Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The core imaging modalities for spinal imaging include radiographs, CT, and MRI. We discuss the advantages and disadvantages of each imaging modality as well as specialized techniques in CT and MRI that can be used in the diagnosis and treatment of neurological spinal disorders.
Back pain, a common indication for performing imaging examinations, and its relationship to degenerative disk disease is poorly understood but likely caused by a combination of degenerative disk disease, mass effect, and biochemical factors. However, imaging findings correlate poorly with patient symptoms, and imaging for uncomplicated acute or subacute back pain in the absence of “red flags” has not been shown to contribute to patient outcome.
MRI is the imaging examination of choice in the evaluation of marrow involvement and paravertebral tumor extension in metastatic disease. Likewise, MRI is the modality of choice in assessment of spinal soft tissue and cord abnormalities.
In cases of acute spinal trauma, CT is the ideal screening test for cervical fracture owing to its time efficiency and sensitivity for osseous abnormalities.
MRI is the screening procedure of choice for detection of vascular abnormalities of the spine. However, catheter-based angiography is considered the “gold standard” for diagnosis of spinal vascular abnormalities.
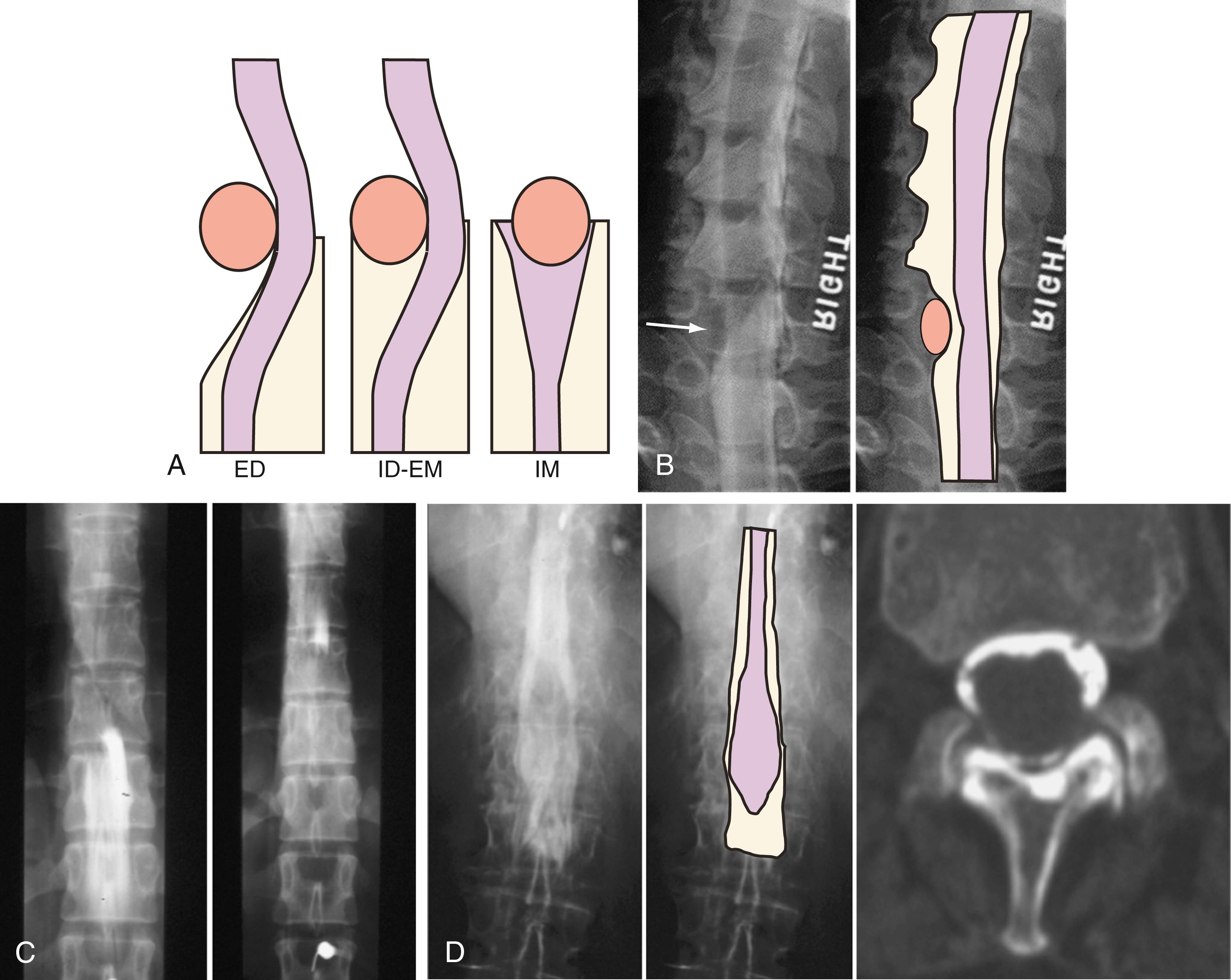
A systematic approach to interpreting spinal imaging examinations requires evaluation of the different compartments of the spine: the paravertebral soft tissues, bone marrow and extradural space, intradural extramedullary compartment, and intradural intramedullary compartment. By localizing the pathology to the compartment of origin, a differential diagnosis can be reached.
The first role of imaging is to provide reliable anatomic or functional information. The second—and perhaps more important—is to provide information that will guide therapeutic decision making. As a rule, patients are referred for imaging for the evaluation of pain syndromes, functional or mechanical alterations, neurological symptoms suggestive of spinal cord or nerve root involvement, trauma, and congenital abnormalities. The space (extradural, intradural, extramedullary, and intramedullary) in which the abnormality exists, although not known a priori, is an important consideration in the differential diagnosis and selection of diagnostic tests.
The different modalities are discussed in terms of their advantages and disadvantages and for which indications imaging is appropriate. Although imaging can be purely diagnostic, it can also play a role in imaging-guided procedures for both diagnostic and therapeutic aims.
Although formally interpreted by radiologists, imaging of the spine can also be considered a tool used by neurosurgeons, and they should have a high level of skill in interpreting imaging examinations. After a discussion of imaging modalities and common imaging indications, a systematic method of interpreting imaging examinations is presented, with a focus on pathology.
The core modalities for spine imaging include conventional radiography, CT (including CT myelography), and MRI. Additional imaging modalities include bone scintigraphy, positron emission tomography (PET), and ultrasonography (US), which may be used alone or in conjunction with radiographs, CT, or MRI.
Conventional radiography is performed primarily in the extradural space, which includes the osseous structures and, to a lesser extent, the immediately surrounding soft tissues. Conventional radiography is less expensive and easier to perform than CT or MRI. It provides a convenient means of assessing alignment and gross bone integrity and can also be used for purposes of localization in procedural planning and evaluation of movement when flexion-extension views are obtained. It is capable of demonstrating the general changes involving various types of arthritis and disk space narrowing. According to appropriateness criteria, radiography is considered sufficient for the initial evaluation of recent significant trauma, osteoporosis, or back pain in individuals older than 70 years.
Digital radiography, as opposed to conventional techniques, has enabled more flexible handling of images (e.g., duplication, transmission, postprocessing), as well as expedited cycle time. The latter advantage is extremely useful in the intraoperative environment because the results are available immediately after exposure, without the need for traditional processing.
The disadvantages of conventional radiographic techniques are that they visualize mainly osseous structures and are relatively insensitive to soft tissue changes within the disk, ligaments, nerves, and paraspinal tissues. Bone marrow involvement must be significant and far advanced before it is demonstrable on conventional radiographs; a 30% to 75% change must occur for the abnormality to become apparent. ,
Conventional radiographs of the spine can be obtained with lateral and frontal projections and with additional views, including oblique, flexion-extension, and weight-bearing. Standing flexion and extension views can be useful in detecting and quantifying instability in spondylolisthesis. Oblique views are useful for detecting pars fractures. In radiography, as well as fluoroscopy, ionizing radiation is used, and so this should be considered when pregnant patients and children undergo radiography.
Since its introduction in the 1970s, CT imaging technology has evolved; currently, multidetector devices can cover larger areas in shorter examination times. , In addition, thinner sections can be used to allow the acquisition of isotropic voxels that can be processed into three-dimensional (3D) data sets, which can be reformatted into any plane without loss of resolution. Subsequent 3D multiplanar reformatted images are particularly useful for the evaluation of trauma, for assessment of fusion postoperatively and pseudoarthrosis, and for instrumentation. Window level and width manipulation can accentuate osseous or soft tissue structures. CT imaging is an important tool and is the first alternative when MRI is contraindicated or unavailable. CT imaging also has the advantage of faster acquisition time in the acute setting of trauma or reducing motion artifact in instances when patients are unable to tolerate the longer scan times required with MRI.
CT imaging without contrast material is most useful for evaluation of the extradural space, including osseous structures and surrounding soft tissues. CT imaging can discriminate among various tissues by differences in attenuation and can provide high-resolution bone detail and enough soft tissue information to accurately assess degenerative disk disease and associated changes or sequelae such as herniation, stenosis, and facet arthritis. CT imaging is equivalent to MRI in the diagnosis of a lumbar herniated disk and stenosis but is less accurate in depicting marrow changes and soft tissue structures such as paravertebral lesions, intraspinal structures, or intraspinal disease. The primary disadvantages of CT imaging are the overlap in attenuation with normal and abnormal soft tissue disease and, as with conventional radiography, ionizing radiation.
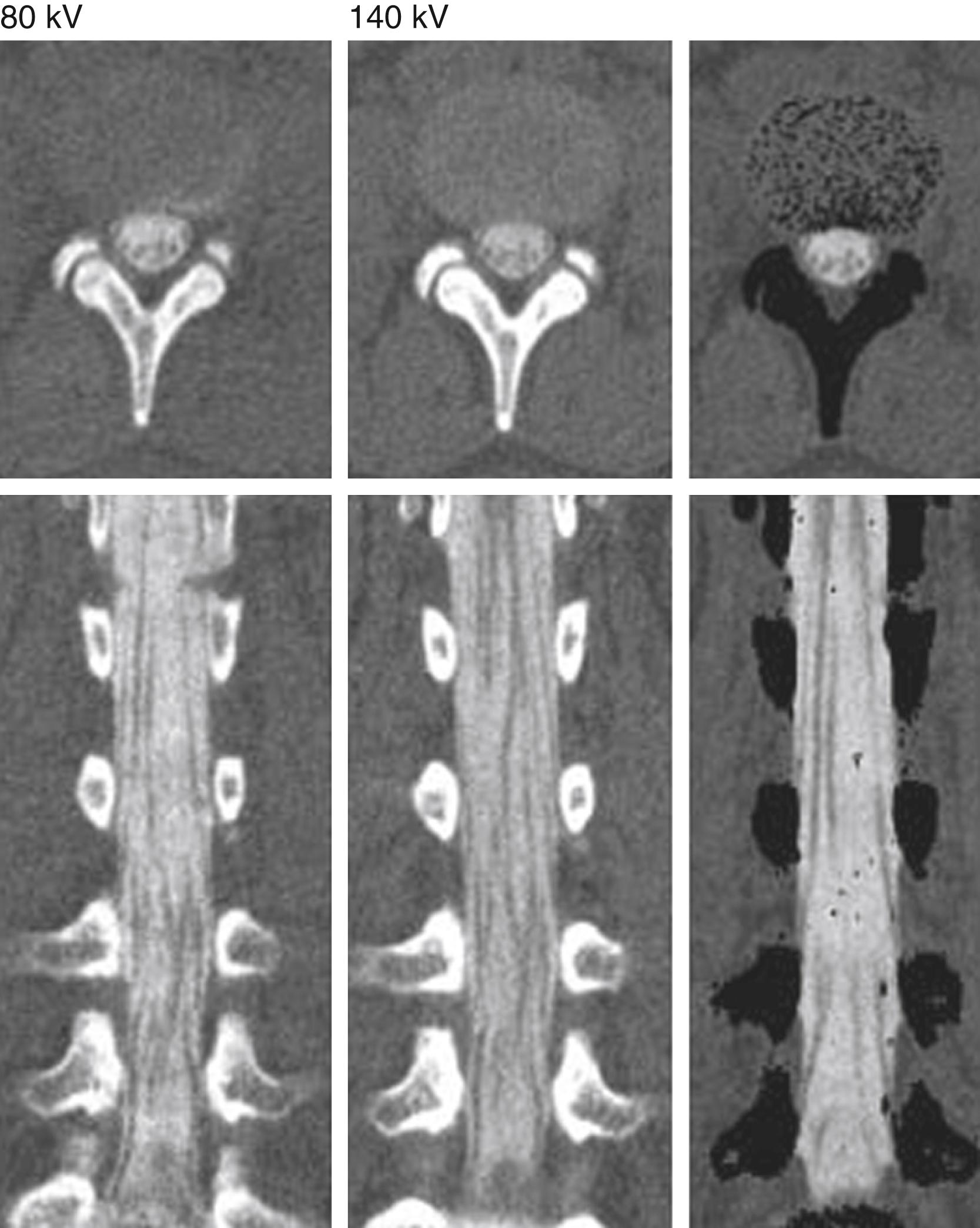
There are emerging applications for dual-energy multidetector CT in spine imaging that are becoming more frequently used. Dual-energy computed tomography (DECT) acquires images by using two beams of different energies, at a higher and lower tube voltage (usually 80 and 140 kV), simultaneously. The attenuation differences between materials and high- and low-energy beams allows DECT to better differentiate tissue types. A clinical application for DECT in spine imaging includes metal artifact reduction techniques, which reduce streak and beam hardening artifacts from metal hardware. This is particularly useful for imaging a postsurgical spine with instrumentation hardware that can obscure the canal, neural foramen, and paraspinal soft tissues. Other emerging applications of DECT include providing a noninvasive way of detecting urate deposits in the spine for diagnosis of early-stage or acute gout, detection of bone marrow edema in the setting of trauma if MRI is unavailable or contraindicated, bone mineral density analysis, and improved CT detection of spinal metastases.
