Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Carcinoma of the lung is the second most common cancer worldwide (over 2 million new cases in 2020) and is the number one cause of cancer death, at 1.8 million worldwide in 2020. , Owing to this frequency, lung specimens, either for diagnosis, or obtained at resection, are common in the pathology laboratory. This chapter is divided into four sections. In the first section, general considerations regarding the epidemiology, clinical presentation, and treatment of lung carcinomas will be introduced, followed by a discussion of specific subtypes of non-neuroendocrine, non-sarcomatoid lung carcinoma in the second section. Neuroendocrine neoplasms and sarcomatoid carcinomas are discussed in separate chapters ( Chapters 14 and 15 , respectively). The third and fourth sections will offer practical approaches to diagnosing lung carcinomas in small biopsies and surgical resections, respectively, discussing general considerations and addressing specific challenges commonly encountered with each of these two specimen types.
Lung cancer today is the number one cancer killer of both men and women, since surpassing the death rate of breast cancer for women 3 decades ago. In 2021, it was estimated that over 235,000 individuals in the United States would be newly diagnosed with lung cancer and 131,000 deaths would be caused by lung cancer. Lung cancer diagnosis approaches 13% of all cancer diagnoses, and over 20 million (6.1%) people in the United States today are expected to develop lung cancer at some point during their lifetime. , Worldwide, lung cancer deaths exceed 2 million per year, accounting for over 20% of cancer deaths globally. ,
Lung cancer is typically a disease of older adults, with over 80% of lung cancer patients being over 60 years of age at diagnosis. Age-adjusted lung cancer incidence rates in the United States parallel smoking prevalence rates, with the highest rates in Kentucky and the lowest rates in Utah. In the past 3 decades, lung cancer incidence has fallen in men in the United States after it peaked in 1984. For women, the incidence did not peak until 1998, but is also declining. , After many years of increasing deaths from lung cancer in the United States, in the last decade mortality rates from lung cancer have finally begun to decline, likely reflecting historical declines in cigarette smoking, though lung cancer remained the leading cause of cancer-related death at the time of this writing. The age-adjusted death rate is higher in men than in women. , The age-adjusted death rate for lung cancer is higher in Black-Americans than in White Americans. , It is almost one-third higher for Black men than for White men, even though Black men have a lower overall exposure to cigarette smoke; and the incidence for Black women is the same as for White women despite a lower overall exposure to cigarette smoke. ,
Cigarette smoking is by far the most common cause of lung cancer, attributable to 90% of cases in men and 80% in women. Compared with people who have never smoked, male smokers are 23 times more likely to develop lung cancer, and female smokers are 13 times more likely. , Second-hand smoke exposure has also been implicated in the development of lung cancer. , Other causes of lung cancer include radon exposure, occupational exposure to carcinogens, and air pollution; these account for approximately 10%, 10%, and 2% of lung cancer cases, respectively. , Some patients may have combined risks. Asbestos-exposed workers who are nonsmokers have an approximately fivefold increased risk of developing lung cancer; however, asbestos-exposed workers who are also smokers have a greatly increased risk. ,
Most patients with lung cancer present with locally advanced or metastatic disease and are symptomatic at the time of diagnosis; only about 10% of patients are asymptomatic at diagnosis. , Symptoms are frequently present for months prior to diagnosis. , Symptoms related to the local effects of lung cancer, such as cough, are frequently attributed to comorbidities such as chronic obstructive pulmonary disease by both patients and their physicians. As a rough approximation, one-third of patients will present with symptoms related to local disease, such as an unrelenting cough; one-third will present with nonspecific symptoms related to metastatic tumor, such as weight loss and fever; and one-third will present with symptoms specifically related to metastatic disease, such as site-specific pain or neurological symptoms and signs.
Local symptoms are dominated by cough, as well as dyspnea, wheezing, chest pain, dysphagia, hoarseness, superior vena cava syndrome, and hemoptysis. Cough is typically caused by bronchial, parenchymal, or hilar nodal involvement with tumor and by postobstructive changes. Wheezing usually indicates airway obstruction by tumor directly or by extrinsic compression of the airway. Dysphagia occurs in lung cancer patients in general because of esophageal compression caused by mediastinal tumor, especially subcarinal lymph node enlargement from tumor. Dysphagia may also indicate esophageal involvement with tumor. Hemoptysis is the most alarming symptom, usually resulting in quicker patient evaluation. Chest pain suggests pleural or chest wall involvement by tumor. A significant minority of lung cancer patients may develop hoarseness, typically due to laryngeal nerve palsy; it is more often seen in left lung tumors due to involvement of the recurrent laryngeal nerve as it loops under the aortic arch. Superior vena cava syndrome is a serious complication in lung cancer patients; it is secondary to local invasion and compression. It involves approximately 5% of non-small cell lung cancer (NSCLC) patients and 10% of small cell lung cancer (SCLC) patients. Patients with superior vena cava syndrome show swelling of the face, neck, arms, and upper chest, and they are frequently dyspneic. Lung cancer is the cause of approximately three-fourths of superior vena cava syndrome cases. ,
Lung cancer in the lung apex may invade local structures, such as the brachial plexus, vertebrae, or ribs, and cause Pancoast syndrome, which manifests as shoulder pain, upper extremity paresthesias and weakness, and Horner syndrome. Horner syndrome—unilateral ptosis and lack of facial sweating on the involved side—is due to tumor compression or involvement of the sympathetic chain. Nonspecific symptoms of metastatic disease include weight loss, fever, weakness, and anorexia. Anemia is suggestive of metastatic disease, and elevated liver enzymes suggest hepatic metastases. Local symptoms of metastatic disease include bone pain and neurologic symptoms such as headache, nausea, mental status changes, and seizures. Over two-thirds of patients who present with brain metastases are found to have lung cancer as the origin of the metastases.
There are numerous paraneoplastic syndromes associated with lung cancer, including endocrine, neurologic, hematologic, rheumatologic, renal, cutaneous, skeletal, and metabolic syndromes. It is important to remember that paraneoplastic syndromes are not in and of themselves indicative of advanced disease. Approximately 10% of lung cancer patients will present with or develop a paraneoplastic syndrome. Frequently paraneoplastic syndromes occur due to hormone or hormone analog secretion by tumor cells. An example of this is the syndrome of inappropriate antidiuretic hormone, which arises in a significant minority of SCLC patients. Ectopic production of adrenocorticotropic hormone may occur in half of lung cancer patients; however, only about 5% develop Cushing syndrome. , SCLC patients with Cushing syndrome have a worse prognosis, frequently due to their increased susceptibility to opportunistic infection during chemotherapy. , Digital clubbing is a skeletal paraneoplastic syndrome that may arise in up to 30% of NSCLC patients; it is characterized by enlargement of the distal fingers and toes caused by a proliferation of connective tissue beneath the nail matrix. Many paraneoplastic syndromes regress upon the initiation of lung cancer treatment.
For patients who may have lung cancer, radiology shares with pathology the need to work as a team to establish a pathologic diagnosis and efficiently stage the disease. Imaging workup of the lung cancer patient by computed tomography (CT) typically includes the chest and upper abdomen, which allows for visualization of the liver and adrenal glands. CT helps characterize the tumor’s clinical stage, specifically features such as tumor size, the existence of satellite nodules, invasion of adjacent tissues, infection or atelectasis, and regional lymph node involvement. However, CT alone is insufficient for lymph node staging. Chest CT may also assist in distinguishing pleural and pericardial masses and effusions, contralateral lung lesions, and metastases. Intravenous contrast assists both in the delineation of hepatic and adrenal lesions and in the differentiation of mediastinal and hilar lymph nodes from blood vessels.
Positron emission tomography (PET) using 18-fluoro-2-deoxyglucose (FDG) is another common noninvasive method of examining lung cancer patients. Its use helps reduce the risk of patient understaging. Metabolic uptake of FDG, suggesting intense hypermetabolic activity, is displayed as a bright image on PET and is suspicious for malignancy. It is important to remember that other causes of intense hypermetabolism such as infection or an area of healing tissue, such as a healing operative site, will cause false positive findings. In contrast, small malignant foci may yield false negative findings on PET. , Physiologic uptake of FDG, such as in the gastrointestinal tract, genitourinary tract, brain, and heart, reduce PET scan precision in those areas. Currently, combined PET/CT scans are used for lung cancer patients to maximize sensitivity and specificity. , For examination of possible intracranial tumors, magnetic resonance imaging (MRI) is useful.
Current lung cancer screening programs are based on low-dose computed tomography (LDCT) performed on patients who meet specific criteria. They must be aged 55 to 74 years, have at least a 30-pack-year smoking history, and be either current smokers or previous smokers who have quit within the past 15 years. , The National Cancer Institute’s National Lung Screening Trial reported a 20% relative reduction of mortality from lung cancer with LDCT screening, and similar benefits of LDCT in this context have also been reported in Europe. ,
Clinical staging is used as an initial assessment of lung cancer tumor burden and extent; however, the pathologic tumor, node, metastasis (TNM) stage is most important for determining the best treatment options for lung cancer patients. Pathologic staging of lung cancer is critical because staging indicates prognosis and determines treatment. , Incorrect or incomplete staging could prevent lung cancer patients from receiving potentially curative therapy. Stage IA, IB, IIA, and IIB NSCLC patients may be candidates for potentially curative surgery, whereas stage IIIA, IIIB, and IV patients are typically candidates for various forms and combinations of chemotherapy and radiotherapy. With small cell carcinoma, surgery is rarely an option; however, staging assists in determining whether chemotherapy along with radiotherapy or chemotherapy alone is the appropriate treatment. The College of American Pathologists’ Protocol for the Examination of Resection Specimens From Patients With Primary Non-Small Cell Carcinoma, Small Cell Carcinoma, or Carcinoid Tumor of the Lung (based on American Joint Cancer Committee [AJCC] Cancer Staging Manual , 8th edition) is a useful online resource for staging lung cancer.
