Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
![]() This chapter includes an accompanying lecture presentation that has been prepared by the authors: .
This chapter includes an accompanying lecture presentation that has been prepared by the authors: .
Minimizing the invasiveness and rate of complications is very crucial in management of already severely disabled epileptic patients.
Low rates of surgical complications and neurological consequences after radiosurgery are the main advantages over open surgery techniques.
Radiosurgery is currently being evaluated as an alternative (or adjunct) treatment modality to open resective microsurgery for intractable temporal lobe epilepsy.
Radiosurgery can be considered a first-line intervention for small to middle-sized hypothalamic hamartoma (HH; types I–III) associated with epilepsy.
Absence of major complications and neurological consequences and availability of a stepwise strategy are the main advantages of radiosurgical callosotomy.
Radiosurgery is the precise application of focused radiation under stereotactic guidance to a targeted volume area within the brain identified on MRI, allowing the neurosurgeon to deliver effective, precise, and accurate doses of radiation to a smaller volume without affecting large portions of normal parenchyma, thereby allowing a powerful radiobiologic effect on the chosen targeted volume. Conceptualized by Lars Leksell for use in functional neurosurgery, radiosurgical treatment of neurological disorders has progressively widened its utility and is now a treatment modality option for several neoplastic and vascular indications.
Epilepsy is one of the most common serious neurological diseases; it has a prevalence of 0.5% to 1.0% in the US population, with approximately 20% of these patients having medically refractory seizures. , Patients with medically refractory seizures may be referred for possible surgical management, and many are found to be suitable candidates for open surgical resection of a seizure focus.
The most common type of open surgery performed for temporal lobe epilepsy is anterior temporal lobectomy. , With modern advances in surgical and anesthetic techniques, microsurgical resection of mesial temporal lobe structures can be performed with low morbidity and even lower mortality. Open invasive surgical procedures, however, have inherent risks, including structural damage, hemorrhage, blood loss, infection, and anesthetic risks. Several clinical studies evaluating the morbidity and mortality associated with open microsurgery for temporal lobe epilepsy have reported that approximately 5% to 23% of patients undergoing open microsurgery experience a symptomatic neurological deficit postoperatively. , , , , In addition, patients may have their epileptic focus in regions that are difficult to access or in eloquent functional regions of the brain where surgical resection could result in irreversible language, motor, or visual impairment. ,
Radiosurgery is currently being evaluated as an alternative treatment modality to open resective microsurgery for intractable temporal lobe epilepsy. Radiosurgery is relatively noninvasive, with frame-based radiosurgery using just frame pins that penetrate only the skin to firmly fix the stereotactic frame to the skull. The highly focused nature of radiosurgery allows stereotactic guidance and sparing of adjacent tissues from the damaging effects of radiation, abrogating many of the risks and limitations of open surgery and permitting patients to return to full activity within 1 to 2 days after treatment. Radiosurgery can also be used as an adjunct therapy to initial open surgical resection of a hippocampal focus, although no formal studies are available on this specific group of patients. Currently, radiosurgery is under investigation as a treatment modality for epilepsy associated with vascular malformations, hypothalamic hamartomas (HHs), and medial temporal lobe epilepsy (MTLE) associated with mesial temporal sclerosis (MTS). , , ,
Radiosurgery is considered to be effective by means of a destructive tool on neural tissues. Since its invention by Leksell in the 1950s, clinical and experimental studies of radiosurgery has demonstrated that for classical indications—for example, arteriovenous malformations (AVMs) and benign tumors—radiosurgery is effective because of its specific histologic effects of thrombotic endothelial proliferation and apoptosis, not simple coagulative necrosis. In functional neurosurgery indications, the strategy is either to target a “small volume” of normal tissue (i.e., ventro-intermediate nucleus, capsulotomy, trigeminal neuralgia) with a high dose (80–140 Gy at maximum) or to target a large volume of tissue (i.e., 5–9 cm 3 in epilepsy radiosurgery) with a moderate dose (17–24 Gy at the marginal isodose).
These radiosurgical procedures have been proposed, technically performed, and evaluated on the basis of the hypothesis that their mechanism of action is purely destructive. However, modern neurophysiologic, radiologic, and histologic analyses are leading us to question this assumption. Tissue destruction is turning out to be either absent or minimal. Therefore one possibility is that radiosurgery is inducing changes in the functioning of the neurons by inducing remodeling of the glial environment and is leading to the modulation of function while preserving basic processing. Thus most radiosurgery procedures may induce the desired biologic effect without requiring the histologic destructive effect for completion of the therapeutic objective. Therefore the concept of “lesioning” radiosurgery may be incorrect, and a completely hidden world of neuromodulation may remain to be discovered.