Computed tomographic angiography (CTA) can also be obtained with the rapid injection of intravenous contrast material for the evaluation of the vasculature of the neck in cases of trauma, for evaluation of vessel dissection, or in cases in which vessels are encased or displaced by neoplasm.
Myelography, in comparison with radiography or cross-sectional imaging, is invasive because exogenous contrast material is administered into the subarachnoid space. Enhancement with contrast material produces both extradural and intradural information. Currently, nonionic contrast material has a lower rate of complication than do previously used contrast media. , Complications include postprocedural headaches (10%–15% of patients) and rare but possible adverse events such as nerve damage, formation of an iatrogenic epidermoid, infection, seizure, allergic reaction, and arachnoiditis. The incidence of headaches severe enough to necessitate treatment (e.g., epidural blood patch) is approximately 1%. ,
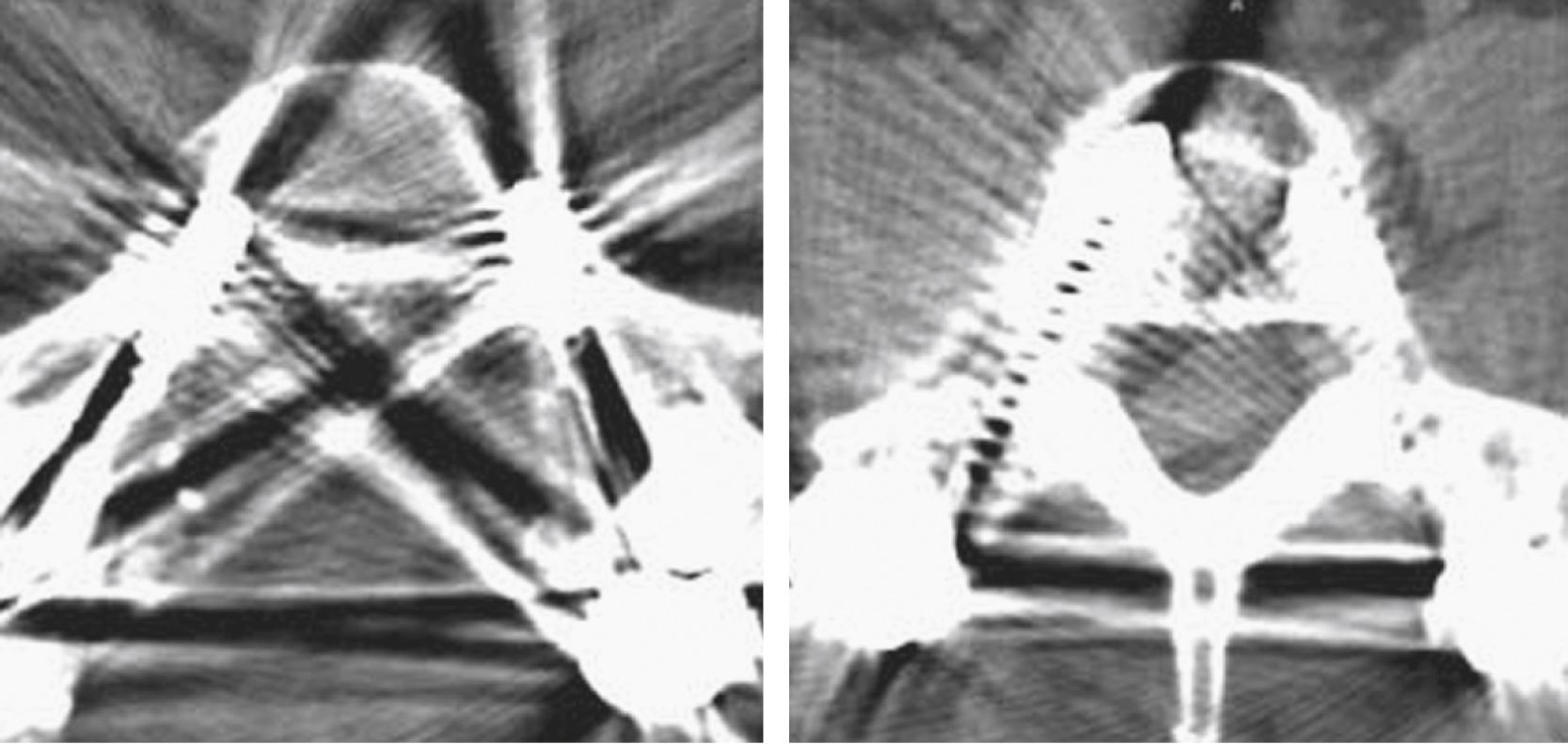
To perform myelography, the patient is placed prone, and access to the thecal sac is achieved in a manner similar to that of a lumbar puncture: typically at the L2–L3 or L3–L4 level from either a midline or an oblique approach. Once access is achieved, the flow of contrast material is monitored under fluoroscopic guidance during the injection. If contrast material fails to extend cranially as a result of mass effect (a myelographic block), it is necessary to obtain access to the subarachnoid space at the C1–C2 level and inject additional contrast material to define the superior aspect of the lesion ( Fig. 11.1 ).
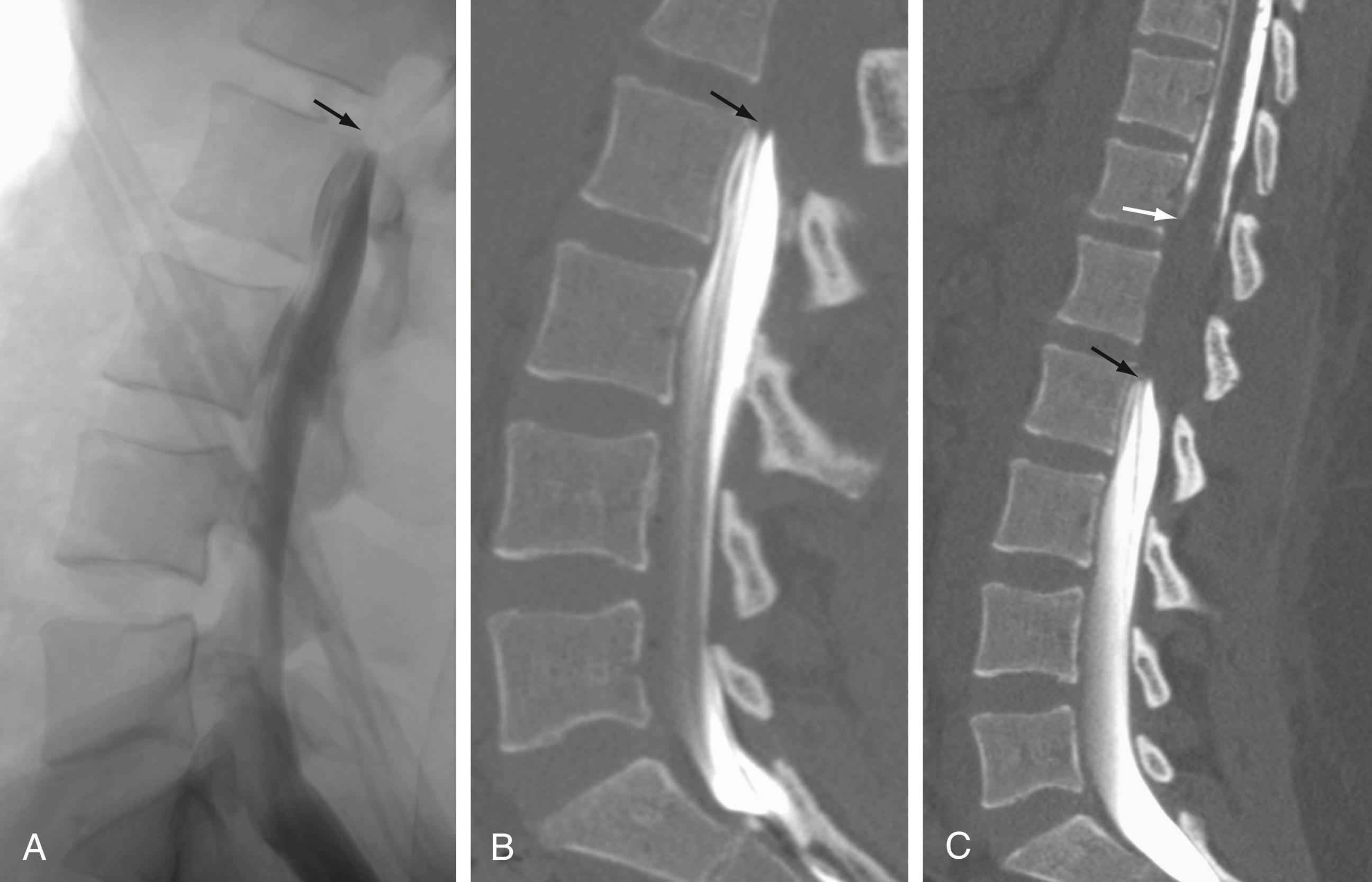
Myelography can outline the nerve roots, spinal cord, and its coverings. Intradural and extradural disease can be identified and characterized on the basis of the character and structure of its effect on the contrast column ( Fig. 11.2 ). Radiographic contrast-enhanced myelography is rarely performed on its own and is almost always used in conjunction with CT imaging. Conventional radiographic myelography may be useful for general anatomic appraisal in patients with complex deformities or when implanted hardware or electronic devices preclude the use of MRI or CT imaging. CT myelography provides excellent delineation of intradural structures such as the spinal cord and nerve roots and their relationship to the soft tissue and bony surroundings. It is useful in distinguishing bone changes from soft tissue changes (e.g., osteophytes and disk herniations), which may be indistinguishable on radiographic myelograms ( Fig. 11.3 ). Multidetector CT imaging of the entire spinal axis can be performed in 20 to 30 seconds. With intrathecal contrast media, subsequent multiplanar reformatted images can provide complex 3D assessment of anatomic and pathologic abnormalities with myelogram-like images. New forms of dual-energy multidetector CT imaging can subtract out bone in both single slices and 3D multiplanar reformatted images ( Fig. 11.4 ).
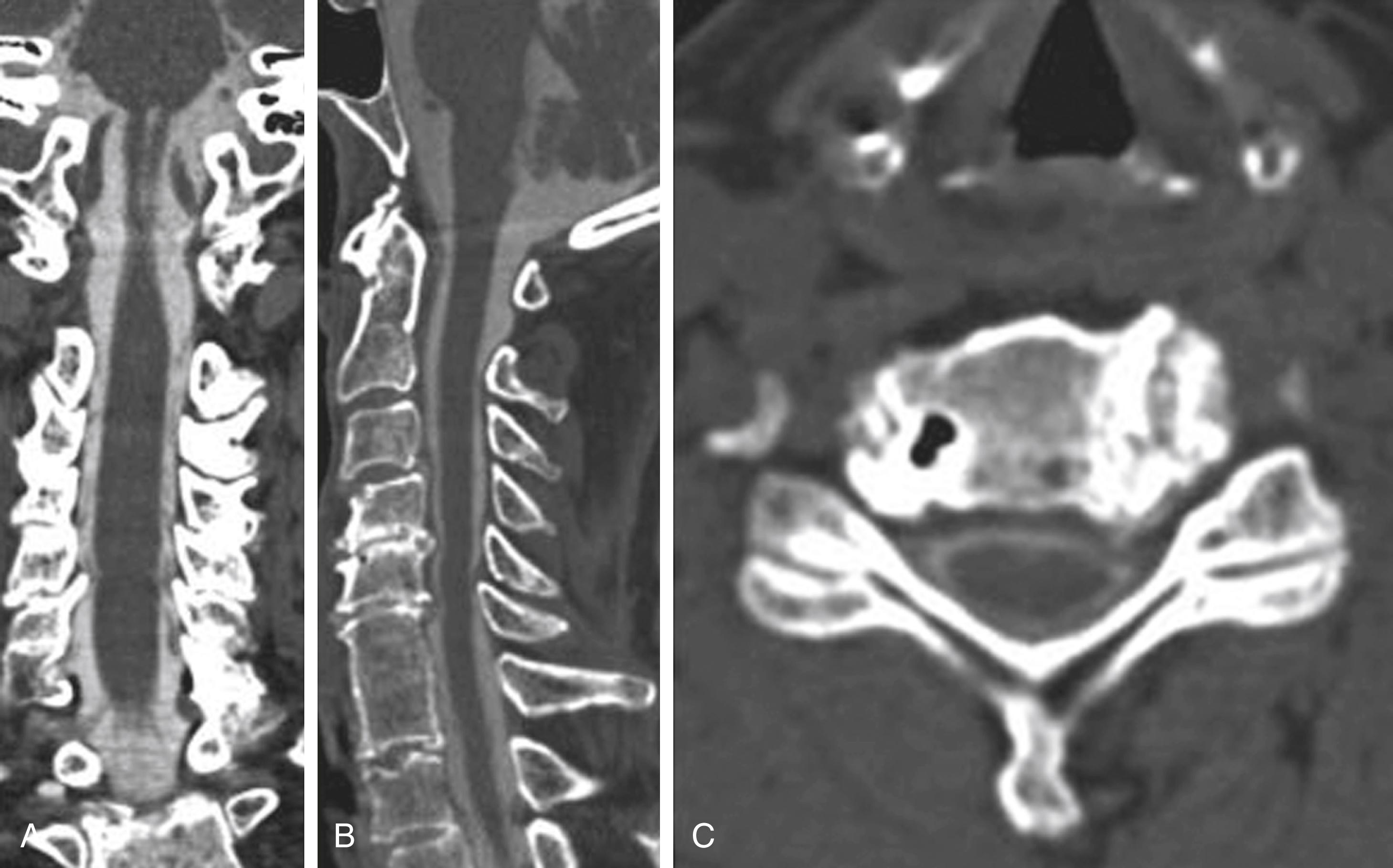
The disadvantages of plain and CT myelography concern primarily the intrathecal introduction of contrast media (toxicity and risk of allergic reaction) into the subarachnoid space, use of ionizing radiation, and limited soft tissue discrimination. Premyelography administration of anticoagulants also poses an issue; aside from aspirin, most must be discontinued.
