Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Small bowel (SB) malignancies account for only 3% of all gastrointestinal (GI) neoplasms and 0.6% of all cancers in the United States. Common primary malignant tumors include carcinoid (40%), adenocarcinoma (31%), lymphoma (17%), and sarcoma (9%). The risk of a specific SB tumor type depends on the exact location in the SB, with adenocarcinomas the most common duodenal tumor and carcinoids the most common ileal tumor; both sarcoma and lymphoma more equally distributed throughout the entire SB. The clinical presentation of SB tumors is nonspecific, with abdominal pain, weight loss, nausea, vomiting, GI bleeding, and SB obstruction being the most common symptoms. Endoscopic evaluation of the SB has been hampered by the long length of the SB, at approximately 5 to 6 m. Novel endoscopic technologies, such as video capsule endoscopy, and device-assisted enteroscopy have allowed evaluation of the entire SB.
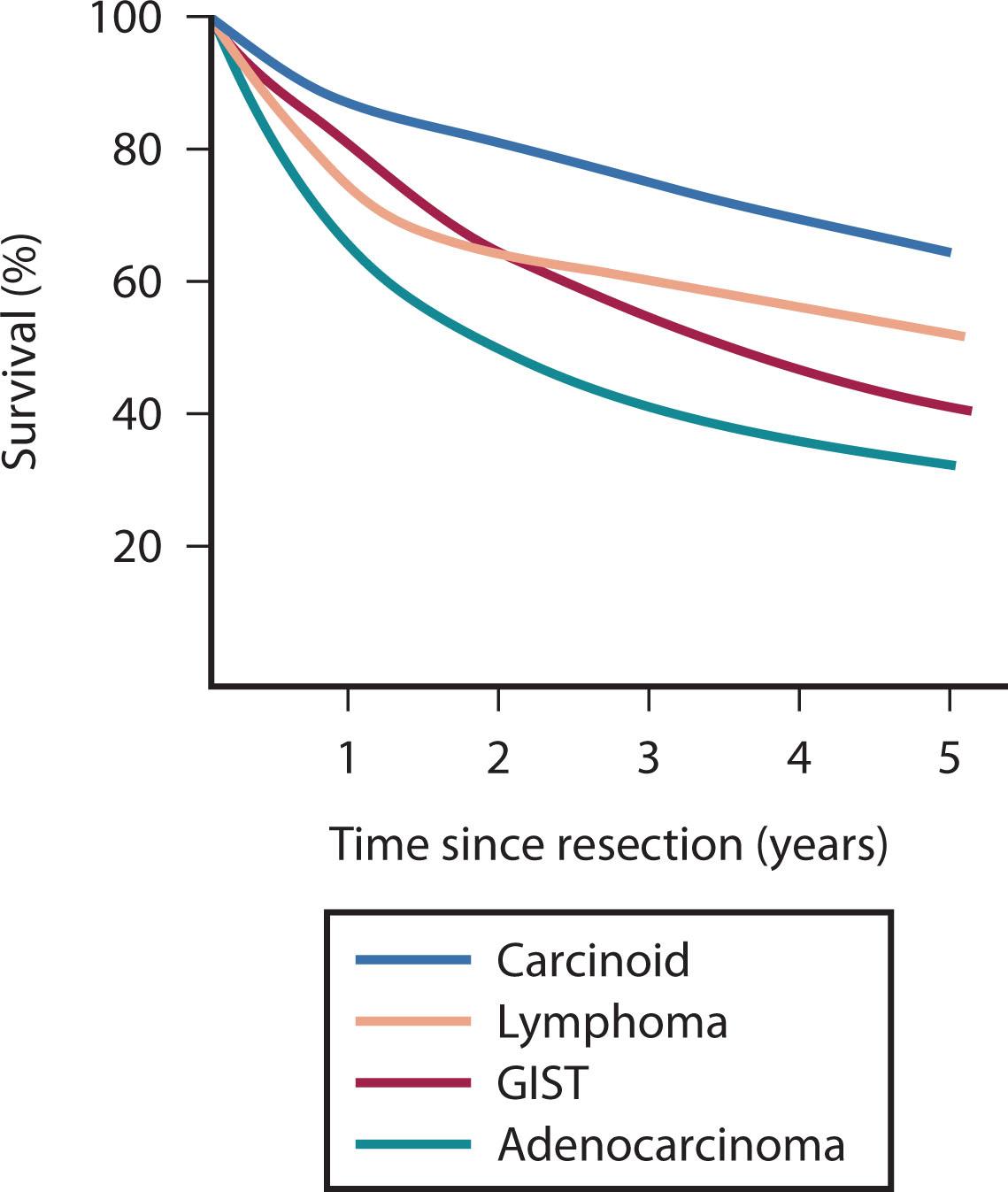
The heterogeneous biology of SB tumors is reflected in survival rates, which are lowest for adenocarcinoma and highest for carcinoid ( Fig. 17.1 ). The most common malignancies of the SB are discussed in this chapter as separate entities.
Carcinoid is the most common SB malignancy, representing 39% to 44% of all primary SB tumors, more commonly affecting Black males in United States, with rising incidence since 2000.
The vast majority of these tumors are sporadic. A small subset is associated with the inherited syndrome MEN I (multiple endocrine neoplasia type I).
