Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Q13.1 Concerning prednisone and cortisone, what is (1) the active form of each drug, (2) the enzyme necessary for this conversion, and (3) the effect of severe liver disease on this conversion? (Pgs. 134, 135)
Q13.2 What are ‘physiologic dose’ levels for (1) prednisone, (2) prednisolone, (3) dexamethasone, (4) methylprednisolone, and (5) triamcinolone? ( Table 13.1 , Pg. 151)
Q13.3 What are several cells that systemic corticosteroid (CS) can induce to undergo apoptosis, and the disease states that may logically be successfully treated because of this apoptosis? ( Table 13.2 , Pgs. 136, 139x2)
Q13.4 Compared with other uses of systemic CS, how do the dose timing, dosing strategy, and drug choice differ when treating patients with androgen excess disorders of adrenal origin? (Pg. 141)
Q13.5 What are several pros and cons of intramuscular (IM) CS injections versus oral dosing? What are several dermatoses for which IM Kenalog has the best risk:benefit ratio and overall logic of use? ( Table 13.5 , Box 13.4 , Pg. 141)
Q13.6 What are the two key issues that determine the rapidity of systemic CS dose tapering? Which of these two key issues matters primarily when dosing is below ‘physiologic’ dose levels? ( Box 13.8 , Box 13.9 , Table 13.14 , Pgs. 142, 149, 151x2)
Q13.7 What are several of the most important adverse effects of systemic CS that can lead to significant, irreversible morbidity (as well as measures to prevent/monitor for these complications)? How do patient comorbidities impact the risk of these conditions? ( Table 13.7 , Pgs. 142, 144)
Q13.8 What are at least four to five of the potentially life-threatening complications of systemic CS, and what are some of the important measures to prevent and/or monitor for these complications? ( Table 13.8 , Pg. 143)
Q13.9 Concerning response to physical stressors, which part of the hypothalamic-pituitary-adrenal (HPA) axis is (1) quickest to be suppressed, (2) quickest to recover, and (3) overall most important to stress responsiveness? (Pg. 148)
Q13.10 What are some of the ‘back-up’ mechanisms the body has to minimize the likelihood of CS-induced HPA-axis suppression? (Pgs. 149x2)
Q13.11 What are some general principles (1) to maximize CS safety ( Box 13.8 ) , (2) to taper CS therapy ( Box 13.9 ) , and (3) to convert CS to alternate-day dosing ( Table 13.14 ) ? What two criteria should be met before making this conversion? (Pgs. 151, 154x2)
Q13.12 What are two to three adverse effects of systemic CS not decreased by comparable doses of systemic CS using alternate-day therapy? (Pg. 154)
Adrenocorticotropic hormone (corticotropin)
Adverse effects/events
Activating protein-1
Bullous pemphigoid antigen-2
Cortisol-binding globulin
Congestive heart failure
Confidence interval (95% CI)
Corticotropin-releasing factor
Corticosteroid
Dual energy X-ray absorptiometry
Dehydroepiandrosterone-sulfate
Glucocorticoid receptor
Hypothalamic-pituitary-adrenal (axis)
Interferon
Inhibitor kappa B
Interleukin
Intramuscular
Intravenous
Mineralocorticoid
Nuclear factor kappa B
Nonsteroidal anti-inflammatory drug
Purified protein derivative
Peptic ulcer disease
Relative risk
Selective glucocorticoid receptor agonist(s)
Stevens–Johnson syndrome
Systemic lupus erythematosus
(Cortico-)Steroid withdrawal syndrome
Toxic epidermal necrolysis
Tumor necrosis factor
Kendall described compound E (cortisone) in 1935. A Mayo Clinic group first described the use of cortisone and adrenocorticotropic hormone (ACTH) in patients with rheumatoid arthritis in 1948. In 1950, Hench and colleagues presented the first report concerning the basic effects and toxicities of corticosteroids (CS).
Sulzberger and associates’ 1951 report described the use of cortisone and ACTH in a variety of inflammatory dermatoses. This report on CS radically changed the therapeutic approach of dermatologists. As the list of CS-responsive dermatoses grew, so did the list of potential adverse effects (AE). In 1961, Reichling and Kligman suggested alternate-day CS use. This was an important step toward reducing AE and yet maintaining significant anti-inflammatory effects over the 48-hour period between doses.
Two major advances in CS therapy occurred during the 1970s and 1980s. Adjunctive therapy with immunosuppressive drugs, such as azathioprine and cyclophosphamide, has been used increasingly to attain a ‘CS-sparing’ effect. These adjunctive drugs allow lower CS doses to be used, which lessens the risk of serious AE while maintaining an adequate immunosuppressive effect. High-dose pulse intravenous (IV) methylprednisolone therapy was increasingly used through the 1980s. Quicker remission with lower risk from therapy is the goal of this method of CS administration. In the 1990s to today, further recognition of efficacy and safety of low-dose CS has led to the primary use today as a ‘bridge’ to alternative CS-sparing treatments.
One population study estimated that 0.5% of patients (prevalence) in industrialized countries with a total population of 1.2 billion are on at least 3 months of continuous CS. Another study estimated that 32 million prescriptions for systemic CS are written annually in the United States. In addition to the cost of AE of CS, the striking frequency of CS use in these countries sets the stage for chance overlap of two relatively frequent events (CS use plus a common AE).
