Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Embryology is the basis for understanding the intimate relation between structures in different organ systems, such as the nervous system and muscle, and is primordial for understanding pathogenesis in disorders of development that in the human may present as one of the congenital myopathies. Demonstration of a defective gene provides an etiology but does not define morphogenesis. The timing and sequence of striated muscle maturation are as precise and predictable as in the nervous system. Interest in neuromuscular ontogeny began with the studies of MacCallum in the late nineteenth century. The account of histologic changes in developing human muscle published in 1917 by Tello in Spain remains as accurate and valid today as any subsequent studies by light microscopy. Ultrastructural studies of developing muscle by transmission electron microscopy began in the 1950s and were supplemented by studies using the scanning electron microscope two decades later. Histochemical techniques to demonstrate biochemical constituents and enzymatic activities in developing muscle were introduced in the 1960s and 1970s; the 1980s was a decade for the introduction of immunocytochemistry to identify other molecules of subcellular components. The late 1980s and early 1990s saw a major breakthrough in the understanding of muscle differentiation by the discovery of myogenic regulatory genes and their transcription products. Studies of the complex interactions of these genes, their translated proteins, and the role of various trophic factors continue to be the focus of current investigations in muscle ontogenesis. Modern embryology, an integration of classic descriptive morphogenesis and the molecular genetic regulation of myogenesis, is the foundation for understanding the pathogenesis of congenital myopathies.
Muscle originates even before the three definitive germ layers have differentiated from the gastrula. The epiblast, the uppermost layer of the bilayered blastula, contains primordial mesodermal cells. Epiblastic cells migrate towards a line formed between parallel ridges that converge at one end; this line is known as the primitive streak, and the convergence of the parallel ridges is designated anterior and termed the primitive node. The axis thus formed establishes bilateral symmetry and a cephalocaudal gradient as the fundamental body plan.
From the primitive node and streak, epiblastic cells stream into the space between the two layers to form paired columns of prospective mesoderm. The primitive node is the future notochord. It is positioned beneath the future neural plate, which is of ectodermal origin. The mesodermal columns are composed of undifferentiated mesenchymal cells extending the entire length of the primitive streak. These cells migrate laterally and rostrally to fill the space between the overlying surface ectoderm, which includes the neural plate, and the deeper endodermal layer, except for an area on either side of the notochord beneath the neural plate.
Further development subdivides the layer of primitive mesenchyme. A thickened band of mesoderm condenses on either side of the primitive streak, from which primordial somites will differentiate. The myotomal plates within these still-unsegmented somite columns are anatomically distinct from the broad lateral expanse and a smaller intermediate strip of mesoderm. Elongation of the embryo is accompanied by regression of the primitive node (notochordal process) and streak caudally. As the node moves posteriorly, paired blocks of somites become segmentally condensed from the originally continuous somitic plate on either side of the developing neural tube. In addition, the lateral mesoderm splits into two layers: the upper layer, or somatopleure, forms the body wall, and the lower layer, or splanchnopleure, forms the mesenteries of the internal organs. A lateral palisading of paraxial mesenchyme against the lateral aspects of the notochord precedes overt segmentation, but once formed, the boundaries between somites are stable and provide no opportunity for cellular mixing.
Segmentation of the myotomal plate occurs along a rostrocaudal gradient. As the more caudal segments are still in the process of separating, segregated anterior somites are already changing in size and arrangement. In transverse section, the somites are composed of high columnar cells arranged radially around a small central cavity. The dorsal part of the somite, the dermatome, retains this columnar structure and forms a flat plate beneath the surface ectoderm. Cells of the ventral portion of each somite disperse to form a loose network known as the sclerotome. The small myocele cavity of the somite is transitory and becomes obliterated by cellular proliferation.
The myotome that differentiates at the medial end of the dermatome as a ventral extension near the neural tube differentiates into the axial (i.e., paraspinal) muscles and also into the muscles of the extremities as the limb buds form. The contractile elements of the developing muscle are of somitic origin, whereas the tendons, interstitial connective tissue, blood vessels, and epimysial sheaths are somatopleural derivatives.
The notochord and floor plate ependyma of the neural plate synthesize retinoic acid and express a gene known in vertebrates as Sonic hedgehog. These factors are important in the dorsoventral patterning not only of the developing neural tube but also of the somites. Overexpression of these factors has a powerful ventralizing influence such that an ectopic length of notochord or a section of neural tube floor plate transplanted ventral to the newly formed segmental somite causes the excessive differentiation of sclerotome (the ventral part of the somite) and deficient formation of the myotome and dermatome, the dorsal part of the somite. As the fetus matures, the loss of balanced antagonism between ventralizing genes, such as Sonic hedgehog, and dorsalizing genes, such as those of the bone morphogenetic protein and paired homeobox families, may result in segmental amyoplasia at the level of the notochordal or floor plate implant, a disturbance of somitic induction . Notochordal signals also regulate the transcriptional cascade of myogenic basic helix-loop-helix (bHLH) genes in the somite for myoblastic differentiation. In a genetic mutant mouse in which somites form after notochordal degeneration, apoptosis of epaxial myotomes is accelerated.
All nonmyofiber components of muscle, except for axons of intramuscular nerves, are derived from neural crest. These components include Schwann cells of nerves, capsules of muscle spindles, perimysial and endomysial collagenous connective and adipose tissues, and intramuscular blood vessels. Thermal stress induces progenitor adipocyte plasticity to produce a distinct form of thermogenic beige fat cell for energy homeostasis.
A family of four proto-oncogenes directs the differentiation of striated muscle from any undifferentiated or incompletely differentiated mesodermal cell by blocking the methylation of DNA. The molecular mechanism of myoblast differentiation requires the activation and suppression of specific genes in a temporal and spatial sequence controlled by a complex array of regulatory transcription factors. Each of these genes can activate the expression of another and, under certain circumstances, can autoactivate as well. The four myogenic regulatory genes are a “family” not only because of their closely related functions in myogenesis but also because they all share encoding transcription factors of the bHLH protein, a molecular structure so fundamental in evolution that it is even found in some bacteria. These four genes, or myogenic regulatory factors (MRFs), that control the specification and differentiation of striated muscle lineage are myogenic factor 5 (Myf5), myogenin, Myf4 (also known as herculin and Myf6 ), and myoblast-differentiating factor, also known as MyoD or MyoD1 . , Several other genes influence these MRFs or interact with them in ways that augment or impair their expression. Myogenin mainly mediates myoblast fusion, and without this factor an abundance of myoblasts may form, but they do not fuse to form myotubes. The expression of Myf5 and Myf4 is transient in early ontogenesis and returns much later in fetal life to persist into adult life. Liver kinase B1 (a serine/threonine kinase) also provides a role in regulating division, self-renewal, proliferation, and differentiation of skeletal muscle progenitor cells.
