Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The term high-risk infant designates an infant at greater risk for neonatal morbidity and mortality; many factors can contribute to an infant being high risk ( Table 117.1 ). High-risk infants are categorized into 4 main groups: the preterm infant, infants with special health care needs or dependence on technology, infants at risk because of family issues, and infants with anticipated early death.
MATERNAL DEMOGRAPHIC/SOCIAL FACTORS
MATERNAL MEDICAL HISTORY
PREVIOUS PREGNANCY
PRESENT PREGNANCY
LABOR AND DELIVERY
NEONATE
|
All high-risk infants require closer evaluation and/or treatment by experienced physicians and nurses. This often starts at delivery and continues through a neonatal intensive care unit (NICU) stay (see Chapter 121 ). Regionalized care for infants is based on the acuity of care that can be provided at hospitals with different levels of care and whether transport should be undertaken (see Chapter 118 ). It is important to note that additional care does not stop at time of NICU discharge, and that many high-risk infants also benefit from additional resources and follow-up after discharge from the hospital (see Chapter 117.5 ).
Approximately 15 million infants are born preterm (before 37 wk gestational age) each year worldwide, accounting for approximately 1 in every 10 babies born, and the overwhelming majority of high-risk infants. The World Health Organization (WHO) defines infants born before 28 wk gestational age as extremely preterm infants, infants born between 28 and  wk as very preterm, and infants born between 32 and
wk as very preterm, and infants born between 32 and  weeks as moderate to late preterm infants. Risk of both morbidity and mortality increases with earlier gestational age. Gestational age, birthweight, and gender are all important factors that impact neonatal mortality ( Fig. 117.1 ). The highest risk of neonatal and infant mortality occurs in infants with birthweight <1,000 g and/or with gestational age <28 wk. The lowest risk of neonatal mortality occurs in infants with birthweight of 3,000-4,000 g and a gestational age of 39-41 wk. As birthweight increases from 400 to 3,000 g and gestational age increases from 23 to 39 wk, a logarithmic decrease in neonatal mortality occurs. Once birthweight exceeds 4000 g and/or gestational age exceeds 42 wk, the incidence of neonatal morbidities and mortality increases.
weeks as moderate to late preterm infants. Risk of both morbidity and mortality increases with earlier gestational age. Gestational age, birthweight, and gender are all important factors that impact neonatal mortality ( Fig. 117.1 ). The highest risk of neonatal and infant mortality occurs in infants with birthweight <1,000 g and/or with gestational age <28 wk. The lowest risk of neonatal mortality occurs in infants with birthweight of 3,000-4,000 g and a gestational age of 39-41 wk. As birthweight increases from 400 to 3,000 g and gestational age increases from 23 to 39 wk, a logarithmic decrease in neonatal mortality occurs. Once birthweight exceeds 4000 g and/or gestational age exceeds 42 wk, the incidence of neonatal morbidities and mortality increases.
acardiac fetus
chimeric
conjoined twins
diamnionic
dichorionic
dizygotic
endoparasitic twins
exoparasitic twins
fission theory
fusion theory
monoamniotic
monochorionic
monozygotic
superfecundation
superfetation
TRAP
TTTS
twin reversed arterial perfusion syndrome
twin-twin transfusion syndrome
Identifying twins as monozygotic or dizygotic is useful in determining the relative influence of heredity and environment on human development and disease. The previous assumption that twins not of the same sex are dizygotic can no longer be held as true. Sex discordance, placentation, and determination of amnionicity and chorionicity are not reliable ways of determining zygosity. Detailed blood typing, gene analysis, or tissue (human leukocyte antigen) typing can be used for zygosity testing (an exception being blood typing in cases of chimeric twins, where one or both twins contain distinct cell lines from multiple zygotes). Physical and cognitive differences may still exist between monozygotic twins because of other factors. The in utero environment may have been different. Additionally, differences may exist in the mitochondrial genome, in posttranslational gene product modification, and in the epigenetic modification of nuclear genes in response to environmental factors.