MRI is currently the preferred modality for most patients with significant signs and symptoms of spinal disorders. It depicts excellent contrast among different types of soft tissues. MRI without contrast material is capable of evaluating the extradural, intradural extramedullary, and intramedullary spaces. MRI is noninvasive and without the risks associated with ionizing radiation. Anatomic imaging can be performed in different planes, and can be used for volume acquisitions. It is the most sensitive modality and most specific technique for identifying abnormalities of the spinal cord, nerve roots, cerebrospinal fluid (CSF) space, and soft tissues, and it also provides direct visualization of bone marrow. When used with gadolinium complexed with a chelating agent such as diethylenetriaminepentaacetic acid (DTPA), MRI has even greater sensitivity for detecting intramedullary disease, inflammatory changes, and reparative processes such as may occur with postoperative changes and trauma. MRI is particularly efficacious for the evaluation of so-called “red flag” diagnoses such as osteomyelitis, neoplasia, and trauma.
For evaluation of degenerative disk disease, T1- and T2-weighted images performed in the axial and sagittal planes are used, although fast spin echo, T2-weighted images have replaced conventional T2-weighted images because acquisition times are shorter. Short tau inversion recovery (STIR) or fat-suppressed T2-weighted images are also used to delineate bone marrow or soft tissue edema ( Fig. 11.5 ). Aside from conventional MRI pulse sequences that focus on morphologic features, techniques that provide physiologic or functional information include dynamic imaging, diffusion imaging, spectroscopy, and MR neurography. , Diffusion-weighted MRI of the spinal cord can be used in some instances for diagnosis of acute spinal cord infarct, when imaging findings of acute ischemia are not straightforward. The finding of restricted diffusion within the cord is more sensitive for acute ischemia than the conventional T2-weighted sequence and can appear earlier than findings on other sequences.
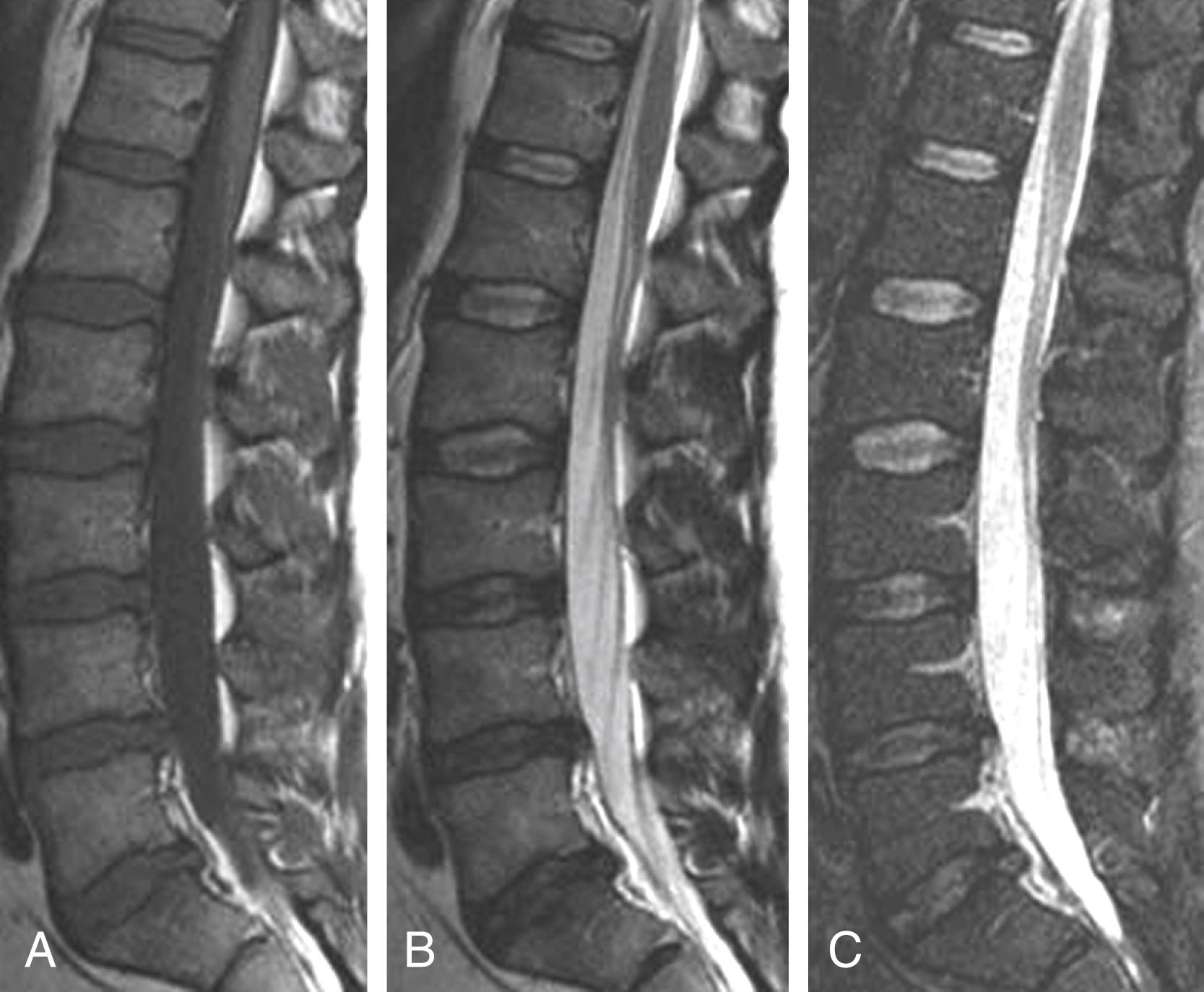
Methods for dynamic imaging of the spine vary, which adds to the difficulty of determining its usefulness. Available methods include axial loading with the patient in the supine position and the use of an upright open MRI system that allows flexion-extension imaging ( Fig. 11.6 ). , The rationale for dynamic imaging is to identify disk protrusions or changes in spinal canal diameter that are not apparent when the conventional supine position is used for imaging. , , Hiwatashi and colleagues evaluated 200 patients with clinical symptoms of spinal stenosis and found in 20 that routine and axial-loaded studies yielded detectable differences in caliber of the dural sac. Management was changed in five of these patients; the surgeons opted for surgery. Although dynamic imaging has benefits, its routine use appears to be outweighed by patient discomfort and added imaging time.
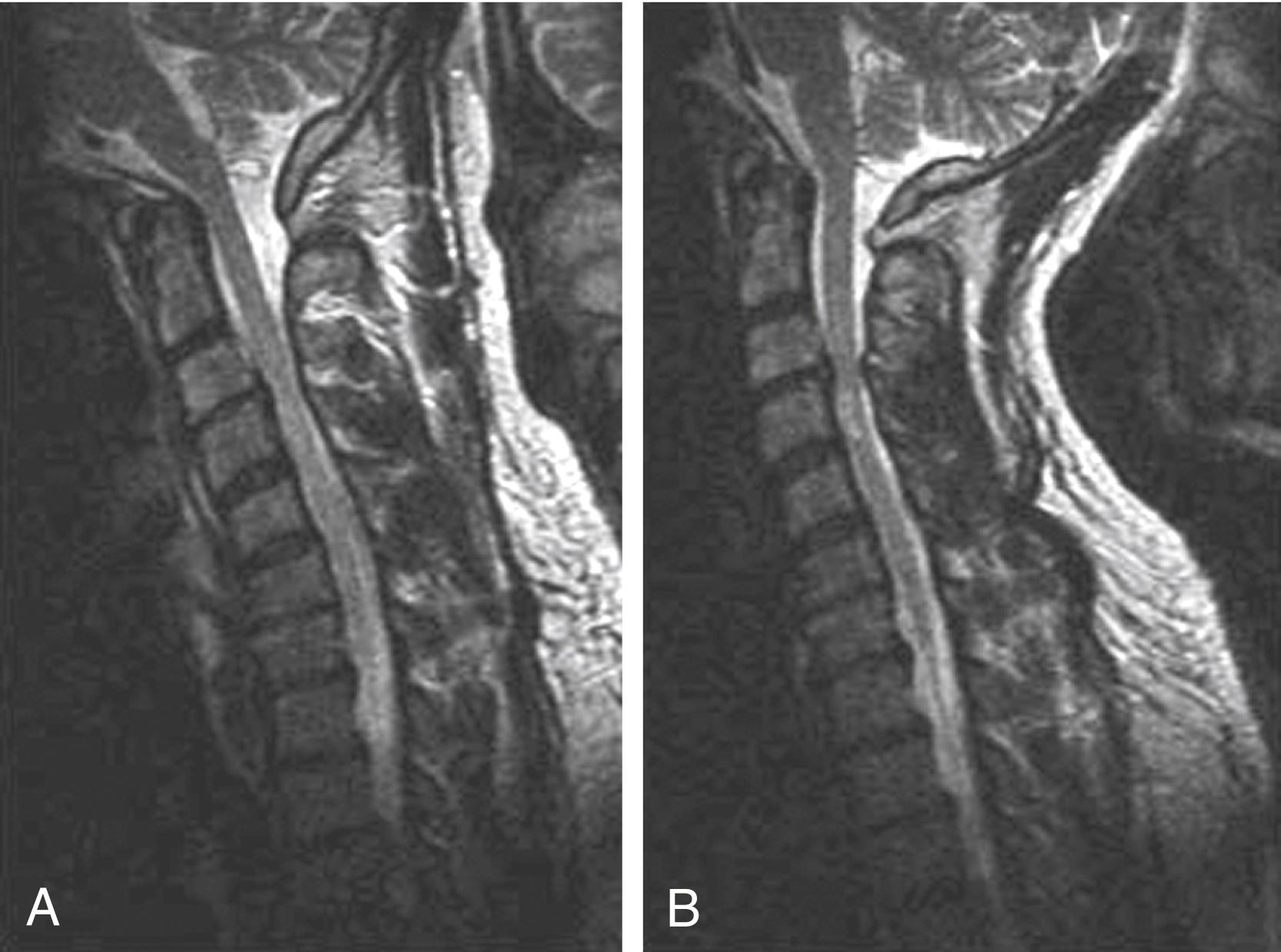
MR neurography is used to evaluate the peripheral nerves, including the brachial and lumbar plexuses, with high-resolution T1-weighted imaging and fat-suppressed T2-weighted or STIR imaging. , , Nerve compression, trauma, hypertrophy, neuroma, and tumor infiltration can be evaluated with MR neurography. ,
CSF flow studies with cine MRI provide cardiac-gated gradient phase-contrast images that are displayed qualitatively as CSF flow images in a closed-loop kinematic format ( ![]() ). This same data set can be displayed quantitatively on a graph or in numerical format as analysis of values of flow velocity and volume flow rate. These images demonstrate the pulsatile motion of CSF, not the bulk flow. Pulsatile flow is a result of expansion of the brain during systole and relaxation during diastole and is therefore bidirectional: CSF flows caudally (downward) during systole and cranially (upward) during diastole. Typically, the signal intensity of normal CSF flow in these studies is demonstrated as hyperintense during systole, when flow is caudal, and hypointense during diastole, where flow is cranial. These images are usually displayed in the sagittal and axial planes. In general, the degree of pulsatile flow diminishes as it proceeds caudally. Clinically, these studies are most often used for the evaluation of CSF flow in patients with Chiari I and II malformations and for the assessment of syrinx cavities ( Fig. 11.7 ). ,
). This same data set can be displayed quantitatively on a graph or in numerical format as analysis of values of flow velocity and volume flow rate. These images demonstrate the pulsatile motion of CSF, not the bulk flow. Pulsatile flow is a result of expansion of the brain during systole and relaxation during diastole and is therefore bidirectional: CSF flows caudally (downward) during systole and cranially (upward) during diastole. Typically, the signal intensity of normal CSF flow in these studies is demonstrated as hyperintense during systole, when flow is caudal, and hypointense during diastole, where flow is cranial. These images are usually displayed in the sagittal and axial planes. In general, the degree of pulsatile flow diminishes as it proceeds caudally. Clinically, these studies are most often used for the evaluation of CSF flow in patients with Chiari I and II malformations and for the assessment of syrinx cavities ( Fig. 11.7 ). ,
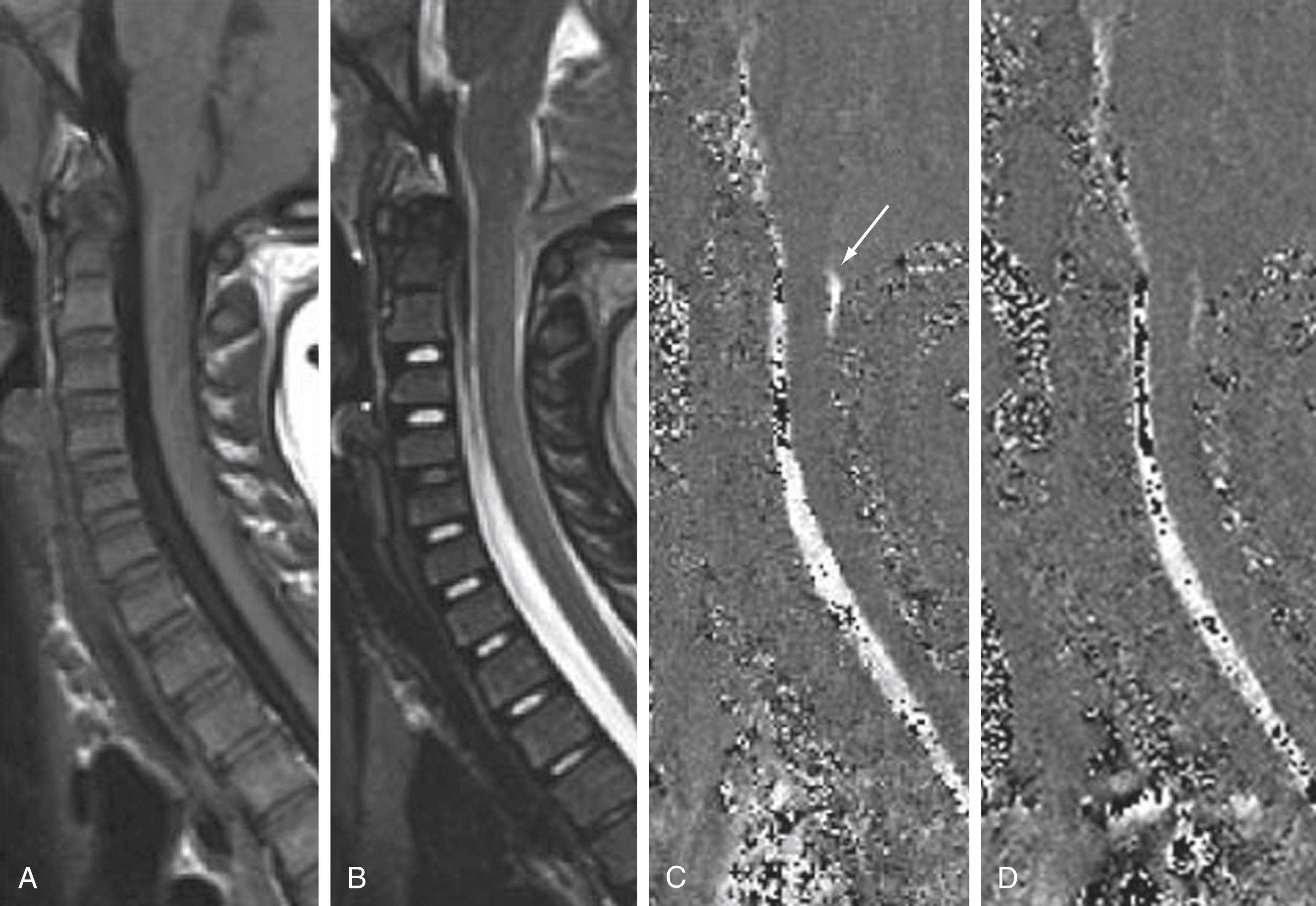
MR myelography using specialized fat-suppressed 3D T2-weighted turbo-spin echo sequences can be used to determine the presence of a CSF leak as well as localize the leak in the spine in cases of spontaneous intracranial hypotension. The heavy T2-weighted sequence allows high contrast differentiation between the bright CSF and dark cord and nerve roots within the canal. MR myelography can detect abnormal epidural or subdural collections in cases of CSF leak and may help localize the origin of a spontaneous CSF leak ( Fig. 11.8 ). Additional uses for these high-resolution fluid-sensitive 3D T2 sequences in the spine include evaluation of intradural filling defects such as septations from arachnoid webs or cysts, vessels in dural fistulas and arteriovenous malformations (AVMs), and other cystic structures such as cystic tumors, Tarlov cysts, or posttraumatic cysts. The advantage of MR myelography over CT myelography is that it does not necessarily require intrathecal contrast injection, is therefore noninvasive, and does not involve ionizing radiation.
