Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Reproductive function and pregnancy are regulated by the complex interaction of a variety of hormones. They are synthesized and secreted by the testis (testosterone), ovary (estradiol and progesterone), pituitary (follicle-stimulating hormone [FSH] and luteinizing hormone [LH]), hypothalamus (gonadotropin-releasing hormone), and placenta (human chorionic gonadotropin [hCG], estrogens, and progesterone).
Laboratory evaluation of reproductive function in the male typically begins with semen analysis. If the results are normal, further evaluation may be unnecessary. If the results are abnormal, then serum hormone measurements, particularly of testosterone, FSH, and LH, are important.
Reproductive dysfunction in the female is often indicated by amenorrhea and/or infertility. Laboratory evaluation, as in the male, involves serum hormone measurements, particularly of hCG, prolactin, thyroid-stimulating hormone, free thyroxine, FSH, LH, and androgens.
Infertility can be treated by several assisted reproductive technologies. These involve a variety of clinical manipulations, all of which are directed at externally controlling as much of the reproductive process as possible to achieve pregnancy and bring it to term. Laboratory monitoring of serum hormone levels (estradiol, progesterone, and hCG) plays an important role in these protocols.
Early pregnancy is monitored by measuring serum hCG concentrations and determining that the pattern of dramatic increase during the first trimester is as expected.
The risks for the most common birth defects, neural tube defects, and chromosomal aneuploidy are determined during the first- and second-trimester screenings.
Fetal hemolytic disease is monitored by spectrophotometric estimation of the level of bilirubin in amniotic fluid or Doppler measurements of peak systolic velocity in the fetal middle cerebral artery.
The status of fetal lung maturation is estimated from the evaluation of pulmonary surfactant in amniotic fluid. This can be accomplished by several methods, including determination of (1) the lecithin/sphingomyelin ratio and phosphatidyl glycerol by chromatography, or (2) microviscosity by fluorescence polarization.
Pregnancy-induced hypertension, or preeclampsia, is characterized by hypertension and proteinuria; consequently, it can be monitored by urine protein measurements.
Risk for preterm delivery and the need for medical intervention can be evaluated by checking levels of fetal fibronectin in the cervical secretions.
Normal reproductive function is mediated by a variety of hormones synthesized and secreted by the gonads (testes and ovaries), adrenals, pituitary, hypothalamus, and placenta. In addition, peripheral nonglandular tissues can contribute to hormone synthesis. Paracrine and autocrine mediators are also involved; however, in almost all cases, their exact roles are not well understood.
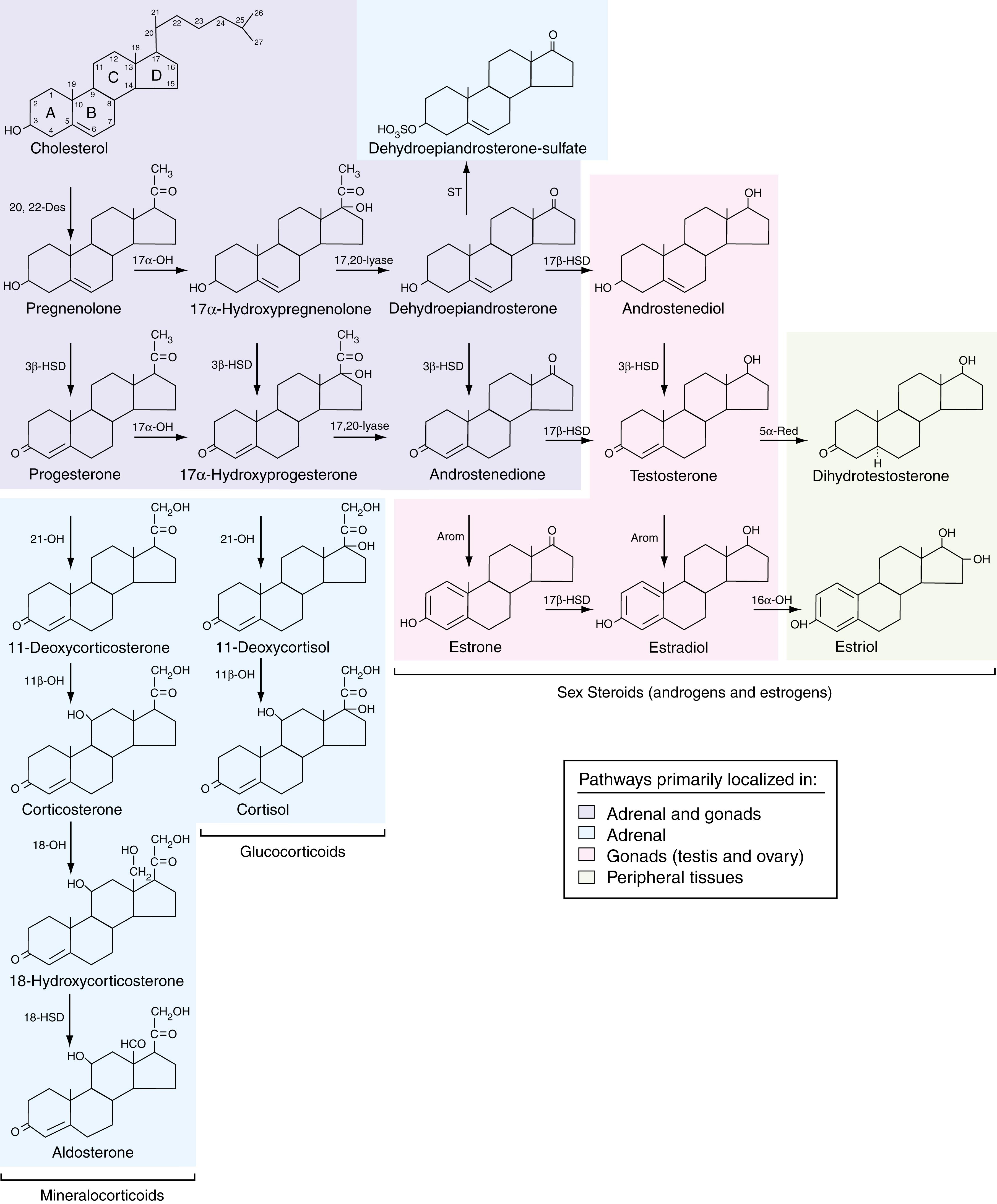
The testes and ovaries synthesize sex steroids (androgens and estrogens) from cholesterol via the same initial pathway used in the adrenal glands to synthesize mineralocorticoids, glucocorticoids, and androgens ( Fig. 26.1 ). The specific hormone secreted by an endocrine gland depends on the presence and relative activities of various enzymes in the steroid pathway. Whereas the adrenal synthesizes androgens such as dehydroepiandrosterone (DHEA), DHEA-sulfate (DHEAS), and androstenedione, the testis metabolizes these steroids primarily to testosterone. The ovary