Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Acknowledgment: The authors wish to recognize the work of previous author Ann E. Van Heest for her contributions to Green’s Operative Hand Surgery and this chapter.
Spinal cord injury is devastating to the patient, the family, and his or her friends. In an instance, life has been dramatically downgraded with respect to ambulation, limb usage, bladder/bowel function, and independence. Spinal cord injury has substantial physical, emotional, and psychosocial ramifications. Approximately 54 people per million sustain injuries to the spinal cord each year (over 17,000 new cases per year), with more than half occurring at the cervical spine and 14% resulting in ventilator dependence.
In the United States, there are approximately 300,000 persons living with disability due to spinal cord injury. Cervical-level spinal cord injuries impair arm and hand function. The majority of spinal cord injury research has focused on restoring ambulatory function; however, patients have consistently rated hand function as the most desirable function to recover, prioritizing hand function over regaining sexual function, trunk stability, bowel and bladder control, and walking. Recovering even partial hand function can have enormous impact on quality of life and independence.
Almost 80% of persons with spinal cord injury are young men, and many require lifelong care to complete their activities of daily living (ADLs) such as feeding, dressing, bathing, bladder/bowel care, and mobility. Maintenance cost estimates for care range from $45,000 to $200,000 per year per patient, depending on the severity and level of the injury. This expensive care places substantial physical and financial stressors on the patient and family.
The most common causes of spinal cord injury are motor vehicle accidents (38%), falls (32%), violence (14%), and sports trauma (8%). Tetraplegia refers to a spinal cord injury sustained to one of the eight cervical segments of the spinal cord, whereas paraplegia refers to spinal cord injury in the thoracic, lumbar, or sacral segment of the spinal cord. The most frequent neurologic categories of spinal cord injury have been incomplete tetraplegia (47%), incomplete paraplegia (20%), complete paraplegia (20%), and complete tetraplegia (12%). This chapter will discuss the evaluation and treatment of patients sustaining spinal cord injuries resulting in tetraplegia.
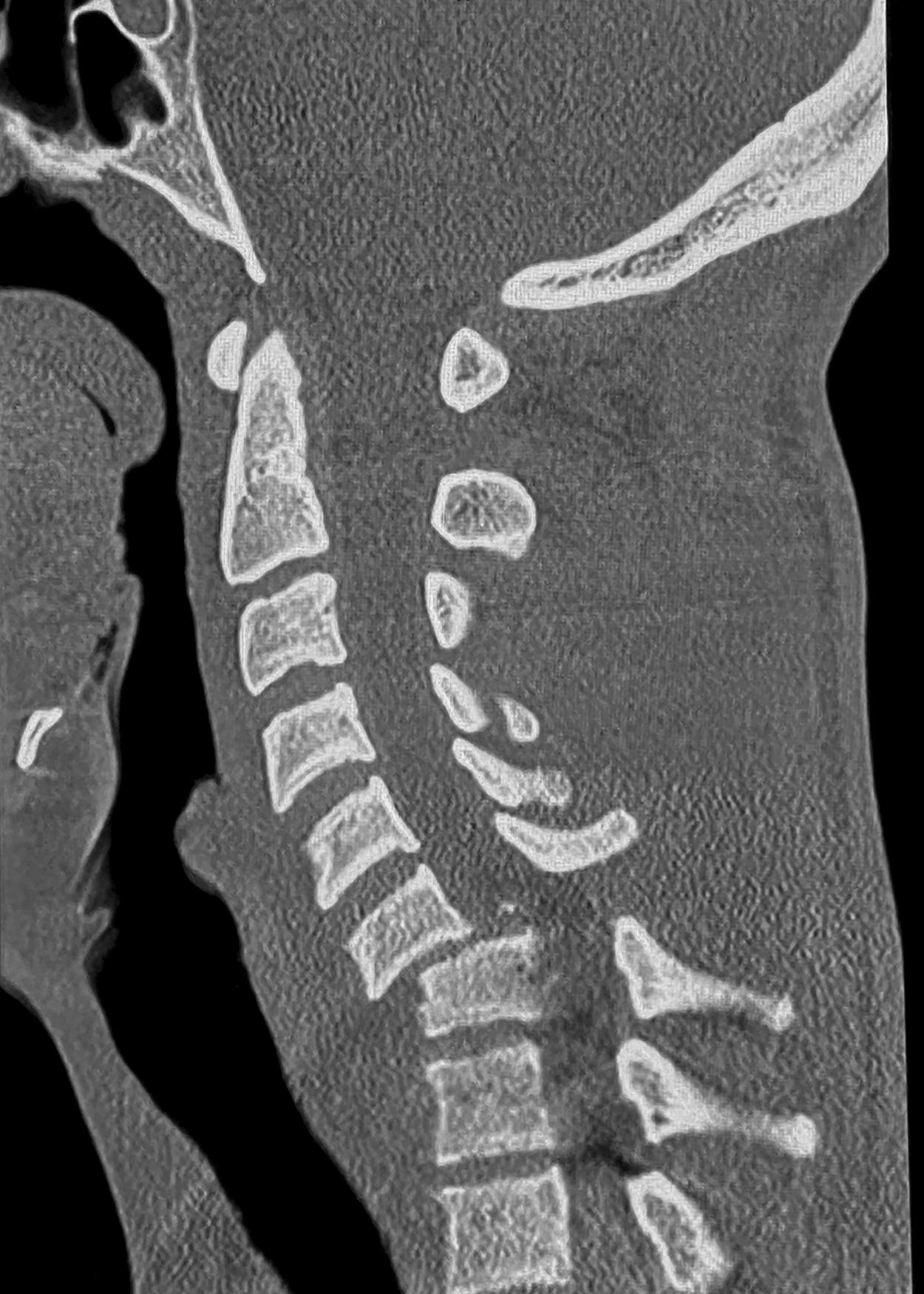
The cervical spinal cord contains eight cervical segments encased in the seven cervical vertebrae. The bone and ligamentous structure of the cervical spine allows a wide range of motion, thus exposing the cervical spinal cord to increased risk of injury. Injury occurs most commonly through a flexion deformity with bone or disk compression of the cord after fracture or dislocation or through traction on the cord with translation due to spine instability ( Fig. 33.1 ). Over days and weeks, acute hemorrhage and edema subside, followed by local reparation and finally scar formation.
Following trauma to the spinal cord, the cord is divided into three basic regions: the supralesional segment, the injured metamere, and the infralesional segment ( Fig. 33.2 ). The supralesional segment refers to the region of the spinal cord cephalad to the site of injury, which retains normal function. The infralesional segment refers to the spinal cord below the region of trauma. The infralesional segment consists of normal spinal cord and normal associated peripheral nerves that have been disconnected from descending cortical control either completely (no intentional movement realized) or incompletely (some degradation of control, typically with substantial spasticity).
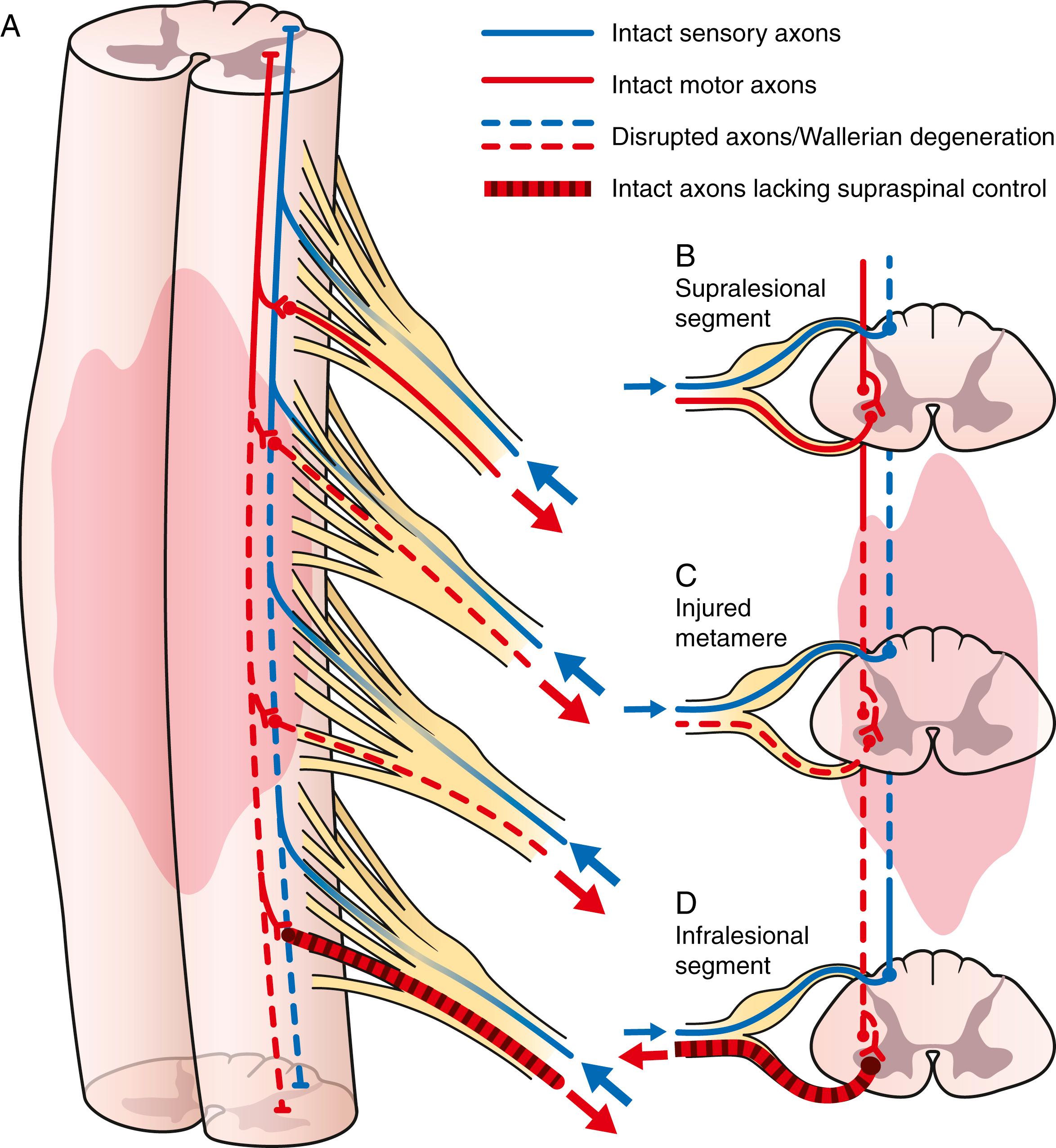
The injured metamere is the region of the cord that suffered the direct trauma. This region can be discrete and well localized, as may occur from a local disk herniation. Alternatively, the region can be lengthy and involve multiple segments, as may occur following a fracture-subluxation of the vertebrae or gunshot wound. This differentiation is important because the most vulnerable portion of the spinal cord is the central gray matter—the home of the cell bodies of the alpha motor neurons. These cell bodies will be lost at the site of injured metamere. Wallerian degeneration occurs and proceeds in a distal direction down the peripheral nerve. Over time, the associated muscles undergo severe atrophy and fatty degeneration. , Similar to a peripheral nerve injury, the associated muscles can only be effectively recovered in the acute period following the injury. In patients who are candidates for nerve transfer, surgery should be undertaken early to optimize the results of the reconstruction. The more time that transpires without innervation in these affected muscles, the less effectively they can recover. After a year from injury, the results following nerve surgery are compromised. After 2 years, useful recovery is not expected. Thus earlier surgical intervention is recommended.
The region of central gray matter destruction may extend partially into an adjacent region, which may not be clinically apparent via denervation atrophy. For example, the cephalad extent can degrade the innervation of the supralesional segment, such that the axon supply to a healthy and normal muscle is reduced. These muscles often have good active control and appear normal. This potential axon depletion must be understood when contemplating nerve transfer surgery and will be discussed in more detail later.