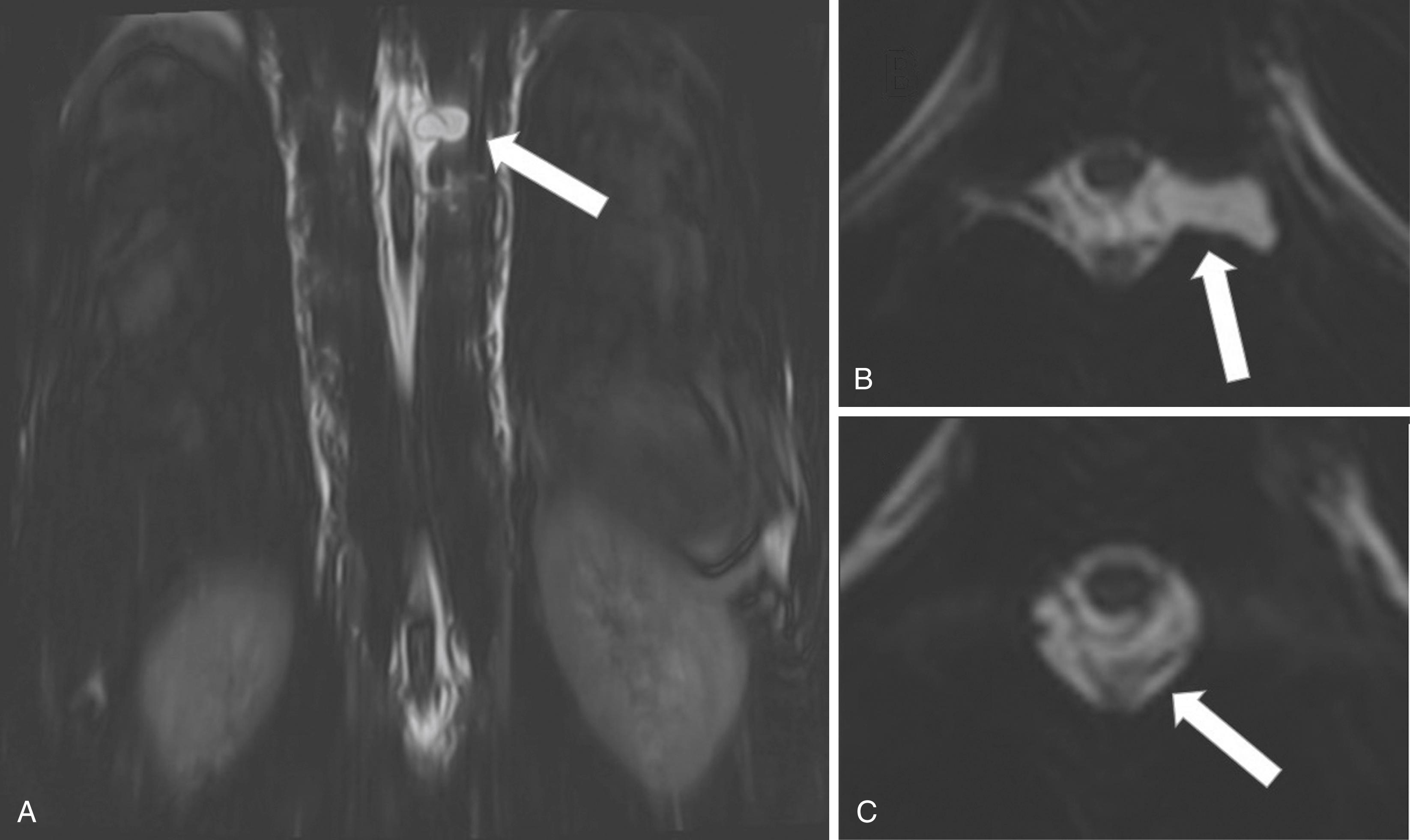
Dynamic contrast-enhanced (DCE) T1-weighted perfusion MRI is a technique that allows assessment and measurement of microvascularization and can be useful in characterizing spinal tumors and metastases. DCE MRI quantifies permeability of certain tissues by measuring parameters of volume and rate of transfer between blood plasma and the extravascular extracellular space. Perfusion measurements in spinal metastases have been shown to reflect tumor responses to radiotherapy, with the potential to predict early treatment responses. , Another use of DCE MRI is characterizing benign versus malignant lesions—for example, differentiating hypovascular or hypervascular metastases from normal bone marrow.
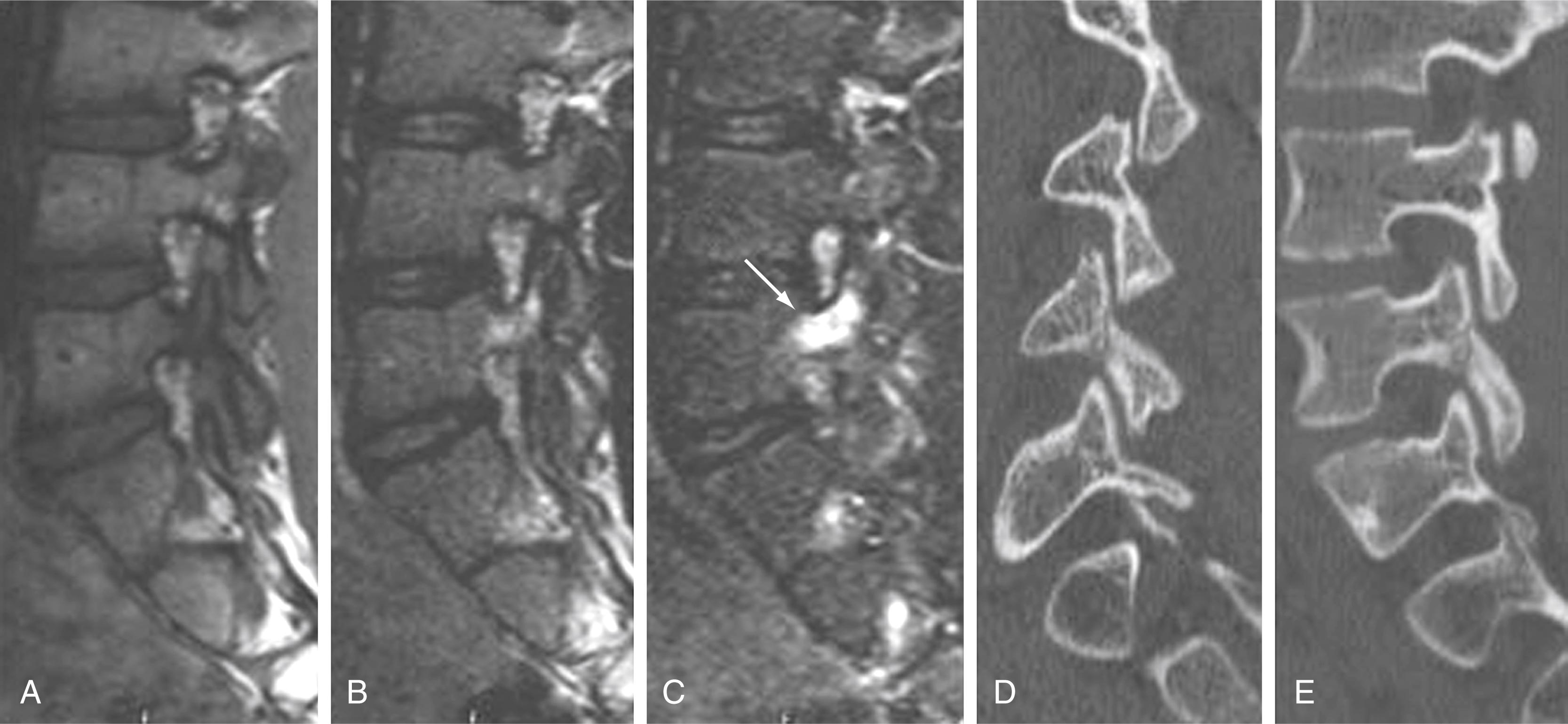
Instrumentation, metal implanted as part of surgical procedures, and implanted electronic devices represent challenges for both CT and MRI. In the case of CT, metal can create beam-hardening artifacts that obscure adjacent soft tissue and osseous structures ( Fig. 11.9 ). Multidetector studies with isotropic voxels and software used to decrease beam-hardening artifact can often decrease the image degradation caused by implanted metal. Different types of metal produce different types of artifacts on MRI that may render the examination uninterpretable. The term magnetic susceptibility refers to the manner and amount by which a material becomes magnetized in a magnetic field. Nonferrous magnetic metals may produce local electrical currents induced by the changing field, which causes distortion of the field and artifacts. These artifacts take two main forms: geometric distortion and signal loss secondary to dephasing ( Fig. 11.10 ). Certain techniques are more susceptible to these artifacts, and gradient echo images in particular are especially sensitive to differences in magnetic susceptibility and field homogeneity. Specialized MRI sequences have been developed to reduce metal artifact from spinal hardware ( Fig. 11.11 ). For example, a technique termed slice encoding for metal artifact correction (SEMAC) encodes each excited slice against metal-induced field inhomogeneities. These specialized sequences can reduce the amount of distortion by metal artifact on MRI, although they cannot completely remove the artifact.