Carcinoid tumor is a well-differentiated low grade malignant neuroendocrine tumor (NET) originating from the Kulchitsky cell, an enterochromaffin cell located in the crypts of Lieberkuhn of the GI tract. Microscopically, carcinoid is composed of uniform small cells containing neurosecretory granules with bioactive products such as serotonin, somatostatin, glucagon, histamine, or gastrin. Macroscopically, carcinoid tumors are present as small submucosal nodules, often subcentimeter in size, not causing obstruction of the lumen per se , with intense desmoplastic response within the adjacent mesentery resulting in shortening and thickening of mesentery and retraction and kinking of nearby vessels. Some 30% of patients with SB carcinoids have multicentric disease at diagnosis. Although much rarer than carcinoid tumors, intermediate- and high-grade NET, characterized by both a higher rate of mitotic activity and tumor necrosis, can also arise in the SB. These tumors have a more aggressive biology, with high-grade NET of the SB behaving like small cell carcinomas of the lung.
Carcinoid is a well-differentiated neuroendocrine tumor.
Microscopically, carcinoid is composed of uniform small cells containing neurosecretory granules, with bioactive products such as serotonin, somatostatin, glucagon, histamine, or gastrin.
Macroscopically, carcinoid tumors are often small submucosal nodules, multifocal in 30% of cases, with intense desmoplastic response within the adjacent mesentery.
Because of their indolent growth, most SB carcinoids are asymptomatic and identified incidentally. One-third of carcinoids present with abdominal pain or bowel obstruction, and only 10% are associated with carcinoid syndrome because of serotonin hypersecretion. The carcinoid syndrome is primarily seen in the context of liver metastases, in which the release of serotonin gains access to the systemic circulation without undergoing hepatic metabolism. Carcinoid syndrome consists of secretory diarrhea, bouts of cutaneous flushing, wheezing, and dyspnea because of bronchospasm. Longstanding carcinoid syndrome may cause fibrotic changes in the cardiac valves predominantly affecting the right heart, typically leading to tricuspid regurgitation and pulmonic stenosis. A 24-hour urinary collection for the serotonin metabolite 5-hydroxyindole acetic acid (5-HIAA) has both good sensitivity and good specificity for the diagnosis of carcinoid syndrome.
Tumorous spread via lymphatics into the mesenteric lymph nodes induces extensive desmoplastic reaction, leading to retraction of the mesentery, kinking and obstruction of the mesenteric veins, and retraction and mechanical obstruction of the surrounding SB loops ( Fig. 17.2 ). This mesenteric nodal metastasis produces a typical mesenteric mass detectable by computed tomography (CT), as opposed to the small primary tumor that is commonly too small to detect by imaging.
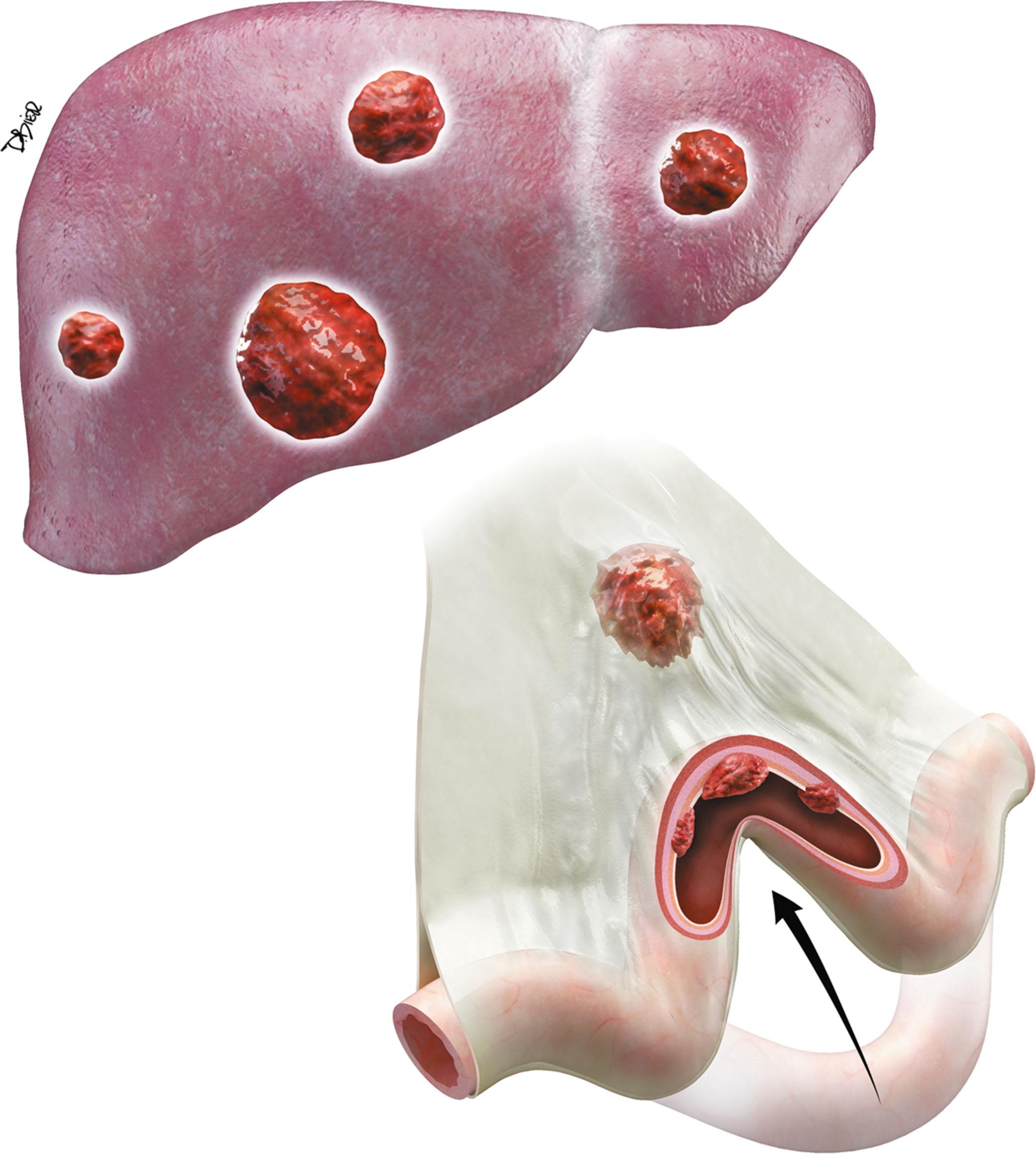
Hematogenous spread to the liver is very common. Liver metastases allow development of carcinoid syndrome. Spread to the bones is rare, producing osteoblastic metastases.
