Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Autoimmune disorders frequently affect the skin, gastrointestinal, and hepatobiliary systems; the kidney; the endocrine system; and the nervous system.
The dominant clinical feature of organ-specific autoimmune disease is chronic inflammation, generally localized in a single organ specific for each individual disease.
In organ-specific autoimmune diseases, an organ-specific, tissue-specific, or cell-specific autoantigen is targeted. Some autoantigens circulate, some are cell surface molecules, and some are intracellular.
Clinical recognition of autoimmunity is dependent on the detection of autoantibodies against relevant autoantigens.
Damage to self can be mediated by humoral and/or cell-mediated autoimmunity, yet the etiology of virtually all organ-specific autoimmune diseases remains elusive.
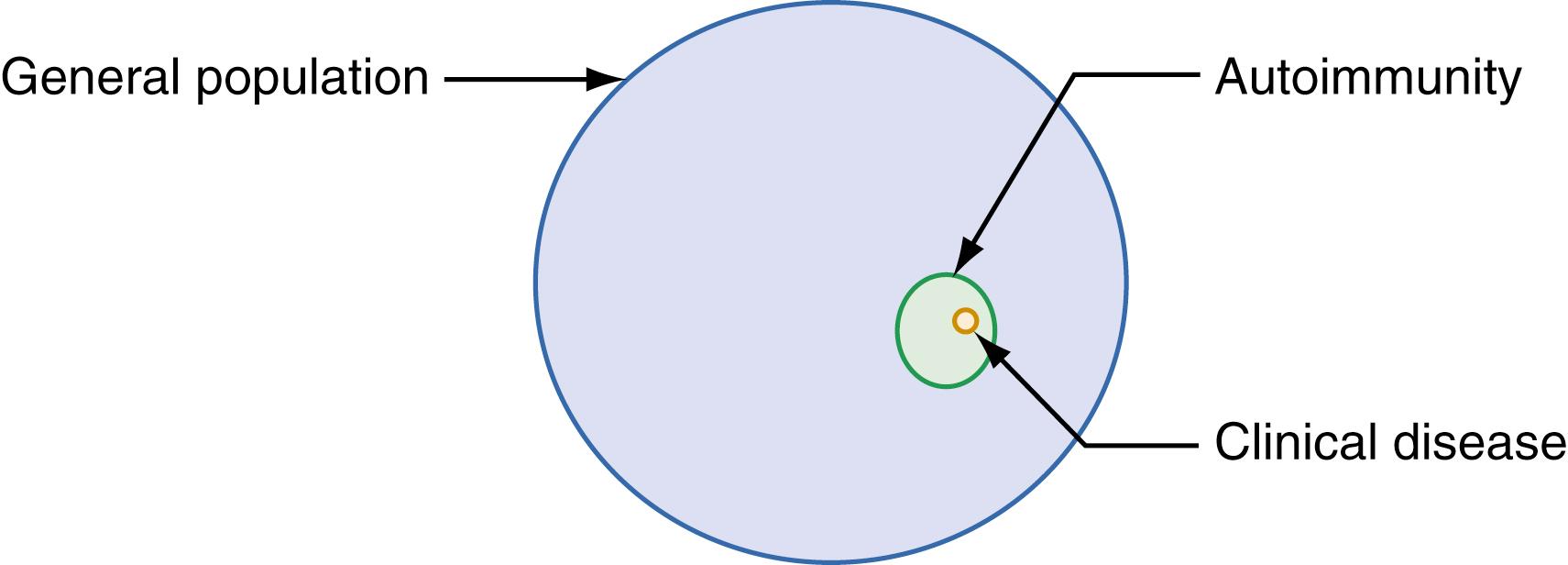
Autoimmune diseases occur in a proportion of people that manifest autoimmunity.
The presence of circulating autoantibodies at significant titers is often useful in establishing the etiology of autoimmune liver disorders.
An expanding universe of microvolume technologies is speeding the development of autoantibody detection that could support screening programs when clinically indicated.
The detection of relevant autoantibodies in the setting of clinical disease can confirm an autoimmune etiology for the disease. Likewise, certain autoantibodies in asymptomatic individuals can predict the later development of disease.
Collectively, organ-specific autoimmune diseases are common and produce significant degrees of morbidity and mortality.
Autoimmunity is aberrant immunity to self ( ; ). To protect the body from infection and neoplasia (when new antigens may be expressed), the immune system must be able to distinguish self from nonself. The innate immune system uses a large number of nonclonotypic cell surface receptors (such as toll-like receptors and the inflammasome) that seek gross differences between self and nonself to recognize the microbiologic world ( ; ). The adaptive immune system uses clonotypic T-cell receptors for peptide–major histocompatibility complex (MHC) identification, while B cells use clonotypic cell surface antibody (i.e., membrane immunoglobulin) to accomplish this task. The adaptive immune system can recognize very subtle differences between self and nonself that may not be “observed” by the innate immune system receptors.
Normally autoimmunity is prevented by central (thymic) tolerance and peripheral tolerance ( ). During T-cell ontogeny in the thymus, at the T-cell receptor double-positive thymocyte stage (i.e., T cells are positive for CD4 and CD8), interaction with class I or class II MHC molecules expressed by epithelial nurse cells “positively” selects such T cells for initial survival. Failure of these double-positive T cells to interact with either class I or class II MHC leads to apoptotic cell death. Once a double-positive T cell is “selected” (aka, “saved”), if it binds extremely tightly to the peptide-MHC complex that positively selected it, the double-positive T cell will undergo apoptosis in a process termed negative selection, which is part of central (thymic) tolerance. In general, if developing lymphocytes [either T or B cell (e.g., naïve immature IgM-positive B cells)] strongly signal through their clonotypic antigen receptor, they will be triggered to undergo apoptosis. After positive selection via class I MHC, the double-positive T cell (if it survives) is programmed to lose CD4 expression and is destined to become a CD8 T cell. Likewise, after positive selection via class II MHC, the double-positive T cell is programmed to lose CD8 expression and is destined to become a CD4 T cell. Therefore single-positive T cells are found in the thymic medulla. Nonetheless, if these single-positive T cells bind extremely tightly to peptide-MHC complexes presented by medullary thymic epithelial cells (mTEC), dendritic cells, or macrophages, they too will undergo apoptosis as part of central (thymic) tolerance. In this way, CD4 and CD8 T cells exiting the thymus will not display very high levels of affinity for self-peptide plus MHC theoretically preventing (in part) autoimmunity. These CD4 and CD8 T cells constitute the naïve T-cell repertoire that leaves the thymus.
Peripheral tolerance develops when naïve T cells encounter MHC plus antigen-peptide without secondary signals. Such “tolerized” T cells are not able to subsequently respond to antigen, even if the proper secondary signals are then made available. Peripheral tolerance ensures that antigen-presenting cells are activating the naïve T cells in an appropriate immune response: Signal 1 is the T-cell receptor’s perception of antigen-peptide presented by MHC (stabilized by MHC-CD4 or MHC-CD8 interactions), whereas signal 2 (a survival signal) is most importantly B7 (from the antigen-presenting cell where B7.1 is CD80 or B7.2 is CD86) engaging CD28 on the T cell. For CD4 T cells, a third set of signals involving cytokines secreted by dendritic cells and natural killer (NK) cells determines whether the newly stimulated CD4 T cell differentiates into a Th1 cell (secreting interleukin 2 [IL-2] and interferon-γ, tumor necrosis factor-α [TNF-α], and granulocyte/monocyte colony-stimulating factor), a Th2 cell (secreting IL-4, IL-5, IL-6, IL-10, IL-13, and eotaxin), a Tfh cell (T follicular helper cell secreting Th1 and/or Th2 cytokines), a Th17 cell (secreting IL-17 and IL-22), or a regulatory T cell (e.g., a CD4+ CD25+ FOXP3+ T cell secreting transforming growth factor-β, IL-4, and IL-10).
Autoimmunity becomes pathologic when clinical disease is manifested ( ; ). A normal immune response is directed against “nonself” antigens such as microorganisms that cause infection. Such immune responses are beneficial. In an autoimmune disease, the immune response is directed against “self” antigens that cause injury. When self-antigens are targeted, cellular, tissue, and organ damage can result ( ). Most autoimmune diseases involve adaptive humoral (types II and III hypersensitivity reactions) and/or cell-mediated (type IV hypersensitivity reactions) mechanisms ( Fig. 55.1 ) that cause disease.