Within the first year following the injury, there is some potential for spontaneous recovery. Surgery must avoid disrupting any natural recovery. There are two types of spontaneous recovery, American Spinal Injury Association (ASIA) grade conversion ( Table 33.1 ) and recovering a cord level or more. Regarding conversion to a different ASIA grade, patients with more favorable injuries have more positive rates of spontaneous recovery. For instance, only about 10% to 15% of patients assessed as ASIA A will convert to ASIA B with an even smaller percentage recovering to ASIA C or D. In contrast, these conversion rates are higher for patients with ASIA B with up to 40% recovering to ASIA C or D. Accordingly, up to 60% to 80% of patients initially categorized as ASIA C will spontaneously convert to ASIA D. In about 80% of patients who demonstrate an improvement in ASIA grade, this recovery will occur within the first 3 months, according to data from the European Multicenter Spinal Cord Injury (EMSCI) Database. Fewer than 20% of patients who improve in ASIA grade first show delayed signs of recovery between 6 and 12 months. After 1 year, this figure drops to only 5%.
| A | Complete | No sensory or motor function is preserved in sacral segments S4-S5 |
| B | Incomplete | Sensory, but not motor, function is preserved below the neurologic level and extends through sacral segments S4-S5 |
| C | Incomplete | Motor function is preserved below the neurologic level, and most key muscles below the neurologic level have a muscle grade of less than 3 |
| D | Incomplete | Motor function is preserved below the neurologic level, and most key muscles below the neurologic level have a muscle grade that is greater than or equal to 3 |
| E | Normal | Sensory and motor functions are normal |
In addition to ASIA grade conversion, persons with tetraplegia can also recover segment levels distal to their site of injury. This recovery can have a major impact on upper extremity function. For example, the difference between a C4 and C5 injury or a C5 and C6 injury translates into tremendous gains in independence. From 57% to 72% of patients with ASIA A injuries will experience recovery of at least one adjacent motor level. Within this subset, 30% recovered two levels. In the setting of complete spinal cord injuries, some authors have described the concept of the “zone of partial preservation,” which designates muscle groups that are partially innervated after injury. Partially innervated muscle groups that possess 1-2/5 British Medical Research Council (MRC) grade power ( Table 33.2 ) or greater are significantly more likely to recover to 3/5 strength versus muscles groups that are initially 0/5 grade. ,
| Grade | Description |
|---|---|
| 0 | No contraction |
| 1 | Flicker or trace of contraction |
| 2 | Active movement with gravity eliminated |
| 3 | Active movement against gravity |
| 4 | Active movement against gravity and resistance |
| 5 | Normal power |
As discussed above, the majority of patients even with sensorimotor complete injuries (ASIA A) will regain one or two motor levels within the first year after injury. Recovery occurs most frequently within the first 3 months. By 9 months, the majority of patients have experienced their maximum spontaneous recovery.
The strength of muscles at the level of injury may also improve over time, most commonly within a year of injury. Ditunno and colleagues and Waters and associates reported upper limb strength recovery rates using manual muscle testing and the MRC grading system (see Table 33.2 ). One-third of muscles classified as grade 0 at 1 month after injury improved to grade 3 at 4 to 6 months after injury; improvement was documented in some cases up to 24 months after injury. All upper limb muscles with an initial strength of grade 1 improved to at least grade 3 by 1 year after injury, with the exception of the triceps. If a muscle’s strength was grade 2 or better at 1 month after injury, the median time for full recovery was 6 months.
Based upon the available evidence, we defer surgery until 6 to 9 months following injury. At that time, patients that fail to demonstrate improvement in ASIA grades or recovery of additional segment levels distal to their site of injury are encouraged to proceed with surgery.
The referral process for persons with spinal cord injury remains disorganized and underutilized. Curtin and colleagues have shown that approximately 65% of the 5000 cervical spine injures per year would benefit from upper limb reconstruction to improve function and increase independence. Despite the potential benefits, less than 500 surgeries are performed annually to improve upper limb function. This disconnect is multifactorial with responsibility levied on physiatrists, surgeons, and institutions.
Published reports of tendon transfers for persons with spinal cord injury have shown improvement in quality of life and overall function. , Despite this evidence, many physicians and even spinal cord rehabilitation facilities remain wary or are unaware of the potential benefits. Many patients that would benefit from surgical reconstruction are never referred for surgical evaluation. With the advent of nerve transfers, this problem will escalate unless knowledge dispersion increases across all subspecialties that manage persons with spinal cord injury. A person with spinal cord injury can typically gain one cervical spinal level of function through tendon transfers. Augmenting the current tendon transfer paradigm with nerve transfers can restore two cervical spinal cord levels of function. However, nerve transfers require a viable muscle target for reinnervation, and irreversible motor endplate demise occurs as early as 18 months from injury. Therefore most nerve transfers have limited success after 12 months from injury. This window of opportunity highlights the time sensitivity and necessity of prompt referral for upper limb reconstruction.
The reconstructive surgeon is less concerned with the specific level of cervical injury than with the remaining strength of each muscle in the upper limb. Classically, tetraplegic patients have been classified by the injured cervical spine segment of C1-C7. Previously, it was assumed that the level of paralysis and sensory loss coincided with the bony injury, producing a precise transverse spinal cord lesion. Careful examination of tetraplegic patients has shown, however, little relationship between the level of the skeletal lesion and the spinal cord lesion. In addition, lesions may be asymmetric with unusual patterns of spared sensory or motor function. A classification for spinal cord injury commonly used by surgeons other than hand surgeons is the ASIA classification (see Table 33.1 ). This classification is based on intact muscle groups.
The classification used in this chapter was developed by an international group of hand surgeons in 1978 in Edinburgh , and modified in 1984 at Giens, France. The International Classification for Surgery of the Hand in Tetraplegia (ICSHT) grades the level of the spinal cord injury based on the number of muscles with grade 4 strength below the elbow and includes ICSHT levels 0 to 9 ( Table 33.3 ). The ICSHT level characterizes the most common patterns of presentation, based on the number of functional muscles below the elbow. The majority of persons with complete spinal cord injury will fall into ICSHT motor groups 2 and 3.
| Group | Sensibility c | Spinal Cord Segment | Key Muscle Below the Elbow Function | Motor Description |
|---|---|---|---|---|
| 0 | O or Cu | ≥C5 | None | Elbow flexion and forearm supination |
| 1 | O or Cu | C5 | + Brachioradialis | Elbow flexion with some supination/pronation b |
| 2 | O or Cu | C6 | + Extensor carpi radialis longus | Wrist extension |
| 3 | O or Cu | C7 | + Extensor carpi radialis brevis | Strong wrist extension |
| 4 | O or Cu | C7 | + Pronator teres | Forearm pronation |
| 5 | O or Cu | C7 | + Flexor carpi radialis | Wrist flexion |
| 6 | O or Cu | C7 | + Extensor digitorum communis | Finger extension (metacarpophalangeal joints) |
| 7 | O or Cu | C7 | + Extensor pollicis longus | Thumb extension |
| 8 | O or Cu | C7 | + Partial digital flexors | Incomplete finger flexion |
| 9 | O or Cu | C8 | Lacks only intrinsics | Complete digital roll-up |
| X | O or Cu | Exceptions | Variable |
a Developed at the First International Conference on Surgical Rehabilitation in the Upper Limb in Tetraplegia, Edinburgh, Scotland, 1978, and modified at the Second International Conference, Giens, France, 1984. This classification does not include the shoulder; it is a guide to the forearm and hand only. Determination of patient suitability for posterior deltoid to triceps transfer or biceps to triceps transfer is considered separately.
b The brachioradialis muscle can supinate and pronate to the neutral position.
c Afferent input, both ocular (O) and cutaneous (Cu), is recorded using the method described by Moberg. When vision is the only afferent available, the designation is “O.” Assuming there is 10-mm two-point discrimination or less in the thumb and index finger, the correct classification is “Cu,” indicating that the patient has adequate cutaneous sensibility. If two-point discrimination is more than 10 mm (i.e., inadequate cutaneous sensibility), the correct designation is “O.” The sensibility designation precedes the motor group (e.g., O2).
Another aspect of patient evaluation includes assessment of the general suitability for surgery. The assessment is based on various factors including age, occupation, level of education, learning capacity, motivation, economic support, family support, and agency support. The patient must have a clear understanding of what can and cannot be accomplished with surgery.
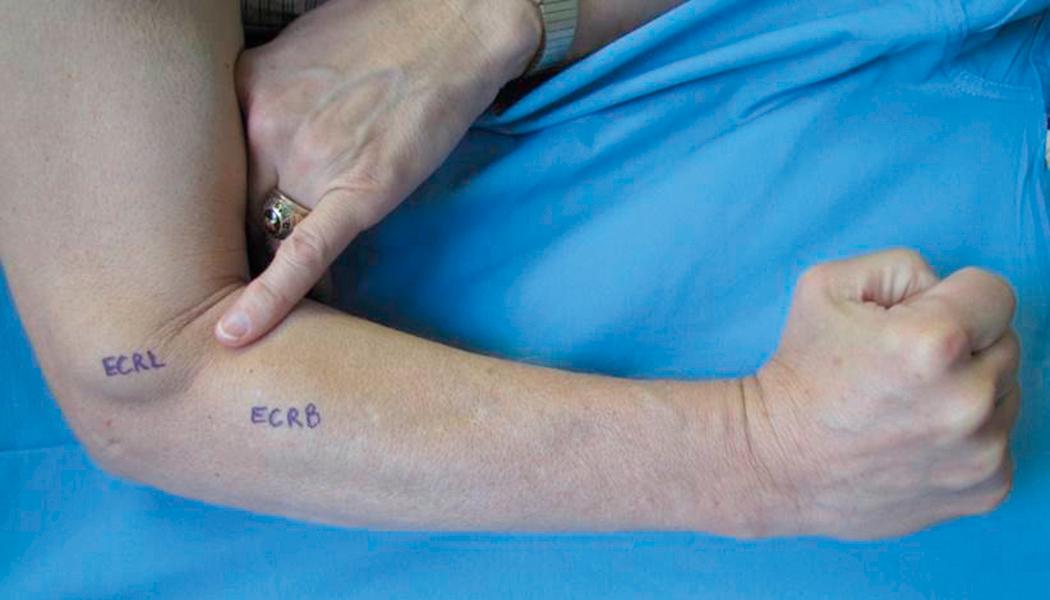
The examination begins with a thorough history of the injury and treatment to date. Subsequently, a meticulous physical examination is performed, including sensory and motor testing to establish the patient’s group (ICSHT level 0 to 9). Manual muscle testing (see Table 33.2 ) is performed on all muscle groups in the upper extremity. The surgeon can make a working list of tendons that are available for transfer (grade 4 strength or higher and expendable) and muscles that are paralyzed (grade 1 strength or lower). Once the muscles with grade 4 strength have been identified, the patient is assigned a group number (see Table 33.3 ). For example, a patient with grade 4 strength of the brachioradialis (BR), both radial wrist extensors, the pronator teres (PT), and the flexor carpi radialis (FCR) would be classified as a group 5 patient. One of the difficulties in physical examination is distinguishing between group 2 and group 3 injuries. In group 2 patients, only the extensor carpi radialis longus (ECRL) has a grade 4 or higher strength, and physical examination reveals substantial radial deviation of the wrist with resisted wrist extension. In group 3 patients, both the ECRL and the extensor carpi radialis brevis (ECRB) have strength of grade 4 or higher. In a group 3 patient, the wrist extends with less radial deviation and often grade 1 to 3 strength is present in the PT, the next most caudal muscle. In thinner patients, the ECRL, a flat muscle, and the ECRB, a more bulbous muscle, can be palpated while muscle strength is being tested ( Fig. 33.3 ).