This chapter covers the pharmacology and clinical use of systemic CS. Particular attention is given to the risks of systemic CS, especially focusing on measures to minimize the risk of these important complications. The physician who is thoroughly familiar with these measures will use systemic CS more comfortably and wisely, to the benefit of many grateful patients.
This chapter includes special sections covering the following areas: (1) normal hypothalamic-pituitary-adrenal (HPA)-axis function; (2) intramuscular (IM) CS administration; (3) pulse IV CS administration; (4) HPA-axis suppression; and (5) therapeutic guidelines for safe and effective CS use.
The basic structure of all CS consists of three hexane rings and one pentane ring. The combined ring structure is known as the cyclopentanoperhydrophenanthrene nucleus. The rings are designated by letters A, B, C, and D, and each carbon is assigned a number from 1 to 21 ( Fig. 13.1 ). The designations alpha (α, away from the CS receptor) and beta (β, toward the CS receptor) refer to the position of any added molecules in reference to the stereochemical plane.
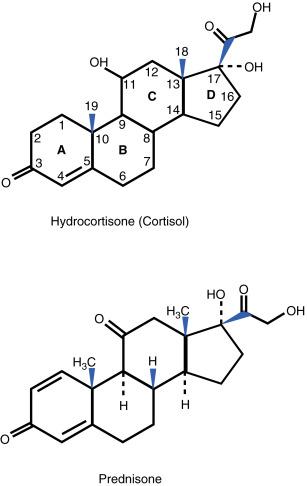
Q13.1 Cortisone and cortisol (hydrocortisone) both possess a 4,5 double bond and a ketone (carbonyl) group at the 3 position. Cortisone, which is an inactive form, has a ketone at the 11 position. The active form, cortisol (hydrocortisone), is formed through hepatic conversion of the 11-ketone to an 11-hydroxyl group catalyzed by 11β hydroxysteroid dehydrogenase. Of note, topical CS must have an 11-hydroxyl group to be effective. The addition of a 1,2 double bond results in increased glucocorticoid activity and decreased rate of metabolic degradation. This results in prednisone (with an 11-ketone group), and through 11-hydroxylation, the active analog prednisolone is formed; with 11β hydroxysteroid dehydrogenase catalyzing this conversion as well. Methylprednisolone is formed through the addition of a 6-methyl group to prednisolone, which leads to slightly increased glucocorticoid potency.
The addition of fluorine to hydrocortisone at the 9-α position leads to increased glucocorticoid, but significant mineralocorticoid (MC) activity. The resulting compound is 9-α fluorohydrocortisone (fludrocortisone). This compound is the basic structure of most fluorinated topical CS. In general, the MC effect is decreased in topical CS by the masking of the 16- or the 17-hydroxyl group with various ester links to side chains (e.g., acetonide, valerate, propionate, dipropionate).
With systemic CS, 9-α fluorohydrocortisone with an added 1,2 double bond is also modified further. By adding a 16-α hydroxyl group (triamcinolone), a 16-α methyl group (dexamethasone), or a 16-β methyl group (betamethasone), three compounds with high glucocorticoid and low MC effects are formed. Because all three drugs have an 11-hydroxyl group, these three compounds are in biologically active forms (without requiring 11β-hydroxysteroid dehydrogenase). All CS have a hydroxyl group at the 17 position (17-hydroxycorticosteroids). Androgenic steroid compounds have a 17-ketone group and a 19-carbon basic ring structure (17-ketosteroids).
Exogenous CS are absorbed in the upper jejunum. More than 50% of prednisone is absorbed. Food delays, but does not reduce, the amount of prednisone absorbed. Peak plasma levels are reached 30 to 100 minutes after the drug is taken.
The primary endogenous carrier protein is cortisol-binding globulin (CBG, transcortin). Overall, 80% to 90% of endogenous cortisol is bound to CBG; the free fraction represents the active form. CBG is a low-capacity, high-affinity binding system. Albumin (CS-binding albumin) represents a low-affinity, high-capacity binding reserve. The avidity with which synthetic CS bind to these carrier proteins is less than the avidity with which endogenous cortisol is bound. Thus, for synthetic CS a greater free fraction is available. Prednisolone is reported to bind to carrier proteins with greater affinity than other synthetic forms, with resultant potential for displacement of endogenous cortisol from the protein-binding sites. There are several systemic conditions which alter the levels of CBG ( Box 13.1 ). Generally, low protein states increase the risk of AE secondary to an increased level of free CS. Additionally, high-dose therapy and prolonged CS treatment results in a greater proportion of free CS in the body.
∗result in increased amount of endogenous and synthetic corticosteroid (CS) free fraction
Hypothyroidism
Liver disease
Renal disease
Obesity
∗result in decreased amount of endogenous and synthetic CS free fraction
Estrogen therapy
Pregnancy
Hyperthyroidism
Overall, CS are widely distributed to most body tissues. All endogenous and synthetic CS are well distributed into fetal tissue, with the exception of prednisone.
Q13.1 Of importance is that the action of 11β hydroxysteroid dehydrogenase in the liver is necessary to convert cortisone to cortisol (hydrocortisone), and to convert prednisone to prednisolone. The type I isoform of 11β hydroxysteroid dehydrogenase catalyzes conversion of endogenous cortisone (inactive) to cortisol (active), and converts exogenous prednisone (inactive) to prednisolone (active). The type II isoform of the above enzyme reverses this conversion, reverting back to the inactive forms. Type II isoform is found in mineralocorticoid tissues. The relative presence of these isoforms can determine the likelihood of (1) inflammatory and autoimmune dermatoses, and (2) response to exogenous prednisone administration. It is important to note that dexamethasone and betamethasone are active independently of this enzyme.