converts testosterone to estradiol, and androstenedione to estrone. In addition, testosterone gets converted to estradiol in peripheral tissues such as adipose cells by the enzyme aromatase. Peripheral tissues (including those targeted by androgens) reduce testosterone to dihydrotestosterone (DHT). Because DHT is two to three times more potent than testosterone, this process yields an enhanced or activated androgenic effect. Peripheral tissues, such as liver, also hydroxylate estradiol to estriol, a metabolite with only one-hundredth the estrogenic potency of its precursor. Thus, this conversion is an inactivation process. During pregnancy, estriol is synthesized via a different pathway and may play a different role than estradiol (see later discussion). Peripheral tissues also convert adrenal androgens to testosterone and androgens to estrone and estradiol, the latter occurring in adipose tissue. Estrone is about one-tenth as potent as estradiol, which likely further modulates estradiol potency. Note that, although progesterone serves as an intermediate in the synthesis of all other steroid hormones, it also functions as a sex steroid in its own right in females.
As with any hormone, sex steroids act at tissue sites distant from their sites of synthesis and secretion. Once in the blood, most of the hydrophobic sex steroids become reversibly and noncovalently bound to plasma proteins. This interaction includes low-affinity, nonspecific binding to hydrophobic sites on albumin and high-affinity binding to specific transport proteins that are synthesized by the liver. Sex hormone–binding globulin (SHBG) transports androgens and estrogens, and corticosteroid-binding globulin (CBG) transports progesterone (as well as glucocorticoids). In blood, only about 1% to 2% of the sex steroids are free (unbound). About half of the remainder are bound to SHBG or CBG, and about half are bound to albumin. Only the free fraction is biologically active because only the free hormone can exit the vascular system (by diffusion) and interact with target cells.
Each steroid binds with high affinity to specific receptor proteins that activate or inactivate genes in the target cell. Thus, the same hormone can elicit a different response in each type of target cell. The potency of a steroid’s hormonal effect (e.g., androgenic effect) is primarily a function of its affinity for the specific receptor protein and the affinity of that complex for the chromatin acceptor sites. Thus, DHT is a more potent androgen than testosterone owing to its high-affinity binding to the target tissue. DHEA and androstenedione bind only weakly, if at all, to the androgen receptor. Hence, their androgenic effect is low and most, if not all, of their androgenic activity derives from their peripheral conversion to testosterone.
Figure 26.2 summarizes the regulation of reproduction in the human male. Gonadotropin-releasing hormone (GnRH) is a decapeptide that is synthesized and secreted by neuroendocrine cells of the hypothalamus, primarily in the arcuate nucleus. GnRH binds to specific cell membrane receptors on gonadotrophs in the anterior pituitary, resulting in the synthesis and secretion of two protein hormones—follicle-stimulating hormone (FSH) and luteinizing hormone (LH)—both named for their effects in females. Both FSH and LH are similar to thyroid-stimulating hormone (TSH) in that all three consist of two subunits and all share the same α subunit. Each has a different β subunit, however, which confers their functional specificity.