In addition to manual muscle testing, the surgeon must also examine the passive range of motion to evaluate joint and muscle contractures along with the resting posture of the hand. The examiner can induce a tenodesis effect by passively flexing and extending the wrist while observing passive myostatic tensioning of the digital extensors and digital flexors, respectively. The hand must have passive mobility and tenodesis to achieve success. For example, if the thumb rests in a fully supinated position and lacks any tenodesis pinch during wrist extension, tendon transfers for pinch will be ineffective without skeletal stabilization (carpometacarpal [CMC] fusion) in a key pinch position.
The management of the wrist and hand in tetraplegia follows the concepts of “hierarchy of hand function” or the “reconstruction ladder.” The primary fundamental movement is wrist extension, which yields tenodesis for grip as the fingers flex into the palm and tenodesis for lateral pinch as the thumb adducts against the index finger. Wrist extension also aligns the finger flexors along Blix’s length-tension curve for maximum active grip. The second most essential movement is lateral pinch, which is necessary to perform numerous ADLs. Most daily activities are accomplished with lateral pinch, such as holding an object, turning a key, or using a fork ( Fig. 33.4 ). The third essential motion is grasp, which allows the holding of objects ( Fig. 33.5 ). The fourth and last movement is digital opening for object acquisition. The reason to place this function lowest on the ladder is that wrist flexion yields passive digital opening, which is often adequate for object procurement. In addition, synchronous digital opening is difficult to achieve via surgery as metacarpophalangeal (MCP) joint extension is mainly an extrinsic function (extensor digitorum communis, extensor indicis proprius, and extensor digiti quinti) and interphalangeal (IP) joint extension is primarily an intrinsic function (interossei and lumbricals). The only way the extrinsic system can elicit IP joint extension is by limiting MCP joint extension (e.g., Zancolli tenodesis of the flexor digitorum superficialis [FDS] or House intrinsic tenodesis). The Zancolli tenodesis of the FDS procedure can cause problems such as limiting flexor digitorum profundus excursion and/or attenuating over time. The House intrinsic tenodesis is difficult to set the appropriate graft tension. Restoring both MCP and IP movements by tendon transfer(s) remains a daunting task replete with complications. A lumbrical bar fabricated by an experienced therapist may be a simpler solution to limit MCP joint extension.


The next step is to determine the patient’s needs and match the needs with the muscles available for transfer. For example, in a group 4 patient, the BR is available because the patient has the biceps and brachialis for elbow flexion (and, therefore, no function will be lost with the transfer); the ECRL is also available because the patient has the ECRB for wrist extension. The PT can also be used for transfer, but this could result in diminished pronator power and loss of strength for manual wheelchair function. To complete the assessment, the muscles available for transfer (BR, ECRL) are matched with the needed functions according hierarchy of hand function or the “reconstruction ladder” combined with other surgical options such as arthrodesis and tenodesis. In a group 4 patient, pinch and grasp are deemed most important, as these patients have active wrist extension. Thus the BR and ECRL are selected for thumb pinch and finger flexion, respectively. The BR will be transferred to the flexor pollicis longus (FPL) because it has (and needs) less excursion. The ECRL will be transferred to the flexor digitorum profundus (FDP) tendons, which is synergistic and provides greater excursion. Surgical adjuncts to be considered include optimizing the thumb position for key pinch with a CMC fusion and augmenting digital extension for object acquisition via extensor pollicis longus (EPL) and extensor digitorum communis (EDC) tenodesis. If necessary, intrinsic reconstruction can be added during a second surgery. By evaluating what the patient has, what the patient needs, and what is available, the surgeon can determine the optimal combination of tendon transfers and adjunctive procedures to restore function (nerve transfers will be discussed later).
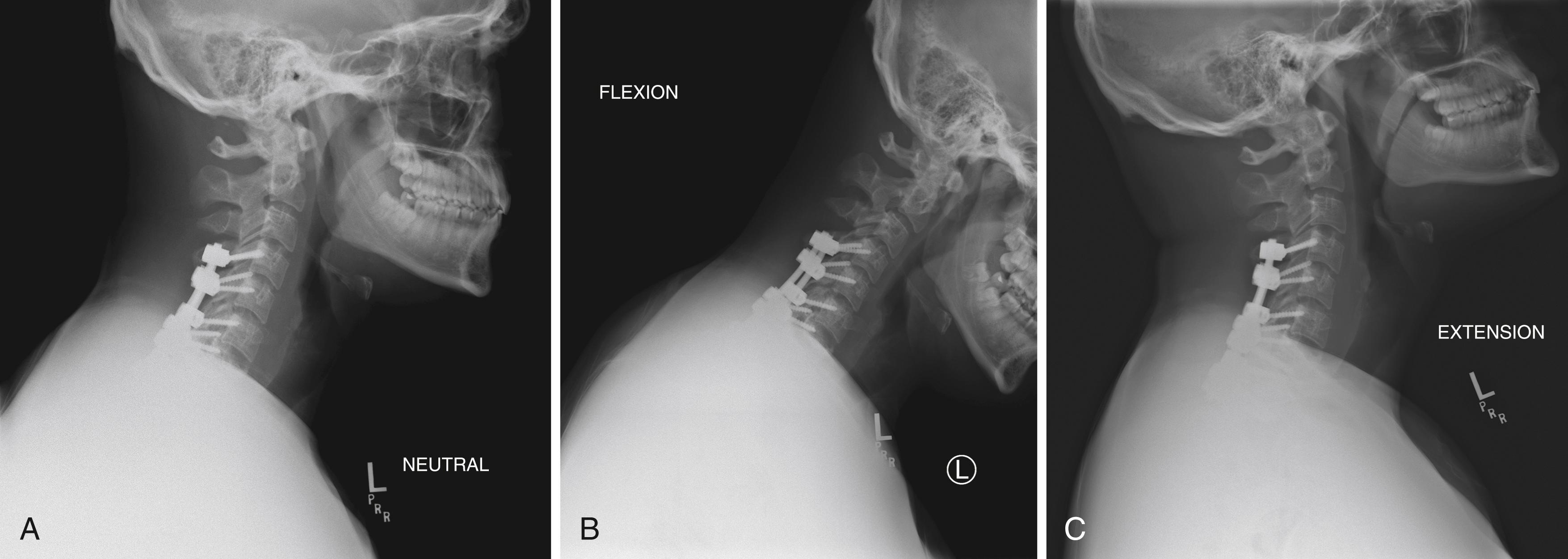
At the time of the acute spinal cord injury, advanced diagnostic imaging is performed to determine the extent of spinal cord and skeletal injury (see Fig. 33.1 ). Subsequent imaging is performed to assess for spinal stability. Prior to upper extremity surgery, flexion and extension lateral x-rays are necessary to ensure stability ( Fig. 33.6 ). Advanced imaging is not routinely performed.
Injuries of the spinal cord produce a different nerve deficit than do brachial plexus injuries or isolated peripheral nerve injuries. The pattern seen with a spinal cord injury is based on segmental innervation. This concept means that the anterior horn cells in the spinal cord are arranged in a pattern from cephalad to caudad. Motor nuclei form longitudinal columns crossing several segmental levels, innervating the peripheral musculature in a predictable manner.
Fig. 33.7 shows the segmental anatomy, based on Zancolli’s clinical experience with tetraplegic patients. When a spinal cord injury occurs, the motor nuclei cephalad to the injury will be functional; the motor nuclei at or caudad to the level of the injury will be nonfunctional.
Before the 1960s, the poor prognosis and low survival rate of patients with spinal cord injury precluded the need for upper extremity reconstruction. As the medical care of patients with spinal cord injury improved, so did the prognosis resulting in surgical advancements within tendon transfer surgery. As shown in Table 33.4 , the basics of tendon transfer surgery that were initiated by Bunnell were applied to tetraplegic patients by Moberg, Lamb, Zancolli, and Freehafer. Patients and physicians became enthusiastic about the potential benefits of a well-designed and well-executed surgical plan for upper limb reconstruction in those with tetraplegia.
| Author | Publication and Year | Importance |
|---|---|---|
| Bunnell | Surgery of the Hand, 2nd ed, 1948 | C6-C7 treated with tendon transfer/tenodesis |
| Nickel, Perry, Garrett | J Bone Joint Surg Am 45:933–952, 1963 | Development of useful function in severely paralyzed hand |
| Wilson | J Bone Joint Surg Am 38:1019–1024, 1956 | Provision of automatic grasp by flexor tenodesis |
| Street and Stambaugh | Clin Orthop Relat Res 13:155–163, 1959 | Finger flexor tenodesis |
| Lipscomb, Elkins, Henderson | J Bone Joint Surg Am 40:1071–1080, 1958 | Two-stage surgical grasp and release for C6-C7 fracture-dislocation patients |
| Freehafer and Mast | J Bone Joint Surg Am 49:648–652, 1967 | First description of brachioradialis to wrist extension in “high” cervical injuries |
| Zancolli | Structural and Dynamic Bases of Hand Surgery, 1968 | Comprehensive review of anatomic bases of tendon transfers and operative options, including two-stage reconstruction for grasp-pinch and release |
| Lamb and Landry | Hand 3(1):31–37, 1971 | Flexor phase surgical technique and results; principles of tendon transfer and results |
| Lamb and Chan | J Bone Joint Surg Br 65(3):291–298, 1983 | Surgical reconstruction of upper limb in traumatic tetraplegia |
| Lamb | J Hand Surg Br 14(2):143–144, 1989 | Upper limb surgery in tetraplegia |
| Zancolli | Clin Orthop Relat Res (112):101–113, 1975 | Surgery with strong wrist extension preserved (97 cases), treated with two-stage reconstructions |
| Moberg | J Bone Joint Surg Am 57(2):196–206, 1975 | Posterior deltoid to triceps transfer and single-stage pinch tenodesis |
| Moberg | The Upper Limb in Tetraplegia: A New Approach to Surgical Rehabilitation, 1978 | Classification based on sensibility and available grade 4 muscles; importance of key pinch position |
| [First] International Conference on Surgical Rehabilitation in the Upper Limb in Tetraplegia (Edinburgh, Scotland, 1978) | Reported in J Hand Surg Am 4(4):387–390, 1979 | First international classification for surgery of the hand based on sensibility, motors available below elbow, and hand function |
| Peckham, Marsolais, Mortimer | J Hand Surg Am 5(5):462–469, 1980 | First description of use of functional electrical stimulation for restoration of key pinch |
| Hentz, Brown, Keoshian | J Hand Surg Am 8(2):119–131, 1983 | Functional assessment of upper limb after reconstructive surgery |
| Second International Conference (Giens, France, 1984) | Reported in J Hand Surg 11:604–608, 1986 | Classification modified; still in use (see Table 33.2 ) |
| Allieu, Benichou, Teissier | Chirurgie 112(10):736–742, 1986 | Report of 52 reconstructions with 28 posterior deltoid to triceps transfers complicated by stretching in 7 cases |
| Waters, Moore, Graboff, Paris | J Hand Surg Am 10(3):385–391, 1985; J Hand Surg, 1987 | Key pinch using BR to FPL and elbow extension transfers to increase pinch strength |
| House and Shannon | J Hand Surg Am 10(1):22–29, 1985 | Opposition transfers vs fusion for thumb control |
| House, Comadoll, Dahl | J Hand Surg Am 17(3):530–538, 1992 | One-stage key pinch with thumb CMC fusion |
| McCarthy, House, Van Heest | J Hand Surg Am 22(4):596–604, 1997 | Intrinsic reconstructions |
In addition to hand surgery reconstruction, noteworthy research on and clinical treatment with a neuroprosthesis was performed from the 1980s to the 2000s. The use of electrical current for muscle stimulation in patients with spinal cord injury led Keith and associates at Case Western University to develop a fully implantable functional neuromuscular stimulation neuroprosthetic system. This technology takes advantage of paralyzed muscles that are no longer under cortical control (due to spinal cord injury) but have intact spinal reflex arcs (infralesional segment). These muscles can be stimulated to contract with weak electrical currents. The stimulation is controlled by normal muscle signals, nerve signals, or volitional movements above the level of spinal cord injury (supralesional segment), typically from the contralateral shoulder or neck.
