Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The anterior and posterior lobes of the pituitary gland control processes vital for survival of the individual and the species. Although growth in infancy and childhood depend on nutrition, genetics, and environment, thyroid hormone and growth hormone (GH) are essential contributors to growth. Thyroid hormone is a master regulator of the metabolic rate and neurologic development in utero, in infancy, and in childhood. The stress response requires the participation of cortisol. Together, cortisol and GH help maintain normal plasma glucose levels. Although prolactin is also a stress hormone, its role in lactation is evolutionarily required for the nutrition, hydration, and survival of the newborn and infant. The survival of the species is dependent upon reproduction and the gonadotropins, which regulate spermatogenesis and ovulation beginning during puberty. The posterior pituitary is no less important than the anterior pituitary. Antidiuretic hormone (ADH) is a key regulator of water balance. Because humans are 60% water (and infants and children have proportionately more total body water than adults), maintenance of intracellular and extracellular volumes is necessary for health and survival. Lastly, oxytocin is involved in breast feeding and parturition. Collectively, the most complex endocrine systems involve the cerebral cortex, hypothalamus, anterior and posterior lobes of the pituitary, pituitary hormones, and target organs and tissues.
This chapter focuses on disorders of the anterior and posterior pituitary that produce deficient or excess hormone activity. In some instances, more specific details are provided in other chapters that concern specific hormone systems. By necessity this chapter provides specific numerical values for reference intervals and diagnostic cut-offs. However, decision thresholds, physiologic ranges, and reference intervals provided in this chapter serve as a general guide. Laboratories should verify that these ranges are appropriate for use in their own settings because values may vary depending on methodologies and other factors.
The pituitary gland (also called the hypophysis) regulates the endocrine system by integrating chemical signals from the brain with feedback from the concentration of circulating hormones to stimulate intermittent hormone release from target endocrine glands. , The pituitary serves as the master gland in maintaining homeostasis by orchestrating the many processes necessary for survival. There are also many important endocrine systems operating independently of the pituitary gland, such as the renin-angiotensin-aldosterone system (RAAS), the calcium-parathyroid axis, and the glucose-insulin axis. Each of these systems and/or axes is far more complex than their simple names. For example, maintenance of normal plasma glucose concentrations involves multiple cells, tissues and organs (e.g., the islets of Langerhans [β, α, and δ cells], liver, adipose tissue, muscle, and intestine), hormones (e.g., insulin, glucagon, growth hormone [GH], cortisol, somatostatin, and the incretins), and various physiologic and biochemical events (e.g., nutrient absorption, glycolysis, glycogen synthesis, gluconeogenesis, glycogenolysis, lipid, and protein metabolism).
The hypophysis is composed of the adenohypophysis (the anterior lobe of the pituitary; ≈75% of the pituitary mass) and the neurohypophysis (the posterior lobe of the pituitary, ≈25% of the pituitary mass—also called the pars nervosa) ( Fig. 55.1 ). In turn, the adenohypophysis has three parts: (1) the pars distalis, where most hormone-producing cells are located; (2) the pars tuberalis, which is part of the hypophyseal stalk; and (3) the pars intermedia. The pars intermedia may be referred to as the intermediate lobe of the pituitary, although it is actually part of the adenohypophysis.
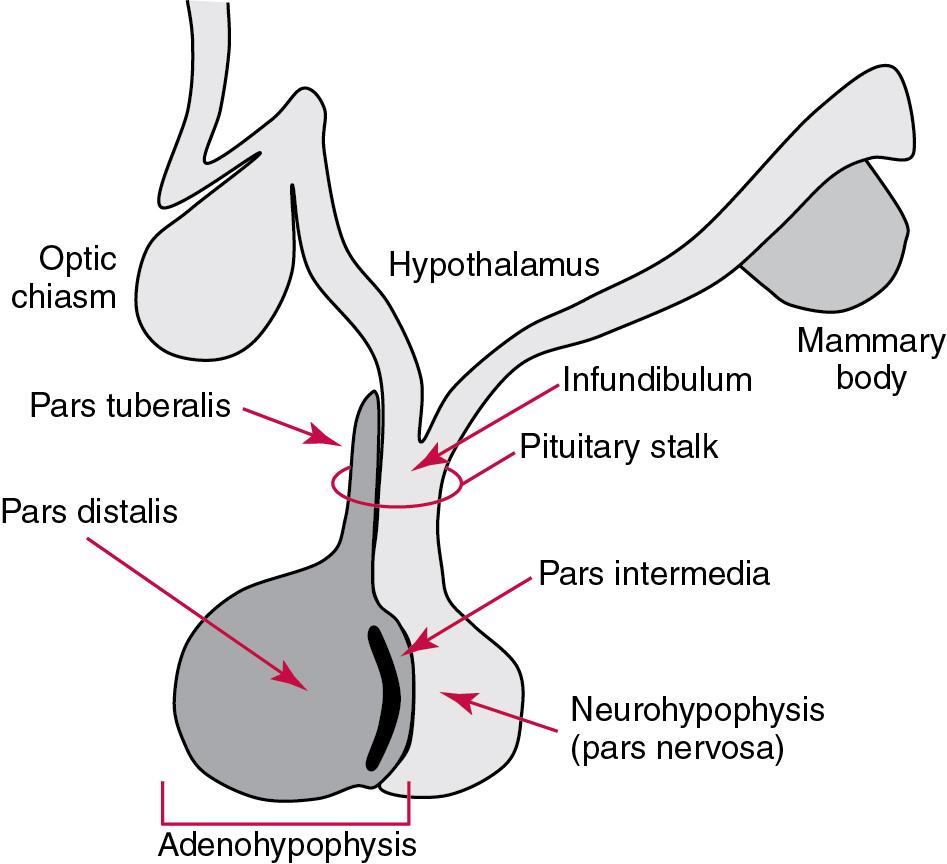
The biology of the adenohypophysis is distinctly different from that of the neurohypophysis; the adenohypophysis is controlled by the hypothalamus via releasing or inhibiting hormones, whereas the cell bodies of the neurohypophysis are anatomically located in hypothalamic nuclei, with oxytocin or ADH reaching the neurohypophysis through neurohypophyseal nerve axons. Thus the neurohypophysis is not a discrete endocrine organ, but rather functions as a reservoir for these two hormones.
The roles of the various hormones secreted by the pituitary are exceedingly diverse and include regulation of (1) the body’s response to stress (adrenocorticotropic hormone [ACTH or corticotropin] and GH), (2) the metabolic rate (thyroid-stimulating hormone [TSH or thyrotropin]), (3) growth (TSH and GH), (4) reproduction (luteinizing hormone [LH] and follicle-stimulating hormone [FSH]), (5) nourishment for the newborn and infant (prolactin), (6) parturition and milk letdown during breast feeding (oxytocin), and (7) fluid balance and blood pressure regulation in states of stress (ADH, or vasopressin, and cortisol). Some of the pituitary hormones have specific targets (e.g., ACTH, TSH, LH, or FSH), whereas other hormones have multiple targets (e.g., GH, prolactin, oxytocin, and ADH). The action of the various hormones is dependent on the expression of the pertinent receptor in the target cell and associated second messenger systems.
A newly recognized product of the pituitary gland detected in some peri- and postmenopausal women is human chorionic gonadotropin (hCG). Usually, hCG is associated with pregnancy or gestational trophoblastic disease. In early pregnancy, hCG doubles approximately every 48 hours, whereas the concentration of hCG originating from the pituitary or gestational trophoblastic disease is relatively stable and does not increase in concentration in the pattern seen in pregnancy. If an elevated hCG of 5 to 14 IU/L is detected in a postmenopausal woman, it is likely to be of pituitary origin if (1) the FSH is elevated (>45 IU/L), (2) the hCG is suppressed after 2 weeks of estrogen replacement, and (3) gestational trophoblastic disease has been excluded.
The placenta produces several hormones similar to pituitary hormones: hCG has functional and structural similarity to LH; human placental lactogen (hPL, or somatomammotropin) has actions similar to prolactin and GH; placental GH becomes the predominant maternal GH during gestation; and placental corticotropin-releasing hormone (CRH) concentration rises in the fetus throughout gestation. Placental GH (GH-V) differs from pituitary GH in 13 of 191 amino acids. Furthermore, GH-V exists in glycosylated and nonglycosylated forms, whereas pituitary GH is not glycosylated.
The pituitary is located at the base of the brain and is protected anteriorly, inferiorly, and posteriorly by a depression in the sphenoid bone called the sella turcica. Inferior and anterior to the sella is the sphenoid sinus, which communicates with the nasopharynx. Neurosurgeons take advantage of the proximity of the sphenoid sinus to the pituitary using it as the preferred surgical access to the pituitary.
The pituitary weighs only 0.5 to 0.6 g. The gland is larger in women than in men; its size increases during pregnancy, and it is larger in multiparous women. At the completion of pregnancy, when the pituitary is largest, it is susceptible to infarction if hypovolemic shock develops from postpartum hemorrhage, producing a state of postpartum panhypopituitarism called Sheehan syndrome.
If the pituitary is greatly reduced in size or is apparently absent on magnetic resonance imaging (MRI) studies, the sella is said to be “empty.” In the empty sella syndrome, the sella may be normal in size or enlarged. An incompetent diaphragma sella with compression of the pituitary gland by a herniating arachnoid can cause an empty sella, or the pituitary may be reduced in size as the result of previous apoplexy (i.e., spontaneous ischemic or hemorrhagic infarction) of a tumor, radiotherapy, or surgery.
Arterial blood is supplied to the pituitary via the superior and inferior hypophyseal arteries, both of which are branches of the internal carotid arteries. The superior hypophyseal arteries supply the anterior pituitary and hypophyseal stalk, whereas the inferior hypophyseal arteries supply the posterior pituitary.
Direct delivery of hypothalamic regulatory hormones to the adenohypophysis occurs through the hypothalamic-pituitary portal system, surrounding the adenohypophysis (pars distalis). A portal system is a vascular apparatus in which blood that initially passes through one capillary network (e.g. the hypothalamus) is collected into vessels that subsequently supply a second capillary network (e.g., the anterior pituitary). Anatomically, this is similar to the nephron where blood from glomerular capillaries is collected into the efferent arteriole to be distributed again to the peritubular capillaries or vasa recta. In this way, the hypothalamus controls the secretion of adenohypophyseal hormones via delivery of hypothalamic venous blood to the anterior pituitary gland. There is also retrograde flow from the pituitary to the hypothalamus via the portal system.
