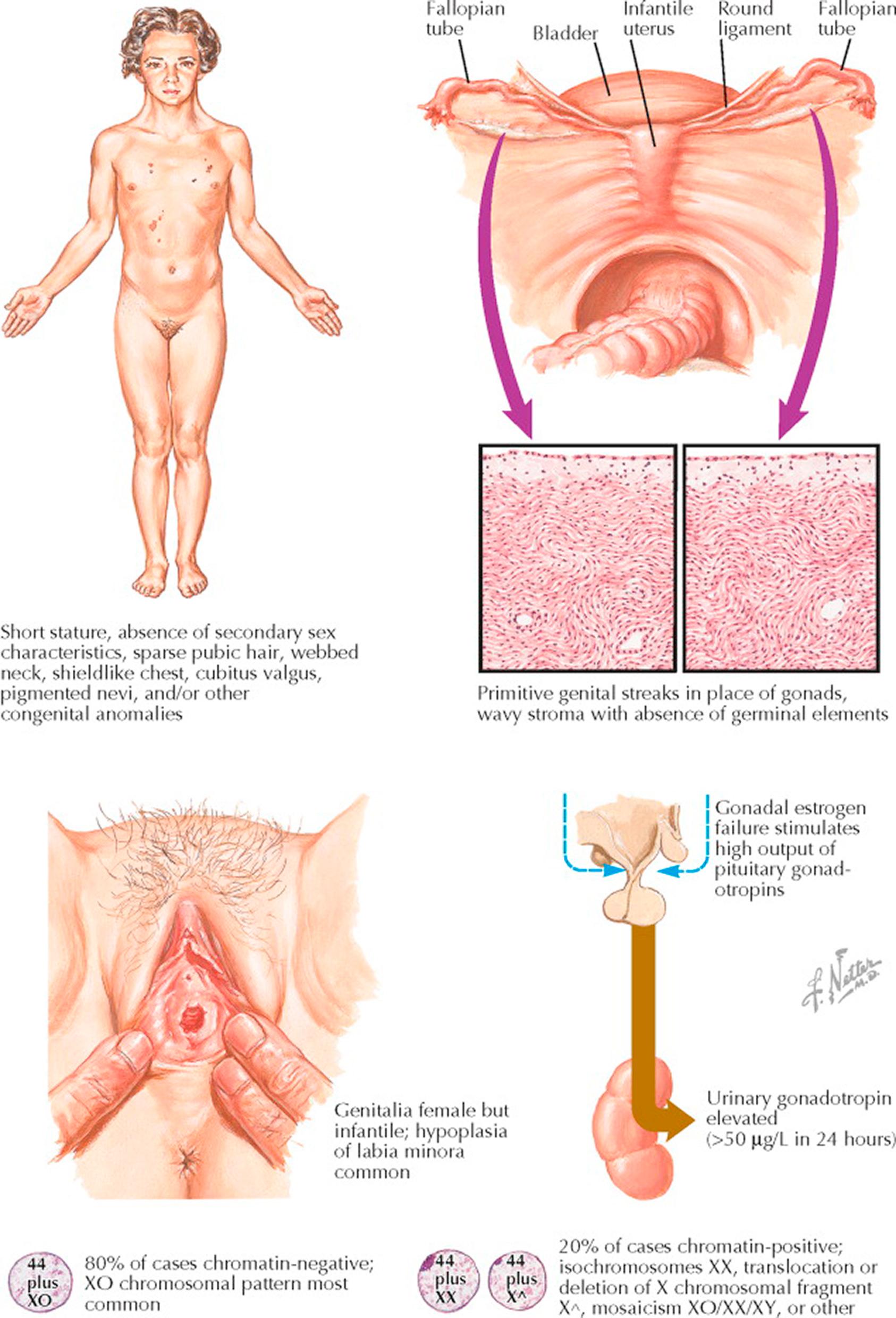
Gonadal dysgenesis is a developmental abnormality of patients who do not carry the stigmata of Turner syndrome but still suffer absent menarche because of chromosomal abnormalities and abnormal (streak) gonads. These patients are generally tall (>150 cm), are more normal in appearance, and are a chromosomally heterogeneous group: 46, XX, 46, XY, or mosaic X/XY karyotypes. Classic Turner syndrome is caused by the absence of one X chromosome. Turner syndrome is a collection of stigmata that include edema of the hands and feet, webbing of the neck, short stature, left-sided heart or aortic anomalies, and gonadal dysgenesis resulting in primary amenorrhea and infertility.