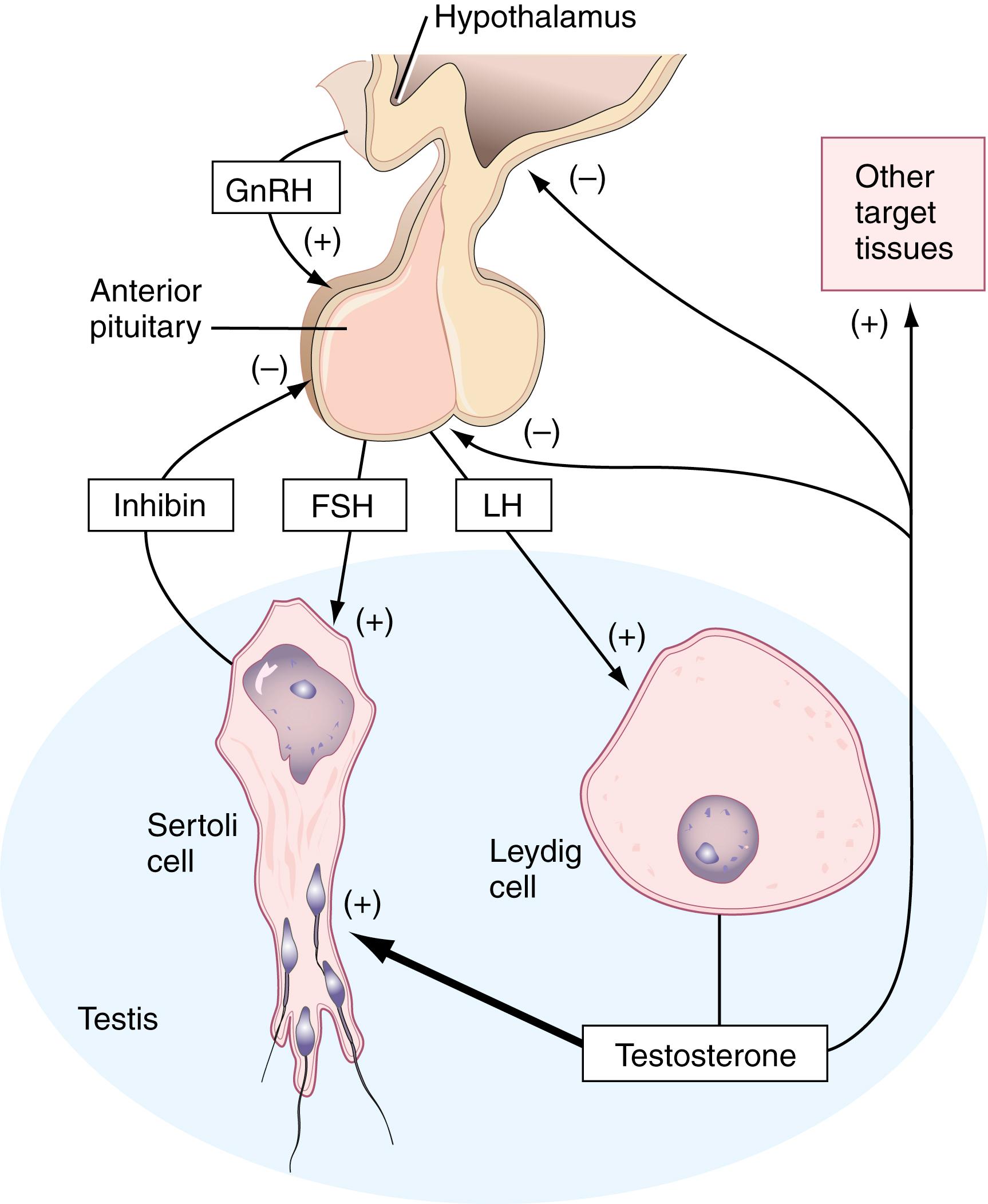
The seminiferous tubules of the testis contain cells in various stages of spermatogenesis (spermatogonia, spermatocytes, spermatids), along with Sertoli cells and Leydig cells. FSH induces Sertoli cells to synthesize and secrete androgen-binding protein into the lumen of the seminiferous tubule, which maintains the high testosterone concentration required for normal spermatogenesis. LH induces Leydig cells to synthesize testosterone; some enters the general circulation and is transported to other target tissues, such as skeletal muscle, where it has an anabolic effect. Some testosterone is also transported to the hypothalamus and anterior pituitary, where it has a negative-feedback effect—that is, it decreases and helps control the synthesis and secretion of GnRH, FSH, and LH. This, in turn, decreases testosterone synthesis by Leydig cells. Sertoli cells also synthesize and secrete another protein hormone—inhibin—which is a dimeric protein composed of an α and β subunit. It interacts with gonadotrophs in the anterior pituitary, where it also has a negative-feedback effect by decreasing the synthesis and secretion of FSH, but not LH. Androgens also stimulate inhibin production, which locally helps regulate spermatogenesis. However, the exact role of inhibin in the regulatory process is not understood.
Figure 26.3 summarizes the regulation of reproduction in the human female. As in the male, GnRH from the hypothalamus increases the synthesis and secretion of FSH and LH from the anterior pituitary. Unlike in the male, however, the regulatory process in the female is cyclic and is referred to as the menstrual cycle . Pituitary, ovarian, and uterine changes that occur during the menstrual cycle are summarized in Figure 26.4 . As illustrated, the cycle begins with menses, or shedding of the uterine endometrium, which is considered day one. During this period, FSH secretion from the anterior pituitary recruits a cohort of about 10 to 12 antral follicles to begin further growth and development within the ovaries. From this cohort, one to two (usually) antral follicles are selected to become the dominant follicle, continuing to grow and develop. The other recruited follicles undergo regression, or atresia . This process of follicular selection and maturation is known as folliculogenesis and occurs during the follicular phase (see Fig. 26.4 ). As the follicle grows, increasing amounts of estradiol are synthesized and secreted from the granulosa cells via FSH-induced aromatase activity. Estradiol acts to restore the endometrium via cell proliferation and growth—hence, the proliferative phase of the uterine endometrial cycle. Estradiol also has a negative-feedback effect on the hypothalamus and the anterior pituitary, decreasing FSH levels during the latter part of the follicular phase.