Peritoneal spread is usually a late development in the course of the disease. Peritoneal metastases commonly remain discrete nodular lesions without ascites, as opposed to peritoneal spread of adenocarcinoma, which causes massive ascites.
Lymphatic spread to mesenteric lymph nodes is associated with desmoplastic reaction causing retraction of the mesentery, potentially leading to bowel obstruction.
Hematogenous spread to the liver may be associated with the development of carcinoid syndrome.
Peritoneal metastases are commonly small and rarely cause ascites.
Bone metastases of carcinoid are osteoblastic.
A revised TNM (tumor, node, metastasis) staging for carcinoid tumors, which is slightly different from the TNM staging of SB adenocarcinoma, has been proposed ( Table 17.1 ).
The TNM (tumor, nodes, metastasis) staging of carcinoid is similar to that of adenocarcinoma, with the exception of T staging including not only the depth of invasion into the bowel wall, but also a size as a separate criterion. Two centimeters serves as a threshold, with tumors larger than 2 cm upstaged to T3 and having a worse prognosis.
| STAGE | CHARACTERISTICS OF TUMOR-NODE-METASTASIS CLASSIFICATION SYSTEM |
|---|---|
|
|
|
|
|
|
| STAGE | GROUPING |
|
|
Mesenteric nodal metastases and liver metastases are usually larger, more easily detectable by imaging, and cause more clinical symptoms than small primary tumors. The primary tumor within the SB is the most challenging for detection by imaging.
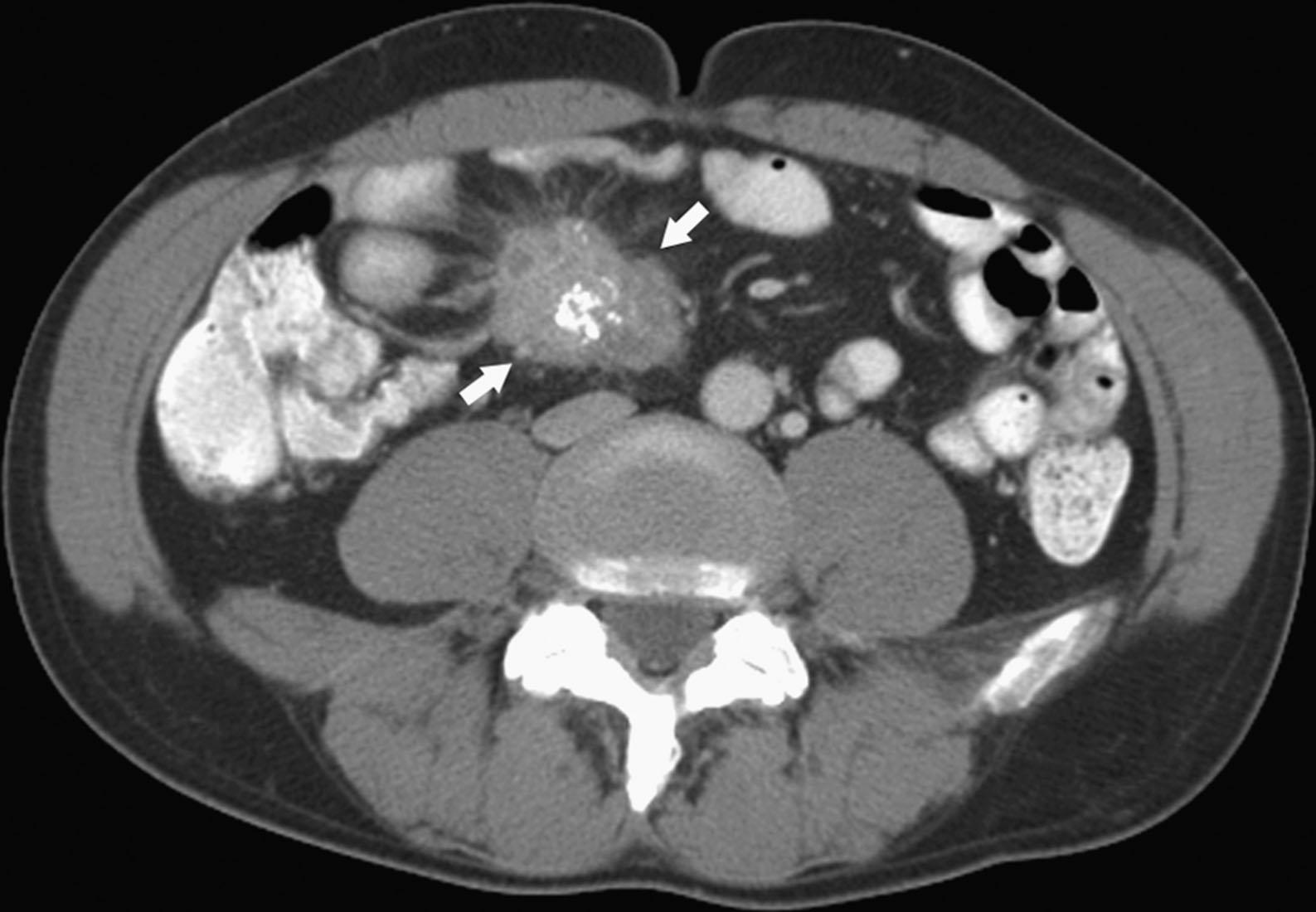
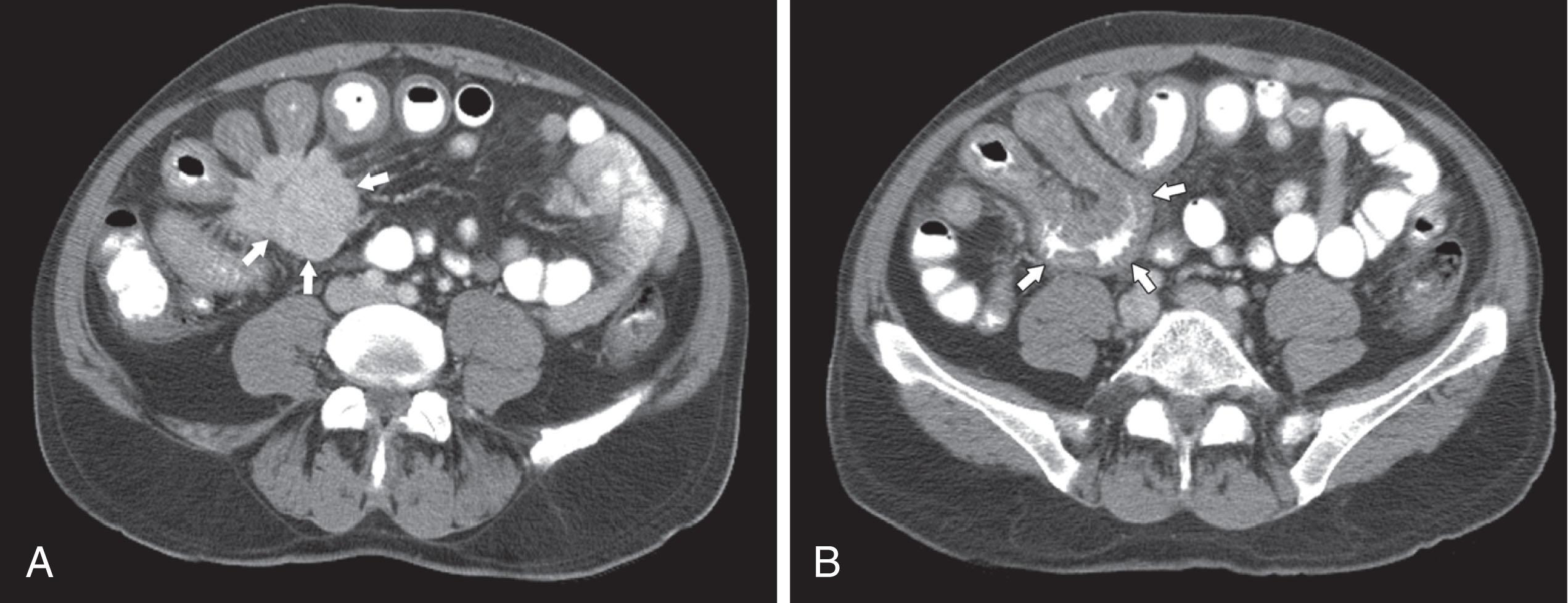
CT may identify the submucosal carcinoid tumor as a small mural mass with early intense contrast enhancement owing to hyperemia. Hyperenhancing tumor can be best appreciated on the background of negative contrast in the bowel lumen in the arterial phase of contrast injection ( Figs. 17.3 and 17.4 ). Classic mesenteric nodal metastasis of carcinoid has a nearly pathognomonic CT pattern as a spiculated soft tissue density mesenteric mass because of desmoplastic reaction ( Figs. 17.5 and 17.6 ). Sometimes, a longer segment of adjacent SB has a thickened edematous wall because of mesenteric venous engorgement (see Fig. 17.6 ). Calcification within the mesenteric extension can be seen in up to 70% of cases (see Fig. 17.5 ). Differential diagnostic considerations for the mesenteric component include sclerosing mesenteritis and treated lymphoma. A minority of mesenteric nodal metastases demonstrate a nonspecific pattern of well-circumscribed ovoid or round masses.