Gonadal dysgenesis occurs in 1 of 2500 female births and Turner syndrome is estimated to occur in 1 of 2700 female births. Most patients with Turner syndrome have a sporadic loss of one X chromosome (45, XO, 60% of cases; others partial losses: amenorrhea with long-arm loss; short stature with short-arm loss). Ninety-eight percent of conceptuses with only one X chromosome abort in early pregnancy. Gonadal dysgenesis can occur with other chromosomal abnormalities including 46,XY gonadal dysgenesis (Swyer syndrome) and 46, XX q5 X chromosome long-arm deletion and with mixed or mosaic states.
The symptoms and stigmata expressed by these individuals depend on the amount of chromatin that has been lost: primary amenorrhea and infertility (95% to 98%) are the most common. (Gonadal dysgenesis is the most common cause of failure to begin menstruation and in approximately 60% of women with primary amenorrhea, an abnormality of gonadal differentiation or function has occurred during the fetal or neonatal period.) These patients have early and accelerated oocyte atresia resulting in few, if any, oocytes remaining in the ovarian cortex at the time of normal puberty. (Germ cell involution occurs soon after they migrate into the undifferentiated gonad. This results in fibrous streak gonads that are hormonally inactive.) Those with complete loss of the X chromosome generally are of short stature (<150 cm) and have a short neck, high palate, low hairline, and widely-spaced, hypoplastic nipples (80%). Seventy to seventy-five percent have a broad (shield) chest, nail hypoplasia, lymphedema, cubitus valgus, prominent anomalous ears, multiple nevi, and hearing impairment. Two thirds of these patients have webbing of the neck and a short fourth metacarpal. Renal and cardiac anomalies are also common. Gonadoblastomas or virilization may occur if the individual is mosaic for 45, X/46,XY.
The diagnosis of gonadal dysgenesis or Turner syndrome is usually established by karyotyping. (Forty percent of those thought to have Turner syndrome have a mosaic karyotype or have an abnormal X or Y chromosome.) Follicle-stimulating hormone (FSH) and luteinizing hormone (LH) levels are high in these individuals, but the elevation is nonspecific.
These individuals generally require hormone replacement therapy, and they may require growth hormone therapy if the diagnosis is established before age 10. When there is a mosaicism involving a Y chromosome, surgical extirpation of the gonads must be performed because of a 25% to 30% risk of malignant gonadal tumors. Timing of gonadal removal in patients with a Y chromosome is controversial: removal as soon as the diagnosis is made versus delaying removal until pubertal changes are complete. (The risk of cancer in XY gonadal dysgenesis is 3% at age 10, 10% at 13, and 75% at 26 years of age.) If hormonal replacement is undertaken, it must be done with care because adolescents are much more sensitive to the effects of estrogen than are postmenopausal women, allowing doses in the range of 0.3 mg of conjugated estrogen, 0.5 mg of estradiol, or their equivalent daily. After 6 to 12 months of therapy at this level, the dose should be doubled and a progestin should be added, or the patient's therapy should be switched to combination oral contraceptives. This generally results in regular menstruation, and normal pubertal development proceeds on its own when the patient reaches a bone age of 13. Growth hormone may be effective if given before age 10.