![Fig. 55.1, The process of target destruction begins with dendritic cells (DC) that present peptides from beta cell autoantigens to CD4 Th1 cells. DCs can be classic (conventional) DCs (cDCs) or plasmacytoid DCs (pDCs). Failure to regulate Th1 cells via defective regulatory T-cell (T reg ) function could contribute to Th1 cell autoimmunity. Activated autoreactive Th1 cells, in turn, activate CD8 T cells via interleukin-2 and DC upregulation. Activated CD8 T cells are functionally T killer cells ( Tk ; aka, cytotoxic T lymphocytes [CTL] ). Via cell-cell contact between Th1 cells and macrophages (via CD40L–CD40 interactions [not shown] ) and interferon-gamma (IFN-gamma) secreted by Th1 cells, macrophages are activated. Via both CD8 Tk cells and macrophages, target cells are triggered to undergo apoptosis. In the case of type 1 diabetes, beta cells die. Fig. 55.1, The process of target destruction begins with dendritic cells (DC) that present peptides from beta cell autoantigens to CD4 Th1 cells. DCs can be classic (conventional) DCs (cDCs) or plasmacytoid DCs (pDCs). Failure to regulate Th1 cells via defective regulatory T-cell (T reg ) function could contribute to Th1 cell autoimmunity. Activated autoreactive Th1 cells, in turn, activate CD8 T cells via interleukin-2 and DC upregulation. Activated CD8 T cells are functionally T killer cells ( Tk ; aka, cytotoxic T lymphocytes [CTL] ). Via cell-cell contact between Th1 cells and macrophages (via CD40L–CD40 interactions [not shown] ) and interferon-gamma (IFN-gamma) secreted by Th1 cells, macrophages are activated. Via both CD8 Tk cells and macrophages, target cells are triggered to undergo apoptosis. In the case of type 1 diabetes, beta cells die.](https://storage.googleapis.com/dl.dentistrykey.com/clinical/OrganSpecificAutoimmuneDiseases/0_3s20B9780323673204000559.jpg)
Autoimmunity is a broader term than autoimmune disease ( Fig. 55.2 ). For example, autoantibodies directed against thyroid self-antigens such as thyroperoxidase and thyroglobulin are relatively frequent in the general population (≥5% prevalence in adults) (Stathatos & Daniels, 2012). However, only a subset of individuals with such humoral autoimmunity will manifest clinical autoimmune thyroid disease (AITD). The most common manifestation of AITD is Hashimoto thyroiditis. When agonist autoantibodies against the TSH receptor are produced, Graves disease results.
Defects in innate immunity can also be harmful, causing self-injury (e.g., C1 esterase inhibitor deficiency), paroxysmal nocturnal hemoglobinuria (PNH), various types of atypical hemolytic-uremic syndrome (aHUS) (e.g., inborn errors in C3, CD46, CFB, CFH, CFHR5, CFI, and THBD), and autoinflammatory diseases (e.g., familial Mediterranean fever [FMF], hyperimmunoglobulinemia D with periodic fever syndrome [HIDS], TNF receptor–associated periodic syndrome [TRAPS], Muckle-Wells syndrome [MWS], familial cold autoinflammatory syndrome [FCAS], and chronic infantile neurologic cutaneous articular syndrome [CINCA]) ( ). While these disorders are fascinating and instructive, we limit our discussion in this chapter to defects involving adaptive immunity causing autoimmune diseases.
An autoimmune disease may be present when one or more of the following findings are associated with the disease: (1) evidence of humoral or cell-mediated autoimmunity (including lymphocytic infiltration of the affected tissue or organ); (2) ability to transfer the disease with antibodies or lymphocytes; (3) beneficial response to immunomodulatory or immunosuppressive therapy; and (4) in the absence of immunosuppression, disease recurrence with organ transplantation. In practical terms, only autoantibody testing is sufficiently robust for routine clinical applications. Therefore most cases of autoimmune disease are substantiated by the finding of autoantibodies that are often directed against the target organ or tissue. However, the detection of autoantibodies, by themselves, indicates a state of “autoimmunity” and not “autoimmune disease.” Through the history, physical examination, imaging studies, histologic reports (if a biopsy were performed), and laboratory findings, the physician can judge whether or not disease is present, promoting the diagnosis from “autoimmunity” to a state of “autoimmune disease” ( .; ).
Traditionally autoimmune diseases are classified as “organ specific” or “non-organ specific” ( ). In an organ-specific autoimmune disease, a single tissue or organ is targeted. In some cases, a single circulating protein or receptor is targeted (e.g., insulin in the autoimmune hypoglycemia syndrome) ( ).
One of the best examples of tissue specificity is type 1 diabetes: Within the islets of Langerhans, beta cells are selectively destroyed ( ; ). Beta cells are the most prevalent of islet cells (∼65%); however, there are ample numbers of alpha cells (producing glucagon) and delta cells (producing somatostatin) and lesser numbers of PP cells (pancreatic-polypeptide producing cells). While there is some evidence of impaired alpha cell function early in the course of type 1 diabetes ( ), hyperglucagonemia is a pathogenic and well-recognized feature of type 1 diabetes that contributes substantially to hyperglycemia, ketogenesis, and ketosis in diabetic ketoacidosis. Examples of organ-specific autoimmune diseases are listed in Box 55.1 . Discoid lupus is not discussed in this chapter because it falls under the heading of lupus (see Chapter 53 ). Autoimmune hemolytic anemia, autoimmune neutropenia, and immune thrombocytopenic purpura are examined in Chapter 33, Chapter 34, Chapter 41 . Multiple organ-specific autoimmune diseases may also occur concurrently in a single patient ( Box 55.2 ). An example of multiple organ-specific autoimmune diseases coexisting is thyrogastric autoimmunity where autoimmune thyroid disease and pernicious anemia (PA) commonly occur together in families ( ). Frequently associated with type 1 diabetes are autoimmune thyroid disease and PA ( ). In hematology, autoimmune hemolytic anemia and immune thrombocytopenic purpura can coexist as Evans syndrome.
Pemphigus vulgaris
Pemphigus foliaceus
Paraneoplastic pemphigus
IgA pemphigus
Bullous pemphigoid
Pemphigoid gestationis
Mucous membrane pemphigoid
Linear IgA dermatosis
Epidermolysis bullosa acquisita
Dermatitis herpetiformis
Cutaneous vasculitis
Autoimmune vitiligo
Autoimmune alopecia
Pernicious anemia
Celiac disease
Inflammatory bowel disease
Autoimmune hepatitis
Primary biliary cholangitis
Primary sclerosing cholangitis
Autoimmune pancreatitis
Membranous nephropathy
IgA nephropathy
Membranoproliferative glomerulonephritis
C3 glomerulopathy
Infection-associated glomerulonephritis
Anti-glomerular basement membrane disease
Pauci-immune glomerulonephritis
Lupus nephritis
Type 1 diabetes mellitus
Acanthosis nigricans with insulin resistance (type B insulin resistance)
Autoimmune hypoglycemia
Insulinomimetic insulin receptor autoantibodies
Chronic lymphocytic (Hashimoto) thyroiditis
Graves disease
Atrophic thyroiditis
Autoimmune Addison disease
Autoimmune disease of the ovary
Autoimmune hypophysitis
Autoimmune diabetes insipidus
Autoimmune hypoparathyroidism due to CaSR agonist autoantibodies
Autoimmune hyperparathyroidism due to CaSR antagonist autoantibodies
Myasthenia gravis
Lambert-Eaton myasthenic syndrome
Multiple sclerosis
Paraneoplastic neurologic syndromes
Stiff-person syndrome
Polymyositis
Autoimmune hemolytic anemia
Immune thrombocytopenic purpura
Autoimmune neutropenia
Autoimmune polyglandular syndrome type I
Autoimmune polyglandular syndrome type II (also known as autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy [APECED])
Thyrogastric autoimmunity
Type 1 diabetes and autoimmune thyroid disease and/or pernicious anemia
Evans syndrome (autoimmune hemolytic anemia and immune thrombocytopenic purpura)
Non–organ-specific autoimmune diseases are also termed systemic autoimmune diseases because more than one organ and/or tissue is damaged ( ). An older term for non–organ-specific autoimmune diseases is collagen-vascular disease because many such autoimmune disorders involve joints, connective tissue, and blood vessels (e.g., vasculitis). However, the term is misleading because collagen itself is not usually an autoantigen; therefore collagen-vascular disease is not the preferred descriptor. Examples of non–organ-specific autoimmune diseases are listed in Box 55.3 .