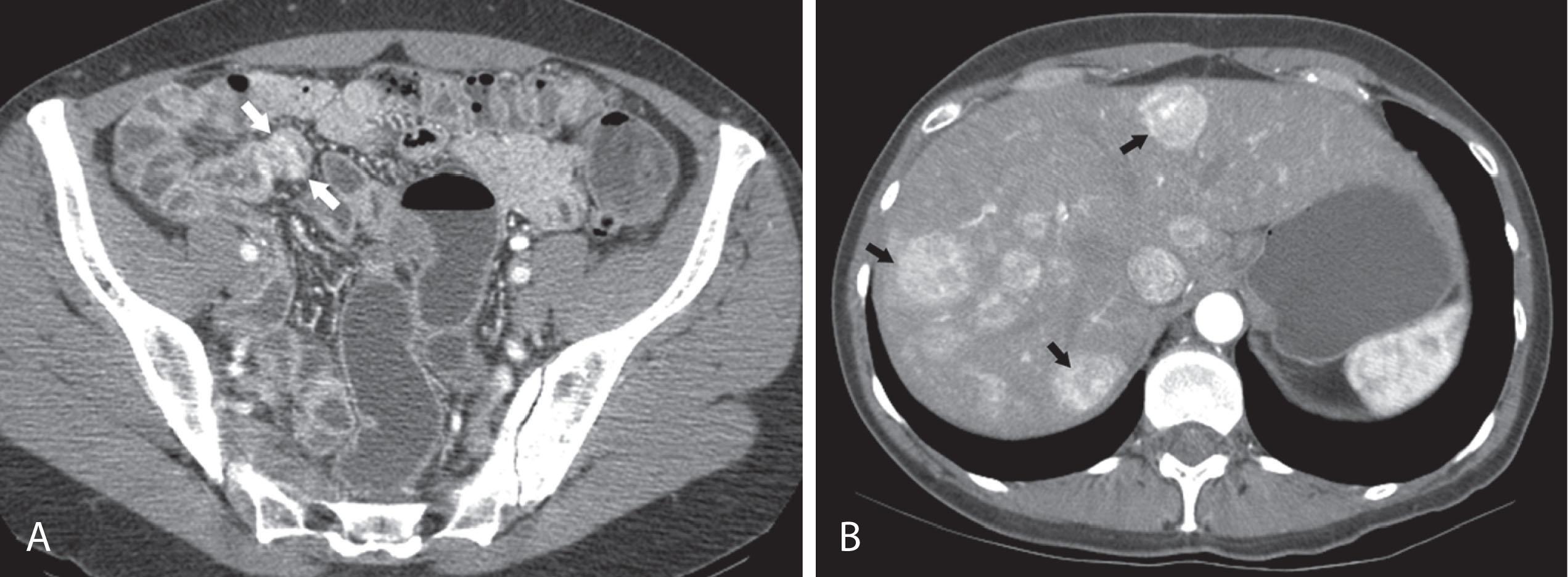
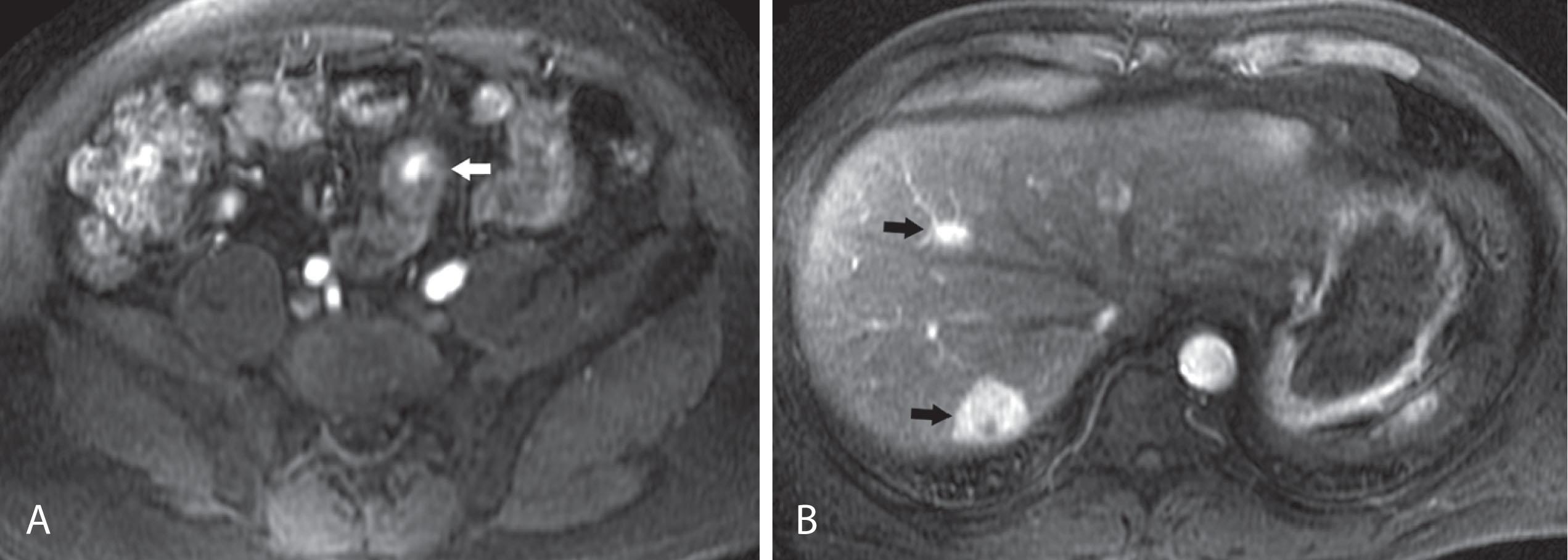
Liver metastases are hypervascular and best detected as hyperenhancing lesions on the late arterial phase of CT (see Figs. 17.3 and 17.4 ) and as hypoenhancing lesions on the delayed venous phase of CT. Lesions may become isodense and least conspicuous in the portal phase. Dual-phase contrast-enhanced CT of the liver should always be performed to detect carcinoid liver metastasis. Noncontrast CT is very helpful for liver metastases detection, unless limited by fatty liver changes. Small carcinoid metastases can be mistaken for benign hypervascular lesions such as hemangiomas or small foci of focal nodular hyperplasia, especially in the absence of a baseline scan. Rarely, liver metastases have a cystic appearance ( Fig. 17.7 ).
