Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
On completion of this chapter, you should be able to:
Describe the effects of hormones on the ovarian cycle
State the characteristics of the simple ovarian cyst and a functional ovarian cyst
Discuss the sonographic findings of the common cystic and complex ovarian masses, common solid ovarian masses, and ovarian neoplasms
List the characteristics of the ovarian syndromes discussed in this chapter
Discuss the benign pelvic masses found in neonates and adolescent girls
Differentiate between mucinous and serous types of tumors
Explain the Doppler parameters used in ovarian torsion
Sonography is clinically useful to characterize adnexal masses, evaluate abnormal bleeding, assess infertility, monitor follicular growth, perform transvaginal needle aspiration and biopsy, and screen for ovarian carcinoma. Both transabdominal and transvaginal sonography are important in these evaluations. Transabdominal imaging furnishes a global survey of anatomy, whereas transvaginal imaging provides better characterization of internal architecture of the ovary, vascular anatomy, and adnexal area. It is important to note that information from the clinical pelvic examination is required for optimal interpretation of the sonographic studies, thus necessitating good communication between the referring physician and the sonologist.
A woman will ovulate nearly 400 times in her reproductive life, and a quarter of a million follicles will be stimulated to varying degrees during this time. It is not surprising that an organ as dynamic as the ovary can form more than 100 different types of tumors, both benign and malignant. These masses are described on the sonographic examination as primarily cystic, complex, or predominantly solid. However, the final diagnosis is left to the pathologist on surgical excision. Precise diagnosis on the basis of sonography alone is highly predictive, although not conclusive until the surgery. The primary role of sonography is to indicate the need for surgical or medical intervention.
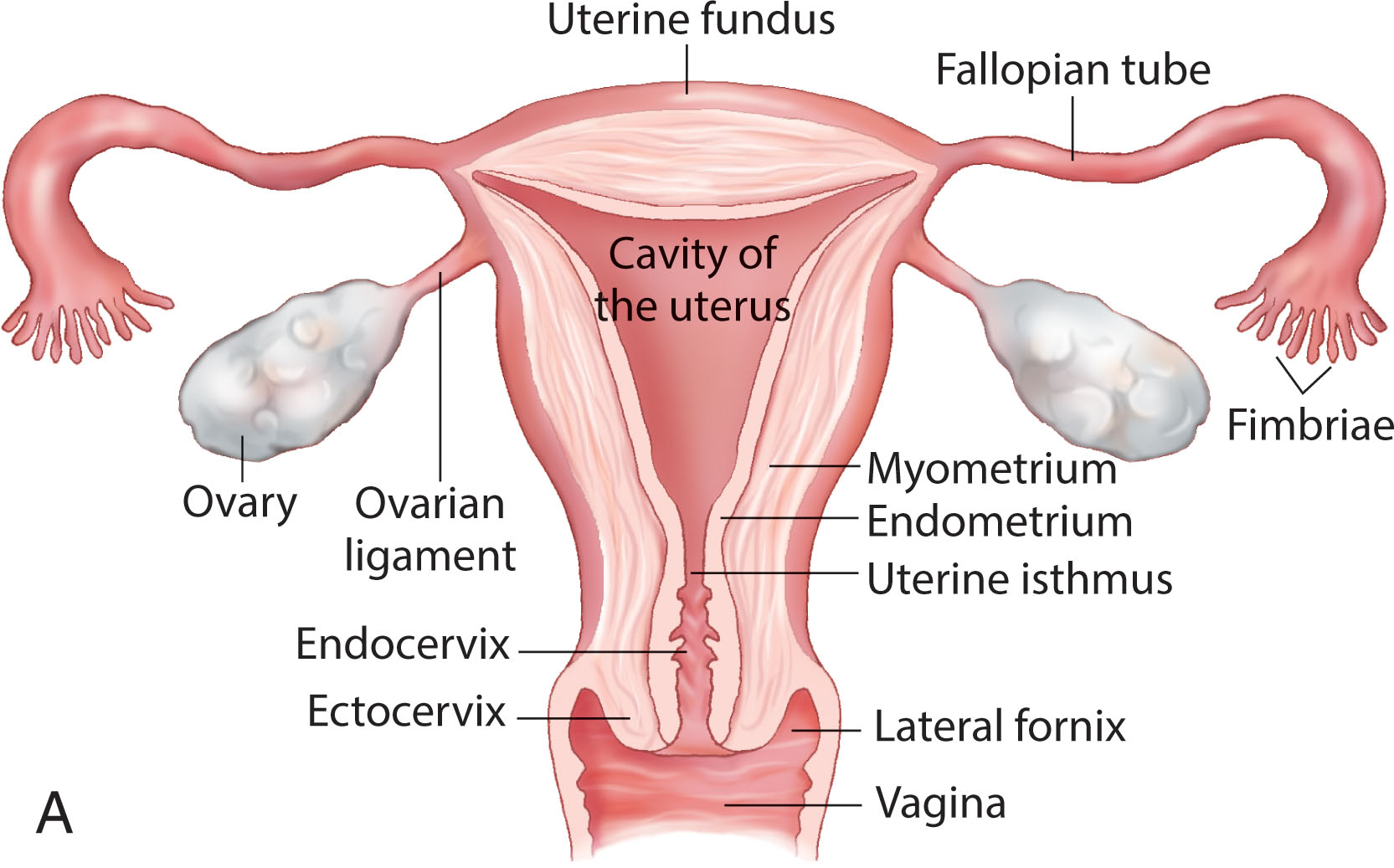
The ovaries are paired, almond-shaped structures situated one on each side of the uterus close to the lateral pelvic wall ( Fig. 44.1 ). The ovaries can vary in position and are influenced by the uterine location and the ligament attachments. In the anteflexed midline uterus, the ovaries are usually identified laterally or posterolaterally. When the uterus lies to one side of the midline, the ipsilateral ovary often lies superior to the uterine fundus. In a retroverted uterus, the ovaries tend to be lateral and superior, near the uterine fundus. When the uterus is enlarged, the ovaries tend to be displaced more superiorly and laterally. Following hysterectomy, the ovaries tend to be located more medially and directly superior to the vaginal cuff. They can be located high in the pelvis or in the cul-de-sac. Superiorly or extremely laterally placed ovaries may not be visualized by the transvaginal approach because they are out of the field of view. Ovaries are ellipsoid in shape, with their craniocaudad axes paralleling the internal iliac vessels, which lie posterior and serve as a reference point.