One of the most remarkable and complex features of all myogenic genes is their intimate interaction with one another. After the discovery of this family of genes, initially it was thought that they all were redundant and any could substitute for another if one were deleted. Some indeed do have overlapping functions, such as Myf5 and MyoD1 , which allow normal muscle development if one, but not both, of these two are defective. , A similar overlap or redundancy exists between Myf4 and MyoD1 . In general, despite these partial redundancies, each gene regulates a different aspect of myogenesis, however, and they do not easily compensate for each other either in the embryo and fetus or in myoblast precursor cells in adults for the regeneration of muscle. MyoD1 cannot compensate for an absence of myogenin expression. MRF4, the human homolog to mouse myogenin (Myf4), but not MyoD1, can substitute for myogenin early in development. Myf5 similarly may be redundant with myogenin but less efficiently. , In Mrf4- or myogenin-deficient mice, particularly severe impairments exist in the development of the thoracic myotomes for intercostal muscle development; rib fusions and sternal defects result, but these are probably secondary because neither of these genes is expressed in rib cartilage or sternum. Immunoreactivity of MyoD1 is now available for application to human fetal and neonatal muscle and is useful for some diagnoses of congenital disorders, such as segmental amyoplasia.
Myf5 is the earliest muscle-specific bHLH gene activated in the developing embryo. , Its expression is restricted to the medial aspect of the myotome, adjacent to the least mature myocytes. Myf5 alone cannot support myogenesis without the other three bHLH genes, however. Myf5 also seems to be mitogenic in inducing the proliferation of myoblast precursors. Myf5 requires some activation by MRF4, and murine Myf4 -null mutants show extremely reduced expression of Myf5, similarly to Myf5 -knockout mice. Myogenin can substitute for Myf5 but is less efficient in this function.
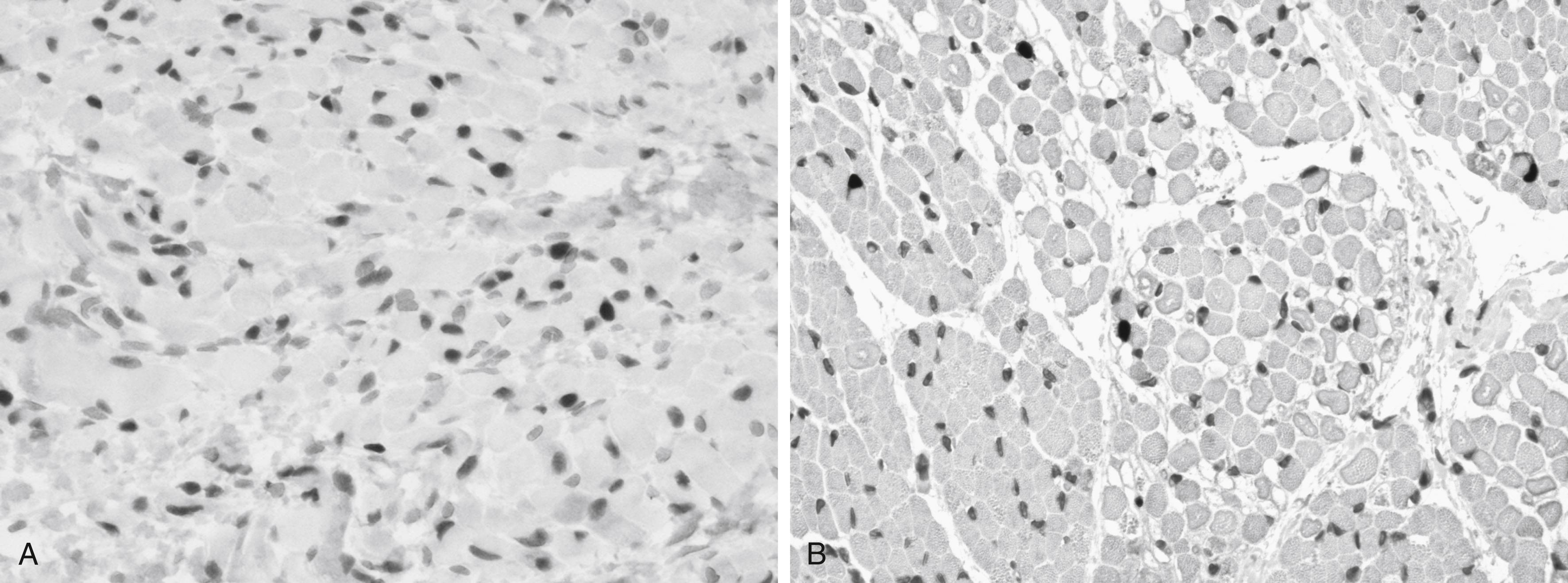
Myogenin does not serve a proliferative function in the genesis of myoblasts but is essential to myoblast fusion in the formation of myotubes, and it is expressed before fusion begins. Myogenin inactivation in mice results in a normal number of myoblasts, but they become arrested in terminal differentiation. Wild-type myoblasts can rescue myogenin-null myoblasts and cause them to fuse in vivo. Myogenin is a specific transcriptional target of mitochondrial activity, unlike Myf5 and MyoD1 . Myogenin can be demonstrated by immunoreactivity in sections of human fetal and neonatal muscle ( Fig. 138.1 ).
MyoD1 is the last of the genes to become activated. , Myf5 expression is normally suppressed by MyoD1 . Despite its late expression, fetal mouse myoblasts express both myogenin and MyoD1 on day 1 in culture, but adult myoblasts (satellite cells) have a delay in expression of these genes and of myoblast fusion. However, satellite cells coexpress proliferating cell nuclear antigen and MyoD1 on entering the mitotic cycle, and MyoD1 is critical for the programming of satellite cells into myogenin-differentiating cells. The transition from proliferation to differentiation is delayed in mice lacking MyoD1. Though coexpressed, MyoD1 and Myf5 act at different times in the cell cycle: Myf5 is maximally expressed in G 2 phase and is minimally expressed in G 1 phase; MyoD1 is maximally expressed in G 1 phase and minimally expressed in S phase; it is reexpressed in G 2 phase but not as strongly as in G 1 phase. Another bHLH muscle–restricted transcription factor, myogenic repressor (MyoR), which antagonizes MyoD1, has been identified. MyoD1 and myogenin are expressed not only during fetal ontogenesis but also in adult life and are essential to the function of myogenic stem cells (i.e., satellite cells) in normal adults and also in the regenerating muscle in muscular dystrophies. , The myogenic regulatory genes, particularly MyoD1, are so dominant that they are able to convert fibroblasts, chondroblasts, and other partially differentiated mesodermal lineages into myoblasts.
The human locus of the MyoD1 gene is on chromosome 11, very near the domain associated with embryonal rhabdomyosarcoma. The genes encoding Myf5 and Myf4 are on chromosome 12 and that for myogenin is on chromosome 1. MyoD1 and myogenin are strongly expressed in human rhabdomyosarcoma cell lines. Furthermore, the MyoD1 gene is closely related to the NeuroD gene, which may explain why some medulloblastomas of the cerebellum in children exhibit striated muscle fibers amongst the tumor cells.
In addition to the family of four bHLH genes, Pax3 has important interactions. It is redundant with Myf5 ; an absence of both Pax3 and Myf5 in embryonic mice results in generalized total amyoplasia and death; an isolated Myf5 mutation causes milder, partial amyoplasia. Up-regulation of Pax3 in chick embryos, by contrast, leads to precocious muscle development and arrested migration of myoblast precursor cells. Pax3 and Lbx1 both induce myoblast proliferation. Other genes that Pax3 induces include Dach2, Eya2, and Six1 . , Myogenin is another key regulator of Six1 . Pax3 is essential for the migration but not differentiation of limb muscle precursors. Additional important functions of Pax3 are the development of the eyes and the differentiation and maintenance of Bergmann glial cells of the cerebellum. Pax7 is critical for the normal function of satellite cells in mature muscle regeneration and is involved in fetal myogenesis as well.
