Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The vasculitides are a group of rare diseases linked by the pathological consequences of vascular inflammation, including bleeding, ischemia, and infarction of downstream organs ( Box 39.1 ). However, the clinical spectrum of these diseases is wide ranging and includes a myriad of clinical and pathological findings. Not all disease phenotypes that occur in the vasculitides are due to true “vasculitis” (i.e., inflammation of vascular structures). Some damage in vasculitis is due to nonvascular inflammation. For example, arthritis, uveitis, and pulmonary nodules are parts of different vasculitides but are not due to interruption of vascular flow. The pathophysiology of vasculitis is covered in Chapter 10 and in individual chapters on large-vessel vasculitis, including giant cell arteritis (GCA) and Takayasu arteritis (TAK) ( Chapter 40 ), medium- and small-vessel vasculitis ( Chapter 41 ), and Kawasaki disease ( Chapter 43 ).
a Most of these diseases can involve vessels of varying sizes but are listed here by the size of the most commonly affected arteries for convenience purposes. This is not an exhaustive list of vasculitides.
by Predominant Size of Vessel Involvement
Giant cell arteritis
Takayasu arteritis
Relapsing polychondritis
Cogan syndrome
Aortitis associated with spondyloarthropathies
Retroperitoneal fibrosis
Idiopathic aortitis
Polyarteritis nodosa
Kawasaki disease
ANCA-associated vasculitis
Granulomatosis with polyangiitis
Microscopic polyangiitis
Eosinophilic granulomatosis with polyangiitis (Churg-Strauss)
Cryoglobulinemic vasculitis
Anti–glomerular basement membrane disease
IgA vasculitis (Henoch-Schönlein)
Rheumatoid arthritis (rheumatoid vasculitis)
Sjögren syndrome
Systemic lupus erythematosus
Systemic sclerosis (scleroderma)
Drug-induced vasculitis
Behçet disease
Central nervous system vasculitis
Drug-induced vasculitis
The diseases outlined in this chapter are rare, and all are each considered “orphan” diseases, with fewer than 200,000 cases in the United States at any time. As with most rare diseases, few or no well-controlled clinical treatment trials have been performed for these disorders. Much of the clinical investigation stems from studies of patient cohorts at large referral centers. However, in the past two decades, increasing international cooperation among vasculitis centers has resulted in several important randomized controlled treatment trials that have had significant impacts on the care and management of patients with several forms of vasculitis, including antineutrophil cytoplasmic antibody (ANCA)-associated vasculitis (eosinophilic granulomatosis with polyangiitis [EGPA; Churg-Strauss], granulomatosis with polyangiitis [GPA], and microscopic polyangiitis [MPA]) and GCA. Similarly, advances in diagnostic imaging and laboratory testing have improved clinicians’ ability to diagnose and evaluate patients with vasculitis.
This chapter reviews the major types of vasculitis, discusses evaluation of suspected cases of vasculitis, and outlines approaches to treatment and management of these disorders. There is a focus on differentiating inflammatory from noninflammatory disease as it relates to the types of patients physicians specializing in vascular medicine are likely to encounter in a consultative practice ( Table 39.1 ). The newest advances in diagnosis and treatment are also reviewed briefly.