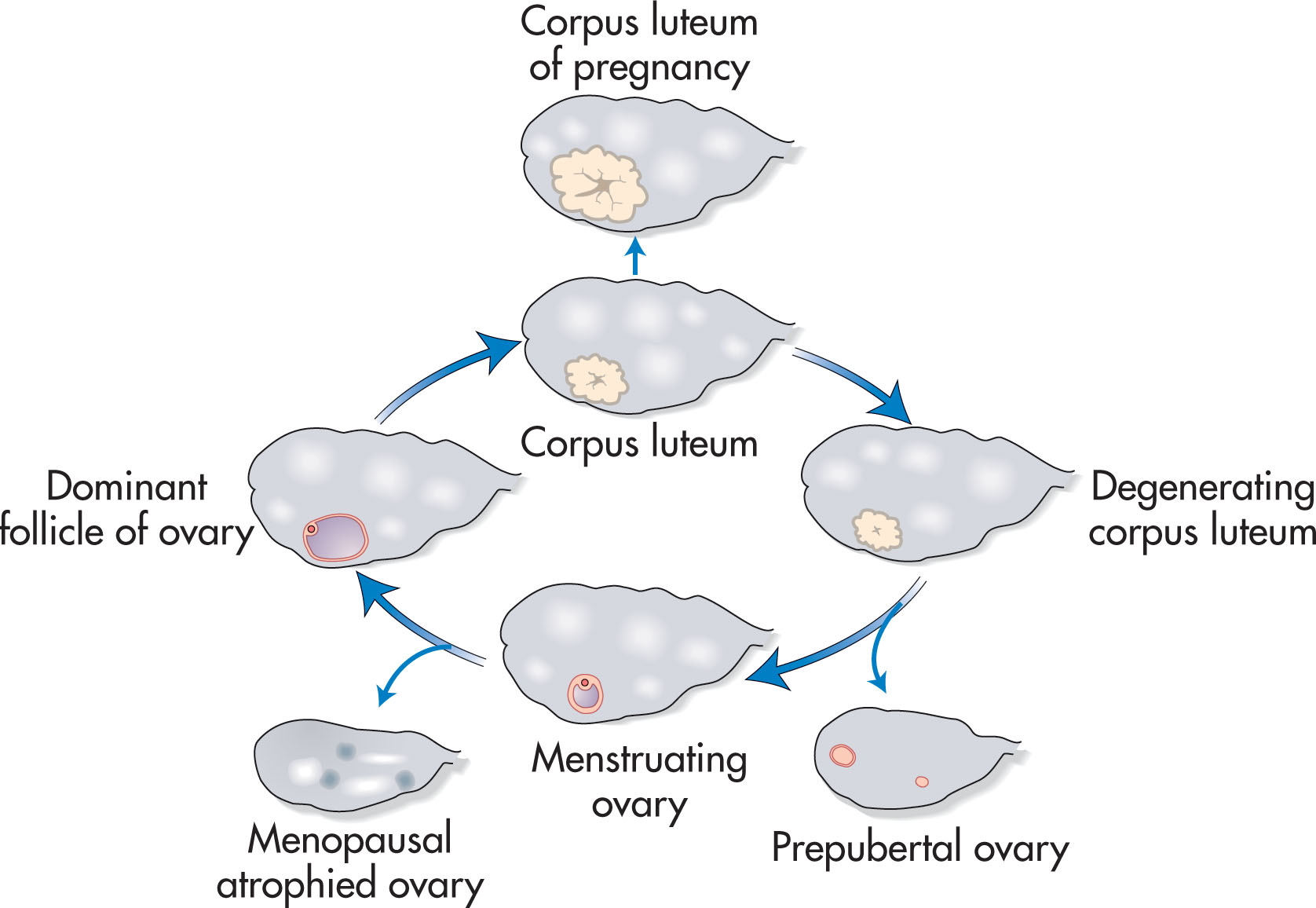
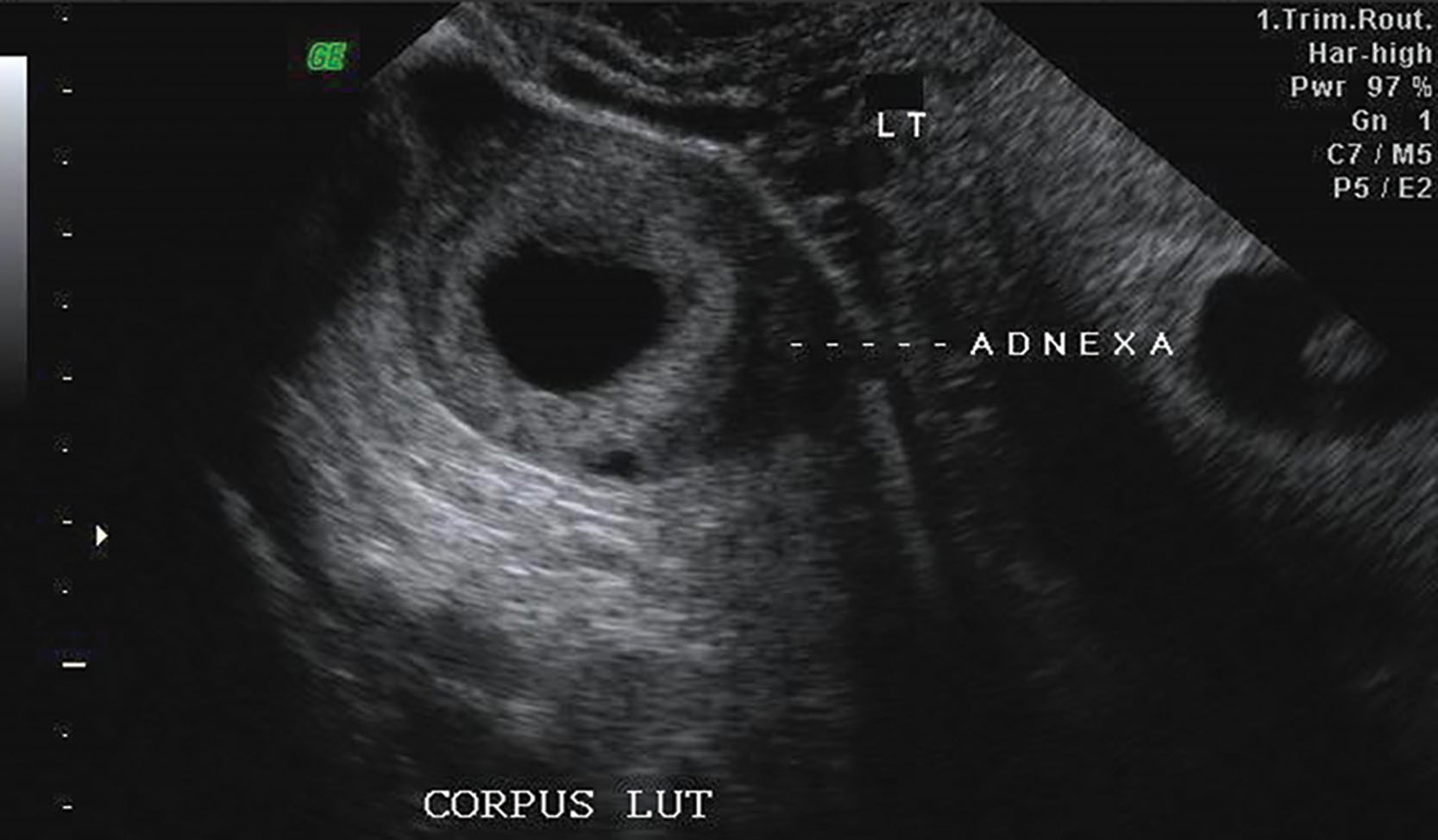
The normal ovary has a homogeneous echotexture, exhibiting a central, more echogenic medulla. Small anechoic or cystic follicles may be seen peripherally in the cortex ( Fig. 44.2 ). The appearance of the ovary varies with age and the menstrual cycle ( Fig. 44.3 ). During the reproductive years, three phases are recognized sonographically during each menstrual cycle. Many follicles develop during the early proliferative phase and increase in size until about day 8 or 9 of the menstrual cycle. This is due to stimulation by both follicle-stimulating hormone (FSH) and luteinizing hormone (LH). At that time, one follicle becomes dominant, reaching up to 2 to 2.5 cm at the time of ovulation. The cumulus oophorus may occasionally be detected as an eccentrically located, cystlike, 1-mm internal mural protrusion. Although visualization of the cumulus indicates a mature follicle and imminent ovulation, no reproducible sonographic sign is reliable. The other follicles become atretic. A follicular cyst develops if the fluid in the nondominant follicles is not reabsorbed. Usually, the dominant follicle disappears immediately after rupture at ovulation ( Fig. 44.4 ). Occasionally the follicle decreases in size and develops a wall that appears crenulated (scalloped). Fluid in the cul-de-sac commonly occurs after ovulation and peaks in the early luteal phase. Following ovulation in the luteal phase, a mature corpus luteum develops and may be identified sonographically as a small hypoechoic or isoechoic structure peripherally within the ovary. It may appear irregular with echogenic crenulated walls and contain low-level echoes ( Fig. 44.5 ). Less frequent appearances include a typical ring color Doppler pattern around the wall of an isoechoic corpus luteum. In the absence of fertilization, the corpus luteum begins to undergo involutional changes on postovulatory day 8 or 9 and disappears shortly before or with the onset of menstruation.
Multiple small, punctate, echogenic foci are commonly seen in the normal ovary. These foci are reported to be a common finding with transvaginal examination. They are generally very small (1 to 2 mm) and located in the periphery. The foci are nonshadowing and can be multiple. A possible source of these foci of specular reflections is from the walls of tiny unresolved cysts below the spatial resolution of ultrasound. More central punctate echogenic foci represent stromal reaction to previous hemorrhage or infection. Because they do not indicate significant underlying disease, no follow-up is necessary.
Following menopause, the ovary atrophies and the follicles disappear with increasing age. For this reason, the postmenopausal ovary may be difficult to visualize sonographically because of the smaller size and lack of discrete follicles. A stationary loop of the bowel may mimic a small shrunken ovary, so scanning must be done slowly to look for peristalsis in the bowel. After a hysterectomy, the ovaries can be difficult to visualize with ultrasound. The use of both transabdominal and transvaginal approaches increases the chance of visualization.