Preclinical studies investigating focused high-dose radiosurgery in animal models of epilepsy have demonstrated the potential utility of radiosurgical treatment applied to nonhuman epilepsy models. Early animal experiments indicated the efficacy of focused irradiation in a feline model of epilepsy in reducing seizure activity. , , Sun and colleagues reported that seizure thresholds in rats treated with 10 or 40 Gy at the 90% isodose line were significantly increased, and the length of afterdischarges was significantly decreased in the group treated with 40 Gy. Corroborating this report, histologic analysis of temporal lobe regions treated with radiosurgery doses of 10 to 40 Gy at the University of Virginia found no necrosis in these tissue specimens. Synaptically driven neuronal firing was reported to be intact in these radiosurgically treated rodent brain slices, suggesting that functional neuronal death was not responsible for the identified reduction in seizures.
Experiments at the University of Pittsburgh were undertaken to evaluate the histopathologic and behavioral effects of “subnecrotic” radiosurgery doses. , In this animal investigation, rats underwent stereotactic injection of kainic acid into the hippocampus to induce seizures, followed by Gamma Knife radiosurgery (GKRS) at doses of 30 or 60 Gy. A statistically significant reduction in seizures was reported in all radiosurgically treated animals, and this antiepileptic effect was observed earlier in the animals treated with the higher radiosurgery dose (weeks 5–9 versus weeks 7–9). No animals treated with radiosurgery were reported to demonstrate a deficit in new memory attainment tasks on water maze testing in comparison with control animals injected with only kainic acid, but both groups showed “cognitive” impairment when compared with rats that did not receive any kainic acid injection or radiosurgical treatment. In histopathologic analysis by two blinded observers, unilateral hippocampal atrophy was also observed in 25 of 46 injected animals. Radiation-induced necrosis matching the target volume of radiation was not reported in any of the animals treated with radiosurgery. These preclinical animal findings suggest that reduction of seizure activity after radiosurgery does not require necrosis or concomitant functional loss of treated neurons.
With the suggestion that necrosis is not necessary for reduction of seizures, the radiosurgery group in Prague reported on their preclinical characterization of a “subnecrotic” dose of radiosurgery in a rat model. , This preclinical investigation evaluated radiosurgery doses of 25, 50, 75, or 100 Gy delivered bilaterally to the rat hippocampus and then assessed the rats with cognitive tests, MRI, and histopathologic examinations at 1, 3, 6, and 12 months after radiosurgery. A progressive time- and dose-dependent response curve was observed in cognitive memory function, edema on MRI, and necrotic histopathology. All animals treated with 75 Gy displayed cognitive memory functional impairments, edema on MRI, and radiation-induced necrotic lesions, whereas only one of the animals treated with the 50-Gy radiosurgery dose had observable edema and necrosis. A second follow-up preclinical study in which a 35-Gy radiosurgery dose was used with a long-term follow-up period of 16 months found that 6 months after radiosurgical treatment, edema was observed on MRI, and this edema was most pronounced at 9 months after radiosurgery. After 16 months, two of six treated animals were reported to have radiation-induced necrotic cavities after treatment with a 35-Gy dose of radiosurgery. The four treated animals without frankly necrotic cavities had other notable histopathologic findings, such as severe atrophy of the corpus callosum (CC), loss of thickness of the somatosensory cortex, and damage to the stratum oriens hippocampi. These preclinical animal studies suggest that the full radiobiologic and histopathologic effects of radiosurgery may manifest several months after radiosurgery.
These preclinical animal studies suggest that the antiepileptic efficacy of radiosurgery is dose dependent, with a dose of approximately 25 Gy being required to induce a therapeutic antiepileptic effect, and the potential full histologic and other toxicity may require several months to fully develop. , , , ,
The first radiosurgical treatments for epilepsy patients were performed by Talairach in the 1950s. As early as 1974, Talairach reported on the use of the radioactive implants (yttrium) in epileptic patients with MTLE without space-occupying lesions and showed a high rate of seizure control and cessation in patients with epilepsies confined to the mesial structures of the temporal lobe. In 1980 Elomaa demonstrated the idea of the use of focal irradiation for the treatment of temporal lobe epilepsy based on the preliminary reports of Tracy, Von Wieser, and Baudouin. , Furthermore, clinical experience of the use of GKRS and linac-based radiosurgery in AVMs and cortico-subcortical tumors revealed an anticonvulsive effect following radiosurgery without tissue destruction or necrosis. , , A series of experimental studies in animals confirmed this effect , and emphasized a relationship to the dose delivered. One of the limitations inhibiting radiosurgery research is the difficulty in recruiting patients to prospective or randomized trials directly comparing radiosurgery with open surgery.