Of these four CS, only cortisol and prednisolone are biologically active. Severe liver disease may impair this conversion, although the traditional teaching to use the active form of these drugs (such as prednisolone) with significant liver disease may be just part of this ‘story’ (plus the liver has tremendous ‘reserve’). Regardless, the administration of prednisolone (rather than prednisone) to patients with advanced liver disease would be appropriate. In addition, liver disease may result in decreased serum albumin, which would increase the free fraction of CS as discussed above.
The plasma half-lives of the various synthetic CS do not correlate well with the duration of biologic activity (see Table 13.1 ). A much more important measure of duration of activity is the duration of ACTH suppression after the administration of a single dose of a given CS. They are generally divided into short, intermediate, and long-acting. This duration of activity correlates well with glucocorticoid and anti-inflammatory effects. There is an inverse correlation between the duration of action and the relative MC effect of the various CS.
| Corticosteroid | Equivalent Dose (mg) Physiologic Dose | Glucocorticoid Potency a | MC Potency | Plasma Half-Life (Min) | Biologic Half-Life (H) |
|---|---|---|---|---|---|
| Short-Acting | |||||
| Cortisone | 25 | 0.8 | 2 + | 30–90 | 8–12 |
| Cortisol (hydrocortisone) | 20 | 1 | 2 + | 60–120 | 8–12 |
| Intermediate-Acting | |||||
| Prednisone | 5 | 4 | 1 + | 60 | 24–36 |
| Prednisolone | 5 | 4 | 1 + | 115–212 | 24–36 |
| Methylprednisolone | 4 | 5 | 0 | 180 | 24–36 |
| Triamcinolone | 4 | 5 | 0 | 78–188 | 24–36 |
| Long-Acting | |||||
| Dexamethasone | 0.75 | 2–-30 | 0 | 100–300 | 36–72 |
| Betamethasone | 0.6–0.75 | 20–30 | 0 | 100–300 | 36–72 |
a Glucocorticoid potency is expressed in a relative scale without specific units of measure; this relative potency number is inversely related to the equivalent dose in the first column.
Q13.3 The most important immunosuppressive and anti-inflammatory effects of systemic CS are listed in Table 13.2 . In addition, the mechanisms responsible for the most important CS AE are listed in later in the chapter in Table 13.7 . The interested reader should take sufficient time to thoroughly understand these two tables in order to have a broad understanding of the overall benefits and risks of systemic CS. There will next be a concise discussion on normal HPA-axis function, glucocorticoid effects, and MC effects. This section concludes with relatively new information on glucocorticoid receptors (GCR), CS resistance and tachyphylaxis, transcription factors, and apoptosis. The reader is encouraged to pursue reviews concerning newer scientific data on the CS mechanisms of action.
| Corticosteroid Action | Mechanism and Biologic Result |
|---|---|
| Effects On Glucocorticoid Receptor (GCR) | |
| Normal response | Upon ligand (CS) binding, the GCR is activated and translocates to the nucleus, binding to glucocorticoid-response elements of multiple genes. CS can function as an agonist or antagonist for these genes—GCR is ubiquitous throughout body. |
| Transcription Factor Effects | |
| NFκB inhibition | Increased IκB production, direct NFκB binding; net result ↓ production of multiple cytokines such as IL-1, TNF-α, adhesion molecules, growth factors, etc. |
| AP-1 inhibition | Decreased production of multiple cytokines; similar cytokine spectrum to NFκB. |
| Apoptosis Induction | |
| Lymphocyte apoptosis | Apoptosis of autoreactive T cells (in autoimmune disorders) and neoplastic T cells (in various lymphomas); AP-1 and caspase cascade probably involved in process. |
| Eosinophil apoptosis | Apoptosis of eosinophils with potential implications for various allergic disorders. |
| Signal Transduction | |
| Phospholipase A2 inhibition | CS effect probably mediated indirectly via ↑ lipocortin-1 (now called ‘annexin’). |
| ↓ ‘Downstream’ eicosanoids | As a result of phospholipase A inhibition, ↓ production of various prostaglandins, leukotrienes, 12-HETE and 15-HETE inflammatory mediators. |
| Cyclo-oxygenase 2 (COX-2) inhibition | ↓ Eicosanoid production generated by this inducible (with inflammation) enzyme; CS effect on COX-2 >> COX-1. |
| Effects on Various WBC Subsets and Other Immunologic Cells | |
| B cells | With higher CS doses significant B-cell effect, reduced immunoglobulin production. |
| T cells | Greater CS effect on T cells (CD4 > CD8 effect) at lower doses compared with above B-cell effect; net result ↑ Tregs and ↓ IL-2 production and resultant amplification effect. |
| Other lymphocyte subsets | ↓ Natural killer (NK) cell activity, ↓ antibody-dependent cellular cytotoxicity mediated by K cells. |
| PMN | ↓ PMN marginization, ↓ chemotaxis, small effect on microbicidal respiratory burst; also ↓ apoptosis of PMN (in contrast with T cells and eosinophils above). |
| Mast cells | Inhibit degranulation, with resultant ↓ release histamine, kinins, other mediators. |
| Monocytes, macrophages | ↓ Monocyte maturation; ↓ access to inflammatory sites, ↓ IL-1 and IFN-γ release. |
| Langerhans cells | ↓ Characteristic surface markers, impaired antigen processing and presentation. |
| Eosinophils, basophils | Reduced numbers and function both cell types, ↓ recruitment to inflammatory sites. |
| Fibroblasts | ↓ Production of collagen, ground substance, fibronectin and collagenase. |
| Membrane stabilization | Both lysosomal and cell membrane stabilization; probable role in mast cell, PMN, other inflammatory cell effects. |
| Bottom line generalizations | CS overall effects—cell trafficking > cellular function; cellular immunity > humoral immunity; major portion of effects mediated via above cytokine alterations. |
| Vascular Effects | |
| Angiogenesis | ↓ Angiogenesis in wound healing and with proliferative lesions (hemangiomas). |
| Vasoconstriction | Net result of vasocortin and vasoregulin, potentiate response to catecholamines. |
| Decreased permeability | Decreased vascular smooth muscle response to histamine and bradykinin. |
It is important to understand the normal function of the HPA axis in order to better understand the potential for HPA-axis suppression by synthetic CS. For further background information, several reviews are particularly helpful.