Systemic lupus erythematosus
Scleroderma
Sjögren syndrome
Rheumatic fever
Rheumatoid arthritis
Goodpasture syndrome
Dermatomyositis
Reactive arthritis (urethritis or cervicitis, arthritis, and conjunctivitis)
Mixed connective tissue disease
Laboratory tests for evaluating autoimmunity focus on the detection of autoantibodies ( ). Tests of T-cell autoreactivity or lymphocyte-proliferation assays are not sufficiently reliable for clinical use. In the setting of clinical disease, the presence of a relevant autoantibody or autoantibodies is highly suggestive that the disease is indeed autoimmune in etiology. For example, goiter and hypothyroidism combined with positivity for thyroperoxidase autoantibodies (TPOA) or thyroglobulin autoantibodies (TGA) establish the diagnosis of Hashimoto thyroiditis.
To detect an autoantibody, the autoantigen must be available in some form whether it is purified (e.g., thyroperoxidase) or not (e.g., thyroid microsomes) ( ). The autoantigen can be present in a tissue section for indirect immunofluorescence (IIF), a cellular fraction, a purified protein or other biomolecule, or a cloned protein or a portion of a protein. Assays for autoantibody detection can be competitive or noncompetitive determinations. As an example of a competitive assay, there is an automated assay for the detection of thyrotropin-binding inhibitory immunoglobulins (TBII). In this assay a labeled monoclonal anti–TSH-receptor antibody competes with the patient’s TSH-receptor autoantibodies (TRAbs) for binding to the TSH receptor.
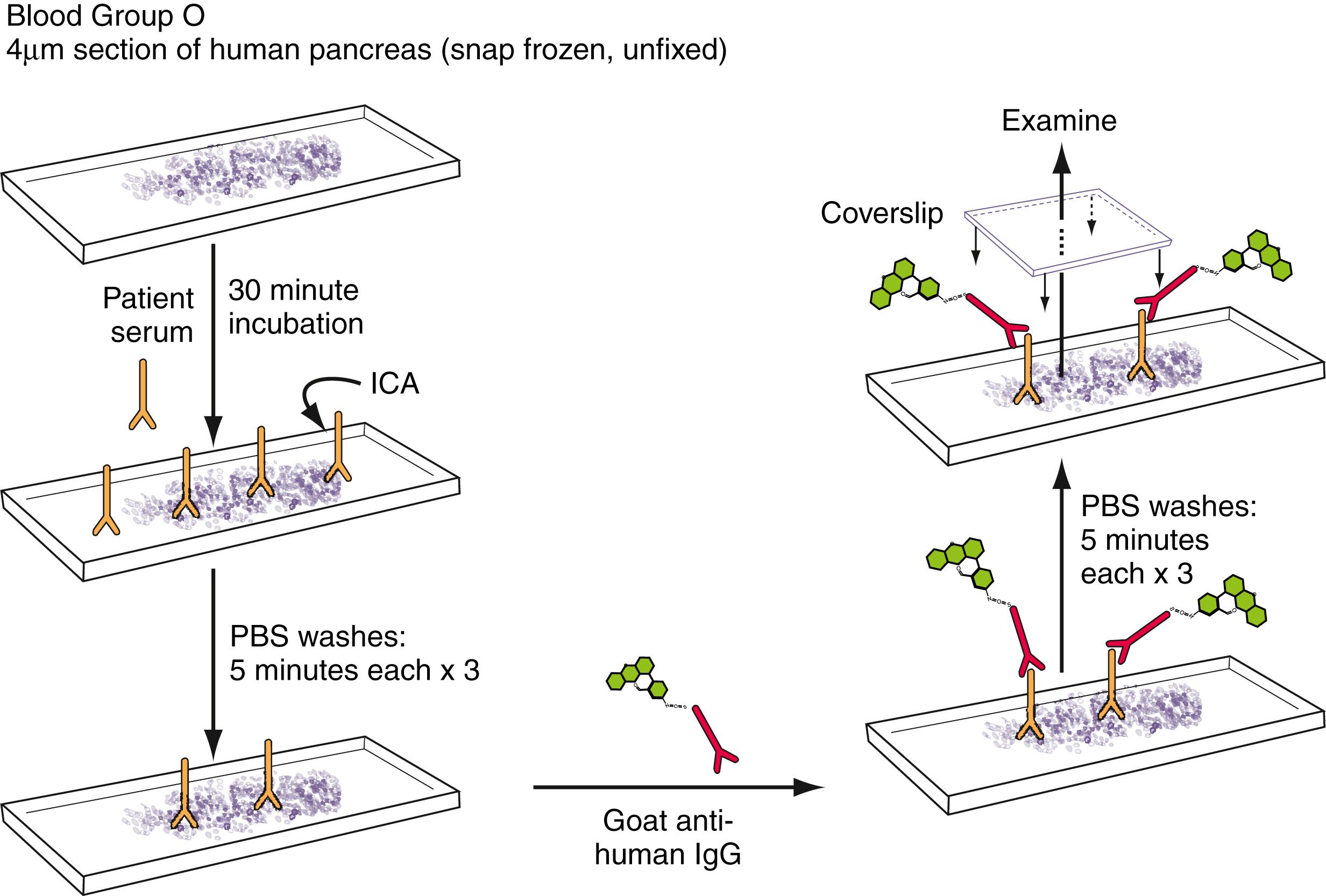
A classic noncompetitive immunoassay method is IIF for islet cell autoantibodies (ICA) ( ). Patient serum is applied to a cryo-cut section of human blood group O pancreas. After incubation and washing, FITC (fluorescein-isothiocyanate)-labeled goat-antihuman IgG is applied. After washing and the application of glycerol and a coverslip, the slide is examined using an epifluorescence microscope. If the islets fluoresce, the patient’s serum contains ICA that bind to islet autoantigens in the tissue that were detected by the FITC-labeled goat-antihuman IgG. ICA testing is illustrated in Fig. 55.3 . Cytoplasmic islet autoantigens include glutamic acid decarboxylase (GAD), the insulinoma-2-associated autoantigen, and an asialoglycoconjugate ( ).
A wide variety of immunoassay formats are available that often utilize nonradioactive detection of the signal. In addition to IIF, classic first-generation monoplex technologies applied to autoantibody detection include Ouchterlony double immunodiffusion, IIF, agglutination, complement fixation, radioimmunoassay, enzyme-linked immunosorbent assay (ELISA), immunoprecipitation, and Western blotting (aka, immunoblotting). A review by Tozzoli and colleagues (2013) provides a well-organized summary of the second- and third-generation monoplex technologies (e.g., radioimmunoassay-immunoradiometric, radioreceptor, immunoenzymatic, immunoenzymometric, immunoblot and dot, chemiluminescence, and fluorimetric), followed by truly multiplex technologies (e.g., “addressable microbead immunoassays”). Recently immuno-PCR (nucleic acid signal amplification involving an antibody-based assay) has been used to detect autoantibodies ( ).