If the placentas are separate, twins are dichorionic , but not necessarily dizygotic. One third of monozygotic twins are dichorionic and diamnionic. An apparently single placenta may be present with either monozygotic or dizygotic twins, but inspection of a dizygotic placenta usually reveals that each twin has a separate chorion that crosses the placenta between the attachments of the cords and 2 amnions. Separate or fused dichorionic placentas may be disproportionate in size. The fetus attached to the smaller placenta or the smaller portion of the placenta is usually smaller than its twin or is malformed. Monochorionic twins are usually diamnionic , and the placenta is usually a single mass.
The incidence of spontaneous twinning is highest among blacks and East Indians, followed by northern European whites, and is lowest in the Asian races. Differences in the incidence of twins worldwide mainly involve dizygotic twins. The incidence of monozygotic twins (3-5 per 1,000) is unaffected by racial or familial factors. Until recently, monozygotic twinning rates remained stable across continents and cultures. In 2014 the U.S. final natality report recorded a twin rate of 33.9 per 1,000 live births, which was a new high for the nation. Increases in monozygotic and dizygotic twinning have been associated with advanced maternal age (AMA) and the use of assisted reproductive technologies (ART). The rate of triplets and higher-order multiple births is 113.5 per 100,000 live births in the United States and continues to decline. The use of single-embryo transfer in ART has decreased the numbers of triplet births and higher-order multiples. However, a doubling of monozygotic twinning and an increase in atypical twinning have been reported. The incidence of dizygotic multifetal gestation is also increasing, attributed to treatment of infertility with ovarian stimulants (clomiphene, gonadotropins).