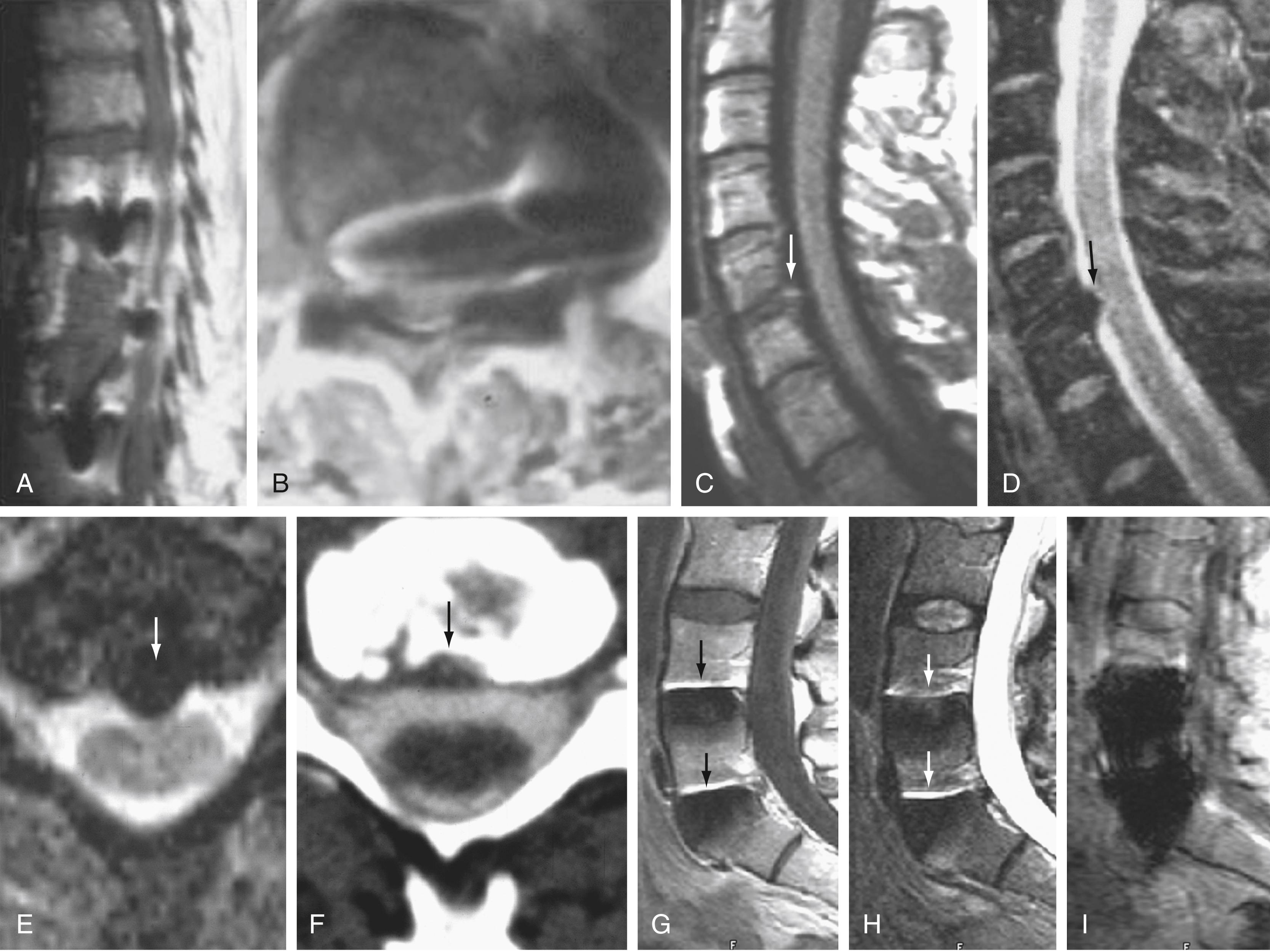
Although metals can cause artifacts that render the examination uninterpretable, certain indwelling devices may be a contraindication to the entire examination. Some types of aneurysm clips, cardiac valves, and vascular devices can result in harm to patients during an MRI examination. Some implantable devices may cease to function properly after an MRI study, and others may be a source of heating with deleterious biologic effects and burns. Publications and websites that track information related to contraindications are available.
The primary disadvantages of MRI are related to its cost and safety concerns with regard to electronic devices or implants. The safety issues include heating, dislodgment, and malfunction, as well as image distortion in the presence of metal. Claustrophobia remains a significant problem that sometimes necessitates the use of machines with a larger bore and sedation or general anesthesia. The acquisition of MRI data sets takes considerably longer than CT imaging; patient motion also decreases the diagnostic quality of the examination.
Intravenous contrast agents are commonly used with both CT and MRI examinations. Both iodinated (CT) and paramagnetic (MRI) substances are cleared by the kidneys and should be used with caution or not at all in patients with impaired renal function. Although this has been common knowledge with the iodinated contrast media used in CT, various complications related to the use of paramagnetic contrast agents have been documented in patients with impaired renal function. Despite being uncommon, nephrogenic systemic fibrosis, a late serious adverse reaction to gadolinium, has been well documented. , In addition, allergic reactions can occur after administration of iodine or gadolinium contrast agents. , Gadolinium-based contrast agents (GBCAs) are composed of gadolinium, a rare earth element, chelated with a ligand, which may be linear or macrocyclic. Used widely in many clinical applications for MRI diagnoses of neurological pathologies, GBCAs increase the specificity of MRI for many disease processes by increasing T1 signal (or T1 shortening) in areas of vascularity or disruption of the blood-brain barrier. There is growing evidence that the gadolinium used in intravenous contrast agents for MRI is deposited in body tissues and persists, particularly in the dentate nucleus and globus pallidus in the brain. Gadolinium deposition appears to be more common with linear GBCAs versus macrocyclic GBCAs, presumably because of increased stability and higher binding affinity of the macrocyclic agents, leading to less dissociation of gadolinium from the chelating ligand. Although the clinical significance of gadolinium deposition in the brain has yet to be determined, many institutions and societies have issued recommendations on limiting the use of GBCAs to those examinations for which they are clinically indicated and providing patients with warnings regarding the unknown risk of gadolinium deposition in the brain.
Last, it may not be possible to perform an examination or procedure within the constraints of table weight limits and maximum open diameters of gantries in CT or MRI machinery.
Spinal angiography can be performed with MRI (magnetic resonance angiography [MRA]), with CTA, and with catheter-based digital subtraction angiography with selective arterial catheterization. , , The last technique is still regarded as the most accurate for the detection and pretreatment characterization of vascular malformations of the spinal cord and meninges. Although it is probably associated with the highest risk among procedures for spinal imaging, the complication rate has been greatly reduced with the use of microcatheters and more experienced users. Definitive treatment of vascular lesions by endovascular means is now more common, and the examination can be better directed by information from diagnostic MRA and CTA studies, which can help focus the catheter examination. , MRA—the addition of angiography to standard MRI—does not have significantly different sensitivity, specificity, and accuracy of detection of spinal malformations, but it often enables detection of the level of a fistula or vascular malformation. Dynamic MRA is a useful noninvasive tool for investigating vascular malformations such as dural arteriovenous fistulas and spinal arteriovenous fistulas. Dynamic MRA can localize the spinal level of the nidus and identify the feeding arteries and draining veins, a useful preoperative tool for planning endovascular treatment. CTA of the spinal axis is relatively easy to perform and is also useful in identifying the level of involvement.
The use of US in the spine is limited because it is difficult for the sound waves to penetrate bone. Its main value is intraoperative, for additional characterization and localization of disease such as spinal cord tumors, and in pediatric applications. High-frequency probes that improve spatial resolution are commonly used intraoperatively because of the ability to directly approach the region of disease.
Most experts believe that thermography findings are too nonspecific to be of significant value for the evaluation of spinal disorders. Diskography is a technique that, by nature of direct stimulation, is thought by some experts to be able to identify painful and concordant disks. , Diskography and CT diskography are still used, especially when other imaging modalities have failed to localize the cause of pain. The morphologic information is not as critical as reproduction of a patient’s characteristic pain. Although some data support its use, prospective well-controlled trials in support of its prognostic value remain absent from the literature.
Technetium 99m methylene diphosphonate bone scans are most useful for evaluation of the extradural space. Bone scans are a moderately sensitive test for detecting the presence of tumor, infection, or occult fractures of the vertebrae but are rarely specific for diagnosis. The yield is very low in patients in whom radiographic and laboratory results are normal and highest in those with known malignancy. These scans are contraindicated during pregnancy. They are an important tool for surveying the entire skeleton for metastatic disease and can visualize as low as a 5% change in bone marrow. In lytic or aggressive lesions, however, there may be an absence of radiotracer uptake, which is indicative of a “cold” lesion; increased radiotracer uptake, in contrast, is indicative of more osteoblast activity. ,
To further evaluate sites that appear positive on bone scans, single-photon emission computed tomography (SPECT) and PET with fluorodeoxyglucose (FDG) are often used in conjunction with CT imaging and are capable of improved specificity and anatomic localization, particularly in patients with malignancies. Whereas PET-CT is commonly used in disease staging, PET-MRI is an emerging technology showing diagnostic promise. Indium 111 octreotide, a somatostatin analogue, demonstrates uptake in neuroendocrine tumors and meningiomas.
Although determining the proper examination to be performed in a particular clinical context is important, clinicians must also recognize that imaging is not necessary in certain situations, such as cases of uncomplicated acute back pain and low-risk trauma. ,
Degenerative disk disease includes desiccation, fibrosis, narrowing of the disk space, diffuse bulging of the annulus beyond the disk space, extensive fissuring and mucinous degeneration of the annulus, end plate changes, and osteophytes at the vertebral apophyses. Conventional radiographs can identify disk space narrowing, bone changes, and malalignment. CT studies can further identify disk herniation and provide better morphologic characterization of various types of stenosis. MRI provides the greatest spectrum of morphologic findings: degenerative changes manifest as disk space narrowing, loss of T2-weighted signal intensity from the intervertebral disk, fissures, vacuum disk changes, disk calcifications, ligamentous hypertrophy or edema, end plate marrow signal changes, osteophytosis, disk herniation, malalignment, and stenosis.
The major cartilaginous joint (amphiarthrosis) of the vertebral column is the intervertebral disk. Each disk consists of the nucleus pulposus and the annulus fibrosus. In degenerative disk disease, there are alterations to disk hydration, collagen, and proteoglycans, with eventual loss of the delineation between the annulus fibrosus and nucleus pulposus. MRI demonstrates this change as low signal intensity on T2-weighted images ( Fig. 11.12 ). Although this low T2 signal intensity of a degenerated disk is commonly referred to as desiccation, absolute measurements of cadaver disks on T2-weighted images are correlated more closely with the glycosaminoglycan concentration than with the absolute water content; this finding suggests that the signal changes are a reflection of the state of water rather than of total water volume.
Annular tears or fissures occur when there is disruption of all or some of the layers of the annular lamellae. Whether these fissures are a manifestation or a cause of degenerative disk disease is not known; however, one theory suggests that once the annulus fibrosus is disrupted, there is subsequent alteration of nucleus pulposus and then the disk is replaced by fibrous tissue and cystic spaces ( Fig. 11.13 ).
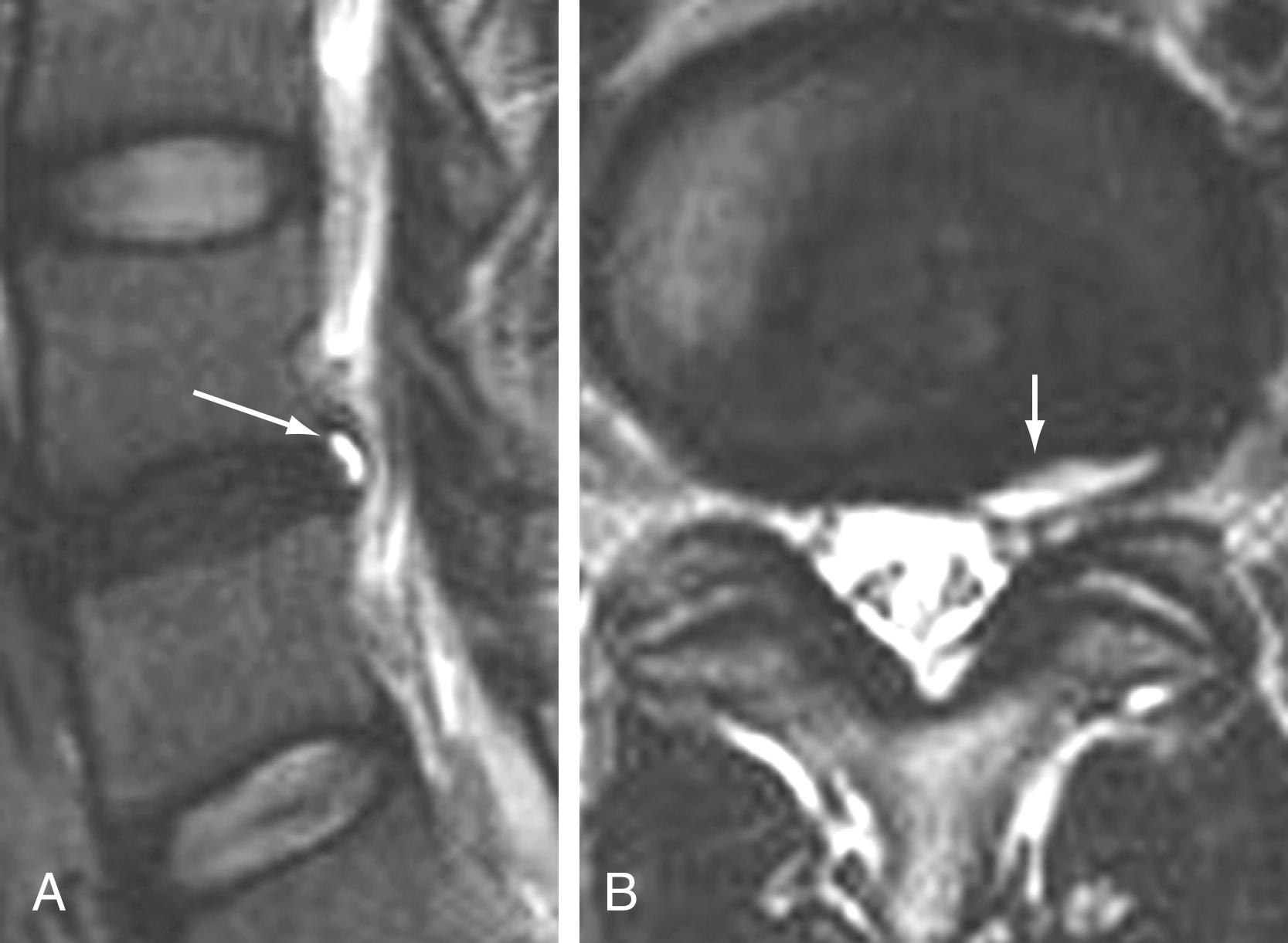
Vacuum disk phenomena representing gas, principally nitrogen, occur at sites of negative pressure in the intervertebral disk, manifesting as lucency on radiographs, low attenuation on CT imaging, or low signal intensity on MRI ( Fig. 11.14 ). Intradiscal or intraosseous air is most commonly degenerative in nature; it can occur with infection, although this is uncommon.