Overall lung cancer 5-year survival in the United States is approximately 22% and over one-half of patients die within a year of diagnosis, , but the prognosis is highly dependent on the stage of disease. The 5-year survival rate for patients with early-stage NSCLC having localized disease confined to the primary site is approximately 60%. In contrast, the prognosis in lung cancer patients with spread to regional lymph nodes or distant metastasis is considerably worse, with a 5-year survival rate of approximately 33% or 6%, respectively.
Traditional lung cancer therapies include surgery, chemotherapy, and radiotherapy. For surgery to be potentially successful in lung cancer patients, it must be appropriate based on evidence-based medical literature, the surgery must be technically feasible, and the patient must be fit enough to tolerate the surgery. Potential surgeries include wedge resection, segmentectomy, lobectomy, and pneumonectomy. Lymph node sampling is also frequently performed at surgery for pathologic staging purposes. In patients with direct chest wall extension, chest wall resection may be appropriate. Palliative resections are rarely indicated because of their significant morbidity; however, palliative resection may be appropriate in patients with massive hemoptysis or superinfection in necrotic tumors.
Chemotherapy has a primary therapeutic role in patients with Stage II and IIIA NSCLCs. External beam radiotherapy also plays a major role in therapy for patients with stage III and IV lung cancer. In patients with marginally resectable tumors, radiotherapy increases the chance for cure. For inoperable lung cancer patients, radiotherapy helps provide local control and increases median survival when combined with chemotherapy. In patients with advanced disease, radiotherapy often provides successful palliation.
Over many decades, the benefits of traditional therapeutic approaches for lung cancer have been modest for many patients and improvements have been incremental. However, in recent years, the identification of genetic alterations in some lung cancers and the development of targeted therapies have together revolutionized the treatment of patients with tumors harboring relevant molecular alterations. This revolution in oncology has been further aided by the advent of immune checkpoint inhibitors that harness the power of the immune system to target lung cancers and a wide variety of other malignancies. For patients with advanced lung cancer harboring an actionable gene mutation, a variety of approved tyrosine kinase inhibitors are now available that target these specific molecular abnormalities, and numerous other inhibitors and monoclonal antibody agents are in development or in clinical trials for these and other potential targets. Depending on the tumor type and stage of disease, such inhibitors may be used as first-line therapy alone or in combination with traditional chemotherapy agents, or as second-line therapy upon tumor progression.
Testing of tissue samples for relevant genetic alterations has become the standard of care for patients with lung cancer requiring systemic therapy, and the College of American Pathologists (CAP), International Association for the Study of Lung Cancer (IASLC), and Association for Molecular Pathology (AMP) have issued guidelines for molecular testing for the selection of lung cancer patients for treatment with targeted tyrosine kinase inhibitors. These guidelines were initially introduced in 2013 and underwent substantial updates in 2018. Since that time, additional recommendations for molecular testing have been added to guidelines published and regularly updated by the National Comprehensive Cancer Network. At the time of this writing, testing of the EGFR , ALK , ROS1 , BRAF , NTRK1/2/3 , MET (exon 14 skipping), and RET