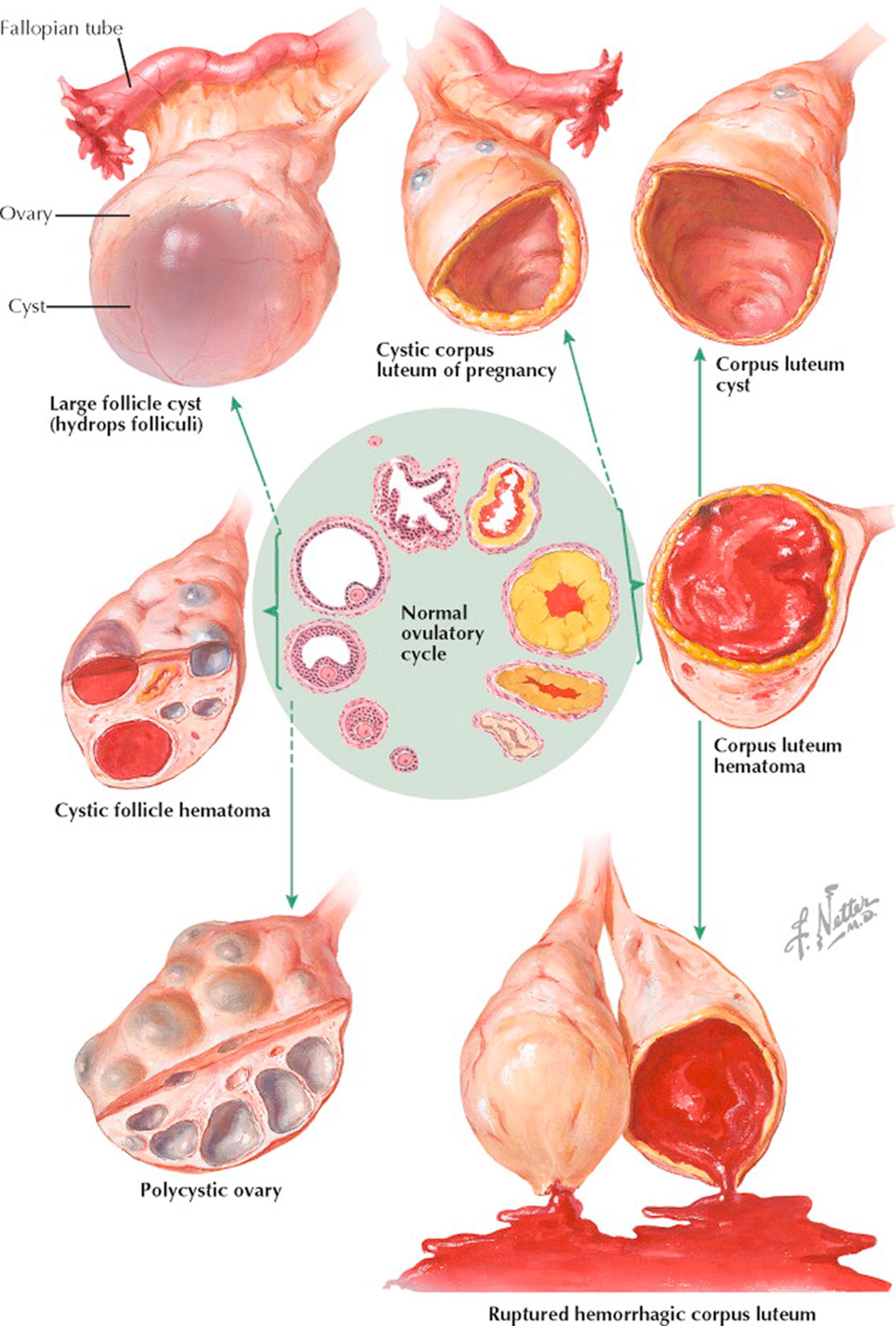
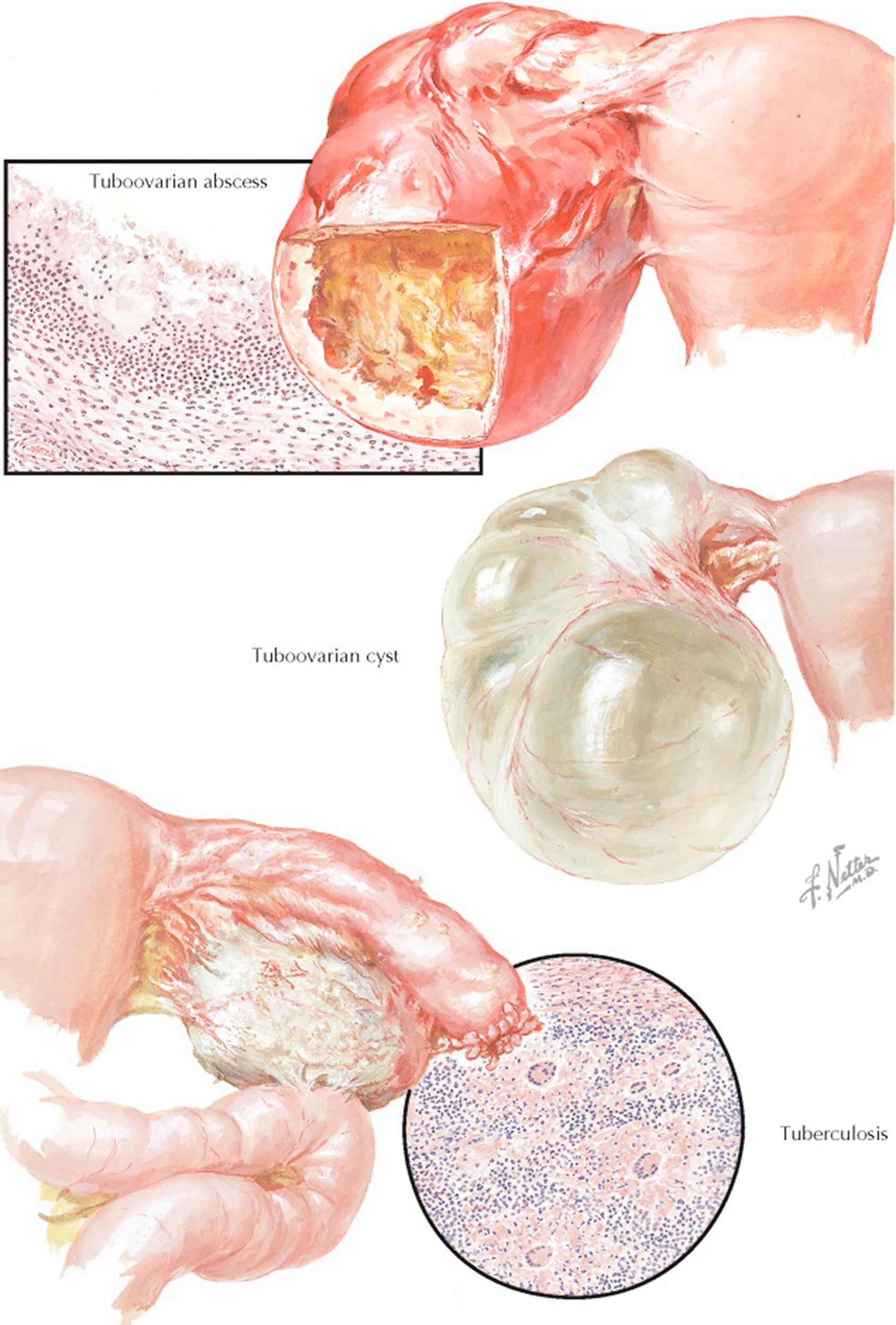
The preponderance of small cystic structures within the ovary represents physiologic variations of the normal ovulatory cycle. These follicle and corpus luteum derivatives are nonneoplastic, that is, they are incapable of autonomous growth. Their clinical recognition and differentiation from true ovarian cysts are most important. A small neoplastic ovarian cyst may be simulated by a single, large follicle cyst, by multiple cystic follicles, or by a corpus luteum cyst. A large or cystic corpus luteum of pregnancy may be mistaken for an ectopic pregnancy or an ovarian cyst. A corpus luteum hematoma may present with signs comparable to those associated with torsion of a small cyst. A ruptured graafian follicle or ruptured hemorrhagic corpus luteum may be misdiagnosed as acute appendicitis or ruptured tubal pregnancy. It is not uncommon to find adnexal cysts during pelvic ultrasonography performed for other reasons, and these are generally not of any clinical significance.
Follicle cysts are distended atretic follicles more than 6 to 8 mm in diameter. They are usually not more than 1 to 2 cm in diameter, thin-walled, translucent, and filled with watery fluid. The cysts may project slightly above the surface of the ovary or may lie more deeply within the cortex. If pricked, the follicle fluid may spurt out under pressure. The inner lining appears smooth and glistening. Microscopically, the granulosa cell lining varies in thickness and may be well preserved or may show evidence of degeneration. On pelvic examination, a unilateral, smooth, cystic, slightly tender, movable, plum-sized ovary may be felt. Therapy is based on the principle that during the reproductive years a cystic ovary up to 6 cm in diameter is presumed, unless proved otherwise, to be a physiologic variation that will undergo subsequent resorption. The patient is reexamined at intervals. If the ovarian enlargement persists or increases in size, surgical intervention may be indicated.
The mature corpus luteum presents a central core filled with blood. With resorption, the cavity may be distended with hemorrhagic or clear fluid, or newly formed connective tissue. Variations in the size of the lumen occur normally. A corpus luteum hematoma is the result of excessive hemorrhage into the corpus cavity during the stage of vascularization. This increased accumulation of blood under pressure may result in local pain, ovarian enlargement, and tenderness. A corpus luteum cyst follows the resorption of a corpus luteum hematoma. It is usually 2 to 4 cm in diameter. Grossly, the yellowish hue of the cyst wall may be evident. A corpus albicans cyst is the sequel to a corpus luteum cyst in which the lutein cells are replaced by a dense, wavy band of fibrous or collagenous tissue.
