Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Blood banking and transfusion medicine are often interchangeable terms for the medical practice of blood product procurement, storage, and administration. It also includes the science of immunohematology, blood component manipulation, and outlining of clinical transfusion guidelines. Hematologic pathologies in neonates and children are vastly different from adults, leading to the varied indications of blood product replacement, further necessitating pediatric-specific strategies for this population.
Of note: the principles in this chapter relate to practices within the United States (US), unless otherwise specified.
Red blood cells (RBCs) express antigens that can lead to immunogenicity with clinically significant responses such as hemolytic transfusion reactions (HTRs) and hemolytic disease of the fetus and newborn (HDFN). See Table 35.1 for some clinically significant major and minor blood groups.
| Antigen (main) | Antibodies in serum (main) | Antibody type (subtype main) | May lead to | ||
|---|---|---|---|---|---|
| HTR | HDFN | ||||
| Major blood groups | |||||
| A | A | Anti-B | IgM (majority) | Yes | Yes |
| B | B | Anti-A | IgM (majority) | Yes | Yes |
| O | H | Anti-A, anti-B, anti-A, B | IgM and IgG | Yes | Yes |
| AB | AB | None | None | Yes | Yes |
| Examples of other minor RBC blood groups | |||||
| Rhesus (Rh) | D | Anti-D | IgG | Yes | Yes |
| Kell | K, k | Anti-K, anti-k | IgG | Yes | Yes |
| Kidd | Jk a , Jk b | Anti-Jk a , anti-Jk b | IgG | Yes | Yes |
| Duffy | Fy a , Fy b | Anti-Fy a , anti-Fy b | IgG | Yes | Yes |
| Lewis | Le a , Le b | Anti-Le a , anti-Le b | IgM | No | No |
The major antigens on red cells are A, B, and H (made up of carbohydrates).
The H antigen is a precursor to A and B antigens leading to the A and B blood types respectively; those with only the H antigen are considered blood group O.
These antigens are attached to proteins and lipids that are attached to the red cell membrane.
Naturally occurring antibodies against ABO antigens are produced by the humoral arm of the immune system and circulate in the serum (commonly IgM subtype):
These antibodies are formed around 4–6 months of age.
The main cause of HTRs is due to ABO incompatibility: for O-individuals, not only are naturally occurring isohemagglutinins formed of the IgM subclass to the A-like and B-like antigens, but there is also the formation of a unique anti-A, B IgG. Since it is of an IgG subtype, it can cross the placenta and bind to either an A or B antigen and cause hemolysis in an infant. This leads to hemolytic disease of the fetus and newborn—ABO type.
Greater than 300 blood groups exist with clinically significant antibodies that form in those who lack an antigen but are exposed to it via transfused RBCs or through pregnancy/miscarriage. They may develop IgG antibodies that can cause acute HTR (AHTR) or delayed HTR (DHTR) in the recipient or pregnant female. Different antigens have different immunogenicity profiles due to their unique composition, the recipient’s lack of the antigen on their own RBCs, and their bodies’ immune response to those foreign antigens.
The formation of antibodies can lead to difficulties with finding compatible RBC units for transfusion and can cause delays in treatment.
Autoantibodies: auto refers to “self”
These antibodies are directed toward a recipient’s own RBCs or other tissue antigens.
For RBCs, these antibodies can be formed in response to an illness or viral trigger.
Other types include antinuclear antibody (ANA) in rheumatologic diseases and antitissue transglutaminase (TTG) in gastrointestinal diseases.
They can be:
Clinically relevant leading to processes such as autoimmune hemolytic anemia (see Chapter 8 : Extracorpuscular Hemolytic Anemia).
Clinically nonsignificant, being observed during the testing process, but not leading to hemolysis in the transfusion recipient.
Alloantibodies: allo refers to “other”
These antibodies:
Are formed in response to exposure to “other” antigens (e.g., via transfusion).
And are then directed at these “other” antigens upon a subsequent exposure.
They can also be clinically or nonclinically significant, with antibody titer and/or avidity being a factor.
Blood donation is an altruistic act, in most cases nonremunerated, with the process starting from donor recruitment and ultimately resulting in transfusion of a recipient. The process is highly regulated by each individual state, the federal Food and Drug Administration (FDA), and other agencies such as AABB (American Association of Blood Banks—a leading organization in transfusion medicine and cellular therapy), the Joint Commission, and the College of American Pathologists.