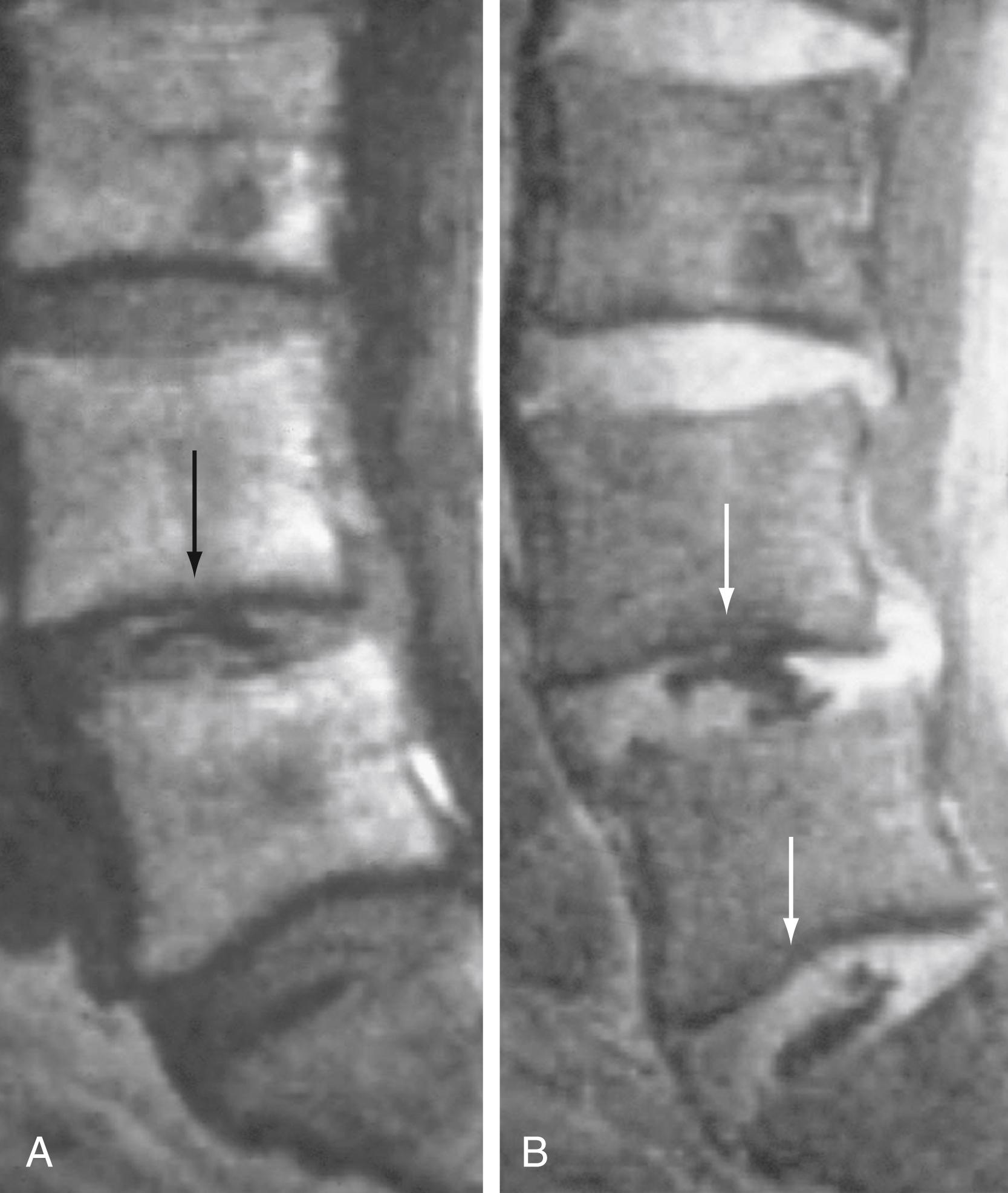
In addition, disks can calcify with degenerative disk disease. Increased density on radiographs or increased attenuation on CT imaging are the most common findings. The appearance of calcification on MRI can be variable, ranging from low in signal intensity (most common) to demonstrating T1 hyperintensity depending on the concentration of calcification ( Fig. 11.15 ). T1 hyperintensity that changes to low signal intensity with fat saturation is thought to represent ossification with fatty marrow in severe disk degeneration or degenerative disk fusion.
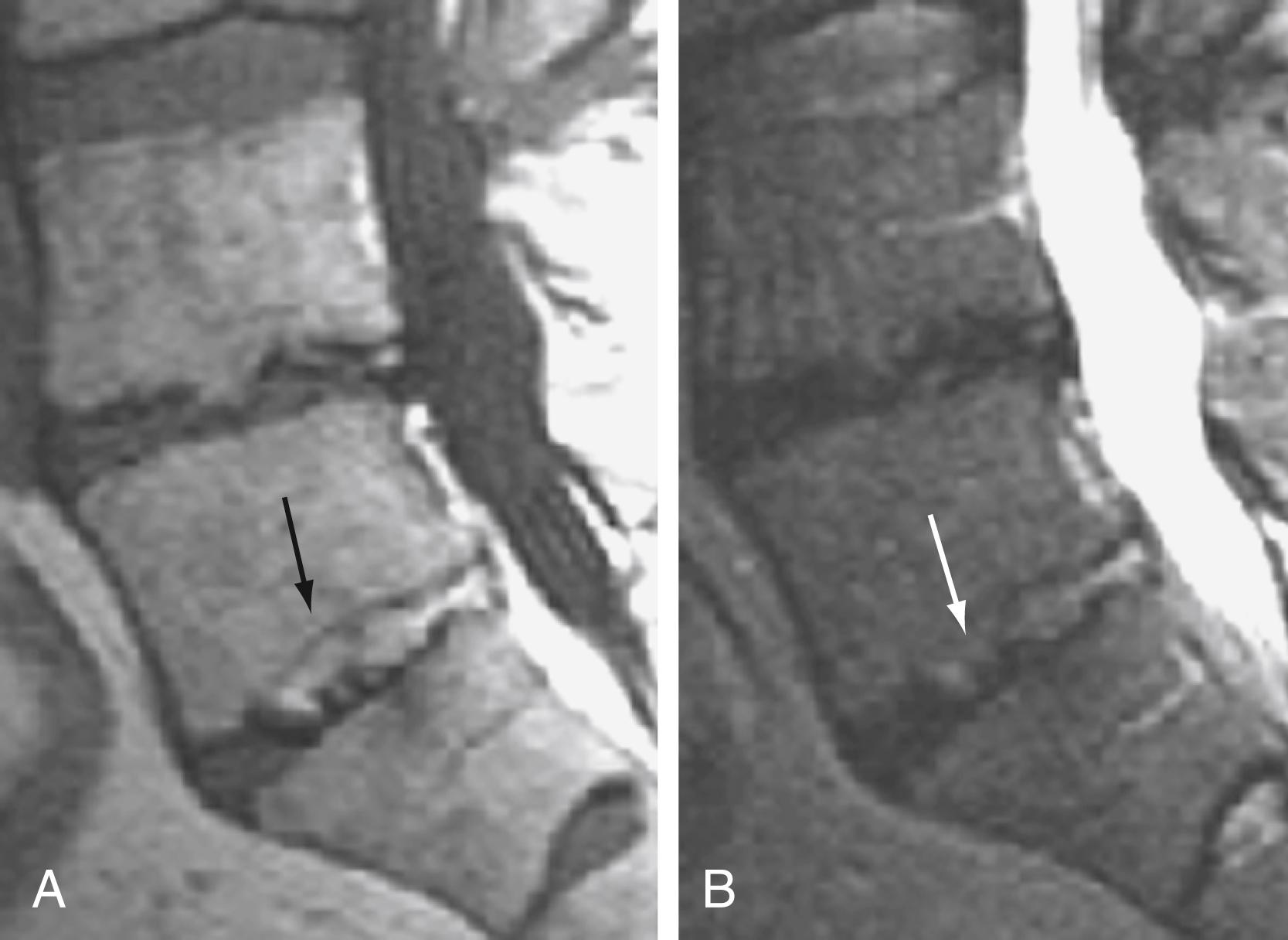
Disk degeneration has been classified in a scheme that has reasonable intraobserver and interobserver agreement. Although MRI demonstrates morphologic alterations in the intervertebral disk with degenerative changes, no clear correlation with symptoms has been identified as yet.
Changes in signal intensity of the vertebral body marrow adjacent to the end plates of degenerated disks are a long recognized and common observation on MRI of the lumbar spine. , These changes are frequently referred to as Modic changes, although they have been independently described by others. However, despite a growing body of literature on this subject, their clinical importance and relationship to symptoms remain unclear.
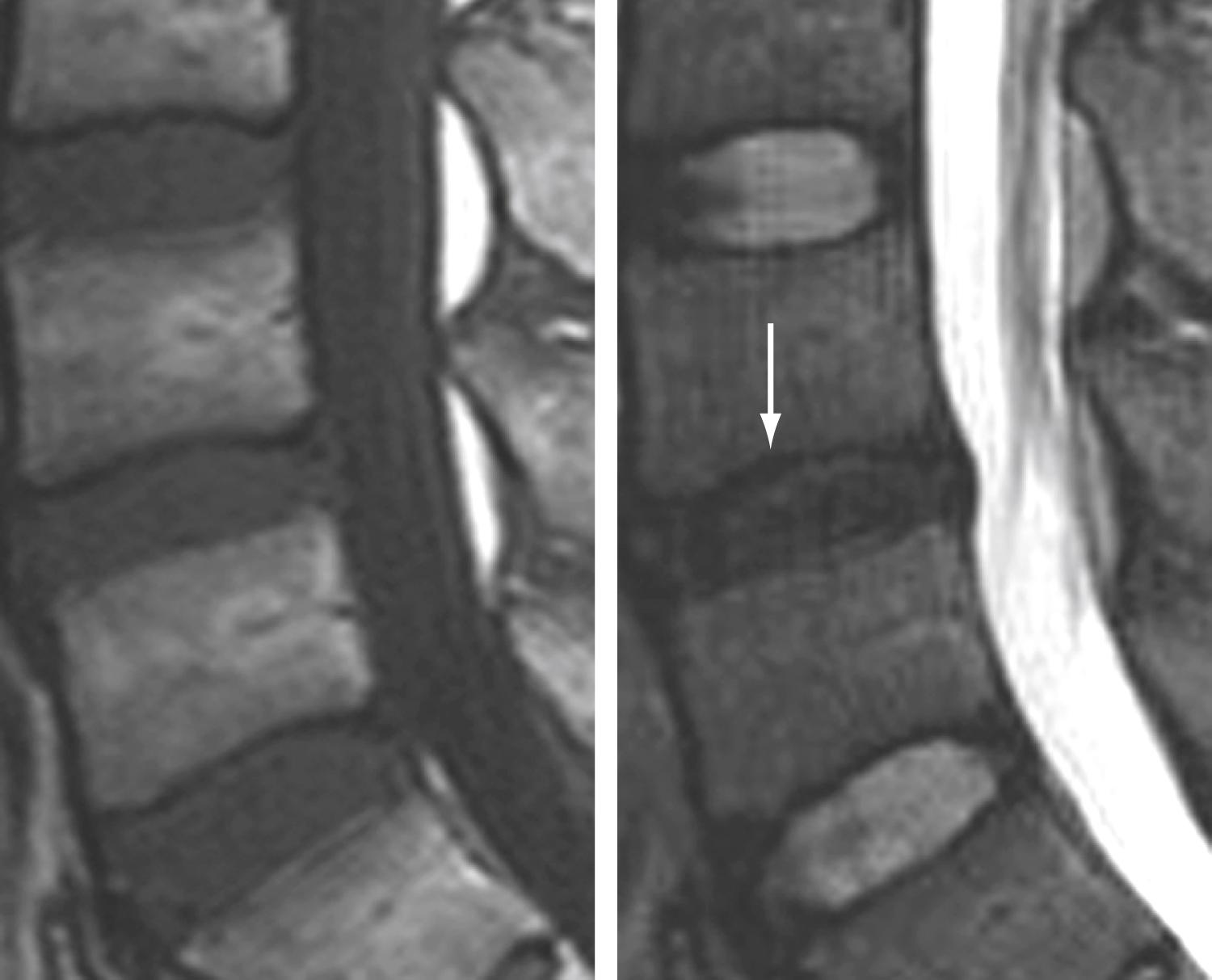
These marrow changes appear to take three main forms and can be simplistically thought of as edema (type I), fat (type II), and sclerosis (type III). Type I changes consist of decreased signal intensity on T1-weighted images and increased signal intensity on T2-weighted images ( Fig. 11.16 ). They have been identified in approximately 4% of patients scanned for lumbar disease, in approximately 8% of patients after discectomy, and in 40% to 50% of chymopapain-treated disks, which may be viewed as a model of acute disk degeneration. Histopathologic analysis of type I changes demonstrates abnormal fibrovascular tissue in the vertebral end plates, which accounts for the MRI findings and also indicates the potential for enhancement. Type II changes are represented by increased signal intensity on T1-weighted images and isointense or slightly hyperintense signal on T2-weighted images ( Fig. 11.17 ). These changes are present in approximately 16% of patients undergoing MRI. Type II changes consist of end plate disruption and fatty marrow replacement, which account for their MRI appearance. Type III changes are characterized by low signal intensity on all pulse sequences and as sclerosis on radiographs. Low signal intensity of type III end plate change represents the sparseness of marrow in areas of advanced sclerosis, which reflects the development of dense woven bone ( Fig. 11.18 ).