Other important genes or gene families that influence the bHLH myogenic genes are bone morphogenetic protein 2, which inhibits terminal differentiation of myogenic cells by suppressing MyoD1 and myogenin, and LIM protein, which promotes myogenesis by enhancing the activity of MyoD1. Wnt signaling is also essential to myogenesis, playing a role in regulating the functions of MyoD1 and myogenin. , WNT3a also up-regulates frizzled class receptor 7 (Fzd7) and induces symmetric expansion of satellite stem cells in developing and regenerating adult muscle. ,
Myostatin is a negative regulator gene of muscle maturation. , It is a member of the transforming growth factor-β family, similar to bone morphogenetic protein. It has the latest expression in somites, after all other myogenic genes and desmin have been expressed. It is also coexpressed with MyoD1 but has a wider extent, also involving nonmuscular tissues such as the dermis. Myostatin inhibits transition from proliferation to differentiation but does not destroy myoblasts or alter mitotic cycling. Myostatin has received attention in the lay press because of its potential use to enhance athletic performance. , There is also a commercial interest in it to augment the muscular bulk of cattle and swine.
In later stages of muscle ontogenesis, the selective accumulation of MyoD1 and myogenin messenger RNA (mRNA) transcripts is not purely under genetic control but is modulated and modified by innervation of the muscle and by hormonal influences. These effects begin earlier than the stage of histochemical differentiation of muscle fiber types at midgestation. Insulin-like growth factor (IGF) regulates normal myogenin expression and influences muscle differentiation both upstream and downstream of myogenin. IGF also augments proliferation that precedes differentiation. Basic fibroblast growth factor similarly has a regulating influence on myoblast proliferation and differentiation. Tumor necrosis factor-α, a cytokine, and basic fibroblast growth factor differentially inhibit expression of myogenin induced by IGF-I in myoblasts. , IGF-I has early inhibitory and late stimulatory effects on myogenin transcription. Other families of genes the transcription factors that influence myodifferentiation include the POU homeodomain, Rho proteins, and Sox proteins. A small number of neuroepithelial cells of the mouse neural tube coexpress neuronal markers and also Myf5 and myosin in vitro, suggesting a potential for myogenesis from the neural tube itself.
MicroRNA expression to detect the posttranscriptional modulation of cellular phenotypes in muscle and particularly in some of the congenital and degenerative diseases of muscle (muscular dystrophies) in humans is now feasible and promises to resolve many questions about the genetic regulation of muscle development, as well as the pathogenetic mechanisms of several developmental myopathies. RNA interference libraries are being established in simple animals, such as the fruit fly Drosophila, which have genes that are phylogenetically conserved and include those of sarcomere formation in mammals.
Other genes essential to muscle development and maintenance are the several genes that program sarcolemmal ion channels, particularly calcium channels. These channels form before innervation of the myofiber at 9 weeks’ gestation. Several additional genes that undergo deletion or mutation are known to be the cause of congenital myopathies. Conformational analysis of mutant proteins may be applied as a tool for the classification of myopathies.
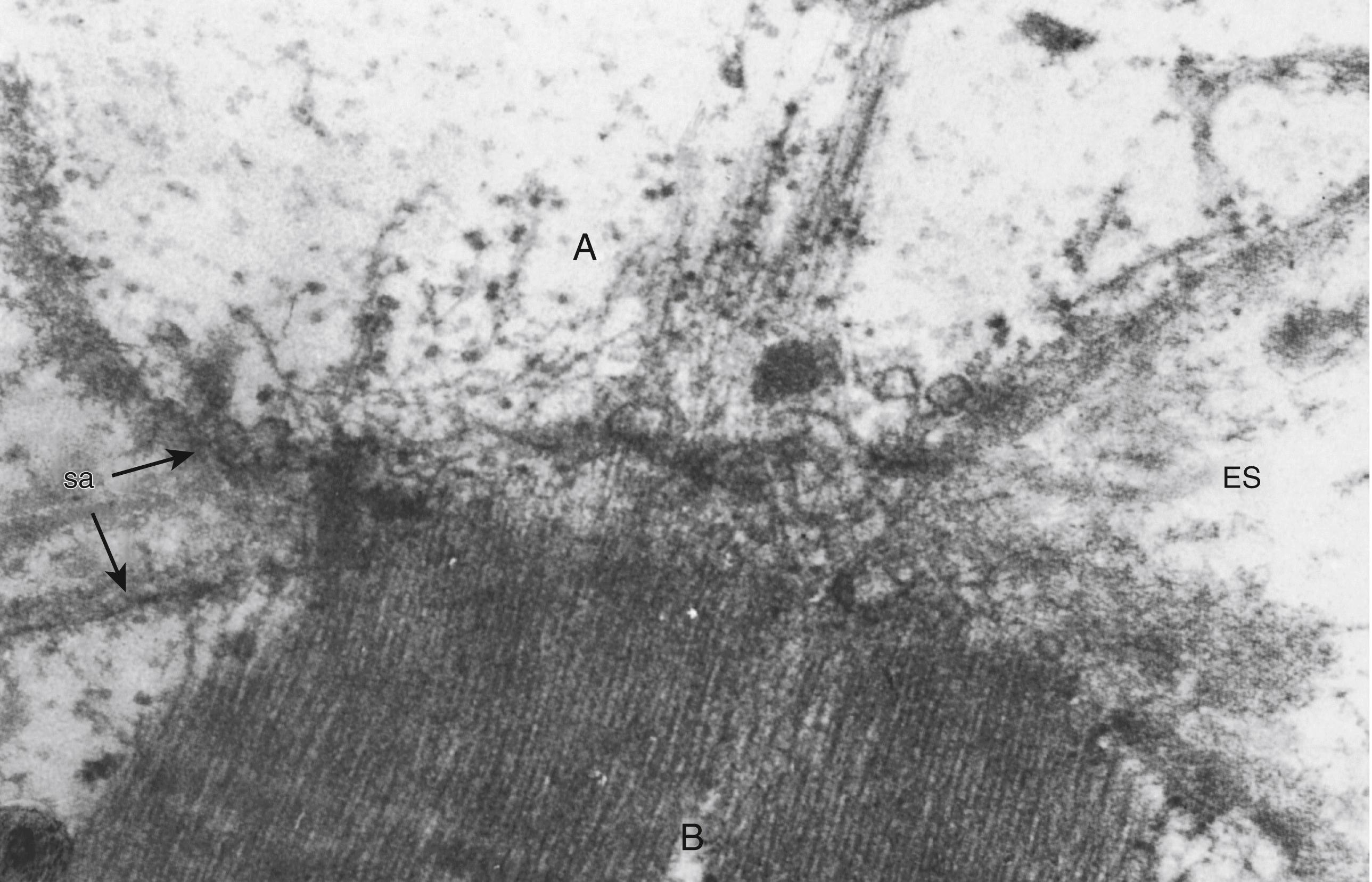
The presumptive myoblast is a morphologically undifferentiated mesenchymal cell that divides mitotically, lacks the distinctive myofilaments of muscle cells, and does not fuse with neighboring cells of the same type. The myoblast differs from its precursor, the presumptive myoblast, by synthesizing myosin and actin and assembling these proteins into thick and thin myofilaments, indicating a commitment to further differentiation as a muscle cell. Although sarcomeres do not form in myoblasts, irregularly scattered clusters of myofilaments are loosely organized into interdigitating arrays. The cytoplasm of myoblasts is sparse. Adjacent myoblasts fuse by extending their contractile myofilaments across the plasma membranes of both cells and aligning the myofilaments produced by a row of several fused myoblasts ( Fig. 138.2 ). Myoblast fusion is enhanced by isoforms of the neural cell adhesion molecule that mediates interactions of apposed plasma membranes. The protein myomaker is a membrane activator of myoblast fusion.