Polyovular pregnancies are more frequent beyond the 2nd pregnancy, in older women, and in families with a history of dizygotic twins. They may result from simultaneous maturation of multiple ovarian follicles, but follicles containing 2 ova have also been described as a genetic trait leading to twin pregnancies. Twin-prone women have higher levels of gonadotropin. Polyovular pregnancies occur in many women treated for infertility.
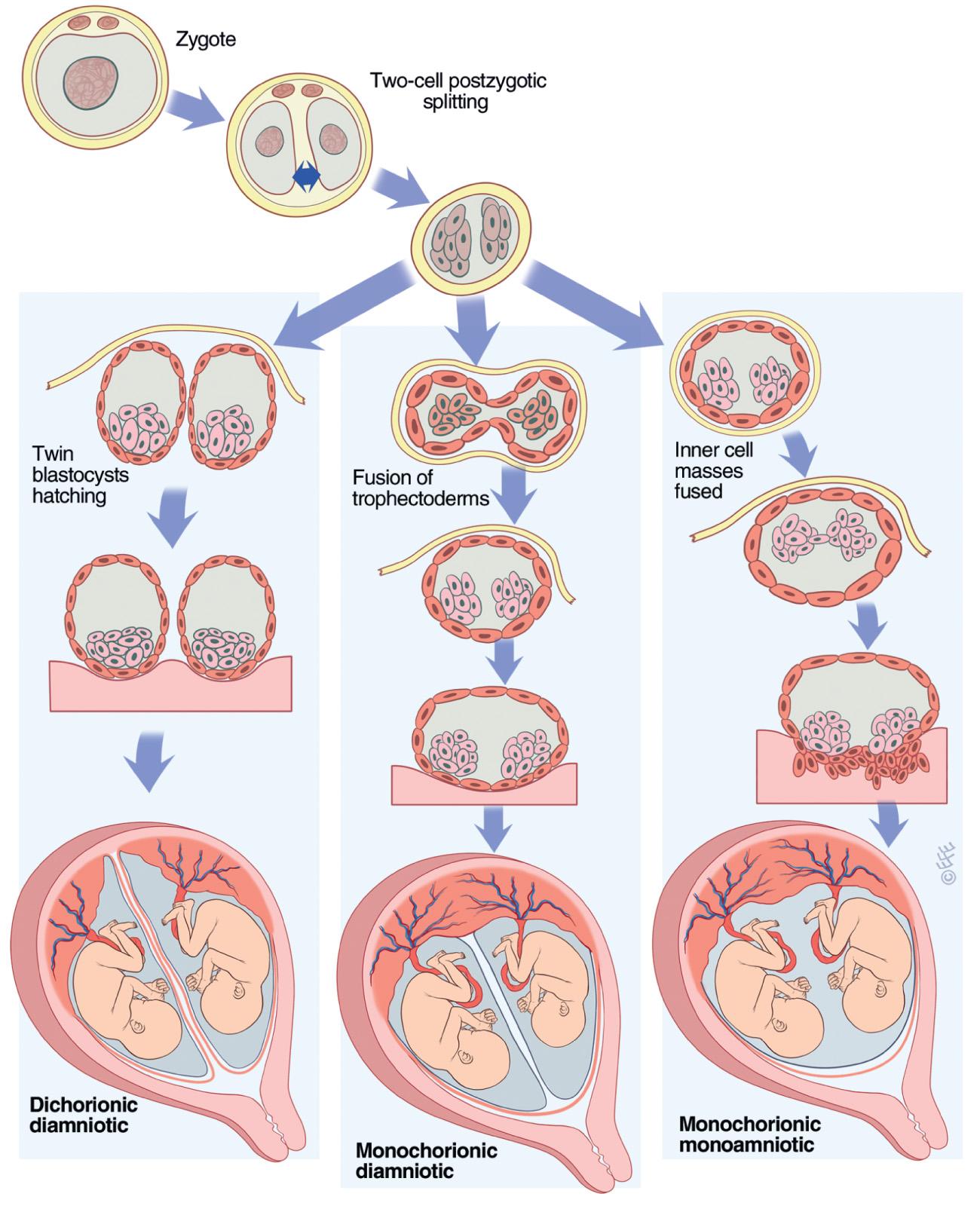
The occurrence of monozygotic twins appears to be independent of heritable factors. The etiology of monozygotic twinning is unknown, but there are 2 prevailing theories. In the classic fission theory , twinning results from the splitting of a single conceptus, with the timing of splitting resulting in differing amnionicity and chorionicity (i.e., the earlier the fission occurs, the more likely the twins are to be diamniotic dichorionic) ( Fig. 117.2 ). However, this theory fails to account for several forms of atypical twinning, including the occurrence of diamniotic dichorionic monozygotic twinning after single-embryo transfer in the late blastocyst state, phenotypically-discordant monozygotic twins, and asymmetrically attached conjoined twins. An alternate fusion theory of twinning has been proposed to account for this discrepancy, in which the inner cell masses of trophectoderm fuse after the initial 2-cell splitting stage ( Fig. 117.3 ).
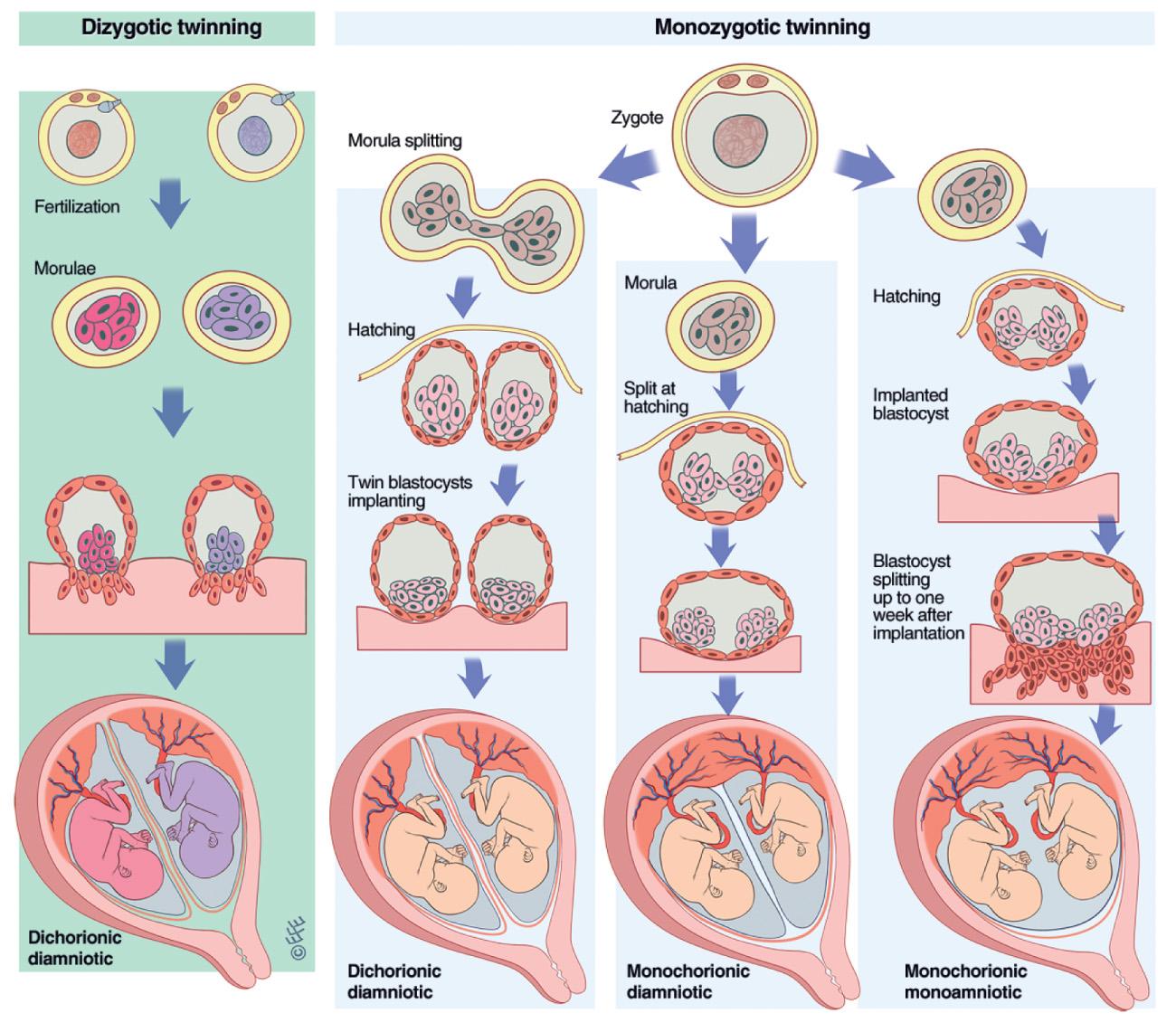
