Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
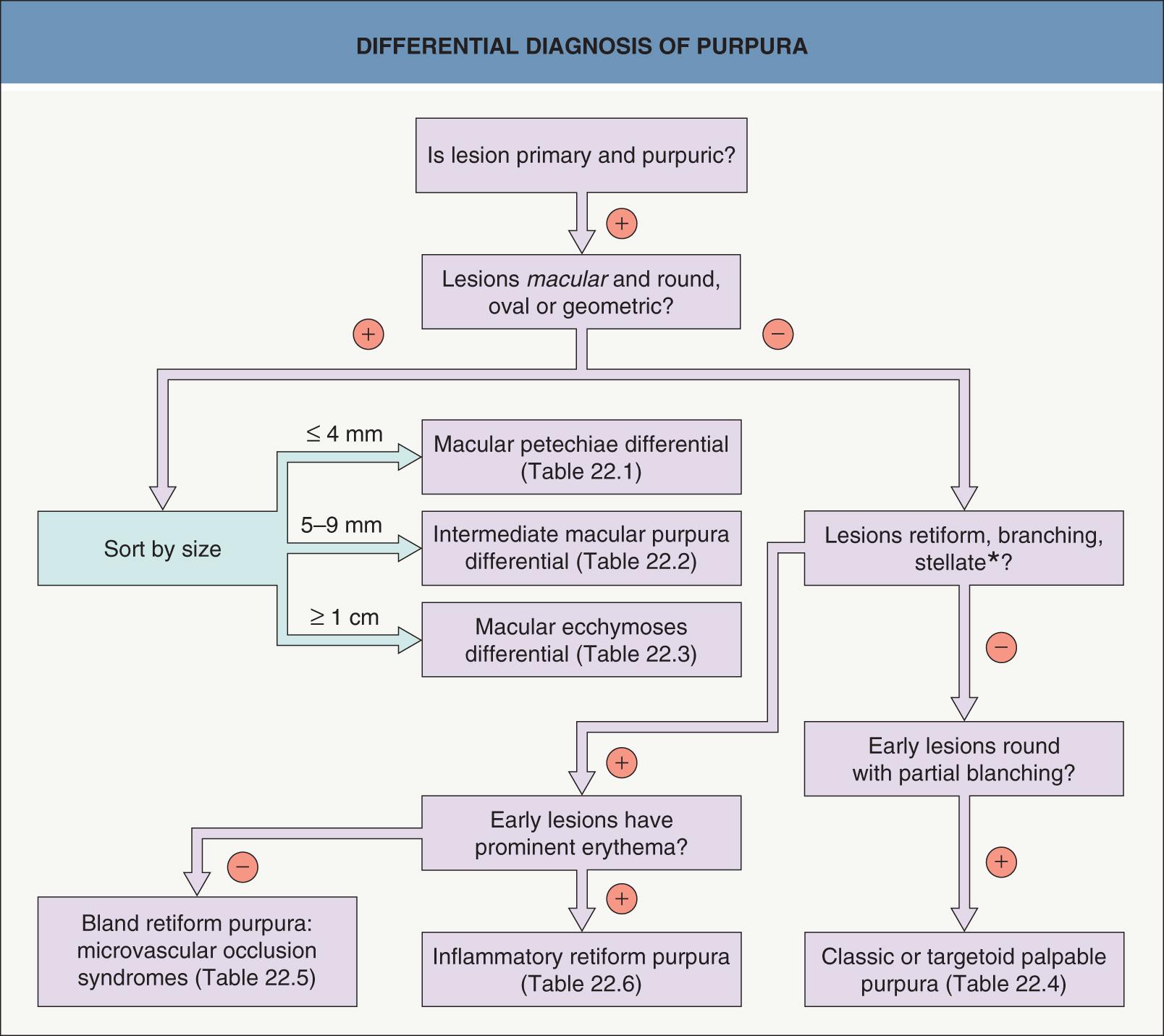
The differential diagnosis of purpura is extensive, but the evaluation of a patient with purpura can be greatly facilitated by categorizing purpuric lesions into morphologic subsets . The number of lesions and their distribution pattern should also be assessed, including whether all the lesions are purpuric or just those located on the distal lower extremities.
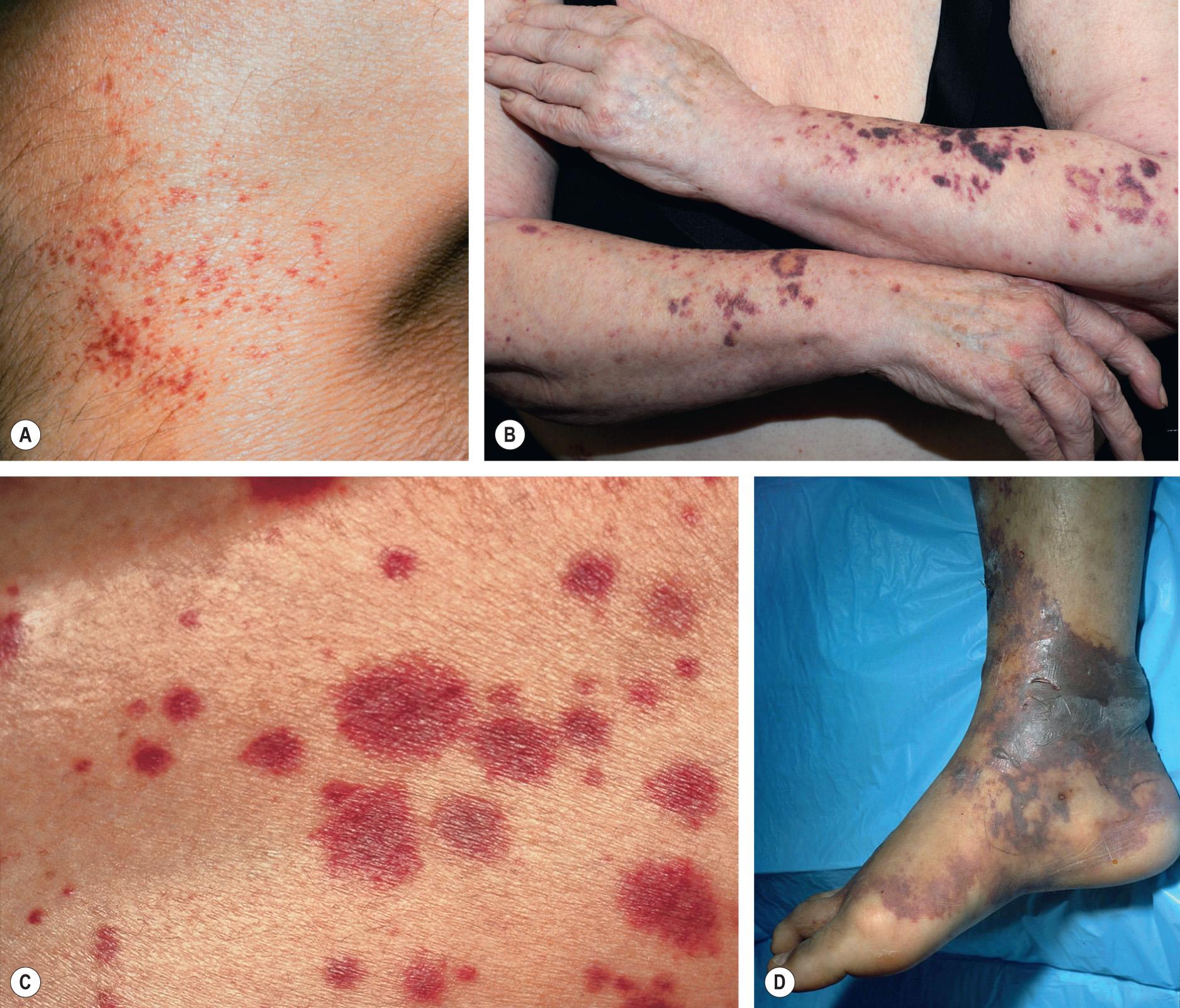
This chapter serves as an introduction to a method for evaluation and classification of patients presenting with purpura, defined as visible hemorrhage into the skin or mucous membranes. The differential diagnosis presented in this chapter is directed toward syndromes of primary purpura, where the hemorrhage is an integral part of lesion formation, rather than secondary hemorrhage into established lesions (e.g. stasis dermatitis, dependent cellulitis, dependent component of a morbilliform drug eruption). Purpuric lesions are divided into six subsets: (1) petechiae ( Fig. 22.1A , Table 22.1 ) ; (2) macular purpura ( Table 22.2 ); (3) macular ecchymoses ( Fig. 22.1B , Table 22.3 ); (4) palpable purpura ( Fig. 22.1C , Table 22.4 ); (5) non-inflammatory retiform purpura ( Fig. 22.1D ; Table 22.5 ); and (6) inflammatory retiform purpura ( Table 22.6 ). Lesions in the first three groups are categorized on the basis of size, while those in the last three groups can range in size from a few millimeters to several centimeters in diameter. The relevance of these morphologic subsets in sorting through the differential diagnosis is outlined in Fig. 22.2 .
| DIFFERENTIAL DIAGNOSIS OF MACULAR PETECHIAE (≤4 mm IN DIAMETER) |
| Hemostatically relevant thrombocytopenia (<10 000–20 000/mm 3 ) * |
| Major etiologies † |
|
| Abnormal platelet function |