| Type of Vasculitis | Thoracic Aortic Disease | Abdominal Aortic Disease | Carotid/Vertebral Arterial Disease | Stroke Due to Small- or Medium-Artery Disease | Upper- and Lower-Extremity Arterial Stenosis | Renal Arterial Disease | Coronary Artery Disease | Mesenteric Arteritis | Myocarditis | Pericarditis |
|---|---|---|---|---|---|---|---|---|---|---|
| GCA | ++ | + | + | + | Rare | Rare | ||||
| TAK | +++ | ++ | +++ | ++ | + | + | Rare | Rare | ||
| Other large-vessel diseases (RPC, CS, RPF, IA) | ++ | + | + | Rare | + | + | Rare | + | Rare | Rare |
| PAN | + | + | + | Rare | +++ | + | + | |||
| Kawasaki disease | Rare | Rare | +++ | ++ | + | |||||
| GPA | Rare | Rare | + | Rare | Rare | + | Rare | + | ||
| MPA | Rare | Rare | Rare | + | Rare | + | ||||
| EGPA | Rare | Rare | + | ++ | ++ | |||||
| CV | Rare | Rare | Rare | +++ | ||||||
| IgAV | +++ | |||||||||
| Small-vessel vasculitis of RA, SS, SLE, or SSc | Rare | + | Rare | + | + | ++ | + | +++ | ||
| Behçet disease | + | ++ | Rare | Rare | + | Rare | Rare | + | ||
| CNSV | ++ | ++ |
The classification and nomenclature of vasculitis can be unnecessarily confusing. The most important first step in approaching these disorders is for clinicians to consider the possibility of “some sort of vasculitis” and, once clinical proof is found, to narrow down the specific type. Nevertheless, knowledge of the classification criteria is quite useful when considering treatment and clinical follow-up. Establishing a treatment plan for a case of vasculitis relies on both an understanding of the prognosis of a specific type and applying results of clinical trials that always include patients who meet specific classification criteria. For example, a patient with arthritis, purpura, and abdominal pain might well be treated with glucocorticoids alone if believed to have immunoglobulin A vasculitis (IgAV; Henoch-Schönlein) but would also receive an additional immunosuppressive drug (e.g., methotrexate, cyclophosphamide, or rituximab) if determined to have GPA. Similarly, the nature of follow-up visits, examinations, and subsequent evaluations is also heavily influenced by the specific type of vasculitis. For example, new-onset hemoptysis in a patient believed to be in remission after treatment for GCA would be concerning for infection or malignancy, whereas the same finding in a patient with GPA would usually prompt immediate reinstitution of high-dose glucocorticoids to treat potential alveolar hemorrhage while further evaluations, including for infection, are put in place.
Two major systems for sorting among the vasculitides exist: the American College of Rheumatology (ACR) classification system and the Chapel Hill Consensus Conference definitions. These systems were not meant to offer diagnostic criteria. The ACR system is used to establish vasculitis and differentiate one vasculitis from another. The Chapel Hill system is designed to standardize the definition of the diseases and was revised in 2012 with inclusion of new names for several entities to move away from eponyms and widely adopt more physiologically appropriate names. The main use of these systems has been for clinical trials and other types of clinical research. Nevertheless, these systems have been adapted for use by clinicians as helpful guides to practice but, when misapplied as diagnostic criteria, may lead to problems. Not all types of vasculitis are included in the ACR or Chapel Hill systems, and the ACR classification criteria are currently undergoing reevaluation and revision.
The practice of differentiating among the inflammatory vasculitides by associated diagnostic antibodies is at this time limited to use of ANCAs. Specifically, many authors refer to ANCA-associated vasculitis , which includes GPA, MPA, renal-limited pauci-immune glomerulonephritis, and EGPA (Churg-Strauss). Although it is convenient to refer to these related diseases as “ANCA-associated” vasculitis, it is important to realize that patients may have any of these diseases and still test negative for the presence of ANCA.
Perhaps the simplest method of sorting out the vasculitides, albeit also incomplete and not fully accurate, is to list them according to the size of artery involved (predominantly but not necessarily exclusively) (see Box 39.1 ). This results in considering small- , medium- , and large-vessel vasculitides and variable-vessel vasculitis . This system, although not applied for clinical trials or clinically for treatment purposes, is an easy one to use as a first approach to describing the diseases and their major manifestations and is used to outline the descriptions of the vasculitides in this chapter. However, when specific diseases and results of treatment trials are mentioned, the ACR and Chapel Hill Consensus systems are applied.
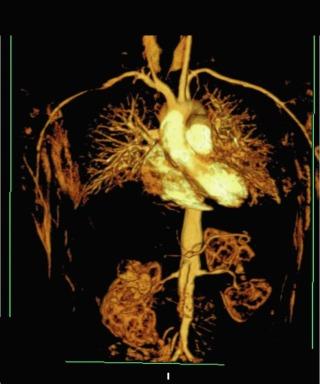
The large-vessel vasculitides are disorders in which the aorta and its main branches, including the subclavian, carotid, vertebral, renal, mesenteric, and iliac arteries, are affected ( Fig. 39.1 ). Because such vessels are so frequently involved in noninflammatory vascular diseases, and patients with these diseases are frequently encountered by specialists in vascular medicine, these disorders are particularly highlighted in this textbook. Also included are individual chapters on GCA and TAK ( Chapter 40 ) and Kawasaki disease ( Chapter 43 ). The vasculitides involving large arteries are briefly described in this section, but it is important to realize that many of them also involve smaller-sized vessels.