A ruptured graafian follicle or hemorrhagic corpus luteum may be associated with varying degrees of intraabdominal bleeding. The former is likely to occur between the 12th and the 16th days and the latter during the last week of an average menstrual cycle. Rupture may occur spontaneously or may follow trauma, pelvic examination, coitus, or exercise. Symptoms and signs include lower abdominal pain, nausea and vomiting, abdominal spasm, tenderness and rebound tenderness, an enlarged, tender ovary, fullness in one adnexal region or the posterior cul-de-sac, and tenderness on manipulation of the cervix. The temperature may be slightly elevated and a leukocytosis may be found, but the leukocyte sedimentation rate remains normal. Ultrasonography will demonstrate the cystic mass and free fluid in the cul-de-sac. Mild cases may be confused with acute appendicitis or torsion. With bed rest the symptoms and signs gradually abate. If rupture is associated with severe bleeding, it may simulate a ruptured ectopic pregnancy. The blood loss, at times, may exceed 1000 mL.
Endometriosis refers to the growth of endometrium outside of its normal intrauterine location (ectopic) that retains the histologic characteristics and biologic response of the endometrium. It is nonneoplastic, that is, incapable of autonomous growth, but it is dependent on estrogenic and progesterone stimulation. Endometriosis may arise by one of several proposed mechanisms—lymphatic spread, metaplasia of coelomic epithelium or müllerian rests, seeding by retrograde menstruation, or direct hematogenous spread. Instances of presumed iatrogenic spread (surgical) have been reported. A role for an immunologic defect is debated but remains to be conclusively established. The greatest incidence occurs between 30 and 40 years of age and may be found in 5% to 15% of women, 20% of gynecologic laparotomies, 30% of chronic pain patients, and 30% to 50% of infertility patients.
Small lesions may be asymptomatic (30%). The diagnosis may be suggested by the presence of infertility, dysmenorrhea, sacral backache, deep thrust dyspareunia, and abnormal uterine bleeding. Pain caused by endometriosis characteristically begins premenstrually and ceases shortly after the menstrual flow is established. Involvement of the rectovaginal septum, cul-de-sac, or rectal wall may be responsible for rectal pain. If bowel endometriosis has penetrated the intestinal wall, the rectum may bleed cyclically. Bladder endometriosis may cause periodic hematuria and bladder irritability.
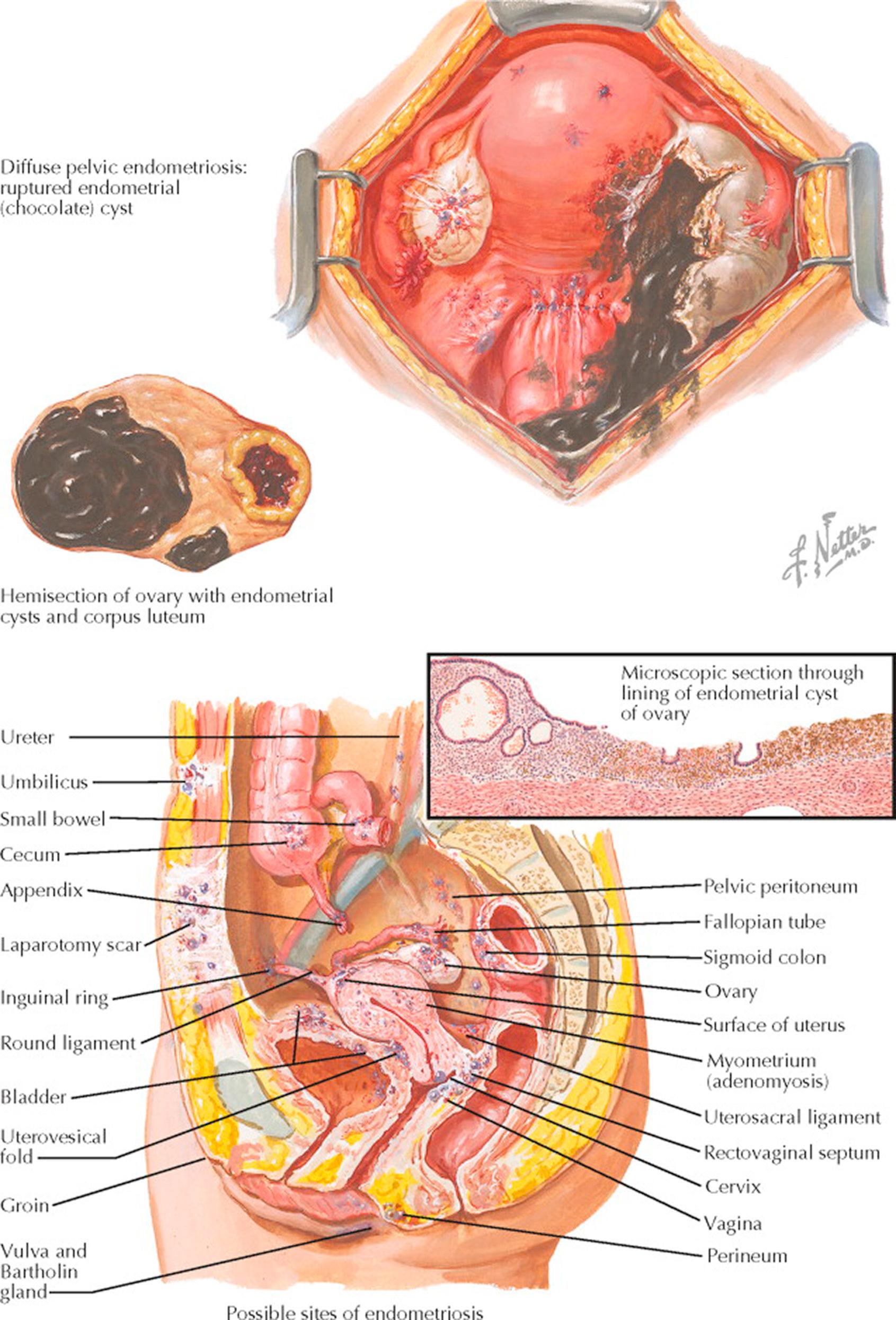