| Major etiologies † |
|
| Non-platelet etiologies |
| Major etiologies † |
|
* Platelets provide factors necessary for hemostasis of endothelial cell junctions. When counts fall below 10 000–20 000/mm 3 , inadequate platelet support leads to degradation of junction function, increased permeability, and hemorrhage .
| DIFFERENTIAL DIAGNOSIS OF INTERMEDIATE MACULAR PURPURA (5–9 mm) |
| Major etiologies * |
|
| DIFFERENTIAL DIAGNOSIS OF MACULAR ECCHYMOSES (≥1 cm) |
| Procoagulant defect plus minor trauma |
| Major etiologies * |
|
| Poor dermal support of vessels plus minor trauma |
| Major etiologies * |
|
| Platelet disorders plus minor trauma |
| Major etiologies * |
|
| Miscellaneous |
|
| PALPABLE PURPURA: INFLAMMATORY PURPURA WITH PROMINENT EARLY ERYTHEMA |
| Leukocytoclastic vasculitis due to immune complex disease (see Fig. 24.1 ) |
| Small vessels only |
|
| Small- and medium-sized (macroscopic) vessels may be involved |
|
| Pauci-immune leukocytoclastic vasculitis (see Fig. 24.1 ) |
| ANCA-associated |
|
| Other |
|
| Not leukocytoclastic vasculitis |
| Small vessels only |
|
| Classic target lesion – usually erythema multiforme, but can be small vessel vasculitis, especially IgA-associated |
| DIFFERENTIAL DIAGNOSIS OF NON-INFLAMMATORY RETIFORM PURPURA |
| Occlusion primarily due to platelet-related thrombopathy |
| Major etiologies * |
|
| Cold-related precipitation or agglutination |
| Major etiologies * |
|
| Occlusion due to organisms growing in vessels |
| Major etiologies * |
|
| Systemic alteration in control of coagulation |
| Major etiologies * |
|
| Vascular coagulopathy |
|
| Embolization or crystal deposition |
| Major etiologies * |
|
| Reticulocyte, red blood cell occlusion |
|
| Miscellaneous |
|
** Also referred to as Coumadin® necrosis.
^ Early lesions primarily thrombotic, but vasculitis can also occur.