The primary stimulus for release of endogenous cortisol originates in the hypothalamus. The tropic hormone is known as corticotropin-releasing factor (CRF). ACTH is subsequently released by the anterior pituitary. ACTH is produced from the prohormone pro-ACTH/endorphin. There are approximately 10 bursts of ACTH release throughout the day. The greatest frequency of these bursts occurs in the early morning hours during a normal sleep cycle. The zona fasciculata of the adrenal cortex is then stimulated to produce and release cortisol. ACTH also stimulates adrenal androgen synthesis. However, ACTH is not significantly involved in the release of the MC aldosterone.
| Glucocorticoid Effects a | MC Effects b |
|---|---|
| Glucose Metabolism | Aldosterone Effects—Endogenous |
| Gluconeogenesis at expense of protein catabolism | Major effect is sodium and water retention |
| Peripheral insulin resistance—reduced glucose entry into cells | This effect is primarily at proximal tubule in kidney |
| Glycogen storage in liver | Potassium is excreted in exchange sodium at this site |
| Lipid Metabolism | Corticosteroid Effects—Exogenous |
| Lipolysis releasing triglycerides as source of ‘energy’ | Endogenous/exogenous cortisol significant MC effects |
| Fat redistribution to central locations | See Table 13.1 for MC effect of various CS |
| Regulation Of Above Processes | Regulation of Endogenous Aldosterone |
| ACTH (pituitary) induces release of cortisol (adrenal) | ACTH has no role in aldosterone production |
| Negative feedback loop to hypothalamus (site of CRF production) | Regulation primarily by renin–angiotensin, potassium |
a Conceptually, all the glucocorticoid effects are prioritized to maintain brain glucose homeostasis.
b Major priority for MC is to maintain sodium and fluid homeostasis, including a normal blood pressure.
There are three main controls of endogenous cortisol production. These are discussed in the summary ‘HPA axis in a nutshell’ ( Box 13.2 ).
Hypothalamus—corticotrophin-releasing factor (CRF)
Pituitary (anterior)—adrenocorticotropic hormone (ACTH)
Adrenal—cortisol (same as hydrocortisone)
Basal production of cortisol —20–30 mg daily
Basal production in prednisone equivalents —5–7.5 mg daily
Maximal stress production of cortisol —300 mg daily
Maximal stress production in prednisone equivalents —75 mg daily
‘Minor stress’ production of cortisol—probably two to three times basal production
Hypothalamus—first to be suppressed, first to recover full function; is most critical component for adequate stress responsiveness
Adrenal—slower to be suppressed, much slower to recover full function
Circadian variations—CRF (and thus ACTH) have innate diurnal variations tied to sleep cycle (highest production mid-sleep, lowest late afternoon)
Negative feedback—increased cortisol levels reduce CRF and ACTH production
Stress response—increased CRF release and subsequently ACTH release
CRF alternate sites of production—cerebral cortex and limbic system, which can be released by acetylcholine and serotonin
Alternative inducers of ACTH release—catecholamines, vasopressin
All the above means serve to maintain glucose homeostasis
CS plays an important teleologic role in maintaining adequate blood glucose levels for brain function. Gluconeogenesis generates glucose at the expense of amino acids derived from endogenous proteins. CS also produces peripheral insulin resistance, which impedes glucose absorption by various body tissues. In addition, glycogen storage in the liver is enhanced. Lipid stores are stimulated to undergo lipolysis, generating increased amounts of triglycerides from which to derive energy.
The net effect is a catabolic state that produces carbohydrates at the expense of protein and fat stores. Through gluconeogenesis, proteins from muscle, trabecular bone (especially vertebral and hip), dermal connective tissue, and vascular proteins are metabolized. Lipolysis results in triglyceride release, with additional fat redistribution (lipodystrophy) to body sites that are characteristic of the habitus for Cushing syndrome.
Aldosterone is the primary endogenous MC hormone. The primary aldosterone effect is sodium reabsorption and resultant water reabsorption at the proximal tubule site in the kidneys. Sodium is exchanged for potassium, which leads to hypokalemia when there is excessive MC effect. ACTH has no direct control on MC production. The primary MC control mechanisms are through the renin–angiotensin system and serum potassium levels. CS with significant MC effects (such as hydrocortisone) have a similar effect on sodium, potassium, and fluid balance as aldosterone ( Table 13.3 ). Long-acting CS (such as dexamethasone and betamethasone) have essentially no MC effect (see Table 13.1 ).