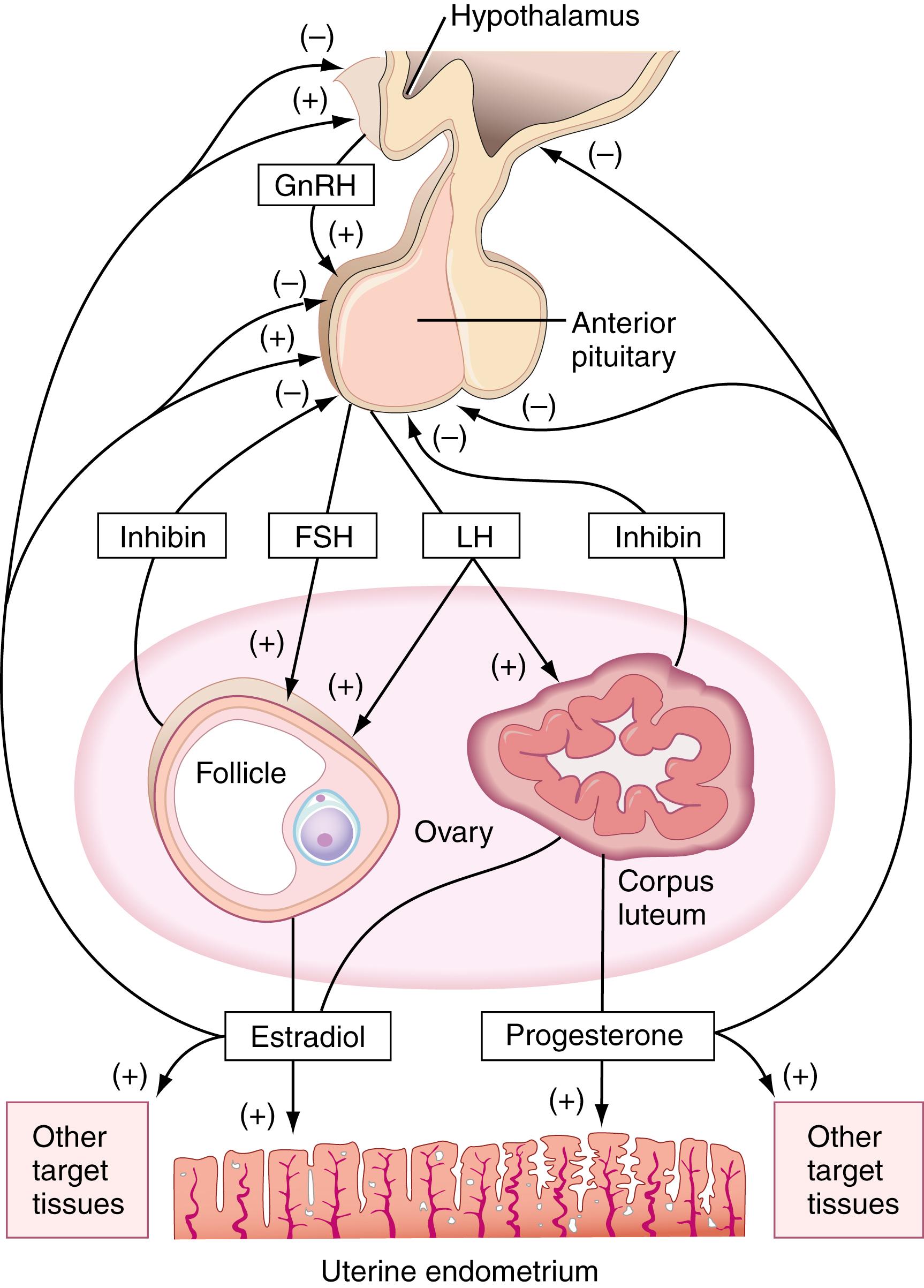
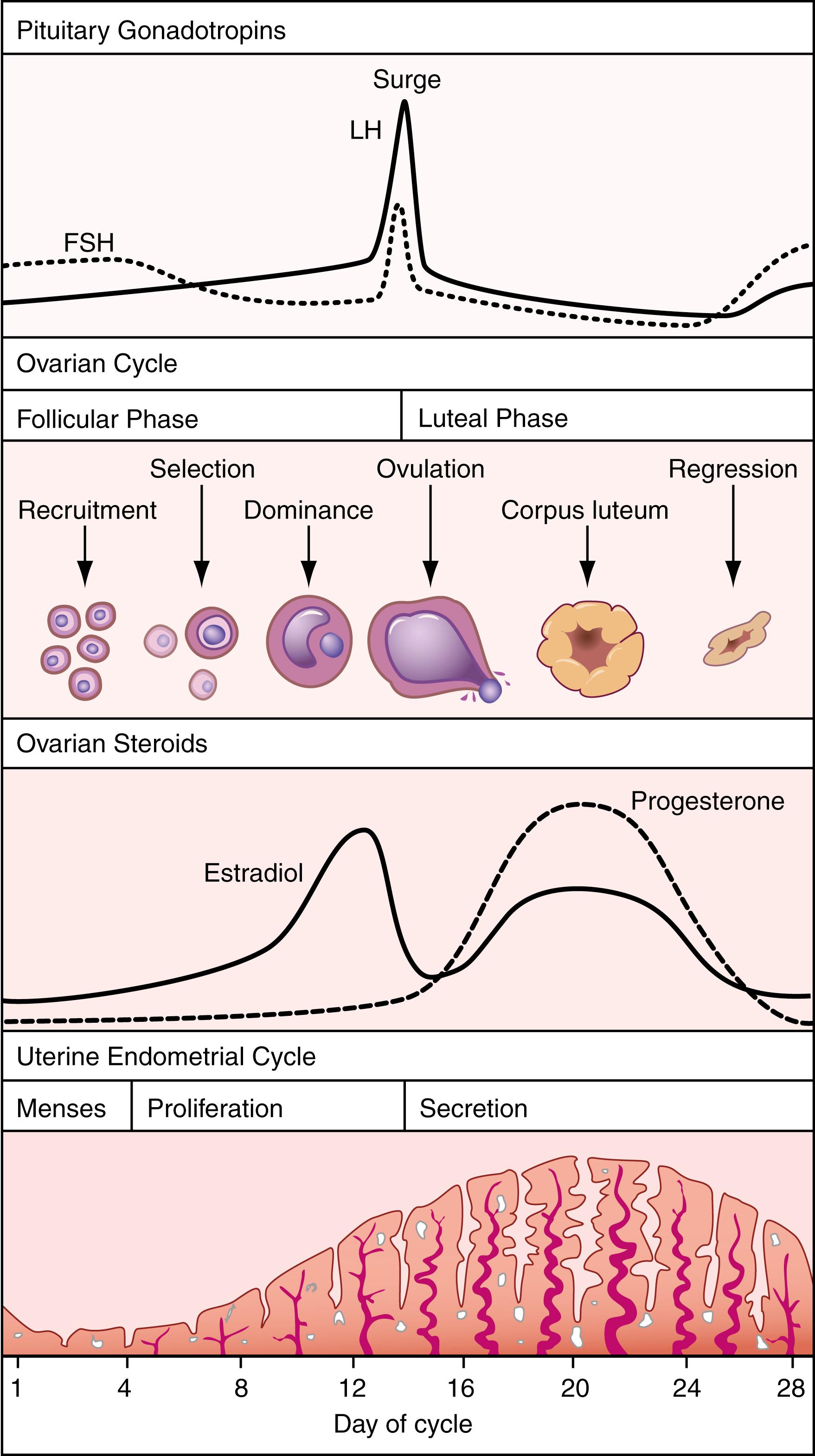
Near the end of the follicular phase, when estradiol levels are at their highest, around 200 to 300 IU, its feedback effect on the hypothalamus and anterior pituitary switches from negative to positive. The exact mechanism for this change to positive feedback is not understood. Its effect, however, is dramatic. It causes a surge in the secretion of GnRH, FSH, and particularly LH, which culminates in ovulation. The LH surge causes final oocyte maturation with the completion of meiosis in the ovaries before the follicle bursts, releasing the oocyte into the fallopian tubes. Owing to disruption of the growing follicle, estradiol synthesis and secretion drop dramatically. The disrupted follicle begins to differentiate into the corpus luteum; thus begins the luteal phase of the ovarian cycle. The corpus luteum synthesizes estradiol and progesterone as a result of the action of LH. The combination of the two steroids acts on the uterine endometrium to cause development of numerous exocrine glands, producing the secretory phase of the uterine endometrial cycle. This phase prepares the endometrium for implantation should fertilization and early development occur. LH levels decline gradually during the luteal phase, indicating restoration of negative-feedback regulation of the hypothalamus and anterior pituitary by estradiol and progesterone.
As in the male, inhibin has a selective negative-feedback effect on FSH. In the female, however, there are two different forms. One is synthesized and secreted by the developing follicles (inhibin B), and the other is from the corpus luteum (inhibin A) ( ). Inhibin B reaches a peak in the early- to midfollicular phase and a second peak at ovulation. Inhibin A reaches its peak in the midluteal phase. Again, the exact regulatory roles they play are not understood.
In a normal fertile cycle, the corpus luteum begins to regress near the end of the luteal phase. This results in a decrease in estradiol and progesterone synthesis and secretion. Because these steroids are required for maintenance of the secretory endometrium, it begins to deteriorate and is ultimately shed during menstruation. With the drop in estradiol and progesterone, their negative-feedback effects decrease, and FSH and LH rise to begin another cycle.
