Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
© 2018 Elsevier Inc. All rights reserved. Please note that the copyright for the original figures submitted by the contributors is owned by Contributors.
Over the past decades, the indications for endoscopic endonasal surgery expanded to include benign and malignant skull base lesions. Many surgical approaches have been described to resect petroclival chordomas and chondrosarcomas; however, expanded endoscopic endonasal approaches (EEA) are the least invasive, have equal or superior resection rates, and fewer complications and adverse sequelae. The progression of EEA for skull base lesions was made possible by advancements in reconstruction of the dura and skull base, and more specifically by the popularization of the nasoseptal flap, use of which has decreased the frequency of postoperative cerebrospinal fluid leak to that of open approaches. In this chapter, we will discuss current options for reconstructing the skull base following resection of petroclival chordomas and chondrosarcomas, whether an open or an endoscopic endonasal approach is used.
Many surgical approaches have been described for resection of petroclival chordomas and chondrosarcomas: anterior transcervical retropharyngeal, transseptal–transsphenoidal, pterional, retromastoid, lateral suboccipital, subfrontal, extended frontal, transbasal, subtemporal–infratemporal, presigmoid–subtemporal, transpetrosal, lateral transcondylar, and transoral–transpalatopharyngeal.
However, over the past two decades, endoscopic endonasal approaches to the skull base have proven to be an effective and safe alternative to open approaches in terms of oncological outcomes, adverse sequelae, complications, and mortality; thus, these techniques have gained wide popularity. Acceptance of endoscopic approaches to expose and resect petroclival chordomas and chondrosarcomas was made possible by the genesis of multidisciplinary teams composed of otolaryngologists and neurosurgeons well trained in endoscopic techniques, and allied specialists (i.e., medical and radiation oncologists, neuroradiologists, neuropathologists, neuroophthalmologists, endocrinologists). Although accumulation of experience with endoscopic endonasal management of benign skull base lesions, acceptance of piecemeal resection of malignant tumors, and technological advances such as surgical navigation and endonasal-powered instrumentation contributed to this evolution, improvements in the success rate for endoscopic repair of cerebrospinal fluid (CSF) leaks secured acceptance of extended endoscopic endonasal resections of chordomas and chondrosarcomas.
Skull base defects can be divided into extradural and intradural. Intradural defects are further subdivided into low- or high-flow leaks. A high-flow CSF leak is defined by the communication of multiple subarachnoid cisterns or a ventricle with the dural defect. Extradural defects are those created by defined by skull base tumor resection that does not out disrupt and cause a CSF leak. Reconstruction following resection of chordomas and chondrosarcomas of the petroclival area is particularly challenging because large defects and high CSF flow rates are often created and because the vector of gravitational force is tangential rather than orthogonal to the interface between closure material and the dura.
Regardless of the approach used to resect the primary tumor, open or endonasal, the goals of reconstruction of skull base defects are the same:
Water and airtight closure to prevent CSF leak, pneumocephalus, meningitis, and other intracranial infections
Complete separation of the cranial cavity and brain from the sinonasal tract
Protection of brain, cranial nerves, and intracranial vessels from desiccation and infection
Acceleration of the healing process, especially if the patient will undergo postoperative external beam irradiation
Preservation and rehabilitation of function
Preservation or restoration of cosmesis
Avoidance of dead spaces that may contribute to hematomas and infection.
Critical factors to be considered when reconstructing defects following resection of skul base lesions are:
Size and location of the bony and dural defect:
Small dural defects are commonly but not universally defined as less than 1 cm in diameter whereas large defects are defined as larger than 3 cm in diameter.
High- versus low-flow CSF leaks
Prior radiation therapy or anticipated postoperative radiotherapy
Previous sinonasal or skull base surgery
Extent of invasion of sinonasal structures
Morbid obesity, obstructive sleep apnea (and pressure required for CPAP), and the possibility of increased CSF pressure as suggested by imaging findings (e.g., dilated ventricles, empty sella, expanded optic nerve sheaths)
Ideally, the route for tumor resection, whether endonasal or open, should be adequate for reconstructing the skull base.