There is only one GCR, but a diverse collection of isoforms, which accounts for endogenous glucocorticoid effects as well as the pharmacological effects of synthetic CS (resulting in both beneficial and AE). This cytosolic receptor is expressed throughout tissues, but there is considerable heterogeneity in sensitivity and biological responses. The GCR functions directly as a transcription factor, which upon translocation to the nucleus binds directly to various glucocorticoid responsive elements of multiple genes in DNA. In addition, the ligand–GCR complex can activate other transcription factors, as detailed in the next section. CS exerts its effects through both genomic and nongenomic actions.
Splice variants, translational isoforms, posttranslational modifications, and polymorphisms give a molecular basis for sensitivity of glucocorticoid responsiveness. There are rare cases of hereditary glucocorticoid resistance, in which there are mutations in the GCR gene. In clinical practice, relative resistance at the GCR in otherwise healthy individuals is much more common than previously recognized. In addition, relative resistance is because of altered CS bioavailability, altered ligand binding to GCR, or altered translocation of the activated GCR complex to the nucleus. Conceptually, this resistance could represent a negative feedback system of sorts, with downregulation of GCR after prolonged or high-dose CS therapy.
There are two well-described transcription factors with a central role in amplification of the inflammatory response ( Fig. 13.2 ). These transcription factors are nuclear factor kappa B (NFκB) and AP-1. NFκB is biologically inactive as long as it is bound to inhibitor kappa B (IκB). Free NFκB translocates to the nucleus, where it induces transcription of numerous cytokines including (1) ‘immunoawakening’ cytokines (interleukin [IL]-1β, tumor necrosis factor [TNF]-α), (2) ‘immunomodulatory’ cytokines (IL-2, IL-8), (3) growth factors, (granulocyte-colony stimulating factor [G-CSF], granulocyte-macrophage colony-stimulating factor [GM-CSF]), (4) adhesion molecules (intercellular adhesion molecule 1 [ICAM-1], E-selectin), (5) receptors (IL-2 receptor), and (6) proinflammatory enzymes (cyclooxygenase-2 [COX-2], phospholipase A 2 ).
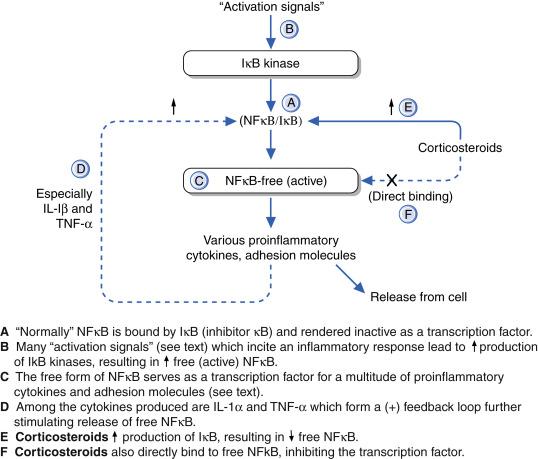
CS reduce the effects of NFκB in two ways. The CS-GCR complex leads to increased IκB formation, leading to subsequent NFκB binding by this inhibitory protein. The GCR/CS complex can also directly bind to NFκB, thus inhibiting this transcription factor. By either means, there can be a dramatic reduction of a wide variety of components of the inflammatory response via this mechanism of CS.
AP-1 consists of either c-jun homodimers or c-jun/c-fos heterodimers, which bind to a common deoxyribonucleic acid (DNA) site, the AP-1 binding site. There is tremendous overlap with the inflammatory response genes induced by AP-1 and NFκB.
In general, transrepression by GCR/CS complex of proinflammatory cytokines (such as IL-1β, TNF-α, interferon regulatory factor 3 [IRF-3]) accounts for most of anti-inflammatory effects of CS. IRF-3 is a member of the interferon regulatory transcription factor family and plays an important role in the innate immune system. In contrast, transactivation by the GCR/CS complex of various regulatory proteins is responsible for most CS AE (such as diabetes, glaucoma). As such, selective glucocorticoid receptor agonist(s) (SEGRA) that favor transrepression were developed as therapeutic agents with reduced AE. However, more recent data show that the transactivation potential of GR is indispensable for its anti-inflammatory properties.
Apoptosis is an orderly process of programmed cell death. The process is a biologically active, noninflammatory sequence of cellular changes that occur with an intact plasma membrane despite nuclear fragmentation. Q13.3 CS can directly induce apoptosis in lymphocytes and eosinophils. CS can induce apoptosis at least in part through downregulation of the CD3 molecule of T cells; this molecule plays an important role in T-cell activation. There can also be an indirect effect on lymphocytes and eosinophils through CS-induced suppression of cytokines essential to cellular survival.
The logical application of these facts is an underlying explanation for CS effects in autoimmune disorders (apoptosis of autoreactive T cells), allergic disorders (apoptosis of eosinophils), and certain neoplastic disorders (apoptosis of malignant T cells). It is doubtful that the ability of CS to induce apoptosis is limited to these cell types.