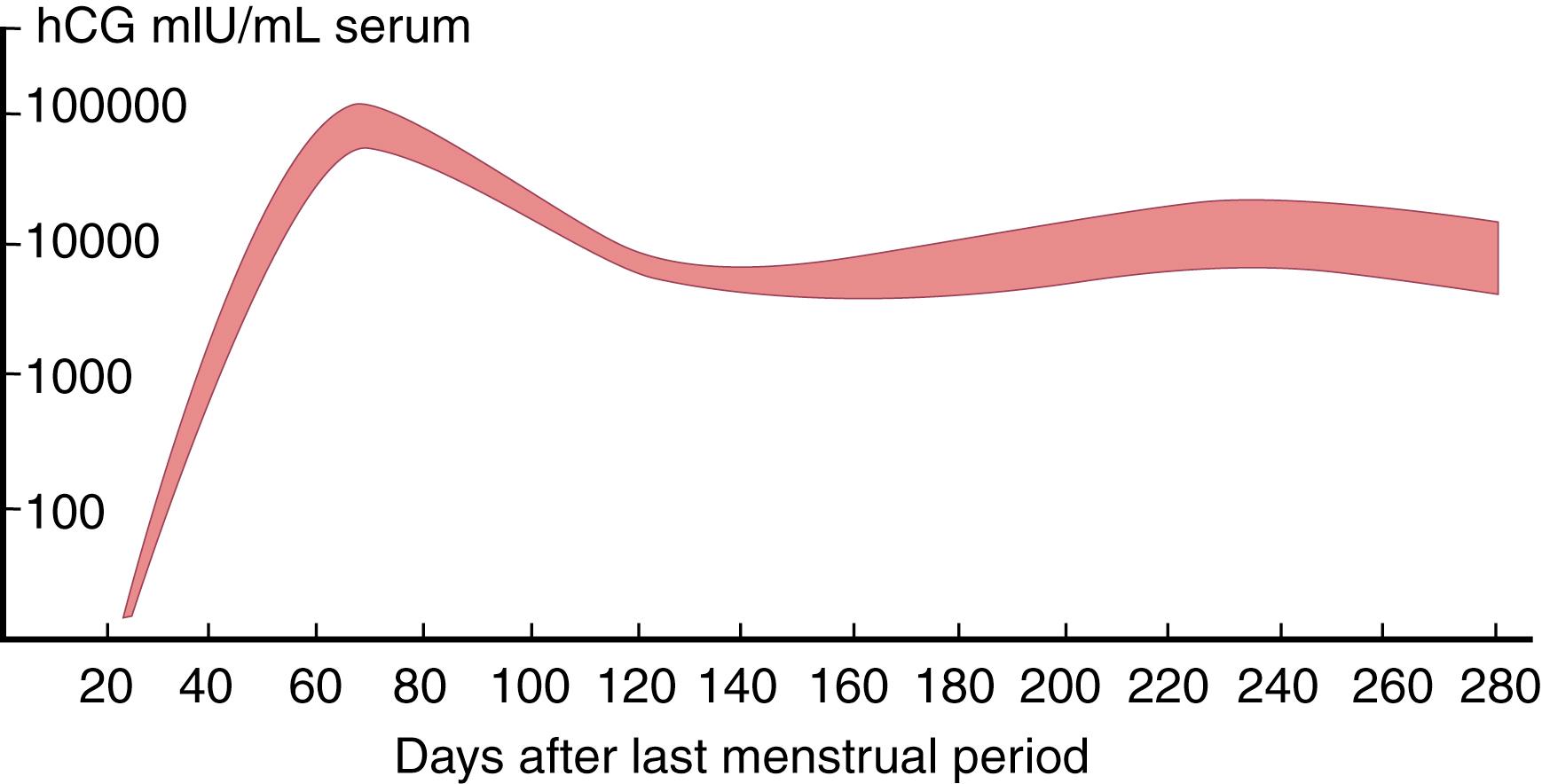
If the oocyte is fertilized during its transit down the fallopian tubes, also known as the oviduct , it develops into a multicellular blastocyst by the time it reaches the uterus. Around the time of implantation (about 7–9 days after ovulation) and before the corpus luteum begins to regress, increasing amounts of an LH-like hormone, human chorionic gonadotropin (hCG), are found in maternal blood. hCG is synthesized and secreted by the syncytiotrophoblast cells of the developing placenta. It is a dimeric protein hormone that has the same α subunit as LH, FSH, and TSH, but a different β subunit. The β subunit of hCG is very similar to that of LH, but larger. Hence, hCG can interact with LH receptors on luteal cells. This interaction prevents regression of the corpus luteum and allows it to continue synthesis and secretion of estradiol and progesterone, both of which are required for appropriate maintenance of the uterine endometrium throughout pregnancy.
During the first trimester of pregnancy, hCG increases from less than 5 mIU/mL serum to possibly more than 100,000 mIU/mL ( Fig. 26.5 ). This increase is responsible for the similarly dramatic increases in the levels of estradiol and progesterone. By the end of the first trimester, however, hCG levels begin to plateau at first and then decline significantly. Estradiol and progesterone continue to increase because, by this time, the placenta has assumed most steroid synthesis, including significant amounts of estrone and estriol. Unlike hCG, these steroid levels increase as placental mass grows. Steroid intermediates from the fetal adrenal gland and the fetal liver also contribute to this process. For example, estriol is synthesized not by hydroxylation of estradiol (see Fig. 26.1 ) but rather by conversion from fetal 16-hydroxy-DHEAS. This is why maternal estriol measurements were historically used to assess fetal well-being in late pregnancy ( ). The estrogenic potency of estriol is only about one-hundredth that of estradiol and only one-tenth that of estrone. However, estriol promotes uteroplacental blood flow as potently as other estrogens, which may be the reason for its dramatic increase during the last two trimesters ( ).
Another hormone synthesized in large quantities by the placenta during the last two trimesters is human placental lactogen (HPL). It is structurally similar to both prolactin and growth hormone and has both lactogenic and growth-promoting activities, although both are relatively weak. In addition, HPL is an insulin antagonist and thus may play a role in maternal glucose utilization. However, its exact role is unclear because apparently normal pregnancies have been described in which HPL is not detectable in maternal blood or placenta ( ). Unlike hCG, the increase in HPL also parallels the steady increase in placental mass ( ).