Pituitary venous drainage is moved to the cavernous and intercavernous sinuses via the lateral hypophyseal veins. The cavernous sinus drains to the superior and inferior petrosal sinuses, which join the transverse sinus to form the jugular vein. This anatomic relationship is clinically important because access to pituitary secretions can be afforded by cannulation of the inferior petrosal venous sinuses. Usually, the neuroradiologist places catheters bilaterally into the femoral veins and passes them via the iliac veins, inferior vena cava, and superior vena cava to the jugular veins to enter the inferior petrosal sinus. Inferior petrosal sinus sampling (IPSS) is used to distinguish Cushing disease (i.e., anterior pituitary corticotroph adenoma) and Cushing syndrome caused by ectopic ACTH production. An important corollary of the plexiform venous drainage of the pituitary is that IPSS cannot be used to determine which side of the pituitary contains a corticotroph adenoma because left-side tumors can have venous drainage to the right and vice versa.
The internal carotid arteries are lateral to the pituitary. Above the pituitary is the diaphragma sellae, which comprises circular (intercavernous) sinuses containing venous blood. Anterior and superior to the pituitary is the optic chiasm. These relations are clinically important because pituitary neoplasms can invade or compress these structures, as well as the sella turcica. For example, superiorly expanding anterior pituitary adenomas can compress the optic chiasm, producing bitemporal hemianopsia, that is bilateral peripheral vision loss.
The adenohypophysis develops in utero from a dorsal evagination of the roof of the stomodeum, which becomes the Rathke pouch. The superior portion of the Rathke pouch constitutes the pars tuberalis (see earlier), whereas the posterior portion of the Rathke pouch develops into the pars intermedia (or intermediate lobe). Transcription factors regulating the development of the anterior pituitary gland include HESX, FGFR1, LHX3, LHX4, SOX3, Pit-1, PROP1, RIEG, and GLI2. Mutations in these transcription factors can cause various types of hypopituitarism, along with other conditions such as septo-optic dysplasia (SOD). , The pars intermedia, which is active only late in pregnancy and in utero, secretes α-, β-, and γ-melanocyte stimulating hormone (MSH), corticotropin-like intermediate lobe peptide, γ-lipotropin, and β-endorphin. MSH is believed to promote melanin synthesis. Lipotropins mobilize fat from adipose tissue, and endorphins are endogenous opioids. The clinical significance of these intermediate lobe products as causes of disease is poorly understood.
The synthesis and release of the following anterior pituitary hormones are stimulated by hypothalamic-releasing hormones: ACTH, TSH, GH, LH, and FSH. Prolactin is the sole anterior pituitary hormone whose release is predominantly regulated through suppression—specifically via dopamine. Corticotrophs secrete ACTH, thyrotrophs secrete TSH, somatotrophs secrete GH, gonadotrophs secrete both LH and FSH, and lactotrophs secrete prolactin. Except for LH and FSH, each hormone is normally produced by a unique cell type. However, some somatotroph adenomas, which cause pituitary gigantism and acromegaly co-secrete prolactin. The molecular composition of the anterior pituitary hormones is summarized in Table 55.1 .
| Hypothalamic Hormone/Abbreviation | Amino Acids | Anterior Pituitary Target Cell | Hormone Regulated | Amino Acids | MW (kDa) |
|---|---|---|---|---|---|
| Corticotropin-releasing hormone (CRH) | 41 | Corticotroph | ACTH | 39 | 4.5 |
| Thyrotropin-releasing hormone (TRH) | 3 | Thyrotroph | TSH a | α: 92 β: 118 |
28 |
| Growth hormone–releasing hormone (GHRH) | 44 | Somatotroph | GH | 191 | 22 |
| 176 | 20 | ||||
| Somatotropin release–inhibiting hormone (SRIH) b | 14 | Somatotroph | GH | 176 | 20 |
| Gonadotropin-releasing hormone (GnRH) | 10 | Gonadotroph | LH a | α: 92 β: 121 |
32 |
| FSH a | α: 92 β: 111 |
30 | |||
| Dopamine | 1 | Lactotroph | Prolactin | 199 | 22 |
a All α-glycoprotein chains are identical, including the α-chain of human chorionic gonadotropin.
Multiple levels of control of the hypothalamic-pituitary-end organ-hormone axis are known ( Fig. 55.2 ). Except for prolactin and LH at the midpoint of the menstrual cycle, negative feedback controls secretion of the adenohypophyseal hormones. The long feedback loop involves suppression of the hypothalamic-releasing hormone and the anterior pituitary trophic hormone by the hormonal product of the target tissue. The major site of negative feedback for cortisol (regulated by ACTH), insulin-like growth factor-I (IGF-I; regulated by GH), and sex steroids and inhibins (regulated by LH and FSH) is the hypothalamus. In contrast, for thyroid hormone (regulated by TSH), the major site of negative feedback is the anterior pituitary. Retrograde flow from the pituitary to the hypothalamus via the portal system permits the existence of short negative feedback loops in which pituitary hormones suppress the secretion of hypothalamic-releasing hormones. Ultra-short feedback loops also exist in which pituitary hormones inhibit their own secretion.
![FIGURE 55.2, Many Anterior Pituitary Trophic Hormones (e.g., Adrenocorticotropic Hormone [ACTH] , Thyroid-Stimulating Hormone [TSH] , Growth Hormone [GH] , Luteinizing Hormone [LH] , Follicle-Stimulating Hormone [FSH] ) Are Regulated by Hypothalamic Releasing Hormones (HRHs) . Releasing hormones secreted by the hypothalamus reach the pituitary via the hypothalamic-pituitary portal system (HPPS) . Long feedback loops involve negative feedback of the target cell hormone at the pituitary gland and hypothalamus. The short feedback loop involves the anterior pituitary trophic hormone feeding back at the hypothalamus, whereas the ultra-short feedback loop involves the anterior pituitary hormone feeding back at the anterior pituitary. [+] , Stimulation; [−] , suppression; TH , trophic hormone. FIGURE 55.2, Many Anterior Pituitary Trophic Hormones (e.g., Adrenocorticotropic Hormone [ACTH] , Thyroid-Stimulating Hormone [TSH] , Growth Hormone [GH] , Luteinizing Hormone [LH] , Follicle-Stimulating Hormone [FSH] ) Are Regulated by Hypothalamic Releasing Hormones (HRHs) . Releasing hormones secreted by the hypothalamus reach the pituitary via the hypothalamic-pituitary portal system (HPPS) . Long feedback loops involve negative feedback of the target cell hormone at the pituitary gland and hypothalamus. The short feedback loop involves the anterior pituitary trophic hormone feeding back at the hypothalamus, whereas the ultra-short feedback loop involves the anterior pituitary hormone feeding back at the anterior pituitary. [+] , Stimulation; [−] , suppression; TH , trophic hormone.](https://storage.googleapis.com/dl.dentistrykey.com/clinical/Pituitaryfunctionandpathophysiology/1_3s20B9780323775724000645.jpg)
Most anterior pituitary hormones are predominantly regulated by stimulatory hypothalamic hormones. The exception is prolactin, which is tonically suppressed by dopamine.
Pulsatile secretion of hypothalamic-releasing hormones is required for normal release of anterior pituitary hormones such as luteinizing hormone and follicle-stimulating hormone.
Either hypo- or hyperfunction can develop from pathologic processes involving the hypothalamus, the hypothalamic-pituitary portal system, or the anterior pituitary.
Reference intervals may depend upon age, gender, time of collection, menstrual cycle and menopausal status.
The hypothalamus is a region of the brain producing hormones that control a number of bodily functions, including the release of hormones from the anterior pituitary gland. The hypothalamus is located in the middle of the base of the brain and encapsulates the ventral portion of the third ventricle.
The hypothalamic hormones regulating the anterior pituitary hormones are listed in Table 55.1 . With the exception of CRH, these are structurally smaller than their pituitary counterparts.
CRH has wide distribution throughout the brain and brainstem. In the hypothalamus, it is released by the paraventricular nucleus (PVN). CRH secretion is stimulated by systemic physiologic stress via (1) neurons of subfornical origin, (2) neurons of the nucleus tractus solitarius, (3) hypothalamic glutamatergic neurons, and (4) 5-hydroxytryptamine–secreting neurons of the raphe nucleus. Neurogenic stress to release CRH also acts via hypothalamic glutamatergic neurons. Stress inhibits hypothalamic GABAergic neurons of the PVN that otherwise would suppress CRH release. Gamma-aminobutyric acid (GABA) serves as an inhibitory neurotransmitter. GABAergic neurons innervating CRH-secreting neurons also originate from the lateral septum and the bed nucleus of the stria terminalis. ACTH release is stimulated by serotonin, endorphins, and acetylcholine but is suppressed by GABA. Physiologically, stress, inflammation, and hypoglycemia stimulate ACTH release.
Thyrotropin-releasing hormone (TRH) is a tripeptide product of the PVN of the hypothalamus. TRH-secreting neurons in the PVN are innervated by axons that release (1) norepinephrine, (2) leptin, (3) neuropeptide Y, (4) agouti-related protein, (5) MSH, (6) CRH, or (7) somatostatin. Leptin is produced by adipose tissue and acts to reduce appetite and raise energy expenditure as body fat stores rise. Leptin receptors are expressed in the ventromedial nucleus of the hypothalamus. Leptin ( LPE gene) deficiency and leptin receptor ( LEPR gene) deficiency are rare causes of severe, early-onset genetic forms of obesity. Neuropeptide Y and agouti-related protein promote food intake.
The energy state and temperature of the organism influence TRH secretion. In addition to TRH regulation of TSH, TSH secretion is suppressed by (1) thyroid hormones, (2) glucocorticoids, (3) estrogens, and possibly (4) GH. Acute inflammatory cytokines such as interleukin (IL)-1β, IL-6, and tumor necrosis factor (TNF)-α stimulate ACTH release but suppress TRH and TSH. Norepinephrine stimulates TSH release, whereas endorphins, serotonin, and dopamine suppress TSH.
GH–releasing hormone (GHRH) is produced by neurons in the arcuate nucleus of the medial basal hypothalamus. Stimulators of GHRH release include dopamine- and galanin-secreting neurons and brain stem neurons with catecholaminergic inputs. Galanin is a neuropeptide that is widely expressed in the endocrine, central nervous, and peripheral nervous systems. There are three unique galanin receptors. Hypothalamic somatostatin suppresses both GHRH release and anterior pituitary GH release. Leptin from adipose tissue and ghrelin from the stomach have the net effect of increasing GHRH secretion and directly increasing GH concentrations. However, the clinical relevance of these influences and of other GH-releasing peptides (e.g., GHRP-6) is not well understood. Ghrelin binds to GH secretagogue receptors, which increases food intake. Ghrelin and obestatin are derived from the ghrelin-obestatin preproprotein. The actions of obestatin may involve anxiety and thirst reduction, recall enhancement, sleep regulation, cell division, and augmented pancreatic enzyme secretion. Hormones affecting GH secretion include estrogen, testosterone, and glucocorticoids. Physiologically, amino acids and hypoglycemia stimulate GH release. Glucagon stimulates both GH and cortisol release (which is why glucagon-stimulation tests are a clinical alternative to the insulin tolerance test [ITT]). In turn, the secretion of IGF-I in response to GH is influenced by nutrition, sex steroids, thyroid hormone, and the presence of chronic disease. Malnutrition, sex hormone deficiency in adolescents and adults, hypothyroidism and chronic disease all produce varying degrees of GH-resistance. Dopamine, endorphins, serotonin, and norepinephrine stimulate GH secretion.