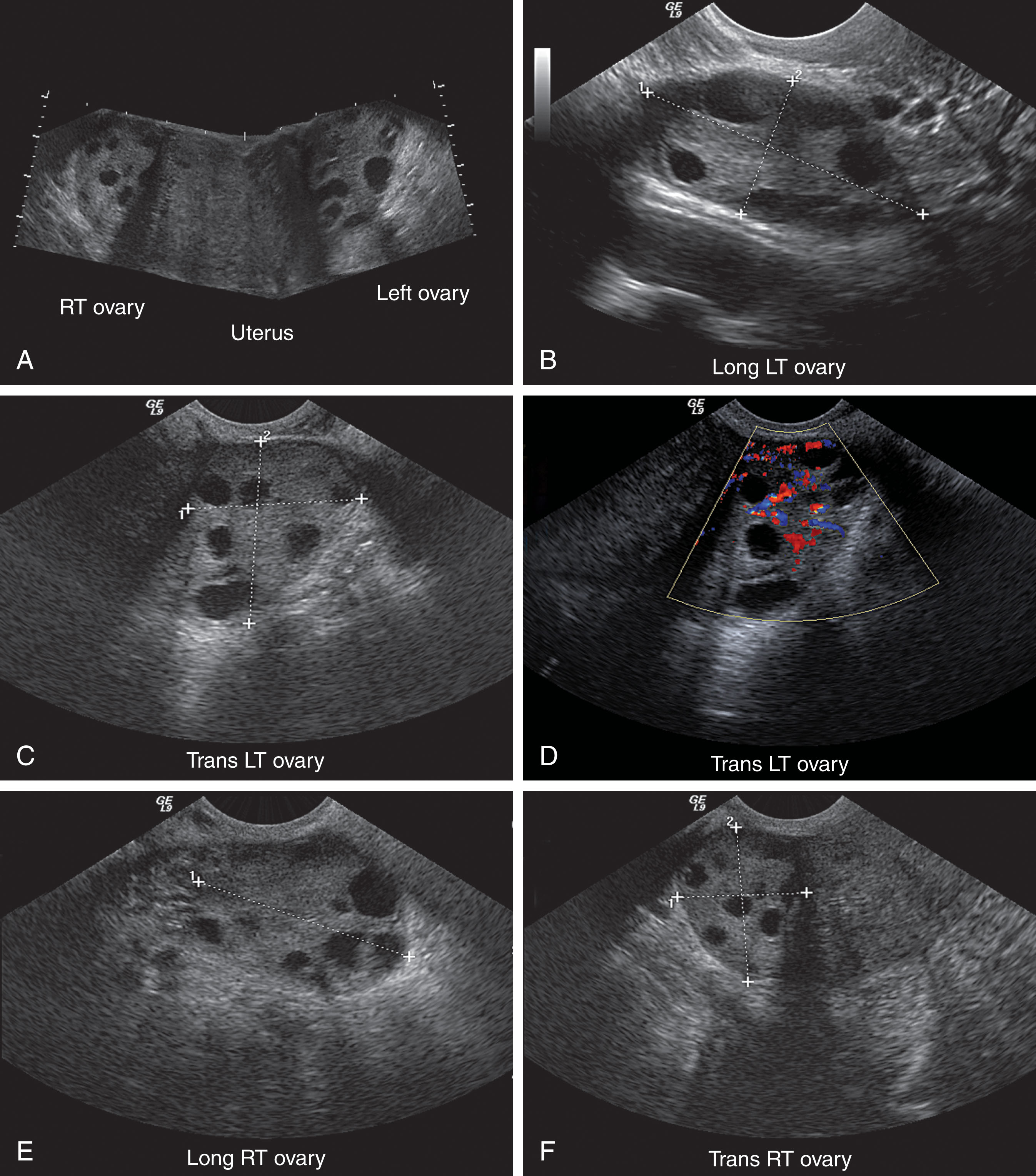
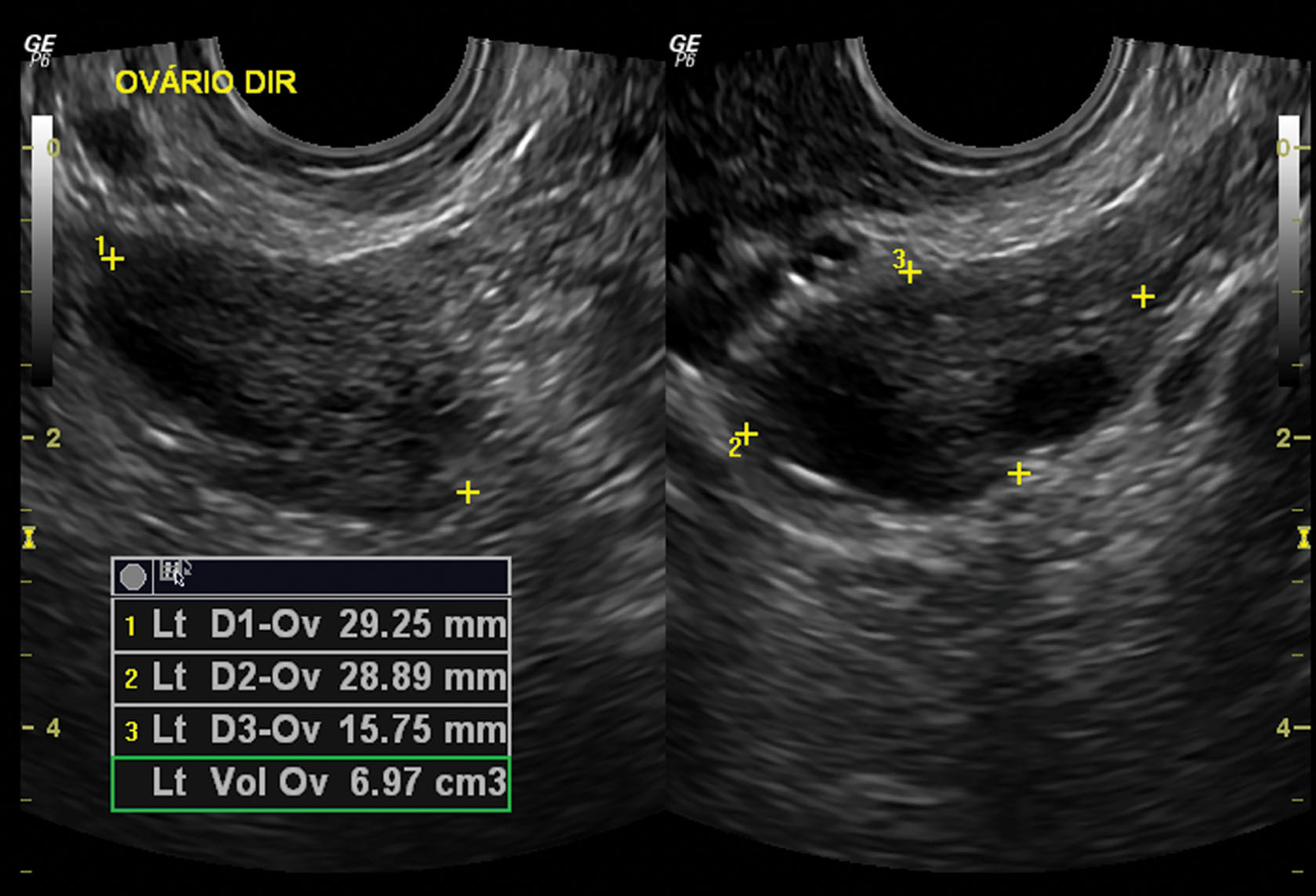
Because of the variability in ovarian shape and size, the volume measurement is the best method for determining overall ovarian size ( Fig. 44.6 ). The volume measurement is based on the prolate ellipse formula (0.523 × length × width × height). In the menstruating adult female, a normal ovary may have a volume as large as 22 mL, 3 with a mean ovarian volume of 9.8 ± 5.8 mL. An ovarian volume of more than 8 mL is definitely considered abnormal for the postmenopausal patient. An ovarian volume more than double that of the opposite side should also be considered abnormal, regardless of the actual size. Three-dimensional (3D) ultrasound may provide the most accurate method of ovarian volume measurement by allowing accurate determination of the ovarian long axis and objective calculation of stromal and cystic volume components. Further development in this technology will define future applications.
An ovary’s function is to mature oocytes until ovulation under the influence of LH and FSH hormone from the pituitary. At the same time, the ovary synthesizes androgens (male hormones) and converts them to estrogens (female hormones). Finally, it produces progesterone after ovulation to sustain early pregnancy until the placenta can do so at 10 to 12 weeks of gestation.
Usually, only one follicle enlarges from 3 mm to approximately 24 mm over about 10 days in the mid- and late-follicular phases of the cycle. This is followed by ovulation. The resulting corpus luteum or an abnormal unruptured follicle can persist as a simple or complex cystic structure from 1 to 10 cm in size. These so-called functional cysts may produce discomfort or delayed menses but can be observed to regress within 8 weeks with serial ultrasound studies. Surgical intervention may be considered if a cyst greater than 6 cm persists more than 8 weeks.