The placenta also synthesizes inhibin. Whereas gonadal (testicular and ovarian) inhibin functions as an endocrine inhibitor of pituitary FSH secretion, placental inhibin may function as a paracrine or autocrine inhibitor of placental hCG secretion ( ). Its exact regulatory role, however, has not been elucidated. It is also sometimes used as a marker in fetal testing (described later).
Parturition is the process by which the fetus is expelled from the internal environment of the maternal uterus to the external environment. This occurs as a result of a change in the activity of the uterine myometrium (smooth muscle), from irregular, long-lasting, low-frequency contractions to regular, high-intensity, high-frequency contractions. The initiation of this process in humans is not well understood and seems to differ from that in other mammals. It has been shown that progesterone inhibits uterine contractions during gestation in most other mammals and that the onset of parturition is preceded by a significant decrease in maternal plasma progesterone. In humans, however, this does not occur. In humans, it has been hypothesized that what is known as a parturition cascade may occur, which involves multiple and redundant endocrine, paracrine, and autocrine mediators. These mediators are thought to include fetal cortisol and DHEAS; placental estriol, oxytocin, prostaglandins, and corticotropin-releasing hormone; and maternal oxytocin. The effects of these mediators may also be modulated by changes in their receptor levels ( ).
Parturition is followed by lactation. Initial development of the mammary gland occurs at puberty, when estradiol causes growth and branching of the ducts and progesterone causes formation of the alveoli. Similarly, during pregnancy, the high concentrations of estrogens and progesterone cause further branching of the ducts and growth of the alveoli. The secretory capability of the alveolar epithelial cells is induced by prolactin, which increases during pregnancy, and by HPL. At parturition, the decrease in estrogen and progesterone eliminates their inhibitory effects on milk secretion, which still requires prolactin. Milk ejection, however, requires oxytocin, acting as part of a neuroendocrine reflex. This reflex is initiated by the suckling stimulus, which generates nerve impulses that travel from the nipple to the hypothalamus. There, they cause secretion of oxytocin from neuroendocrine cells in the posterior pituitary. The oxytocin travels to the mammary gland, where it stimulates the contraction of smooth muscle cells surrounding the alveoli, causing milk ejection. Prolactin also participates in this neuroendocrine reflex, in that nerve impulses reaching the hypothalamus also inhibit the synthesis and secretion of dopamine, which functions as a prolactin release–inhibiting hormone. This promotes the release of prolactin from the anterior pituitary, which then travels to the mammary gland, where it stimulates milk secretion, to replace that lost by ejection. As long as lactation continues, plasma prolactin concentrations remain elevated. This postpartum hyperprolactinemia causes postpartum amenorrhea due to interference with normal regulation by GnRH of FSH and LH secretion. Although this hypogonadotropic state will prevent pregnancy, it is not considered a reliable means of contraception, particularly in modern societies where breastfeeding is not consistent.
Table 26.1 describes laboratory findings in various male and female reproductive disease states typically categorized according to (1) hormone deficiency or excess and (2) primary (gonad) or secondary (pituitary) dysfunction. In primary disease states, gonadal steroid levels are inversely related to pituitary gonadotropin levels, whereas in secondary disease states, they are directly related (e.g., both high or both low), as discussed in the endocrine section of Chapter 9 and in Chapter 25 . These changes occur because gonadal steroids provide negative feedback to gonadotropins. For example, in primary ovarian failure or menopause, the decrease in estradiol reduces its negative-feedback effect on the hypothalamic-pituitary axis, resulting in increases in FSH and LH.
| DISEASE STATE | HORMONE LEVEL | ||||
|---|---|---|---|---|---|
| Classification | Example | FSH | LH | Testosterone | Estradiol |
| Male | |||||
| Primary deficiency | Klinefelter syndrome | High | High | Low | – |
| Secondary deficiency | Panhypopituitarism | Low | Low | Low | – |
| Primary excess | Testicular tumor | Low | Low | High | – |
| Secondary excess | Precocious puberty | High | High | High | – |
| Other | Seminiferous tubule failure | High | Normal | Normal | – |
| Other | Partial androgen insensitivity | Normal | High | High | – |
| Female | |||||
| Primary deficiency | Menopause | High | High | – | Low |
| Secondary deficiency | Sheehan syndrome | Low | Low | – | Low |
| Primary excess | Feminizing ovarian tumor | Low | Low | – | High |
| Secondary excess | Gonadotropin-producing tumor (rare) | High | High | – | High |
| Other | Polycystic ovarian syndrome | Normal | High | High | – |
| Other | Masculinizing ovarian tumor | Low | Low | High | – |
The evaluation of reproductive dysfunction in the male usually begins with semen analysis because it is a cost-effective and relatively simple procedure. In addition, if the results are normal, further evaluation is often unnecessary. If semen analysis is abnormal, then hormone analyses are performed, which include FSH, LH, and testosterone levels.
In addition to its use in the evaluation of reproductive dysfunction, particularly infertility, semen analysis is used to select donors for therapeutic insemination and to monitor the success of surgical procedures, such as varicocelectomy and vasectomy. Semen analysis consists of microscopic and macroscopic components; the latter include measuring physical (e.g., volume) and chemical (e.g., pH) properties. Useful guides and references for the procedure are available ( ; ; ; ; ; ).