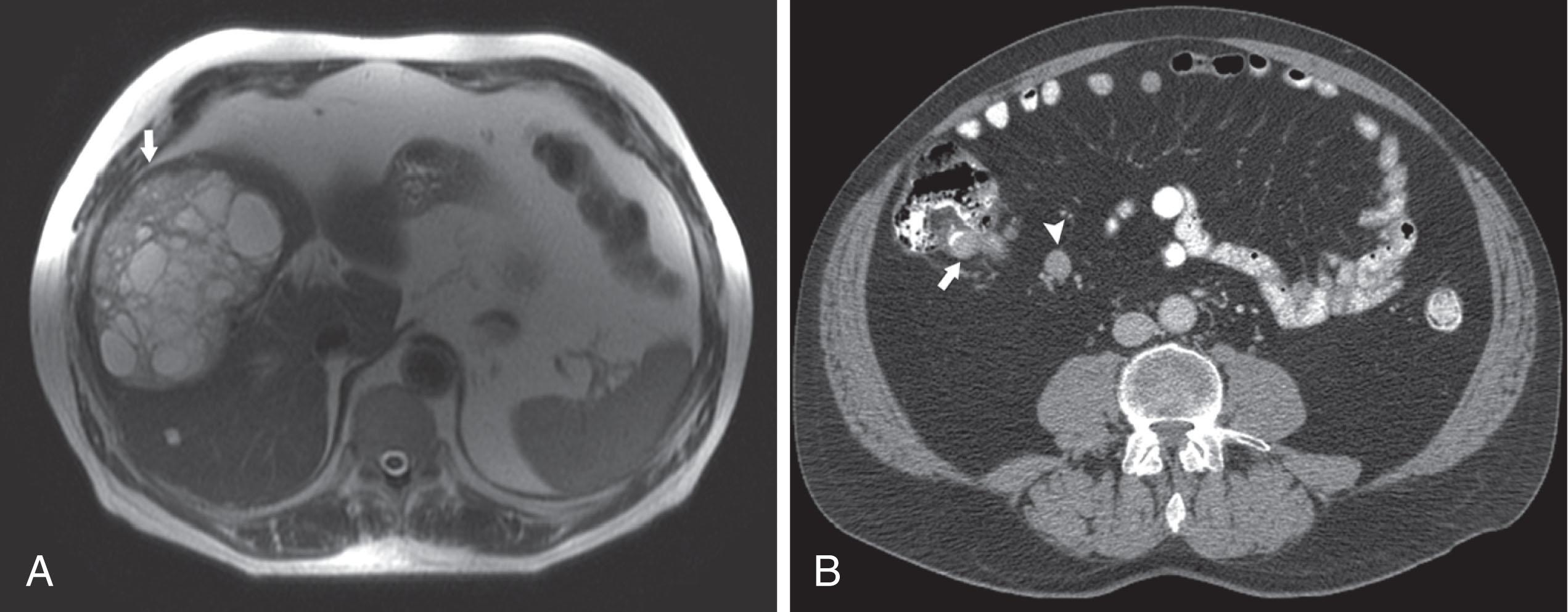
Magnetic resonance imaging (MRI) with gadolinium can demonstrate the primary hyperenhancing carcinoid in the bowel on fast dynamic sequences with T1 contrast (see Fig. 17.4 ). Owing to increased sensitivity to gadolinium enhancement, MRI can detect more liver metastases than can CT. MRI is a preferred modality when there are no extrahepatic metastases. Unlike CT, MRI evaluation of liver metastases is not limited by fatty liver. Whereas gadolinium is necessary for initial characterization of liver lesions in a patient with carcinoid, follow-up MRI examinations maintain diagnostic quality even in the absence of contrast, so they can be used in patients with severely impaired renal function or lack of intravenous access.