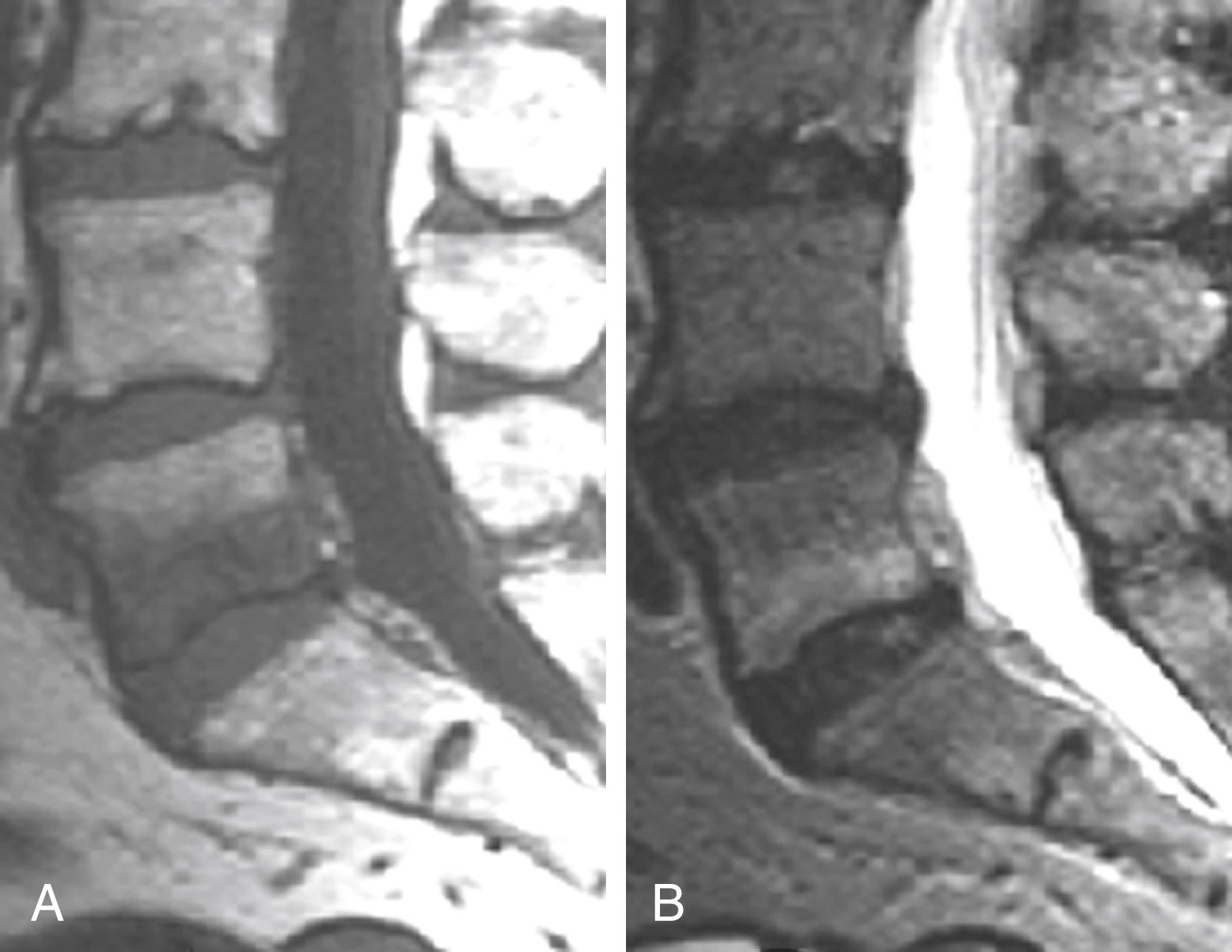
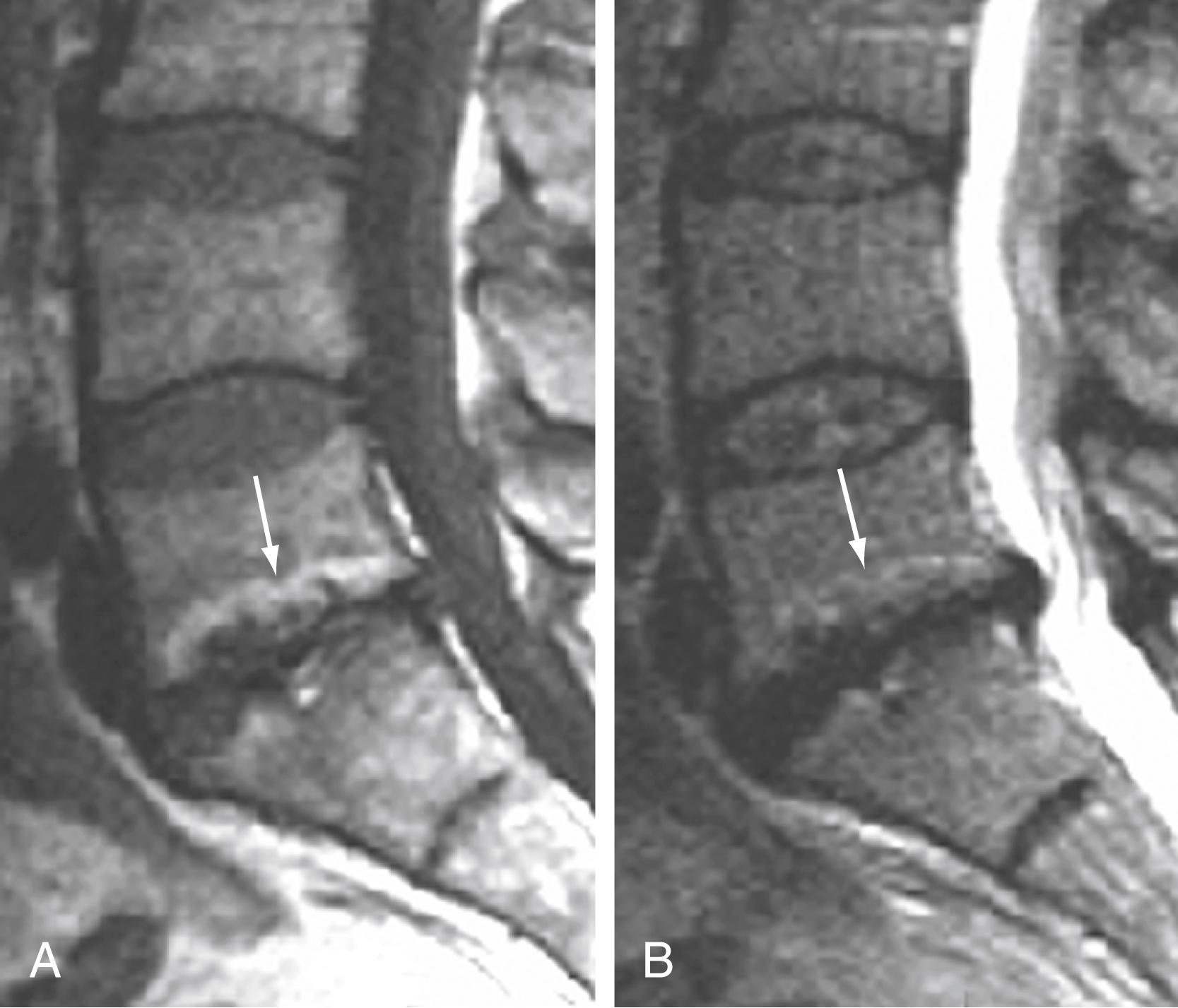
Similar marrow changes in end plates have also been noted in the pedicles ( Fig. 11.19 ). Although originally described as being associated with spondylolysis, they have also been noted in patients with degenerative facet disease and pedicle fractures. , The exact mechanism by which these marrow changes occur is not known. The association of these marrow changes with degenerative disk disease, facet changes, and pars and pedicle fractures suggest that they are a response to biomechanical stress.
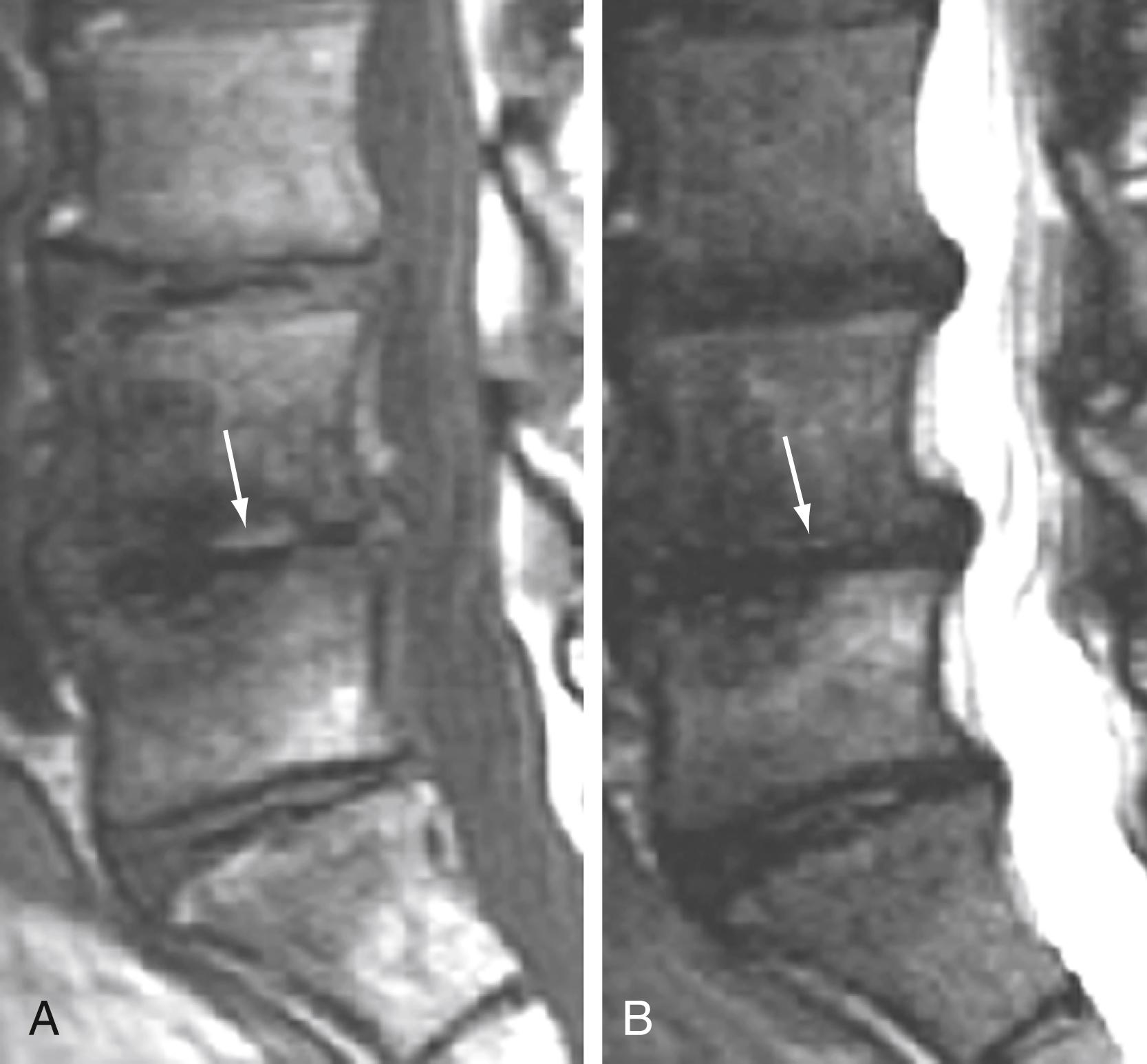
Of these three types of changes, type I changes appear to be most dynamic, affected by ongoing underlying pathologic processes such as continuing degeneration with associated changing biomechanical stresses. Type I changes are most often associated with ongoing low back symptoms. In a longitudinal study, the incidence of new degenerative marrow changes was 6% over a 3-year period, most being type I. In a study of patients with nonoperated low back pain, Mitra and associates found that 92% of type I changes converted either wholly or partially to type II changes (52%), became more extensive (40%), or remained unchanged (8%). There was an improvement in symptoms in patients in whom type I changes converted to type II.
Some diskography studies in patients with degenerative marrow changes have suggested that type I marrow changes are invariably associated with painful disks. , Other studies have failed to reproduce this association, and thus the relationship between degenerative marrow changes and diskogenic pain remains unproven. ,
Multiple authors have observed a variety of inflammatory mediators in association with degenerative marrow changes. Burke and colleagues observed an increase in proinflammatory mediators in the disks of patients with type I marrow changes who were undergoing fusion for low back pain. Ohtori and coworkers found that the cartilaginous end plates of patients with type I marrow changes had more protein gene product (PGP) 9.5–immunoreactive nerve fibers and more tumor necrosis factor (TNF)–immunoreactive cells than did normal end plates. PGP 9.5 immunoreactivity occurred exclusively in patients with diskogenic low back pain. TNF immunoreactivity in end plates with type I marrow changes was higher than in those with type II marrow changes. Rahme and Moussa concluded that type I marrow changes represent a more active inflammation mediated by proinflammatory cytokines, whereas type II and type III changes are more quiescent. In a study of infliximab, a monoclonal antibody against TNF-α, Korhonen and associates found that it was most effective when degenerative type I marrow changes were symptomatic. Nevertheless, the relationship of degenerative marrow changes to immunobiologic and cellular response mechanisms, although probably important, remains unclear.