Gonadotropin-releasing hormone (GnRH) regulation is complicated by the fact that GnRH must differentially control LH and FSH secretion, which vary greatly during the menstrual cycle in women. GnRH-secreting neurons are not located in a discrete nucleus but are diffusely distributed throughout the hypothalamus. Embryologically, these neurons are unusual because they originate outside the central nervous system (CNS). GnRH secretion is stimulated by neurons that secrete (1) galanin-like peptide, (2) kisspeptin, (3) glutamate, (4) neuropeptide Y, and (5) norepinephrine. Kisspeptin, derived from Kiss 1 gene expression, is a neuropeptide that regulates puberty and reproduction. Hyperprolactinemia inhibits Kiss 1 gene expression and leads to diminution of GnRH and gonadotropin secretion. Neurons secreting GABA, β-endorphins, and CRH inhibit GnRH. Gonadotropin release is stimulated by norepinephrine, GABA, and acetylcholine, and is suppressed by endorphins, dopamine, and serotonin.
GnRH pulsatility is essential to gonadotroph responsiveness. Tonic release of GnRH downregulates GnRH receptors on gonadotrophs causing hypogonadism. Therapeutically, downregulation is accomplished with a long-acting GnRH agonist, such as leuprolide acetate in the treatment of central precocious puberty in children or the induction of hypogonadism in men with prostate cancer. Conversely, pulsatile GnRH administration is used to initiate puberty and to induce ovulation or spermatogenesis in states of GnRH deficiency. The rate of pulsatility may influence the relative secretion of LH and FSH. In primate studies, GnRH at one pulse per hour preferentially released LH, whereas one pulse every three hours caused a decline in LH and a mild rise in FSH.
In the anterior pituitary, (1) CRH receptors are expressed on corticotrophs, (2) TRH receptors are expressed on thyrotrophs, (3) GHRH receptors are expressed on somatotrophs, (4) GnRH receptors are expressed on gonadotrophs, and (5) prolactin-inhibiting hormone (PRIH, dopamine) receptors are expressed on lactotrophs. There are two CRH receptors (CRHR1 and CRHR2) that are G-protein–coupled receptors. The gene for CRHR1 is located on chromosome 17q21.31. The gene for CRHR2 is located on chromosome 7p14.3. The TRH receptor is also G-protein coupled. The gene for the TRH receptor is located on chromosome 8q23.1. Chromosome 7p14.3 is the location of the GHRH receptor gene. The gene for the G-protein–coupled GnRH receptor is chromosome 4q. In pathologically high concentrations, TRH stimulates the release of LH and prolactin which is why primary hypothyroidism is a cause of prolactin elevation. Otherwise, TRH does not appear to play a major role in regulating LH or prolactin secretion.
Corticotrophs are stimulated by high concentrations of proinflammatory cytokines, such as IL-1, IL-6, and TNF-α. This emphasizes the interrelationship of the endocrine and immune systems in a hypothalamic-pituitary-adrenal-immune system axis. Through vasopressin type 3 receptors (also known as the arginine vasopressin receptor 1B, V1b receptors, gene location: chromosome 1q32), high concentrations of ADH stimulate corticotrophs to release ACTH.
The hormonal products of each anterior pituitary target cell (if applicable) are listed in Table 55.2 together with a summary of each system. Many of these hormones display circadian (daily), ultradian (more than daily), or infradian (less than daily) variation that reflects changes in hypothalamic control. Deficiency of an individual pituitary hormone is typically called hypopituitarism, whereas deficiency of all anterior pituitary hormones is termed panhypopituitarism.
| Hypothalamic Hormone | Anterior Pituitary Hormone | Target Organ/Tissue | Target Hormone |
|---|---|---|---|
| CRH | ACTH | Adrenal cortex: zona fasciculata and zona reticularis | Cortisol |
| TRH | TSH | Thyroid follicular cell | Thyroxine (T 4 ) and 3,5,3′-triiodothyronine (T 3 ) |
| GHRH and SRIH | GH | Liver and many tissues of the body | IGF-I, IGFBP-3, and ALS |
| GnRH | LH, FSH | Gonad | Sex steroids and inhibins |
| Dopamine | Prolactin | Breast | Not applicable |
Linear growth is the consequence of (1) genetic potential, (2) nutrition, (3) the presence or absence of disease, and (4) hormonal effects. Many hormones influence growth, but the most important are GH, thyroid hormone, and sex steroids. Excess glucocorticoids can impair growth in children. GH deficiency can be symptomatic in adults, and so GH appears to be essential for health throughout life.
The GHRH gene is located on chromosome 20q11.2. Prepro-GHRH is a polypeptide chain of 108 amino acids (12.4 kDa). Removal of the 20 amino acid signal (leader) sequence peptide yields the 88 amino acid pro-GHRH. Cleavage of the 11 amino acid N-terminal pro-sequence and the 31 amino acid C-terminal pro-sequence, with release of two free amino acids (positions 76 to 77 with reference to prepro-GHRH), produces the 44 amino acid mature GHRH. The terminal leucine of GHRH is amidated. Alternative splicing of the mRNA (isoform 2) produces a prepro-GHRH protein of 107 amino acids, which is missing amino acid 103.
Somatostatin is also known as somatotropin release–inhibiting hormone (SRIH). It is widely expressed throughout the body (CNS, gut, and δ-cells of the islets of Langerhans) and produces multiple physiologic effects. Somatostatin receptors are likewise widely distributed (see Table 55.3 ). Somatostatin functioning as SRIH is produced by the PVN of the hypothalamus. In pancreatic islets, somatostatin suppresses both glucagon and insulin secretion, whereas somatostatin release is stimulated by both of these hormones. In this way, δ-cell somatostatin modulates islet function by smoothing out extremes in the secretion of glucagon and insulin to maintain a stable blood glucose concentration. Somatostatin in the gut is found in highest concentration in the duodenum and jejunum.
| SSTR | Gene Location | Amino Acids/Molecular Weight | SST Binding | Distribution |
|---|---|---|---|---|
| SSTR1 | 14q1 |
|
SST-14 >SST-28 | Fetal kidney, fetal liver, adult pancreas, brain, lung, jejunum, stomach |
| SSTR2 | 17q2 |
|
SST14 and SST-28 | Cerebrum, kidney |
| SSTR3 | 22q13.1 |
|
SST-14 and SST-28 | Brain, pituitary, pancreas |
| SSTR4 | 20p11.2 |
|
SST-14 | Fetal and adult brain, lung, stomach, less in kidney, pituitary, adrenals |
| SSTR5 | 16p13.3 |
|
SST-28 >SST-14 | Adult pituitary, heart, small intestine, adrenal, cerebellum, fetal hypothalamus |
The somatostatin gene is located on chromosome 3q2. Expression of the somatostatin gene produces the 116 amino acid polypeptide prepro-somatostatin. Cleavage of the signal sequence (24 amino acids) produces pro-somatostatin (92 amino acids), and subsequent cleavage of the N-terminal pro-sequence (64 amino acids) yields a 28 amino acid form of somatostatin (SST-28). SST-28 has an intrachain disulfide bond between amino acids 17 and 28. In many tissues, SST-28 undergoes cleavage to a 14 amino acid form (SST-14) through removal of the N-terminal 14 amino acid sequence by the enzymes prohormone convertase 1/prohormone convertase 2 (PC1/PC2) and carboxypeptidase E (CPE). SST-14 is the major form of somatostatin in the CNS and δ-cells, whereas SST-28 is the major form in the gastrointestinal tract. SST-28 is also the major circulating form of somatostatin. Therefore somatostatin measurements in peripheral blood do not reflect SRIH secretion. Somatostatin is highly conserved in nature; all vertebrates have the identical sequence for SST-14.
In addition to GH suppression, somatostatin also suppresses TRH, TSH, CRH, and ACTH. However, the effect of somatostatin on the regulation of the adrenal cortical and thyroid axes is usually minor. In the gastrointestinal tract, somatostatin reduces the secretion of multiple hormones, including (1) gastrin, (2) secretin, (3) cholecystokinin, (4) vasoactive intestinal polypeptide, (5) motilin, (6) neurotensin, and (7) pepsin, and reduces gastric pH, intestinal motility, ion and nutrient absorption, and proliferation of the mucosa (see Chapter 52 ). This motivates the clinical use of octreotide (a synthetic somatostatin analogue) in the medical management of VIPoma and glucagonoma. Calcitonin, catecholamines, renin, and pancreatic exocrine function and insulin release are also suppressed by somatostatin. Indium-111 and gallium-68 labeled octreotide have been used for imaging, and lutetium-177 labeled octreotide has been used in radiotherapy of tumors expressing somatostatin receptors (e.g., neuroendocrine tumors). For more information on neuroendocrine tumors, refer to Chapter 53 .
The anterior pituitary somatotroph GHRH receptor (GHRHR) is a member of family B-III of the G-coupled receptor superfamily (the “secretin” family). Receptors for (1) secretin, (2) vasoactive intestinal polypeptide, (3) parathyroid hormone (PTH), and (4) calcitonin share partial sequence identity with GHRHR.
Pre-GHRHR is a 423-amino-acid polypeptide that is converted to the mature 401-amino-acid form of GHRHR by removal of the 22-amino-acid signal peptide. The N-terminal extracellular domain is 110 amino acids. GHRHR has seven transmembrane domains and a 42-amino-acid cytoplasmic domain. Amino acid 50 may be glycosylated.
Throughout the body, there are five receptors for somatostatin (SSTR1 through SSTR5; Table 55.3 ). Each receptor is encoded by a gene located on a separate chromosome. SSTR2 has two alternatively spliced isoforms. All of the SSTR receptors have seven transmembrane domains and are coupled with a pertussis toxin–sensitive G-protein. SSTR2, SSTR3, SSTR4, and SSTR5 are expressed in the pituitary.