GCA, also commonly known as temporal arteritis and described in detail in Chapter 40 , is the most common of the idiopathic vasculitides. , GCA affects men and women aged 50 and older but is especially prevalent after age 70. Many vascular and systemic manifestations are seen in this disease. Vascular disease occurs in the aorta and its branches, with predilection for the branches of the carotid arteries, especially the ophthalmic artery, with resulting headaches, jaw claudication, and visual impairment. Rapid-onset irreversible monocular blindness is the most feared complication, but stroke, limb ischemia, and aortic disease can occur, the latter much more common than generally appreciated, especially several years after the initial presentation. Common systemic manifestations include fever, anemia, proximal arthralgias (polymyalgia rheumatica), and fatigue. Diagnosis is often established by finding arteritis on temporal artery biopsy, but this is not required for a diagnosis. Elevated acute phase reactants are seen in 90% of cases. Treatment with high-dose glucocorticoids is highly effective but often results in significant drug-related morbidity. There is now good evidence from randomized controlled trials for the efficacy of methotrexate or tocilizumab (interleukin-6 [IL-6] receptor inhibitor) to treat GCA and act as “steroid-sparing” agents.
Takayasu arteritis, described in detail in Chapter 40 , is a vasculitis that involves the aorta and all its major branches and the pulmonary arteries, including but not limited to, the brachiocephalic, carotid, vertebral, subclavian, renal, femoral, and coronary arteries. This disease often results in stenoses, occlusions, and ischemic damage to end organs and limbs. , Stroke, myocardial infarction, limb claudication, and severe renovascular hypertension are all complications well known to occur in this disease. It is mostly seen in women and usually first presents clinically in the second or third decade, but it can occur at older ages. Many patients have associated systemic symptoms of fever, arthralgias, and malaise. The disease has a waxing and waning course, and delay in diagnosis is common. Treatment involves glucocorticoids in almost all patients and often the addition of immunosuppressive medications. Surgical bypass procedures may be necessary in some cases.
Relapsing polychondritis is a rare connective tissue disease that predominantly affects the cartilaginous structures of the eyes, ears, nose, and subglottis/trachea but may also affect a wide variety of other organ systems and is associated with vasculitis, especially of large vessels. The cardinal feature of polychondritis is auriculitis, inflammation of the outer ear, usually sparing the noncartilaginous lobe. Auriculitis, which is also a feature of GPA and EGPA but virtually of no other diseases, is readily treated with glucocorticoids and can result in disfigurement if allowed to go untreated. Other common manifestations include inflammatory eye disease that can lead to blindness, destruction of nasal cartilage leading to internal derangement and external disfigurement, sensorineural hearing loss and vertigo, arthritis, and subglottic inflammation with resulting stenosis, a life-threatening condition. Each of these features can also be seen in GPA, although auriculitis is rare in this disease, and relapsing polychondritis is not associated with parenchymal pulmonary manifestations.
The vasculitis seen in relapsing polychondritis can affect vessels of any size, but large-vessel vasculitis is the most common. Aortitis with associated aortic valvular dysfunction and accompanied by thoracic or abdominal aortic aneurysms is fairly common and can lead to heart failure, aneurysmal rupture or dissection, and involvement of branch arteries. Small-vessel disease can affect nerves, eyes, kidneys, and other systems.
The histopathology of relapsing polychondritis includes destructive inflammation of various types of cartilage, necrotizing aortitis, vasculitis in small vessels (e.g., skin, glomeruli), and direct inflammatory infiltration of eye structures, heart valves, pericardium, skin, and other tissues.
Relapsing polychondritis has been associated with various other primary autoimmune diseases, such as inflammatory bowel disease, lupus, and others. The rarity of this syndrome has precluded comprehensive research that might help to both better differentiate cases from other conditions and learn more about the pathophysiology. Treatment almost always involves systemic glucocorticoids, and immunosuppressive agents are frequently prescribed for this often rapidly progressive disease.
Cogan syndrome is a rare disorder characterized by inflammatory eye and inner ear/vestibular disease that can also involve inflammatory vasculitis. It is a disease of young adults, usually first affecting patients before age 40, although both children and older patients have also been affected.