Seizure control and cessation may be generated by a specific neuromodulatory action of radiosurgery technique, without induction of a significant degree of histologic necrosis or destruction. The selection of the appropriate radiosurgical parameters (e.g., dose, target volume) allowing us to accurately obtain the desired functional outcome and effect without histologic damage remains a very important challenge. A detailed review of these cases, as well as other clinical and experimental studies, suggests that radiosurgery is beneficial only to those patients in whom a clear preoperative definition of the extent of the epileptogenic zone and network has been achieved, and where strict rules of dose planning have been followed. The strategy must be to define the patients in whom the safety-efficacy ratio makes radiosurgery advantageous or at least comparable to craniotomy and surgical cortical resection.
MTLE associated with MTS is perhaps the most well-defined epilepsy syndrome responsive to structural intervention. MTS is an idiopathic process associated with extensive loss of neurons and an increase in astrocytes in the mesial temporal structures, which include the amygdala and hippocampus in the temporal lobe. When temporal lobe epilepsy is due to underlying MTS, improvements in seizures with open microsurgical structural resections can be expected in 65% to 90% of patients. , , , , This form of temporal lobe epilepsy is particularly amenable to structural interventions, such as radiosurgery, because 80% to 90% of these patients show detectable changes on MRI. ,
Radiosurgery has also been explored as an alternative to open microsurgery for MTS-associated MTLE. , Until the 1990s, curative epilepsy surgery was limited to open microsurgery, with which abnormal epileptogenic tissue was physically removed. In a small series of patients with MTLE treated with GKRS, Régis and colleagues , initially pioneered this technique in the mid-1990s and reported clinically effective amelioration of seizures with minimal morbidity. A prospective multicenter European study evaluating GKRS for MTS showed comparable efficacy rates (65%) for reduction of seizures by conventional microsurgery and radiosurgery after 2 years of follow-up.
After use of a marginal dose of 24 Gy, Régis and colleagues reported the first comprehensively evaluated series and demonstrated that radiosurgery can be used as an alternative to conventional open microsurgery to treat MTLE associated with MTS effectively and to improve quality of life with comparable rates of morbidity and mortality in a small case series. Several prospective trials from this group have demonstrated advantages of radiosurgery:
Safety and efficacy profile , ,
Specific clinical and radiologic timetable of events ,
Importance of the anterior parahippocampal cortex for seizure control and cessation ,
Importance of the marginal dose (24 Gy) for high efficacy
Feasibility of sparing verbal memory in dominant-side epilepsy ,
Nonlesional mechanism of action , ,
All these findings have been reconfirmed by a prospective trial in the United States. To date, the Marseille group has treated 287 patients with epilepsy surgery using GKRS among a total of 21,000 GKRS procedures since 1992. The majority of these patients were treated for MTLE (95 patients) or HH (124 patients), underwent a callosotomy (25 cases), or had severe epilepsy associated with small benign lesions (43 patients) for which an epileptic zone was considered to be confined to the surrounding cortex.
The international Radiosurgery or Open Surgery for Epilepsy (ROSE) trial was conducted comparing the efficacy and safety of radiosurgical doses and radiosurgery versus open temporal lobectomy in patients with MTS. This trial was based on a pilot clinical trial examining radiosurgical dosing in a direct comparison of 20 and 24 Gy of radiosurgery, finding that 67% of all patients were free of seizures for at least 12 months after treatment at the 36-month follow-up examination, with 10 of 13 in the 24-Gy group finding seizure freedom compared with 10 of 17 in the 20-Gy group. In comparison, historically, open resection was found to have a seizure reduction rate of 58% in a prospective trial by Wiebe and colleagues, whereas a multicenter parallel group controlled trial by Engel and colleagues in 2012 comparing anteromesial temporal lobe resection versus antiepileptic drug therapy confirmed these findings, with 11 of 15 patients treated with surgery becoming seizure free at 2 years compared with 0 of 23 in the medical group. A systematic review and meta-analysis showed an average pooled seizure reduction with anterior temporal lobectomy of 66%. , This pilot trial further examined the safety and efficacy of two dosing approaches and the effects, timing, and risks associated with this therapy.
Although the pilot trial demonstrated that radiosurgery is effective in improving MTLE-associated seizures, the beneficial effects of radiosurgery are not demonstrated immediately. Typically, patients with MTLE treated with radiosurgery can achieve improvement in seizures between 9 and 12 months, with dramatic improvement in seizures between 18 and 24 months after treatment.