| DIFFERENTIAL DIAGNOSIS OF INFLAMMATORY RETIFORM PURPURA |
| Vasculitis |
| Primarily dermal vessels |
|
| Dermal and subcutaneous vessels usually involved |
|
| Dermal vessel inflammation/occlusion/constriction |
|
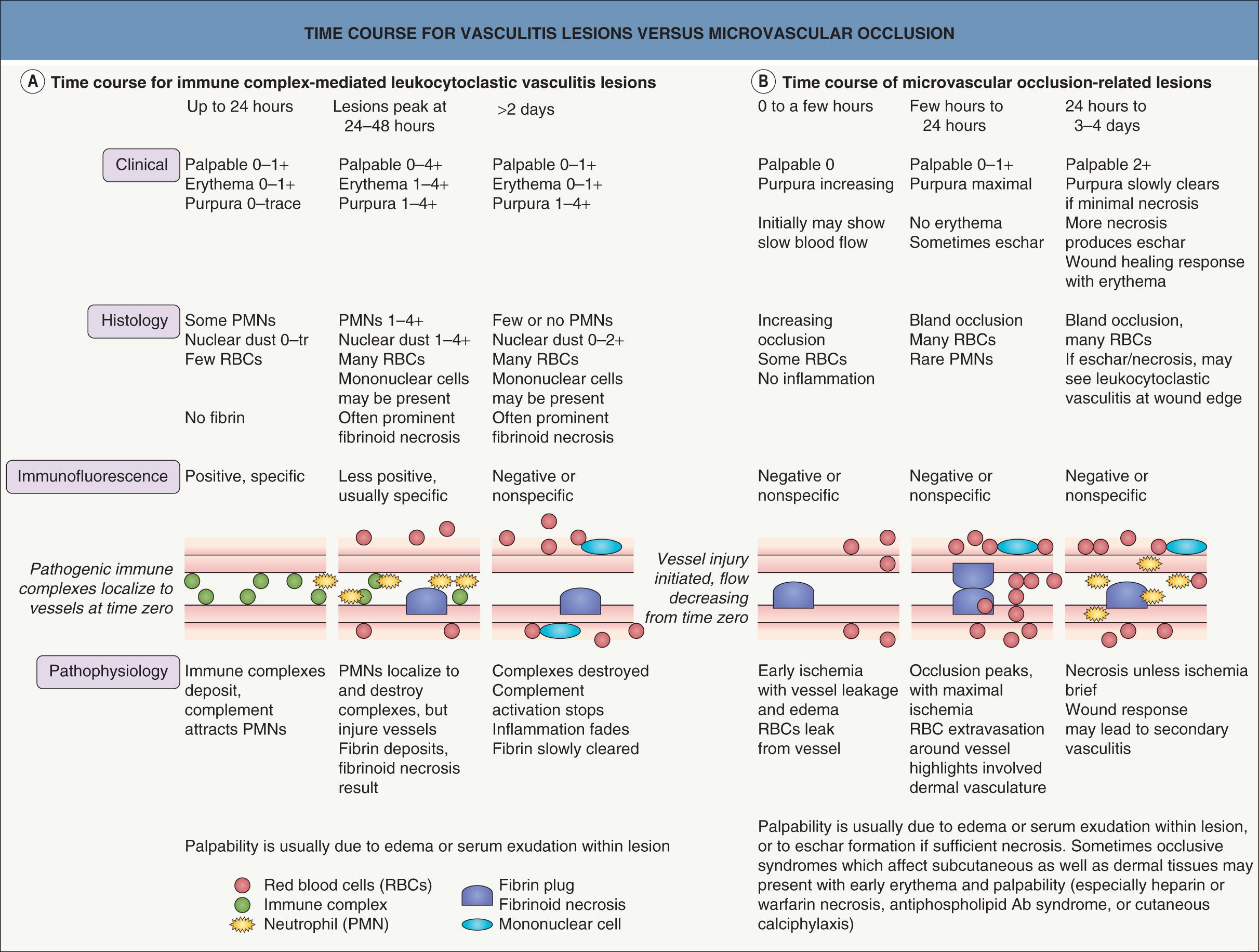
Two major causes of purpura, microvascular occlusion syndromes and vasculitis, are discussed in Chapters 23 and 24 . The former are important to recognize because they may mimic vasculitis but require a very different approach to diagnosis and therapy. The most typical presentation of microvascular occlusion syndromes is non-inflammatory retiform purpura (see Table 22.5 ) . Early lesions seldom show much erythema, and in the uncommon instance in which early erythema is present, purpura or necrosis typically comprises at least two-thirds of the lesion.
In the skin, livedo reticularis is a reflection of the physiologic anatomy of slow flow states (see Ch. 106 ). It is the three-dimensional structure and flow regulation of the dermal and subcutaneous vasculature that gives rise to the net-like pattern of livedo reticularis. The diameter of the almost-circular individual units within the net-like grid varies from 2 cm or larger on the back to 5 mm or less on the palms or soles.
Retiform purpura morphology results from occlusion of the vessels that produce the livedo reticularis pattern, but the two entities can be distinguished based upon the presence or absence of purpura, respectively, hence the term “retiform purpura” . Given the size of dermal vessels, the clot within the vessel is often too small to be seen grossly. What is actually observed is hemorrhage around the vessel within the dermis, presumably due to ischemia with hemorrhage prior to complete occlusion of the vessel. The shape of such a hemorrhagic lesion is determined by the anatomy of the slow flow network, though a complete reticulate pattern is very rarely seen. Instead, the morphology of retiform purpura is composed of “puzzle pieces” of the livedo reticularis pattern. While some lesions have a branching appearance, very few are truly stellate, i.e. characterized by a central area of necrosis or hemorrhage with radiating extensions.
Retiform lesions should be carefully examined to confirm the presence of purpura. Inflammatory retiform purpura (see Table 22.6 ) may also have a reticulate or branching morphology, but it is accompanied by peripheral erythema. However, just as the wound healing response can eventually lead to erythema and leukocytoclasis in lesions initiated by non-inflammatory occlusion, vasculitis sometimes produces lesions that show minimal early inflammation ( Fig. 22.3 ). Among the inflammatory disorders that can result in retiform purpura, the ANCA-positive vasculitides (especially granulomatosis with polyangiitis and microscopic polyangiitis) are the ones most likely to present with lesions that are difficult to distinguish from retiform purpura due to occlusion. Despite this overlap of clinical presentations, careful examination of early lesions to determine whether there is a substantial inflammatory component can greatly aid in focusing the differential diagnosis and directing the evaluation.
This approach to the differential diagnosis of purpura represents a departure from traditional categorization by pathophysiology. Because the pathophysiology of purpuric syndromes is what the clinician is attempting to ascertain, sorting by pathophysiology is of limited clinical utility. A method based primarily on the morphology of purpuric lesions (in addition to number and distribution) is designed to streamline the process of generating clinical hypotheses and most likely diagnoses, thereby facilitating a rapid, efficient and accurate evaluation to prove or disprove the suspected diagnosis.
