Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
When chronic pain becomes refractory to medications and all available medical arsenal, one can have recourse to functional neurosurgery.
Chronic pains are of many kinds. Main varieties include pain due to the alteration of the musculoskeletal apparatus, complex regional pain syndromes (CRPSs), pain from visceral or vascular origins, pains related to neoplasia (frequently the consequence of the association of several of the previously stated mechanisms), neuropathic pain, central poststroke pain syndrome, and others.
Neurosurgical armamentarium is wide, from transcutaneous electrical neuro-stimulation (TENS) to lesioning surgery. Neuromodulation methods are the first options to be considered. Among the electrical neuromodulation techniques, spinal cord stimulation (SCS) of the dorsal columns is in the forefront. Others can be used: stimulation of peripheral nerves, dorsal root ganglion stimulation, and stimulation of the brain either with deep brain stimulation or motor cortex stimulation (MCS). Also intrathecal infusion of not only opioids but also more recent analgesic substances—that is, pharmacological neuromodulation—slowly increases in use. Lesioning techniques, especially the ones directed to the dorsal root entry zone (DREZ)—can be a valuable recourse for some well-selected pain syndromes, in particular the ones linked to a deafferentation phenomenon and related to hyperactivity in the dorsal horn neurons ( Fig. 38.1 ).

This chapter will mainly focus on pain of the neuropathic type. According to the International Association for the Study of Pain, “Neuropathic pain is defined as pain related to a lesion or a disease of the somatosensory system” .
Not all neuropathic pain syndromes can be alleviated by SCS. To be effective, SCS needs functional integrity of the dorsal column fibers (i.e., the axons from the cell bodies situated in the dorsal root ganglion up to the brain stem, Figs. 38.2–38.4 ). Their integrity can be assessed prior to deciding for the intervention by using somatosensory-evoked potentials (SEPs) and checking the central conduction time (CCT), namely, the N13–N20 interval for the upper limb and the N22–P39 interval for the lower limb . However, study of SEPs (from stimulation of the median/ulnar nerves for the upper limb and the tibial nerve for the lower one) requires that the peripheral fibers be intact.



The lesioning procedures at the spinal cord level are mostly indicated for irreducible pain due to malignancies. Anterolateral cordotomies of the spino-reticulo-thalamic tract, posterior commissural myelotomies for interrupting the decussating nociceptive fibers or central myelotomies can be performed through open or percutaneous computerized tomography (CT) guided approaches . Spinal DREZ-lesioning necessitates an open approach through hemi- or complete laminectomy. For “cancer pain” indications, obey the following criteria: well-defined pain territory and circumscribed tumor extension, “sufficiently good” general condition with a “not too short” life expectancy when pain results from evolving diseases. Choice of the most appropriate technique depends on the topography of the cancer ( Fig. 38.5 ).