genes should be included as part of broad molecular profiling, in addition to programmed death ligand-1 (PD-L1) testing, on all pulmonary adenocarcinomas, large cell carcinomas, and NSCLCs not otherwise specified. At the present time, pulmonary squamous cell carcinomas only require PD-L1 testing and broad molecular profiling is not yet mandated but should still be considered. A detailed review of these guidelines is beyond the scope of this chapter, but it should be noted that testing guidelines and discovery of new drugs and corresponding predictive biomarkers are constantly evolving and the interested reader should refer directly to these living documents for the most current guidelines.
Although the degree of involvement of pathologists in molecular test selection varies by practice location and local oncologists’ preferences, appropriate selection and proper management of lung cancer tissues for predictive biomarker testing is a key responsibility and practical matter that always falls on the pathologist’s shoulders. These tissues include resection specimens, but they most often consist of small biopsies and cytology specimens. The relatively small amount, and sometimes paucity, of tumor tissue for testing requires the pathologist to be frugal in the handling of samples. At the present time, selection of tumors for molecular testing is largely focused on a diagnosis of adenocarcinoma or inability to exclude a diagnosis of adenocarcinoma. Thus making the diagnosis of adenocarcinoma is important in identifying which tumors require broad molecular profiling, a task that may require ancillary testing to accomplish, but sufficient tissue should also be preserved for molecular testing. For tumors showing morphologic features enabling a definitive diagnosis (i.e., intracytoplasmic mucin or gland formation indicating adenocarcinoma, or squamous pearl formation or intercellular bridges indicating squamous cell carcinoma), immunohistochemistry (IHC) to confirm the diagnosis is unnecessary and should be avoided, to preserve tissue for molecular testing. In cases where a definitive diagnosis cannot be reached on morphologic grounds alone, IHC should be used judiciously, always with an eye to preserve as much tissue as possible for subsequent molecular testing. Most cases can be resolved with TTF-1 and p40 immunostains alone, and additional immunostains should only be performed when the clinical setting or morphologic features require them. Methods, technology, and recommendations for handling and testing of small biopsies and cytology specimens showing lung cancer are constantly evolving , , but commonly used techniques are discussed in greater detail in the third section of this chapter.
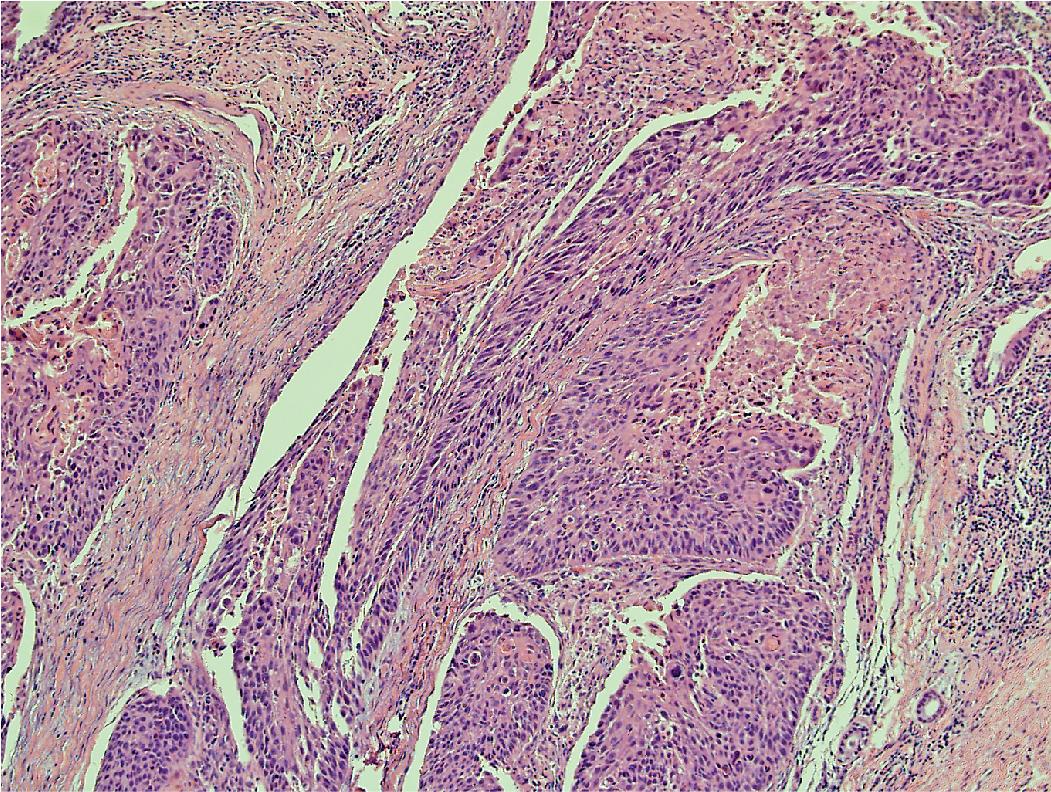
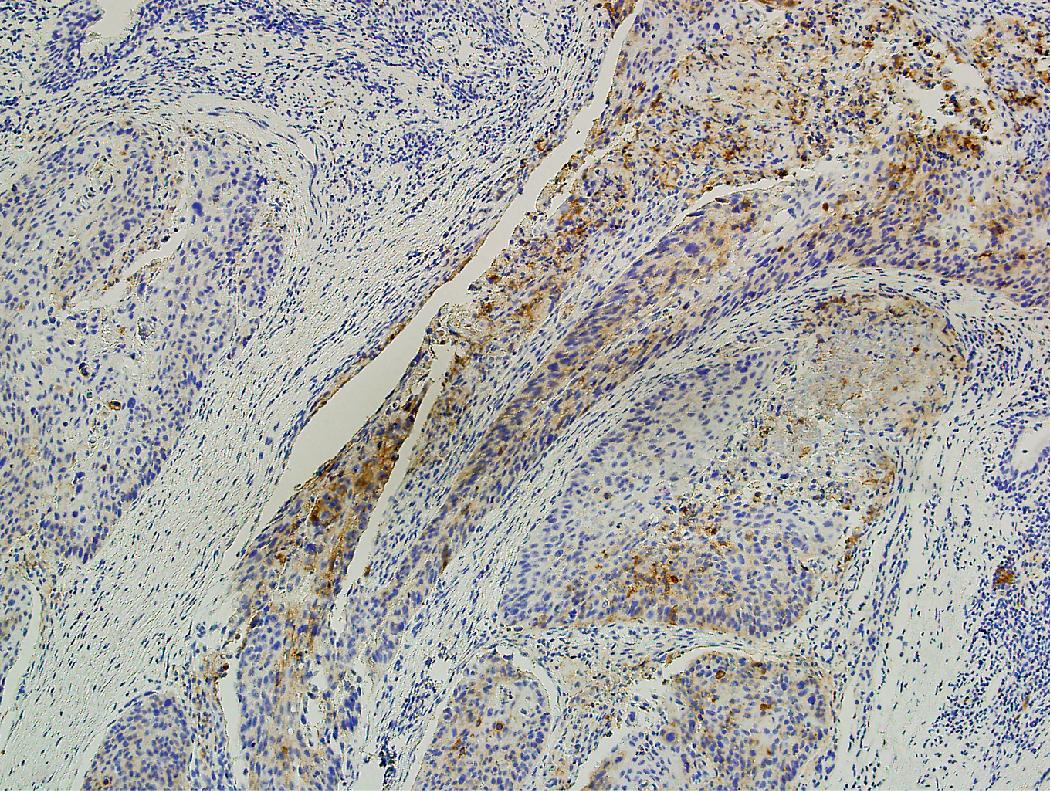
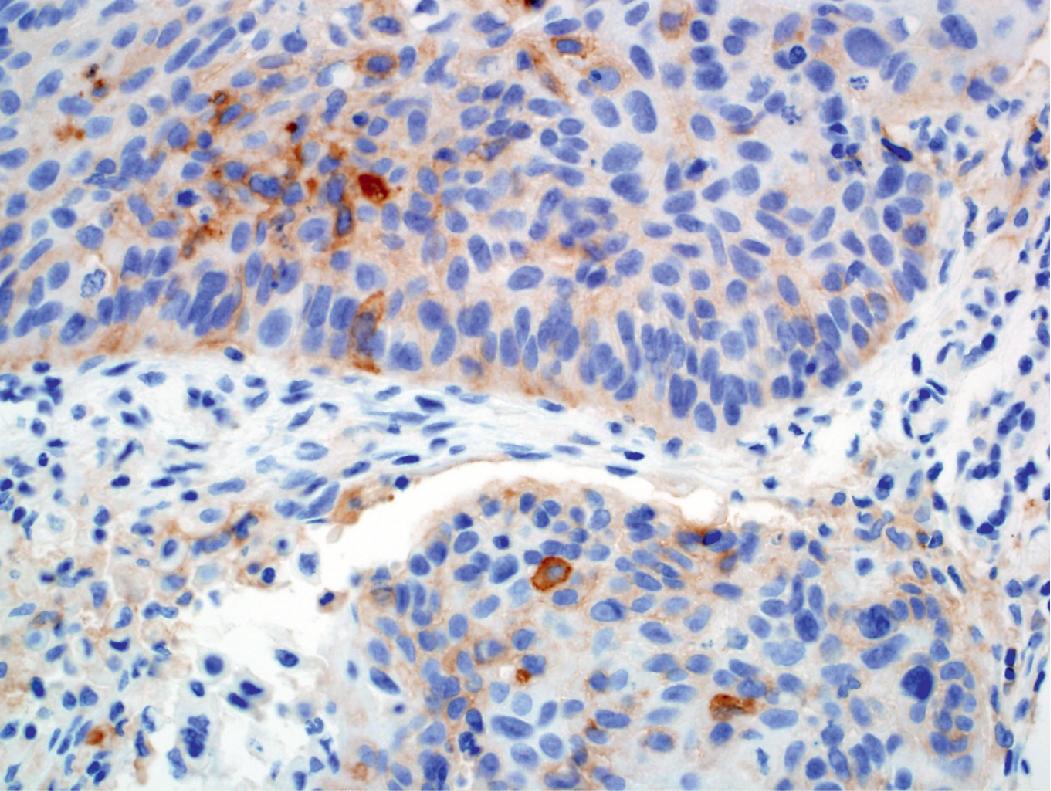
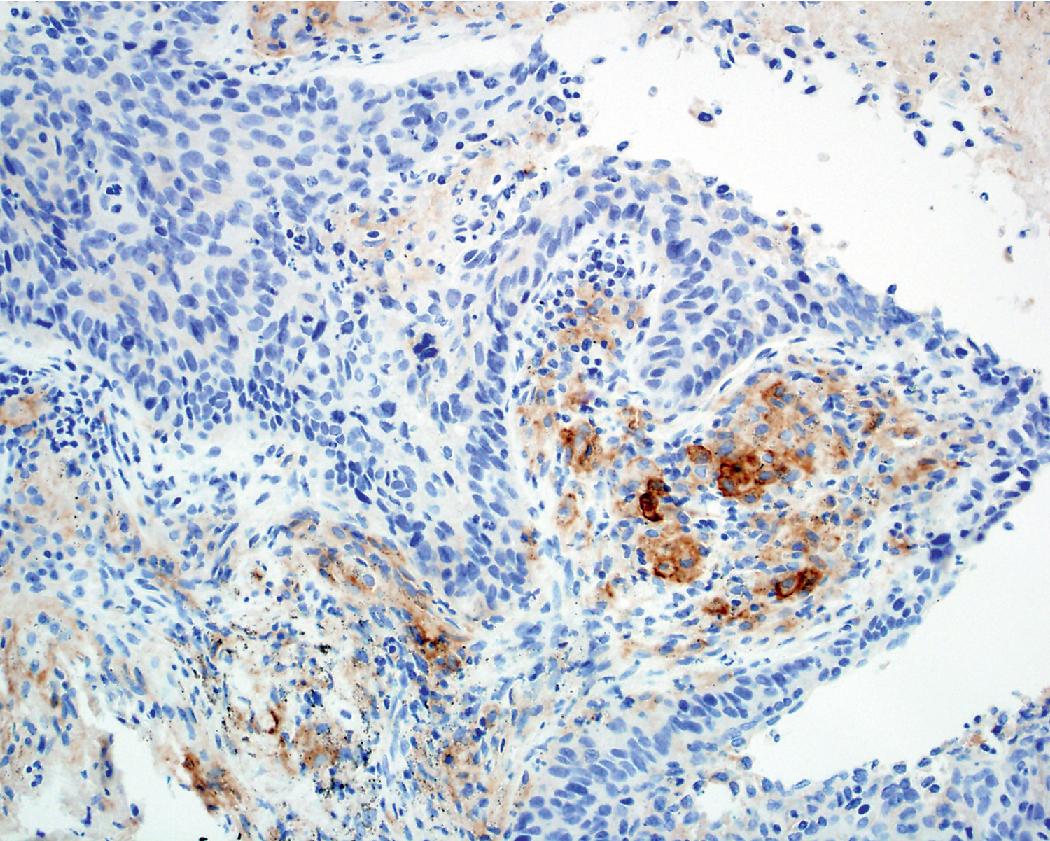
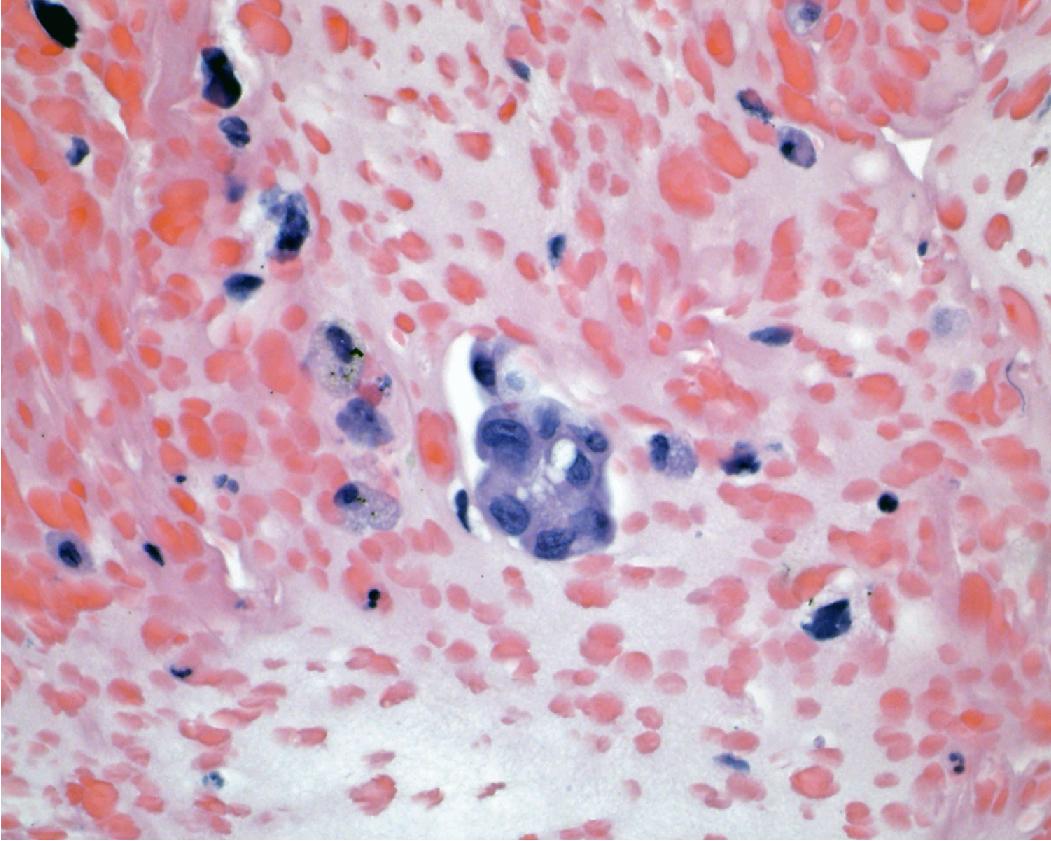
PD-L1 testing has also emerged as a critical element in the workup of NSCLCs and should be performed on all tumors requiring systemic therapy, regardless of histologic subtype. Challenges exist with PD-L1 testing, as a variety of immunohistochemical testing platforms are currently available, significant staining heterogeneity exists within tumors ( Figs. 17.1–17.3 ), and “cutoff” values constituting a positive result are variable and continue to evolve. In addition, the POPLAR trial suggests PD-L1 expression in inflammatory cells ( Fig. 17.4 ) may also be predictive of tumor response. New data will likely continue to emerge with regard to immunohistochemical evaluation as this field develops. It is also worth mentioning that other immune checkpoint molecules and pathways exist, and pathologists may be asked to evaluate their expression profiles as new immune checkpoint therapies are developed.