The three subsets of macular (non-palpable) purpura (see Tables 22.1–22.3 ) typically develop via simple hemorrhage, with extravasated red blood cells and minimal inflammation observed in biopsy specimens. As such, these lesions have a very simple evolution, from initial hemorrhage to steady clearing of red blood cells and hemoglobin. Clinically, this correlates with fading of lesions and, in larger lesions, transition of color from red–blue or purple to green, yellow or brown before complete fading.
In syndromes of inflammatory hemorrhage, such as cutaneous small vessel vasculitis, as well as in microvascular occlusion, the evolution and clearing of lesions is more complicated. This is outlined in Fig. 22.3 . Because of the different evolution patterns of these two processes, it is possible for a biopsy specimen of a late lesion of vasculitis to resemble an early lesion of occlusion histologically. Conversely, a late lesion of occlusion with some resulting dermal necrosis may on histologic examination show features characteristic of leukocytoclastic vasculitis. This means that the biopsy specimen of a purpuric lesion must be interpreted within the context of the lesion's age and clinical setting. The clinical and histologic assessments of purpuric lesions are equally important in properly evaluating and diagnosing a purpuric syndrome.
Primary hemostasis consists of the formation of a platelet plug, and this is sufficient for the many daily minor injuries to the microvascular system. When a platelet plug is inadequate due to the size of the vessel or the size of the injury, secondary hemostasis with clot formation is needed. The control of clot formation is critically important: too little clotting can lead to death by hemorrhage; inappropriate clotting produces thrombosis, embolus or necrosis; and uncontrolled clotting with fibrinolysis can produce both thrombosis and hemorrhage, as in disseminated intravascular coagulation. Therefore, a thrombus must form rapidly at sites of injury, but it should not extend beyond where it is needed, and clotting factors that escape the local injury site must be prevented from triggering clotting at distant sites. As might be expected for a system that requires exquisite regulation to perform properly, the control of coagulation is multifaceted and incompletely understood . Basal coagulation encompasses the constant low-level activation of some components of the procoagulant, natural anticoagulant, and fibrinolytic pathways, so that these systems are ready for a rapid response when needed. An overview of the controls and interconnections of these pathways is shown in Fig. 22.4 .
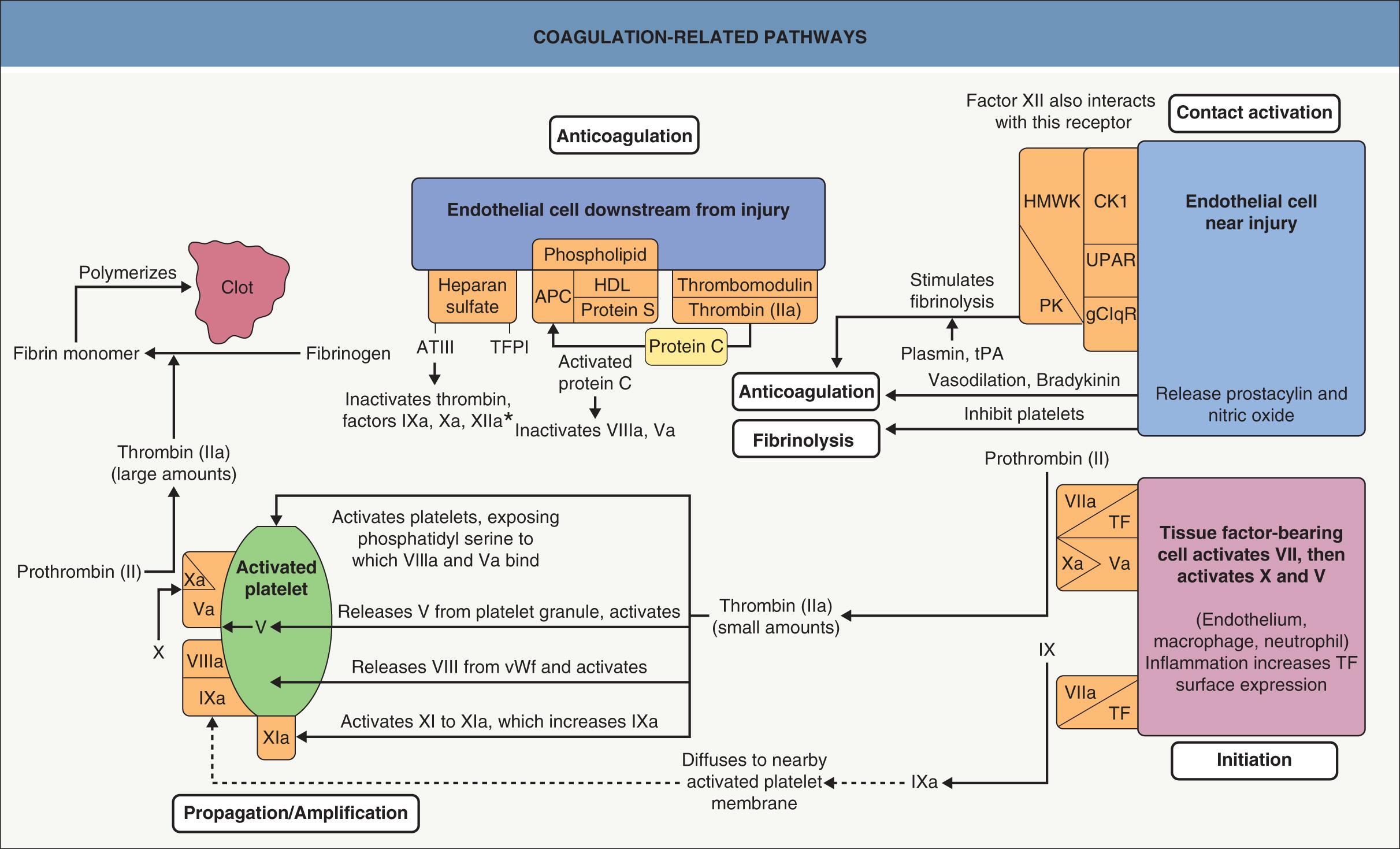
The initiation stage of clot formation generates small amounts of thrombin (factor IIa) via formation of a tissue factor–factor VIIa complex that activates factor IX to IXa and factor X to Xa (a process formerly referred to as the extrinsic pathway of coagulation) . This phase may take place on any cell that expresses tissue factor and is exposed to plasma; inflammatory cells and injured endothelial cells are common sites. Only small amounts of these factors are released in this phase, usually insufficient to produce a clot. However, these small amounts are critical for triggering the propagation/amplification phase of coagulation. The thrombin released during initiation activates platelets. Platelet membrane surfaces are then inverted to expose phospholipids (especially phosphatidyl serine) critical for localizing membrane procoagulant complexes, starting with the binding of factors VIIIa and Va (cofactors for factors IXa and Xa, respectively). Thrombin molecules also stimulate the release of factor V from platelet granules and activate factor V as well as factor VIII, freeing the latter from von Willebrand factor. Finally, thrombin activates factor XI to XIa (which leads to further activation of IX to IXa). As shown in Fig. 22.4 , the activated platelet surface is now the site of complexes capable of rapid amplification of thrombin generation, which then catalyzes the cleavage of fibrinogen to fibrin with subsequent polymerization of fibrin monomers into a clot.