A transient increase in partial seizures (auras) has been reported at approximately the same time that the complex seizures decrease, about 1 year after treatment. In addition, higher radiosurgical dosing increased steroid prescribing (temporary), new headaches (temporary), and visual field defects (permanent). Many patients require a temporary course of corticosteroids to treat the delayed radiation-induced edema associated with the initial radiosurgical effect, commonly 10 to 15 months after treatment, with 62% of those studied in the this pilot trial requiring steroids. In addition, the patients are still exposed to the risks of sudden death of epilepsy during this latent period; Srikijvilaikul and colleagues reported two posttreatment deaths during this latency period following radiosurgery, presumed to be due to sudden death from epilepsy, although these patients were treated with a lower dose of 20 Gy. There was no evidence that the radiosurgical treatment per se was responsible for these deaths.
One of the potential pitfalls of radiosurgical treatment of intracranial lesions is that it can expose the optic nerves to harmful radiation, causing posttreatment visual deficits, not unlike those potentially caused by open resection. Régis and colleagues found prospectively that 9 of 21 patients treated with radiosurgery for MTLE experienced new visual field defects. Hensley-Judge and colleagues prospectively found that 15 of 24 patients (62.5%) had postoperative visual field defects, all homonymous superior quadrantanopias, among pilot trial participants. These findings were similar to historical controls for open resection, demonstrating that this risk is still present even without brain retraction or true resection, and they did not significantly vary whether 20 Gy or 24 Gy was used. However, a greater proportion of patients who became seizure free developed postoperative visual field defects, possibly owing to a connection between wider resection margins and higher doses leading to greater destruction of the optic radiations, although this has not been proven.
One of the advantages of radiosurgery is its potential for relative preservation of memory in comparison with open resection. Open surgery for temporal lobe epilepsy entails risks of significant verbal memory impairment ranging from 10% to 60%. Radiosurgery also has some effects on verbal memory, although this is largely limited and has shown signs of improvement over time. When the Wechsler Memory Scale and the California Verbal Learning Test were used, significant verbal memory impairment was seen in 25% of dominant-hemisphere surgery patients and in 7% of nondominant-hemisphere surgery patients, for an average of 15%. In addition to these new deficits, however, significant improvement was seen in 16% of dominant-hemisphere and 7% of nondominant-hemisphere radiosurgery patients in this pilot study, although the mechanism for this improvement is not well understood. Patients more rigorously analyzed with the Boston Naming Test, California Verbal Learning Test, Wechsler Memory Scale, Trail Making Test, and Beck Depression Inventory at 24 months after radiosurgery were found to have no significant neuropsychological changes from their preoperative baseline.
The targets in functional radiosurgery (capsulotomy, thalamotomy of ventral intermediate [VIM] or the centromedian nuclei, pallidotomy) were treated using a high dose (300–150 Gy nuclei) delivered in very small volumes (3–5 mm in diameter). Barcia-Salorio et al. reported a small and heterogeneous group of patients treated with different types of radiosurgery and variable dosimetry plans according to the patient. Some of these patients were treated with very large volumes and very low dosage (10 Gy). These results led several other investigators to consider using very low doses, as low as 10 to 20 Gy at the margin, but to expect the same efficacy as the 24-Gy protocol (at the margin). This was the dose that was used for first series of patients with MTLE in Marseille. However, the real rate of seizure cessation and control was only 36% (4 of 11) among the 11 patients reported by Barcia-Salorio et al., and this was much lower than what we would expect with resection in MTLE. , ,
In another publication, Yang et al. confirmed that only a very low rate of seizure cessation is achieved when low doses (9–13 Gy at the margin) are used, based on the results in a heterogeneous group of 176 patients. Again, a deescalation study has demonstrated poorer results in patients receiving doses of 18 to 20 Gy at the margin compared with 24 Gy. , This finding is significant because any radiosurgical management strategy associated with a much lower rate of seizure control in MTLE is unacceptable because of the high rate of seizure freedom achievable by surgical resection. Fractionated stereotactically guided radiotherapy was used by Grabenbauer et al. in 12 patients; none of these patients become seizure free, and only seizure reduction has been obtained in this series. ,
Efforts to use lower doses to reduce radiographic changes consistent with tissue damage and edema have shown that there seems to be an effective dose threshold at or greater than 20 Gy. Recent dose studies have also suggested that a dose of 20 Gy or less at the margins may be less effective than higher marginal doses in reducing seizure activity. Cmelak and colleagues reported unsuccessful reduction of seizures with a 15-Gy marginal radiosurgery dose. Similarly, Kawai and colleagues reported two cases of radiosurgery with an unsuccessful antiepileptic effect at a marginal radiosurgery dose of 18 Gy. Finally, Srikijvilaikul and colleagues from the Cleveland Clinic also reported their series of ineffective radiosurgical treatment for seizure control with a 20-Gy marginal dose. Chang and colleagues showed that a significant radiographic change was correlated with a better seizure-free outcome, further corroborating these data.