Q13.3 Many of the common CS-responsive dermatoses are listed in Box 13.3 . A few disorders are discussed selectively to illustrate various principles of CS therapy. Reference citations for many other dermatoses not discussed specifically in the text are listed in Box 13.3 as well.
Cutaneous
Systemic
Pyoderma gangrenosum
Behçet disease/aphthous ulcers
Sweet syndrome
Contact dermatitis
Atopic dermatitis
Exfoliative erythroderma
Lichen planus
DRESS syndrome
Sarcoidosis
Sunburn or photodermatitis
Urticaria (severe a )
Androgen excess (acne/hirsutism)
Prevention of isotretinoin-induced acne fulminans
Overall, well-controlled studies of CS use in the following dermatoses are quite uncommon. In clinical practice, it is actually quite easy in most cases to have a relatively high level of certainty about the benefits of CS in an individual patient through tapering the dose (the disease flares) and cautiously raising the dose (disease control returns). Definitions of importance to both the Clinical Use and Therapeutic Guidelines sections are listed in Table 13.4 .
| Dosing Level Definitions | |
| Decrement | Amount of reduction in CS dose—either a fixed percentage or fixed interval decrease |
| Increment | Amount of increase in CS dose, guided by the urgency to attain disease control |
| Induction | Initial CS dose focused on quickly attaining disease control |
| Maintenance | Relatively constant dose of CS to maintain disease control attained by induction dose |
| Minimal effective | The lowest dose of CS just adequate to almost completely control the disease process |
| Pharmacologic | Generally considered to be any dose above physiologic levels (see text) |
| Physiologic | Dose of exogenous CS which is similar to the quantity of endogenous CS produced |
| Replacement | Is a synonym of physiologic dose—term also used to refer to endogenous MC levels |
| Supraphysiologic | Is a synonym of pharmacologic dose |
| Tapering | Any effort to reduce the CS dose, given that reasonable disease control is attained |
| Dosing Frequency or Duration Definitions | |
| Alternate-day | CS doses given every other day—result is an ‘on’ day and an ‘off’ day |
| Burst | Short course of CS (generally 2–3 weeks or less) to control self-limited disease |
| Consolidation | Change from a divided dose to a single daily dose without changing the daily dose; necessary step before tapering |
| Divided | Any dosing frequency which is more frequent than daily dosing; usually BID or QID |
| ‘Off’ day | With alternate-day therapy, day in which CS is omitted (or lower dose is given) |
| ‘On’ day | With alternate-day therapy, day in which CS is administered (or higher dose is given) |
| Pulse | Usually represents a very brief course (5–7 days) of very high dose (10–15 mg/kg per day) of intravenous methylprednisolone (see text for other usage of this term) |
The best-studied purely dermatologic indication for systemic CS therapy is pemphigus vulgaris. The emphasis here is high-dose CS therapy, with use of adjunctive ‘steroid-sparing’ immunosuppressive therapy. CS are appropriate at the start of therapy for any relatively severe case of pemphigus vulgaris that has no absolute contraindications to CS use. Adjunctive therapy is generally used with a choice of azathioprine, mycophenolate mofetil, methotrexate, cyclosporine, cyclophosphamide, rituximab, intravenous immune globulin (IVIg), or plasmapheresis. Anti-inflammatory antibiotics, such as the tetracyclines, as well as CS-sparing immunosuppressive medications have been used for milder cases of pemphigus vulgaris and for pemphigus foliaceus. Pulse methylprednisolone as well as rituximab are other options, which may be indicated to attain rapid disease control in more severe cases of pemphigus vulgaris.
Current management includes prednisone doses no greater than 2 mg/kg daily in divided doses. In general, it is reasonable to start prednisone at 1 to 1.5 mg/kg daily, increasing to the above dose range very selectively as indicated for more severe cases of pemphigus vulgaris. Disease control is usually attained by 4 to 6 weeks. Given this adequate disease control, the divided dose should be consolidated into a single daily dose and tapered rapidly to the 40-mg daily range. Azathioprine or related immunosuppressive drugs can be added at the time of prednisone tapering in many cases. For more severe cases it is wise to add the ‘CS-sparing’ agent at the start of therapy. Management of oral involvement needs to be reasonably aggressive, both to limit progression to more serious cutaneous involvement and to maintain adequate fluid and nutrition intake. Juvenile pemphigus vulgaris is overall similar to that just described, with CS doses adjusted for body weight. The challenge of managing paraneoplastic pemphigus has been reviewed by Anhalt.
In patients with bullous pemphigoid, moderate doses of CS up to 1 mg/kg daily are used. The CS course is typically given for a defined duration of time (generally 3–6 months or less), ideally achieving a physiologic dose range within 1 to 2 months. Nonsteroidal immunosuppressive drugs (‘CS-sparing’ drugs) should be the mainstay of therapy if the disease persists beyond this time. As with most indications for systemic CS, randomized controlled trials are few in number. One such trial noted that topical CS was overall equivalent to systemic CS for patients with bullous pemphigoid. It is indeed counterintuitive that patients with widespread intact blisters could benefit from topical CS alone: experience dictates that systemic CS and appropriate ‘CS-sparing’ measures are still of central importance in this setting.