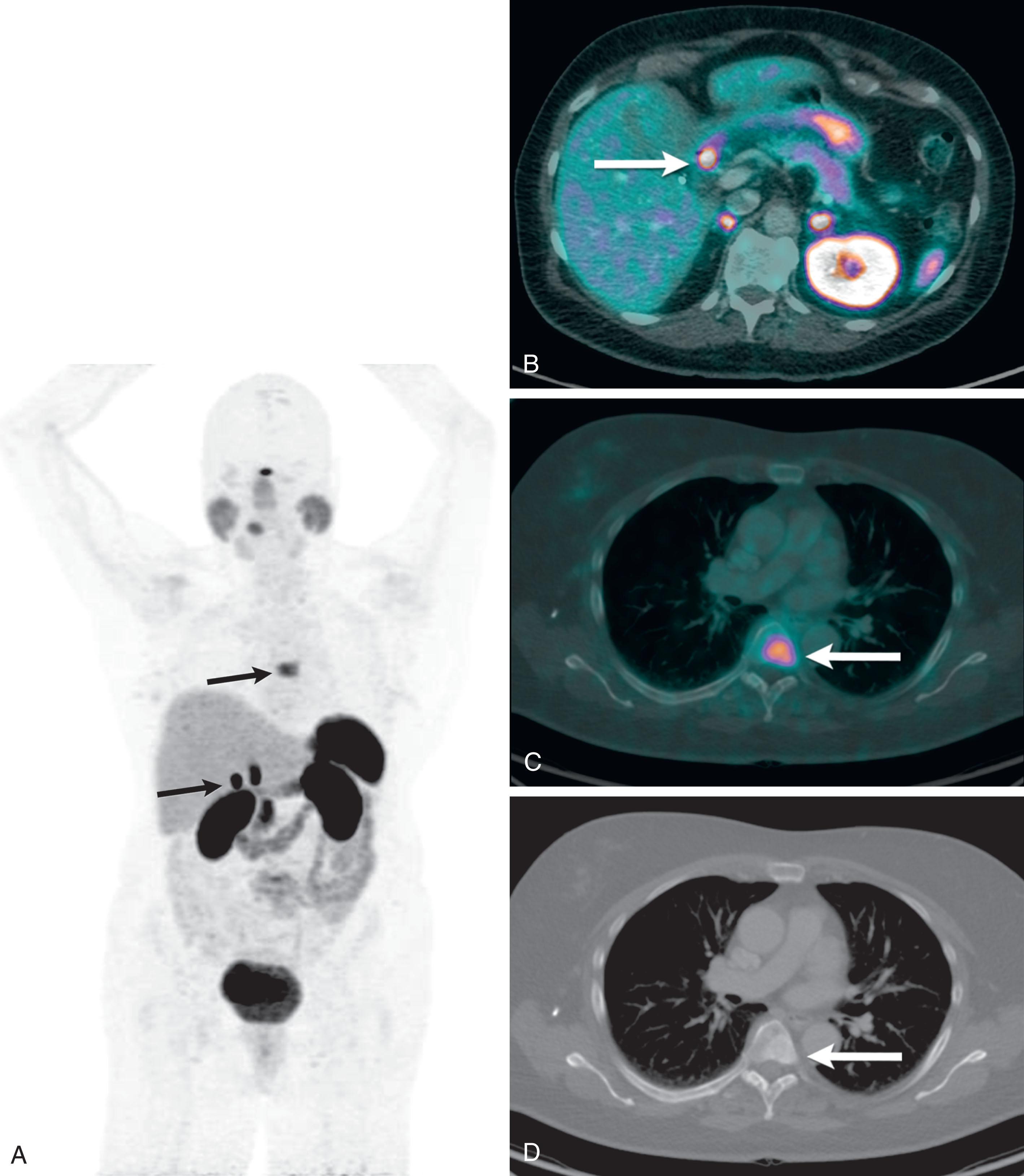
Carcinoid tumors tend to express somatostatin receptors (SSTR), allowing for imaging with radiolabeled somatostatin analogues. Somatostatin scintigraphy using 111 indium-DPTA-D-Phe-1-octreotide (Octreoscan) allows whole-body planar images and single photon emission computed tomography (SPECT) or SPECT/CT images. More recently, SSTR positron emission tomography (PET)/CT with 68 Ga-DOTATATE (Netspot) has come into use. In addition to identifying more lesions than somatostatin scintigraphy, these scans are better for the patient as they can be done faster (in a few hours rather than 2–3 days) and therefore expose the patient to less radiation. , SSTR PET/CT has a complementary role to CT and MRI not only in identifying occult primary SB carcinoid, but also in detecting mesenteric nodal metastasis and distant metastases ( Fig. 17.8 ). Although SSTR PET/CT performance in the detection of liver metastasis is inferior to that of CT and MRI owing to decreased spatial resolution, it can help characterize indeterminate hypervascular lesions. Moreover, it may provide valuable information for predicting the efficacy of somatostatin analog (octreotide) treatment.
Location of primary small bowel carcinoid if possible (rarely visualized on computed tomography [CT] or magnetic resonance imaging [MRI] as a hyperenhancing mural nodule).