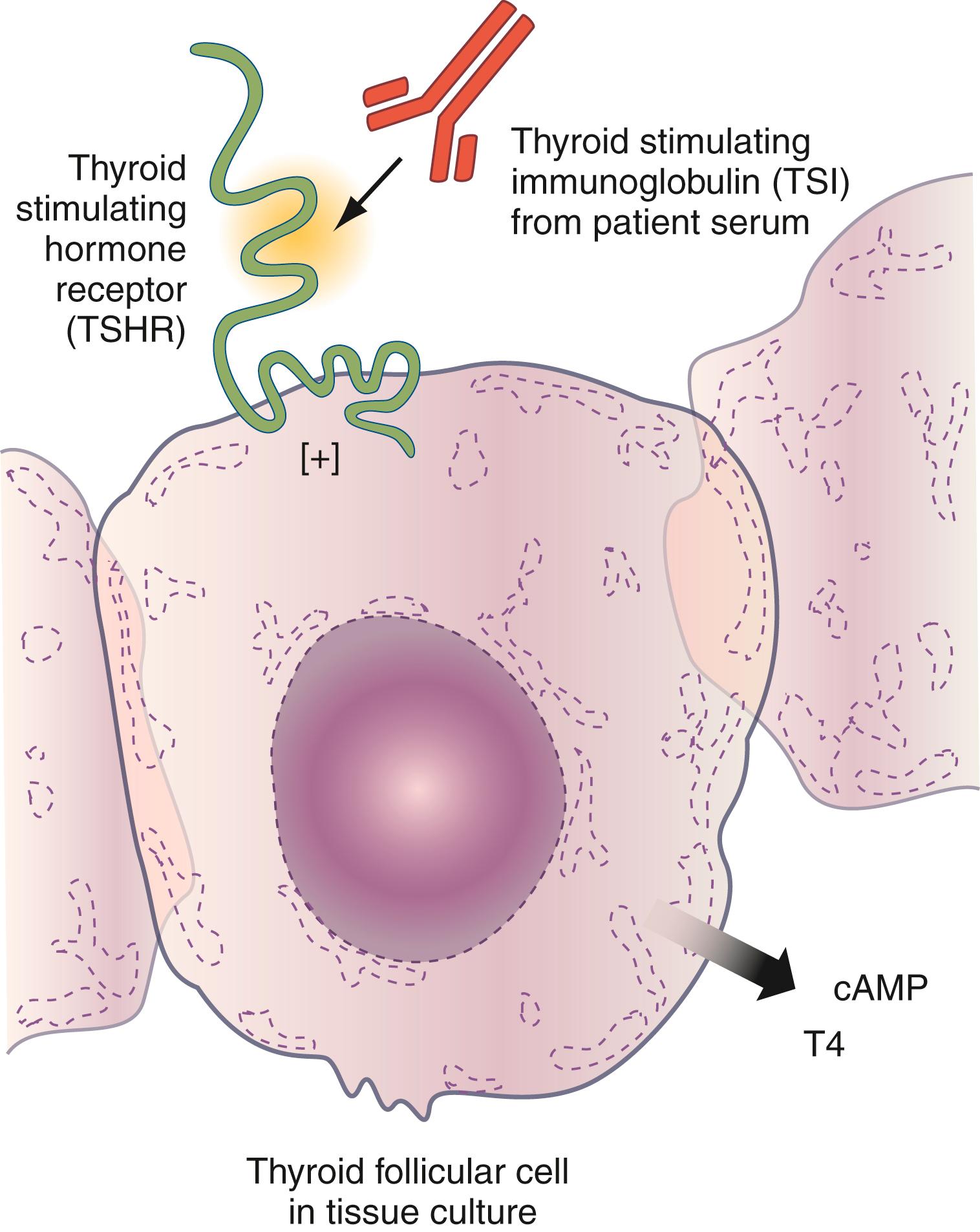
Bioassays require living cells such as the detection of thyroid-stimulating immunoglobulins (TSIs) ( ; ) ( Fig. 55.4 ). Chemiluminescent, electrochemiluminescent, bead, and flow cytometric technologies are being applied to autoantibody detection. The latest adaptations to autoantibody detection involve matrix and solid-chip technologies theoretically testing for hundreds, if not thousands, of autoantibodies simultaneously.
For many diseases, especially autoimmune blistering diseases of the skin, direct and IIF are key technologies. Biopsy of normal skin adjacent to a skin lesion allows examination of the tissue for in situ autoantibody and/or complement deposition. IIF using patient serum applied to normal tissues can reveal autoantibody reactivity to autoantigens. The salt-split skin (SSS) technique is especially useful in autoimmune diseases affecting the skin.
In the SSS technique, the patient’s skin biopsy is initially incubated in the cold in 1 M sodium chloride for 1 day. Using forceps, the epidermis is then gently separated from the dermis. The specimens are next histologically processed using standard methods. For IIF, the SSS can be used as substrate.
The two questions that must be answered are “How are positive/negative cutoffs established for autoantibody assays?” and “Does the titer (i.e., concentration) of the autoantibody provide useful clinical information about the natural history, severity, prognosis, or therapy of disease?” Positive/negative cutoffs for autoantibody tests are established using criteria similar to other tests intended to diagnose disease ( ). If the analyte’s concentration versus prevalence (frequency) in a population is distributed in a bell-shaped (normal) pattern (e.g., a histogram is developed), a positive (abnormal) test might be defined as the mean concentration of the autoantibody in the healthy population, plus either 2 or 3 standard deviations. If the mean plus 2 standard deviations is chosen as the cutoff, approximately 2.5% of the healthy population will test “positive.” A more stringent definition of positivity could employ the mean plus 3 standard deviations as the cutoff, where only approximately 0.15% of the healthy population should test “positive.” Furthermore, to ensure fewer “false positives” (at the expense of less sensitivity), some researchers have set autoantibody cutoffs as high as the mean plus 4 or 5 standard deviations. Because sensitivity and specificity are always at odds with one another in choosing a positive/negative cutoff, the best approach to defining a cutoff is to construct a receiver-operator characteristic (ROC) curve (see Chapter 8 ).
For distributions of results in a healthy population that do not follow a bell-shaped (normal) distribution, a large number of healthy controls need to be tested to define the desired cutoff—that is, the 97.5th percentile (e.g., the upper limit of the central 95% of the distribution) or the 99.85th percentile (e.g., the upper limit of the central 99.7% of the distribution). In such cases, defining the 97.5th percentile in a healthy population likely requires study of 200 or more healthy controls. Defining the 99.85th percentile in a healthy population whose results are not normally distributed is likely beyond the ability (or finances) of any laboratory except, possibly, major reference laboratories.
The answer to the second question—“Does the titer (i.e., concentration) of the autoantibody provide useful clinical information about the natural history, prognosis, severity, or therapy of disease?”—is solely dependent on the specific autoimmune disease and autoantibody under discussion. For example, in Graves disease patients, the titer of TSIs is inversely correlated with the likelihood of remission following pharmacologic antithyroid therapy (e.g., the likelihood of remission following propylthiouracil [PTU] or methimazole [MM] treatment) ( ). Also, the titer of TSIs in maternal Graves disease correlates directly with the likelihood of neonatal Graves disease in the newborn ( ). In nondiabetic individuals with ICA, higher ICA titers do predict an increased risk for the subsequent development of type 1 diabetes ( ). This is also true for glutamic acid decarboxylase autoantibody (GADA). In latent autoimmune diabetes of adulthood (LADA—a late-onset form of type 1 diabetes), higher GADA levels predict the need for earlier insulin treatment, which is beneficial ( ). Rarely, the autoantibody concentration provides information differentiating two diseases. In typical cases of type 1 diabetes, the GADA titer is only mildly to modestly elevated above the cutoff; however, in type 1 diabetes that is part of stiff-person syndrome, the GADA titer is much higher ( ).
As opposed to autoantibody concentrations near the cutoff value, higher concentrations of an autoantibody ensure that the autoantibody is truly present. Also, when studying an antibody that can be induced by an environmental exposure (such as insulin), the assay specificity should be considered. For example, the insulin autoantibody (IAA) assay does not discriminate autoantibodies from antibodies against exogenous insulin ( ). Exogenous-insulin-induced antibodies are typically in much higher concentration than spontaneous IAAs that predict type 1 diabetes. Thus if the patient has been exposed to insulin for more than 14 days with the development of insulin antibodies, IAA should not be determined as an index of islet autoimmunity. Likewise, because therapy-induced insulin antibodies bind insulin and can raise the measured insulin concentration, total insulin measurements in insulin-treated individuals do not reflect the biologically active insulin ( ). In short, in insulin-treated patients, total insulin measurements do not reflect either the amount of secreted or injected insulin. This becomes an issue in insulin-treated patients who are (1) hyperglycemic and noncompliant with insulin injections suspected or (2) hypoglycemic and excessive insulin administration is suspected. In such cases, C-peptide can be measured to assess endogenous insulin secretion.
As opposed to these examples where the autoantibody concentration influences the prediction, clinical course, or therapy of the disease, in other circumstances the degree of autoantibody positivity appears to be irrelevant. For example, it is highly controversial whether the persistence of GADA after diagnosis relates to beta cell function following the diagnosis of type 1 diabetes ( ). Whereas generalizations are subject to many exceptions, in most autoimmune diseases the detection of an autoantibody is more important than the concentration of the autoantibody when that concentration is above the threshold of positivity. Consistent results require strict attention to the principles of quality assurance and quality control.
To investigate autoimmune diseases, both in the clinical diagnostic setting and for research purposes, the ability to analyze biological samples for a myriad of potential autoantibodies, antigens, and other biomarkers represents a significant advance ( ; ; ). The diagnostics revolution in developing new autoantibody immunoassays also involves the identification of new autoantigens in each of the autoimmune diseases discussed in this chapter. Candidate autoantigens can be identified by Western blotting, two-dimensional electrophoresis, mass spectroscopy, automated immunoprecipitation of cell or tissue extracts with sera from affected individuals, proteomic approaches, and immunoassays ( ).
Traditional noncompetitive or “sandwich” immunoassays detect a single analyte; assays that detect multiple analytes (antigens or antibodies) in a single reaction are termed multiplex assays ( ). Fluorescence is a common label in multiplex assays. Hundreds if not thousands of potential purified autoantigens can be investigated by the application of patient and control sera separately or in competition with one another.