The neuroprosthesis is indicated for tetraplegic patients for whom standard surgical procedures or orthotic devices cannot provide useful improved function. Most commonly, the neuroprosthesis is implanted in patients in groups 0, 1, 2, and 3. More than 250 adults and children have received the neuroprosthesis system worldwide. As of 2008, the company that manufactured the device discontinued its production. The second-generation 12-channel devices with telemetry and myoelectric control are only available under research protocols; however, they have shown promise in achieving independent two-handed use.
The goal of tetraplegic hand reconstructive surgery is to increase the patient’s independence. Candidates for surgery include patients (1) whose recovery from injury has stabilized, (2) have an unchanging upper extremity motor examination, and (3) whose functional impairments can be improved by surgical reconstruction. Ideally, the patient is free from contractures and pain with spasticity well controlled. The patient must have the ability to comply with the postoperative rehabilitation regimen, have appropriate postoperative support services, and be highly motivated to improve function. It is also critical that the patient be medically stable, including bowel and bladder function and blood pressure control, and be without urinary tract infection or decubitus ulcers.
Two factors have had a uniformly adverse effect on the results of surgical treatment: spasticity and psychological problems. Spasticity that is uncontrollable is a strong contraindication to surgery. Freehafer and colleagues and Moberg stated that some spasticity might be helpful, but judging the degree of spasticity that is compatible with good results is very difficult. In fact, Moberg noted that even unappreciated spasticity leads to poor results. Psychological impairment, unrealistic expectations, and insufficient motivation to complete the operative and postoperative protocols will also result in disappointing results. The surgeon, patient, and therapist must be able to communicate effectively and share realistic expectations of the benefits of surgery. Patient-reported outcome measures, such as the Canadian Occupational Performance Measure (COPM), are particularly useful. The COPM assesses an individual’s perceived occupational performance in the areas of self-care, productivity, and leisure. Allowing a prospective patient to visit a previous patient with a similar ICSHT classification that has undergone surgical reconstruction is particularly useful.
Cervical spine injury with upper limb partial paralysis
Stabilized motor recovery
Functional deficits that can be improved with surgery
Medically stable (blood pressure, bowel and bladder function)
Infection-free (decubitus ulcers, bladder)
Full passive range of motion
Realistic goals with good motivation and desire
Personal and social stability to carry out rehabilitation
Uncontrolled spasticity
Contractures
Chronic pain problems
Psychological instability
Four types of surgical procedures are most commonly performed: tendon transfer, tenodesis, arthrodesis, and nerve transfer (discussed later).
Tendon transfers are preferred over tenodesis or arthrodesis because these restore active control and strength. However, the primary problem after spinal cord injury is a paucity of adequate donor muscles available for transfer. It is critical to ensure that sacrifice of a potential donor muscle for transfer will not create another functional deficit. The available muscles are prioritized first for wrist extension, then for pinch, then for grasp, and finally for release functions. Tendon transfer principles, including donor availability, donor strength, amplitude of excursion, synergism, passive mobility of recipient joints, and condition of the soft tissue bed, are prerequisites. When tendon transfer options are exhausted, additional hand functions may be achieved through tenodesis and/or arthrodesis.
Tenodesis is defined as the movement of one joint produced by the motion of an adjacent (usually proximal) joint. For instance, passive extension and flexion of the wrist prompts digital flexion and extension, respectively. Although the finger and thumb muscles are paralyzed, there is sufficient remaining myostatic tension to induce reciprocal action of the digital flexor and extensor tendons that cross the wrist joint. Spinal cord injury patients learn to use the tenodesis effect naturally and commonly have functional but very weak pinch or grasp owing to the tenodesis effect alone. The tenodesis effect can be enhanced surgically without the need to transfer an active muscle. Passive tenodesis procedures anchor the paralyzed tendon proximal to the wrist joint to decrease effective tendon excursion and thus increase digital movement with active wrist movement. Common tenodesis techniques include anchoring the finger flexors and extensors for grasp and release, respectively.
Thumb CMC joint arthrodesis is useful to stabilize this multiarticular joint for pinch. The index finger proximal IP (PIP) joint can undergo arthrodesis to stabilize the finger as a firm post for pinch. The wrist is never fused because the tenodesis effect would be lost. Surgical techniques for arthrodesis are no different in the paralytic hand. When possible, rigid fixation is preferred to allow earlier weight bearing and expedient mobilization.
Nerve transfers have supplemented and even supplanted the use of tendon transfers in some clinical scenarios. The ICSHT is applicable to nerve transfer with important considerations. Nerve transfers will be discussed in detail following the section on tendon transfers. Tendon transfer reconstruction is based on the patient’s international classification group (see Table 33.3 ). Elbow procedures are discussed first, followed by procedures for grasp, pinch, and release.