Growth hormone has two disulfide bridges (amino acids 54 and 165, and amino acids 182 and 189). Structurally, GH has four main α-helices, and within the connecting loops, it has three mini-helices. Two circulating forms of GH are present: a 22-kDa form that is a 191 amino acid chain (full-length GH) that represents 85 to 90% of circulating GH, and a 20-kDa GH that lacks amino acids 32 through 46. The 20-kDa form of GH results from alternative splicing of the GH mRNA transcript. In addition to the 22- and 20-kDa forms, circulating GH exists as aggregates and oligomers. “Big GH” is a dimer of GH monomers, and “big, big GH” is GH associated with its binding protein (GHBP). GHBP is the external domain of the GH receptor (GHR), which binds GH with high affinity and is produced by cleavage of the GHR. Approximately 55% of all circulating GH forms are monomeric; big GH and big, big GH represent approximately 27% and approximately 18% of circulating GH, respectively. Approximately 50% of GH is not bound to GHBP; approximately 45% is bound to GHBP, and the remaining 5% of GH is bound to low-affinity binding proteins. Considering the multiple forms of GH, it is not surprising that significant analytical biases were historically observed between different immunoassays for GH. With the availability of recombinant standardized reference materials, this problem is expected to improve.
The gene for GH (chromosome 17q24.2) is a member of the GH subfamily that includes (−5′ to 3′ direction) (1) GH (the GH1 gene), (2) a chorionic somatomammotropin (hPL) pseudogene designated CSHP, (3) a chorionic somatomammotropin-A designated CSH1, (4) the placentally produced 22-kDa GH variant (GH-V; gene designation GH2), and (5) chorionic somatomammotropin-B (gene designation CSH2). Somatomammotropin (hPL) is a placental hormone with growth-promoting properties. Prolactin (199 amino acids) shares a homologous amino acid sequence with GH, but prolactin, encoded on chromosome 6p22, is not part of the GH complex on 17q24.2.
GH has both direct and indirect activity. Its direct actions will be described in the following sections. The indirect activity of GH is mediated by IGF-I. To initiate its direct and indirect activity, GH binds to receptors (GHR) that appear to be expressed by all tissues.
The GHR is a member of the class 1 hematopoietic cytokine family. Other members of this family include receptors for erythropoietin, granulocyte-macrophage colony-stimulating factor, and various interferons. Structurally, the GHR is a single-chain, 620-amino-acid protein (130 kDa). Pre-GHR includes an 18 amino acid leader sequence. The GHR structure includes an extracellular domain (246 amino acids), a transmembrane domain (24 amino acids), and a cytoplasmic domain (350 amino acids). When the extracellular portion of the GHR is shed, the 55-kDa GHBP moiety is released into the circulation.
Four isoforms of the GHR are expressed by alternative splicing of the nascent mRNA. Isoform 1 is the full-length receptor. Isoform 2 differs in the sequence of amino acids 292 to 297 (with reference to pre-GHR) and lacks amino acids 298 to 638. Isoform 3 differs in the sequence of amino acids 292 to 294 and lacks amino acids 295 to 638. An alanine at position four is replaced by aspartic acid, and amino acids 25 to 46 are missing in isoform 4.
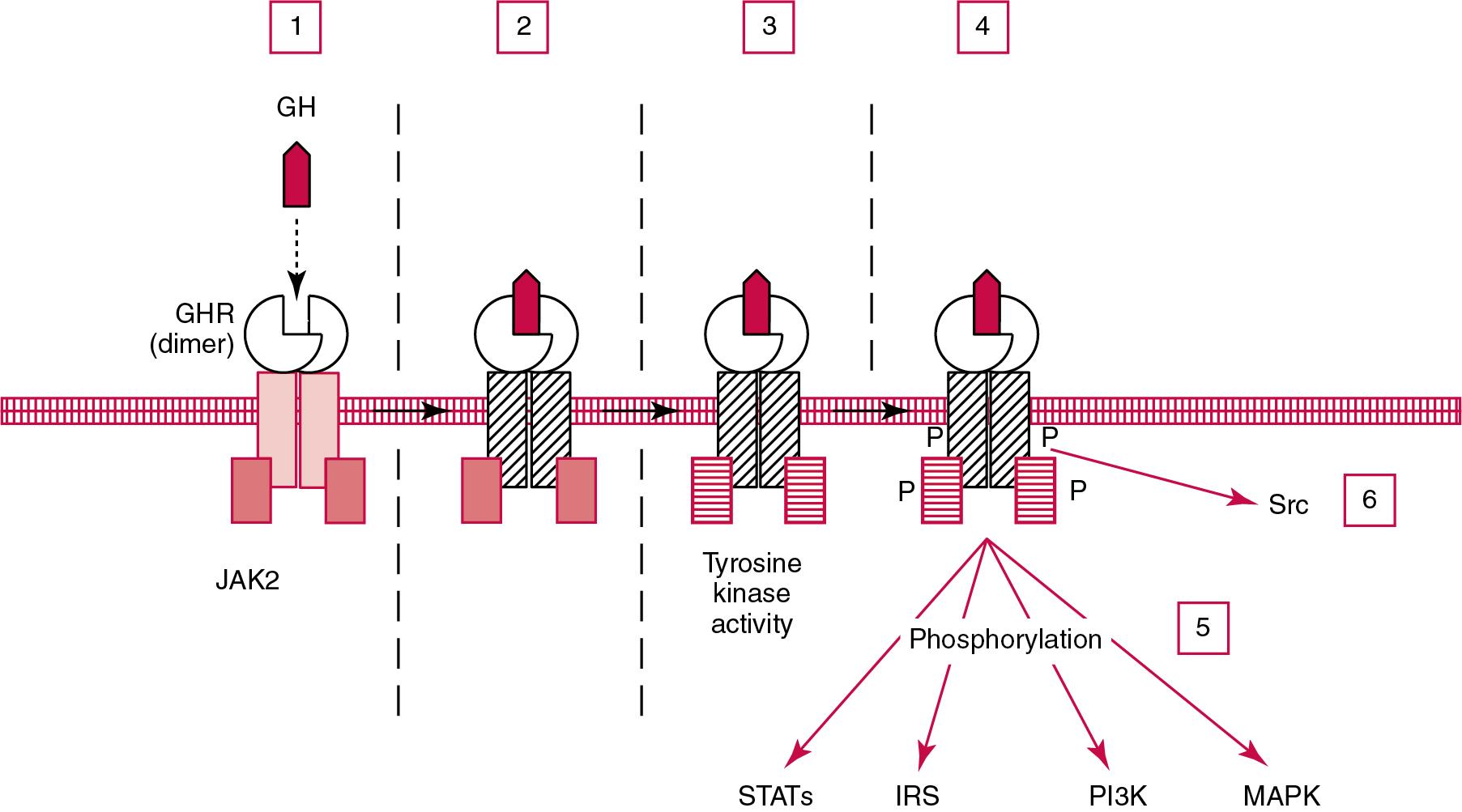
The GHR exists as a cell surface dimer in its inactive state ( Fig. 55.3 ). When GH binds to the GHR, the receptor recruits or activates 120-kDa Janus-associated kinase enzymes (JAK2; a type of adapter tyrosine kinase). JAK2 then exerts tyrosine kinase activity by phosphorylating itself and the GHR. , Phosphorylation activates JAK2, which triggers several intracellular pathways involving (1) signal transducers and activators of transcription (STATs), (2) the insulin receptor substrate, (3) phosphatidylinositol 3′-kinase (PI3K), and (4) a mitogen-activated protein kinase (MAPK). Via receptor-associated kinases, members of the STAT family are phosphorylated to permit the formation of homodimers or heterodimers that act as transcriptional activators once they translocate to the cell nucleus. STAT5b is involved, but it is unclear whether STAT5a is also involved. In its role as a transcription factor, phosphorylated, dimerized STAT5b enters the nucleus to promote gene transcription. Independent of JAK2, GHR signaling can be effected via Src , which is a tyrosine kinase. (Note: Src is the Rous sarcoma virus protooncogene).
IGF-I is a member of the insulin-related peptide family whose other members include IGF-II, insulin, and relaxin. Stimulated by hCG during pregnancy, relaxin is produced by the corpus luteum verum (the corpus luteum of pregnancy), the decidua (the uterine lining during pregnancy), and the placenta. Relaxin increases collagenase activity to soften and lengthen the cervix and pubic symphysis and facilitate parturition. Relaxin also reduces uterine contractility by inhibiting myosin kinase activity. In humans, there are three nonallelic relaxin genes: RLN1, RLN2, and RLN3.
Proinsulin, IGF-I, IGF-II, and relaxin are all composed of two domains (A and B) joined by a connecting domain. The connecting domains vary in sequence and length much more than the A and B domains. The connecting domain of proinsulin is cleaved to release C-peptide (connecting peptide), producing insulin A and B chains. Insulin, IGF-I, IGF-II, and relaxin have two disulfide bridges. IGF-I and IGF-II share 62% homology, and they each share 50% homology with insulin.
The 110 amino acid sequence of preproinsulin includes a signal peptide (amino acids 1 to 24), the insulin B chain (amino acids 25 to 54), C-peptide (amino acids 57 to 87), and the insulin A chain (amino acids 90 to 110). The two interchain disulfide bonds are between amino acids 31 and 96, and 43 and 109. The single intrachain disulfide bond is between amino acids 95 and 100.
Two forms of prepro–IGF-I are expressed as a consequence of alternative mRNA splicing: IGF-IA and IGF-IB. The IGF-IA preprohormone is 153 amino acids, including a signal peptide (amino acids 1 to 21), an N-terminal propeptide (amino acids 22 to 48), IGF-I (70 amino acids), and a C-terminal propeptide (the E domain, amino acids 119 to 153). The IGF-I polypeptide includes the B domain (amino acids 49 to 77), the C (connecting) domain (amino acids 78 to 89), the A domain (amino acids 90 to 110), and the D domain (amino acids 111 to 118). Three disulfide bonds are present between amino acids 54 and 96, 66, and 109, and 95 and 100. IGF-I is not glycosylated. A relatively common (∼0.6%) single nucleotide polymorphism in IGF-I (A70T) has been identified in the screening population for acromegaly which is clinically relevant since some commercial immunoassays do not detect this variant form leading to factitiously decreased IGF-I concentrations.
The 195 amino acid IGF-IB preprohormone is composed of a signal peptide (amino acids 1 to 21), a propeptide (amino acids 22 to 48), IGF-I (70 amino acids), and another propeptide region (the E domain, amino acids 119 to 195). Differences in the D domain of the prepro–IGF-I distinguish IGF-IA from IGF-IB; the tertiary structure of the IGF-I proteins and the placement of disulfide bonds are identical between IGF-IA and IGF-IB.