In the microsphere assay , antibodies are linked to microspheres. A case in point is the BioPlex 2200 Multiplex Testing System ( ; http://www.bio-rad.com/en-us/category/bioplex-2200-multiplex-testing?ID=a82cd166-67ca-4696-91a8-a98a550936f2 ). This utilizes the Luminex xMap technology. The 8-μM microspheres are labeled with different fluorophores, and antibodies are coupled to the microspheres. Every antibody to a different antigenic target is covalently attached to a bead with a unique fluorescence emission wavelength.
The beads are first incubated with a serum sample. The beads are washed and are then incubated with biotin-labeled antibodies against all the antigenic targets. After another wash, the beads are incubated with the reporter streptavidin-phycoerythrin conjugate ( ; http://www.bio-rad.com/en-us/category/bioplex-2200-multiplex-testing?ID=a82cd166-67ca-4696-91a8-a98a550936f2 ). Phycoerythrin is a fluorescent pigment-protein complex. A “sandwich” is generated that is composed of the bead with attached antibody + antigen + biotinylated antigen-specific antibody + streptavidin-phycoerythrin. Analysis is by flow cytometry. The beads are run through a flow cell where analysis is achieved by two lasers: the “classification” laser (635 nm excitation), for identifying the beads, and the “reporter” laser (532 nm excitation), for detecting the target (BioPlex 2200 Multiplex Testing, http://www.bio-rad.com/en-us/category/bioplex-2200-multiplex-testing?ID=a82cd166-67ca-4696-91a8-a98a550936f2 ). The system detector performs a minimum of 150 measurements per analyte at the rate of 100,000 measurements per minute.
The CytoBead test system (GA Generic Assays GmbH) ( , ) uses an IIF reaction on cells in one reaction compartment and multiplex microbead immunoassays in another compartment. For example, the CytoBead ANCA ( ) employs ethanol-fixed human neutrophils and in a separate section fluorescent microbeads coated with recombinant antigens PR3 or MPO. The AKLIDES is an automated digital IIF system ( ) for standardized immunofluorescence (IF) imaging and data processing. The system uses a sequential, multistage process, including image acquisition by a charge-coupled device (CCD) camera and software-controlled quality control.
The Luciferase Immunoprecipitation System (LIPS) ( , ) is a versatile technique that has been adapted to antibody identification. Antigens are expressed in COS1 cells as recombinant luciferase-antigen fusions. Specifically, the gene encoding luciferase (Ruc), from the coral Renilla reniformis , is fused in-frame with a variety of recombinant protein targets ( ). These can be full-length proteins or protein fragments ( ). The latter are reacted with the test sera; if the sera contain antibodies to the antigen, the immunoglobulins will complex with the fusion protein. Protein A/G beads are used to capture IgG molecules that have bound the luciferase-antigen. Addition of a luciferase substrate generates a light signal that permits antibody quantitation.
A completely different technique for analyzing biomarkers is a form of mass spectroscopy called multiple reaction monitoring (MRM) (also referred to as selected reaction monitoring ) ( ; ). This has been adapted to the analysis of proteins in plasma; MRM is especially useful in quantification of specific proteins within highly complex biological mixtures. Unique peptides representing proteins of interest are predefined together with their fragment ions ( ). Such peptides are termed proteotypic peptides . In this procedure, three quadrupole mass spectrometers are linked in tandem. The first quadrupole selects the precursor ion of interest. Fragmentation occurs in the second quadrupole, usually by collision with a neutral gas ( ; ). The third quadrupole then analyzes the fragments. Stable synthetic isotope ( 13 C or 15 N)–labeled internal standard peptides are the key to quantification. This is achieved by comparing labeled to unlabeled peak areas. 13 C/ 15 N standards are otherwise identical to the unlabeled peptide and are distinguishable by m/z only. The overall technique is sometimes referred to as stable isotope dilution (SID) selected reaction monitoring multiple reaction monitoring or SID-MRM-MS analysis ( ). Each fragment ion from an analyte needs to be sampled for only a few milliseconds to obtain interpretable spectra. More than 100 precursor product ion pairs (referred to as transitions) can thus be recorded per second in MRM ( ). To apply this technique after extraction, proteins are digested into peptides. Internal standards are then added. In some cases, depletion of abundant proteins using immunoaffinity absorption may be additionally used.
Phage immunoprecipitation sequencing (PhIP seq) uses the T7 bacteriophage, which is designed to express over 400,000 overlapping peptides of all known human protein sequences ( ). The engineered phage is called a T7 peptidome phage display library (T7-Pep). The T7-Pep library is mixed with patient samples (e.g., cerebrospinal fluid). Autoantibodies present in the biological specimen will bind to the phage-expressed protein. These antibodies and bound phage are captured on a solid phase system. In this way, deoxyribonucleic acid (DNA) from the immunoprecipitated phage can be isolated and sequenced, permitting identification of the antigen to which the autoantibody binds.
The answer to the question “Can autoantibodies predict disease?” is dependent on the specific disease under review. In the proper circumstances, the detection of an autoantibody can predict the subsequent development of disease ( ). This is most evident in the current research effort to prevent type 1 diabetes ( ; ). In persons with multiple islet autoantibodies (e.g., three or more), there is conservatively more than a 50% risk of developing type 1 diabetes over the span of 5 to 10 years of follow-up. If there is evidence of beta cell dysfunction with even one islet autoantibody (e.g., ICA), the 10-year risk for type 1 diabetes approaches 100%.
There are many other autoimmune diseases where autoantibody positivity predicts disease ( ). This has been extensively studied in the organ-specific endocrinopathies of AITD and Addison disease developing in persons with type 1 diabetes who are positive, respectively, for thyroid autoantibodies (e.g., TPOA) or adrenal cytoplasmic autoantibodies (ACA) ( ; ). Likewise, gastric parietal cell autoantibodies (PCA) in persons with type 1 diabetes predict the development of PA ( ).
If autoantibodies can predict certain diseases, is it prudent that we screen the general population for various organ-specific autoantibodies? The answer to this question is complex. The answer depends on the following factors:
Can the disease be prevented, or does early detection of disease only allow earlier treatment of disease?
How common is the autoimmune disease?
What is the cost of treatment versus the cost of screening?
Does screening actually change outcome?
What is the morbid burden to the individual and society of delayed diagnosis?
Considering these issues there are few circumstances where screening for autoimmune diseases should be undertaken in the absence of a preexisting autoimmune disease or a family history of autoimmune disease.
In the case of type 1 diabetes, because of the high prevalence of associated AITD, PA, and Addison disease (which if untreated can be rapidly fatal), it is recommended that persons with type 1 diabetes be screened for thyroid autoimmunity with TPOA testing, chronic lymphocytic gastritis (the most common cause of PA) with gastric PCA, and adrenal autoimmunity with adrenocortical cytoplasmic autoantibodies (ACA) or 21-hydroxylase autoantibodies ( ). If a person is autoantibody positive, periodic testing would follow: In TPOA-positive individuals, TSH is measured yearly; in PCA-positive individuals, vitamin B 12 and ferritin are measured yearly; and in ACA-positive individuals, yearly cosyntropin stimulation testing is performed together with supine renin measurements ( ). The prediction of Addison disease is likely the most important screening to be undertaken because Addisonian crisis can be fatal ( ).
Because some autoimmune diseases are strongly familial, it may be beneficial to perform autoantibody screening in first-degree relatives of affected individuals. The best example of this situation is thyrogastric autoimmunity. AITD is strongly familial and is common. Although no single gene causes AITD, it appears to be inherited in a dominant fashion. If the proband has Hashimoto thyroiditis or Graves disease, their first-degree relatives can be screened for TPOA to anticipate AITD. If TPOA were negative, thyroglobulin autoantibodies (TGA) could be sought, but TGA testing is less sensitive that TPOA testing. TPOA-positive or TGA-positive relatives would then have yearly thyroid function testing, including, at a minimum, the measurement of TSH. Gastric autoimmunity (e.g., PCA and PA) is commonly associated with AITD, so PCA testing is worthwhile to pursue. If the person is PCA positive, vitamin B 12 and ferritin are measured yearly.
As a frequent target of the immune response, the skin presents us with an excellent visual picture of autoimmune disease pathology. Immunobullous disorders, connective tissue disease, vasculitis, and vitiligo each present with characteristic clinical features. However, diagnosis of many of these disorders often relies on utilization of light microscopy and IF testing. Direct immunofluorescence (DIF) and immunoassays are methods used to help establish a diagnosis and are especially critical for classifying the histologically similar autoimmune bullous diseases. Examination of the pattern of immune complex deposition at various locations within the skin may be specific for a particular disorder or may need to be interpreted within the setting of its clinical and histologic findings ( Box 55.4 ). Rapid and definitive differentiation of these autoimmune skin diseases is important as the disorders can differ in their prognosis and treatment decisions.