Donors must provide proof of identity and reliable contact information (so they may be notified of pertinent test results) to donate. Donation criteria may vary marginally by state; see Table 35.2 for common deferrals. Refer to regional blood bank websites for a complete list of deferrals, time-based details, and exceptions.
| Deferral time frame | Category |
|---|---|
| Temporary | Age younger than 16 years |
| Weight <110 pounds | |
|
|
| Temperature >99.5°F or >37.5°C | |
| Heart rate: >100 or <50 beats/min (unless approved by medical doctor) | |
|
|
| Indefinite | Positive for HIV or diagnosed with AIDS (at any point in time) |
| Hepatitis B or C diagnosis (at any point in time) | |
| Ebola virus infection (at any point in time) | |
| Resident of the United Kingdom between 1980 and 1996 (with total time spent equaling 3 months or more)—concern for exposure to variant Creutzfeldt–Jakob disease | |
| Resident of France or Ireland between 1980 and 2001 (with total time spent equaling 5 years or more)—concern for exposure to variant Creutzfeldt–Jakob disease | |
| 3 years | Resident of malaria-endemic region (with total time spent >5 years) |
| Malaria diagnosis and subsequent treatment | |
| 12 months | Living with a person with hepatitis B or symptomatic hepatitis C |
| Sexual contact with a person with hepatitis B or symptomatic hepatitis C | |
| Incarcerated for >72 h consecutively in the last 12 months | |
| 3 months | Recipient of a transfusion |
| Recipient of a transplant (organ, tissue, or marrow) | |
| Contact with another person’s blood (open wound or needle stick injury) | |
Sexual contact with:
|
|
| Tattoo placement (deferral not necessary if sterile needles and nonreused ink are utilized—see state-specific guidance) | |
| Piercing placement (ear or body) | |
| Treatment for syphilis or gonorrhea | |
| Use of needles to inject nonprescribed products (such as drugs) | |
| Receipt of payment (monetary or otherwise) for sex | |
| Travel to malaria-endemic region | |
| 8 weeks | Whole blood donation |
| 6 weeks | Pregnancy |
| Varied (refer to local blood bank guidance) | Medical conditions |
| Medications | |
| Vaccinations |
Extensive pathogen testing of blood products is necessary to prevent transfusion-transmitted infections (TTIs) and to maintain a safe blood supply. A high-sensitivity test such as nucleic acid testing (NAT) is used to detect ribonucleic acid or deoxyribonucleic acid (DNA) specific to a pathogen. Table 35.3 shows the pathogens currently tested for nationwide and the residual risk estimates for transfusion-transmission and/or the reported number of cases in the US. Babesia testing (not shown in the table) has been added in certain regions of the US where it is prevalent.
| Pathogen | Estimated residual risk of transfusion-transmission/number of reported cases in the United States |
|---|---|
| HIV | 1 case per 2 million (transfused products) |
| HCV | 1 case per 2 million |
| HBV | 1 case per 1.7 million |
| CMV | Rare; addressed by leukoreduction |
| HTLV | 1 case per 3 million |
| West Nile virus | Rare after screening; about 1 case per year |
| Zika virus | 4 cases reported in Brazil; none in the United States |
| Trypanosoma cruzi (Chagas disease) | 10 reported cases in the United States and Canada before testing was instated |
| Treponema pallidum (syphilis) | Last reported transfusion-associated case in the United States in 1966 |
With the improvement of blood product screening and testing, directed donation requests have decreased over the last two decades. These are initiated by the recipient or the prospective donor (usually a family member or close friend), with the blood product collected, processed, and then transfused to the intended recipient. Although noble in nature, directed donations are not inherently safer than volunteer donations. Furthermore, the process is laborious and cannot be used in emergency situations due to the time required to ensure that ABO blood types are compatible with the intended recipient and mandated infectious disease testing is complete prior to blood component release. Furthermore, there is an increased risk of transfusion-associated graft-versus-host disease (TA-GVHD) due to human leukocyte antigen (HLA) similarities in family members, if they are the chosen directed donors.