The multinucleated syncytium of the row of fused myoblasts forms the myotube. Early myotubes continue to fuse with myoblasts and also with other early myotubules ( Fig. 138.3 ). Circumferentially distributed myofilaments align themselves parallel to the longitudinal axis of the row of fused myoblasts as the filaments grow in length to extend through several fused myoblasts. Myotubular nuclei are enclosed within the center of the newly formed myotube as a single row within a central core of cytoplasm containing the membranous organelles and cytoplasmic particles. Cytoplasm is abundant, and contractile myofilaments are sparse in myotubes in comparison with more mature myocytes, but myofilaments and myofibrils are much more numerous in late myotubes than in early myotubes. Early myotubes predominate until the time of innervation at approximately 11 weeks’ gestation, and late myotubes are dominant during the second half of the myotubular stage from 11 to 16 weeks’ gestation.
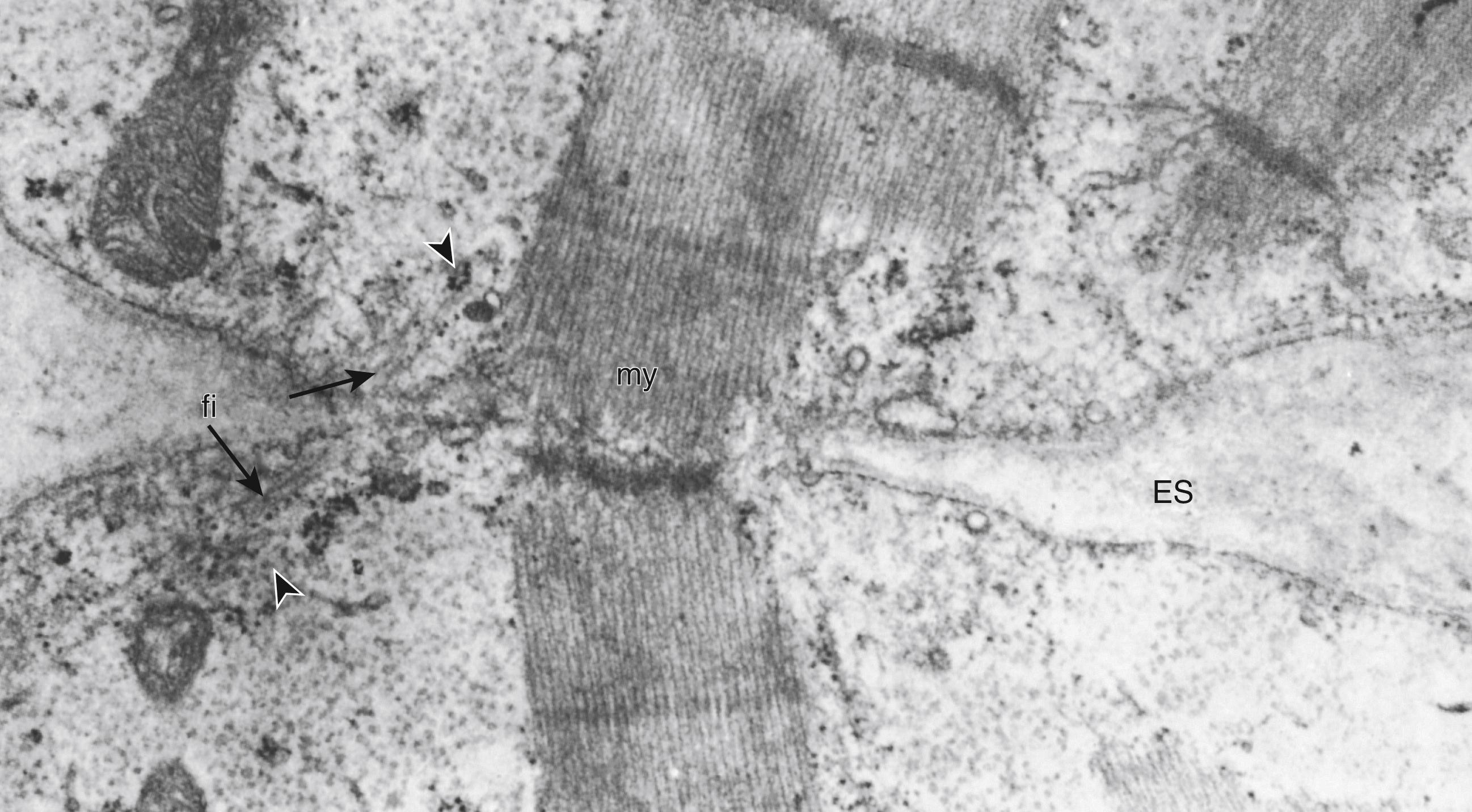
Intermediate filaments, thinner but longer than the contractile myofilaments, appear in fusing myoblasts and myotubes. These intermediate filaments, such as vimentin and desmin, are cytoskeletal proteins that help organize the cytologic structure of the muscle cell and spatial relationships of organelles, including the orientation and alignment of myofilaments (see later).
A basal lamina (i.e., basement membrane) appears as an indistinct, electron-dense thickening around the developing myotube. It usually encloses several myotubes and a number of less-differentiated myoblasts and presumptive myoblasts in the vicinity. , , Mast cells, fibroblast-like cells, and other “unclassified” cells may also be included within the basal lamina. Isolated myoblasts lack a basal lamina. In this regard, they are similar to isolated fibroblasts and mast cells within the perimysium. Further maturation of the myotube to become a myocyte involves the synthesis of more contractile myofilaments and more numerous myofibrils, displacing the central nuclei to the peripheral subsarcolemmal region and dispersing the sarcoplasm (i.e., cytoplasm) and its organelles between myofibrils. The Z bands of several myofibrils within each maturing myotube are not “in register” with each other and do not become aligned consistently until approximately 30 weeks’ gestation. The sarcotubular system begins forming in the late myotube but is not mature until after 30 weeks’ gestation.
After the myofiber has achieved morphologic maturity in the relative positions and orientations of the various subcellular components, the next phase consists of the myofiber achieving greater length, an increase in the number of myofibrils in a cross-sectional field of the myofiber, and the development of histochemical fiber types. Although the number of myofibers and their morphologic maturation are mainly genetically determined, metabolic differentiation into fiber types is principally under neural control. Metabolic fiber types also exhibit subtle morphologic differences in the relative number of mitochondria and glycogen granules and in variation in the width of the Z band. Single fiber proteomics by mass spectrometry now enables comparison, within the same myofiber, of proteins associated with different subcellular structures: plasma membrane, mitochondria, sarcoplasmic reticulum (SR), myofibrils, and nucleus.