Recent years have seen substantial changes to the histologic classification of lung carcinoma. Lung cancer is divided into two major categories based on histologic cell type: small cell lung cancers or SCLC (~15% of lung cancers) and non-small cell lung cancers or NSCLC (~85% of lung cancers). NSCLC are further subdivided into two major histologic types: adenocarcinomas and squamous cell carcinomas. Adenocarcinoma is further subdivided, on complete resection, into adenocarcinoma in situ (AIS), minimally invasive adenocarcinoma (MIA), lepidic predominant adenocarcinoma (LPA), and invasive adenocarcinoma. The rarer variants of NSCLC covered in this chapter include lymphoepithelial carcinoma, adenosquamous carcinoma, large cell carcinoma, nuclear protein in testis (NUT) carcinoma, and salivary gland-type carcinomas. In the following sections on lung cancer histology, we describe the histologic and cytologic features of NSCLC based on the 2021 WHO Classification. The 2021 WHO definitions for these lesions are provided in Box 17.1 . Beginning in 2015 and continuing with its updated classification of lung tumors in 2021, the WHO has also included recommended diagnostic criteria and terminology based on the specimen type received, recognizing that many lung carcinoma diagnoses are based on small specimens and many of the lesions defined above require complete histologic assessment for definitive diagnosis ( Table 17.1 ). ,
Adenocarcinoma : Non-small cell lung carcinoma with histologic (gland formation, mucin production) or immunohistochemical evidence of glandular differentiation.
Adenocarcinoma in situ: Small (≤30 mm) localized adenocarcinoma with malignant cells growing across preexisting alveolar walls.
Minimally invasive adenocarcinoma: Small (≤30 mm) solitary adenocarcinoma with a lepidic predominant pattern and <5 mm of invasion.
Lepidic predominant adenocarcinoma: Lepidic predominant adenocarcinoma that is >30 mm in size (regardless of the presence of invasion) or with >5 mm of invasion.
Invasive adenocarcinoma: Invasive adenocarcinoma of any size and any sub-type (aside from lepidic) with >5 mm of invasion
Squamous cell carcinoma: Non-small cell carcinoma with histologic (keratinization, intercellular bridges) or immunohistochemical evidence of squamous differentiation.
Adenosquamous carcinoma: Carcinoma showing at least 10% of both adenocarcinoma and squamous cell carcinoma.
Lymphoepithelial carcinoma: Squamous cell carcinoma with an associated lymphoplasmacytic infiltrate and frequent association with Epstein-Barr virus.
Large cell carcinoma: Undifferentiated non-small cell carcinoma not meeting criteria for adenocarcinoma, squamous cell carcinoma, or sarcomatoid carcinoma.
NUT carcinoma: Poorly differentiated carcinoma defined by the presence of nuclear protein in testis ( NUTM1 ) gene rearrangement.
Salivary gland-type carcinomas: Carcinomas arising in the lung that morphologically and genetically mimic their salivary gland counterparts, presumably arising from similar precursor cells.
| Suggested Cytology and Small Biopsy Terminology | Cytologic/Histologic Findings and Immunohistochemical Staining Patterns | Suggested Resection Specimen Terminology |
|---|---|---|
| Adenocarcinoma (architectural patterns can be discussed as applicable) | Cytologic/histologic features of adenocarcinoma (gland formation, mucin production) clearly present | Adenocarcinoma (list predominant pattern and subpatterns in 5% increments: lepidic, acinar, papillary, solid, micropapillary) |
| Adenocarcinoma with lepidic pattern (comment that invasion cannot be excluded if pure lepidic pattern is seen) | Adenocarcinoma in situ, minimally invasive adenocarcinoma, invasive adenocarcinoma with lepidic component | |
| Invasive mucinous adenocarcinoma (can comment on lepidic pattern if applicable) | Invasive mucinous adenocarcinoma | |
| Adenocarcinoma with colloid features | Colloid carcinoma | |
| Adenocarcinoma with fetal features | Fetal adenocarcinoma | |
| Adenocarcinoma with enteric features (consider clinical history and use immunostains to exclude metastasis) | Enteric adenocarcinoma | |
| Non-small cell carcinoma, favor adenocarcinoma | Defining cytologic/histologic features absent, but TTF-1 positive supportive of adenocarcinoma | Adenocarcinoma |
| Squamous cell carcinoma | Cytologic/histologic findings of squamous cell carcinoma (keratinization and intracellular bridges) | Squamous cell carcinoma |
| Non-small cell carcinoma, favor squamous cell carcinoma | Defining cytologic/histologic features absent, but p40 positive supportive of squamous cell carcinoma | Squamous cell carcinoma (given cytomorphologic features are absent, the predominant pattern may be nonkeratinizing) |
| Non-small cell carcinoma, with squamous and adenocarcinoma patterns present (comment that this may represent adenosquamous carcinoma) | Cytologic/histologic findings of squamous cell and adenocarcinoma and/or positive adenocarcinoma and squamous markers in two distinct cell populations | Adenosquamous carcinoma (providing both components are ≥ 10% of the tumor) |
| Non-small cell carcinoma, not otherwise specified | No defining cytologic/histologic findings or staining pattern | Large cell carcinoma (unless a defining histotype is present after extensive tumor sampling) |
Adenocarcinomas are the most frequent cell type of lung cancer and include in situ (preinvasive), minimally invasive, and invasive neoplasms. Several basic histologic patterns or cell subtypes are observed with adenocarcinomas: lepidic, acinar, papillary, micropapillary, and solid. Over 90% of tumors have a mixed histologic pattern or combination of growth patterns, but in the majority of cases, one growth pattern predominates. These subtypes are important to recognize for purposes of differential diagnosis, prognosis, and some associations with molecular alterations and biomarkers. Atypical adenomatous hyperplasia is an incidentally identified lesion that is thought of as a potential precursor to lung adenocarcinoma. We begin with a review of general cytologic features of this family of lesions, followed by a discussion of individual recognized histologic subtypes and related lesions.
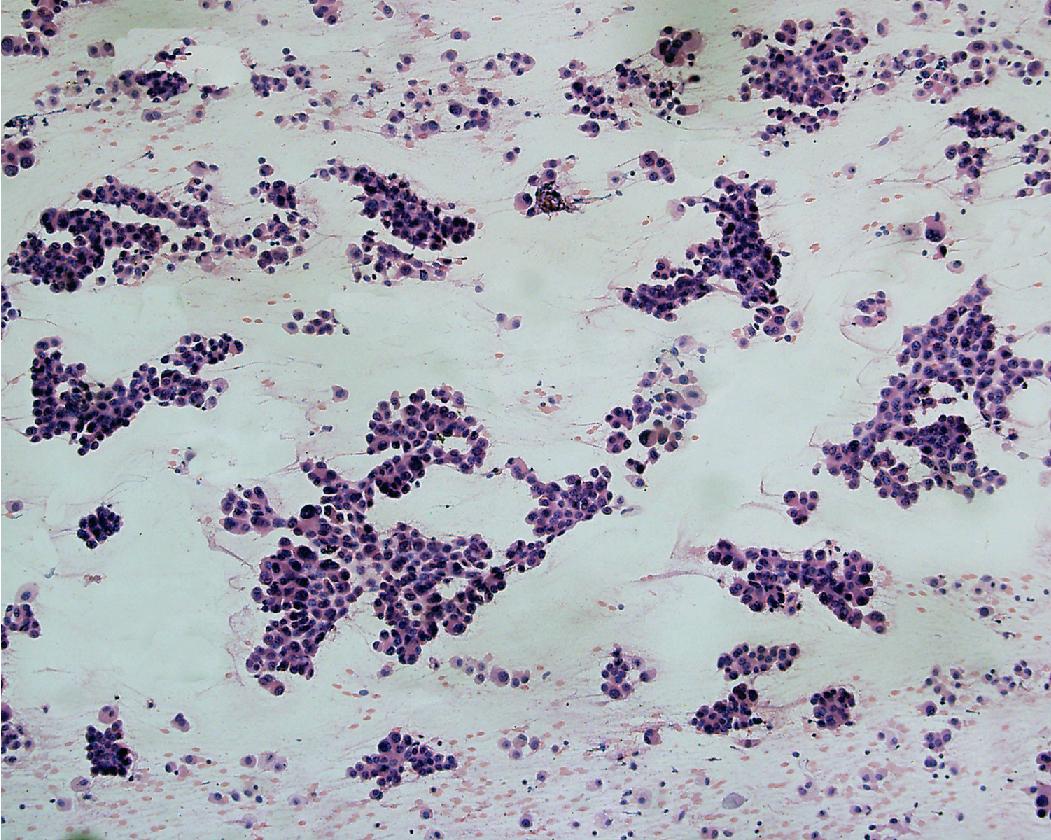
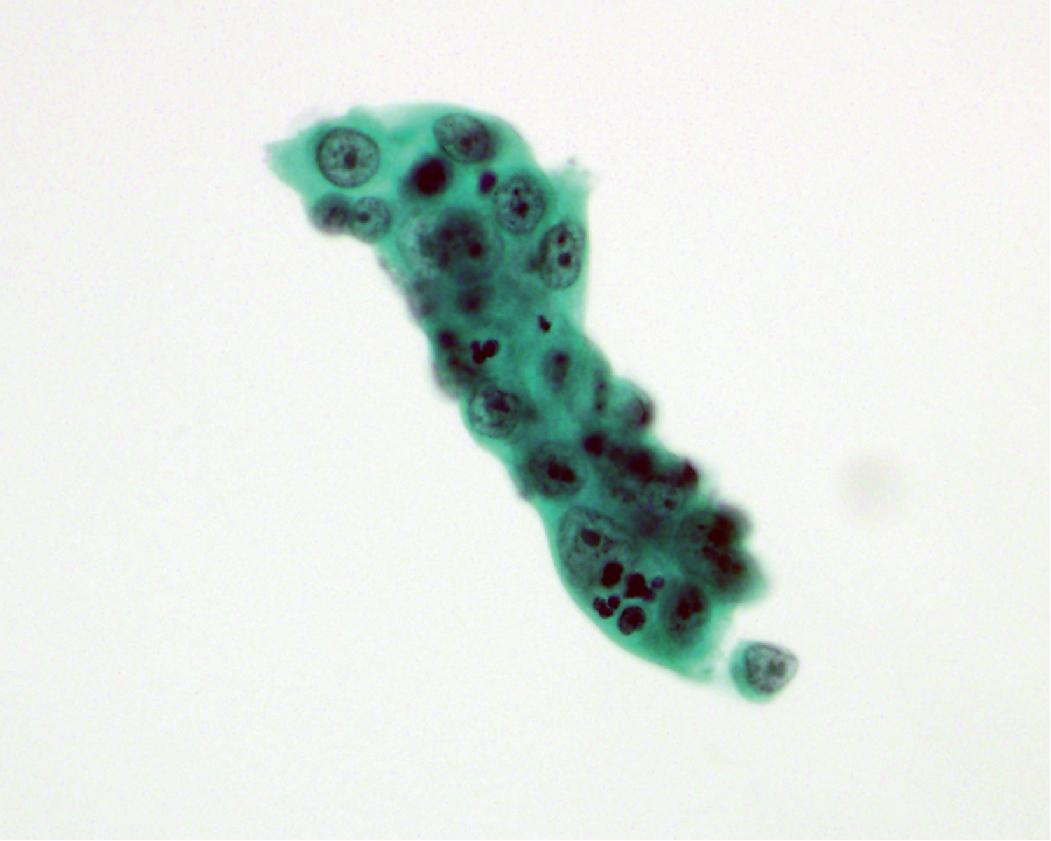
In general, the cytologic features of adenocarcinoma can be extremely variable. Background features can include necrosis and/or mucin. The cells are cuboidal to columnar in shape and often maintain some degree of nuclear to cytoplasmic polarity (referring to basally situated nuclei) ( Figs. 17.5 and 17.6 ). The cytoplasm can be relatively homogeneous, foamy to vacuolar, or contain mucin. Some tumors have a large cytoplasmic vacuole that peripherally displaces the nucleus (signet ring morphology). The nuclei are often enlarged, open, and frequently contain a prominent nucleolus. Chromatin features include irregular clumping and hyperchromasia; however, the chromatin alterations are less pronounced compared with those seen in squamous cell carcinoma.