Tendon transfer procedures will be discussed first, followed by nerve transfer procedures. Discussion of procedures will begin with the elbow and proceed in a distal direction. Procedures for grasp, pinch, and release will be discussed based on reconstructive priorities for the patient’s ICSHT group (see Table 33.3 ). Finally, principles of combined nerve and tendon transfer reconstruction will be discussed.
Most persons with cervical spine injury lack triceps function and elbow extension. Restoration of elbow extension carries a high priority. Active elbow extension assists the patient in reaching objects above the shoulder level, improves driving ability, aids in wheelchair propulsion, permits pressure relief, and facilitates independent transfer. Additionally, active elbow extension provides an antagonist to elbow flexion, which facilitates improved function through tenodesis after hand reconstructions that use the BR as a tendon transfer. Pinch strength has been shown by Brys and Waters to improve from 0.15 to 3.9 pounds in wrist extension in patients after elbow extension tendon transfers that improve elbow extension strength. Two options exist for tendon transfer: deltoid to triceps transfer and biceps to triceps transfer. , ,
The biceps and the deltoid are innervated from the spinal cord at a higher level than the triceps. The posterior deltoid to triceps transfer has been used extensively over the past 30 years, but more recently, the biceps to triceps transfer has been used more frequently.
The biceps to triceps tendon transfer is our preferred method for establishing elbow extension ( Fig. 33.8 ). The biceps is used as the donor muscle. To ensure that function will not be lost with transfer of the tendon, the strength of the brachialis as an elbow flexor and of the supinator as a forearm supinator must be verified before transfer. Manual muscle testing for elbow flexion is performed, attempting to isolate the brachialis by supinating the forearm, which relaxes the biceps. This can be verified by palpating the groove between the anterior tubular biceps and the flatter brachialis that resides posterior to differentially test strength. In reviewing the segmental innervations of the upper limb from the spinal cord (see Fig. 33.7 ), note that the biceps, brachialis, and supinator are all innervated at about the same level (C5-C6). If the patient has strong wrist extension, the spinal cord lesion should be below C6, sparing the biceps, brachialis, supinator, and wrist extensors, but above the innervation of the paralyzed triceps. Such findings on clinical examination verify that transfer of the biceps will not lead to a functional loss. Electromyography is not part of the routine evaluation. If there is any doubt about the strength of the brachialis or supinator, the biceps muscle can be injected with bupivacaine, which induces temporary paralysis. Subsequently, the strength of the brachialis and supinator can be assessed.
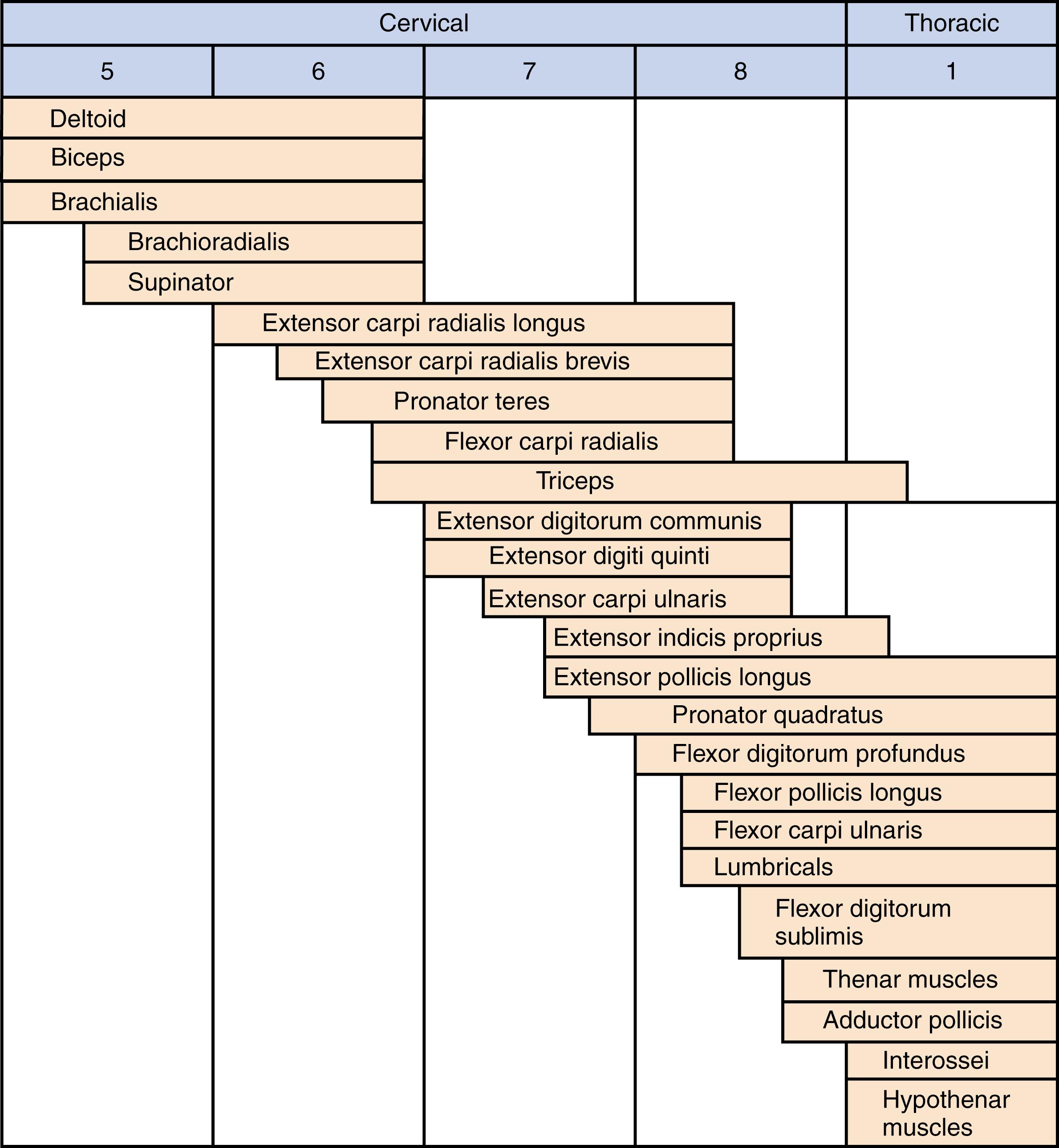
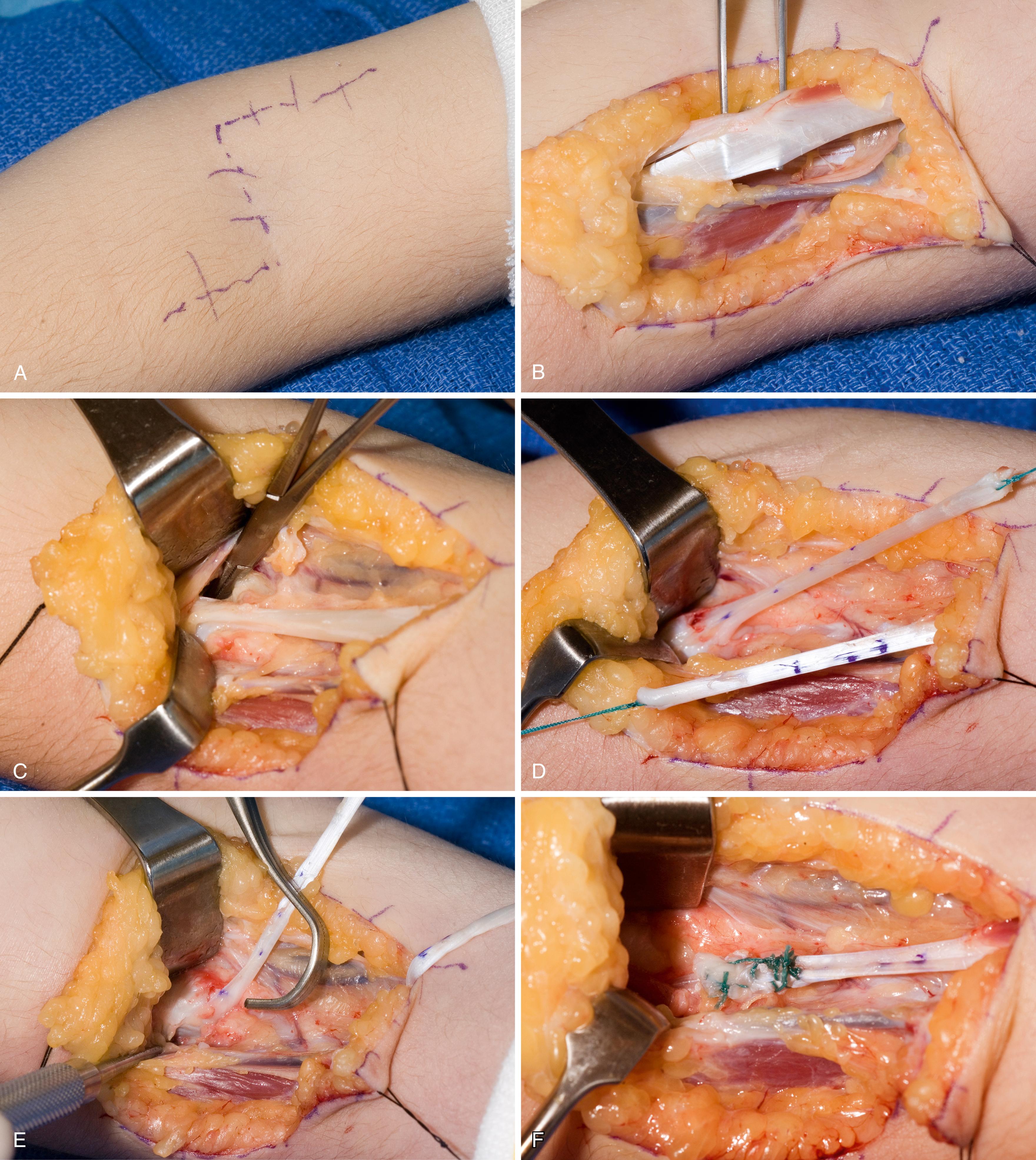
The biceps to triceps tendon transfer can be performed using a medial , or a lateral routing technique. The lateral technique was first described by Friedenberg in 1954 and later reported by Zancolli. , No loss of active elbow flexion was noted, although flexor strength was diminished by 24%. In 1988 Ejeskär reported his results using the lateral routing technique in five patients, including the first complication of radial nerve palsy. Injury to the radial nerve and medial routing is strongly preferred.
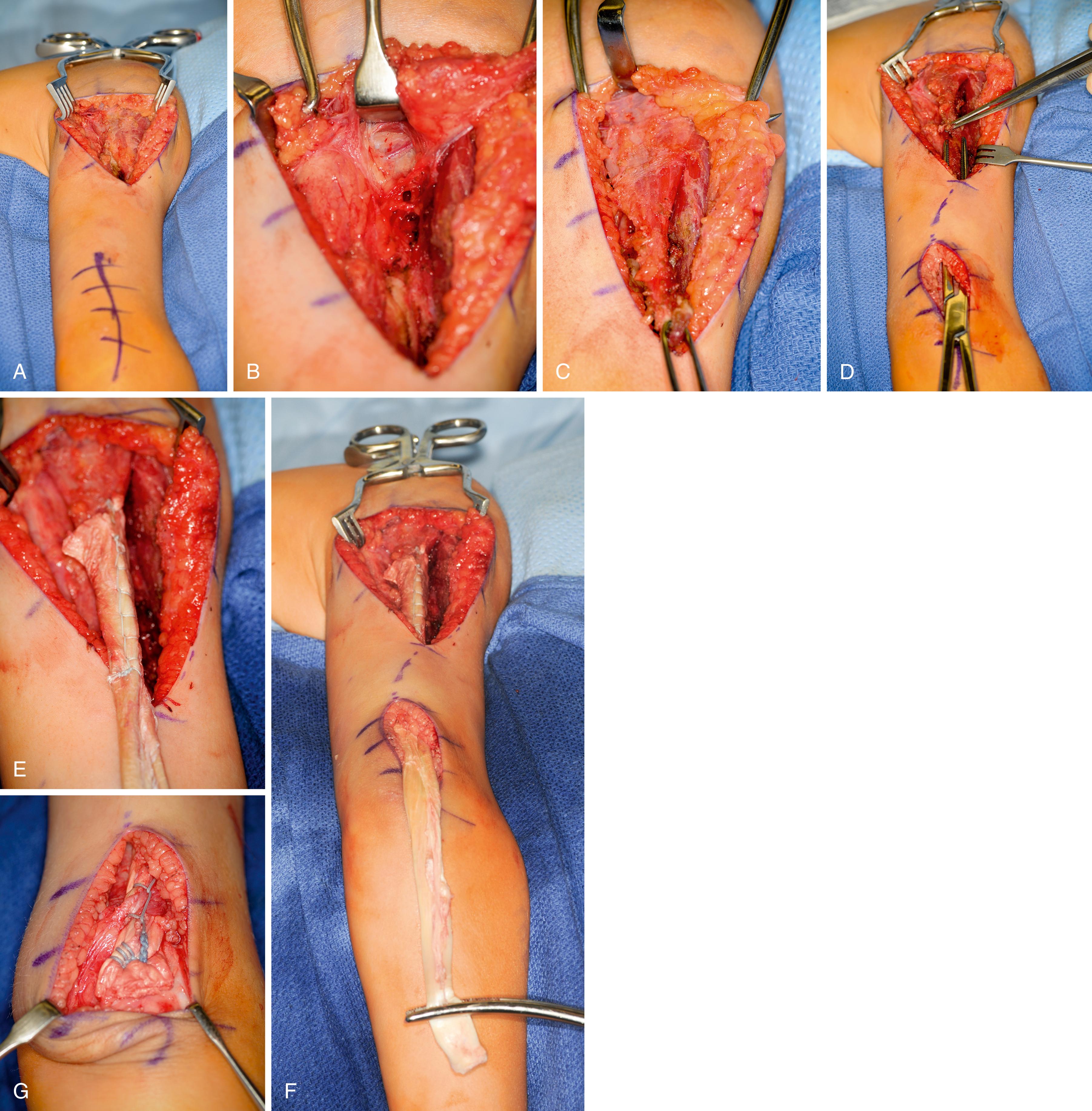
The procedure is performed with the patient supine, a roll under the operative shoulder blade, and the limb draped free with a sterile tourniquet high on the arm. The incision extends from the midhumerus on the medial side, transversely across the antecubital crease, and courses distally, centered over the biceps insertion on the radial tuberosity. The lateral antebrachial cutaneous nerve is identified and protected as the biceps tendon is dissected free from its medial and lateral fascial attachments in the arm and then sharply released from its insertion on the bicipital tuberosity. The lacertus fibrosus is freed from the forearm fascia during the dissection and can be preserved as a second tail of the biceps tendon for subsequent weaving. The muscle is raised in a proximal direction, with care taken to preserve the musculocutaneous nerve running on its undersurface and along the brachialis muscle.
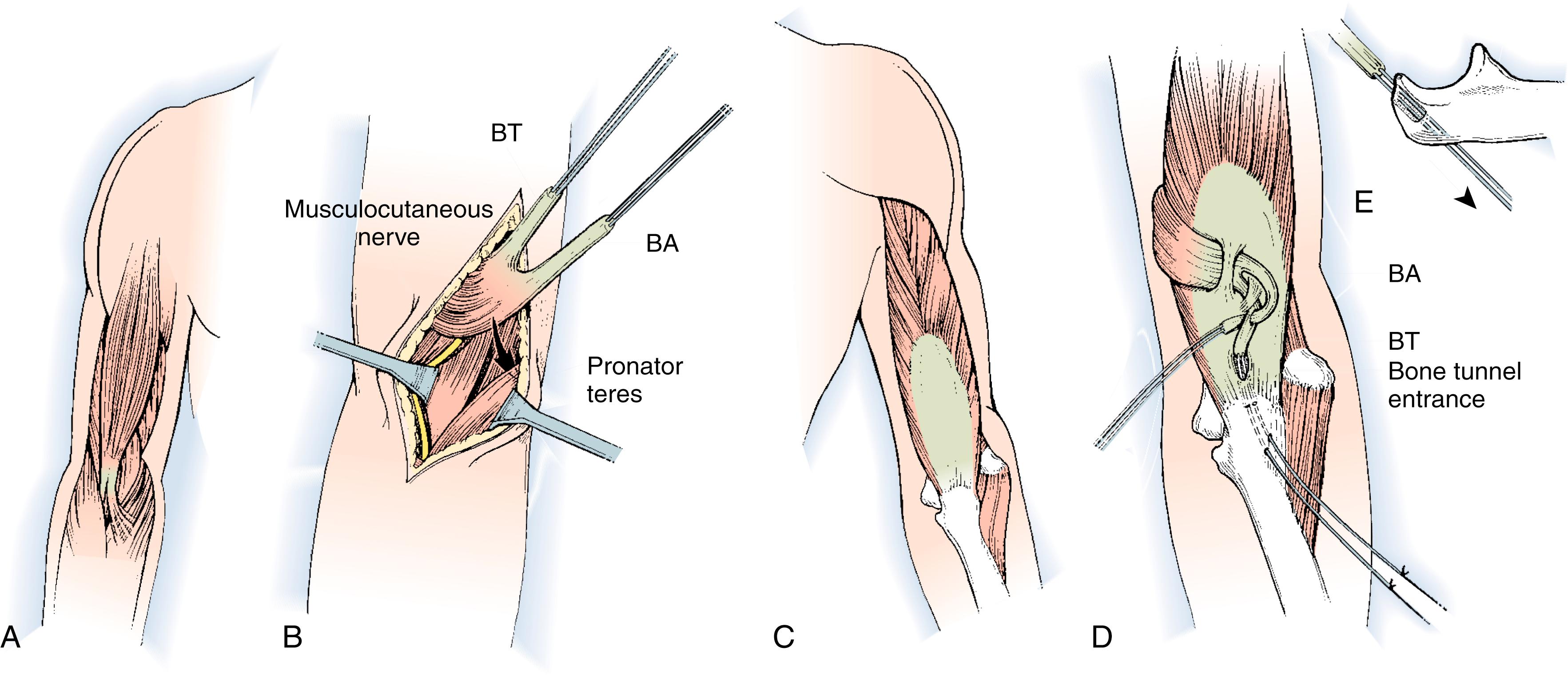
A second (posterior) incision is performed over the distal one-third of the triceps tendon, passing lateral to the olecranon to avoid subsequent olecranon pressure ulceration and allowing an adequate skin bridge with the medial incision. A subcutaneous tunnel is made around the medial elbow from the anterior wound to the posterior wound, creating a line of pull that is straight and free for medial rerouting of the biceps ( Fig. 33.9A ). The medial intermuscular septum may need to be partially resected, and care is taken to protect the neighboring ulnar nerve. The biceps tendon is passed from the anterior wound into the posterior wound. The tendon can be routed superficial or deep to the ulnar nerve. We prefer deep to avoid iatrogenic compression of the ulnar nerve, particularly if there are future advances in neuroregeneration (see Fig. 33.9B ). A No. 5 nonabsorbable grasping suture is placed into the end of the biceps tendon.