In a study by Toyone and colleagues, segmental hypermobility was present in 70% of patients with type I marrow changes but in only 16% with type II changes. The greatest evidence that these marrow changes, particularly type I, are related to biomechanical instability is probably based on observations after fusion. Chataigner and collaborators suggested that patients with type I marrow changes have much better outcomes with surgery than do those with isolated degenerative disk disease and normal or type II marrow changes. In addition, conversion of type I marrow changes to either normal or type II was associated with higher fusion rates and better outcomes. Other studies support the contention that persistence of type I changes after fusion suggests pseudoarthrosis and is associated with persistent symptoms. Conversely, conversion of type I marrow changes to either normal or type II changes is associated with higher fusion rates and better outcomes ( Fig. 11.20 ). The conclusion is that fusion produces greater stability, reduces biomechanical stress, and accelerates the course of type I marrow changes toward improvement.
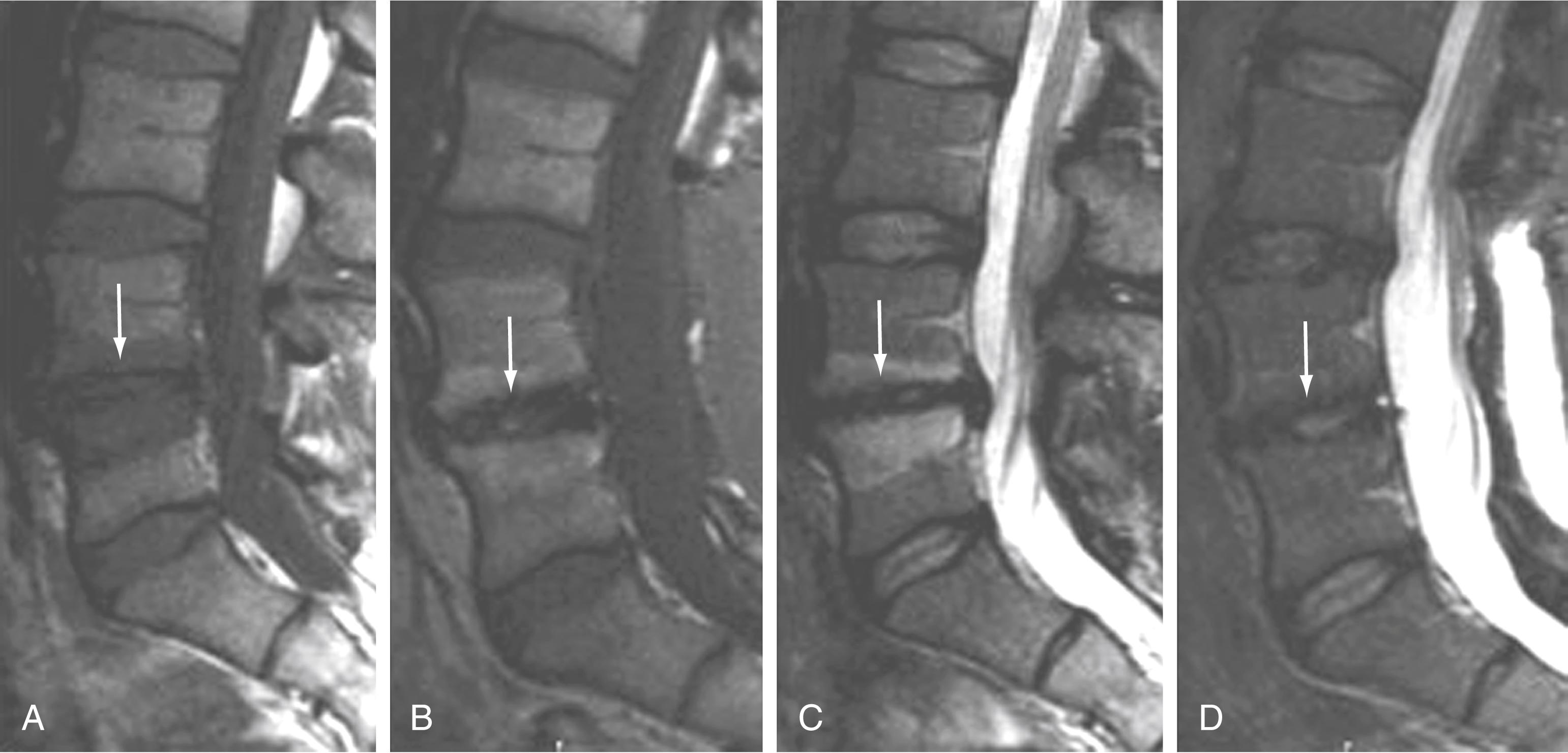
As further support that these fluid marrow changes reflect biomechanical stress, there is similar marrow conversion in the pedicles of vertebral bodies associated with symptomatic pars and pedicle fractures, as well as in severe degenerative facet joint disease. Pedicle marrow change to a normal or type II appearance is associated with improvement in symptoms.
Additional research has yielded findings that type I end plate changes can be secondary to infection. Stirling and colleagues found positive cultures in 19 of 36 disks obtained after microdiscectomy. In 16 of those 19 disks, Propionibacterium acnes was isolated. Albert and associates found similar results by studying patients undergoing surgery for degenerative disk disease. Of the patients who underwent discectomy, 43% had positive anaerobic cultures, and 80% of those patients went on to develop new type I end plate changes. Because these findings suggested that disk herniations can provide conditions favorable for low-grade anaerobic infections, a study was performed with long-term antibiotic therapy; the treatment produced improvements over placebo.
In summary, type I end plate changes are more strongly associated with pain and underlying instability and also are predictive of a favorable outcome after stabilization surgery. Type I changes can progress to type II changes, which are more static and less associated with symptoms. Conversion of type II changes to type I changes would raise concern for infection or progression in degenerative changes. The origin of end plate changes on MRI is thought to be secondary to mechanical stresses or instability, possibly in combination with biochemical or infectious factors, although additional research is needed.
The zygapophyseal joint is composed of the superior and inferior articulating facets and, like diarthrodial synovium-lined joints, it is susceptible to arthropathy ( Fig. 11.21 ). Loss of disk space height and altered biomechanics result in facet joint arthrosis and osteophytosis with consequent canal, foraminal, and lateral recess stenosis. Alternatively, it has been proposed that facet arthropathy can occur independently and be inherently symptomatic. , Aside from facet arthrosis, joint effusions can occur, with pain caused by irritation of nerve fibers of the synovium and joint capsule. Probably as a result of osteoarthritis and instability of the facet joints, 2.3% of synovial cysts are in anterior or intraspinal locations and 7.3% are in posterior or extraspinal locations ( Fig. 11.22 ).
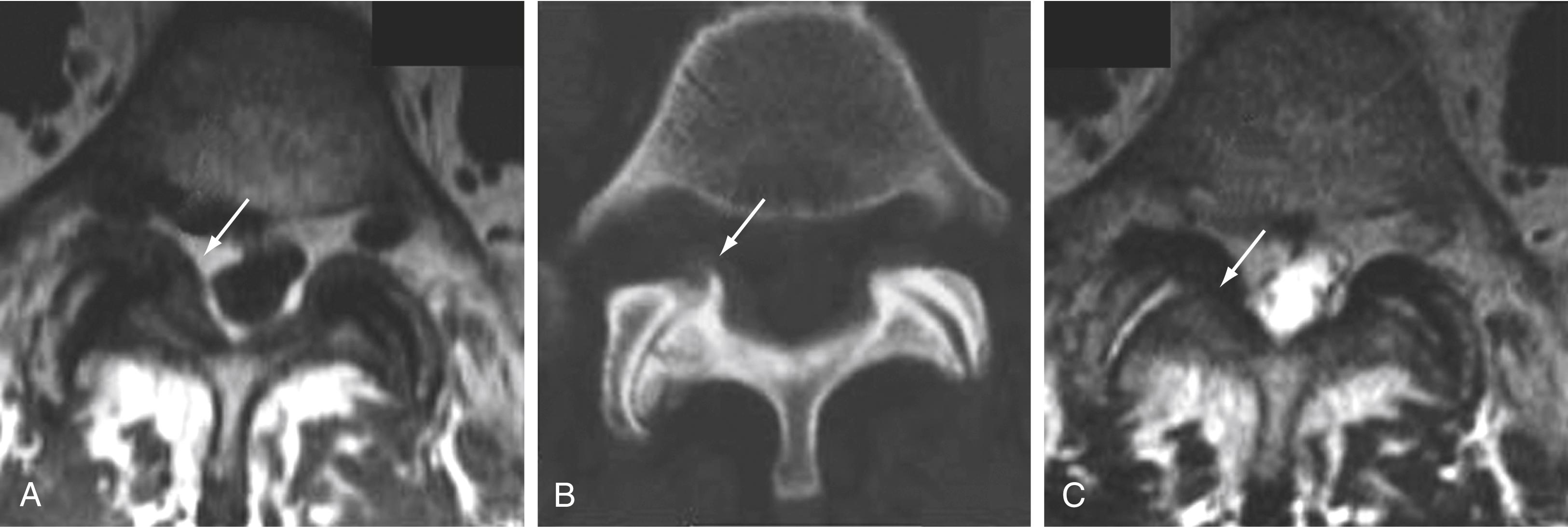
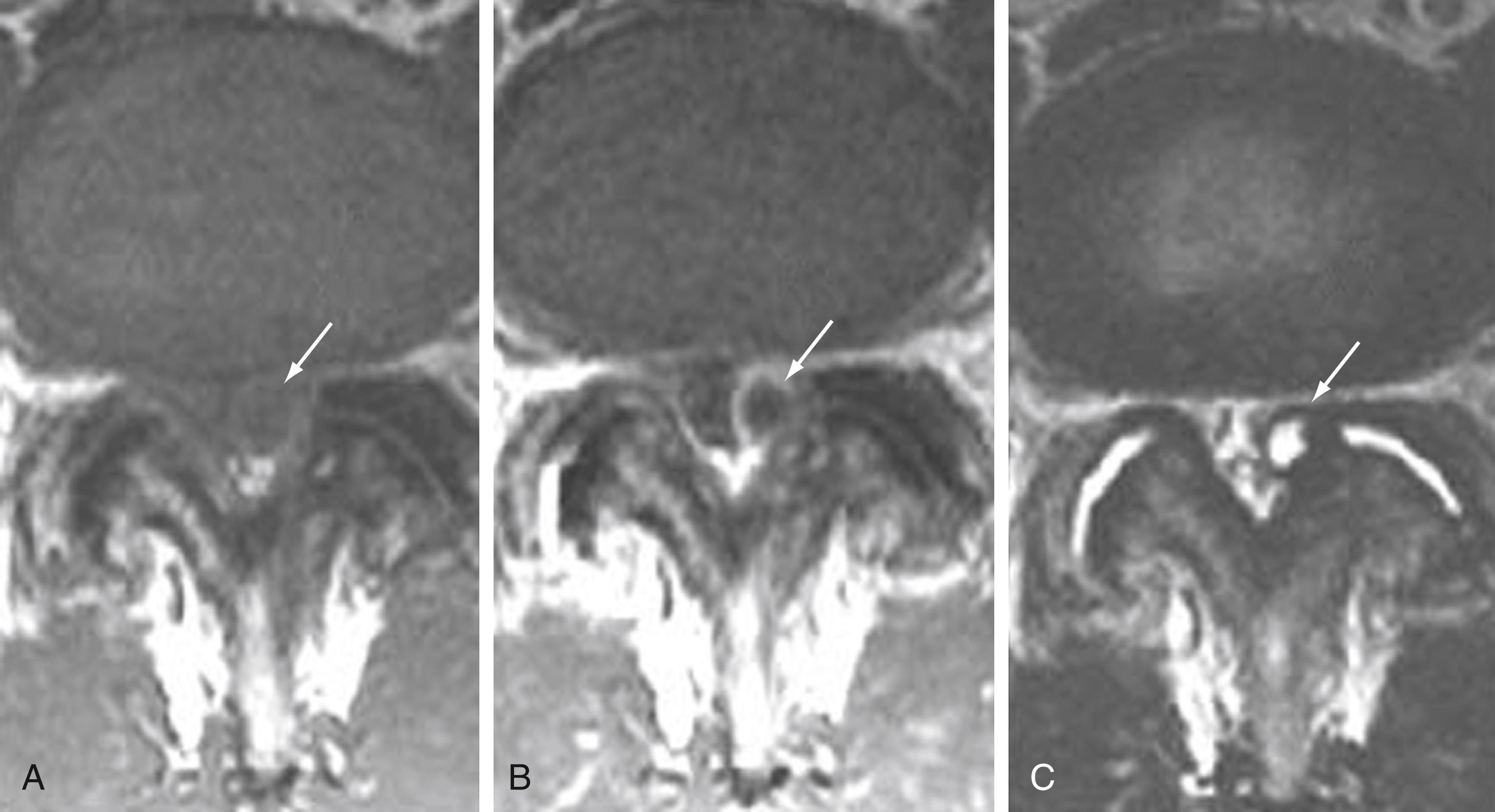
Ligaments of the spine are the anterior longitudinal ligament, the posterior longitudinal ligament, the paired sets of ligamenta flava, the intertransverse ligaments, and the unpaired supraspinous ligament. Alterations in alignment can lead to ligamentous laxity, followed by subsequent deterioration with loss of elasticity, calcification, and ossification. Exaggerated lordosis or severe disk space loss in the lumbar spine leads to close approximation and contact of the spinous processes and to degeneration of intervening ligaments. ,
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here