The authors favor reconstruction of skull base defects using pedicled vascular flaps, multilayer reconstruction techniques, and the aforementioned principles, especially for central skull base defects. However, multiple options available to reconstruct the skull base bone and dura can be divided as discussed below.
Primary closure is used to reconstruct small dural defects and when resection of the chordoma or chondrosarcoma is performed through a lateral open approach, which provides ample room to suture the dura using the microscope. With rare exceptions, primary closure cannot be accomplished for large dural defects and in endoscopic endonasal approaches.
Free autografts involve the harvesting of tissue from a donor site and implanting it in a recipient site. They lack their own vascularization as there is no attachment to the donor site; therefore, a well-vascularized recipient bed is needed for high probability of graft survival. Commonly used free autografts include the following:
Free mucoperiosteal/mucoperichondrial grafts harvested from the nasal cavity can be used to reconstruct small to moderate size skull base defects.
They are usually used as the final layer of a multilayer closure and can only be placed in onlay fashion on the nasal side of the reconstruction. The graft should include periosteum or perichondrium to accelerate neovascularization and improve survival of the graft. Most commonly, they are harvested from the middle turbinate, the inferior turbinate, the nasal floor, or the nasal septum. Their availability, accessibility, ease of placement, low donor-site morbidity, and high survival rate make free mucoperichondrial/mucoperiosteal grafts a useful option, provided that the graft is larger than the dural defect.
Dandy was the first to describe repair of dural tears using autologous fascia lata grafts. Autologous fascia lata grafts are commonly used for duraplasty. Its texture and consistency resemble dura. Fascia lata is often effective when used as a simple onlay, when sutured to the dura, or as part of a multilayer reconstruction. It is easy to harvest from the lateral lower thigh and a large graft can be obtained along with fat, if needed. However, it requires a separate skin incision carrying the risks of donor-site morbidity.
Free-fat autografts are extremely versatile and can be used as a primary graft filling the defect to obliterate dead spaces, as a bolster, or as biological dressing to a multilayered reconstruction promoting formation of vascularized granulation tissue.
For defects resulting from resection of chordomas and chondrosarcomas, fat is used as part of a multilayer reconstruction as a radiologic spacer, to obliterate dead spaces and bony tunnels and to contour the vascularized flap ( Case 2 ).
Fat is harvested most commonly from the abdomen through a periumbilical incision, which is cosmetically acceptable and allows harvest of a significant amount of tissue through a small skin incision. Other donor sites include the left lower quadrant and the lateral thigh. Disadvantages include donor-site morbidity such as unsightly scar, infection, hematoma, and seroma.
Free-cartilage autografts can be harvested from either the septum or the auricle. They provide rigid support as an intracranial-extradural inlay in a multilayer reconstruction of moderate-sized defects. They are often used to reconstruct the middle cranial fossa floor in lateral approaches. They are rarely used in endonasal approaches.
Bone autografts are usually not used in the reconstruction of the skull base. Exceptions include significant brain herniation or prevention of such in morbidly obese patients with sleep apnea. Bone can be harvested as a split calvarial bone graft or from the vomer and perpendicular plate of the ethmoid during the septectomy. However, bone grafts are prone to osteoradionecrosis.