The triceps tendon is split and the tip of the olecranon is exposed. A large-bore blind bone tunnel is made in the olecranon for acceptance of the biceps tendon. The tendon is passed through the medial leaflet of the triceps tendon in preparation of docking into the bone tunnel (see Fig. 33.9C ). The tendon is docked using two posterior unicortical holes and suture retrievers (Suture retriever, Smith Nephew, Andover, MA) (see Fig. 33.9D ). The elbow is placed in full extension and the two ends are drawn into the bone tunnel using suture retrievers (see Fig. 33.9E ). The sutures are tied over the posterior olecranon cortex. Additional suturing is performed during closure of the triceps split with incorporation of the biceps tendon. The lacertus fibrosus can be interwoven through the biceps to triceps weaves to further secure the tendon weave. Following closure, a well-padded long-arm cast is applied with the elbow in extension (see Fig. 33.9F ). The wrist is included within the cast and the hand position depends on concomitant procedures performed for hand function.
Tendon rehabilitation requires supervised therapy for months. The biceps to triceps can be mobilized early (7 to 10 days) or delayed (3 weeks) depending on the patient’s age, patient’s compliance, and the strength of the tendon transfer repair. The rehabilitation is divided into three phases: early mobilization, mobilization, and strengthening/functional retraining. Mobilization is initiated in gravity-eliminated plane within a limited arc of flexion. Flexion is progressively increased 15 degrees per week as long as no extension lag develops. Strengthening and wheelchair propulsion are prohibited until 12 weeks from surgery. Transfers and weight shifts are initiated 16 weeks from surgery.
The strength of the biceps to triceps transfer continues to improve for at least a year, resulting in substantial improvements in performing ADLs. Antigravity elbow extension is obtainable in the vast majority of cases ( Fig. 33.10 ; ![]() ). Published series have reported functional improvements for overhead, reaching, and driving activities. , , , , No clinical loss of active flexion has been reported, although flexor strength was diminished by 24% in one series. Mulcahey and coworkers published a randomized prospective comparison of posterior deltoid to biceps transfer versus biceps to triceps with medial routing transfer. These authors reported that at 2 years, seven of eight arms treated with biceps to triceps transfer had antigravity use of the arm, whereas only one of eight arms treated with posterior deltoid to triceps transfer had antigravity use of the arm.
). Published series have reported functional improvements for overhead, reaching, and driving activities. , , , , No clinical loss of active flexion has been reported, although flexor strength was diminished by 24% in one series. Mulcahey and coworkers published a randomized prospective comparison of posterior deltoid to biceps transfer versus biceps to triceps with medial routing transfer. These authors reported that at 2 years, seven of eight arms treated with biceps to triceps transfer had antigravity use of the arm, whereas only one of eight arms treated with posterior deltoid to triceps transfer had antigravity use of the arm.
Availability of biceps as donor (adequate brachialis and supinator strength)
Full passive range of motion or an elbow flexion contracture of less than 30 degrees
Absent triceps function
Verify brachialis and supinator strength.
Obtain elbow passive range of motion (therapy, splinting, or serial casting if necessary).
Medial routing avoids the possible devastating complication of radial nerve palsy.
Anterior and posterior incisions are used, with an adequate skin bridge.
Mobilize the biceps tendon (can include the lacertus fibrosus).
Use No. 5 nonabsorbable grasping suture grasping suture in the biceps tendon.
Ensure a straight line of pull around the medial distal one-third of the humerus.
Perform weave of biceps tendon into triceps tendon.
Insert the terminal end of the biceps tendon into the bone tunnel at the tip of the olecranon.
A posterior incision over the olecranon is a potential pressure sore area.
An elbow contracture >30 degrees will result in a biceps tendon with inadequate length to reach the olecranon.
The biceps to triceps can be mobilized early (7 to 10 days) or delayed (3 weeks) depending on the patient’s age, patient’s compliance, and the strength of the tendon transfer repair.
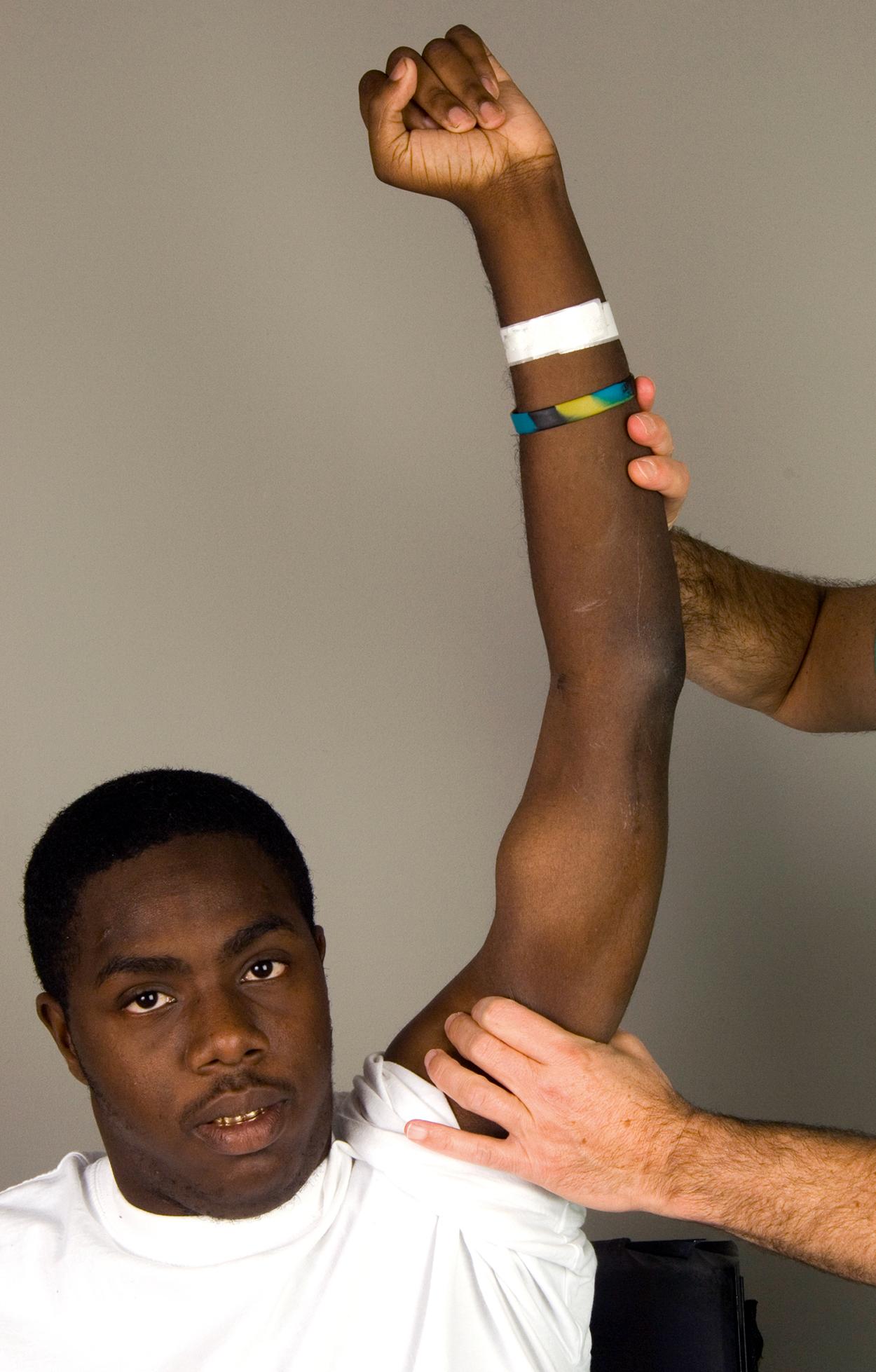
The posterior deltoid must have adequate strength to motor elbow extension. Shoulder extension and palpation best assesses posterior deltoid strength and turgor. The examination can be performed with the patient seated, although placing the patient prone provides a more reliable assessment. Similar to biceps to triceps transfer, a supple elbow with near complete range of motion is also necessary. Patients with an elbow contracture require resolution of the contracture prior to surgery.
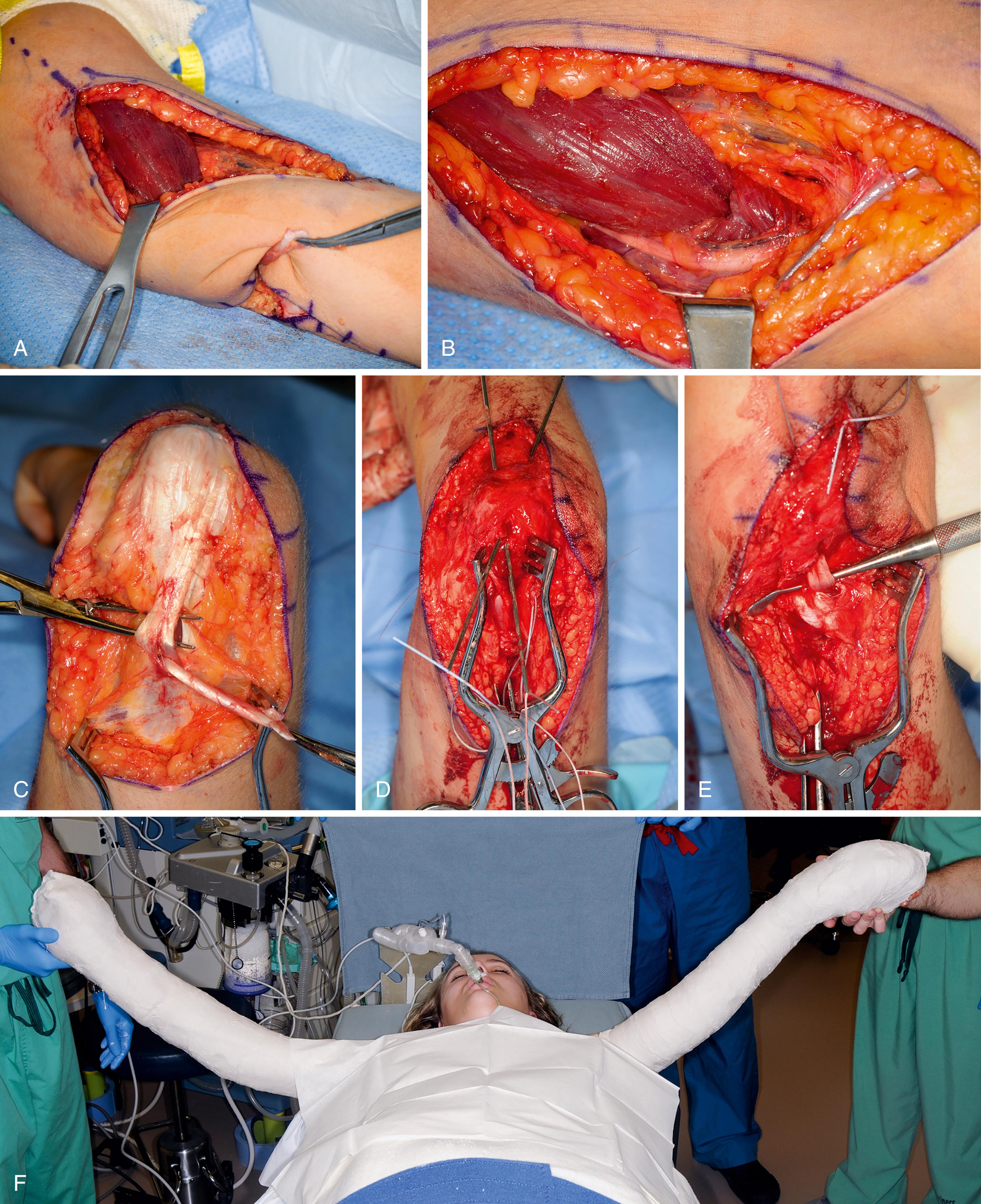
The posterior deltoid to triceps transfer uses the posterior third and the posterior half of the middle third of the deltoid muscle as a donor to provide active elbow extension ( Fig. 33.11 ). An interposition tendon graft is used, and the triceps tendon serves as the insertion.