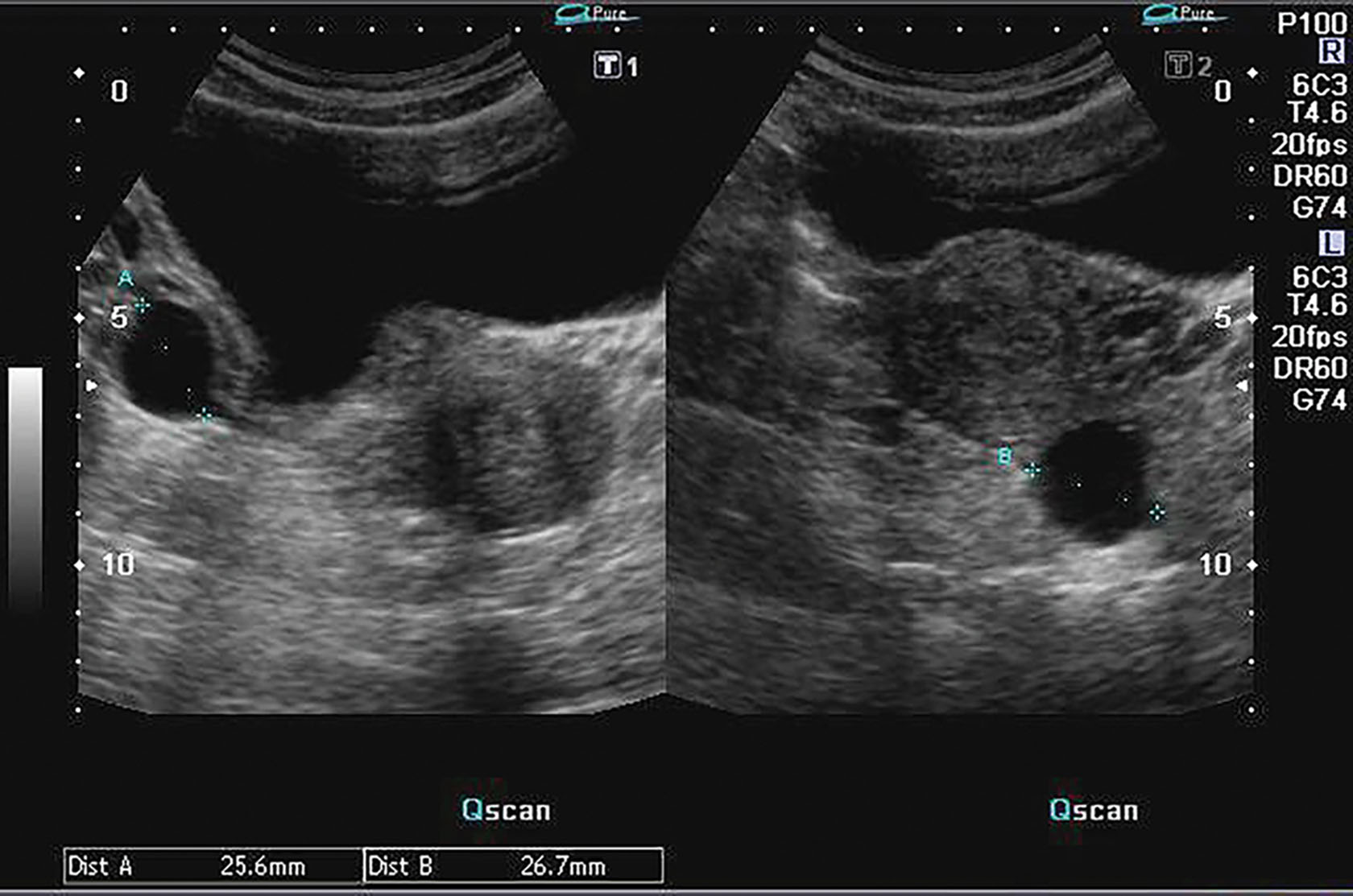
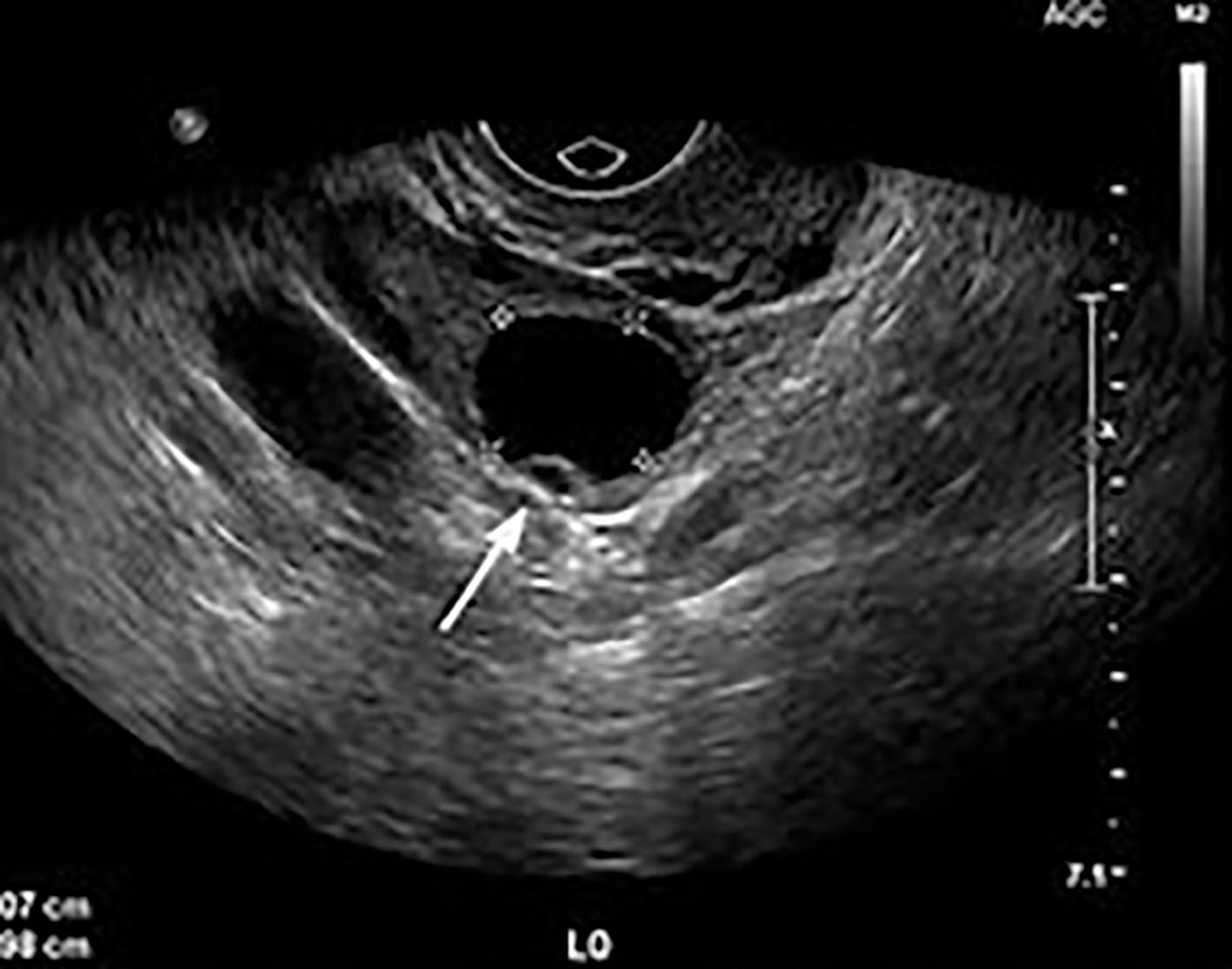
Ultrasound-guided needle aspiration has become another option for reducing recurrent simple ovarian cysts in carefully selected cases. Most ovarian masses are simple cysts, most of which are benign. Sonographic criteria for a simple cyst include a thin, smooth wall, anechoic contents, and acoustic enhancement ( Fig. 44.7 ). In premenopausal women, these cysts usually are functional. The differential considerations of simple adnexal cysts include functional cyst, paraovarian cyst, cystadenoma, cystic teratoma, endometrioma, and rarely tubo-ovarian abscess ( Box 44.1 ).
Follicular cyst
Corpus luteum cyst of pregnancy
Cystic teratoma
Paraovarian cyst
Hydrosalpinx
Endometrioma (low-level echoes)
Hemorrhagic cyst
Small anechoic cysts may be seen in postmenopausal ovaries. They can disappear or change in size over time. Serial sonographic studies can monitor the size and document any changes. Surgery is generally recommended for postmenopausal cysts greater than 5 cm and for those containing internal septations or solid nodules ( Fig. 44.8 ).
Any simple cyst that hemorrhages as it involutes may appear as a complex mass ( Box 44.2 ). In patients of reproductive age, the classic differential considerations of a complex adnexal mass are ectopic pregnancy, endometriosis, and pelvic inflammatory disease (PID) ( Fig. 44.9 ). Dermoids and other benign tumors can appear similarly.