Classically, factor XII and the contact activation complex have been considered part of the coagulation cascade (formerly referred to as the intrinsic pathway). However, while patients deficient in factor XII, high-molecular-weight kininogen, or prekallikrein may have prolonged activated partial thromboplastin times (aPTT), they do not bleed excessively and may actually be prone to thrombosis. These findings, plus other investigations, have challenged traditional concepts of the role of the contact activation system . Factor XII may play a role in clot propagation, particularly in pathologic states, but the contact activation system seems more targeted toward other physiologic functions.
The contact activation complex is formed by the assembly of high-molecular-weight kininogen (HMWK) and prekallikrein (PK) on a multiprotein receptor complex on endothelial cell membranes; this complex consists of cytokeratin 1, the globular head of the C1q receptor, and the urokinase plasminogen activator receptor (see Fig. 22.4 ). Binding of HMWK, PK, and sometimes factor XII to this receptor complex results in the production of the powerful vasodilator bradykinin and other vasoactive mediators. In addition, fibrinolytic activity is generated through both activation of single- and two-chain urokinase-like plasminogen activator and release of tissue plasminogen activator, which subsequently convert plasminogen to plasmin. C1 esterase inhibitor, well known in controlling complement reactions, also has a major role in regulating this pathway. Of note, the episodic edema of hereditary angioedema is probably due more to deficient C1 esterase inhibitor-mediated down-regulation of bradykinin production than to deficient inhibition of complement (see Ch. 18 ). The contact activation system is likely responsible for some of the fibrinolysis and refractory hypotension seen in sepsis, following cardiopulmonary bypass (“post-pump” systemic inflammatory response syndromes), and in other settings where contact activation is likely to be extensive.
Natural anticoagulant control of clot extension is likewise complex . The initiation phase of clotting is down-regulated by tissue factor pathway inhibitor (TFPI) and antithrombin III (ATIII). TFPI and ATIII are bound to heparan sulfate molecules on the surface of endothelial cells, and their presence in close proximity to sites of desired clot formation allows them to capture activated clotting factors and prevent them from leaving the area. TFPI can inactivate factor Xa, and the TFPI–factor Xa complex can efficiently bind to and inhibit factor VIIa (which is found in a complex with tissue factor); in the absence of factor Xa, TFPI reacts with factor VIIa at a much slower rate. ATIII can neutralize thrombin as well as factors IXa, Xa, and XIIa; this process is greatly enhanced by heparan sulfate or heparin binding to ATIII. However, the factor IXa produced during initiation is not inhibited by TFPI and is only slowly bound by ATIII.
The thrombomodulin/protein C/protein S system is the other major natural anticoagulant pathway. While both ATIII and protein C pathways are important in large vessels, the protein C system seems to be more critical for normal function of the microvasculature. Thrombin that escapes the clot site can bind to thrombomodulin on the endothelial cell surface. Once bound to thrombomodulin, thrombin loses its ability to activate procoagulant factors, and instead avidly binds and activates protein C, a vitamin K-dependent anticoagulant protein. The anticoagulant function of activated protein C is enhanced by its binding to a phospholipid surface as well as a high-density lipoprotein (HDL) and protein S (another vitamin K-dependent anticoagulant). This complex then inactivates factors Va and VIIIa. The factor V Leiden mutation results in a single amino acid change at the site of cleavage by activated protein C, and this change renders it much more resistant to inactivation, thus explaining the associated prothrombotic state.
The prothrombin 20210A mutation is a polymorphism located at the polyadenylation site in the 3′ untranslated region of the prothrombin gene that does not affect the final structure of prothrombin. However, it increases the levels of prothrombin, leading to a prothrombotic tendency in some patients.
Hemostasis is one aspect of the host response to injury. It is clear that inflammatory injury can lead to fibrin deposition in vessels, as exemplified by fibrinoid necrosis in vasculitis (see Fig. 22.3 ). Conversely, coagulation factors may play an important role in inflammatory responses and wound healing . It can be argued that the vertebrate coagulation system is an extension of the innate immune response system observed in the living fossil the horseshoe crab . In this arthropod, 99% of the circulating cells are hematocytes that express pathogen-recognition receptors; upon activation, these cells release multiple proteins with both clotting and complement-like functions which localize and immobilize invading pathogens. The serine proteases involved in blood clotting in vertebrates appear to have diverged from primitive complement precursors, providing a link between the inflammatory and coagulant pathways.
Thrombin not only is a procoagulant factor, but it also can act to promote monocyte, fibroblast, and endothelial cell migration into an area of recent injury in order to begin removal of damaged tissue and wound repair. Thrombin bound to thrombomodulin activates protein C, which (in addition to its potent anticoagulant action) also appears to have important anti-inflammatory properties. Of note, administration of activated protein C has been shown in some, but not all, studies to decrease mortality and improve organ function in patients with severe sepsis. In addition, the anti-inflammatory and antithrombotic effects of the protein C pathway may play a role in multiple disorders, from inflammatory bowel disease and rheumatoid arthritis to asthma and atherosclerosis.