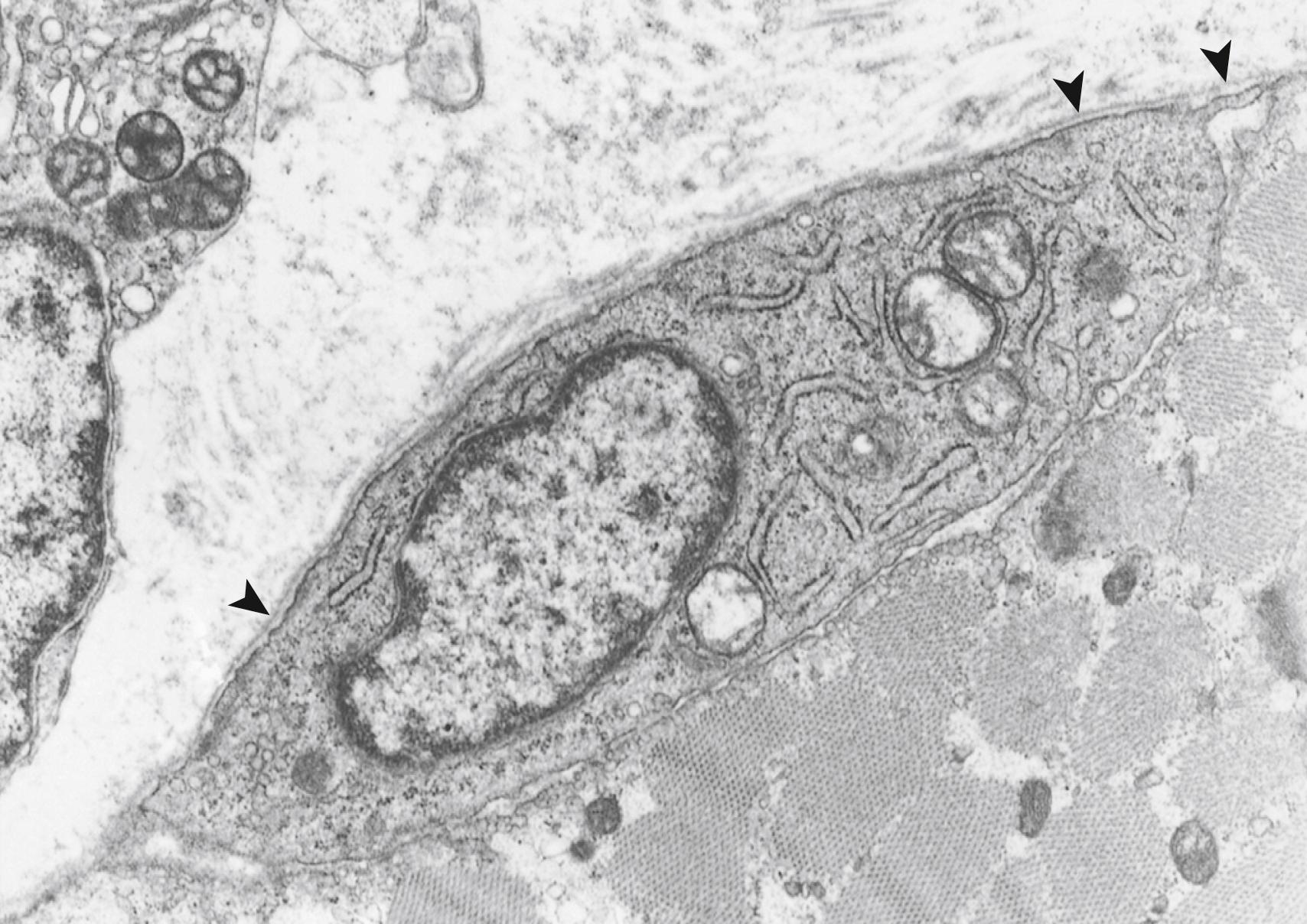
Spindle-shaped mononucleated cells with abundant polyribosomes and endoplasmic reticulum persist in close apposition to the myotube and myocyte (i.e., multinucleated myofiber) enclosed within the same basal lamina. Such cells are presumptive myoblasts ( Fig. 138.4 ). In mature muscle, they are redesignated satellite cells ( Fig. 138.5 ). Although they are dormant and inactive, they are capable of mitotic proliferation, migration, fusion, and myogenesis even in the adult and are the basis of regeneration of injured muscle. In normal mature muscle, about 7% of the nuclei at the periphery of the myofiber are really satellite cells; this percentage is higher in immature muscle.
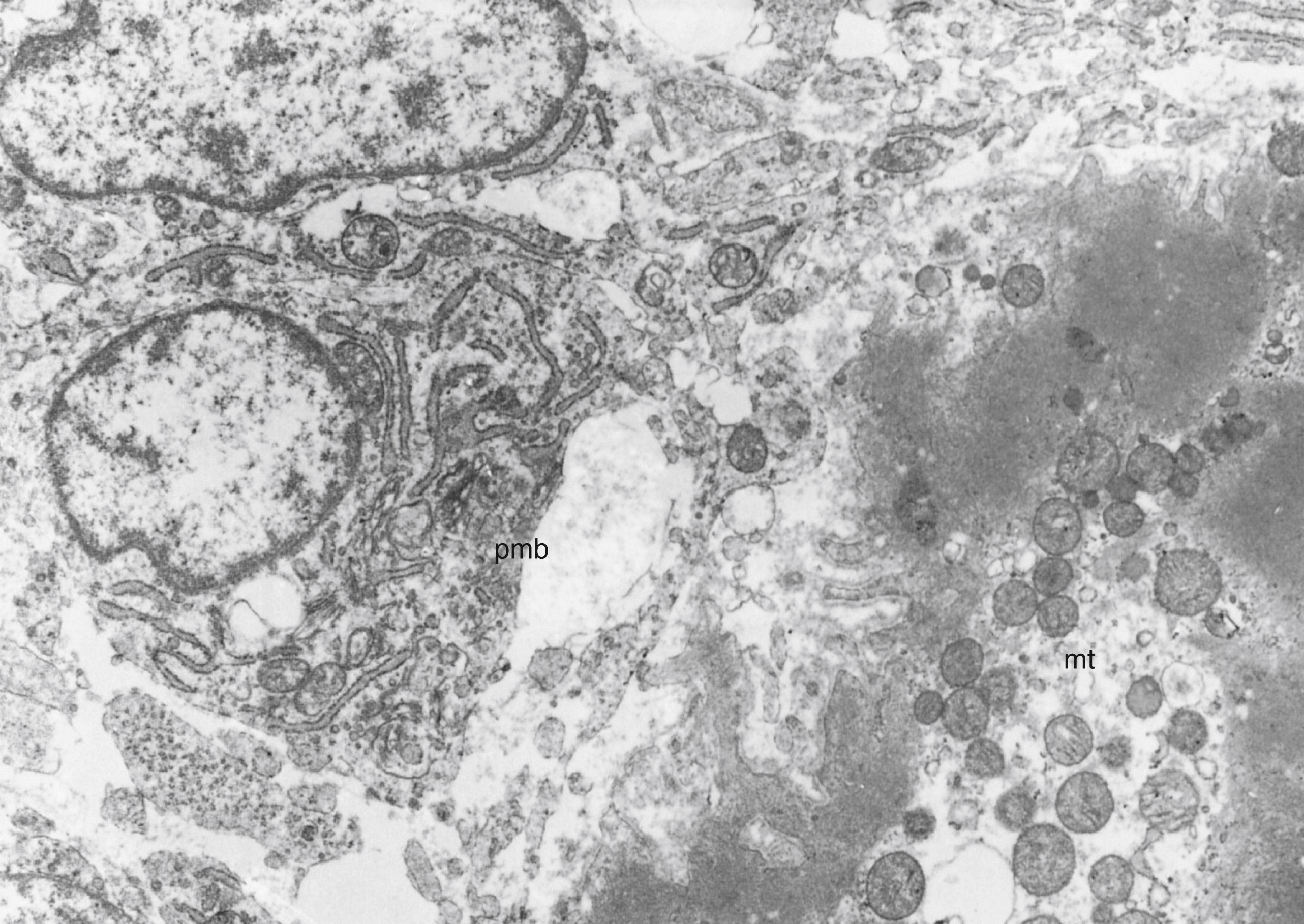
No stage of fetal muscle development is “pure” in the sense of muscle cells exhibiting the same degree of maturation uniformly. Transitions among periods of development are gradual. At 5 to 8 weeks’ gestation, presumptive and true myoblasts predominate, but scattered myotubes are already evident at 6 weeks’ gestation. The period from 8 to 16 weeks’ gestation is regarded as the myotubular stage of development, and myotubes are indeed the most numerous types of muscle cells. However, many myoblasts are still identified, presumptive myoblasts frequently undergoing mitosis are encountered, and a few maturing myocytes beyond the myotubular stage are scattered during the latter weeks of this period. From 16 to 20 weeks’ gestation, a progressive maturation of myotubes to the cytologic structure of the mature myocyte occurs, but a few myotubes persist. Histochemical differentiation of muscle occurs mainly between 20 and 30 weeks gestation. From 30 weeks’ gestation to term, myofibers undergo several more subtle morphologic adjustments to maturity, such as alignment of Z bands of adjacent myofibrils to attain “register” (i.e., parallel alignment) and further development of the sarcotubular system.