Conjoined twins (1 in 50,000 pregnancies and 1 in 250,000 live births) are obligate monozygotes . Theoretically, they result from later fission of a single zygote (10-14 days) or from fusion of 2 zygotes (as proposed for asymmetrically attached conjoined twins). The majority of conjoined twins are female. The prognosis for symmetrically conjoined twins depends on the possibility of surgical separation, which in turn depends on the extent to which vital organs are shared. The site of connections varies: thoracoomphalopagus (28% of conjoined twins), thoracopagus (18%), omphalopagus (10%), craniopagus (6%), and incomplete duplication (10%). The term parasitic twin has historically been used to describe the smaller and less completely developed member of a pair of conjoined twins; this parasitic twin has typically had embryonic demise but remains vascularized by the surviving independent twin (the autocyte ). For asymmetrically attached conjoined twins in whom one twin is dependent on the cardiovascular system of the intact autocyte ( exoparasitic twins , 1 in 1 million live births) survival of the autocyte depends on the feasibility of excising the exoparasitic twin. For endoparasitic twins ( fetus in fetu, 1 in 500,000 live births) in whom one (or more) fetus exists as a benign mass in the autocyte, survival of the autocyte is unaffected.
Superfecundation, or fertilization of an ovum by an insemination that takes place after one ovum has already been fertilized, and superfetation, or fertilization and subsequent development of an embryo when a fetus is already present in the uterus, have been proposed as explanations for differences in size and appearance of certain twins at birth.
Problems of twin gestation include polyhydramnios, hyperemesis gravidarum, preeclampsia, premature rupture of membranes (PROM), vasa previa, velamentous insertion of the umbilical cord, abnormal presentation (breech), and premature labor. Monoamniotic twins have a high fatality rate because of obstruction of the circulation secondary to intertwining of the umbilical cords.