In general, 60 to 80 mg daily (∼1 mg/kg daily) of prednisone in divided doses is successful in eliminating new blister formation within several weeks. Should the patient not respond to this dose, or require a high maintenance dose, adjunctive immunosuppressive therapy can be added. In the absence of new blisters over 5 to 7 days, the prednisone dose can be gradually tapered. Contrary to prior reports, Schmidt and coworkers reported that disease activity can be successfully monitored by following bullous pemphigoid antigen (BPAg2) 180 titers. Deaths still occasionally occur in older patients with more extensive involvement; more conservative management using alternatives to CS may help lessen the risk of sepsis in this age group.
There is still significant controversy regarding the use of systemic CS for the spectrum of Stevens–Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN). Trends toward the use of cyclosporine for these patients has taken some of the heat off the debate. A recent meta-analysis and review of 96 studies found CS and cyclosporine were the most promising systemic immunomodulating therapies for SJS/TEN, although all analyses had limitations. A significant number of studies support routine CS therapy. A majority of studies, however, present data supporting routine use of burn unit care in the absence of systemic CS therapy. These studies report a higher fatality rate, particularly from sepsis, in CS-treated patients, than in patients managed in a burn unit without CS therapy.
Proponents of routine systemic CS use suggest that for SJS and TEN patients, systemic CS treatment early in the disease course (before significant sloughing of skin), followed by rapid tapering of CS, may be beneficial and even life saving. After widespread sloughing occurs (>10% of total surface area), the risk of infection clearly outweighs the potential CS benefits. Of importance is that drug and infectious precipitators be sought and eliminated if possible. Should CS therapy be indicated, up to 2 to 2.5 mg/kg daily of IV methylprednisolone in divided doses is generally used initially, with relatively rapid tapering to more moderate doses when new blister formation ceases.
Severe acute contact dermatitis caused by poison ivy/poison oak is a classic situation in which a 2- to 3-week burst of systemic CS therapy is usually successful at minimal risk to the patient. The potential for ‘rebound’ disease activity as a result of prednisone courses of less than 10 to 14 days is important to consider. Cases with widespread cutaneous involvement (or significant facial involvement) treated early in their course typically respond rapidly. Doses up to 1 mg/kg prednisone daily (generally 40–60 mg daily) tapered over 2 to 3 weeks yield adequate improvement with minimal risk of rebound flare after cessation of therapy. A simple approach that is easy for patients to follow uses just 20 mg prednisone tablets. The patient receives 5 days each of 60, 40, and 20 mg of prednisone. Various ‘dose packs’ of prednisone and methylprednisolone typically do not provide an adequate dose (to rapidly attain disease control) for an adequate duration (to avoid a rebound flare).
Acute flares of chronic atopic, nummular, or contact dermatitis can be managed in a similar fashion, although a recent systematic review did not find evidence strong enough to determine optimal delivery or duration of systemic CS in atopic dermatitis and discouraged their use because of short- and long-term AE and an unfavorable risk–benefit profile. Because of rebound flaring, use of CS should ideally be limited to short courses as a bridge to CS-sparing agents. Maintenance CS therapy is best avoided in these settings. Doses of prednisone significantly less than 1mg/kg daily will commonly suffice for acute flares of chronic dermatitis subsets.
Exfoliative erythroderma management commonly requires systemic CS therapy. Given that psoriasis has been excluded, exfoliative erythroderma refractory to aggressive topical management or to phototherapy may respond to prednisone up to 1 mg/kg daily. This dose is tapered rapidly to low-dose alternate-day therapy. In erythroderma patients, a low-dose daily or alternate-day therapy at or near physiologic levels for an additional 2 to 3 weeks may be required for patients to normalize the epidermal barrier function.
Q13.4 For hirsutism and recalcitrant acne vulgaris due to elevated adrenal androgens (most commonly mild dehydroepiandrosterone-sulfate [DHEA-S] elevations), a unique CS approach is often indicated. In these patients, night-time suppressive therapy with low-dose dexamethasone (below physiologic dose levels) is predictably successful. Most cases can be controlled with 0.125 to 0.375 mg of dexamethasone at bedtime. This timing is important to suppress the early morning peak of ACTH, which stimulates adrenal androgen production. A reasonable approach is to start with dexamethasone 0.125 mg nightly, with the repeat androgen laboratory test in 6 to 8 weeks. If the test result has not normalized, dose increases of 0.125 mg up to a maximum of 0.375 mg may be used, with follow-up DHEA-S 6 to 8 weeks after dose increments. For a more complete discussion on androgen excess syndromes, see Chapter 34 . Additionally, short-term CS therapy can be considered to prevent acne fulminans from flaring during initiation of isotretinoin for treatment of severe nodulocystic acne.
Another controversial area of systemic CS therapy is prevention of postherpetic neuralgia. A 2013 Cochrane review concluded that systemic CS is beneficial in treating acute pain related to herpes zoster infection, but does not prevent postherpetic neuralgia. It is reasonable to treat (1) patients with facial involvement, (2) patients with severe acute pain during the cutaneous eruption, and (3) patients over 55 to 60 years of age, using combined antiviral and CS therapy, ideally initially very early in the disease course. Although disseminated herpes zoster from CS therapy is a theoretical concern, this is a distinctly uncommon complication in patients with normal baseline immunity and simultaneous antiviral therapy. Recent evidence suggests that systemic CS may have a greater role in treating the acute pain of herpes zoster than for preventing postherpetic neuralgia.