Pemphigus vulgaris
Pemphigus foliaceus
Paraneoplastic pemphigus
IgA pemphigus
Bullous pemphigoid
Mucous membrane pemphigoid
Pemphigoid gestationis
Epidermolysis bullosa acquisita
Bullous lupus erythematosus
Linear IgA dermatosis
Lupus erythematosus
Dermatitis herpetiformis
Vasculitis
The autoimmune bullous disorders are a heterogeneous group of mucocutaneous blistering diseases characterized by autoantibodies to specific adhesion proteins of the epidermis and dermoepidermal junction ( Table 55.1 ). Classification of the blister location as intraepidermal or subepidermal divides the immunobullous disorders into the pemphigus and pemphigoid group, respectively. The pemphigus group is associated with antibodies against epidermal desmosomal proteins and intercellular immune deposits between keratinocytes. In pemphigoid, the hemidesmosomal complex and basement membrane zone (BMZ) is targeted. Because there can be considerable overlap in the clinical and histologic features of these blistering disorders, accurate diagnosis of an autoimmune bullous disease requires the detection of tissue-bound and circulating autoantibodies. The detection of circulating autoantibodies can be made by different tests, including IIF, ELISA, immunoblot, and immunoprecipitation. In addition to help with diagnosis, ELISA is also being used to monitor disease activity.
| Diagnosis | Blister Site/Ultrastructural Location | Autoantigen | DIF | IIF |
|---|---|---|---|---|
| Intraepidermal | ||||
| Pemphigus vulgaris | Suprabasal | Dsg 3, Dsg 1 | Intercellular IgG and C3 | Intercellular IgG (monkey esophagus) |
| Pemphigus foliaceus | Subcorneal or within granular layer | Dsg 1 | Intercellular IgG and C3 | Intercellular IgG (guinea pig esophagus or lip) |
| Paraneoplastic pemphigus | Suprabasal | Dsg 3, Dsg 1, plakin family (desmoplakin 1 and 2, envoplakin, periplakin, BP230, plectin, A2ML1) | Intercellular IgG +/− C3 and at DEJ | Intercellular IgG (rat bladder) |
| IgA pemphigus | Subcorneal | Desmocollin 1, Dsg 3 | Intercellular IgA | Intercellular IgA |
| Subepidermal | ||||
| Bullous pemphigoid | Hemidesmosome | BP180, BP230 | Linear IgG and C3 at BMZ | IgG at BMZ SS: epidermal |
| Pemphigoid gestationis | Hemidesmosome | BP180, BP230 | Linear C3 at BMZ | |
| Mucous membrane pemphigoid | Lamina lucida | BP180, laminin 332, α6β4 integrin, laminin 311, BP230 | Linear IgG, C3, and IgA at BMZ | IgG at BMZ SS: epidermal or mixed SS: dermal laminin 332 |
| Epidermolysis bullosa acquisita | Anchoring fibrils | Type VII collagen | Linear IgG and C3 at BMZ, +/− IgA and IgM | IgG at BMZ SS: dermal |
| Linear IgA dermatosis | Lamina lucida | LAD-1 | Linear IgA at BMZ, +/− C3 | IgA at BMZ SS: epidermal, dermal, or mixed |
| Dermatitis herpetiformis | Papillary dermis | Epidermal transglutaminase | Granular IgA at tips of dermal papillae, +/− C3 | IgA endomysial antibody (monkey esophagus) |
For skin biopsies, site selection and technique are important considerations in the interpretation of histologic and IF results. For blistering disorders, biopsy for DIF should be taken from nonbullous lesional, erythematous skin or uninvolved perilesional skin about 1 cm from a blister ( ). Blistered, ulcerated, and uninvolved skin are associated with false-negative results, and it is recommended that biopsies be taken from above the waist if possible ( ). The DIF specimen should not be placed in formalin. Michel or Zeus medium is recommended for preservation; however, some laboratories may require fresh specimens be received during working hours ( ). The biopsy for light microscopy should include an intact vesicle or the edge of a larger bulla. That specimen should include both intact and blistered skin.
Pemphigus describes a rare group of potentially life-threatening, mucocutaneous blistering diseases characterized by the production of antibodies to epidermal adhesion molecules, resulting in loss of cellular cohesion between keratinocytes (acantholysis) and intraepidermal bullae formation ( ). This intraepidermal acantholysis is considered the histologic hallmark of the pemphigus group and is responsible for the clinical presentation of flaccid blisters and erosions ( ). Through the direct interference in desmosomal function, pathogenic autoantibodies to desmoglein 3 (Dsg3) and/or Dsg1 on epidermal keratinocytes play an active role in disease activity ( ). Four major variants have been described in this group and include pemphigus vulgaris (PV), pemphigus foliaceus (PF), paraneoplastic pemphigus (PNP), and IgA pemphigus. Review of their clinical, histopathologic, and immunopathologic findings may provide differentiating features that are helpful in the diagnosis of the pemphigus subtype ( ; ).
PV is the most common variant of pemphigus. The mean age of disease onset is between 40 and 60 years of age, with no gender bias ( ; ). Dsg1 and Dsg3 are the target autoantigens for circulating IgG autoantibodies in this disease. Because of differences in the expression of the desmogleins in cutaneous and mucosal epithelium, patients may present with one of two clinical variants. For example, an anti-Dsg3 immune profile will present with mucosal-dominant disease, but the presence of antibodies to both Dsg1 and Dsg3 will result in the mucocutaneous phenotype ( ).
Patients with PV often present with painful oral mucosal lesions as their first manifestation of disease, and it is the most frequent site of involvement. Skin involvement classically consists of flaccid bullae that rupture easily and multiple painful erosions. Skin lesions may present on any part of the body, but the scalp, head, neck, and flexural surfaces are more favored sites. There is a positive Nikolsky sign in perilesional skin in both PV and pemphigus foliaceus. In the Nikolsky sign, when the skin is slightly rubbed, the top layers of skin slip apart from the lower layers. Of PV cases, 5% to 10% are fatal, and morbidity/mortality is related to the extent of disease and the complications from immunosuppressive treatment ( ).
The intraepidermal acantholysis that characterizes the histology of the pemphigus group of blistering disorders is found in a classic suprabasalar location. Cleavage above the basal layer keratinocytes has been said to resemble a “row of tombstones” sitting on the basement membrane.
DIF evaluation reveals an intercellular pattern of IgG deposition and C3 characteristics of all types of pemphigus, with the exception of IgA pemphigus ( Fig. 55.5 ). Examination of perilesional skin in PV reveals linear and granular staining. This pattern may be described as “chicken wire” because of the hexagonal mesh outline created by the continuous deposition around individual keratinocytes ( Fig. 55.6 ). Increased intensity of the staining pattern may be seen at the level of blister formation within the affected skin (i.e., lower epidermal for PV and superficial layers for pemphigus foliaceus) ( ). The basement membrane zone does not stain in PV patients. Essentially, all patients with PV display IgG staining, whereas complement staining varies between 50% and 100% of cases. IgM or IgA staining is observed in 30% to 50% of cases of pemphigus.