Cystadenoma
Dermoid cyst
Tubo-ovarian abscess
Ectopic pregnancy
Granulosa cell tumor
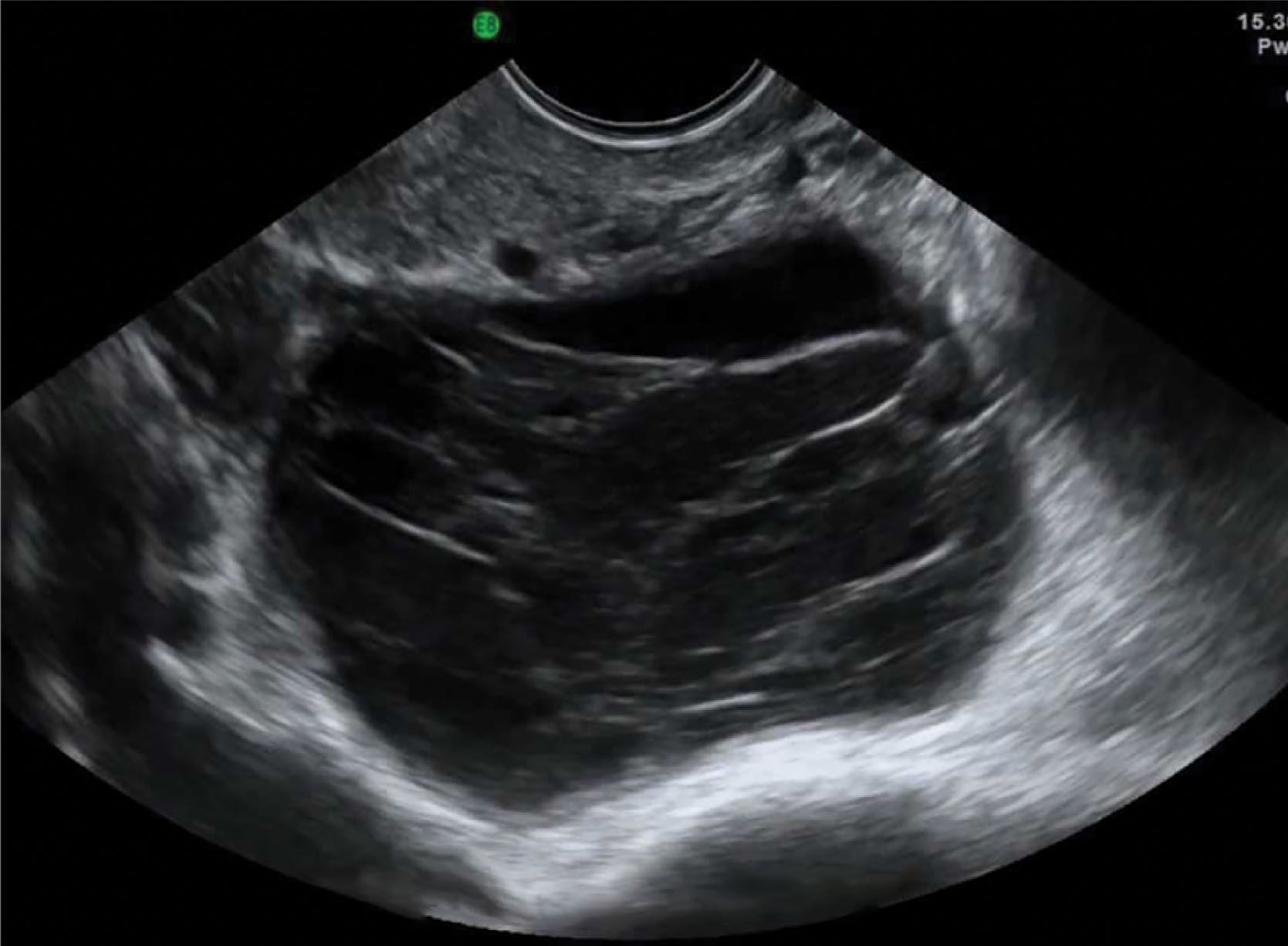
Mixed solid to cystic ovarian masses are typical of all the epithelial ovarian tumors; the most common are the serous types: cystadenoma and cystadenocarcinoma . During the peak fertile years, only 1 in 15 is malignant; this ratio becomes 1 in 3 after age 40 years.
The more sonographically complex the tumor, the more likely it is to be malignant, especially if associated with ascites. The epithelium of serous tumors is tubal in type, and there may be one or multiple cysts. One-fourth of them are bilateral, and most occur in women over age 40. They are large and often fill the pelvic cavity.
The differential considerations of a solid-appearing adnexal mass include pedunculated fibroid, dermoid, fibroma, thecoma, granulosa cell tumor, Brenner tumor, and metastasis. Tubo-ovarian abscess, ovarian torsion, hemorrhagic cysts, and ectopic pregnancy also may appear solid. Solid adnexal masses are often difficult to diagnose because normal ovarian size varies widely. However, as previously noted, an ovary with a volume double that of the opposite side is generally considered abnormal. When a solid mass is found, care should be taken to identify a connection with the uterus to differentiate an ovarian lesion from a pedunculated fibroid ( Box 44.3 ). The use of color Doppler can be helpful, as color can be used to identify a vascular pedicle between the uterus and the mass, as can often be identified with pedunculation.
Solid teratoma
Adenocarcinoma
Arrhenoblastoma
Fibroma
Dysgerminoma
Torsion
When any abnormality of the ovary is detected, Doppler examination should be performed. In the case of a suspected cystic lesion, color Doppler helps differentiate a potential cyst from adjacent vascular structures. Color also can be used to localize flow to further determine flow velocity with pulsed Doppler, which can be obtained on all ovarian masses. Pulsed Doppler interrogation of the adnexal branch of the uterine artery, the ovarian artery, or intratumoral flow is performed to determine the resistive index (RI) or pulsatility index ( Fig. 44.10 ). Patients with normal menstrual cycles are best scanned in the first 10 days of the cycle to avoid confusion with normal changes in intraovarian blood flow because high diastolic flow occurs in the luteal phase.
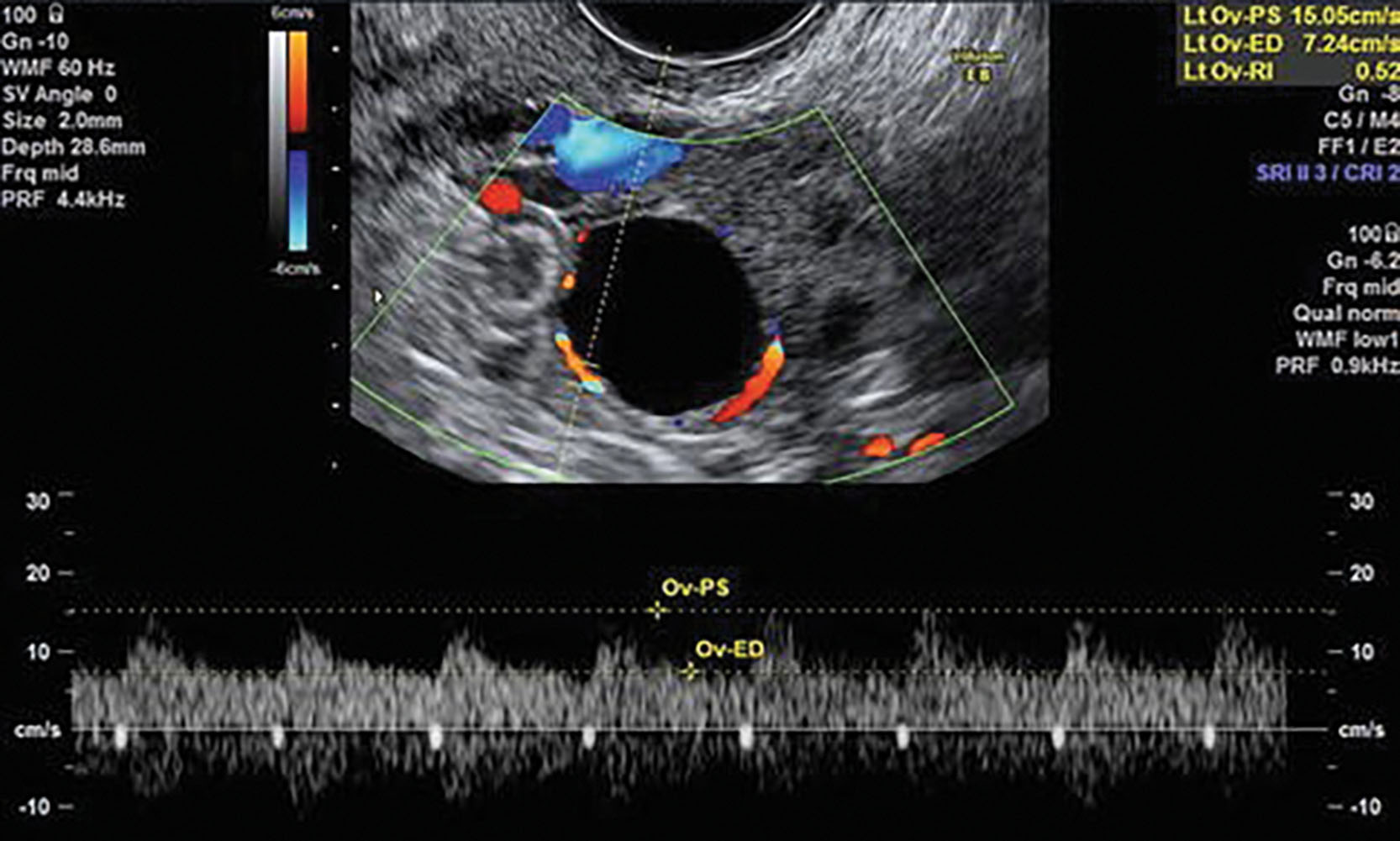
A debate in the literature exists regarding the value of an RI in distinguishing between benign and malignant adnexal masses. The largest study in the literature uses a cutoff of greater than 0.4 as a normal RI in a nonfunctioning ovary. 7 Other investigators employ a pulsatility index of greater than 1 as normal. 1,5 Intratumoral vessels, low-resistance flow, and absence of a normal diastolic notch in the Doppler waveform are signs that may be worrisome for malignancy. In addition, abnormal waveforms can be seen in inflammatory masses, metabolically active masses (including ectopic pregnancy), and corpus luteum cysts. The most significant problem in using an RI is that it is not a sensitive indicator of malignancy. One study found a low RI in only 25% of malignant lesions. 8
The color Doppler central distribution of the small arteries within an ovarian mass may be important in malignancy. Two pulsed Doppler indices have been analyzed, comparing the relative amount of diastolic to systolic components of their arterial waveforms. The pulsatility index (PI) is calculated as peak systolic velocity minus end-diastolic velocity divided by the mean velocity. The resistive index (RI) is the peak systolic velocity minus the end-diastolic velocity divided by the peak systolic velocity. Although these indices have different cutoff values, increased diastolic flow suggests neovascularity and the likelihood of malignancy. The cutoff value for the PI is 1, and the value for the RI is 0.4, with malignancy considered more likely below and benign disease more likely above these values. Inflammatory masses, active endocrine tumors, and trophoblastic disease (ectopic pregnancies) may give low indices, thus mimicking cancer.