The characteristic clinical manifestations of Cogan syndrome are interstitial keratitis, sensorineural hearing loss, and vestibulatory dysfunction. Although interstitial keratitis is the most common eye problem in Cogan syndrome, uveitis, scleritis, and many other types of ophthalmological inflammation can occur. The eye and ear damage is often permanent and can be quite debilitating. The combination of inflammatory eye disease and inner ear problems is required for a diagnosis of Cogan syndrome, but these findings can occur in other diseases as well, such as infections, malignancies, sarcoidosis, and various autoimmune diseases, including other vasculitides (e.g., GPA, relapsing polychondritis, Behçet disease). Other organ systems are less commonly involved.
Vasculitis occurs in up to 15% of patients with Cogan syndrome and is mostly large-vessel disease, with some medium-vessel manifestations reported. The large-vessel disease in Cogan syndrome is similar to that of TA and includes aortitis with aortic insufficiency, stenoses of the carotid, subclavian, and other aortic branch arteries, and even coronary artery disease (CAD). Treatment of Cogan syndrome includes both glucocorticoids and immunosuppressive drugs, appropriate rehabilitation (e.g., vestibular retraining), surgical correction of eye damage, and use of hearing aids or surgical correction of hearing loss.
Aortitis may be found in the absence of any other manifestations of a systemic inflammatory disease. These cases often come to the attention of vascular medicine specialists when patients undergoing surgical repair of aortic aneurysms and dissections are found to have inflammation consistent with aortitis on pathological specimens. Autopsies and studies of large numbers of surgical specimens have demonstrated that noninfectious aortitis occurs in 4% to 15% of cases. Although, with detailed investigation, many of these patients are retrospectively found to have had evidence of GCA, TAK, relapsing polychondritis, GPA, or another definable vasculitis, it is common among these cases to find no evidence of more systemic inflammatory disease. The majority of cases of so-called idiopathic aortitis involve thoracic lesions, in contrast to the overall predominance of abdominal aortic lesions for noninflammatory disease.
It is possible that cases of isolated inflammatory aortic aneurysms will be increasingly identified earlier as magnetic resonance imaging (MRI) technology continues to improve and helps to demonstrate inflammation in the arterial wall. However, it can be difficult to differentiate inflammation due to true idiopathic aortitis and vasculitis from the vascular and periaortic inflammations seen in association with atherosclerotic disease. Currently, in the absence of pathological specimens or other evidence of a vasculitis, MRI alone is not diagnostic for inflammation. The emergence of positron emission tomography (PET) scanning for large-vessel disease may also help in the evaluation of such patients.
The approach to treatment of idiopathic aortitis is unclear; many patients never develop other findings of vasculitis. However, new aneurysms and significant vascular disease do occur in some cases. Comprehensive evaluation of evidence of systemic disease is necessary and should include a detailed physical examination, diagnostic imaging, laboratory studies, and other approaches outlined later in this chapter. Appropriate treatment should be given if inflammatory disease other than that seen in the surgical specimen is found, but not all patients require glucocorticoids, especially in the postoperative period. Furthermore, regular follow-up of such patients by a specialist knowledgeable about vasculitis is imperative because lesions may develop subtly and only years after the initial pathological diagnosis is made.
Although large-vessel vasculitis is rarely seen with other systemic inflammatory conditions, it is important to recognize these potential associations. Aortitis is rarely associated with long-standing seronegative spondyloarthropathies (ankylosing spondylitis, reactive arthritis, psoriatic arthritis, and inflammatory bowel disease) and can result in aortic insufficiency. Retroperitoneal fibrosis, a rare disease of proliferating fibroblasts usually causing ureteral obstruction and at times aortic stenosis and periaortitis, is also associated with true inflammatory aortitis. Rarely, IgG4-related disease can cause aortitis and is also frequently the etiology of retroperitoneal fibrosis. There have been a few case reports of large-vessel vasculitis in patients with rheumatoid arthritis, systemic lupus erythematosus (SLE), and GPA.
Among the inflammatory vasculitides, the medium-vessel diseases have the greatest variety in clinical manifestations, which result from the broad range of vessel sizes actually involved in the process. As stated earlier, classifying the vasculitides by affected vessel size can be problematic but particularly so with the medium-vessel disorders.
Specialists in vascular medicine need to be aware of protean presentations of active medium-vessel disease and the lasting damage they can cause. As with large-vessel disease, these disorders can mimic noninflammatory cardiac, renal, cerebral, and other vascular disease. This fascinating set of diseases comprises the vasculitides for which the highest quality and quantity of clinical trial data are available to help guide therapy.