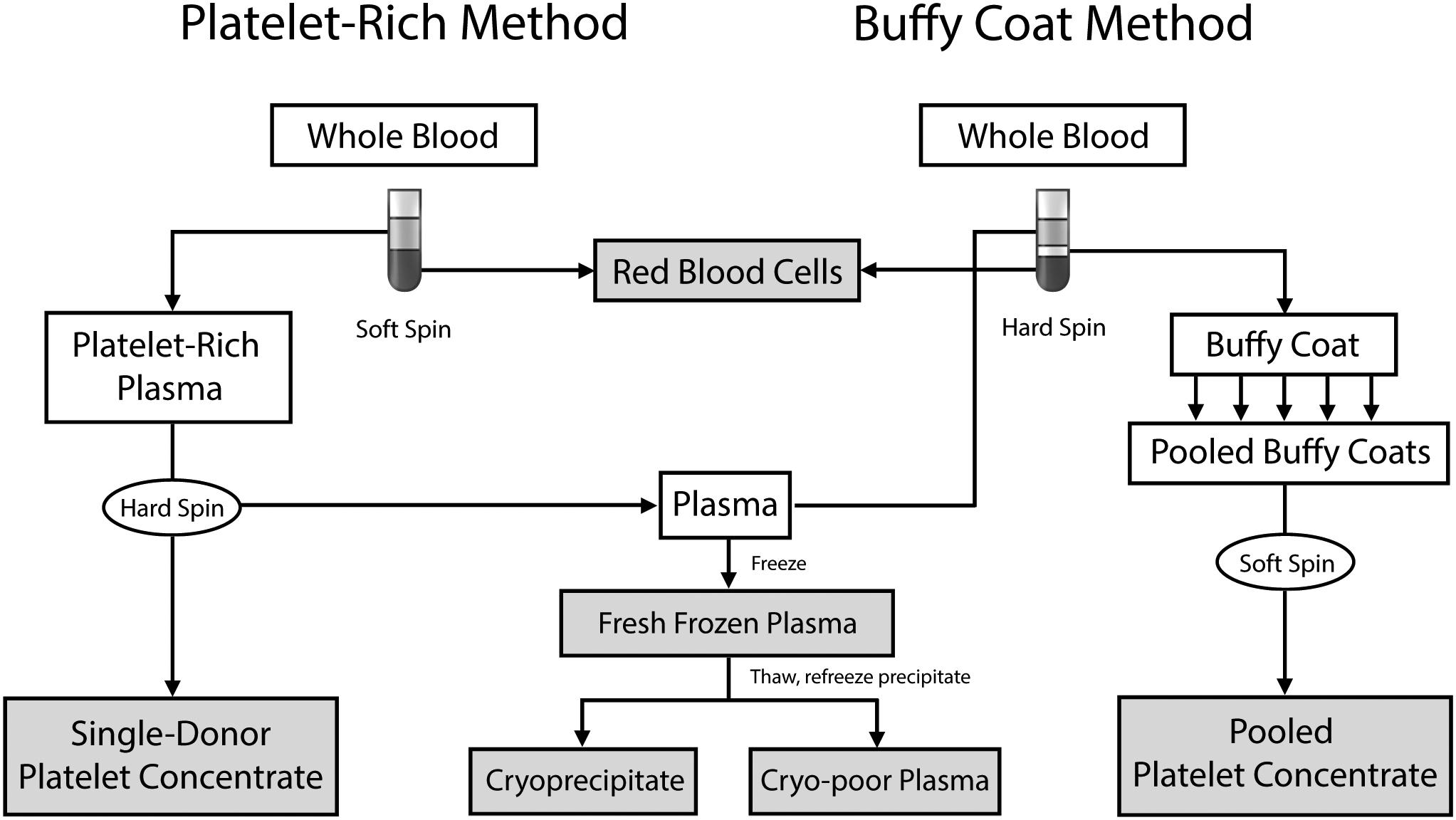
Donation of whole blood (WB) or specific blood components via apheresis are the two primary methods of collecting blood. Approximately 450–500 mL of WB is collected that then undergoes centrifugation with subsequent processing to separate it into RBCs, platelets, and plasma. Fig. 35.1 shows the various methods by which WB can be processed, which is country and blood center dependent (the buffy-coat method is not used in the US). WB can produce a unit of RBCs, a unit of plasma (which may yield a unit of cryoprecipitate and resultant cryo-poor plasma), and a unit of platelet concentrate. Typically, 4–6 units of platelet concentrate are pooled to make a unit of platelets.
Apheresis is the process whereby blood is separated into specific components, via centrifugation or filtration, with the remainder of the blood components returned to the donor. This allows for the collection of a single or double unit of RBCs; single, double, or triple units of platelets or plasma (depending on donor specifications). Various combinations of apheresis products may also be obtained from a single apheresis donor session, such as 1 unit of RBCs and 1 unit of platelets or 1 unit of RBCs and 1 unit of plasma.
In the US, around 85% of RBCs are obtained via WB collection, with 90% of platelets and plasma collected via apheresis.
Collected WB is placed in bags with an anticoagulant–preservative, commonly containing:
Citrate, phosphate, dextrose:
Citrate–phosphate–dextrose (CPD)
Citrate–phosphate–dextrose–dextrose (CP2D)
Citrate–phosphate–dextrose–adenine (CPDA-1)
Acid–citrate–dextrose (ACD)
ACD, CPD, and CP2D allow a shelf life of 21 days, while CPDA-1’s shelf life is 35 days.