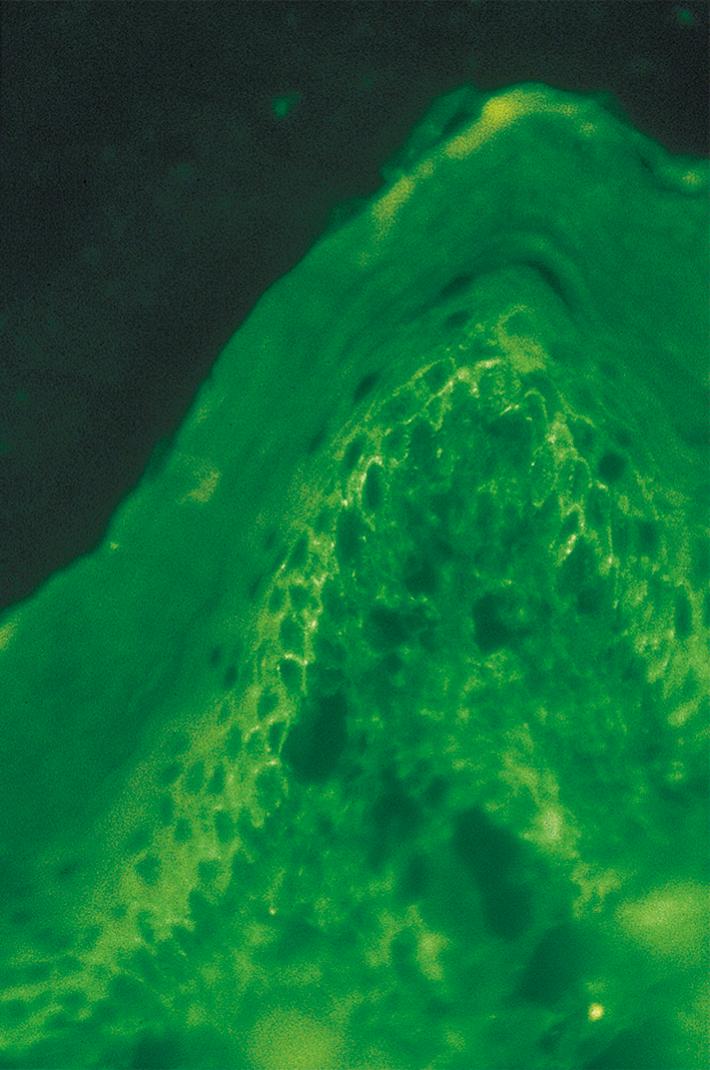
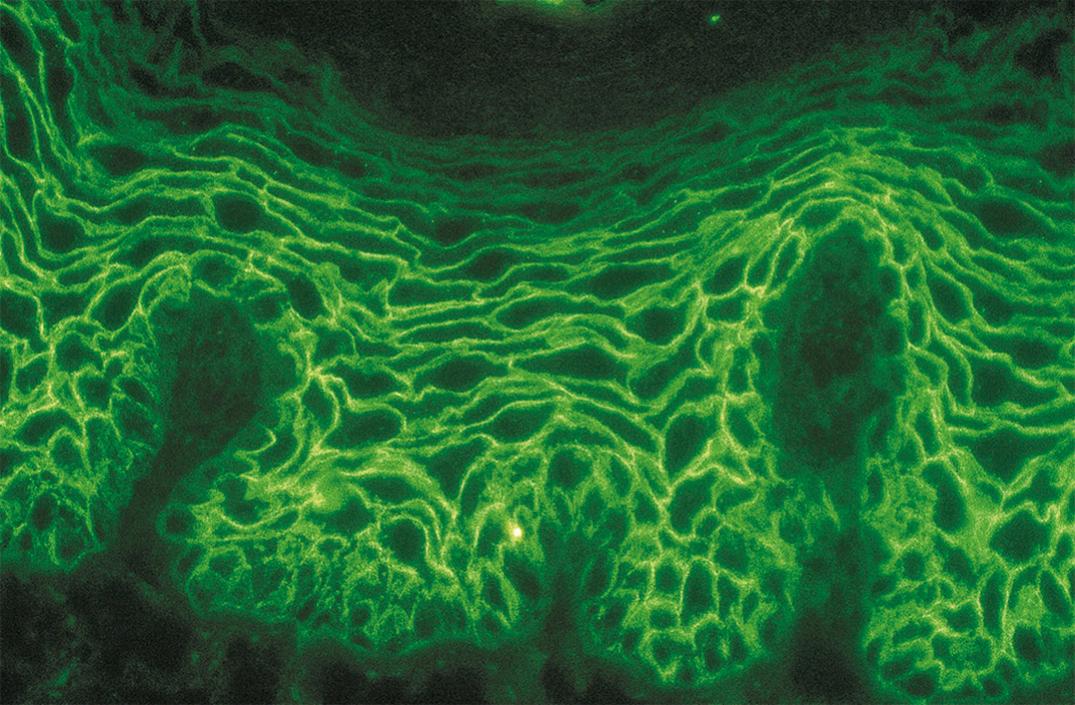
Circulating IgG autoantibodies are detected on the surfaces of the squamous epithelial cells by IIF. For all investigations by IIF, the appropriate choice of tissue substrate is important because different substrates exhibit different autoantigens ( ). Monkey esophagus is the tissue of choice when testing for PV. On the other hand, guinea pig esophagus is the tissue of choice in cases of pemphigus foliaceus. Reference laboratories offer tests such as “Cutaneous Immunofluorescence Antibodies (IgG), Serum” (see http://www.mayomedicallaboratories.com/test-catalog/Overview/8052 ).
Reference laboratories provide ELISA-based testing for anti-Dsg1 and anti-Dsg3 autoantibodies. One reference laboratory provides a helpful interpretive summary of skin IF testing (see http://www.mayomedicallaboratories.com/test-catalog/Overview/83680 ).
PF is characterized clinically by small and superficial flaccid blisters that immediately rupture, evolving into crusted erosions found in a seborrheic distribution (upper torso, scalp, and face). In contrast to PV, there is no mucosal involvement. Antibodies to Dsg1 are responsible for the findings in PF. Although PF is considered to have a less severe clinical course, the eruption still requires treatment with prolonged systemic immunosuppressive therapies ( ). The serologic hallmark of PF is the demonstration of IgG autoantibodies against Dsg1, and serum levels have been found to correlate with disease severity ( ). Fogo selvagem (endemic pemphigus) and pemphigus erythematosus (Senear-Usher syndrome) are two distinct clinical variants within PF.
Compared to PV, the epidermal split in foliaceus lesions is found in the most superficial layers of the epidermis. Acantholysis can be found within the granular layer or in a subcorneal location and may result in loss of the stratum corneum. The differences in intraepidermal blister location correlate with the differences in desmoglein expression within the skin between the pemphigus subtypes. Eosinophilic spongiosis has been reported in some cases of PF. Spongiosis is the descriptive term for increased intercellular fluid in the epidermis (e.g., intercellular edema) that separates keratinocytes from one another, highlighting their desmosomal connections.
DIF of biopsy tissue will show the same intercellular pattern of fluorescence as described in PV. Guinea pig esophagus is recommended as the most sensitive IIF substrate, and testing should detect circulating IgG autoantibodies to the epithelial cell surface in 90% of specimens ( ). In addition, serologic titers reported in PV and PF may correlate with disease activity and treatment response ( ).
PNP is a unique form of pemphigus that is characterized by a refractory stomatitis and an association with an underlying malignancy. Non-Hodgkin lymphoma, chronic lymphocytic leukemia, Castleman disease, and thymoma are the most commonly reported neoplasms ( ). In addition to painful mucosal erosions, a polymorphic skin eruption and internal organ involvement are possible (Hertl et al., 2014). The prognosis of the mucocutaneous disease is linked to the presence of the associated neoplasm. In addition to the clinical distinctions from classic pemphigus, the autoantibody profile is more diverse and includes antibodies to the desmogleins and plakin family proteins (i.e., envoplakin, periplakin, plectin, desmoplakin 1 and 2) ( ).
The histopathologic features in PNP combine the acantholysis characteristic of pemphigus with changes of interface dermatitis with necrotic keratinocytes. Antibodies in PNP are directed against both desmosomal and hemidesmosomal proteins and explain why DIF shows immunoreactivity within the epidermis and at the BMZ. BMZ deposition of IgG and complement can be found in a linear or granular pattern along the dermoepidermal junction. If the histologic features and DIF findings suggest the diagnosis of PNP, IIF studies should be pursued. IIF testing using rat bladder epithelium is considered the best screening test for PNP ( ). Additional laboratory tests such as immunoprecipitation, immunoblotting, and ELISA are helpful in confirming the diagnosis after initial workup.
The hallmark of IgA pemphigus is the intercellular deposition of IgA as the target antigen is present in desmosomes. Neutrophil infiltration follows the presence of tissue-bound IgA and results in epidermal acantholysis and vesiculation (Zone et al., 2004).
Subcorneal acantholysis with intraepithelial neutrophilic collections characterize IgA pemphigus histopathology. DIF will show that intercellular IgA deposits are present within the epidermis in a typical pemphigus pattern. Positive IIF for circulating IgA is found in 50% of patients ( ).
The pemphigoid group of autoimmune bullous disorders is characterized by subepidermal blister formation due to the loss of adhesion between the basal layer keratinocytes and the underlying basement membrane. Autoantibodies are directed at one or more structural proteins found in the hemidesmosome, lamina lucida, lamina densa, or anchoring fibrils that make up the BMZ. This group of disorders includes bullous pemphigoid (BP), pemphigoid gestationis (PG), mucous membrane pemphigoid (MMP), and linear IgA dermatosis (LAD).