A mass showing a complete absence or minimal diastolic flow (very elevated RI and PI values) is usually benign. A diastolic notch in early diastole may also signify benign disease. This finding is not often noted, and its absence has no diagnostic value.
The PI and RI values may vary considerably in the fertile patient during the menstrual cycle, complicating the pulsed Doppler analysis. In the first 7 days, the flow to the ovaries has the greatest resistance with the lowest diastolic flow, and the indices are at their highest. Later in each cycle, the diastolic flow increases, particularly to the dominant ovary, and may lower the indices sufficiently to falsely suggest a malignant process. A Doppler study can be performed at any time during the cycle. If the indices are in the benign range, they do not need to be repeated. However, if a suspicious mass is present and the indices suggest possible malignancy and are expected to affect management, a repeat study should be performed to confirm the abnormal indices in the first week of another cycle.
Functional cysts result from the normal function of the ovary. They are the most common cause of ovarian enlargement in young women. Functional cysts include follicular, corpus luteum, hemorrhagic, and theca-lutein cysts. Hormonal therapy is sometimes administered to suppress a cyst. Most cysts measure less than 5 cm in diameter and regress during the subsequent menstrual cycle. A follow-up sonographic examination in 6 weeks usually documents change in size.
A follicular cyst forms when a mature follicle fails to ovulate or involute post-ovulation ( Box 44.4 ). These cysts are usually unilateral, asymptomatic, and less than 2 cm in size, but they can be as large as 20 cm in diameter. They regress spontaneously and are frequently detected incidentally on sonographic examinations.
Occur when a dominant follicle does not succeed in ovulating and remains active though immature
Usually unilateral
Thin-walled, translucent, watery fluid; may project above or within surface of the ovary
May grow 1–8 cm
Usually disappear spontaneously by resorption or rupture
Clinical findings: asymptomatic to dull, adnexal pressure and pain, abnormal ovarian function, torsion of the ovary resulting in severe pain
Sonographic findings: simple cyst
Corpus luteum cysts result from failure of resorption or excess bleeding into the corpus luteum. These cysts usually are less than 4 cm in diameter and are unilateral. They are prone to hemorrhage and rupture. The presenting feature is often pain. If the ovum is fertilized, the corpus luteum continues as the corpus luteal cyst of pregnancy during the first trimester of pregnancy, when maximum size is reached by 10 weeks, and resolution occurs by 12 to 16 weeks ( Box 44.5 ).
Result from hemorrhage within a persistently mature corpus luteum
Filled with blood and cystic fluid
May grow 1–10 cm in size
May accompany intrauterine pregnancy
Clinical findings: irregular menstrual cycle, pain, mimic ectopic pregnancy, rupture
Sonographic findings: “cystic” type of lesion; may have internal echoes secondary to hemorrhage and increased color
Because of the hemorrhagic nature of these cysts, they usually appear as complex masses with central blood clot and echogenic septations ( Fig. 44.11 ). This appearance is difficult to distinguish from ectopic pregnancy and endometriosis. They may exhibit posterior acoustic enhancement, depending on the content.
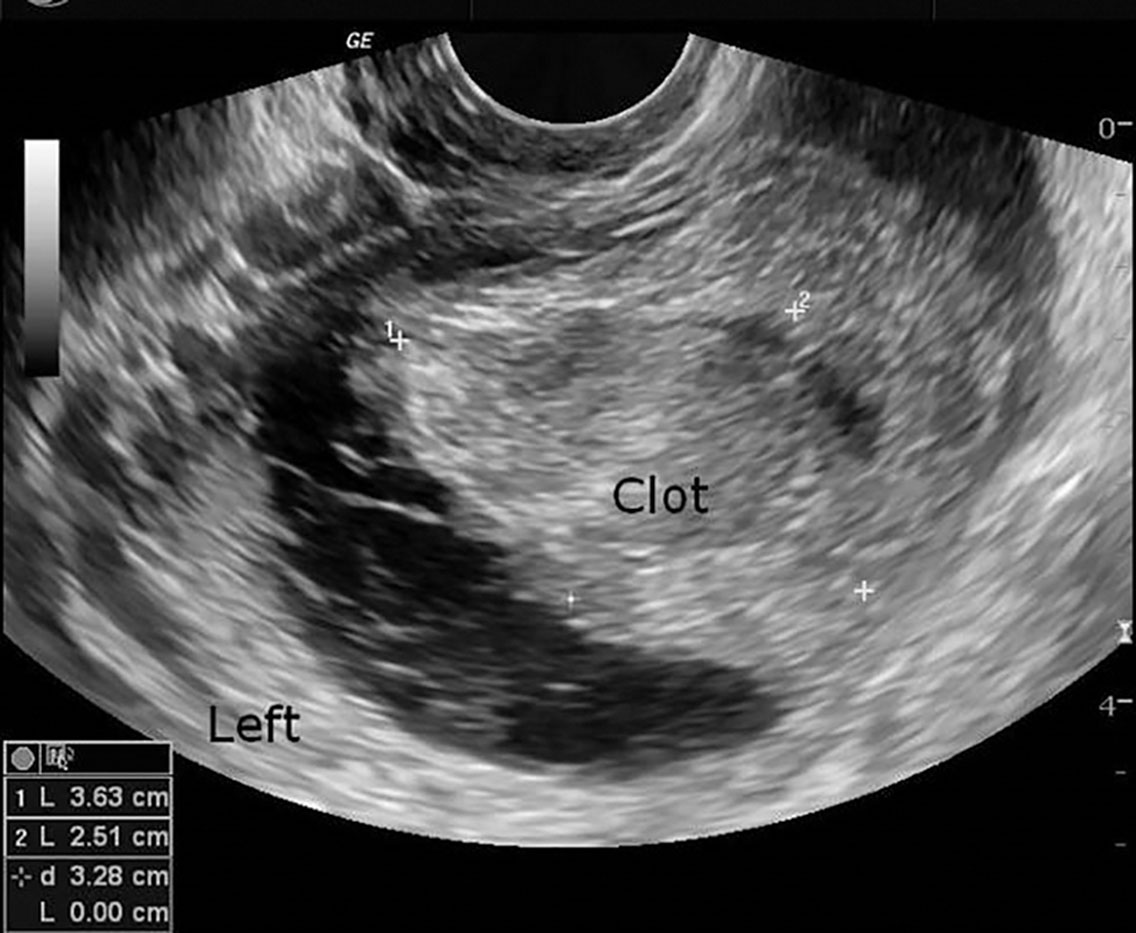
Duplex Doppler reveals prominent diastolic flow in corpus luteum cysts. This low-velocity waveform is present throughout the luteal phase of the cycle. They may also have a peripheral rim of color around the wall of color Doppler, sometimes termed the “ring of fire.”
Internal hemorrhage may occur in follicular cysts or, more commonly, in corpus luteal cysts. The patient may present with an acute onset of pelvic pain.
The sonographic picture is variable depending on the amount of hemorrhage, clot formation, and time passed since hemorrhage ( Fig. 44.12 ). The internal characteristics are better visualized by transvaginal scanning, but transabdominal scanning should also be performed for a global view. An acute hemorrhagic cyst is usually hyperechoic and may mimic a solid mass. It usually has a smooth posterior wall and shows posterior acoustic enhancement indicating its cystic component. Diffuse low-level echoes may be seen but are more commonly seen in endometriomas. As time goes on, the internal pattern becomes more complex. The clotted blood becomes more echogenic and may show a fluid level. Echogenic free intraperitoneal fluid in the cul-de-sac can help confirm the diagnosis of a ruptured or leaking hemorrhagic cyst. This may mimic a ruptured ectopic pregnancy, so it is critical to know if the patient has a positive pregnancy test.
Theca-lutein cysts are the largest of the functional cysts and appear as very large, bilateral, multiloculated cystic masses. They are associated with high levels of human chorionic gonadotropin. They are seen most frequently associated with gestational trophoblastic disease (30%). Similar cysts occur in normal pregnancies, especially multiple gestations, and in some patients being treated with infertility drugs, particularly Pergonal ( Box 44.6 ). These cysts may undergo hemorrhage, rupture, and torsion ( Fig. 44.13 ).