Incorporation of additive solutions (ASs) to RBC units allows extension of unit shelf life to 42 days. Commonly licensed ASs in the US are AS-1, AS-3, AS-5 with variable concentrations of its constituents: mannitol–adenine–sodium chloride–dextrose (with/without a phosphate buffer). Other ASs used around the world include sodium chloride–adenine–glucose–mannitol, phosphate–adenine–glucose–guanosine–gluconate–mannitol.
Table 35.4 summarizes the important characteristics of each blood product. The needs of special patient populations, including neonates and patients with hemoglobinopathies, are discussed in “Special Populations.” It is important to note that institutional policies regarding transfusion thresholds vary greatly; therefore prompt consultation with departmental transfusion guidelines is necessary prior to clinical decision-making.
| Collection method (in the US) | Component | Storage | Volume/unit (mL) | Indications | Administration | |
|---|---|---|---|---|---|---|
| RBCs | Whole blood | Red blood cells | 4°C for up 42 days | 300 |
|
10–15 mL/kg (maximum 1 unit) over 2–4 h |
| Platelets |
|
|
20°C–24°C for 5–7 days (requires constant agitation) |
|
|
10 mL/kg over 1–4 h |
| Whole blood | Platelet concentrate | 50 | ||||
| Granulocytes | Apheresis | White blood cells | 4°C for 24 h | Variable | Prolonged severe neutropenia with severe infection | 1–8×10 10 neutrophils per dose (age dependent) |
| Plasma | Apheresis | Coagulation factors, fibrinogen, protein C, protein S, antithrombin, albumin, immunoglobulins | ≤−18°C for up to 12 months | 200 | Multiple factor deficiencies | 15 mL/kg (maximum 1 unit) over 2 h |
| Cryoprecipitate | Whole blood | Fibrinogen, fibronectin, von Willebrand factor, factor VIII, factor XIII | ≤−18°C for up to 12 months | 20 |
|
1–2 units per 10 kg (maximum of 5 units) |
| Whole blood | Whole blood | RBCs, platelets, plasma | 4°C for 35 days | 500 | Trauma | Limited pediatric experience |
Indications for RBCs transfusions include:
Acute blood loss (e.g., trauma);
Chronic blood loss (e.g., heavy menstrual bleeding);
Decreased RBC production:
Acquired (e.g., due to chemotherapy) or
Congenital (e.g., bone marrow failure syndromes).
Increased red cell mass ultimately improves end-organ oxygenation. The trigger for transfusion can vary by clinical situation (such as neonates) and particular predefined thresholds (that are institution specific). Patients’ hemodynamic stability should be taken into account and blood alternatives considered when feasible.
For those with acute blood loss (approximately 15 mL/kg—which is 25% of the total blood volume) with hemodynamic instability (such as hypotension and tachycardia) should be rescuscitated with colloid fluids followed by RBC transfusion at 15 mL/kg. In well-compensated chronic blood loss (due to causes such as heavy menstrual bleeding), RBC transfusion can be avoided with a course of oral (PO) or intravenous (IV) iron, based on the degree of anemia.
For a child with severe anemia (hemoglobin <5 g/dL) of an acute-on-chronic nature (e.g., leukemia) requiring transfusion, smaller aliquots of 5–10 mL/kg should be transfused consecutively, each over 2–4 hours. Careful monitoring of cardiopulmonary examination should occur to detect potential pulmonary edema from transfusion-associated circulatory overload (TACO) that may occur due to high cardiac output failure. This may require intervention with a diuretic during the first aliquot or between aliquots. If this occurs, a transfusion reaction form should be reported to the hospital blood and transfusion medicine service immediately.
A unit of RBCs can be redistributed into multiple aliquots in a process known as sterile docking, which keeps the aliquots' original expiration date due to it being produced in a closed system. This allows repeat patient transfusion from the same donor unit, limiting the risk of exposure to multiple antigens from multiple blood donors, and thus potentially decreasing alloimmunization and infectious risks. Another factor in decreasing alloimmunization is the transfusion of a single unit of RBCs as opposed to a double unit in patients receiving prophylactic transfusions (such as those undergoing chemotherapy). It has been shown that a single unit of RBCs (in adult patients) decreases overall red cell usage (and, therefore, alloimmunization side effects due to decreased exposure), without leading to increased bleeding or the need for additional transfusions of other blood products, such as platelets. This may have application in older children and adolescents but has not been studied extensively as it has been in adults.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here