Platelets release many growth factors and cytokines that affect wound healing (e.g. platelet-derived growth factor, transforming growth factor-β; see Fig. 141.3 ) . A powerful angiogenesis inhibitor, angiostatin, is a cleavage product of plasminogen. These and many other findings suggest that coagulation, inflammation, and wound healing pathways are richly and importantly integrated.
A thorough history (including family history) and physical examination are crucial in the proper selection and interpretation of tests measuring coagulation and anticoagulation parameters. A basic laboratory assessment of clotting includes a platelet count, prothrombin time (PT), and aPTT. A prolonged PT with a normal aPTT suggests a factor VII deficiency or the use of an oral anticoagulant (at a relatively low dose) . A prolonged aPTT with a normal PT can result from the use of heparin, a lupus anticoagulant, an acquired factor VIII inhibitor, von Willebrand disease (which results in a functional deficiency of factor VIII as well as defective platelet adhesion), or a significant deficiency of one of the following: factor VIII, IX, XI or XII, high-molecular-weight kininogen (HMWK), or prekallikrein (PK). If both the PT and the aPTT are prolonged, there may be a deficiency (or inhibitor) of fibrinogen, prothrombin, factor V, or factor X versus a combined deficiency of multiple procoagulant factors, e.g. due to severe vitamin K deficiency or high doses of an oral anticoagulant. Platelet function tests (e.g. bleeding time, ristocetin-induced platelet aggregation studies) are performed in settings suggesting platelet function defects unexplained by medications (e.g. aspirin, nonsteroidal anti-inflammatory drugs [NSAIDs]) or metabolic disorders such as renal failure. An example would be the platelet dysfunction observed in patients with Hermansky–Pudlak syndrome.
If the PT or aPTT is prolonged, repeating the test using a 1 : 1 mixture of the patient's plasma and normal plasma can be helpful in distinguishing between a deficiency state and the effects of an inhibitor. A 1 : 1 dilution will usually normalize the time if there is a factor deficiency, but the time will remain prolonged if an inhibitor is present. Other tests, such as the dilute Russell viper venom time, may be helpful in confirming the presence of lupus anticoagulant activity.
Laboratory tests that are useful in the evaluation of a patient suspected to have a hypercoagulable state are presented in Table 105.9 .
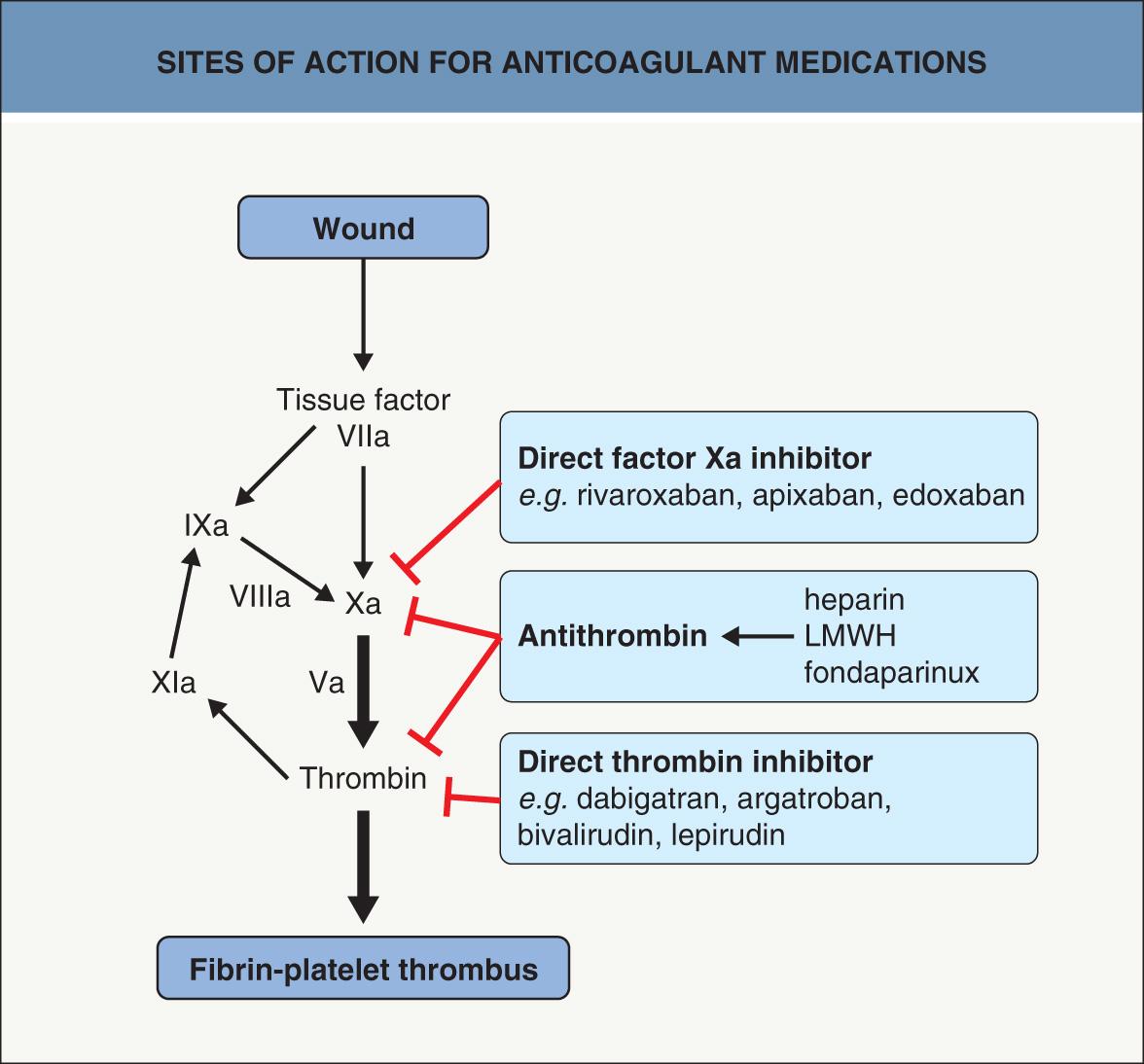
In a number of clinical settings, antiplatelet agents are used to reduce the risk of thrombosis and these agents are outlined in Table 22.7 . Also, newer agents have joined heparin, low-molecular-weight heparins, and warfarin as useful anticoagulant medications ( Table 22.8 ) . For example, direct oral anticoagulants (DOACs) that inhibit thrombin or Factor Xa have been approved by the FDA ( Fig. 22.5 ), and they do not require monitoring of the international normalized ratio (INR), i.e. prothrombin ratio. Although DOACs have reduced the rate of major bleeding by ~30% and the rates of intracranial and fatal hemorrhages by 50%, if a major bleed occurs only one of them (dabigatran) currently has a specific reversal agent (idarucizumab) . However, additional specific antidotes (e.g. andexanet) are under review at the FDA at the time of writing , and antisense oligonucleotides that target coagulation factors are in the development phase. Lastly, treatment options for safe anticoagulation of patients developing heparin-induced thrombocytopenia continue to evolve .