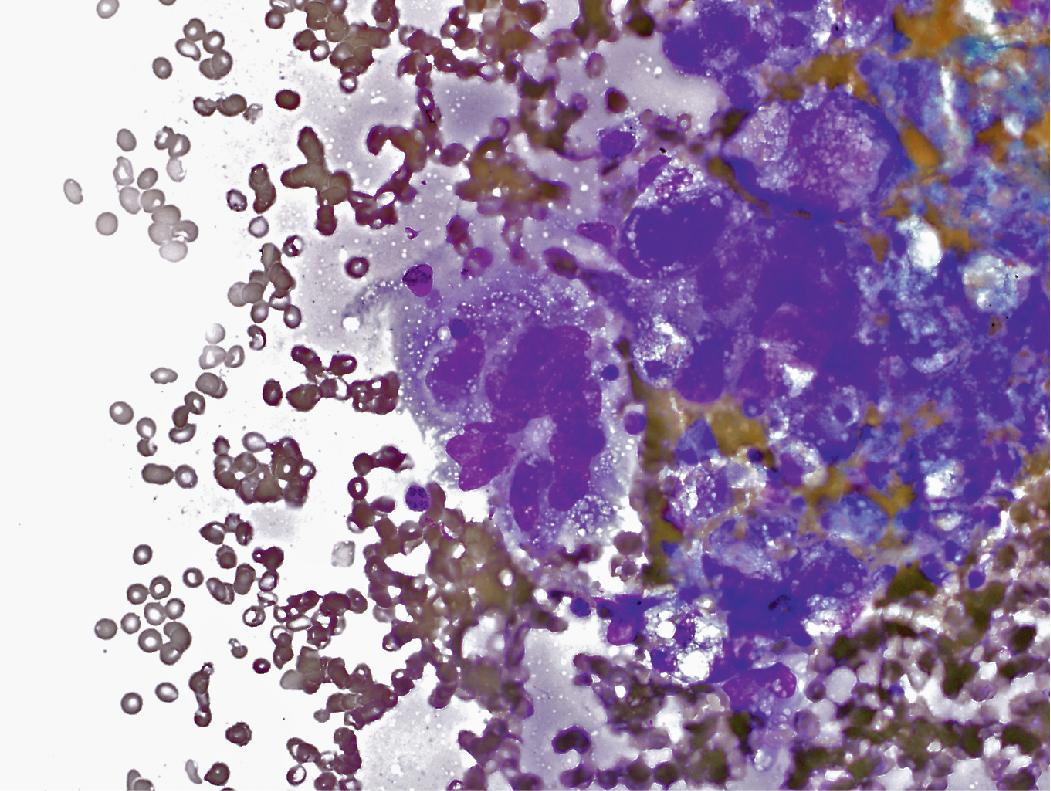
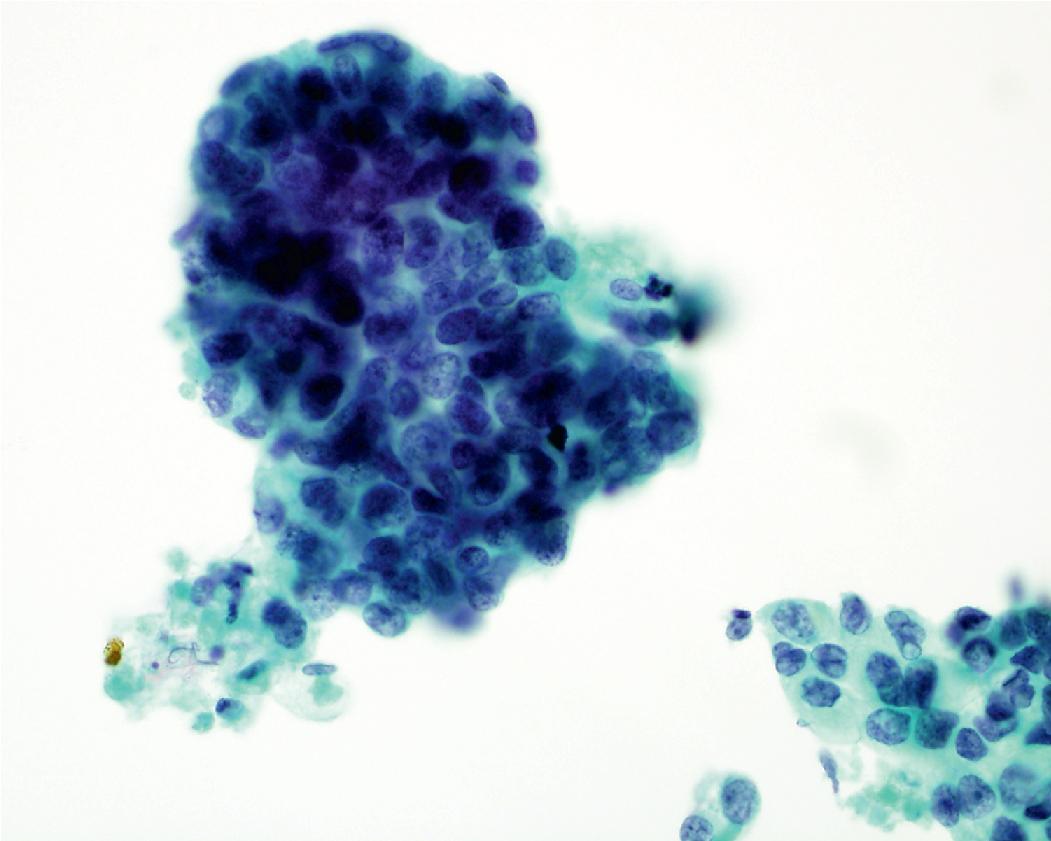
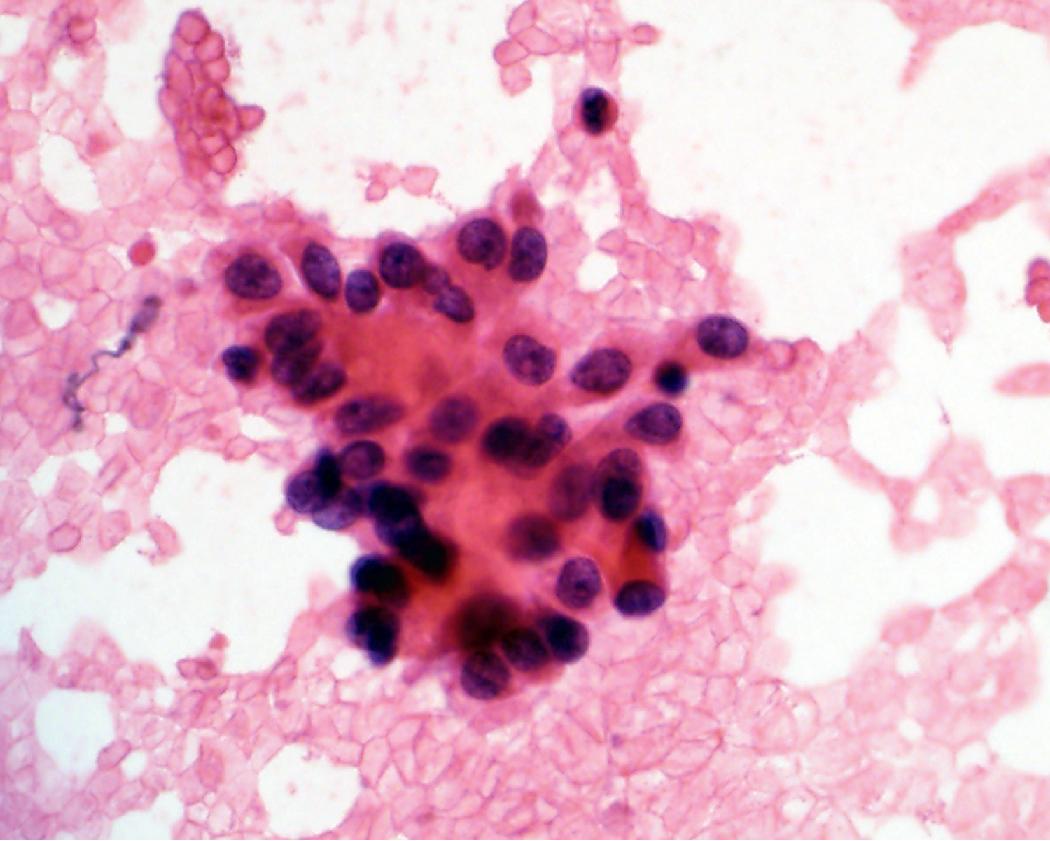
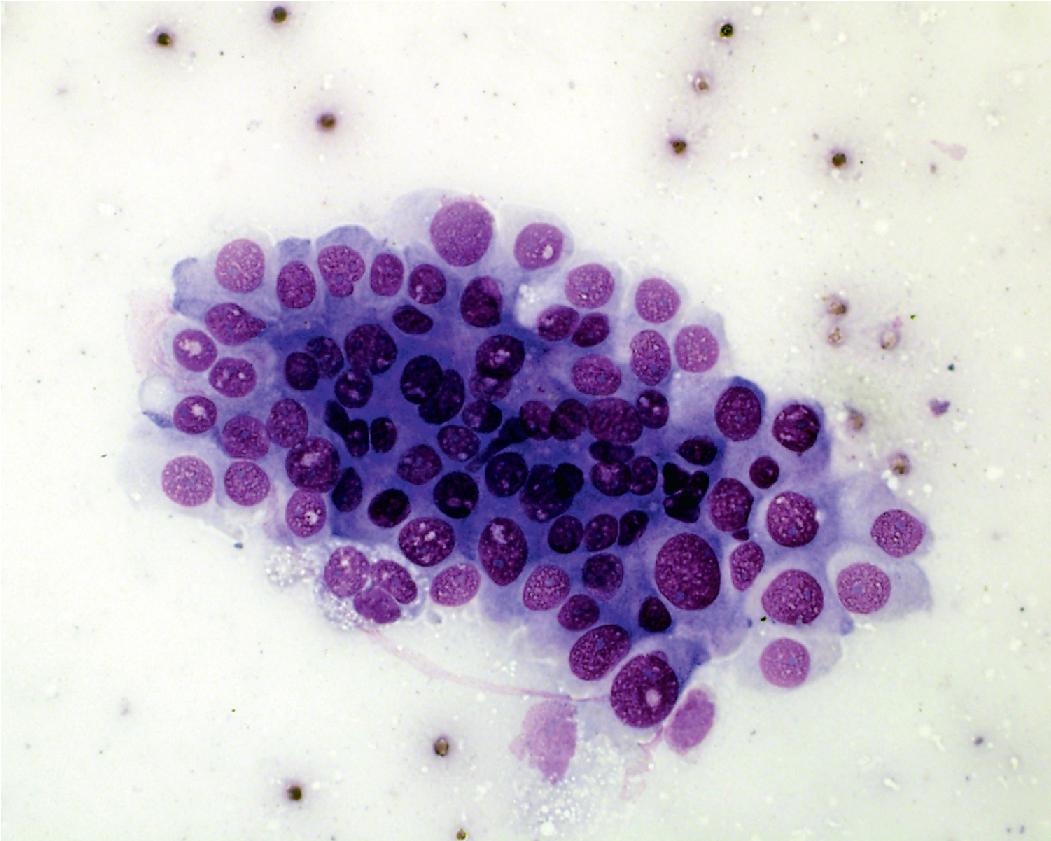
In cytology specimens, the architectural pattern and cell-to-cell organization can be particularly helpful in identifying adenocarcinoma, as the cells are often arranged in three-dimensional groups ( Fig. 17.7 ), papillary clusters, or acinar structures. It is important to realize that often significant tumoral heterogeneity exists and patterns observed on cytology may not correlate with the predominant pattern seen histologically on larger resection specimens. As such, the terminology used for cytology specimens includes adenocarcinoma with lepidic pattern ( Fig. 17.8 ) or adenocarcinoma with colloid features . Certain cytomorphologic findings are more common with certain subtypes of adenocarcinoma. For example, lepidic pattern tumors can have nuclear grooves and inclusions. Micropapillary variants may contain numerous small, rounded clusters of malignant cells.
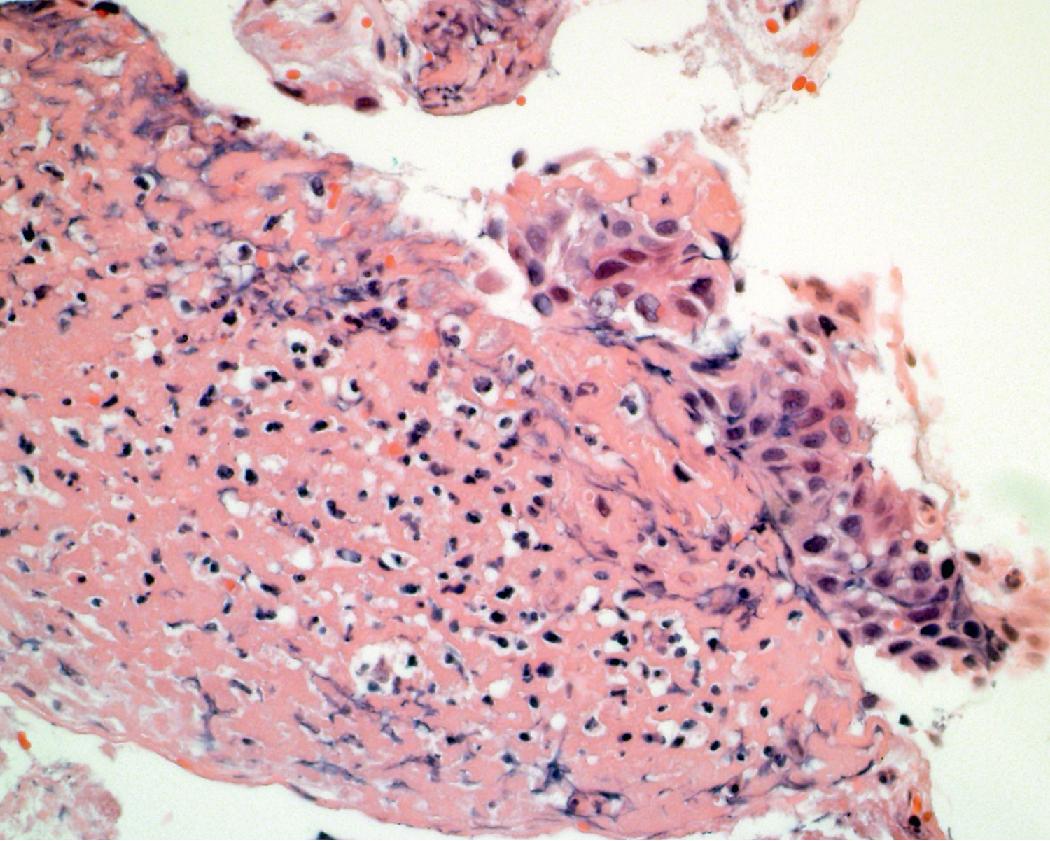
One should always correlate cytologic findings with the patient’s history, as carcinoma mimics can be encountered (i.e., reactive atypia [ Figs. 17.9 and 17.10 ] and therapy effects). A benign neoplasm that can mimic adenocarcinoma is sclerosing pneumocytoma ( Figs. 17.11 and 17.12 ). This is a rare adenoma that can have a papillary, solid, and/or sclerotic architecture. , Cytologically, an epithelial and stromal component can be seen. The