Polyarteritis nodosa (PAN) is among the “purer” vasculitides in that most of its manifestations are due to true vascular inflammation. With the identification of other types of vasculitis, the spectrum of what is now diagnosed as PAN has narrowed over the past 60 years. Although characterized as a medium-vessel disease, PAN may also involve small vessels such as those in the skin. PAN frequently involves inflammation leading to multiple small aneurysms that often appear angiographically as a “string of beads.” Ischemia and infarction of kidneys, intestines, and skin are common in PAN, with arthralgias, myalgias, and fevers also frequently seen. Diagnosis is based on angiographic appearance ( Fig. 39.2 ) or tissue pathology, often from surgical specimens such as a resected ischemic bowel segment.
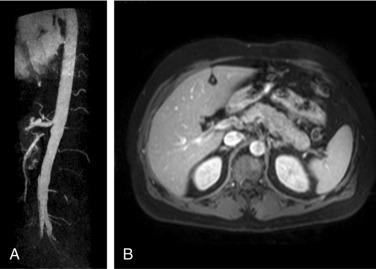
After accurate diagnostic testing for hepatitis B virus (HBV) became widely available and implemented in clinical practice, it became apparent that many cases of PAN were caused by HBV infection. With the widespread use of highly effective vaccines to prevent HBV in many countries, the prevalence of PAN in these locations has fallen precipitously.
PAN is also associated with infection with hepatitis C virus but not as often as with HBV. , Importantly, there is a difference between hepatitis C–associated PAN and hepatitis C–associated cryoglobulinemic vasculitis (CV, see later section).
Cardiac manifestations of PAN are due to coronary arteritis or malignant hypertension (secondary to renal artery disease) and include myocardial ischemia, heart failure, and arrhythmias.
Treatment of PAN almost always involves high-dose glucocorticoids followed by a slow tapering of the dose. In more severe cases, an immunosuppressive agent is added. Hepatitis-associated PAN is now often treated with short courses of glucocorticoids and prolonged courses of antiviral agents. The rate of disease relapse in PAN is lower than that for many other types of vasculitis, and this relatively good prognosis is another important factor to take into consideration when deciding on a therapeutic regimen. Due to the rarity of the disease, controlled clinical trials for PAN are unlikely to occur; treatment is based on case series and expert opinion.
Kawasaki disease, a vasculitis of young children involving medium and small arteries, is a leading cause of acquired CAD in children. The disease manifests as a systemic illness with high fevers, conjunctival injection, erythematous oropharyngeal lesions, erythematous rashes and skin desquamation, lymphadenopathy, and other signs and symptoms. Cardiac involvement is frequent in Kawasaki disease and can result in long-term morbidity. Myocarditis and pericarditis are common and can be serious, but coronary artery aneurysms are the most feared aspect of the disease. Both panarteritis and granulomas can be seen in the vessels, with subsequent scarring and aneurysm formation. Treatment includes aspirin and intravenous immunoglobulin; such regimens have been shown to markedly reduce the incidence of coronary complications. Kawasaki disease is described in detail in Chapter 43 .
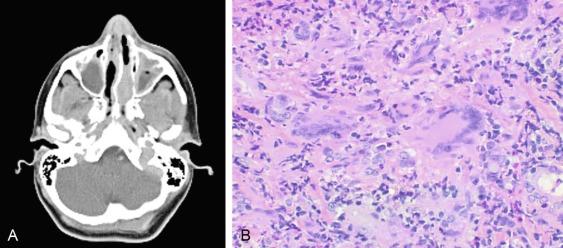
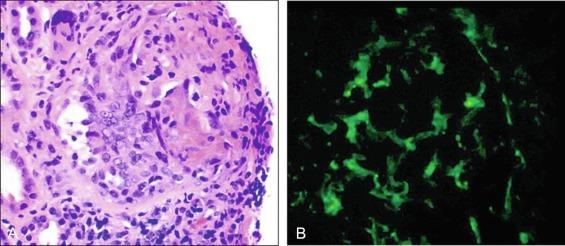
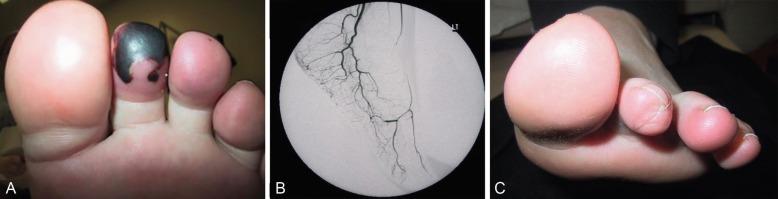
GPA, previously named Wegener granulomatosis, is characterized by the triad of inflammation and destruction of tissue in the upper airway and sinuses ( Fig. 39.3 ), lower airway ( Fig. 39.4 ), and kidneys ( Fig. 39.5 ), as well as the development of ANCAs. , More than 70% of patients with GPA are positive for ANCA at diagnosis, although some will develop the antibodies later in the course of their illness. Among patients with GPA and glomerulonephritis, more than 90% are positive for ANCA. Although the combination of these features is common in GPA, many patients present with only a subset of these findings. GPA also frequently involves many other