The presumptive diagnosis of pelvic endometriosis is based on the history, the absence of a previous pelvic infection, and characteristic findings on bimanual vaginal and rectal examinations. A final diagnosis requires laparoscopy, laparotomy, or histologic confirmation. Pelvic examination may reveal the presence of small, firm, tender, fixed nodules in the region of the uterosacral ligaments, the posterior cul-de-sac, and the posterior surface of the uterus. The uterus is not infrequently retroverted, retroflexed, and fixed. Endometrial cysts are usually bilateral, rarely larger than a lemon or orange, cystic, and firmly fixed behind the uterus.
At laparotomy, endometriosis may be found incidentally to other pelvic lesions, particularly uterine fibroids and uterine retrodisplacements. Peritoneal implants may be seen as small, scattered, scarred puckerings or irregular, brown (“tobacco-stained”) areas anywhere on the pelvic peritoneum. The peritoneum may be the site for atypical endometriosis, vesicles from clear, to red, to the classic dark-brown lesion. The ovarian or uterosacral ligaments may contain single or multiple discrete or confluent cicatrized nodules, with partially enveloped, minute, dark-blue or brown hemorrhagic blebs.
Endometriosis of the ovary may be manifested by minute surface “implants,” small hemorrhagic cysts within the cortex, or by large “chocolate” cysts, which may practically replace the substance of the ovary. In surface endometriosis, tiny red, purple, or dark-brown hemorrhagic blebs are encompassed within puckered, cicatricial tissue. Endometrial cysts (chocolate cyst) vary in size but are rarely larger than 10 cm in diameter. They are frequently bilateral. The outer surface appears irregular, puckered, and adherent. Black or brown hemorrhagic areas may be evident. Along with its corresponding tube, the ovary is usually found adherent to the posterior surface of the broad ligament, uterus, lateral pelvic wall, and rectosigmoid. An attempt to free the adnexa usually results in rupture of the cyst with escape of large quantities of thick, chocolate-colored fluid. The cyst wall appears thick, irregularly convoluted, and yellow-white in color. The inner lining has a dark, hemorrhagic stain. Microscopically, typical endometrial stroma and glands may line the cyst wall. Older lesions, presumably due to repeated desquamation and pressure of retained blood, may show little evidence of endometrial tissue. The cyst may be lined by a broad zone of pseudoxanthoma cells, containing a hemoglobin derivative (hemosiderin). Hyalinization and fibrosis are seen in other areas.
Therapy depends upon the age, parity, location, and extent of the lesions, severity of symptoms, the desire for children and the possibility of pregnancy, the patient's attitude toward loss of menstrual function or premature castration, and the coexistence of other pelvic pathology such as uterine fibroids.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here