The IGF-II preprohormone comprises 180 amino acids. The first 24 residues constitute the signal peptide. After their removal, IGF-II is derived from pro–IGF-II after cleavage of the C-terminal E peptide (amino acids 92 to 180). Therefore amino acids 25 to 91 include the B region (amino acids 25 to 52), the C region (amino acids 53 to 64), the A region (amino acids 65 to 85), and the D region (amino acids 86 to 91) of IGF-II. Isoform I is the full-length prepro–IGF-II (180 amino acids). Formed by alternative splicing, isoform II lacks amino acid 25 (alanine) and is therefore 179 amino acids in length. IGF-II is glycosylated at amino acid 99 and has three intrachain disulfide bonds between amino acids 33 and 71, 45 and 84, and 70 and 75. A potential glycosylation site is amino acid 163.
Prorelaxin is a 185-amino-acid protein that contains a signal sequence (amino acids 1 to 22), a B chain (amino acids 23 to 53), a connecting propeptide (amino acids 56 to 158), and an A chain (amino acids 163 to 185). A–B interchain disulfide bonds can occur between amino acids 35 and 172, and 47 and 185. An additional disulfide bridge may occur between amino acids 171 and 176.
IGF-I and IGF-II circulate together with an IGF binding protein (IGFBP) (most importantly IGFBP-3), and the acid-labile subunit (ALS) to form a 150-kDa trimeric protein complex. Approximately 75 to 80% of IGF-I/IGFBP-3 complexes are trimeric; the remaining IGF-I/IGFBP-3 complexes are dimeric and may include other IGFBPs. Less than 1% of the total IGF-I is free (the biologically active form). The binding affinity of IGF-I for the insulin receptor is low (≈7% of insulin affinity) but circulating IGF-I concentrations exceed insulin by three orders of magnitude. Without binding proteins, therefore IGF-I could cause potentially devastating hypoglycemia.
The trimeric IGF-I-IGFBP-3-ALS complexes do not normally cross capillary membranes because of their size. However, the 50-kDa binary complex and free IGF-I are able to enter the interstitium, where binding to type I IGF receptors can occur.
Most of the circulating IGF-I is produced by hepatocytes. However, IGF-I is also produced locally throughout the body and thus acts as a paracrine and an autocrine hormone. The possible endocrine (systemic) influence of IGF-I on growth is discussed later.
Similar to IGF-I, IGF-II does not normally produce hypoglycemia. However, tumors that secrete a larger than normal form of IGF-II have been described. These are usually more than 0.5 kg in size; nonislet cell tumors are most often of mesenchymal or hepatic origin. Since big IGF-II does not bind normally to IGFBP-3, the free IGF-II concentration is greatly elevated, and the molar IGF-II to IGF-I ratio is greater than 10. As a result of IGF-II binding to the insulin receptor, the clinical syndrome is similar to that of hypoglycemia caused by hyperinsulinism (i.e., absence of ketonemia with no elevation of free fatty acids, lactate, or alanine), and insulin itself is suppressed because β-cells are normal. Physiologically, hypoglycemia stimulates the release of counter-regulatory hormones such as GH, but in this scenario IGF-II suppresses the GH axis. Removal of the tumor leads to the resolution of hypoglycemia. ,
Because of their high affinity (K d 10 −10 to 10 −11 M) for IGFs, IGFBPs are regarded as inhibitors of IGF action. IGFBPs have higher affinity for IGFs than do the IGF receptors. IGFBP-3 has biological actions independent of the IGF/IGF-I receptor axis. Receptors for IGFBPs have been described. Proteolysis of IGFBPs releases IGF-I; therefore IGFBP proteases can influence free IGF-I concentrations. Table 55.4 summarizes the features of the seven known IGFBPs. From a clinical standpoint, only IGFBP-3 has relevance as it is used as an adjunctive tool in addition to GH and IGF-I in the diagnosis of GH deficiency.
| IGFBP | Chromosome Gene Location | Amino Acids | Affinity for IGF-I vs. IGF-II | Specific Features |
|---|---|---|---|---|
| 1 | 7p13 | 234 | 1 = 2 | RGD sequence a |
| 2 | 2q3 | 289 | 1 < 2 | RGD sequence a |
| 3 | 7p13 | 264 | 1 = 2 | N-glycosylation |
| 4 | 17q | 237 | 1 = 2 | Extra cysteines |
| 5 | 2q3 | 252 | 1 < 2 | Ternary complex with ALS |
| 6 | 12q13 | 216 | 1 < 2 | O-glycosylation |
| 7 | 4q12 | 282 | b | Stimulates prostacyclin production |
Two types of receptors for IGFs have been identified: the type I IGF receptor and the type II IGF receptor ( Fig. 55.4 ). Structurally, the type I IGF receptor is similar to the insulin receptor. Because this receptor does not exclusively bind IGF-I, the terminology “IGF-I receptor” is not recommended. The type I receptor is derived from a single precursor protein of 1367 amino acids that include the 30 amino acid signal peptide. The 706 amino acid (130 kDa) α-chain is extracellular and is bound by a disulfide bond to the transmembrane 90-kDa (627 amino acids) β- chain. Cleavage of the α- and β-chains releases a tetrapeptide (707 to 710: arginine-lysine-arginine-arginine). Beta-chain amino acids 906 to 929 form a transmembrane domain. The receptor exists as a homodimer (β-α-α-β) with the two α-chains bound to each other by two disulfide bonds. Similar to the type I IGF receptor, the insulin receptor is a homodimer of two 135-kDa α-chains and two 95-kDa β-chains.
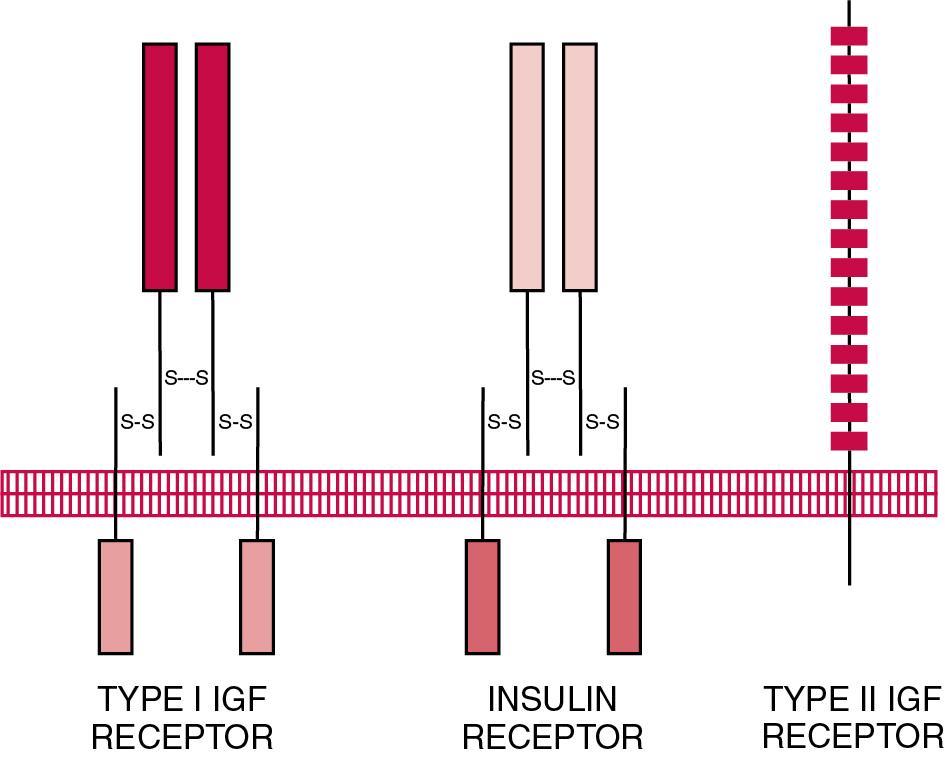
Tyrosine kinase activity in the cytoplasmic portion of the type I IGF receptor β-chain results from binding of an IGF molecule to the cysteine-rich portion of the α-chains (amino acids 148 to 302), which causes conformational changes in both α- and β-chains. Intracellular signaling involves autophosphorylation and phosphorylation of the 185-kDa insulin receptor substrate 1, which is the predominant target of the active type I IGF receptor. The type I IGF receptor binds IGF-I with higher affinity than IGF-II, and affinity for insulin is lower than the affinity of the receptor for IGF-I or IGF-II. The affinities of the insulin receptor are the opposite: insulin ⪢ IGF-II > IGF-I.
The type II IGF receptor is structurally dissimilar from the type I IGF receptor and the insulin receptor. The 270-kDa, 2451-amino-acid, type II IGF receptor is a monomeric protein that is similar to the epidermal growth factor (EGF) receptor. (Note: The leader sequence is 40 amino acids.) The EGF receptor itself is also known as ErbB1 or HER1. The external portion of the receptor is 2264 amino acids, the transmembrane domain is 23 amino acids, and the cytoplasmic domain is 164 amino acids. Beginning at the N terminus, there are 13 amino acid repeats of approximately 150 amino acids, and a 47 amino acid fibronectin type II domain, followed by 2 more repeats. Evidence indicates at least five glycosylation sites: amino acids 112 (with preference for the pre–type II IGF receptor), 581, 626, 747, and 1246. Two disulfide bonds are found between amino acids 1903 and 1927 and between amino acids 1917 and 1942.
Ligand binding to EGF receptors results in dimerization and tyrosine kinase activation. The external ligand-binding domain of EGFs is composed of numerous short amino acid repeats. The type II IGF receptor removes IGF-II from the circulation. The type II IGF receptor binds mannose-6-phosphate, in addition to IGFs, permitting the uptake and intracellular movement of mannose-6-phosphate–containing lysosomal enzymes. The binding sites for IGFs and mannose-6-phosphate are found on different parts of the receptor. The affinities of the type II IGF receptor are as follows: IGF-II ⪢ IGF-I > insulin. Because this receptor does not exclusively bind IGF-II, the terminology “IGF-II receptor” is not recommended.
A hybrid receptor consisting of the α–β-chain of the insulin receptor and the α–β-chain of the type I IGF receptor has been described. It has been suggested that these hybrid receptors may allow cancers to respond to insulin. In cancers, insulin is also thought to act through the insulin signaling pathway via insulin receptors and also probably acts through receptors that bind IGF-I. Thus IGF-I also plays a role in cancer biology. Increasing evidence suggests that IGF-I may enhance the growth of many tumors, and it is known that acromegaly confers increased risk for both colorectal and thyroid cancer. Thus the casual use of IGF-I injections in children to enhance growth in short but otherwise normal children is problematic and potentially carcinogenic.
Growth hormone (GH) deficiency in children should be considered only when other causes of low growth-velocity short stature have been excluded.
The diagnosis of GH deficiency usually requires stimulation testing, although some endocrinologists will diagnose GH deficiency based solely on the measurement of insulin-like growth factor-I (IGF-I).
Defects in IGF-I generation and response are rare compared to GH deficiency.
GH deficiency does occur in adults, and accordingly, they may benefit from GH replacement.