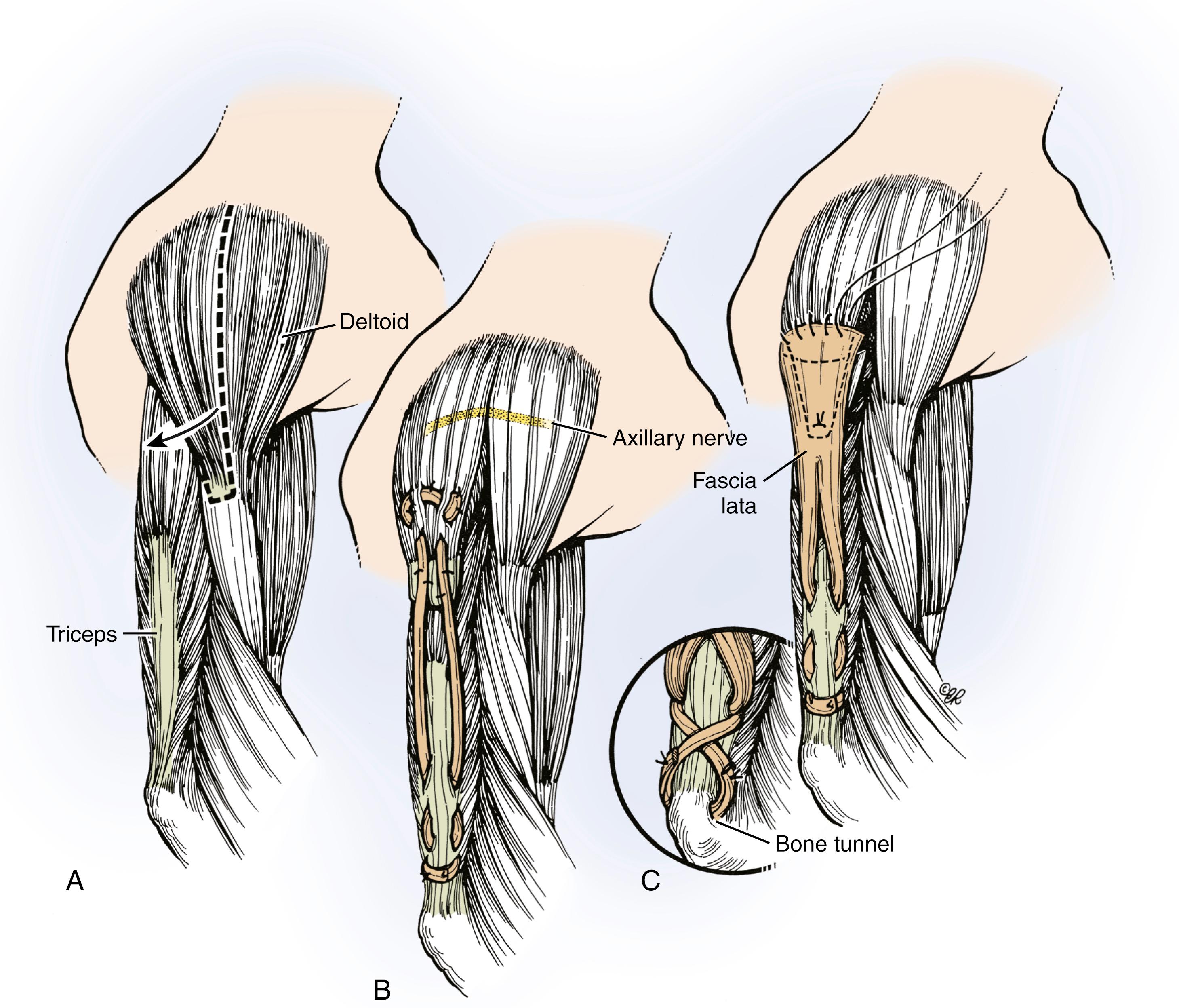
Prior to prepping and draping, Marcaine with epinephrine is instilled in the planned incision sites. The upper extremity and hemithorax are then prepped and draped in usual sterile fashion. A gently curved incision is made over the deltoid muscle ( Fig. 33.12A ). Skin flaps are elevated. The axillary nerve is identified entering the deltoid muscle (see Fig. 33.12B ). The posterior half of the deltoid is selected for transfer (see Fig. 33.12C ). A periosteal sleeve is elevated with the posterior deltoid. Proximal dissection is performed to improve the excursion and line of pull.
A distal incision is made over the distal third of the triceps tendon. The triceps’ tendon is identified. The ulnar nerve is isolated and retracted out of harm’s way. A subcutaneous tunnel is then created between these two incisions using sequential hemostats (see Fig. 33.12D ). An allograft is favored to bridge the distance between the deltoid muscle and triceps’ tendon. The allograft is thawed and the proximal end is sutured to the posterior deltoid and underlying periosteum (see Fig. 33.12E ). The remaining tendon is then passed through the subcutaneous tunnel to the distal incision (see Fig. 33.12F ). The elbow is placed in full extension and the allograft woven into the triceps tendon and/or bone for additional augmentation (see Fig. 33.12G ).
Several alternatives exist for the interposition graft. Moberg originally used toe extensors from the second, third, and fourth toes to allow at least three weaves at each attachment site. Alternative graft materials have included fascia lata, tibialis anterior, , and extensor carpi ulnaris (ECU) Alternatively, Castro-Sierra and Lopez-Pita used the central one-third of the triceps. In this method, a 1-cm strip from the central one-third of the triceps is harvested from its insertion in a retrograde manner (proximally based), mobilizing it sufficiently to graft back to the distal end of the posterior deltoid. Reflecting the central third of the triceps with a bone block and internally fixing this to a bone block from the deltoid insertion has also been described.
After the interpositional graft has been placed, the transfer is sewn into place and tensioned with the shoulder in 30 to 40 degrees of abduction and no forward flexion so that the elbow can flex to 60 degrees with moderate tension. Most commonly, a well-padded long-arm cast is applied to hold the elbow in about 10 degrees of flexion. Forward flexion and shoulder adduction are avoided during the 6 weeks of cast immobilization. Gentle active exercises are performed to slowly gain elbow flexion for the next 3 months with a rate of 5 to 10 degrees of increased flexion per week.
Posterior deltoid to triceps transfers are compromised by the prolonged shoulder and elbow immobilization required postoperatively, as well as tendon graft elongation over time. Elongation of the tendon graft leads to decreased strength over time. Fridén and colleagues used intraoperative metal markers at the ends of the tendon grafts and measured an average of 2.3 cm of elongation after 2 years. They changed their postoperative regimen to include longer elbow extension immobilization and increased shoulder abduction. Follow-up of five patients using this regimen using the same markers averaged 0.8 cm.
The forearm is the “forgotten joint” with regards to movement and balance. The forearm is articulated at both its proximal and distal ends (proximal and distal radioulnar joint joints). The strong interosseous ligament tightly links the radius and ulna and participates in load transfer. Forearm rotation is balanced by two muscles that supinate (biceps and supinator) and two muscles that pronate (PT and quadratus). Supination is primarily controlled by the C5 and C6 nerve roots (biceps and supinator muscles). Pronation is principally motored by the C7 (PT) and C8 (pronator quadratus) nerve roots. Midcervical tetraplegia frequently results in preservation of the supinators and loss of the pronators. The resultant imbalance will result in considerable deformity over time. The deformity is initially supple, but over time, the contracture(s) becomes fixed ( Fig. 33.13 ).
Pronation is important to patients who have only active wrist extension (groups 2 and 3). These patients use the automatic or tenodesis effect for grasp, but if the hand cannot be pronated, gravity cannot be used to provide a tenodesis effect for digital extension and release. Those patients using a tenodesis brace need pronation for the same reason.
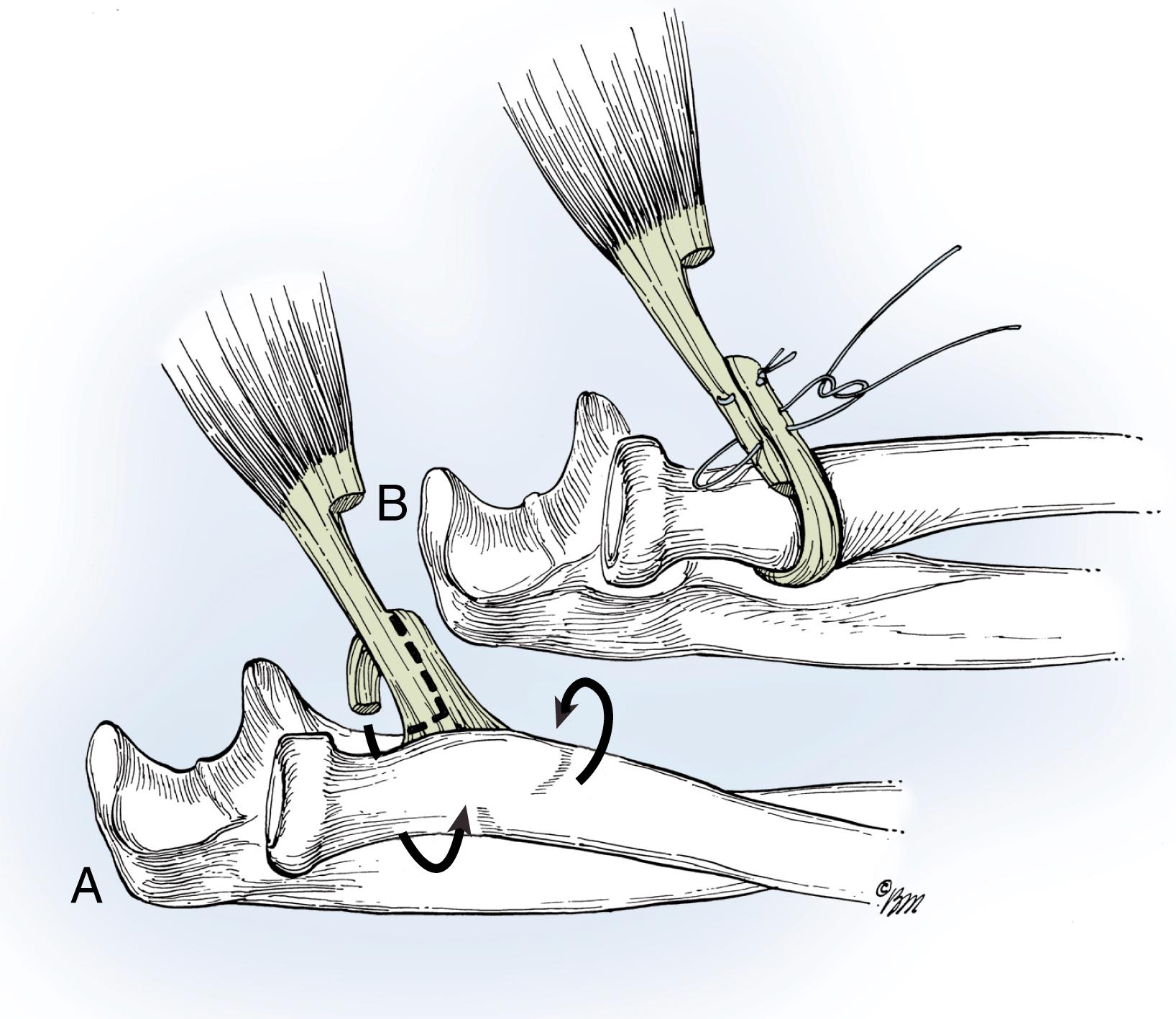
Zancolli produced pronation by converting the biceps into a forearm pronator. He rerouted the tendon around the radius, converting the biceps from a supinator to a pronator.