All these procedures, especially the percutaneous ones, need to be guided by imaging (fluoroscopy or intraoperative CT-guidance) and controlled by neurophysiology (impedance measurements, electrical stimulation with neurological-induced effects, in awake patients) .
In spinal percutaneous cordotomy, use of neurophysiological testing is particularly essential to check proper location at the interior of the spinal canal. Impedance during the passage through the CSF, the pia mater and the medullary substance itself will increase from roughly 100 to 200 Ω in the CSF, 400 to 500 Ω at the point of touching the spinal cord, to 1000 Ω inside the nervous tissue. Once inside the spinal cord, stimulation is crucial to check effects before performing the lesion. Initially, this is performed at a low frequency (2 Hz, 60 µs, and intensities of 0.3–0.5 mA) in order to eliminate the possibility that the needle tip is close to the motor pathways. Next, parameters should be increased (60 Hz, 1000 µs, and 0.5 mA max) in order to elicit a response in the desired pathway that is the spinothalamic tract. This will generally result in a warm often pleasant sensation in the targeted half of the body. Rarely this stimulation will elicit painful sensations. The height of the level where the stimulation is perceived is predictive of the medullary level of analgesia that will be achieved.
Similarly in mesencephalic tractotomy , stimulation can be used to refine the location of the probe. Stimulation protocols for this procedure are more complex since several adjacent structures must be avoided; however, the general protocol is similar with low-frequency stimulation initially to ensure absence of motor response and increasing frequency of stimulation thereafter.
In both situations, precise location can be greatly improved by using electrophysiologic stimulation and ensure a desired effect (analgesia) as well as and above all avoid neurologic complications.
In 1965 the Gate Control Theory drew neurosurgeons’ attention to the dorsal horn as a possible target for pain surgery . On this basis neurostimulation of the primary afferent neurons was developed to enhance the inhibitory mechanisms of the spinal cord . Conversely in 1972, the senior author undertook anatomical studies and preliminary surgical trials in the human DREZ and dorsal horn to determine whether a destructive procedure of the pain fibers and pain generators at this level was feasible . Soon after, in 1976, Nashold and his group started to develop DREZ lesions using the Radiofrequency (RF)-thermocoagulation as the lesion maker . Then DREZ procedures were performed with the laser beam or an ultrasound probe or by simple aspiration , especially for pain after brachial plexus avulsion.
The procedure consists of a longitudinal incision of the dorsolateral sulcus, ventrolaterally at the entrance of the rootlets into the sulcus, followed by bipolar microcoagulations performed continuously inside the sulcus, down into the dorsal horn, all along the spinal segments selected for surgery. The lesion penetrates the lateral part of the DREZ and the medial part of the tract of Lissauer (TL) and extends to the dorsal horn. Lesioning is 2–5 mm deep according to the desired effects ( Fig. 38.6 ).
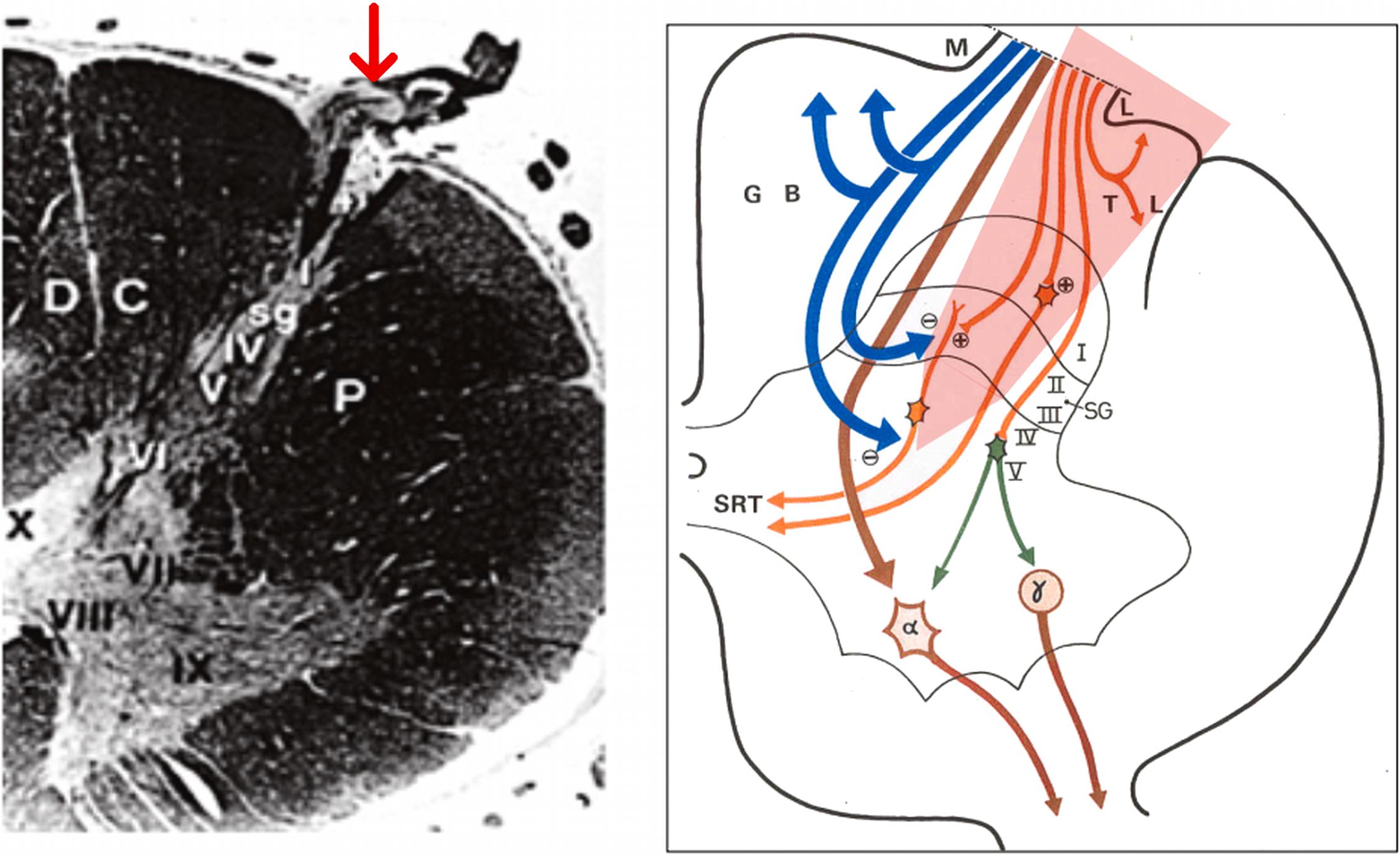
The procedure is presumed to preferentially destroy the small mainly unmyelinated (nociceptive) fibers regrouped in the lateral bundle of the dorsal rootlets, as well as the (excitatory) medial part of the LT. The dorsal-most layers of the dorsal horn are also destroyed provided bipolar coagulations are made inside the dorsal horn, known to be the site of “hyperactive” neurons especially in the case of peripheral deafferentation. The procedure is presumed to at least partially preserve the most medial structures of the DREZ (i.e., the large myelinated fibers that reach the dorsal column, as well as their—inhibitory—recurrent collaterals to the dorsal horn). This method, named microsurgical DREZotomy (MDT), was conceived in order to avoid complete abolition of the tactile and proprioceptive sensations and prevent from further deafferentation phenomena .
Working in the DREZ requires knowledge of the morphological anatomy at the dorsal roots and the dorsal horns of the various spinal levels ( Fig. 38.7 ). Details have been given in previous publications . The axis of the dorsal horn in relation to the sagittal plan crossing the dorsolateral sulcus will condition the angulation of the DREZotomy. According to 82 measurements performed by Young , the mean DREZ angle is 30 degrees at C6, 26 degrees at T4, 37 degrees at T12 and 36 degrees at L3. The site and extent of the DREZ lesion will also be determined by the shape, width, and depth of the TL and dorsal horn.