GH excess produces the clinical syndrome of gigantism in children and acromegaly in adults.
GH excess is diagnosed by elevated IGF-I levels and the failure of GH suppression after an oral glucose load.
Reference intervals for IGF-I are influenced by methodology, age, sex, and pubertal stage.
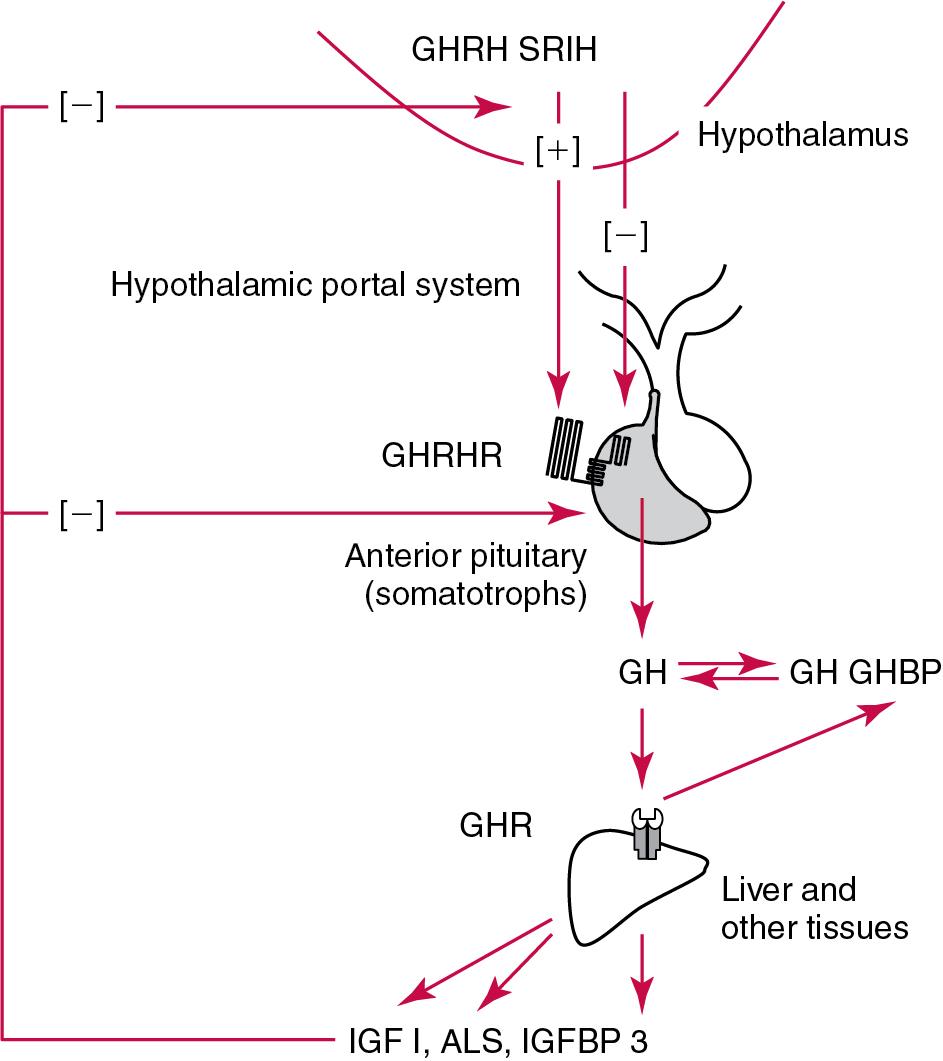
Growth hormone ultimately stimulates release of IGF-I, which negatively feeds back to regulate GH release via two hypothalamic hormones: somatostatin (or SRIH; from the hypothalamic PVN) and GHRH (from the hypothalamic infundibular nucleus) ( Fig. 55.5 ). These hypothalamic GH-regulating hormones are carried to the anterior pituitary via the specialized hypothalamic-pituitary portal vascular system. Somatotrophs of the anterior pituitary gland have receptors for both hormones. Somatostatin inhibits GH release, whereas GHRH promotes the release of GH. However, GH release is the predominant hypothalamic effect because surgical interruption of the pituitary stalk with destruction of the hypothalamic-pituitary portal system leads to GH deficiency, not excess. In addition to its hypothalamic negative feedback effects, IGF-I directly suppresses pituitary release of GH.
Physiologically, GH secretion is episodic and pulsatile. Consequently, random measurements can neither exclude GH deficiency nor confirm its excess; between pulses, GH concentrations can be quite low and do not distinguish GH insufficiency from normal production of the hormone. During daytime hours, the plasma concentration of GH in healthy adults remains stable and relatively low (<2 ng/mL; <2 μg/L) with several secretory spikes occurring approximately 3 hours after meals (particularly meals high in protein and arginine) and after exercise. In contrast, during the evening hours, adults and children show a marked rise in GH secretory activity approximately 90 minutes after the onset of sleep; GH concentrations reach a peak value during the period of deepest sleep. This pattern of GH secretion may be important for anabolic and repair processes and for proper skeletal growth. GH is also increased by psychologic or physical stress and hypoglycemia. Normal GH secretion requires thyroxine and age-appropriate concentrations of testosterone or estrogen.
GH is suppressed by elevations in blood glucose. One of the tests for GH excess measures GH after an oral glucose load (e.g. 75 g in adults and 1.75 g/kg in children); the normal response is a GH concentration of less than approximately 0.4 ng/mL (0.4 μg/L) for a modern analytically specific chemiluminescent sandwich assay. , Some research further suggests that body mass index should be considered when interpreting GH results after a glucose load ( Box 55.1 ). GH also declines with increases in free fatty acid concentrations, rapid eye movement sleep, and aging. In the presence of abnormally high concentrations of glucocorticoids, GH secretion is suppressed. In addition, circulating GH is thought to influence the release of hypothalamic hormones through the short feedback loop. Other hypothalamic hormones, such as TRH and GnRH, do not affect GH release in normal subjects but may provoke GH release in patients with acromegaly.
Normal subjects show suppression of serum growth hormone (GH) concentrations after oral administration of glucose. Subjects with acromegaly fail to exhibit appropriate GH suppression.
The test should be performed after an overnight fast with the patient maintained at bed rest. After a baseline blood specimen is collected for GH and glucose measurement, a solution of 75 g of glucose is given orally (in children, 1.75 g/kg to a maximum dose of 75 g). Glucose and serum GH are measured again on specimens collected 30, 60, 90, and 120 min later.
Serum GH concentrations in normal individuals fall to less than 0.4 ng/mL (0.4 μg/L) using a modern chemiluminescent sandwich assay. Subjects with acromegaly fail to show this suppression and sometimes show a paradoxical increase in GH concentration. Patients with liver disease, uremia, or heroin addiction may have false-positive results with this test (failure to suppress serum GH concentrations after oral glucose load).
Age-associated decline in GH production, as can be appreciated through age-dependent IGF-I reference intervals, has spawned an industry of dietary supplements purported to “support” GH secretion. These supplements are amino acid preparations that theoretically stimulate release of the subject’s own GH. Such dietary supplements have no proven medical value. Use of GH by athletes to enhance strength or promote recovery from injury is prohibited in most sports.
The role of non-GHRH GH secretagogues in the physiologic control of GH and growth is highly debated. One such secretagogue is ghrelin (28 amino acids, 3.4 kDa; gene name: GHRL; chromosome 3p2). Although ghrelin is produced in the hypothalamus, its highest concentration is found in gastric tissue. In addition, ghrelin is widely distributed in the rest of the gastrointestinal tract, heart, lung, and adipose tissue. Ghrelin appears to stimulate food intake and obesity.
Ghrelin binds to the somatotroph GH-secretagogue receptor (gene name: GHSR; isoform 1A: 366 amino acids, 41 kDa; isoform 1B: 289 amino acids, 32 kDa; chromosome 3q26.31), which is distinct from the GHRHR. GHRL, the gene that encodes ghrelin, also encodes the 23 amino acid peptide obestatin. As noted previously, obestatin may decrease food intake and increase satiety, but this has been debated. Obestatin is a ligand for the orphan GPR39 receptor (453 amino acids, 51 kDa; chromosome 2q21). However, despite obestatin binding to the GPR39 receptor, receptor activation may not follow.
Growth hormone effects can be classified as indirect or direct. GH directly raises blood glucose by stimulating gluconeogenesis and reducing insulin sensitivity. Also, it causes adipose tissue lipolysis, and the resulting GH-induced elevations in free fatty acids provide an alternative energy source that serves to spare glucose for CNS use. Therefore when glucose and free fatty acid concentrations are raised at times of stress, in partnership with epinephrine, glucagon, and cortisol, fuels for the fight-or-flight response are provided. GH has other effects on intermediary metabolism: GH stimulates the uptake of nonesterified fatty acids by muscle and accelerates the mobilization and metabolism of fat from adipose tissue to the liver.
At the epiphysis of growing bone, GH promotes epiphyseal prechondrocyte differentiation. GH directly stimulates the production of the ternary complex of IGF-I, IGFBP-3, and the ALS. In turn, after entering the interstitium, unbound IGF-I binds to type I IGF receptors.
Indirect effects of GH are mediated through IGF-I production, which (together with GH) is necessary for linear growth in childhood. IGF-I is mitogenic and antiapoptotic. Epiphyseal prechondrocyte differentiation stimulated by GH, along with the local effects of IGF-I (also under the control of GH), stimulates the clonal expansion of differentiating chondrocytes.
Thus the overall effect of GH is to promote growth in soft tissue, cartilage, and bone. This action results from stimulation of protein synthesis that is induced in part by an increase in amino acid transport through cell membranes. The effects of GH on bone and muscle are exerted both directly and through the effects of IGF-I under the influence of GH. Increased growth of soft tissue and the skeleton is accompanied by changes in electrolyte metabolism, including positive nitrogen and phosphorous balance, a rise in plasma phosphorous concentration, and a fall in blood urea and amino acid concentrations. Additional responses to GH include increased intestinal absorption of calcium and decreased urinary excretion of sodium and potassium. The metabolic changes most likely are caused by increased uptake of these ions by growing tissue.
IGF-I increases glucose oxidation in adipose tissue and stimulates glucose and amino acid transport into diaphragmatic muscle and heart muscle. Synthesis of collagen and proteoglycans is enhanced by IGF-I, which also has positive effects on calcium, magnesium, and potassium homeostasis. The insulin-like effects of this growth factor have been ascribed in part to its structural similarity to insulin.
Evidence indicates that the local effects of IGF-I (autocrine or paracrine) are predominant in stimulating growth when compared with the systemic effects of IGF-I produced by the liver. When the hepatic IGF-I gene was knocked out in mice (although nonhepatic tissues expressed IGF-I), growth was normal, and IGFBP-3 and ALS concentrations were low. IGF-II may function as a growth factor in utero; however, its secretion in utero is not under the control of GH.