Large, bilateral, multiloculated cysts
Associated with high levels of human chorionic gonadotropin
Seen in 30% of patients with trophoblastic disease
Clinical findings: nausea and vomiting
Sonographic findings: multilocular cysts in both ovaries
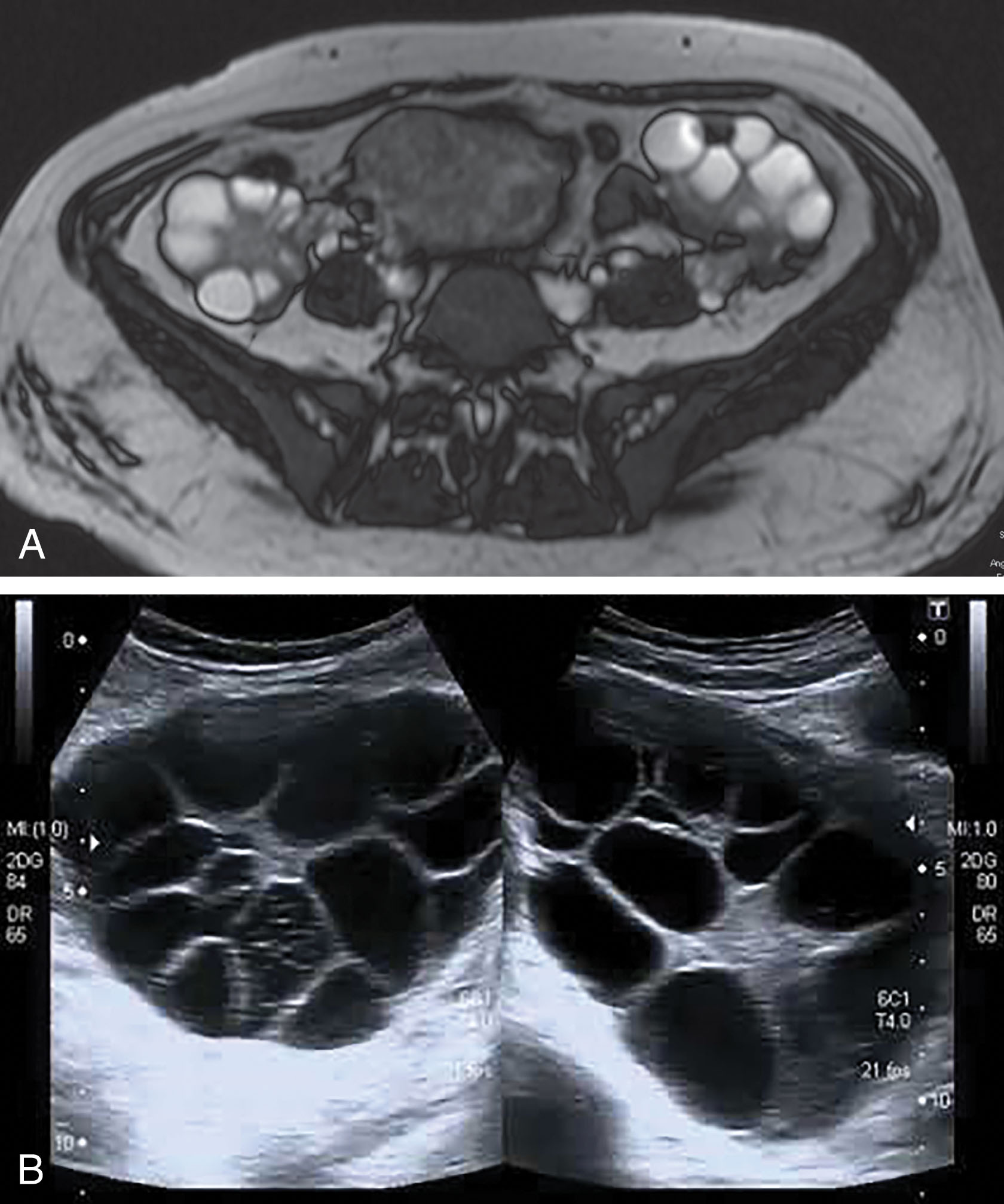
Ovarian hyperstimulation syndrome is a frequent iatrogenic complication of ovulation induction. This hyperstimulation can result in mild, moderate, and severe forms. In the mild form, the patient presents with pelvic discomfort but no significant weight gain. The ovaries are enlarged but measure less than 5 cm in diameter ( Fig. 44.14 ). The patient has severe pelvic pain, abdominal distention, and notably enlarged ovaries with severe hyperstimulation, measuring greater than 10 cm in diameter. There can also be associated ascites, pleural effusions, and numerous large, thin-walled cysts throughout the periphery of the ovary. When treated, this condition usually resolves within 2 to 3 weeks.
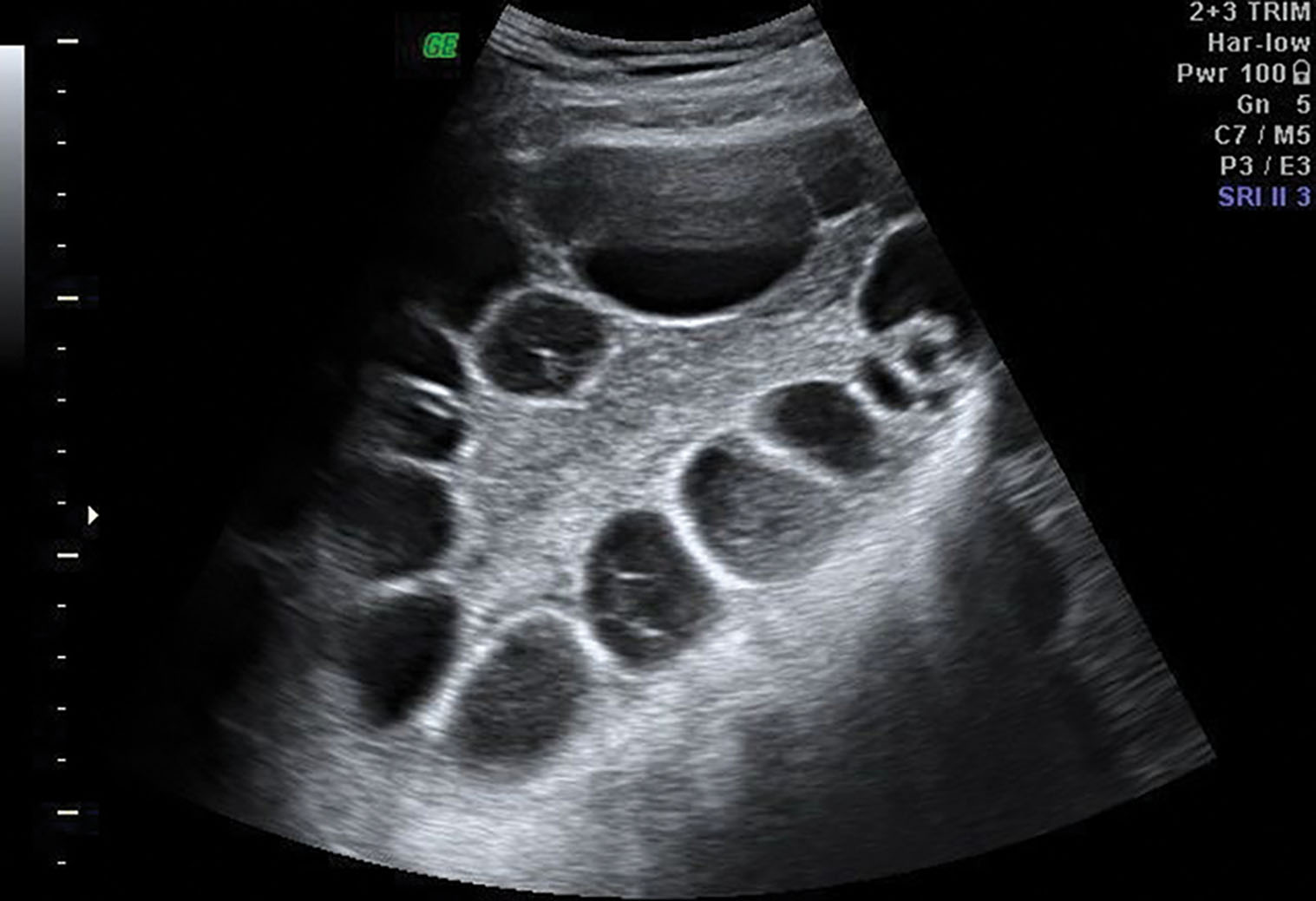
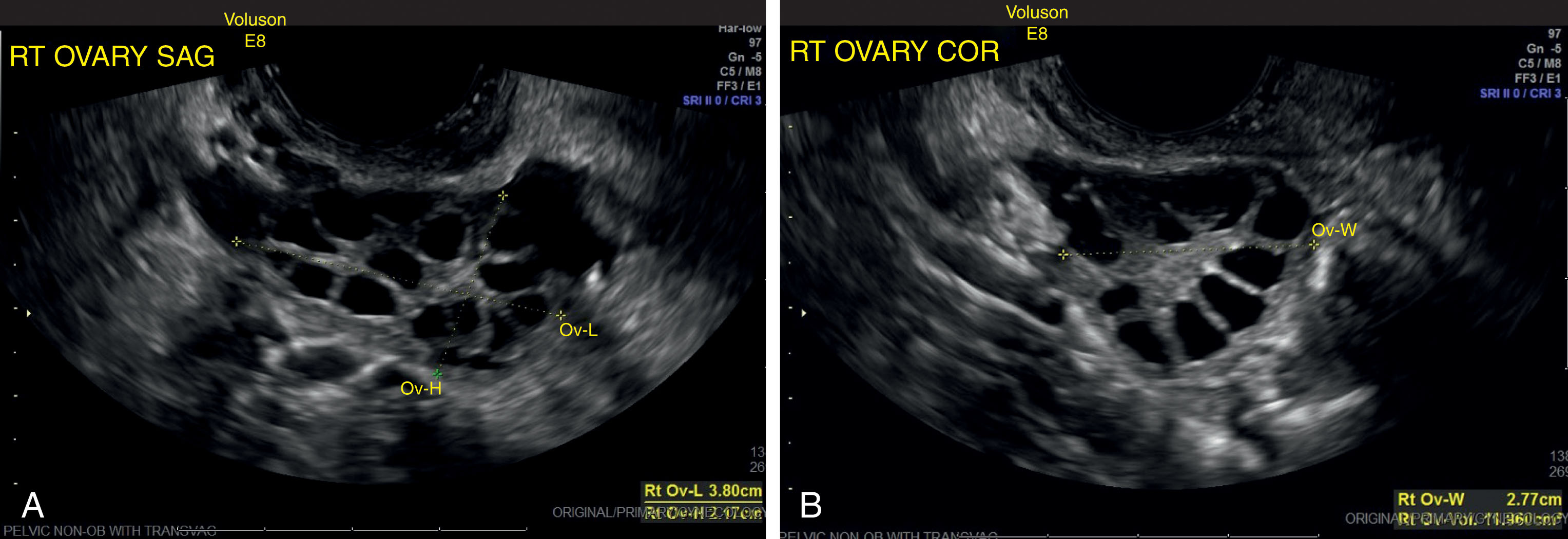
Polycystic ovarian syndrome (PCOS) , which includes Stein-Leventhal syndrome (infertility, oligomenorrhea, hirsutism, and obesity), is an endocrine disorder associated with chronic anovulation ( Box 44.7 ). An imbalance of LH and FSH results in abnormal estrogen and androgen production. The serum LH level is high, and the FSH level is low. An elevated LH/FSH ratio is characteristic. Pathologically, the ovaries are rounded, usually two to five times normal size, with an increased number of follicles. Clinically, PCOS encompasses a spectrum of findings from hyperandrogenism in lean, normally menstruating women to obese women with severe hirsutism and oligomenorrhea or amenorrhea, as originally described by Stein and Leventhal. Manifestations of unopposed estrogenic hyperplasia and endometrial carcinoma occur in a significant proportion of patients, so long-term follow-up is recommended. PCOS is a common cause of infertility and a higher-than-usual rate of early pregnancy loss.
Includes Stein-Leventhal syndrome
Bilaterally enlarged polycystic ovaries
Occurs in late teens through twenties
May have endocrine imbalance
Spectrum of ultrasound appearances
Clinical findings: amenorrhea, obesity, infertility, hirsutism