When the target volume is a lesion, then it can be precisely defined radiologically and the question of the selection of the marginal dose can be easily addressed by correlating safety and efficacy with individual outcome to the marginal dose and can be refined based on stratification according to volume, location, age, and so on. However, this is not the case in patients with MTLE for two main reasons: there is no consensus regarding the required extent of mesial temporal lobe resection for optimum seizure control, and the concept of MTLE syndrome with a stable extent of the epileptogenic zone that can be defined as surgical target is controversial. , The volume, in association with marginal dose, is well known to be a major determinant of the tissue effect in radiosurgery, as shown in integrated risk/dose volume formulas. Therefore target volume definition and determination are crucial in the effectiveness of GKRS in patients with MTLE. In the first series of patients treated in Marseille, the marginal isodose volume was approximately 7 mL (range, 5–8.5 mL). In a published study, authors tried to correlate dose and volume, degree of MRI changes, and seizure control rates. In this study it was found that the higher the dose and the volume were, the higher the risk was of having more severe MRI changes, as well as the higher the chance was of achieving seizure control and cessation.
It is clear that effective dose planning with smaller prescription isodose volumes needs more precise definition of the essential targets in the mesial temporal lobe. This is a difficult main task, however. There is growing evidence in the current literature that defines the organization of the epileptogenic zone as a network. According to this hypothesis, the epileptogenic zone includes several different and possibly distant structures that discharge simultaneously at the onset of the electroclinical seizures. This perspective helps to explain the high failure risk in a simple lesionectomy without preoperative investigations in the management of severe drug-resistant epilepsies associated with a benign lesion. , This has been also reported in MTLE cases. , Therefore, precise definition of the target in radiosurgery is very critical. Wieser et al. analyzed postoperative MR images of patients who were operated on by Yasargil and underwent amygdalohippocampectomy. In this study they were able to correlate the degree of the resection of each substructure of the mesial temporal lobe and correlate the result with the seizure outcome cessation. The authors reported that only the resection amount of the anterior parahippocampal cortex was correlated strongly with a higher chance of seizure control and cessation. We in the Marseille group also tried to perform a similar study in patients treated with GKRS.
Whang and Kwon reported seizure cessation and control in 39% (12/31) of the patients who were treated with radiosurgery for epilepsy associated with slowly growing lesions. This observation as well as the arguments we summarized previously emphasize the importance of preoperative clear definition of the epileptic zone and network and its relationship with the target lesion. , Therefore the optimum philosophy is to choose appropriate preoperative investigations and management strategies in each case individually. In some patients the electroclinical information, structural and functional imaging, and neuropsychological examination may be sufficiently concordant, and surgery of the temporal lobe is proposed without depth electrode recording. In other cases preoperative assessment results may be discordant to define MTLE reliably, and a stereo-electroencephalographic (stereo-EEG) study is performed. Stereo-EEG implantation is used to assess the reliability of the primary hypothesis (mesial epileptogenic zone) or alternative hypothesis (early involvement of the temporal pole, lateral cortex, basal cortex, insular cortex, or other cortical areas). The aim is to record the patient’s seizures to define the temporospatial pattern of cortical involvement during these seizures. In these patients, depth electrode recording allows precise tailoring of the extent of surgical resection according to the temporospatial course of the seizures. Furthermore, if depth electrode investigation enables us to define a particular subtype of MTLE, then further tailoring of the treatment target volume, even reducing it, becomes feasible. Because the main limitation of radiosurgery is the size of the target (prescription isodose volume), the requirement for precision and accuracy to define the epileptogenic zone and network is higher in this technique. Although this requirement makes the GKRS planning for MTLE very challenging, it also makes it the most selective surgical modality for this group.