In the absence of GH, IGF-I is not as effective a growth stimulant as it is when GH and IGF-I both are present. IGF-I treatment alone is not recommended as therapy for GH deficiency; IGF-I therapy is reserved for cases of GH resistance due to GHR deficiency (e.g., Laron syndrome). ,
GH (through IGF-I) and insulin induce growth in a similar manner because both have protein anabolic effects and stimulate the transport of amino acids into peripheral cells. Their respective effects on glucose homeostasis, however, oppose one another—chronic GH excess induces diabetes mellitus. Most growth-promoting GH effects are delayed rather than immediate and are exerted primarily through IGF-I.
IGF-I concentrations vary widely with age and gender. IGF-I rises during childhood, and during puberty, IGF-I concentrations can be two to three times the adult concentration. After adolescence, IGF-I concentrations show steep decline until age 30, followed by a gradual decline until old age.
GH is not the only determinant of IGF-I concentration in the circulation. Transformation of the GH stimulus to IGF-I production and secretion is modulated by (1) nutrition, (2) the presence or absence of chronic inflammation, (3) thyroid function, (4) glucocorticoids, and (5) sex steroids. IGF-I secretion is reduced by (1) malnutrition, (2) malabsorption (e.g. inflammatory bowel disease, celiac disease), (3) obesity (4) cystic fibrosis, (5) chronic disease, (6) sex hormone deficiency in adolescence. Therefore a decreased IGF-I concentration is not necessarily synonymous with GH deficiency.
In cases of acquired GH resistance and genetic GH resistance (GHR mutation or signaling disorder), GH concentrations will rise, and high concentrations of GH produce hyperlipidemia and hyperglycemia. Cases of acquired GH resistance are not treated with GH or IGF-I, but instead are treated by addressing the underlying disorder.
The actions and regulation of IGF-II have been debated. IGF-II is believed to be important for intrauterine growth. Mice display intrauterine growth retardation when the IGF-II gene is knocked out. Although IGF-II–producing tumors are rare, such tumors can produce hypoglycemia attributable to incompletely processed variant of IGF-II. , Both IGF-I and IGF-II are of great interest to oncology researchers; the reader is referred to the literature for a detailed discussion of this topic.
Diagnosis of clinically important states of GH excess or deficiency are uncommon and may go unrecognized for years. GH concentrations vary widely under normal circumstances; therefore random measurements of GH, in general, are not diagnostically useful. A single GH measurement cannot distinguish between normal fluctuations and the low or high concentrations that are typical of various diseases. GH measurements are best determined as part of dynamic function testing: physiologic or pharmacologic provocative stimuli are used to help diagnose GH deficiency, whereas GH suppression (or lack thereof) following glucose administration is used to identify GH excess. IGF-I concentrations often correlate better with the clinical severity of acromegaly than with glucose-suppressed or basal GH concentrations.
In contrast to GH, a single measurement of IGF-I is considered to be an accurate reflection of GH-IGF-I production, irrespective of the time of the day or meals. IGF-I has a much longer half-life than GH and accordingly has more stable plasma concentrations. The half-life of GH is slightly longer than 15 minutes, whereas the half-life of the trimeric IGF-I-IGFBP-3-ALS complex is 17 to 22 hours. The half-life of unbound IGF-I is only 10 to 20 minutes, but the unbound form of the hormone accounts for less than 1% of the total concentration. Serum concentrations of IGF-I are influenced by (1) age, (2) sex, (3) degree of sexual maturity, (4) thyroid status, (5) nutritional status, and (6) body mass index. As mentioned previously, IGF-I concentrations are low in GH deficiency and in patients with acute or chronic protein or caloric deprivation and, in the other extreme, obesity. In pediatric endocrinology, measurements of IGFBP-3 have been used in addition to IGF-I measurements to assess GH; however, the additional value of IGFBP-3 measurement over and above IGF-I measurement has not been firmly established. The diagnostic use of GH to stimulate IGF-I production is controversial and is not currently included in standard medical practice.
Acromegaly is the rare clinical syndrome in adults resulting from GH excess. , Even less common is pituitary gigantism, which results from GH excess in childhood. The clinical features of acromegaly involve overgrowth of the skeleton and soft tissue, producing (1) acral enlargement (enlargement of the extremities), (2) organomegaly (enlarged heart and/or liver), (3) facial coarsening, (4) intestinal polyposis and attendant increased risk of colorectal cancer, (5) premature cardiovascular disease, (6) hyperhidrosis (increased sweating), (7) skin tags, (8) bone and joint disorders, (9) myopathy with weakness, (10) insulin resistance, and often (11) diabetes mellitus. Premature cardiovascular disease is the most common cause of death in individuals with acromegaly. Gigantism is characterized by extreme tall stature, in addition to the clinical features of acromegaly, as pathologic GH excess occurs before epiphyseal fusion is complete (e.g., in children or adolescents).
Most cases of acromegaly (≈95%) result from anterior pituitary GH-secreting tumors (“somatotroph adenomas” or “somatotropinomas”). Somatotropinomas are usually macroadenomas (>10 mm in diameter) by the time they come to clinical attention; the vast majority of these can be visualized by computed tomography (CT) or MRI. Some anterior pituitary tumors secrete both GH and prolactin (somatomammotropinomas). GH-secreting anterior pituitary adenocarcinomas are exceedingly rare. Approximately 5% of GH-secreting tumors are familial and caused by disorders such as multiple endocrine neoplasia type 1 syndrome, familial acromegaly, Carney syndrome, McCune-Albright syndrome, and familial isolated pituitary adenoma. Rare causes of acromegaly include GHRH-secreting hypothalamic tumors, extrapituitary somatotropinomas, and GHRH-secreting islet cell tumors, or those of lung or breast.
In severe or advanced cases of GH excess, the diagnosis may be nearly certain on the basis of physical appearance alone. However, in less severe or early cases, the physical changes may be subtle and gradual, so careful attention to clinical findings is required to make an early diagnosis. The reversibility of tissue changes depends largely on the duration of the disease. In addition to soft tissue changes, acromegaly may cause severe disability or death from cardiac, pulmonary, and/or neurologic sequelae. The most important requirement for the diagnosis of acromegaly is the demonstration of inappropriate and excessive GH secretion.
As many as 10% of patients with active acromegaly have random serum GH concentrations that fall within the reference interval. Essentially all patients with acromegaly have an abnormal GH response to an oral glucose load (see Box 55.1 ). Patients with acromegaly typically show modest or no change in their basal concentration of GH or demonstrate a paradoxical increase in GH ; in contrast, normal individuals show suppression of GH concentrations to less than 0.4 ng/mL (0.4 μg/L) after a 75-g oral glucose. , IGF-I is considered to be the most important diagnostic tests for the initial diagnosis of acromegaly. , Additionally, because of the development of GH-receptor antagonist therapies for acromegaly (e.g. pegvisomant), IGF-I is also the only clinical tool available for monitoring patients receiving these therapies.
In children, short stature with a normal growth velocity (≥4 to 5 cm/year) results from (1) familial short stature, (2) primordial growth failure (prenatal-onset growth failure), or (3) constitutional delay in growth and adolescence (delayed maturation). Short stature with a low growth velocity (<4 to 5 cm/year) results from genetic short stature, chronic illness, malnutrition, deprivation (nutritional or psychological), Turner syndrome, or endocrine disorders (e.g., hypothyroidism, disorders of the GHRH-GH-IGF-I axis, extremely poorly controlled diabetes, rickets, pseudohypoparathyroidism, pseudopseudohypoparathyroidism, and Cushing syndrome).
Idiopathic short stature is the term that is used when children with short stature and a low growth velocity lack evidence of pathology. It has been increasingly recognized that 5 to 15% of these children have a mutation in the SHOX (short stature homeobox) gene located on chromosome Xp22.33. Forearm anomalies are common in such children, and they become more evident during puberty.
GH deficiency is not a common cause of growth retardation. Approximately one-half of children evaluated for growth retardation have no specific organic cause; approximately 15% have an endocrine disorder, of which approximately half (approximately 8% of all children with short stature) have GH deficiency. Children with significantly reduced height and low growth velocities with no clear explanation should be screened for GH deficiency once other endocrine disorders have been excluded.
GH deficiency in children is characterized by (1) short stature, (2) low growth velocity, (3) immature facial appearance, (4) delayed bone age on radiologic examination, and (5) increased adiposity. In cases of congenital GH deficiency, size at birth is usually normal because in utero IGF-I does not appear to be under GH control. Micropenis is evident in some boys with congenital GH deficiency and will resolve with GH replacement in childhood. Micropenis suggests hypopituitarism, although there are other causes for micropenis. Adults with GH deficiency experience (1) reduced muscle mass (“sarcopenia”), (2) increased central adiposity, (3) osteoporosis with decreased bone density, (4) increased fracture risk, (5) decreased quality of life, (6) dyslipidemia, and (7) increased risk of cardiovascular disease. GH deficiency is probably the most common endocrine abnormality in adults with large (non-somatotroph) pituitary adenomas and in patients who have undergone pituitary irradiation. Recovery of normal hormonal production after surgery for these types of pituitary adenomas occurs uncommonly. GH replacement therapy forms an important part of the clinical care of GH-deficient children. Whether GH therapy is required in GH-deficient adults remains controversial.
GH insufficiency can be a consequence of (1) hypothalamic disease, (2) disruption of the portal system between hypothalamic nuclei and the anterior pituitary, (3) GHRHR loss-of-function mutations, or (4) somatotroph disease. GH deficiency can occur in isolation (isolated GH deficiency) or together with other pituitary deficiencies (multiple pituitary hormone deficiencies [MPHDs] or combined pituitary hormone deficiencies [CPHD]). Patients with isolated GH deficiency should be followed clinically for the development of other pituitary hormone deficiencies because MPHD can evolve over time. Biochemical stimulation testing is necessary to establish the diagnosis of GH deficiency, GH resistance, or MPHD. In most affected children, the cause of GH deficiency is unknown (idiopathic GH deficiency). An organic cause is identified in about one in four children with proven GH deficiency; half of these children will be diagnosed with a CNS tumor.