Surgery is performed with the patient under general anesthesia, but with only an initial short-lasting muscle relaxant to allow intraoperative observation of the motor responses to bipolar electrical stimulation of the roots, for their topographical identification. Stimulated dorsal roots have a motor threshold at least three times higher than the ventral roots. Standard microsurgical techniques are used with 10×–25× magnification.
The prone position with the head fixed with a three-pin head holder and neck flexed in the “Concorde” position has the advantage of avoiding brain collapse caused by CSF depletion.
The level of laminectomy is determined after the identification of the prominent spinous process of C2 by palpation. A hemilaminectomy, generally from C3 to C7 (included), with preservation of the spinous processes, allows sufficient exposure to the posterolateral aspect of the cervical spinal cord segments that correspond to the upper limb innervation, that is, to the rootlets of (C4) C5–T1 (T2).
After the dura and the arachnoid are opened longitudinally, the exposed roots and rootlets are dissected free by separation of the tiny arachnoid filaments that bind them to each other. The radicular vessels are preserved.
Each of the dorsal roots from C4 to T1 when not avulsed is electrically stimulated at the level of their corresponding dural sheath to check their muscular innervation and functional value. Responses are: in the diaphragm for C4—with a clinical response palpable below the lower ribs and more importantly a respiratory pressure wave that is detectable by the anesthesiologist , in the shoulder abductors for C5, the elbow flexors for C6, the elbow and wrist extensors for C7, and the muscles of the hand for C8 and T1.
The microsurgical lesions are performed at the selected levels that correspond to the pain territory. The incision is made with a microknife of the ophthalmological type. The microcoagulations are made in a “chain” (i.e., dotted) manner with a sharp, graduated, bipolar forceps specially designed for MDT and using it at low intensity. Depth and extent of the lesion depend on the degree of the desired therapeutic effect and the preoperative functional status of the limb.