The patient should be instructed to collect semen after 2 to 5 days of sexual abstinence to ensure that the sperm count will be at its highest and improve the reliability of the test. Longer periods of abstinence usually result in a higher sperm concentration but reduced sperm motility. For semen collection, the laboratory should provide a sterile plastic (polypropylene) container with a screw top. The semen specimen should be delivered to the laboratory within 1 hour of collection and kept warm during transportation. A postejaculate urine sample may be collected at this time if retrograde ejaculation is suspected. Two specimens collected within 2- to 3-week intervals should be used for evaluation. If they are markedly different, additional specimens should be collected. Ideally, semen specimens should be collected in the privacy of a room adjacent to the laboratory, because some samples require sperm to be separated from seminal plasma as soon as possible. Assisted reproductive technologies (ARTs; see later discussion), such as in vitro fertilization (IVF), require that motile sperm be isolated from seminal plasma within 1 hour of ejaculation to protect sperm from the inhibitory effects of seminal plasma on fertilization. Semen should be obtained by masturbation; if circumstances preclude such collection, special Silastic condoms should be made available for semen collection with intercourse. Incomplete semen specimens should not be analyzed because they can give inaccurate values.
Macroscopic examination should be performed after liquefaction, which usually occurs in less than 20 minutes at room temperature. Failure to liquefy may indicate inadequate prostate secretion. Semen should be thoroughly mixed before examination and its viscosity recorded. The volume of ejaculate can be measured by weighing the collection cup before and after specimen collection. The appearance of a yellow hue in a semen specimen is associated with pyospermia; a rust color is due to small bleedings in the seminal vesicle. The pH ranges from 7.2 to 7.8; however, it may be 8.0 or higher with acute infection in the prostate, seminal vesicle, or epididymis. The pH will be 7.0 or lower if there is contamination with urine, an obstruction in the ejaculatory ducts, or if the specimen consists of mainly prostatic fluid.
Microscopic examination should be performed to obtain estimates of sperm concentration, motility, morphology, and agglutination. Other cellular elements, such as polygonal cells of the urethral tract and round cells such as spermatogenic cells and leukocytes, can also be observed when sperm are counted in a hemocytometer or Makler chamber. Because sperm motility and velocity are temperature dependent, these parameters must be assessed on a microscope with a warm stage. At least four different fields of two specimen aliquots should be counted and the mean of the eight separate readings recorded. Total sperm count is then calculated by multiplying the dilution factor (normal concentration range, 15–50 million/mL) by its volume (normal range, 2–5 mL).
Total motility (normal range, 40% or above) is expressed as the percentage of sperm that move, including twitch. Progressive motility (normal range, 32% or above) is expressed as the percentage of sperm that move in a forward, linear motion. In addition, forward movement is graded. Sperm that move rapidly in a straight line with little yaw and lateral movement are grade 4 or, if they move more slowly, grade 3. Grade 2 sperm move even more slowly and with substantial yaw. Grade 1 sperm have no forward progression. Zero progression denotes absence of any motility. If motility is less than 30%, a viability stain of eosin Y with nigrosin as a counterstain is done. In bright-field microscopy, dead sperm will stain red, whereas live sperm will exclude the dye and appear unstained. In samples with no visible sperm, such as postvasectomy semen, the entire sample should be centrifuged and the pellet examined for intact or damaged sperm fragments. The analysis should be repeated in 4 to 6 months.
Agglutination occurs when motile sperm stick to one another in an orientation that is reproducible within a given specimen, such as head to head, tail to tail, midpiece to midpiece, or mixed ways, depending on the specificity of sperm antibodies. Agglutination suggests an immunologic cause of infertility, and a description of the type of agglutination should be recorded. This can usually be distinguished from clumping due to bacterial infection or tissue debris, which typically involves nonspecific orientation of the sperm.
Round cells should be differentiated into two classes: immature germ cells with a single or double highly condensed nucleus with a relatively large area of cytoplasm, and polymorphonuclear leukocytes, which are smaller than the germ cells and have a lower nuclear/cytoplasmic ratio. Peroxidase staining specifically identifies the polymorphonuclear leukocytes in the presence of lymphocytes and other cells that normally occur in semen. Bacterial contamination should be noted, as should the presence or absence of epithelial cells. If no sperm are seen in association with a low semen volume, a fructose test should be performed to confirm the presence of fluid from the seminal vesicle; the same test should be performed on postejaculate urine to rule out retrograde ejaculation. However, the significance of a fructose test result has declined over the years because of the availability of more direct diagnostic tools, such as transrectal ultrasonography.
Sperm morphology can predict fertility ( ). In 2010, the World Health Organization (WHO) standardized the new normal values based on data from men with proven fertility. These men were defined as men who were known to help their partners conceive in the previous 12 months. Typically, more than 4% of the sperm in a semen specimen should exhibit normal morphology. Kruger and colleagues (1988) developed strict criteria for normal sperm morphology. Morphologically abnormal sperm usually have multiple defects; the average number of defects per sperm, designated as the teratozoospermic index , is a significant predictor of sperm function both in vivo and in vitro. A strict morphology score of greater than 4% of normal indicates excellent fertilizing capacity. Scores between 0% and 3% predict probable inability to fertilize. Wide variability in the size of the acrosomal cap is the most obvious characteristic of abnormal sperm. An acrosomal cap less than one-third of the head surface is considered abnormal, as are retention of a cytoplasmic droplet greater than one-half of the head size and a tail less than 45 μm long. Of particular note is the direct relationship between acrosome size and the frequency of fertilization or pregnancy.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here