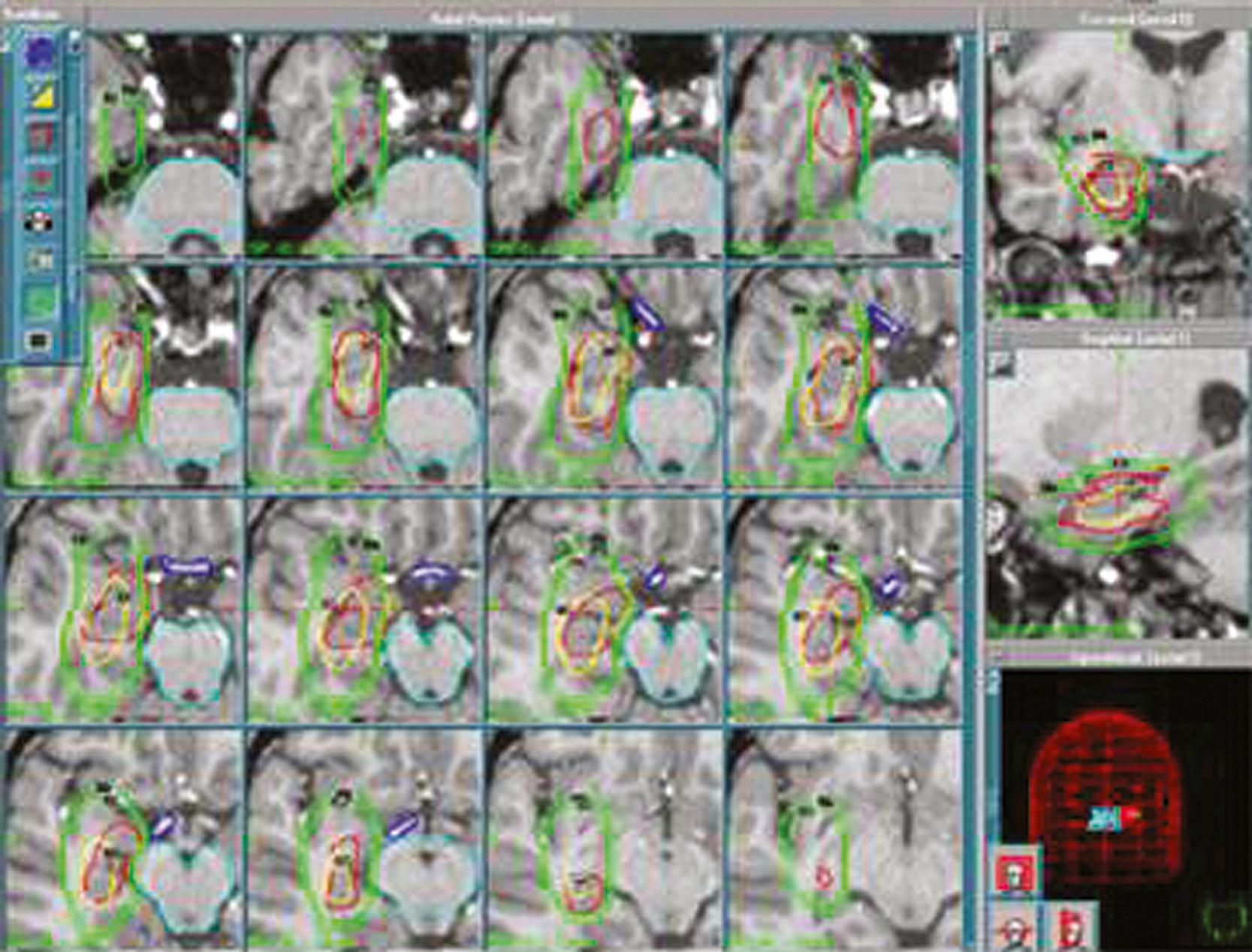
One of the difficulties in applying radiosurgery broadly as an application for intractable MTLE is the definition of the radiosurgical target because of a lack of clear boundaries of MTS ( Fig. 97.1 ). For example, Régis and colleagues radiosurgically targeted the mesial temporal lobe structures in their series, whereas Kawai and colleagues restricted their treatment to the amygdala or hippocampus structures, and each series reported varying rates of successful amelioration of MTLE. , , , Although target definition can vary among different neurosurgeons, radiosurgery for MTS-associated MTLE remains an attractive therapeutic option because of its effectiveness, low morbidity and mortality, and the consistent manifestations of this disease with identifiable imaging characteristics on MRI. Moreover, conventional open microsurgical temporal lobectomy is still possible if the initial radiosurgical treatment is ineffective after sufficient time has elapsed for the delayed radiosurgical antiepileptic effect.
Histologic examination of radiosurgically treated human mesial temporal tissue for MTLE has been limited because of the efficacy of radiosurgery for MTS-associated MTLE. However, histologic analysis of radiation-treated tissues has been reported in patients who underwent resection because of ineffective seizure control after radiosurgery. , , Histopathologic studies after subtherapeutic doses of 15 and 18 Gy found one of three patients with a necrotic focus with some prominent vascular changes consisting of vessel wall thickening and fibrinoid and hyaline degeneration. , When treated with a larger but subtherapeutic dose of 20 Gy, all five patients from a series reported from the Cleveland Clinic demonstrated histopathologic necrosis, perivascular sclerosis, and macrophage infiltration on resection and evaluation.