If the laxity of the root is sufficient, the dorsolateral incision is performed continuously, along all the rootlets of the targeted root. If not, the incision is made successively on each rootlet of the root, after the surgeon has isolated each one by separation of the tiny arachnoid membranes that hold them together.
For pain due to brachial plexus avulsion, dotted microcoagulations inside the dorsal horn, in the order of 5 mm in depth, are performed after incision of the dorsolateral sulcus at the level of the avulsed roots. The ventrolateral DREZ lesioning is extended to the root remaining above and below the level of the avulsed roots especially if they are atrophic. In brachial plexus avulsion dissection of the spinal cord is sometimes difficult to achieve safely because of scar tissue and arachnoiditis adhering to the cord. Atrophy and/or gliotic changes of the spinal cord at the level of the avulsed roots can make identification of the dorsolateral sulcus hazardous. In such cases, it is necessary to start from the root remaining above and below. The presence of tiny capillaries that enter the sulcus may help determine its location. Yellow areas corresponding to old hemorrhages on the surface of the cord and/or microcavities in the depth of the sulcus and in the dorsal horn provide some guidance for the tracing of the sulcomyelotomy. When the dorsolateral sulcus is difficult to find, intraoperative monitoring of the dorsal columns SEPs evoked by stimulation of the tibial nerve and motor evoked potentials (MEPs) to the ipsilateral lower limb can be helpful.
The patient is positioned prone on thoracic and iliac supports with the head placed 10 cm below the level of the surgical wound to minimize CSF loss. The desired vertebral levels are checked by a lateral fluoroscopy that starts from S1 counting upward. A laminectomy either bilateral or unilateral according to the painful territory—from T11 to L1 (or L2) for conus medullaris - is performed. The dura and arachnoid are opened longitudinally and the filum terminale is isolated. Identification of the root levels is then performed by electrical stimulation with observation of corresponding muscle responses.
The L1 and L2 roots are entirely visible and can be identified at their penetration into the dural sheaths; on the contrary the lower lumbar and the sacral roots—and consequently the corresponding spinal cord segments—cannot be so well ascertained because the roots are not visible on their entire length and most importantly to their passage through the dural foramen. Adding to the difficulty of identification are the facts that the dorsal rootlets enter the DREZ along an uninterrupted line, and the ventral ones are hidden by the dentate ligament. Furthermore, these roots have a tendency to have an important overlap in their motor innervation making decision difficult. In general, stimulation of the L2 produces a response of the iliopsoas and adductor muscles, L3 produces a preferential response in the adductors and quadriceps, L4 in the quadriceps, L5 in the extensor digitorum, and S1 in the triceps muscles. One must take note of the high variability of distribution of the innervation passing through the lumbosacral plexus as demonstrated by Schirmer et al. .
Another option is the use of surface SEPs as described in Fig. 38.11 . Because neurophysiologic investigations are time-consuming, it is (perhaps) preferable in patients with severe neurologic deficits to use simple anatomical landmarks from measurements along the conus. The technique is described in detail in Chapter 35 , Neurosurgical lesioning-procedures for spasticity and focal dystonia, by Sindou, Brinzeu, and Georgoulis.

MDT at the lumbar and sacral levels has the same principles as the ones at the cervical level. However at the lumbosacral level the procedure may be difficult and dangerous because of the rich vasculature of the conus. The dorsolateral spinal artery courses along the posterolateral sulcus. Its diameter is between 0.1 and 0.5 mm and is fed by the posterior radicular arteries. It joins caudally with the ascending branch of the Adamkiewicz artery through the conus medullaris anastomotic loop of Lazorthes. In patients with no or minor deficits, these arteries need to be dissected and preserved.
Neuropathic pain after spinal cord/root injury touches 35% of patients for the conus medullaris/cauda equina location. Differentiation of the pain that is segmental from the infralesional one (i.e., below the lesion) is of practical importance ( Fig. 38.9 ). In our experience, MDT is only effective in patients in whom the main component of the pain corresponds with the level and extent of the injured segments of the spinal cord, in contrast to the pain located in the territory below the lesion (especially the one located in the perineosacral region) which is not influenced even when DREZ-lesioning is performed at the lower medullary segments . Anatomically, segmental pain is the one that resides in the territories corresponding to the injury and the altered neighboring segments. Mechanisms may result from nerve root contusion, entrapment or scarring, and to the development of central dysfunction due to deafferentation and/or direct damage of the spinal cord neurons that increase(s) their intrinsic excitability and lead to abnormal spontaneous patterns of discharge in the dorsal horn . Such hyperactivities could be recorded during surgery in patients with deafferented segments of the spinal cords . A neighboring hypothesis, based on the fact that cordectomy improves pain only if performed rostral to the level of the lesion , suggests that the origin of the segmental pain comes from the spinal cord segments just rostral to the site of injury.

In paraplegic patients with complete motor, sensory and sphincter deficits, MDT can be done extensively on the selected segments. On the contrary, in the patients with incomplete paraplegia DREZ-lesioning should be performed more restrictively to avoid creating additional neurologic deficits. In patients with spine fractures not previously treated, surgery must start with liberation of the neural structures from the bony fragments that may occupy the intraarachnoidial and the intradural spaces. Frequently, there may be the need for an intradural freeing from an adhesive arachnoiditis. Such a preparatory approach can be long and somewhat bloody; in that eventuality surgery may be stopped and MDT performed in a second stage, around 2 weeks later.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here