Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Competency of the atrioventricular valves allows blood to enter the ventricles, where pressure is generated. When adequate systolic blood pressure is generated, the aortic and pulmonary valves open, allowing blood to enter the arterial system. The atrioventricular valves close, preventing the flow of blood into the atria. During diastole, the aortic and pulmonary valves close, the atrioventricular valves open, the ventricles fill, and ultimately begin the cycle of pulsatile blood flow through the systemic and pulmonary vascular tree.
Malfunction of any of the cardiac valves results in a less efficient circulatory system. Valvular dysfunction causes work overload in one or both ventricles. In extreme cases, this results in heart failure.
Valvular heart disease treatment is evolving with the addition of new technologies. Valve replacement technology has changed with the development of more durable tissue valves and new mechanical valves. The addition of transcatheter therapies has led to a paradigm in the treatment of valvular heart disease.
Initial attempts to duplicate valve leaflets with flexible, nonbiological materials failed. The leaflets of these valves were too stiff in comparison with normal valve leaflets. Efforts at using nonflexible leaflets by constructing hinged-valve leaflets resulted in hinge thrombosis and malfunction. Design engineers then focused on free-floating occluders, such as disks or balls retained in a cagelike housing. This general valve design produced the first clinically useful valves. In 1958, the Starr-Edwards valve was used in the first clinically successful valve replacement.
Although these early designs functioned as intended, the first caged-ball valves had major shortcomings: (1) they were bulky in design and did not fit well into a small ventricle or aorta; (2) they had a small internal orifice, making them relatively stenotic; and (3) they stimulated thrombus formation, which precipitated thromboembolic events, which then necessitated long-term anticoagulation therapy.
The disadvantages of early prosthetic valves led to the development of two divergent lines of valve design using synthetic materials (mechanical valves) or biological tissue (bioprosthetic valves). The caged-ball valves were modified, and pivoting hingeless disk valves, such as the Lillehei-Kaster, Medtronic-Hall, and Björk-Shiley valves, were developed. The St. Jude and Carbomedics valves were the first successful hinged-leaflet valves ( Fig. 50.1, top ).
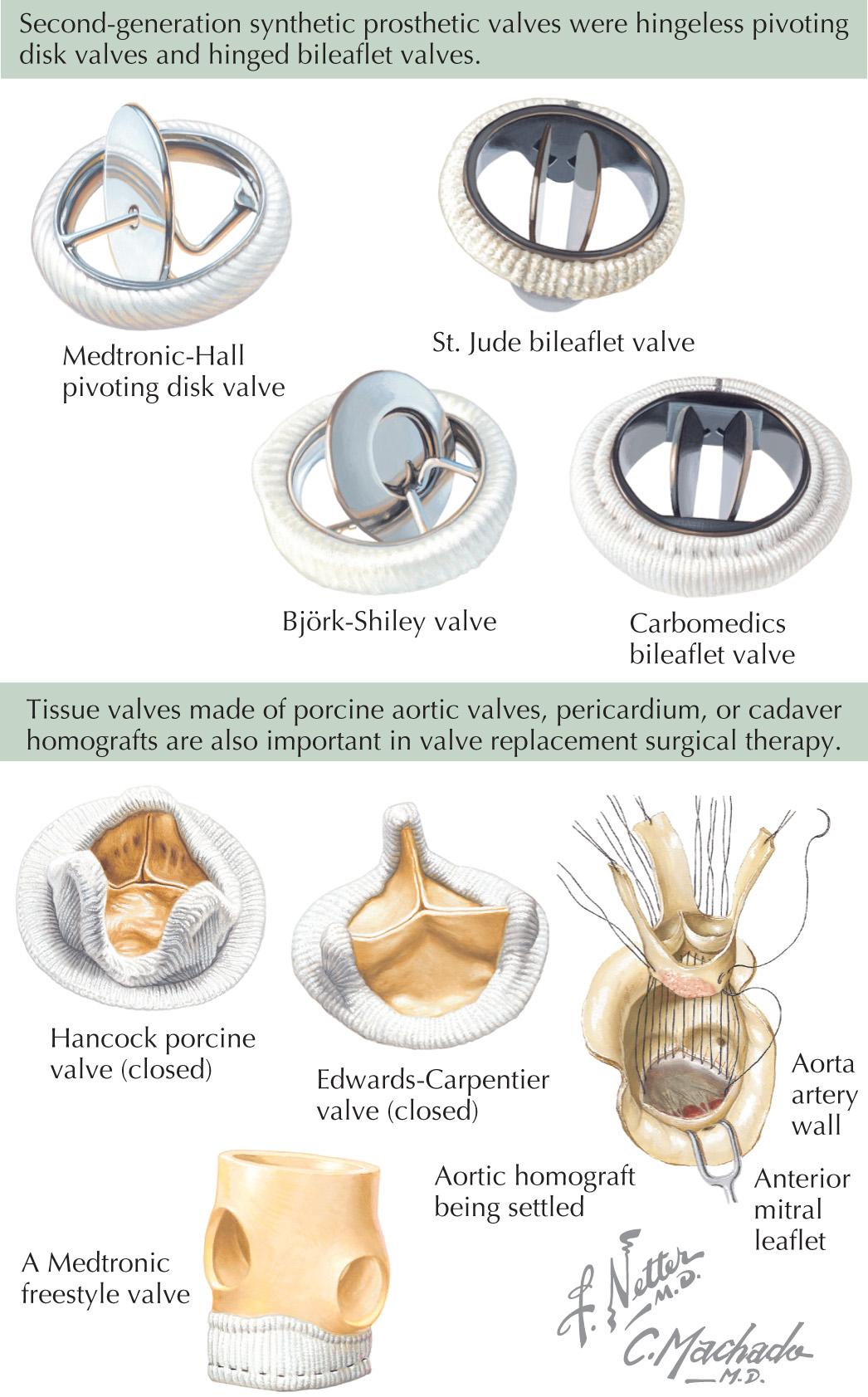