organ systems. The upper airway lesions include destructive rhinitis, often leading to nasal bridge collapse and the “saddle nose” deformity, sinusitis, and subglottic inflammation that can lead to life-threatening tracheal stenosis. The most severe form of pulmonary disease in GPA is alveolar hemorrhage, and this is a common cause of early death. Other common pulmonary lesions include nodules, with or without cavitation, and tracheobronchitis. Additional common features of GPA include retroorbital pseudotumor with resulting proptosis, conductive and sensorineural hearing loss, mononeuritis multiplex, arthritis, and purpura. Peripheral vascular involvement with gangrene is seen in GPA and may be the presenting feature ( Fig. 39.6 ).
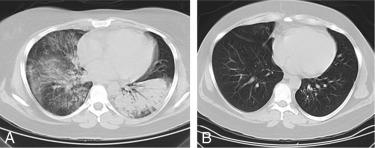
Inflammatory cardiac disease is rare in GPA but can include myocarditis and pericarditis. Aortic or large-vessel involvement in GPA is extremely uncommon.
Venous thromboses, including both deep vein thromboses and pulmonary emboli, occur frequently in GPA and may be associated with active disease. , Although some of the pathology in GPA is indeed granulomatous with histiocytes, piecemeal necrosis, and occasional giant cells and eosinophils, other manifestations of inflammation are also seen in the disease. True vasculitis occurs and includes capillaritis. The renal disease of GPA is identical to other ANCA-positive diseases, and the pathology is that of rapidly progressive glomerulonephritis.
Untreated, GPA most often leads to death or serious damage. Glucocorticoids are always used for treatment, but the prognosis of GPA changed considerably when a protocol using cyclophosphamide was introduced in the 1970s at the National Institutes of Health. The morbidity and mortality of GPA was markedly improved by cytotoxic therapy: 1-year mortality changed from more than 80% to less than 20%. , , However, serious side effects are common with the use of cyclophosphamide, and the rate of recurrent disease in GPA after therapy is greater than 50%. In recent years, new treatment protocols have been tested in open and controlled trials that incorporate less toxic immunosuppressive drugs, including methotrexate and azathioprine. Two multicenter randomized controlled trials have demonstrated that treatment with rituximab, a monoclonal antibody directed against the CD20 receptor on B cells, was equivalent to cyclophosphamide for induction of remission in ANCA-associated vasculitis (GPA and MPA). , In 2011 the US Food and Drug Administration approved rituximab for the treatment of GPA and MPA, and it has quickly become an established, and increasingly preferred, alternative to cyclophosphamide.
With the publication of the Chapel Hill Consensus Conference classification system, recognition of MPA as a separate entity gained acceptance. , MPA is a mostly small- to medium-vessel ANCA-associated vasculitis with manifestations that strongly overlap with GPA. Its key features include glomerulonephritis, alveolar hemorrhage, skin lesions, and mononeuritis multiplex, but many other organ systems may also be involved. Unlike GPA, the pathology of MPA is nongranulomatous and does not involve the type of nonvascular disease seen in GPA or EGPA. The glomerulonephritis of MPA is identical to that seen in GPA. Most patients with MPA are positive for ANCA, and the predominant ANCA antigen specificity is myeloperoxidase (MPO). Cardiac manifestations of MPA are uncommon, but peripheral artery disease (PAD) and gangrene are seen and may be confused with noninflammatory vascular disease. MPA should be differentiated from classic PAN. PAN is more of a medium-vessel disease and does not include glomerulonephritis or pulmonary capillaritis. MPA does not produce the microaneurysms seen in PAN. Treatment of MPA is essentially identical to that for GPA, although MPA is less likely to relapse than GPA.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here