cells are typically bland; however, atypia and intranuclear cytoplasmic invaginations may be present. Both cell types express TTF-1; however, the epithelial cells are typically positive for cytokeratin markers, while the stromal cells are negative. Findings favoring adenocarcinoma include marked cytologic atypia, pleomorphism, prominent nucleoli, and necrosis.

Atypical adenomatous hyperplasia is a preinvasive lesion that is usually an incidental finding (≤5 mm in most cases) discovered on microscopic examination of lung resections, particularly in lung cancer resections. The relationship of atypical adenomatous hyperplasia to adenocarcinoma is believed to be comparable to that of squamous dysplasia for squamous cell carcinoma. It was originally conceived as a precursor of adenocarcinoma, analogous to the association between tubular adenomas and adenocarcinoma in the colon, and described as bronchioloalveolar adenoma by Dr. Roberta Miller. , Because there is no invasion, when completely excised, the 5-year survival rate for this lesion is 100%.
Lesions are usually 5 mm or less in size, but this is not an exact diagnostic criterion. The lesions are most often discovered within the parenchyma as incidental findings, and they may sometimes be observed grossly as a faint, poorly defined yellow-tan nodule. Atypical adenomatous hyperplasia consists of dome-shaped to cuboidal to columnar epithelial cells with mild to moderate atypia growing in a lepidic pattern along intact alveolar septa ( Fig. 17.13 ).