A 427-kDa cytoskeletal protein known as dystrophin is encoded by a large gene at the Xp21.2 locus. This subsarcolemmal protein attaches to the sarcolemmal membrane overlying the A band and M band of the myofibrils and consists of distinct regions or domains: the amino terminus contains 250 amino acids and is related to the binding site of α-actinin; the second domain is the largest, with 2800 amino acids, and contains many repeats, giving it a characteristic rod shape; a third domain is rich in cysteine and is related to the carboxyl-terminus domain of 400 amino acids, unique to dystrophin and to a dystrophin-related protein encoded by chromosome 6. Dystrophin is part of a large tightly associated glycoprotein complex containing other proteins called dystrophin-related proteins. Dystrophin may be demonstrated in striated muscle by its immunoreactivity, either by immunoperoxidase reaction ( Fig. 138.6 ) or by fluorescence microscopy, and is detected in developing human fetal muscle at 11 weeks gestation. , Dystrophin mRNA is normally detected in cardiac and smooth muscle in striated muscle and in the brain. The dystrophin gene encodes a 14-kilobase mRNA of approximately 80 exons. Defective dystrophin is the biologic basis of Duchenne and Becker muscular dystrophies.
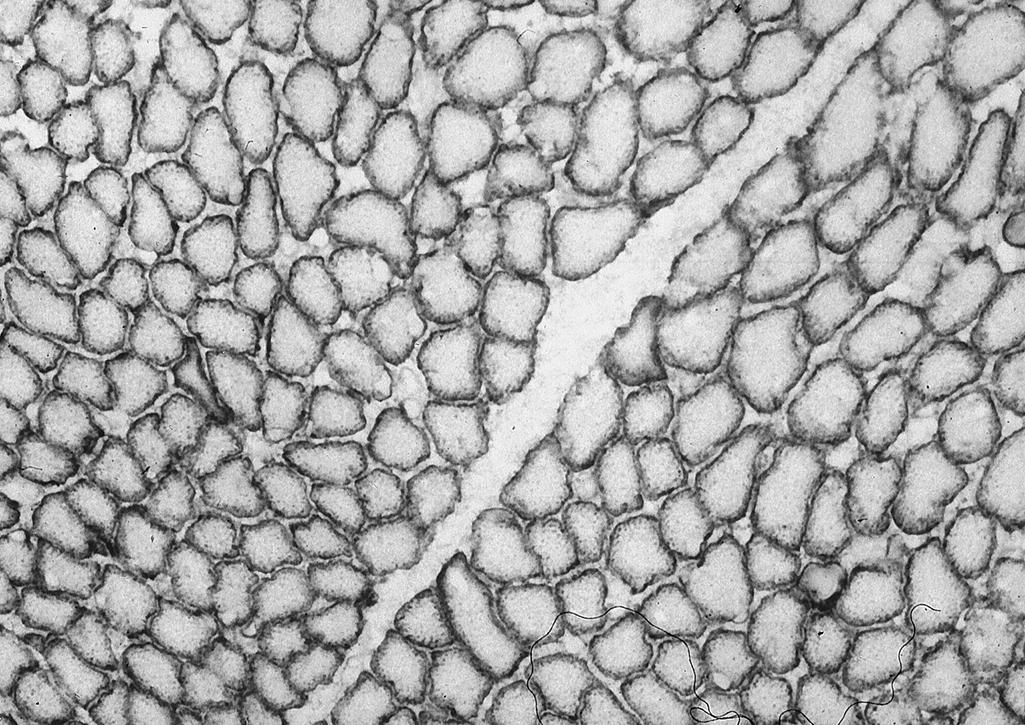
Several dystrophin-related glycoproteins also form in this same general period. α-Sarcoglycan (formerly adhalin ) becomes expressed before 14 weeks gestation. The other sarcoglycans (β, γ, and δ) are expressed shortly thereafter. Dysferlin and caveolin 3 are other sarcolemmal membrane proteins at sites different from those of dystrophin and the sarcoglycans. Dysferlin deficiency is the basis of a well-documented progressive myopathy in children and adults, but dysferlin overexpression in striated muscle can also produce a progressive myopathy; hence the genetic regulation of expression, including its binding proteins, must be precise. Caveolin 3 is first detected on day 10 in developing human somites.
α-Dystroglycan is another sarcolemmal regional protein. O -Glycosylation of this protein may be altered by mutations of several genes during development, and hypoglycosylation is the molecular basis for some of the congenital muscular dystrophies.
The precise timing of the earliest expression of most sarcolemmal region proteins is uncertain, but collagen VI, which is deficient in a congenital muscular dystrophy known as Ullrich disease , is probably first detected at approximately 10 to 12 weeks, which is similar to other proteins that attach to the sarcolemma. The principal source of collagen VI synthesis in striated muscle is the interstitial resident fibroblasts in the muscle. It is noteworthy that this timing of the appearance of sarcolemmal regional proteins coincides with the time of innervation of muscle at 9 to 11 weeks’ gestation. Knowledge of this timing is important in medicine because fetal muscle biopsies may now be performed during the course of pregnancy. Therefore if a fetus is at risk for one of these diseases because of involvement of a sibling, and the family wishes to know whether the present fetus is similarly involved, an accurate diagnosis can be confirmed as early as possible by immunoreactivity of sections of the fetal muscle or by haplotype analysis. The various sarcolemmal regional proteins of striated muscle and their genetic defects in various myopathies were reviewed by Sewry.