In addition to the pemphigoid group, epidermolysis bullosa acquisita (EBA) and dermatitis herpetiformis (DH) are other immunobullous disorders that share the histologic hallmark of subepidermal blister formation. Because of clinical and histologic overlap among these disorders, IF studies can play a critical role in the distinction among disorders (see Table 55.1 ). The SSS technique is an additional test that can be used in the evaluation of the subepidermal blistering disorders to increase the sensitivity of antibody detection and gain further information on the autoantibody binding site ( ; ).
BP is the most common type of autoimmune bullous disorder, and it occurs most frequently in the elderly. Clinically, BP patients present with urticarial lesions and tense blisters on the trunk and extremities, with associated pruritus. Mucosal involvement is uncommon and is reported in 10% to 30% of BP patients ( ). The Nikolsky sign is negative in BP.
In BP, autoantibodies target the two hemidesmosomal proteins: bullous pemphigoid antigen 1 (BPAg1 [BP230]) and bullous pemphigoid antigen 2 (BPAg2 [BP180]). BPAg2, also known as type XVII collagen, is a transmembrane protein that is determined to be the target autoantigen for pathogenic autoantibodies in BP ( ). In it is unclear if autoantibodies to BPAg1, a cytoplasmic plakin family protein, relate to the pathogenesis of BP ( ). Complement activation is believed to follow autoantibody binding, causing the physical loss of adhesion between the basal cell layer and the basement membrane ( ).
A subepidermal blister with a superficial dermal inflammatory infiltrate, including numerous eosinophils, describes the typical histopathologic findings in bullous lesions of BP ( Fig. 55.7A ). Eosinophilic spongiosis may serve as a clue in early lesions ( ). Neutrophil predominant and cell-poor variants also exist and may pose a diagnostic challenge.
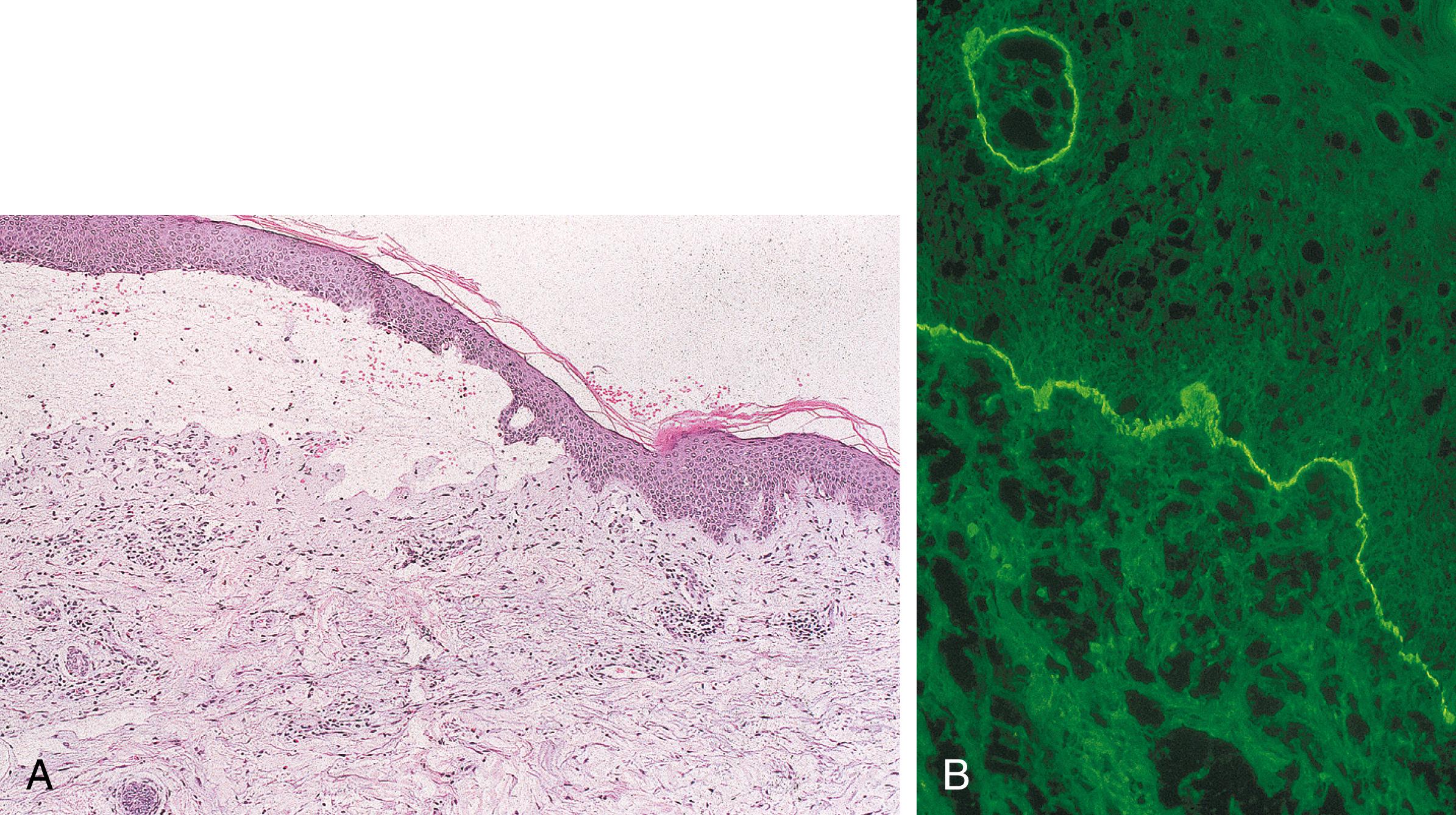
DIF of perilesional skin reveals linear IgG and/or C3 deposition at the BMZ in BP, PG, and MMP (see Fig. 55.7B ). The finding of C3 deposition with a stronger intensity than IgG favors a pemphigoid group disease ( ). Complement may sometimes be found as the sole immunoreactant in BP, but more often in PG. IgA and/or IgM staining can also be present but will stain with less intensity. IIF shows circulating IgG anti-BMZ autoantibodies in 75% of patients ( ).
Serum IgG autoantibodies to BPAg1 and BPAg2 can be detected using ELISA testing. The sensitivity of ELISA BPAg1 autoantibodies in cases of BP is approximately 60%, and the sensitivity of ELISA BPAg2 autoantibodies for BP is approximately 80% ( ).
PG, previously known as herpes gestationis, is considered to be a form of BP that develops during pregnancy. PG usually develops in the second or third trimester and typically resolves within weeks to months after delivery. Because this is a humoral autoimmune disease, the newborn can be transiently affected through passive maternal transfer of IgG autoantibodies ( ).
Linear deposits of C3 are present in 100% of PG patients with IgG deposits found less frequently in 30% to 40% of cases. IIF detects anti-BMZ autoantibodies in only 10% to 20% of patients ( ).
MMP, also known as cicatricial pemphigoid, is a rare, chronic variant of pemphigoid characterized by predominant mucous membrane disease. Erosions, blisters, and scarring may involve oral, conjunctival, nasal, laryngeal, esophageal, and anogenital mucosa. Lesions often heal with scarring, which can interfere with the normal function of the affected area, producing significant morbidity. For example, ocular cicatricial pemphigoid presents with progressive subconjunctival scarring that leads to decreased vision and eventual blindness ( ).
Several target autoantigens have been identified with the recognition of a few clinically important variants. MMP with autoantibodies against laminin 332 is clinically indistinguishable from other forms of MMP but is associated with an increased risk of internal malignancy ( ).
Perilesional skin and mucosal biopsies reveal continuous IgG and C3 deposits, with occasional IgA present along the basement membrane by DIF. IIF studies are usually negative. Because the numerous target autoantigens in MMP vary in their location within the BMZ, the site of autoantibody binding on SSS microscopic exam may be epidermal, dermal, or both. IIF SSS studies do increase the sensitivity of detection and can be positive in approximately 80% of cases of MMP.
LAD is a rare subepidermal blistering disease due to IgA autoantibodies directed at autoantigens in the BMZ. Activated complement and neutrophil infiltration result in the disruption of the dermoepidermal attachments and blister formation. The clinical lesions of LAD can be polymorphic, but annular patterns of tense vesicles or blisters are classically described. Most cases of LAD are considered idiopathic, but drug-induced cases are reported. Chronic bullous dermatosis of childhood is considered a childhood form of linear IgA dermatosis.
The strong presence of IgA linear deposition along the BMZ is characteristic of LAD ( ). These features help separate it from the DIF findings in DH. In addition, only LAD shows IgA anti-BMZ autoantibodies by IIF examination ( ).
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here