Homograft valves harvested at autopsy and preserved in antibiotic solution or frozen were the first nonsynthetic valves to be implanted successfully. Their limited availability prompted the use of porcine valves procured from slaughterhouses. Porcine valves were preserved with glutaraldehyde and mounted on modified nylon-covered plastic or metal stents. Valves made of pericardium were also developed and used successfully ( Fig. 50.1, bottom ). Porcine and bovine pericardial valves are commonly used bioprosthetic valves today, whereas the hinged leaflet valves are the commonly used mechanical valves. No valve has proven to be the perfect replacement, but durability has dramatically improved over the years.
Cardiac valve pathology consists of two broad categories: congenital valve deformity and acquired valvular dysfunction. Congenital deformity can occur in one or more cardiac valves (see Section VIII). Patients with severe congenital valvular dysfunction can die if prompt surgical intervention is not undertaken. In patients with normally developed hearts, infection can cause valvular dysfunction at any age. Rheumatic heart disease secondary to untreated streptococcal infection and bacterial endocarditis can destroy a normal heart valve. Generalized inflammatory illnesses, such as lupus erythematosus, rheumatoid arthritis, and eosinophilic endocarditis, as well as carcinoid disease, similarly can cause valvular dysfunction. Connective tissue diseases, such as Ehlers-Danlos syndrome and myxomatous degeneration, can cause valve deformity and dysfunction. Severe myocardial ischemia and injury can cause papillary muscle dysfunction, which can result in mitral valve insufficiency. Finally, aging often results in atherosclerotic changes and calcium deposition in arterial walls, and aging can also affect the aortic valves, sometimes with severe calcification of the leaflets. The mitral valve annulus can also be severely calcified, with or without valvular dysfunction.
The presenting symptoms in patients with dysfunctional valves vary considerably, depending on the type and severity of dysfunction and the location of the affected valves. Diseased valves can become incompetent, stenotic, or both. Young patients with moderate aortic valve stenosis are often asymptomatic. Likewise, many patients with moderate mitral valve stenosis or insufficiency may be asymptomatic. In general, patients whose valve dysfunction progresses eventually experience dyspnea on exertion. Syncope or angina pectoris, alone or in association with dyspnea, can develop in patients with aortic stenosis.
In patients presenting with dyspnea and fatigue, noncardiac causes, such as anemia, hypertension, pulmonary pathology, and hypothyroidism, should be excluded. Primary cardiac myopathy should be considered. Coronary artery disease must be ruled out if angina pectoris is a symptom.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here