Cadherins are another group of transmembrane proteins that mediate calcium-dependent cell-cell adhesion by means of homophilic binding; M-cadherin is a cell type–specific member of this family. In regenerating muscle and in developing fetal muscle, reactivity for M-cadherin is restricted to the plasma membrane of myoblasts and satellite cells and is most intense at the membrane areas facing adjacent myotubes or myofibers. M-cadherin is genetically expressed but appears to be neurally regulated ; it is not expressed in denervated muscle. The mechanism by which increased cadherin expression promotes early steps in muscle differentiation is by up-regulation of myogenin. ,
Merosin is an extracellular matrix protein forming the heavy chain of the skeletal muscle basement membrane protein laminin and is important for maintaining the integrity of the sarcolemma. Defective merosin is implicated in the pathogenesis of one form of congenital muscular dystrophy, and the chromosomal site of the disease gene is at the 6q2 locus. The merosin (laminin subunit α 2 ) gene maps to locus 6q22-23. , Merosin may be detected before birth in the human placenta. Type XIX collagen is a transient extracellular matrix protein that contributes to the formation of fetal basal lamina and closely follows the expression of Myf5 . Extracellular matrix is required for muscle differentiation and for the fusion of myoblasts to form myotubes independently of myogenin. Inhibition of fibrillar collagen production in avian and mammalian embryos results in abnormal somite formation and perturbed myogenesis.
The nuclear envelop proteins Lamin-A and Lamin-C, encoded by the lamination’s A/C (LMAC) gene, are normally expressed in both striated and cardiac muscle during development and modulate actin dynamics. Their mutation results in Emery-Dreifuss muscular dystrophy with cardiomyopathy and a few other rarer myopathies.
The innervation of muscle fibers occurs at approximately 11 weeks’ gestation, in the middle of the myotubular stage. Muscle spindles and Golgi tendon organs form after the myotubular stage as the general fascicular organization of the muscle evolves.
The development of joints in the extremities begins at approximately 5 weeks’ gestation within the early mesenchymal condensations of precartilaginous bone. Joint spaces are already apparent by 7 weeks. Motion is probably essential for the development of joints, and some articular motion is evident as early as 8 weeks, before myotubes are innervated. These small-amplitude movements probably result from spontaneous contractions of newly formed sarcomeres. Active fetal movements are well established after 11 weeks, with the innervation of skeletal muscle, and increase progressively in both force and range of motion around articulations until birth.
In addition to the various structural proteins of the myofiber and especially of the sarcolemma, certain “chaperone proteins” are essential for intracellular folding and assembly of polypeptides to achieve the final molecular structure of cellular structural proteins. However, they remain chaperones because they do not themselves become incorporated into the molecular structure of the protein they regulate in development. One of the most recognized of these chaperone proteins is the small heat shock protein α-B-crystallin, closely related to heat shock protein 27, which is found in all tissues and is phylogenetically