Currently the radiobiology of radiosurgery in the setting of MTS-associated MTLE is not completely understood. Although some preclinical studies have suggested an antiepileptic effect of radiation with subnecrotic doses, human clinical studies have suggested that a certain amount of tissue necrosis and histopathologic changes may be required to produce significant amelioration of MTS-associated seizures. The importance of this issue on biologic effect is that radiosurgical treatment of eloquent brain regions would be possible if an effective subnecrotic dose could be found.
Although radiosurgery has been shown to reduce seizures in various forms of medically intractable epilepsy, the mechanism by which this abatement occurs is not well understood. It has been suggested that radiation itself has a direct antiepileptic effect that can operate through several mechanisms. Because glial cells are more radiosensitive than neurons, Barcia-Salorio proposed that low-dose radiosurgery may reduce glial scar formation, allowing increased dendritic sprouting and improved cortical reorganization that results in fewer seizures. Elomaa theorized that the antiepileptic effect of radiation is further mediated through the effects of somatostatin. Although the clinical results of the most recent human studies suggest that the therapeutic efficacy of radiosurgery is linked to histopathologic changes and identifiable necrosis of mesial temporal structures, proof of this theory would need to come from direct observation and histologic evaluation of tissue samples from patients in whom radiosurgery has effectively controlled the seizures. This is unlikely to occur because only patients with persistent seizures after radiosurgery are likely to undergo further open resective microsurgery. Among patients who underwent open resection after radiosurgery, Kawai and colleagues and Srikijvilaikul and colleagues found necrotic foci with vessel wall thickening and fibrinoid and hyaline degeneration, perivascular sclerosis, and macrophage infiltration on resection on histopathologic analysis in two patients treated with 18 and 20 Gy, although no radiation-induced histopathologic changes were found in tissues treated with 15 Gy of radiosurgery, suggesting that some histologic damage may be needed for effective seizure control. a
aReferences 39, 44, 45, 48, 94, 109.
Animal studies have shown mixed results regarding this hypothesis, with some animal studies demonstrating improvement in seizures without evidence of necrosis, whereas others have shown direct structural, destructive lesions in the tissue zone to correlate with seizure remission. The mechanisms may be some combination of neuromodulation and neuronal destruction, with ischemic factors likely playing a role.
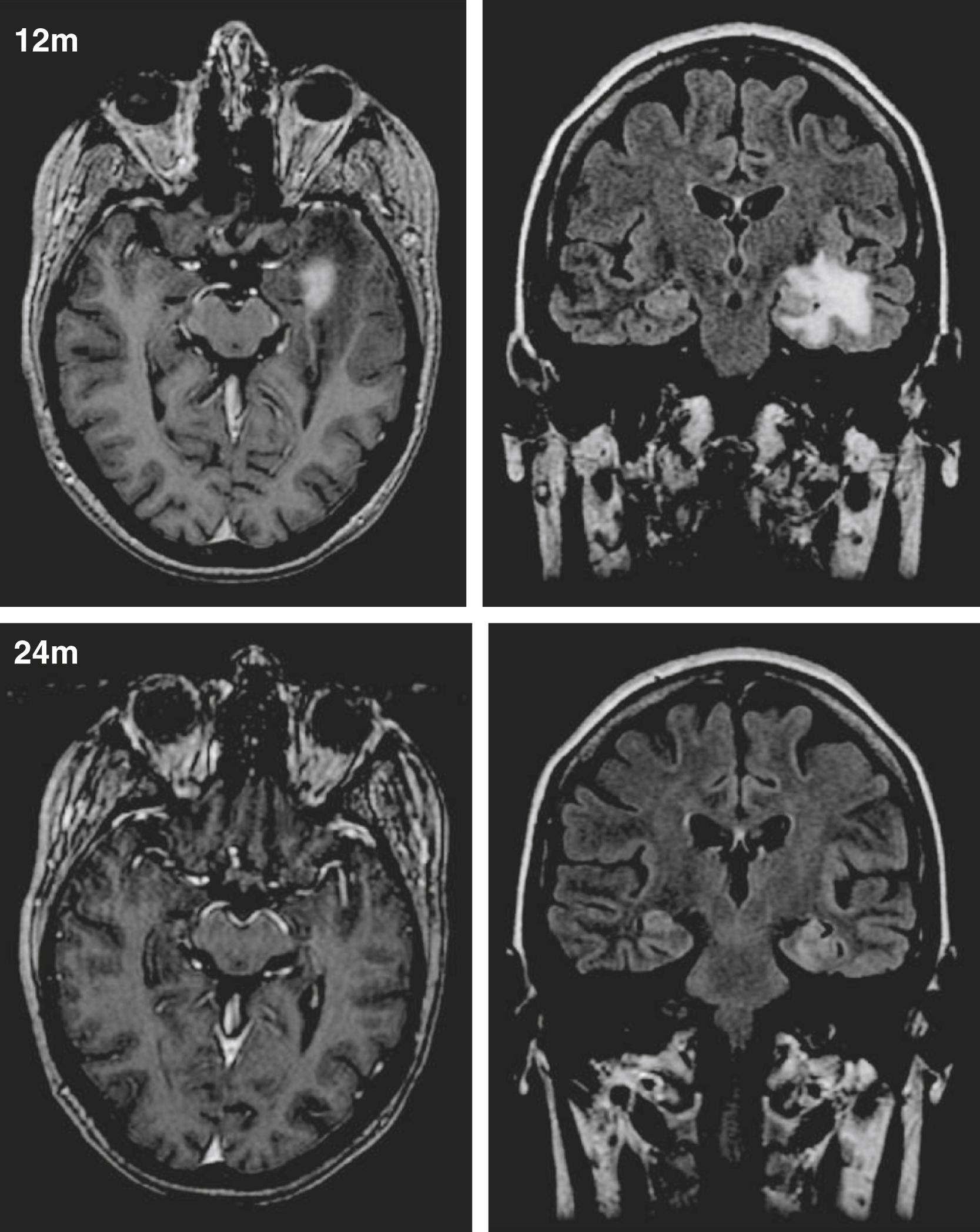
Surrogate markers of radiation effect and radiobiology, such as changes on MRI, are also showing some promising results. Radiation-induced edema typically becomes evident in most patients 9 to 15 months after radiosurgery ( Fig. 97.2 ). T2 hyperintensity volumes of edema were found to be closely related to seizure remission such that no seizure remission was found between 24 and 36 months in patients who had volumes of edema less than 200 mL at 12 months. These imaging findings, however, are usually time limited and are often followed by focal atrophic changes. Thus changes on MRI might not be diagnostic or indicative of true radiation necrosis. Furthermore, our pilot clinical trials have shown that MRI changes and peak MRI effects are poorly correlated with posttreatment symptoms. The actual biomechanism by which high-dose radiation and radiosurgery reduce neuronal hyperexcitability to ameliorate seizures will probably not be found or elucidated from human studies.