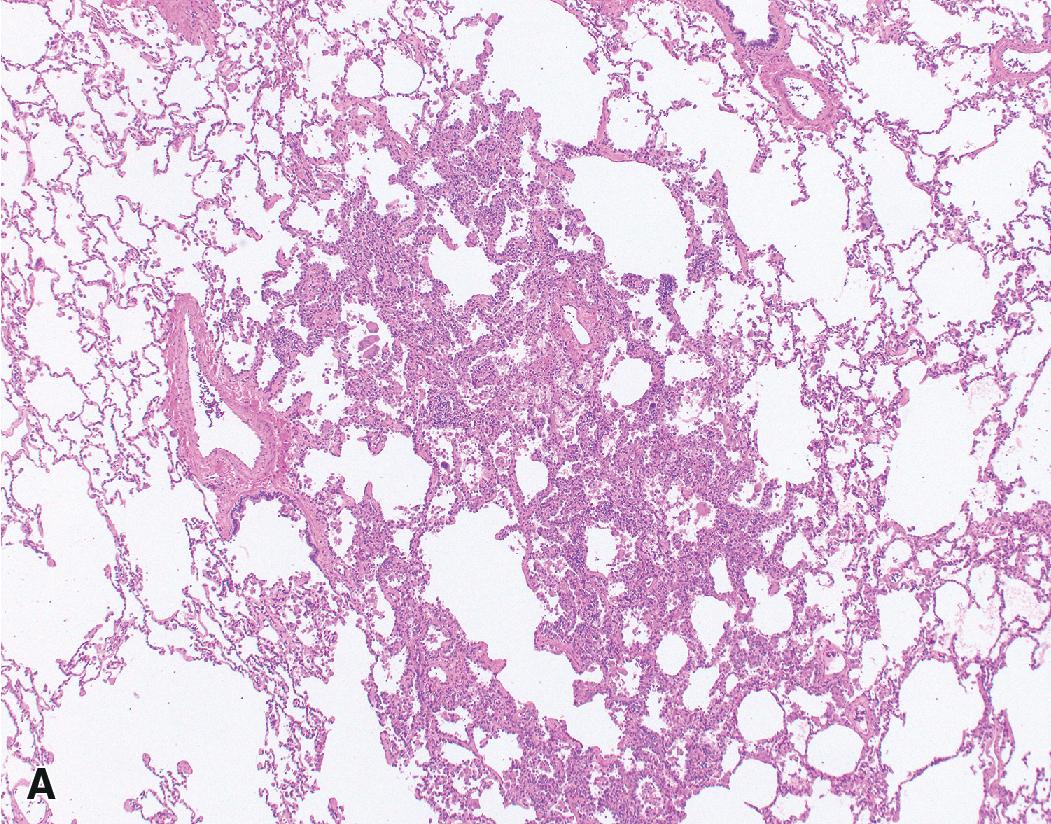
The alveolar septa are almost always thin and delicate without fibrosis or inflammation ( Fig. 17.14 ).
Histologically, the cytologic atypia tends to be more uniform in atypical adenomatous hyperplasia than in most reactive pneumocyte hyperplasias and, in contrast, the epithelial proliferation is not overshadowed by the fibrosis and inflammation seen in a reactive process. The degree of cellularity and cytologic atypia is generally less than that of AIS, which typically shows a more uniform moderate to high grade atypia. However, it should be noted that atypical adenomatous hyperplasia ( Fig. 17.15 ) is indistinguishable from AIS and other well-differentiated adenocarcinomas on cytology specimens, as this diagnosis requires interpretation of atypia in the broader architectural context. It should also be remembered that such lesions are typically very small and are almost never biopsied, and this diagnosis is typically made only on a resection specimen performed for other reasons. From a practical standpoint, by the time a suspicious lesion is large enough to biopsy, the likelihood that it represents atypical adenomatous hyperplasia is extremely low and this diagnosis should generally be avoided on a small biopsy specimen.
AIS is a preinvasive lesion and is defined as a small (≤3 cm) solitary adenocarcinoma with pure lepidic (in situ) growth and no stromal, vascular, air space, or pleural invasion. , , AIS is usually an incidental finding on CT where it classically appears as a small ground-glass opacity in the lung parenchyma. PET generally shows low activity in contrast to other types of lung cancer. Grossly, AIS is an ill-defined pale nodule or may grossly blend into the surrounding parenchyma, making it difficult to identify even when CT indicates its presence. Since there is no invasion, similar to atypical adenomatous hyperplasia, when completely excised, the 5-year survival rate for AIS is 100%.
The vast majority of AIS tumors are nonmucinous. Although mucinous AIS may exist in concept and has been formally recognized as a distinct entity by the WHO, it is vanishingly rare. Almost all mucinous adenocarcinomas are aggressive malignancies that are recognized late and typically present at higher stages, despite their deceptively bland histologic appearance and predominantly lepidic growth. Because of this fact, pathologists should use the term mucinous AIS with extreme caution and only after complete resection of a lesion, as this term may not accurately reflect the aggressive biologic behavior of most tumors.
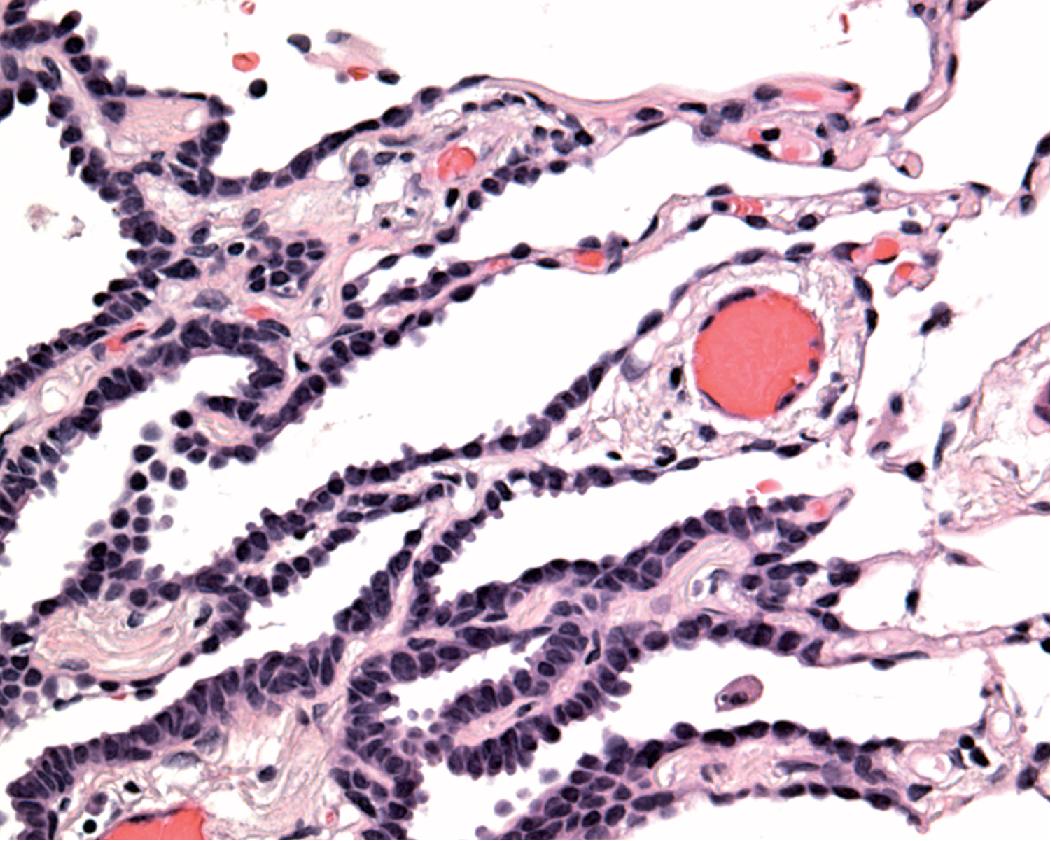
Nonmucinous AIS consists of cuboidal to columnar cells in a compact linear arrangement along intact alveolar septa ( Fig. 17.16 ). These cells have features of alveolar type II pneumocytes or Clara cells, but differentiation of these has no known clinical significance. The neoplastic cells display at least moderate atypia and have a monotonous appearance from cell to cell. The cells may have intranuclear inclusions or loss of polarity with apical nuclei. The alveolar septa may be widened with fibrous tissue but are architecturally intact, and there is no distinct scar formation that would suggest invasion. Nonmucinous AIS is sharply demarcated from the surrounding alveolar parenchyma as a circumscribed lesion ( Fig. 17.17 ). , , ,
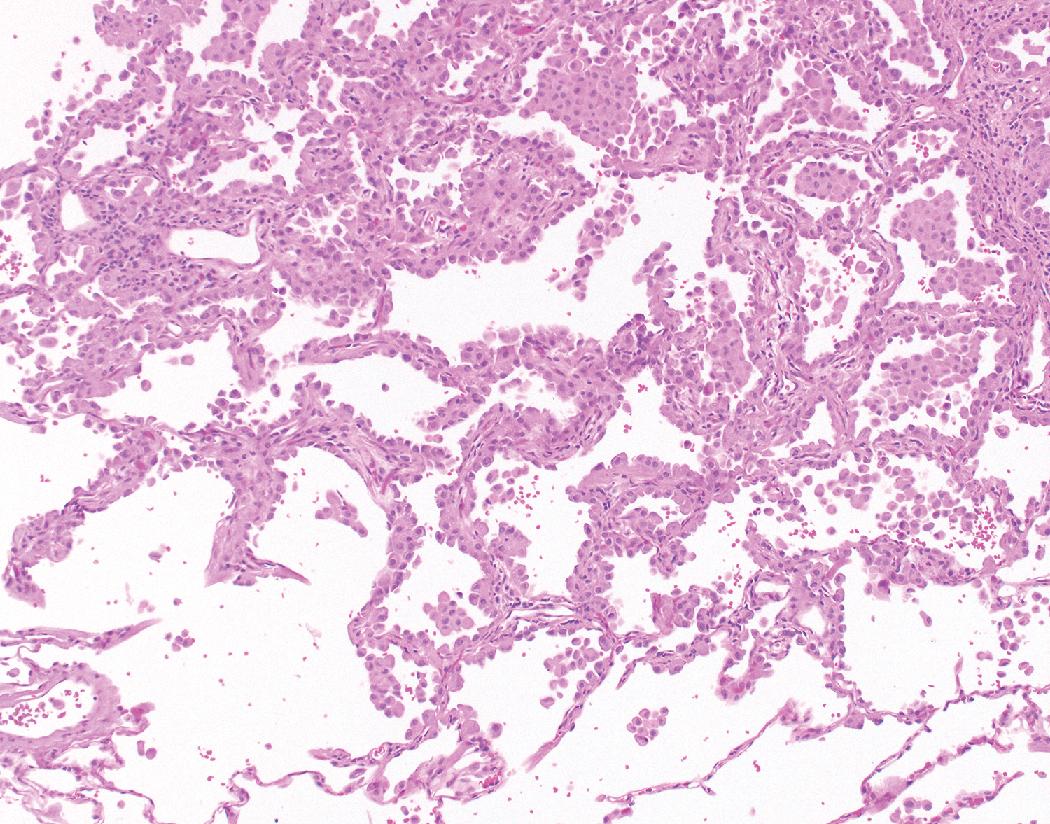
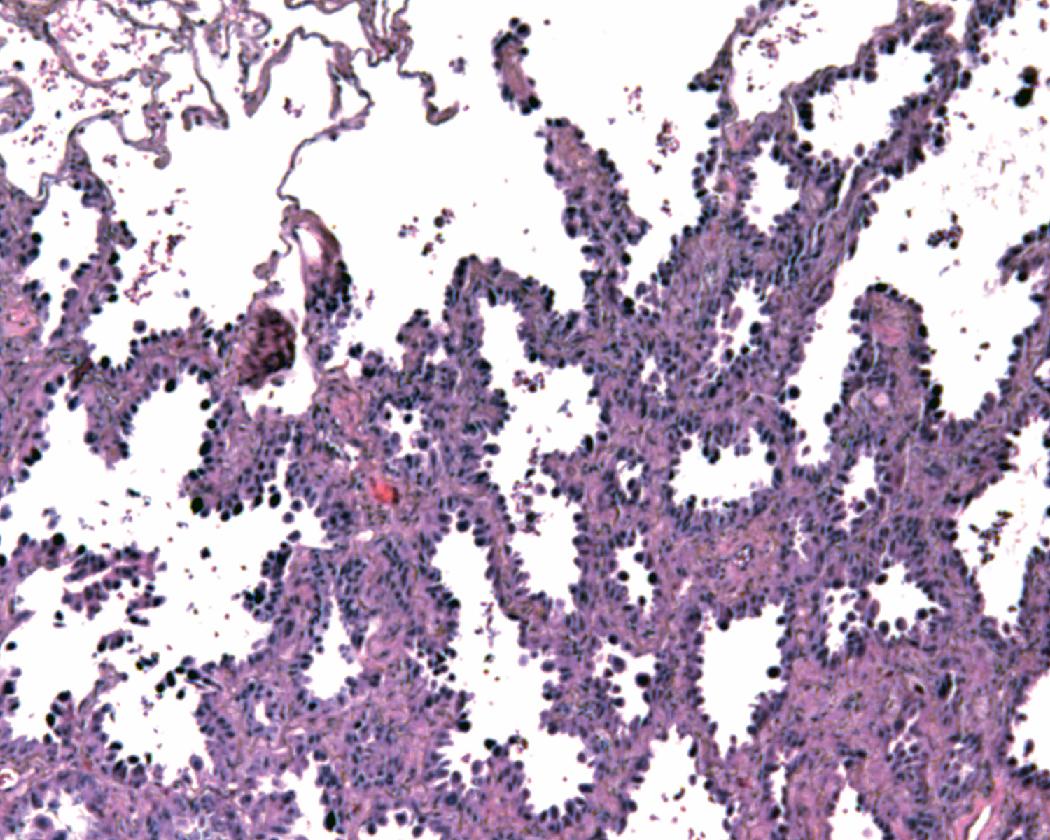
This histology contrasts with reactive type II pneumocyte hyperplasia in which cells of varying degrees of reactive atypia are admixed together with normal lining cells. This produces a variegated appearance as opposed to the generally homogeneous appearance of the neoplastic cells seen in AIS. With reactive hyperplasia, the fibrosis, perhaps accompanied by inflammation or other features, overshadows the epithelial proliferation, particularly when viewing the entire section at low magnification, whereas with AIS the epithelial proliferation predominates over the septal widening. Reactive hyperplasia tends to blend gradually into the surrounding alveolar parenchyma rather than being sharply demarcated from adjacent normal parenchyma as is characteristic of AIS. , , ,