very old, expressed even in bacteria and simple worms. It is demonstrated in both cytoplasm and nucleoplasm. It inhibits aggregation and precipitation of denatured proteins and is up-regulated in a variety of tissues during various adverse or stressful conditions, such as fever, chronic ischemia, acidosis, X-irradiation, exposure to toxins, and in epileptic activity in the brain. , A close association of α-B-crystallin with the Z band of striated muscle implies that it is particularly important in protecting the Z band from the disintegration that results in myofibrillar breakdown and also protects myosin from thermal denaturation.
In muscle, the natural form of α-B-crystallin is overexpressed in myofiber atrophy, associated with a decrease in the amount of the cytoskeletal microtubule protein tubulin. It is also overexpressed, but as a mutant form, in certain diseases of muscle known as myofibrillar myopathies and may cause severe weakness and muscle stiffness leading to early infantile death. The α-B-crystallin may have an additional role in muscle in regulating connective tissue proliferation, as it does in the dermis of the skin during wound healing. In normal human fetal muscle, this protein is not demonstrated by immunoreactivity at any gestational age, perhaps because the concentrations are too low to detect by this method.
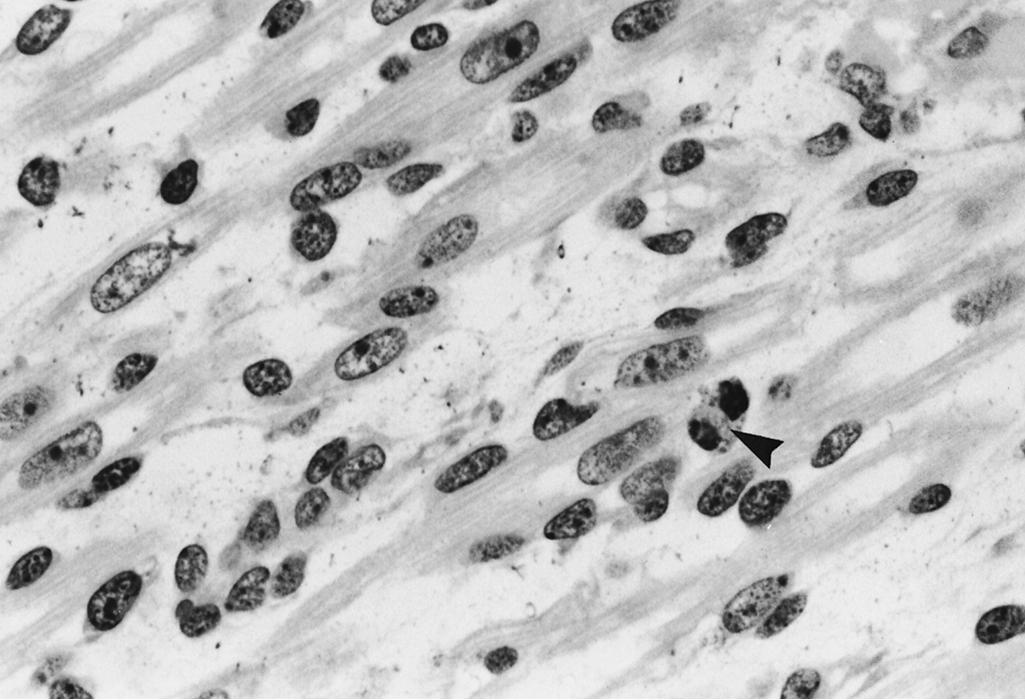
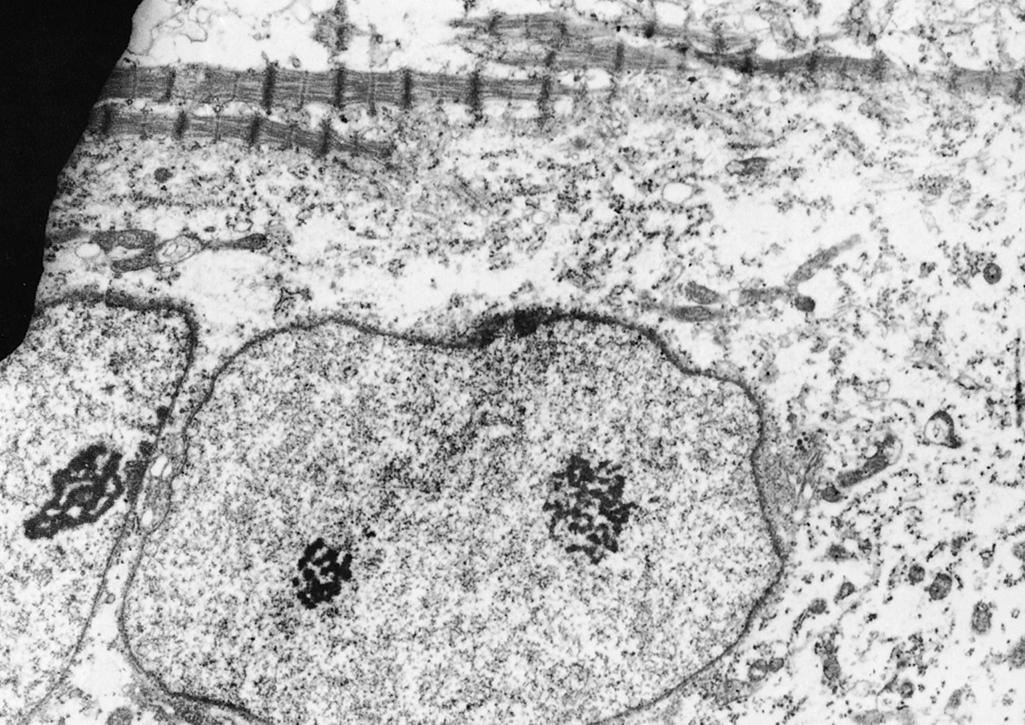
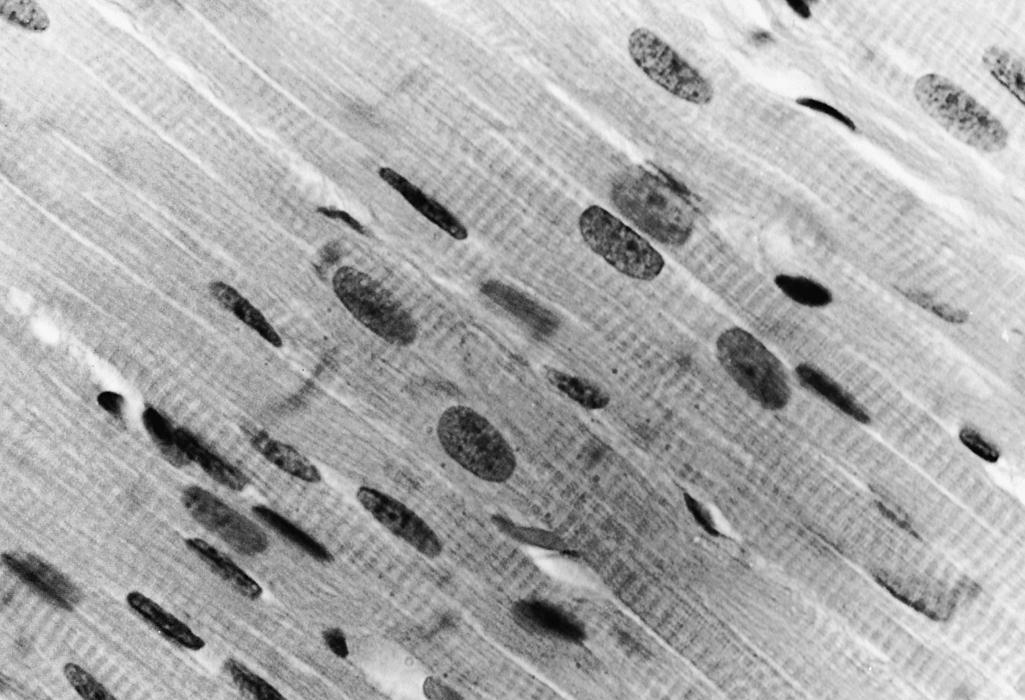
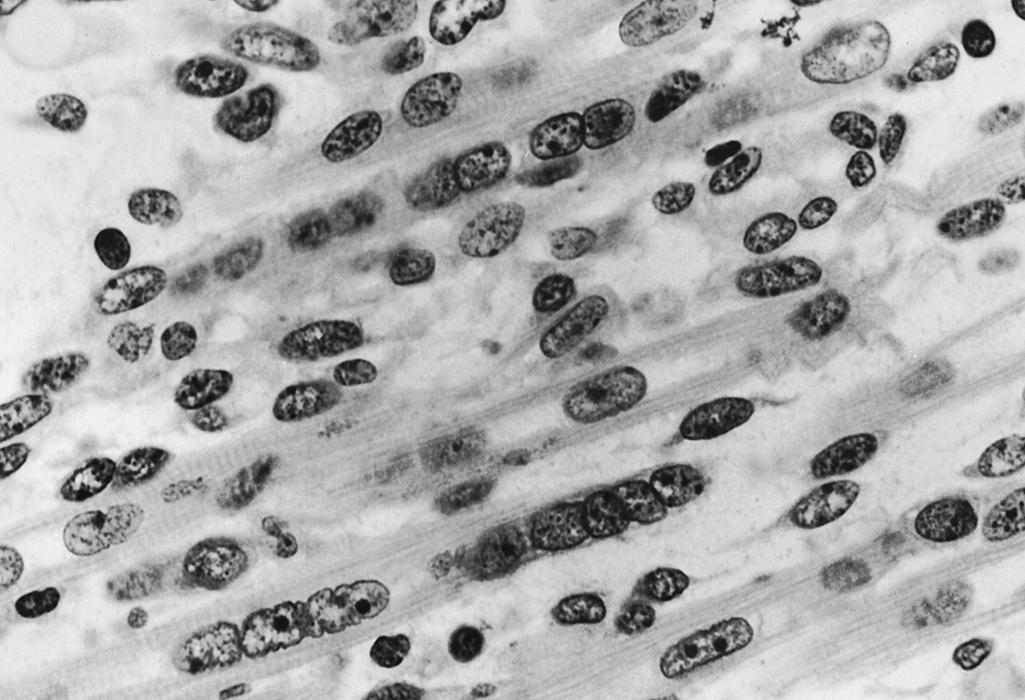
Myoblasts and myotubes have large, vesicular, ovoid nuclei. The chromatin is finely dispersed throughout the nucleoplasm, with a thin condensation against the nuclear membrane. One or often two prominent nucleoli are present ( Fig. 138.7 ). Ultrastructurally, the ribonucleoprotein of these nucleoli forms coarse, electron-dense strands (nucleolonemata) rather than the more massive nucleolar sphere of the mature sarcolemmal nucleus ( Fig. 138.8 ). These features of the nucleoli and of the finely dispersed DNA are characteristic of active transcription of RNA needed for the synthesis of contractile proteins. The nuclei of regenerating myofibers of children and adults have a similar appearance. The nuclear membrane of fetal myotubes contains pores, similarly to the mature state. In the term neonate, some sarcolemmal nuclei are still vesicular and ovoid as are seen in less mature stages, but most nuclei have acquired the elongated shape with dense chromatin that characterizes the mature state ( Fig. 138.9 ). The nuclei of myotubes are arranged in a single-file row within the central core of sarcoplasm. Nuclei may be tightly packed and in contact with neighboring nuclei, or they may be separated by intermediate segments of sarcoplasm of variable lengths ( Fig. 138.10 ). Both arrangements occur simultaneously within the same muscle, and contiguous as well as widely spaced nuclei occur within different segments along the same myofiber. Closely spaced nuclei are generally more characteristic of early myotubes than of late myotubes. Intranuclear lamin A/C is expressed in differentiating myoblasts. Lamin A/C, lamin B1, and emerin are defective in certain hereditary myopathies, highlighting the importance of myonuclear membrane proteins, as well as sarcolemmal plasma membranes, in the integrity of normal muscle development and maintenance.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here