Although preclinical evidence and the results from early clinical human trials suggest that control of seizures might be possible with doses of radiosurgery that are lower than those typically applied to tumors, , recent case reports also demonstrate the failure of low-dose radiosurgery to control seizures. , , Although failure of seizure control is easy to identify, it is a much more difficult task to determine that a lack of seizure control is caused by an insufficient radiation dose. The time dependence of radiosurgical effects is also a confounding factor that has not been fully elucidated, and a consensus among different treating radiosurgical centers of when radiosurgical treatment has “failed” has not yet been reached. Furthermore, radiosurgery patients with inadequate reduction of seizures commonly received radiation doses of 20 Gy or less, and these patients showed little evidence of radiation-induced necrosis or histopathologic changes in their tissue specimens. , , Thus the best evidence from human and animal preclinical experiments suggests that there is a steep dose-response curve for seizure reduction and that some neuronal necrosis is required to produce abatement of seizures. This evidence suggests that the radiosurgery dose required to reduce seizures is close to the absolute tolerability threshold of human brain tissue.
Radiosurgery for MTLE is still an investigational option. The advantages are the comfort and no-invasiveness of the technique; avoiding general anesthesia and severe surgical complications, including mortality; the very short hospital stay; and finally the immediate return to the previous function level, activity, and employment. Whether or not radiosurgery provides a better result in sparing memory function is still a matter of debate and needs to be confirmed with further comparative analysis and studies. Long-term efficacy and safety of radiosurgery also need to be established. Microsurgery of MTLE provides very satisfactory results because of the rarity of surgical complications and the high rate of seizure control. Therefore the most appropriate treatment strategy should be chosen carefully, and the patient should clearly understand the advantages, disadvantages, and potential limitations of both strategies. The patient should be able to understand the limits and constraints of radiosurgery very well. In our opinion, the most important selection parameter is the demonstration of the purely mesial location of the epileptogenic zone.
Another good candidate for GKRS is the patient with proven MTLE who has had a surgical failure because of insufficient extent of resection. Overall, the best candidates are young patients with moderately severe epilepsy and socially well-adapted people with a high functioning level and quite a high risk of memory deficit with open surgery techniques (such as MTLE on the dominant side with a subtle hippocampal atrophy and slight preoperative deficit in verbal memory). Postoperative memory deficit exposes these patients to potentially huge social and professional consequences; therefore GKRS constitutes a very good alternative strategy for this patient group.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here