Description of mesenteric nodal metastasis that may have the pathognomonic CT pattern of a spiculated soft tissue density mesenteric mass because of desmoplastic reaction, with a 70% calcification rate.
Evaluation of the liver for presence of hypervascular metastases best seen on the late arterial phase of CT or MRI. Small liver metastasis is difficult to distinguish from hemangioma by either CT or MRI.
CT (most commonly the first modality to lead to detection of mesenteric and liver metastases).
SSTR PET/CT (for identification of occult SB primary or distant metastases, also for SSTR expression before some therapies).
MRI of the liver for follow-up when no significant extrahepatic disease is present (advantage of sparing radiation dose for long-term survivors).
Localized disease requires treatment with wide en bloc surgical resection that includes the adjacent mesentery and lymph nodes. Resection of a primary tumor should also be considered in patients with liver metastases, to prevent the development of fibrosing mesenteritis and possible mechanical obstruction, bleeding, and perforation.
Liver metastases are the most common site of metastatic disease and can become symptomatic owing to hormone secretion or pain. Local modalities addressing the liver metastases have been shown to result in improved overall survival. Therefore, in patients with resectable liver disease, metastasectomy should be favored. When resection is either not feasible or incomplete, other local ablative techniques such as chemoembolization, radiofrequency ablation, and cryotherapy should be considered.
Owing to the slow-growing nature of carcinoid tumors, systemic chemotherapy is not effective in the treatment of this malignancy. The primary medical therapy for metastatic carcinoid tumors is with an analog of the potent inhibitory GI hormone somatostatin, such as octreotide or lanreotide. Their use is extremely effective in controlling the symptoms of carcinoid syndrome, slowing the growth of carcinoid tumors, and inducing biochemical marker responses in approximately 50% of patients. Actually, tumor shrinkage from octreotide treatment is extremely rare. Octreotide-resistant disease can be addressed by treatment with the radiolabeled somatostatin analogue 177 Lu-Dotatate or by molecular targeting therapy with the mTOR kinase inhibitor everolimus. SSTR PET/CT is required before 177 Lu-Dotatate treatment to confirm that the tumor activity is greater than that of the liver.
Resection of the primary tumor is indicated, regardless of the presence of liver metastases, to prevent bowel obstruction.
Local modalities such as resection or ablation for the treatment of liver metastases improve disease-free survival.
The somatostatin analog octreotide is of value in symptomatic relief from carcinoid syndrome, although actual tumor responses are extremely rare.
Two effective markers are available for monitoring patients with metastatic carcinoid tumors:
Serotonin metabolite 5-HIAA (in 24-hour urine collections).
Plasma chromogranin A.
Imaging monitoring of tumor response to treatment is usually performed by CT, allowing detection of both liver metastasis and peritoneal metastasis, as well as evaluation of potential risk for an SB obstruction. MRI is preferred over CT for monitoring of intrahepatic target lesions. SSTR PET/CT is helpful for problem-solving in cases of clinical or laboratory progressive disease with no evidence of progression on CT or MRI, and for characterization of new lesions of indeterminate significance.
SB carcinoid is the most common SB malignancy of neuroendocrine origin, commonly with indolent clinical course, often requiring long-term follow-up by imaging. The hypervascular nature of this tumor requires late arterial-phase cross-sectional imaging to detect metastatic disease. The small primary tumors commonly remain undetected by preoperative imaging. Surgery or ablative therapy of this tumor is the preferred treatment.
Adenocarcinoma is the second most common primary malignancy of the SB (31% of all primary SB tumors). One of the more interesting aspects of small intestine adenocarcinoma is its rarity in comparison with large intestine adenocarcinoma. Despite the small intestine representing approximately 70% to 80% of the length and over 90% of the surface area of the alimentary tract, the incidence of SB adenocarcinoma is 30-fold less than that of colon adenocarcinoma.
Most cases of adenocarcinoma are sporadic, with a male predominance and peak incidence in the seventh and eighth decades. An increased incidence is associated with the genetic cancer syndromes of hereditary nonpolyposis colorectal cancer, Peutz–Jeghers, familial adenomatous polyposis, and Lynch syndrome. Inflammatory bowel disease, and in particular Crohn’s disease, is a risk factor, with risk correlated with both the extent and the duration of SB involvement.
Most frequently, the tumor occurs within the duodenum (49%), particularly around the papilla of Vater, and with decreasing frequency in the jejunum (21%) and ileum (15%). In Crohn’s disease–associated cases, 70% of tumors present in the distal ileum.
There are four histologic types of adenocarcinoma: well, moderately, and poorly differentiated, and undifferentiated. Prognostic factors consistently associated with poor outcome include the presence of metastatic disease, noncurative surgical resection, poor differentiation, and advanced age. In patients who have had surgical resection, the pathologic factors associated with increased risk of relapse include lymph node involvement, positive surgical margins, poor tumor differentiation, T4 tumor stage, and lymphovascular spread. The genomic profiles of SB adenocarcinomas show common mutations in the KRAS oncogene.
Symptoms of SB adenocarcinoma are nonspecific and frequently do not occur until advanced disease is present. The most commonly reported symptoms are abdominal pain, nausea, vomiting, weight loss, and GI bleeding. Adenocarcinoma of the proximal duodenum involving the ampulla of Vater may present with obstructive jaundice.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here