Sonographic findings: multiple tiny cysts around the periphery of the ovary; ovary may be normal size or enlarged
On sonography, the ovaries appear normal or enlarged with echogenic stroma ( Fig. 44.15 ). Multiple small follicles are seen, often bilaterally, in the size range of 5 to 8 mm. The reported number of immature follicles varies in the literature from 11 to less than 15. The ovaries have a more rounded shape, with the follicles usually located peripherally, commonly referred to as the “string of pearls.” Small cysts of variable size can also occupy both the subcapsular and stromal parts of the ovary. The multitude of these small cysts contributes to the enlarged size of the ovary. Transvaginal sonography is more sensitive for detecting these small follicles than is transabdominal scanning. The diagnosis of PCOS is usually made biochemically, but sonography is useful. Serial studies show the follicles persist because ovulation does not occur.
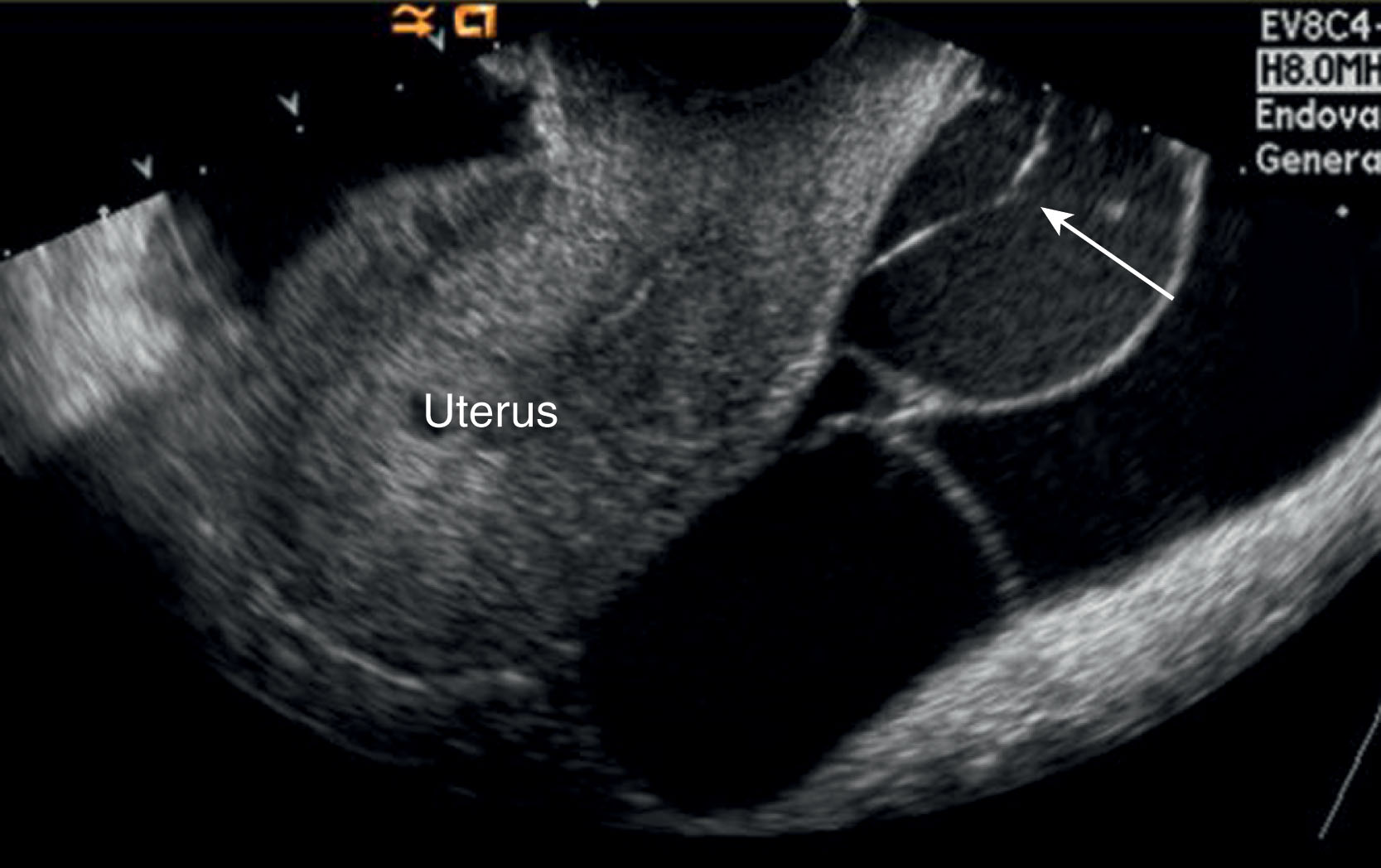
Infrequently, a cystic mass may be seen in a patient who has a history of bilateral oophorectomy. This usually results in a technically difficult surgery (because of adhesions), in which a small amount of residual ovarian tissue has been unintentionally left behind. The residual ovarian tissue can become functional and produce cysts with a thin rim of ovarian tissue in the wall.
Peritoneal inclusion cysts are lined with mesothelial cells and are formed when adhesions trap peritoneal fluid around the ovaries, resulting in a large adnexal mass. Clinically, most patients have pelvic pain or a pelvic mass.
Peritoneal inclusion cysts (benign cystic mesothelioma) are multiloculated cystic adnexal masses. The diagnosis must include the presence of an intact ovary either within or on the margin of the cyst. The fluid may contain echoes resulting from hemorrhage or proteinaceous fluid ( Fig. 44.16 ). Predominantly these occur in premenopausal women with a history of abdominal surgery. The cyst may also occur in patients with a history of trauma, PID, or endometriosis. The risk of recurrence after surgical resection is 30% to 50%. Do not confuse this condition with hydrosalpinx, which appears as a tubular or ovoid cystic structure with visible folds, where the ovary lies outside the cystic structure.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here