| ANTIPLATELET AGENTS | |||||
|---|---|---|---|---|---|
| Agent | Route of administration and dosage | Dose adjustment | Half-life | Management of life-threatening bleeding | Comments |
| Thromboxane inhibition and related mechanisms | |||||
| Aspirin (acetylsalicylic acid; irreversible inhibitor of COX-1, which is required for the biosynthesis of thromboxane A2) | Oral: 81 mg daily (low-dose) | None if low-dose | Acetylsalicylic acid: 15 min Salicylate: 2 hours |
Platelet transfusions may be helpful | More limited antiplatelet effect because COX-1 is an intermediate step in thromboxane A2 synthesis so that the latter can still be produced by other cyclic endoperoxidase precursors |
| Triflusal (available in Europe, Asia, and South America) | Oral: 300 mg every 8 hours or 600 mg every 12 hours | NA | Triflusal: 30 min Major metabolite: 34 hours |
NA | Pharmacodynamics are comparable to aspirin Safety profile may be superior to aspirin with a lower risk of hemorrhagic events |
| Inhibitors of P2Y12, the platelet receptor for adenosine diphosphate (ADP) | |||||
| Clopidogrel (second generation; prodrug) | Oral: 300 mg loading dose, then 75 mg daily in combination with low-dose aspirin | None | 6 hours | Platelet transfusions may be helpful | 85–90% inactivated by liver/gut esterases Remaining 10–15% undergoes 2-step metabolism to a molecule that irreversibly binds to P2Y12 |
| Prasugrel (thienopyridine; third generation) | Oral: 60 mg loading dose, then 10 mg daily in combination with low-dose aspirin | None | Active metabolite: 7 hours | Platelet transfusions may be helpful | Irreversible inhibitor of P2Y12 More bioavailable than clopidogrel |
| Ticagrelor (nonthienopyridine cyclopentyltriazolopyrimidine) | Oral: 180 mg loading dose, then 90 mg twice daily in combination with aspirin (75–100 mg daily) | Avoid if severe hepatic impairment | 7 hours Active metabolite: 9 hours |
No known treatment Not dialyzable |
Reversible antagonist of P2Y12 Rapid onset |
| Cangrelor | IV: 30 mcg/kg bolus, followed by 4 mcg/kg/min for at least 2 hours or duration of procedure (whichever is longer) | Avoid if CrCl <30 ml/min | Recovery of platelet function is within 60 min of discontinuation |
Reversible P2Y12 antagonist Rapid onset of action: within 3–6 min |
|
| Glycoprotein IIb/IIIa inhibitors – inhibit fibrinogen and von Willebrand factor-mediated platelet-to-platelet cross-linkage (the final pathway of platelet aggregation) | |||||
| Eptifibatide | IV: 180 mcg/kg bolus, then 2 mcg/kg/min | If CrCl <50 ml/min, reduce to 1 mcg/kg/min | 2.5 hours | Bleeding time is 1.4× normal within 6 hours of discontinuation | FDA approved for acute coronary syndrome |
| Tirofiban | IV: 25 mcg/kg within 5 min, then 0.15 mcg/kg/min for up to 18 hours |
If CrCl ≤60 ml/min, reduce to 0.075 mcg/kg/min | 2 hours | Adjustment of infusion rate or cessation; removal via hemodialysis if necessary | FDA approved for acute coronary syndrome |
| Abciximab | IV: 0.25 mg/kg bolus, then 0.125 mcg/kg/min for up to 12 hours | Not renally excreted | <10 min, with second phase of ~30 min | Bleeding time returns to <12 min in 90% of patients after infusion discontinued | FDA approved for unresponsive unstable angina and for percutaneous coronary intervention |
| Protease-activated receptor (PAR) inhibitors (thrombin mediates platelet activation via PAR-1) | |||||
| Vorapaxar (antagonist) | Oral: 2.08 mg daily | Avoid if severe hepatic impairment | 165–311 hours Terminal elimination half-life: 8 days |
No known treatment | Significant inhibition of platelet aggregation remains 4 weeks post-discontinuation |
| Phosphodiesterase inhibitors | |||||
| Cilostazol (quinolinone derivative) | Oral: 100 mg BID | Avoid if congestive heart failure Reduce to 50 mg BID if receiving CYP3A4 or CYP2C19 inhibitors |
Terminal half-life: 10 hours | Not dialyzable | FDA approved for claudication |
| Dipyridamole | Oral: 75–100 mg TID to 4 times daily | May increase effect of adenosine infusionMay decrease effect of cholinesterase inhibitors and worsen myasthenia gravis | 10 hours | Not dialyzable | FDA approved as adjunct to warfarin to prevent postoperative thromboembolic complications of valve replacement |
| ANTICOAGULANTS | |||||
|---|---|---|---|---|---|
| Agent | Route of administration and dosage | Dose adjustment | Half-life | Management of life-threatening bleeding | Comments |
| Act with antithrombin to primarily inhibit IIa (thrombin) and/or Xa * | |||||
| Unfractionated heparin | IV (treatment): bolus of 5000 U vs 80 U/kg followed by continuous infusion SC (prophylaxis): 5000–7500 U BID |
Keep aPTT at 1.5 to 2 times normal | IV: 0.5 hours SC: 1.5 hours |
Protamine sulfate | With the exception of patients with CrCl <30 ml/min, used less commonly nowadays Greater frequency of anti-heparin Ab than with LMWH Inhibits thrombin and Xa (see Fig. 22.5 ) |
| Enoxaparin (LMWH) | SC (treatment): 1 mg/kg BID or 1.5 mg/kg daily SC (prophylaxis for DVT): 40 mg daily |
Avoid if CrCl <30 ml/min | 4.5 hours (unless renal dysfunction) 7 hours with repeated dosing |
Partially reversible with protamine sulfate | Inhibits Xa (see Fig. 22.5 ) |