Compared with the 1st-born twin, the 2nd twin is at increased risk for respiratory distress syndrome and asphyxia. Twins are at risk for intrauterine growth restriction, twin-twin transfusion syndrome, and congenital anomalies, which occur predominantly in monozygotic twins. Anomalies are a result of compression deformation of the uterus from crowding (hip dislocation), vascular communication with embolization (ileal atresia, porencephaly, cutis aplasia) or without embolization (acardiac twin), and unknown factors (conjoined twins, anencephaly, meningomyelocele).
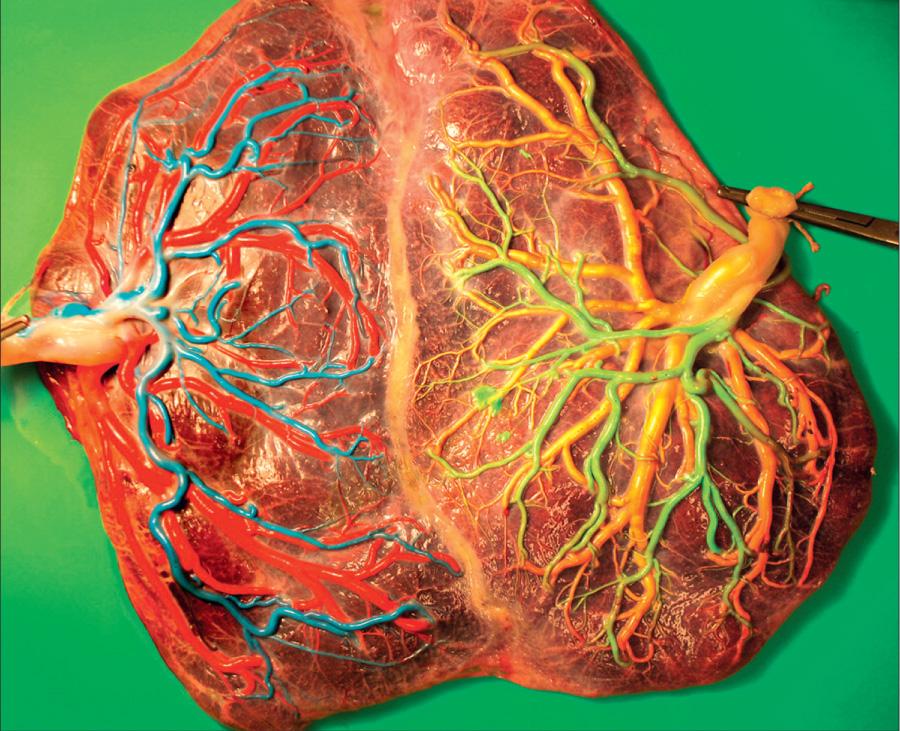
Placental vascular anastomoses occur with high frequency in monochorionic twins. In monochorionic placentas, the fetal vasculature is usually joined, sometimes in a very complex manner. They are usually balanced so that neither twin suffers. Artery-to-artery communications cross over placental veins, and when anastomoses are present, blood can readily be stroked from one fetal vascular bed to the other. Vein-to-vein communications are similarly recognized but are less common. A combination of artery-to-artery and vein-to-vein anastomoses is associated with the condition of acardiac fetus . This rare lethal anomaly (1 in 35,000) is secondary to the twin reversed arterial perfusion (TRAP) syndrome . In utero radiofrequency or laser ablation of the anastomosis or cord occlusion can be used to treat heart failure in the surviving twin. However, death of the autocyte is reported in up to 75% of cases. In rare cases, one umbilical cord may arise from the other after leaving the placenta, and the twin attached to the secondary cord usually is malformed or dies in utero.