Q13.5 Dermatologists have long held widely divergent viewpoints regarding the pros and cons of IM CS therapy. Table 13.5 attempts to summarize both sides of the ‘argument.’ In addition, the relatively unique complications of IM CS are listed in Box 13.4 .
| Issue | Oral Administration | Intramuscular Administration |
|---|---|---|
| Absorption | Reasonably predictable | Highly variable from patient to patient |
| Compliance | Variable based on patient reliability | Guaranteed that dose is administered |
| Duration of therapy | Any duration possible | Must use short-, intermediate-, and long-acting IM versions |
| Patient illness affecting dosing | Requires ‘cooperative’ GI tract | Can be given with nausea/vomiting |
| Patient participation in dosing | Requires active patient participation | Patient in a passive role |
| Physician level of control | Can vary the doses based on disease activity and adverse effects | Can be certain the patient received the medication |
| Reproduces diurnal variation | With a.m. dosing reproduces diurnal variation somewhat | Constant levels without diurnal variance |
| Tapering | Precise tapering possible | Gradual tapering as drug metabolized |
A pivotal point of debate is the effect of IM CS on the HPA axis. Kusama and associates detected HPA-axis suppression up to 3 to 4 weeks after each injection of triamcinolone acetonide, as measured by plasma cortisol and urine 17-hydroxycorticosteroids. Mikhail and colleagues studied patients receiving IM triamcinolone acetonide every 6 weeks for 1.5 to 5 years. Roughly half the patients had impaired response to the insulin hypoglycemia test. The authors noted that the interval between doses is a more important factor in HPA-axis suppression than the actual dose administered. Low-dose IM CS at 2 to 4-week intervals produced greater suppression than did higher doses at 6-week intervals. Using the metyrapone test, Carson and associates found evidence of HPA-axis suppression up to 10 months after treatment. Droszcz and colleagues detected abnormal ACTH stimulation in 6 of 48 patients (13%) receiving IM triamcinolone acetonide every 2 to 6 weeks. In a related study, Carson and associates evaluated triamcinolone acetonide 40 mg given every 3 weeks for four doses. This resulted in anovulatory menstrual cycles in women because of decreased gonadotropin levels. Traditionally it has been taught that CS-induced menstrual abnormalities were generally due to IM CS. More recently, relatively small studies have demonstrated that up to 40% of premenopausal women on at least 20 mg prednisone daily experience significant menstrual abnormalities.
After the arguments and data just given, a reasonably balanced viewpoint follows. Serious AE are rare with either a single IM CS injection or a burst of oral prednisone. Rarely do oral ‘bursts,’ single IM injections, or long-term use of either route of administration result in clinically relevant, significant HPA-axis suppression with CS use for dermatologic indications. Neither route of administration has a clear-cut advantage in withdrawal from chronic CS use. It is important to focus on altering disease precipitators and providing adequately aggressive topical therapy when either oral or IM CS are given. Should repeated IM CS therapy be desired by clinicians, short- to intermediate-acting products such as Celestone and Aristocort should be used. When a long-acting form such as Kenalog is used, a reasonable limit would be three to four injections per year. Each clinician must make up his or her own mind concerning the relative advantages and disadvantages of IM versus oral CS therapy. As is often the case, the correct answer depends on the clinical situation; neither form has a clear-cut advantage over the other.
In general, the authors favors the precision of dosing and the active patient participation that oral CS regimens require for most dermatoses. Given the very thick stratum corneum on the palms (and soles) and the general challenge of successfully treating various subsets of hand dermatitis topically, our most common use of IM CS is for these patients (Kenalog 80 mg IM ideally three to four times yearly at most, ideally tapering to one to two injections yearly over time).
Pulse CS therapy has been proposed as a means to rapidly control life-threatening or serious conditions with minimal toxicity, allowing for less aggressive long-term maintenance CS therapy. Typically, 500 to 1000 mg of methylprednisolone (roughly 10–15 mg/kg daily) is given IV over at least 60 minutes. This dose is repeated on a daily basis for 3 to 5 consecutive days. Pulse methylprednisolone is traditionally administered in an inpatient setting, with cardiac and electrolyte monitoring highly recommended. Alternate-day CS or a nonsteroidal immunosuppressive (‘CS-sparing’) drug such azathioprine and cyclosporine is used to maintain the improvement from the pulse IV CS.
Sudden death of presumed cardiac origin is a notable complication of IV pulse CS therapy. Atrial fibrillation has been reported as well. Furthermore, anaphylaxis attributed to pulse IV CS is a potentially life-threatening complication. Acute electrolyte shifts have been postulated to explain the rare cases of sudden cardiac death. Careful potassium infusions may minimize the risk of these potentially serious cardiac AE. Given that the vast majority of cardiac complications have occurred outside of dermatologic settings, some authors have questioned the need for hospitalization and cardiac monitoring for dermatologic purposes. The recent trend for pulse IV CS therapy is administration in an ambulatory setting if there is no significant renal or cardiac disease present.
The suppression of various lymphocyte subsets is greater with pulse CS therapy than with standard doses of oral CS therapy. CS-induced apoptosis likely plays a key role in this greater effect of IV pulse CS therapy. In addition, there appears to be a persistent decrease in natural killer cell activity. Other immunologic effects are qualitatively similar to those of oral administration.
The overall interest in pulse CS therapy with IV methylprednisolone has waned over the recent decades. This modality should be used only when the severity of the patient’s condition and the lack of response to alternative modes of therapy indicate its appropriateness. Pulse IV methylprednisolone or dexamethasone therapy should be considered experimental and used very selectively in an individualized fashion.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here