If there are any findings of invasion, including any acinar, papillary, micropapillary, solid, cribriform, or other variant pattern of invasive growth, or individual infiltrating cells within adjacent desmoplastic tissue, vascular invasion, lymphatic invasion, pleural invasion, metastasis, or air space invasion including aerogenous spread or spread through airspaces (STAS), the tumor is not AIS. To exclude the possibility of invasion, the entire lesion should be examined. Therefore, a diagnosis of AIS cannot be rendered on a small biopsy or cytology specimen since the failure to sample foci of invasion cannot be excluded. If a pure lepidic pattern is seen in a small biopsy, it is recommended to use the terminology adenocarcinoma with lepidic pattern , followed by a disclaimer stating an invasive component cannot be excluded.
Pure AIS is rare in comparison to MIA and LPA. In a large study of over 1000 stage I completely resected lung adenocarcinomas, only two AIS lesions were identified on retrospective histologic classification, compared to 34 and 103 examples of the latter, respectively.
MIA is defined as a small (≤3 cm) solitary adenocarcinoma with predominantly lepidic (in situ) growth and an invasive component that is 5 mm or smaller in greatest dimension. , , , , Similar to AIS, MIA is usually an incidental finding on CT where it appears in the peripheral lung parenchyma. Classically, on a CT scan, a ground-glass opacity with a solid component represents a lepidic tumor with invasion. Grossly, MIA resembles AIS but has a small solid focus or foci representing the invasive component. Similar to atypical adenomatous hyperplasia and AIS, when completely excised, the 5-year survival rate for MIA is 100%.
Similar to AIS, the majority of MIA are nonmucinous and a few cases are mucinous. The lepidic component of MIA, which makes up 95% of the tumor, has the same histologic features as nonmucinous (or occasional mucinous) AIS. If there is a single focus of invasion, the focus must be less than 5 mm to qualify for MIA. If there are multiple foci, the recommendation is to multiply the percentage of surface area of invasive growth by the overall tumor size. If this results in less than 5 mm, the lesion meets criteria for MIA. For example, if a 15 mm lesion has three foci of invasion representing 20% of the overall lesion by surface area, it would meet criteria for MIA (20% of 15 mm is 3 mm). By definition, the invasive component consists of a histologic subtype other than lepidic (in situ), including acinar, papillary, micropapillary, solid, cribriform, or a variant pattern of invasion, or tumor cells infiltrating a myofibroblastic stroma ( Fig. 17.18 ). MIA should have no lymphatic, blood vessel, or pleural invasion, no tumor necrosis, and no STAS.
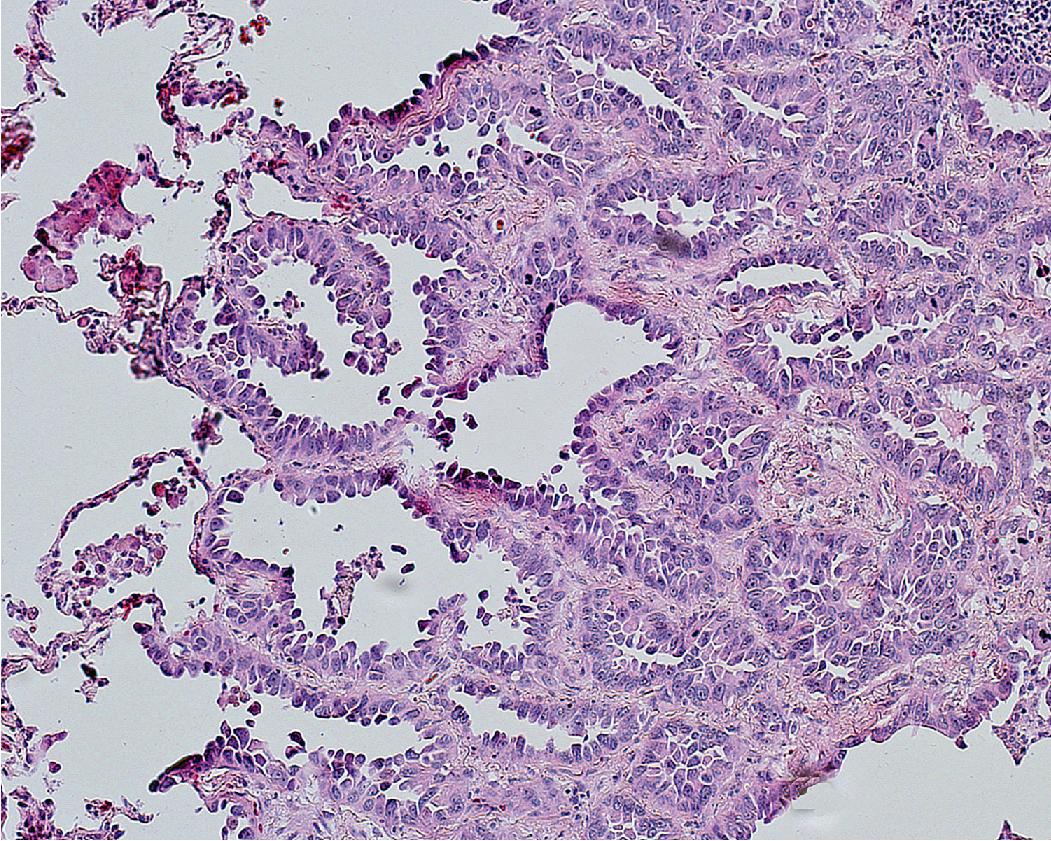
The majority of adenocarcinomas and, hence, the majority of lung cancers are invasive adenocarcinomas. As described in the section on staging, about 70% of invasive adenocarcinomas present in advanced disease stages, and tissue samples typically consist solely of small biopsies or cytology specimens, with resection reserved primarily for the less common early stage tumors. , , On CT, adenocarcinoma is most often a nodule or mass in the peripheral lung parenchyma, but exceptions occur. Most are solid, indicating an invasive tumor, and the presence of ground-glass opacity around the periphery suggests a lepidic (in situ) component. Calcifications may be seen, but cavitation is not characteristic of invasive adenocarcinoma, although, again, exceptions occur. Invasive adenocarcinomas show high activity on PET scan.
Over 90% of invasive adenocarcinomas are histologically heterogeneous with mixed growth patterns on microscopic examination. , This is primarily noted on resection specimens where there is the opportunity to sample the tumor more extensively. It may not be apparent on the limited tissue provided by a small biopsy or cytology specimen. One of the histologic patterns or subtypes often predominates. Several histologic subtypes or patterns of invasive adenocarcinomas are recognized (lepidic, acinar, papillary, micropapillary, and solid) and some additional histologic variants (invasive mucinous, colloid, fetal, and enteric).
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here