In twin-twin transfusion syndrome (TTTS) , an artery from one twin acutely or chronically delivers blood that is drained into the vein of the other. The latter develops polyhydramnios, plethoric and large for dates, and the former has oligohydramnios, anemic and small ( Fig. 117.4 ). TTTS is more common in monozygotic twins and affects up to 30% of monochorionic twins. Maternal polyhydramnios in a twin pregnancy suggests TTTS. Anticipating this possibility by preparing to transfuse the donor twin or bleed the recipient twin may be lifesaving. Death of the donor twin in utero may result in generalized fibrin thrombi in the smaller arterioles of the recipient twin, possibly as the result of transfusion of thromboplastin-rich blood from the macerating donor fetus. Disseminated intravascular coagulation (DIC) may develop in the surviving twin. Table 117.2 lists the more frequent changes associated with a large shunt. Treatment of this highly lethal problem includes maternal digoxin, aggressive amnioreduction for polyhydramnios, selective twin termination, and more often, laser or fetoscopic ablation of anastomosis ( Fig. 117.5 ).
| TWIN ON: | |
|---|---|
| Arterial Side—Donor | Venous Side—Recipient |
| Prematurity Oligohydramnios Small premature Malnourished Pale Anemic Hypovolemic Hypoglycemic Microcardia Glomeruli small or normal Arterioles thin walled |
Prematurity Polyhydramnios Hydrops Large premature Well nourished Plethoric Polycythemic Hypervolemic Cardiac hypertrophy Myocardial dysfunction Tricuspid valve regurgitation Right ventricular outflow obstruction Glomeruli large Arterioles thick walled |
A prenatal diagnosis of pregnancy with twins is suggested by a uterine size that is greater than that expected for gestational age, auscultation of 2 fetal hearts, and elevated maternal serum α-fetoprotein (AFP) or human chorionic gonadotropin (hCG) levels. It is confirmed by ultrasonography. Physical examination of twins is necessary but not sufficient to determine zygosity of twins. In the event that congenital anomalies are present or there are transfusion or transplantation considerations, genetic testing of zygosity should be performed. While noninvasive prenatal testing (NIPT) is becoming more common, the results should be interpreted with caution in multiple-gestation pregnancies until more findings are better established.
Most twins are born prematurely, and maternal complications of pregnancy are more common than with single pregnancies. The risk for twins is most often associated with twin-twin transfusion, ART, and early-onset discordant growth. Because most twins are premature, their overall mortality is higher than that of single-birth infants. The perinatal mortality of twins is about 4 times that of singletons, with monochorionic twins being particularly at risk. Monoamnionic twins have an increased likelihood of cord entanglement, which may lead to asphyxia. Twins are at greater risk for congenital malformations, with up to 25% of monozygotic twins being affected. Theoretically, the 2nd twin is more subject to anoxia than the 1st because the placenta may separate after birth of the 1st twin and before birth of the 2nd. In addition, delivery of the 2nd twin may be difficult because it may be in an abnormal presentation (breech, entangled), uterine tone may be decreased, or the cervix may begin to close after the 1st twin's birth.
Triplet or higher-order births are associated with an increased risk of death or neurodevelopmental impairment compared with extremely-low-birthweight (ELBW) singleton and twin infants after controlling for gestational age. The mortality for multiple gestations with ≥4 fetuses is excessively high for each fetus. Because of this poor prognosis, selective fetal reduction has been offered as a treatment option. Monozygotic twins have an increased risk of one twin dying in utero. The surviving twin has a greater risk for cerebral palsy and other neurodevelopmental sequelae.
Prenatal diagnosis enables the obstetrician and pediatrician to anticipate the birth of infants who are at high risk because of twinning. The risk of multiple-gestation pregnancies using ART may be reduced by elective single-embryo transfers. In addition, elective delivery of twins at 37 wk (or earlier for monochorionic , monoamniotic twins) reduces the complication rate for the fetuses and the mother. Furthermore, in twin pregnancies between 32 and 39 wk of gestation, planned vaginal delivery is preferred if the 1st twin is in the cephalic presentation. Close observation and attendance by a pediatric team are indicated in the immediate neonatal period so that prompt treatment of asphyxia or fetal transfusion syndrome can be initiated. The decision to perform an immediate blood transfusion in a severely anemic “donor twin” or a partial exchange transfusion of a “recipient twin” must be based on clinical judgment.
Ballard scoring system
extremely low birthweight
extremely low gestational age newborn
extremely preterm
kangaroo care
low birthweight
neutral thermal environment
very low birthweight
Traditionally, a delivery date is determined 280 days after the last menstrual period (LMP). However, only 4% of pregnant women actually deliver at 280 days, and only 70% deliver within 10 days of the estimated delivery date.