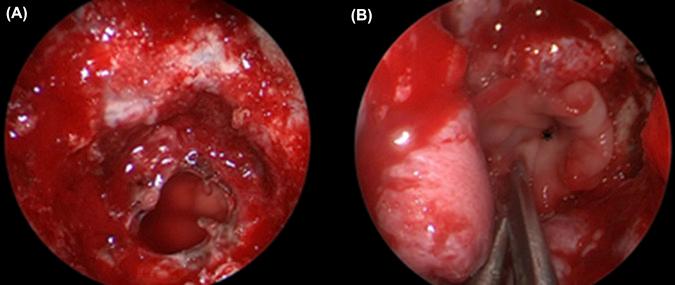
Heterologous materials are commonly used to reconstruct the dural and skull base defects. They can either be used as a single layer or as part of a multilayer closure. Germani et al. described the reconstruction of large skull base defects (>2 cm) using a single layer of acellular dermal graft placed in inlay or onlay fashion with a 97% success rate. We favor the use of collagen matrix (e.g., DuraGen, Integra Life Sciences, Plainsboro, NJ, USA) ( Fig. 22.1 ). The texture and pliability of collagen matrix permit its safe placement around critical neurovascular structures. It is used to obliterate intradural dead space or as the subdural inlay of a multilayer closure ( Fig. 22.1 ). The most commonly used synthetic heterologous materials in skull base surgery are summarized in Table 22.1 .
| Generic Name | Ultra Pure Collagen | Acellular Dermal Allograft | Small Intestinal Submucosa (SIS) |
|---|---|---|---|
| Brand Name | DuraGen Plus | AlloDerm | Cook Biodesign |
| Composition | Ultra Pure Collagen (Type I) sourced from bovine Achilles tendon | Acellular dermal matrix processed from banked human cadaver skin | Nonporous, absorbable, multilayer sheet of extracellular collagen matrix derived from porcine small intestinal submucosa |
| Thickness | 3–4.5 mm | Thin: 0.23–0.51 mm | |
| Medium: 0.53–1.02 mm | |||
| Thick: 1.04–2.28 mm | |||
| Extra Thick: 2.30–3.30 mm | |||
| Advantages | Expandable with fluids preventing CSF pockets formation and dead spaces | Can be used as a sole graft material for large dural defects (>2 cm) |
Vascularized flaps for reconstructing the ventral skull base are divided into intranasal or local and extranasal or regional.
The intranasal/local flaps include:
Posterior pedicled nasoseptal or Hadad–Bassagaisteguy flap
Bilateral nasoseptal flap (“Janus flap”)
Reverse nasoseptal flap (Caicedo flap)
Posterior pedicle inferior turbinate flap
Posterior pedicle middle turbinate flap
Lateral nasal wall flaps—anterior pedicled or Hadad–Bassagaisteguy flap 2 (HBF2) and posterior pedicled or Carrau–Hadad flap (CHF)
The extranasal/regional flaps include:
Temporoparietal fascia flap
Pericranial and galeopericranial flap
Occipital galeopericranial flap
Temporalis muscle flap
Palatal flap (Oliver pedicled palatal flap)
Pedicled facial buccinator and facial artery musculomucosal flap
Salpingopharyngeus flap (Dicle flap)
The main intranasal flaps suitable for reconstruction of petroclival defects include the nasoseptal flap, the posteriorly based lateral nasal wall flap, and the inferior turbinate flap. The main extranasal flaps are the temporoparietal fascia and the pericranial flaps. We will discuss all the available vascularized flaps with a special focus on the main ones.
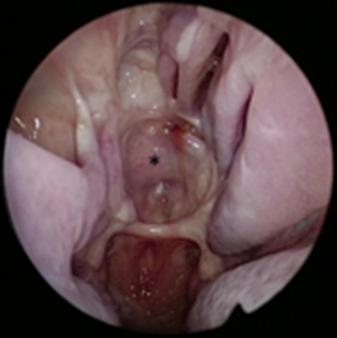
Many prefer mucoperichondrial/mucoperiosteal nasoseptal flap (NSF) ( Figs. 22.2 and 22.3 ; Case 1 ; Case 2 ) for reconstructing skull base defects following an endoscopic endonasal tumor resection. It is a vascular pedicle flap based on the posterior nasoseptal artery, a branch of the posterior septal artery, and a terminal branch of the sphenopalatine artery. The NSF can reconstruct large skull base defects. Overall rates of postoperative CSF leaks dropped to less than 5% after adopting this flap for endoscopic reconstruction of the ventral skull base defects. Postoperative CSF leaks rates for high-flow leaks remain slightly higher, ranging from 5% to 7%.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here