The biceps is rerouted from a supinator moment arm that wraps counterclockwise around the proximal radius to a pronator moment arm by wrapping the tendon clockwise around the proximal radius ( Fig. 33.14 ). This procedure can be done in isolation or in combination with one of the hand reconstructive procedures listed below. Indications for surgery are passive mobility of the forearm into pronation, without active pronation, as well as a biceps tendon of grade 5 strength that is a deforming force as a supinator leading to dysfunctional supination posturing. The biceps needs to be available for transfer, but not for transfer to the triceps to provide elbow extension strength.
The patient is placed supine on the operating room table and the entire extremity is prepped and draped. A sterile circular tourniquet that exsanguinates during application is applied (HemaClear, OHK Medical Devises, Grandville, MI). An S-shaped incision is made along the medial arm, across the antecubital fossa, and over the BR muscle ( Fig. 33.15A ). The lateral antebrachial cutaneous nerve is identified and protected lateral to the biceps tendon (see Fig. 33.15B ). The biceps tendon is traced to its insertion into the radial tuberosity (see Fig. 33.15C ). There are large crossing veins that require ligation. The lacertus fibrosis is incised from the biceps tendon protecting the underlying neurovascular structures. The entire length of the biceps tendon is isolated. A long Z-plasty is performed leaving one limb left attached to the radius and the other limb attached to the muscle (see Fig. 33.15D ). The limb attached to the radius is rerouted around the radius through the interosseous space to yield a pronation force. A curved clamp, such as a Castaneda pediatric clamp (Pilling Surgical, NC), facilitates tendon passage (see Fig. 33.15E ). The posterior interosseous nerve must be protected to prevent iatrogenic injury.
The elbow is positioned in 90-degrees of flexion with the forearm in pronation. The rerouted distal tendon is woven back through the proximal tendon that remained attached to the biceps muscle (see Fig. 33.15F ). The subcutaneous tissue and skin are closed in routine fashion. A long-arm cast is applied with the elbow in 90-degrees of flexion and the forearm in pronation for 5 weeks.
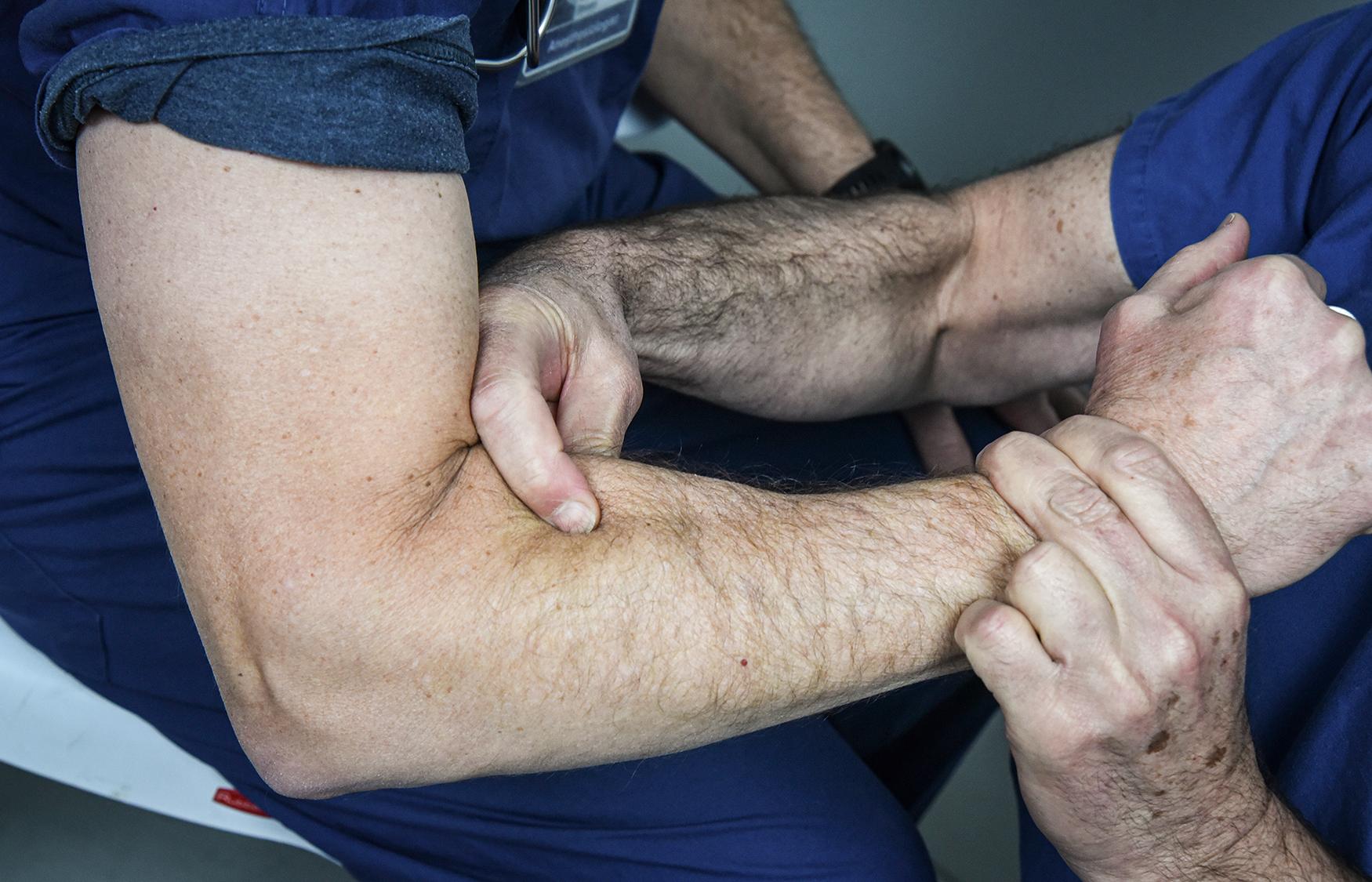
By definition, patients in this group have no muscle groups below the elbow with grade 4 strength to provide restoration of pinch function. Elbow extension transfers should be performed as described earlier. Some group 0 patients may have both a weak BR and a weak ECRL. Assessing the strength of the BR muscle is difficult and requires an experienced examiner. Examination is best performed by having the patient flex the elbow against resistance with the forearm in neutral position while palpitating the BR muscle belly and assessing its strength and turgor ( Fig. 33.16 ). Transfer of the BR to the radial wrist extensors may provide sufficient strength to extend the wrist against resistance. This tendon transfer technique is described later for group 1 patients. Because the muscle’s strength is no better than grade 3, the patient needs good elbow extension to serve as an antagonist to the BR transfer to enhance wrist extension function. If sufficient wrist extensor strength is obtained, a wrist-driven hinged tenodesis splint can be used ( Fig. 33.17 ), or a passive pinch tenodesis reconstruction can be considered.
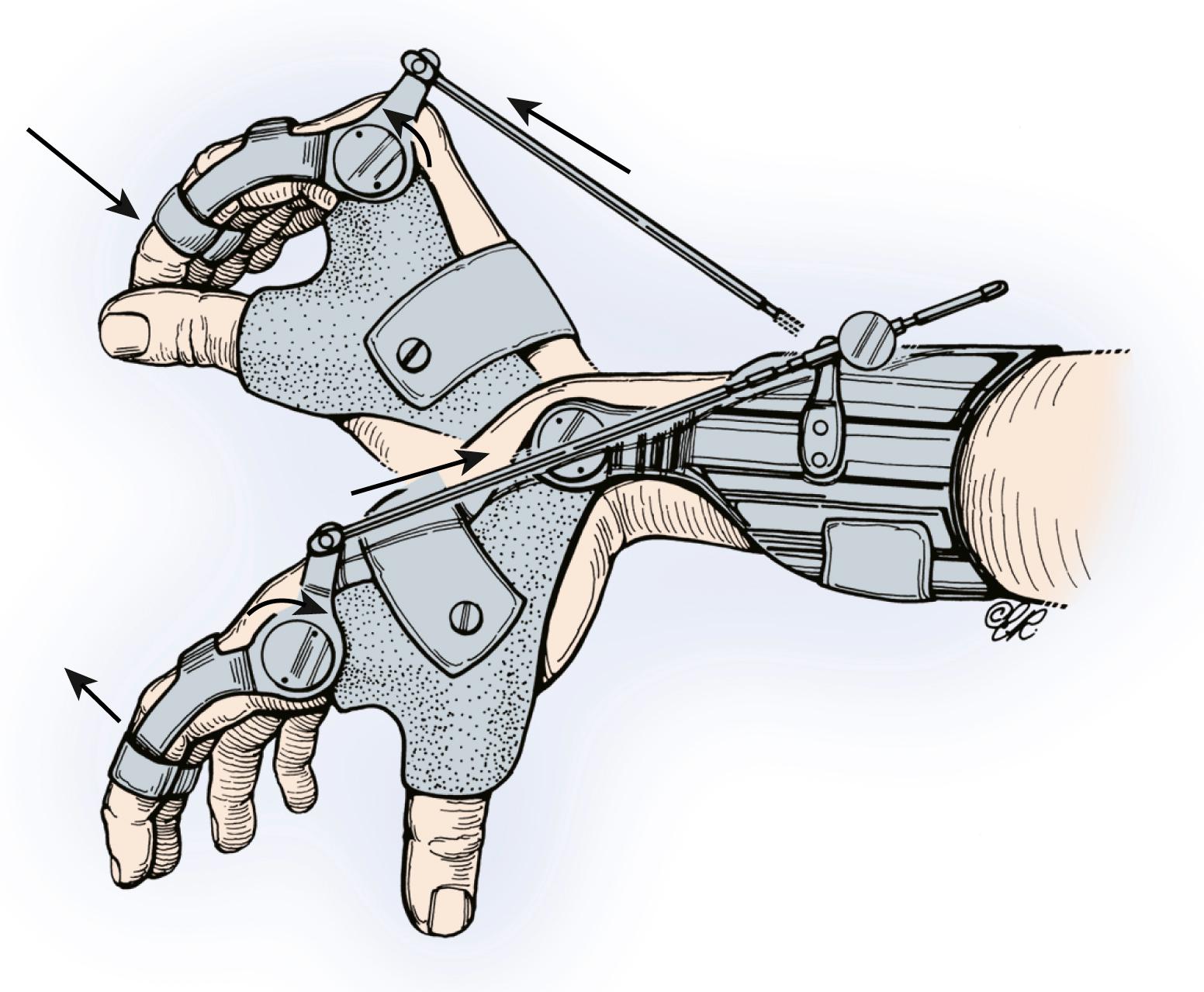
In group 1 patients, the BR has grade 4 or greater strength, and all other muscle groups below the elbow have less than grade 4 strength. Elbow flexion is provided by the biceps and brachialis (innervated above the level of the BR), which makes the BR is available for tendon transfer to provide wrist extension (the most necessary according to the hierarchy of hand function reconstruction ladder). Lateral key pinch can be provided by tenodesis using a wrist-driven tenodesis splint (see Fig. 33.17 ) or by surgical reconstruction. If a tenodesis splint is not used, two procedures are necessary for group 1 patients, and both can be done simultaneously: BR to ECRB tendon transfer for active wrist extension and passive key pinch reconstruction.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here