Infants born before 37 wk from the 1st day of the LMP are termed premature by WHO. Infants born before 28 wk gestation are extremely preterm, also referred to as extremely low gestational age newborns (ELGANs) ; whereas infants born between 28 and  are very preterm . Moderate and late preterm infants (born between 32 and
are very preterm . Moderate and late preterm infants (born between 32 and  wk gestation) are discussed in Chapter 117.3 .
wk gestation) are discussed in Chapter 117.3 .
In addition to classification by gestational age, classification is also based on birthweight. Extremely low birthweight (ELBW) is used to describe infants with a birthweight <1000 g, very low birthweight (VLBW) describes infants <1500 g, and low birthweight (LBW) describes infants <2500 g at birth. Birthweight in general is a proxy for gestational age, but in the cases of intrauterine growth restriction (IUGR) and small-for-gestational-age (SGA) infants, birthweight can sometimes be misleading for true gestational age (see Chapter 117.4 ).
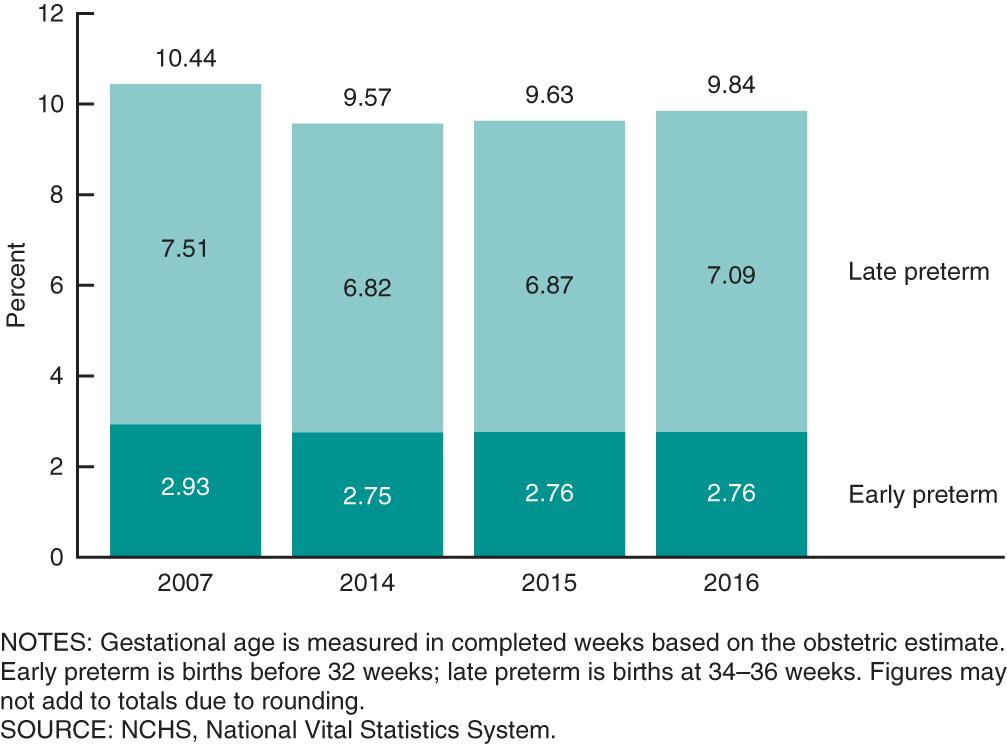
Preterm birth , or birth before 37 wk of gestation, is fairly common. Worldwide, approximately 15 million preterm births occur annually. In the United States, approximately 10% of all births are preterm. After a prolonged period of increasing rates of preterm birth, preterm births in the United States peaked at 10.44% in 2007. From 2007 until 2014, a slow but steady decline occurred in preterm births, to 9.57% in 2014. Preliminary data show that preterm births have slightly increased since 2014, with 9.84% of all U.S. births being preterm in 2016 and a disproportionate increase in late preterm births ( Fig. 117.6 ). Of preterm births in 2016, the majority were late preterm infants, approximately 72% of preterm births, with the remaining 28% being extremely or early preterm.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here