| Fondaparinux (synthetic pentasaccharide) | SC (treatment): 5–10 mg daily, based on body weight SC (prophylaxis): 2.5 mg daily |
Avoid if CrCl <30 ml/min | 17–21 hours | No known antidote | Inhibits Xa (see Fig. 22.5 ) |
| Inhibit gamma carboxylation of factors II, VII, IX and X, protein C, and protein S (vitamin K-dependent proteins) ** | |||||
| Warfarin (racemic mixture; 50% R and 50% S enantiomers) | Oral: 1–7.5 mg daily, depending upon INR | Keep INR between 2 and 3 | S-warfarin: 24–33 hours R-warfarin: 35–58 hours |
Vitamin K Urgent reversal: PCC, FFP, or recombinant activated factor VIIa |
Requires monitoring of INR Multiple drug–drug interactions (see Ch. 131 ) |
| Direct thrombin inhibitors | |||||
| Dabigatran | Oral: 150 mg BID | Renal impairment Cr clearance <30 ml/min: 150 mg orally BID Cr clearance 15-30 ml/min: 75 mg orally BID Cr clearance <15 ml/min: not recommended |
12–17 hours | Idarucizumab (specific antidote) Hemodialysis PCC Recombinant activated factor VIIa |
Does not require monitoring of INR FDA approved for: treating (and reducing recurrence of) venous thromboembolism ^ ; prevention of both venous thrombosis post orthopedic surgery and thromboembolism in nonvalvular atrial fibrillation |
| Argatroban | IV: 2 mcg/kg/min (steady state within 1–3 hours) | Keep PTT at 1.5 to 3 times normal If severe hepatic impairment, 0.5 mcg/kg/min |
39–51 min | No specific antidote PTT returns to baseline 2–4 hours after discontinuation (longer if hepatic impairment) |
FDA approved for HITS or if patient at risk for HITS and undergoing percutaneous coronary intervention |
| Bivalirudin (synthetic hirudin analog) | IV: 0.75 mg/kg bolus followed by 1.75 mg/kg/hour | Renal impairment – dose adjust | 27–57 min, depending on renal status | No known antidote Can be hemodialyzed |
FDA approved for unstable angina in patients undergoing percutaneous coronary intervention |
| Direct factor Xa inhibitors | |||||
| Rivaroxaban | Oral (treatment): 15 mg BID for 1 st 21 days, then 20 mg daily Oral (prophylaxis): 20 mg daily |
Avoid if CrCl <15 ml/min | 5–9 hours young, 11–13 hours elderly | PCC Recombinant activated factor VIIa Andexanet (specific antidote) ^^ |
Does not require monitoring of INR FDA approved indications – see dabigatran |
| Apixaban | Oral (treatment): 10 mg BID × 7 days then 5 mg BID Oral (prophylaxis): 2.5 mg BID (postoperative) or 5 mg BID |
Reduce to 2.5 mg BID if patients on strong CYP3A4 or P-gp inhibitors or have at least 2 of following: age ≥80 yr, weight ≤60 kg, Cr ≥1.5 mg/dl | 12 hours | PCC Recombinant activated factor VIIa Andexanet (specific antidote) ^^ |
Does not require monitoring of INR FDA approved indications – see dabigatran |
| Edoxaban | Oral (treatment): 60 mg daily post 5–10 days of parenteral anticoagulant Oral (prophylaxis): 60 mg daily |
Avoid if CrCl ≥95 ml/min Reduce to 30 mg daily if CrCl = 15–50 ml/min or if coadministered with certain P-gp inhibitors |
10–14 hours | PCC Recombinant activated factor VIIa Andexanet (specific antidote) ^^ |
Does not require monitoring of INR FDA approved for: treatment of venous thromboembolism ^ ; prevention of thromboembolism in nonvalvular atrial fibrillation |
* Danaparoid, composed of heparan, dermatan, and chondroitin sulfates, is not available in the US and is indicated for the treatment of HIT.
** Phenprocoumon and acenocoumarol are available in Europe and dosage adjustments are based on INR.
Practice standards are evolving with respect to discontinuing antiplatelet and anticoagulant agents prior to cutaneous surgical procedures. While previous standard practice was to withdraw these agents prior to surgery, multiple studies have shown that the risk of bleeding is low. Therefore there has been a movement toward continuing these medications in order to reduce the risk of stroke, coronary occlusion, or thrombosis .
Some syndromes that are not traditionally discussed in chapters on microvascular occlusion (see Ch. 23 ) or vasculitis (see Ch. 24 ) will be addressed here.
▪ Pigmented purpuric eruptions ▪ Capillaritis ▪ Purpura pigmentosa chronica ▪ Purpura simplex ▪ Majocchi–Schamberg disease
Variants:
Schamberg disease: Schamberg purpura, progressive pigmentary dermatosis of Schamberg, purpura pigmentosa progressiva
Purpura annularis telangiectodes of Majocchi: Majocchi's disease
Pigmented purpuric lichenoid dermatitis of Gougerot and Blum
Eczematid-like purpura of Doucas and Kapetanakis: eczematoid purpura, itching purpura
Lichen aureus: lichen purpuricus
Clustered petechial hemorrhage
Often a background of yellow–brown discoloration due to hemosiderin deposition
The location and pattern depends on the particular variant
Pigmented purpuric eruptions represent a group of diseases characterized by petechial hemorrhage thought to be secondary to capillaritis . These diseases have no systemic findings, but they occasionally lead to a patient evaluation to exclude thrombocytopenia or vasculitis because of the purpuric (usually petechial) nature of the lesions and clinical misdiagnosis, especially of the rarer forms.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here