Any type of hypothalamic disease or dysfunction can lead to or be associated with GHRH deficiency, including (1) tumors, (2) inflammation, (3) previous infection, (4) trauma (including previous surgery), (5) hemorrhage, (6) irradiation, and (7) malformations (e.g., SOD). Low-dose irradiation of the hypothalamus and/or pituitary can cause idiopathic GH deficiency, whereas higher doses of irradiation can cause MPHD. SOD is defined by the triad of (1) midline brain defects, such as agenesis of the septum pellucidum and/or corpus callosum, (2) hypoplasia of the optic nerve, and (3) anterior and/or posterior pituitary hormone abnormalities. A small number of cases of SOD are explained by mutations in HESX1, SOX2, and SOX3, all of which are transcription factors. HEXS1 (chromosome 3p14.3) is a paired-like homeobox gene, SOX2 (chromosome 3q26.3) is the SRY (sex-determining region on the Y chromosome) box 2 gene, and SOX3 (chromosome Xq27.1) is the SRY box 3 gene. Midline brain tumors, such as craniopharyngiomas, meningiomas, gliomas, germinomas, third ventricle colloid cysts, ependymomas, and optic nerve gliomas, also affect the hypothalamus. Disruptions in the hypothalamic-pituitary portal system can result from tumors, inflammation, previous infection, trauma (including previous surgery), and irradiation.
Congenital GH deficiency from pituitary disease has many causes, including GHRHR gene mutations (idiopathic GH deficiency type IB), GH1 mutations (idiopathic GH deficiency types IA, IB, II, and III, and bioinactive GH), and transcription factor mutations (which usually cause MPHD: LHX3, LHX4, PROP1, Pit-1, RIEG) and malformations (anencephaly and holoprosencephaly) ( Table 55.5 ). Homozygous LHX3 mutations have caused panhypopituitarism, with the exception that ACTH was not affected. Heterozygous LHX4 mutations have caused deficiencies of GH, TSH, and ACTH. PROP1 (name derived from PROphet of Pit-1; POU1F1; Pit-1 stands for “paired-like homeodomain transcription factor”) mutations cause deficiencies of GH, prolactin, TSH, and gonadotropins. ACTH deficiency has also occurred in some cohorts. PROP1 gene mutations are inherited as autosomal recessive traits. Pit-1 gene mutations cause GH, prolactin, and variable degrees of TSH deficiencies. These transcription factor mutations may be inherited as autosomal recessive or dominant traits. Heterozygous RIEG gene mutations (PITX2 , a paired-like homeobox gene) are the cause of Rieger syndrome, which may include GH deficiency. Features of Rieger syndrome encompass developmental abnormalities of the teeth, the anterior chamber of the eye, and the umbilicus. Mutations in GLI1, GLI2, Shh, ZIC2, SIX3, tgif, PATCHED1, DGF1, and FAST1 have variously been cited as causes of holoprosencephaly. Children with midline facial clefts (cleft lip, cleft palate, or combined) or a single central incisor can exhibit GH deficiency. A number of GH1 gene mutations have been identified to cause bioinactive GH. In these mutations, GH does not have full biological activity but may retain its immunoreactivity. Therefore in contrast to other forms of pituitary or hypothalamic disease, the measured GH concentration has been observed to be high. Reference laboratories can provide GHRHR , GH1 , and GHR gene sequencing services.
| Gene/Classification/Inheritance | Mutation | Phenotype |
|---|---|---|
| GH1; IA; AR | Deletion, FS, NS | Absent GH expression; immune resistance to GH treatment is common |
| GH1; IB; AR | Splicing? | Reduced GH; responds to GH treatment a |
| GHRHR; IB; AR | Possible MS | Reduced GH; responds to GH treatment a |
| GH1; II; AD | DN | Reduced GH; responds to GH treatment; MPHD is possible |
| Unknown; III; XLR | — | Reduced GH; responds to GH treatment; agammaglobulinemia is possible |
Acquired causes of pituitary disease include (1) tumors (anterior pituitary adenoma, craniopharyngioma), (2) congenital cysts (Rathke cleft cyst, arachnoid cyst), (3) infiltrative disease (amyloidosis, histiocytosis, hemochromatosis), (4) inflammation (autoimmune, granulomatous or IgG4-related hypophysitis), (5) infection, (6) trauma (including surgery), (7) bleeding, (8) irradiation, (9) infarction (from pituitary apoplexy and Sheehan syndrome). Hemochromatosis does not cause hypopituitarism until many decades have passed. Some investigators believe that all patients with “idiopathic” hypopituitarism should be screened for hemochromatosis.
The diagnosis of GH deficiency in adults and children is different, the latter being a more complex topic. In adults, initial screening is performed by the measurement of IGF-I and followed up with stimulation testing. A definitive diagnosis of GH deficiency in children usually requires the performance of a GH stimulation test, though biochemical testing is not always required for the diagnosis depending on the prior medical history. All forms of GH testing should be performed after the subject has fasted overnight. Common provocative agents include insulin, glucagon, clonidine, arginine, and l-dopa. However, the ITT requires both good communication skills and emotional resolve on the part of the patient, which are often lacking in children. This makes it a less popular option in pediatric settings. In peripubertal children, the likelihood of a falsely abnormal GH screening test can be reduced by pretreatment of both boys and girls with a short course of sex steroids (ethinyl estradiol [40 μg/m 2 daily] for 2 days before testing). The mechanism of sex-steroid priming is unclear; however, sex steroids appear to play a major role in increasing the response of IGF-I to GH at the time of puberty. From clinical experience, GH deficiency is overdiagnosed in some peripubertal children who are tested without the benefit of sex hormone priming.
Exercise physiologically enhances GH release. Typically, in the fasting state, the child exercises vigorously for approximately 20 minutes (e.g., running up and down stairs, running on a treadmill). At the completion of the exercise, when the child is tachycardic and sweating, a venous sample is collected for GH measurement ( Box 55.2 ). A baseline GH measurement is not required. A GH concentration may also be obtained 40 minutes after exercise, in case of a delayed GH release.
Brisk exercise normally causes an increase in serum growth hormone (GH) concentrations.
The test is best performed in the morning after an overnight fast but may be done at any time. Vigorous physical exercise (running or calisthenics) is performed for 20 min. A venous blood specimen for determination of GH is drawn immediately after termination of exercise.
If the serum GH concentration is 7–10 μg/L or greater (depending on the specific GH immunoassay used), GH deficiency is unlikely in children. A normal response in adults is a GH concentration of ≥5 μg/L. In children, a single subnormal response is not diagnostic for GH deficiency and should be confirmed with a second provocative test.
It has been argued that measuring GH during sleep is a physiologic assessment of the hypothalamic-somatotroph-GH axis. Some authorities suggest electroencephalographic monitoring with GH measured during deep sleep (e.g., stage III, stage IV). More simply, GH could be measured 1 hour after the onset of sleep. However, in reality, these types of sleep studies are cumbersome because they require hospitalization or overnight boarding in a sleep laboratory. In practice, they are rarely performed. Furthermore, the high cost of hospitalization or the sleep laboratory suggests that an exercise tolerance test or a simple pharmacologic stimulus would be a more cost-effective approach to initial GH testing.
As discussed, reference intervals for IGF-I and IGFBP-3 are age- and sex-dependent. , If IGF-I is squarely within its reference interval for age and sex in children, GH deficiency is excluded. If IGF-I is low, definitive GH testing is required. Because IGF-I concentrations can be depressed in states of (1) malnutrition/malabsorption, (2) obesity, (3) chronic disease, (4) hypothyroidism, and (5) sex hormone deficiency, a low IGF-I concentration does not confirm GH deficiency. IGFBP-3 is less dependent on good nutrition to achieve normal concentrations, so it may have advantages over IGF-I measurement. However, one study failed to demonstrate that measuring IGFBP-3 alone or together with free IGF-I was superior to measuring IGF-I alone as a screening test for GH deficiency. In another study, only approximately 50% of GH-deficient children had a low IGFBP-3 concentration; this finding calls into question its value as a sensitive screening test.
Analytical advantages of IGFBP-3 measurements over IGF-I measurements include the following: (1) IGF-I must be separated from its binding proteins to be measured, whereas IGFBP-3 does not require a dissociation step; (2) IGFBP-3 is present in higher concentrations than IGF-I; and (3) less age dependency is seen for IGFBP-3 compared with IGF-I. Because of stable concentrations, IGF-I and IGFBP-3 measurements may be obtained at any time of day. However, some researchers have concluded that IGFBP-3 measurements are too nonspecific to be used for the evaluation of GH deficiency. Furthermore, reports indicate that IGF-I and IGFBP-3 exhibit imperfect sensitivity and specificity for the diagnosis of GH deficiency.
IGF-I measurements in adults are often diagnostically unhelpful. For reasons that are unclear, IGF-I concentrations can be normal in GH-deficient adults. Therefore a normal IGF-I does not rule out adult GH deficiency. If the IGF-I concentration is low and suspicion for GH deficiency is high (MPHD or childhood-onset severe GH deficiency), some experts would diagnose GH deficiency in the absence of GH testing.
GH responses to insulin-induced hypoglycemia (ITT) ( Box 55.3 ) and GH responses to centrally acting pharmacologic or biological agents ( Box 55.4 ) are considered definitive tests. The stimuli can be sequential or administered on different days. The classical diagnosis of pediatric GH deficiency requires that GH responses to two different stimuli ( Table 55.6 ) be deficient. Note that many variations of these protocols exist because endocrinologists often customize these tests.
The stress of insulin-induced hypoglycemia triggers the release of growth hormone (GH) and adrenocorticotropin hormone (ACTH) from the pituitary gland in normal subjects. The GH response is measured directly. Cortisol is measured as the indication of the ACTH response.
The test is done after an overnight fast with the patient at bed rest. An indwelling intravenous (IV) line is inserted. Sampling begins after a 30-min rest period. Baseline samples are drawn for determination of glucose, GH, and cortisol. Regular insulin, 0.1–0.15 U/kg body weight, is injected intravenously. Samples are then obtained at +10, +20, +40, and +60 min for glucose, GH, and cortisol determinations. Optional time points are +30, +75, +90, and +120 min. To be confident that adequate stress has been applied, the patient must become symptomatic (exhibit sweating or tremor), or the glucose concentration must fall to less than 40–45 mg/dL (2.2–2.5 mmol/L). Additional IV insulin may be given if this has not occurred by 30 min, in which case sampling should be prolonged by 30 min. The physician should be in attendance throughout the test, and 50% dextrose for IV administration should be kept on hand to be used in the event of severe hypoglycemic reaction and after adequate hypoglycemia has been documented. Glucagon (1 mg) should be available for parenteral administration in case IV access is lost. The test is contraindicated in older adult patients and those with a seizure disorder, ischemic heart disease, or cardiovascular insufficiency.
The serum cortisol concentration should increase to a peak value of 15–20 μg/dL (414–552 nmol/L) or greater. The serum GH concentration should rise to a peak value of 5–7 ng/mL (5–7 μg/L) or greater. No response or inadequate response may be due to pituitary hormone deficiency or a hypothalamic lesion.
In normal subjects, intravenous (IV) administration of arginine hydrochloride stimulates growth hormone (GH) release.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here