Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Much has occurred since the 2009 edition of this book was published. Terms like causalgia, described by Mitchell in 1864 ( ), and reflex sympathetic dystrophy (RSD), coined by , were replaced with the umbrella taxonomy of complex regional pain syndrome (CRPS) by Stanton-Hicks in , and laid the foundation for symptoms and signs that describe this syndrome. CRPS, which embodies Type I and Type II, representing RSD and causalgia respectively, is currently used in more than 80% of citations worldwide ( ).
The diagnostic criteria for CRPS developed at a consensus meeting in Budapest were first published in 2007 ( ), validated in 2010 ( ), and accepted by the International Association for the Study of Pain (IASP) Committee on Chronic Pain Terms in 2012. They have been responsible for an explosive growth in research which has, among other things, yielded pathophysiological bases amenable to neurostimulation. Four basic characteristics, sensory, vasomotor, sudomotor/edema, and motor/trophic, constitute the sign-and-symptom complex that defines a diagnosis of CRPS ( Fig. 48.1 ).
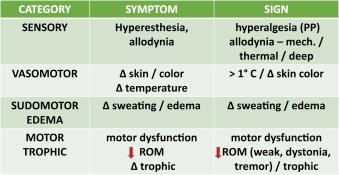
Sympathetically maintained pain ( ), an integral and prior requirement for a diagnosis of CRPS, can be demonstrated by a successful sympathetic block and is found in a significant number of patients with CRPS. In contrast, sympathetically independent pain reflects the failure of a positive analgesic response to a sympathetic block.
The clinical course of CRPS begins with pain, which often follows a minor injury but is disproportionately greater than one would expect from the nature of the real or perceived tissue damage. Patients frequently describe a constant burning sensation in deep and/or superficial tissues, usually in the palm or plantar surface of the extremity. However, where the injury may be proximal, for example in a shoulder or knee, the symptoms will begin in these regions. The course of the condition may be accompanied by changes in hair and nail growth, abnormal color changes, mottling of the skin, hyper/hypohidrosis, changes in sensitivity such as hyper/hypoesthesia, mechanical or thermal allodynia, and/or hyperalgesia.
A movement disorder, represented by tremor, weakness, or dystonia, is present in 80% of patients with CRPS ( ). One mechanism of dystonia seems to be a dysregulation of force feedback vis-à-vis peripherally initiated conditions (peripherally induced movement disorders). These may change both anatomical and functional connectivity of spinal and supraspinal sensorimotor circuits, which in turn may lead to pain chronicity, abnormal centrally mediated motor responses, and sensory impairment. Also, dystonia has a relationship to some human leukocyte antigen gene complexes 1
1 The human leukocyte antigen system, the major histocompatibility complex in humans, is controlled by genes located on chromosome 6. It encodes cell surface molecules specialized to present antigenic peptides to the T-cell receptor on T cells. Taken from the internet: https://www.google.com/#q=what+is+an+hla+gene& ∗.
( ). Dystonia may precede pain, appear spontaneously, or even occur on the contralateral side. Early sympathetic block with local anesthetics may alleviate these motor symptoms ( ).
Central sensitization may arise de novo or as a consequence of the peripheral inflammatory process sensitizing Aδ and C-fiber afferents. Chronic C-fiber afferent substance P signaling activates spinal neuroglia, maintaining central sensitization ( ). In addition, increased glutamate activity at the N-methyl-D-aspartate (NMDA) receptors facilitates transduction of afferent signals, setting up afferent–efferent loops and centralizing spinal segmental and suprasegmental levels.
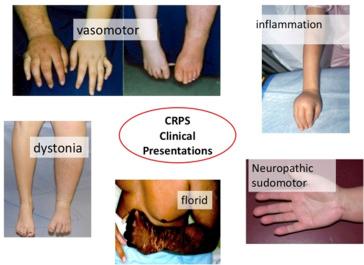
Structural changes may affect bones, joints, integumentary tissues, and dermis, leading to atrophy or dystrophic changes ( Fig. 48.2 ).
The differential diagnosis of CRPS requires consideration of local pathology, neuropathic pain syndromes, peripheral neuropathies, vasculopathies, inflammatory and infectious diseases, and arthritidies ( ). Differentiating CRPS from trauma is critical to a correct diagnosis ( ). Specifically, motor signs, trophic changes, and increased sweating distinguish CRPS from trauma. The majority of patients develop CRPS after injury or surgery: 29% after sprain or strain, 24% after surgery, 23% due to spontaneous or unknown causes, 15% after fracture, and 8% after contusion or crush injuries ( ).
The Neuromodulation Therapy Access Coalition found excellent evidence supporting the use of spinal cord stimulation (SCS) to treat CRPS ( ). The authors identified 3 randomized controlled trials (RCTs), 6 long-term follow-up studies, 6 short-term follow-up studies, and 10 case studies.
In the first RCT of SCS for CRPS, all patients met IASP CRPS Type I criteria ( ). All patients had their pain for more than 6 months and all had failed conventional treatment, consisting of medical management and physical therapy (PT). Patients were randomized to receive SCS plus PT (n = 36) or PT alone (n = 18). Only those patients who had a successful trial underwent an SCS implant. Intention-to-treat analysis demonstrated a significant reduction in pain for patients in the SCS group when compared to those in the PT group ( P < .001): 39% had an improved global perceived effect (GPE) compared to 6% in the control group. The quality of life (QOL) improved 11% overall in the 24 patients who received a permanent SCS system. In a 2-year follow-up the authors found the SCS/PT group maintained their significant GPE improvement compared with the PT-only group ( P ≤ .001 Neither group showed any clinically important improvement of functional status. The authors concluded that SCS provides long-term pain reduction and improved QOL in patients suffering from CRPS. A 5-year follow-up found no difference between pain scores, but the GPE results were significantly better in the SCS plus PT group ( P ≤ .02 ( ), and 95% of all SCS-treated patients stated they would repeat the treatment for the same result. undertook an interesting RCT in which the analgesic effects of carbamazepine were compared with sustained-release (SR) morphine in patients with CRPS. All patients were pretreated with SCS, and 43 had their SCS systems switched off before receiving their medication or placebo. Compared with placebo, those patients who received carbamazepine had a delayed onset of pain, but there was no effect in patients receiving morphine SR. Two patients receiving carbamazepine and one patient receiving morphine SR preferred to continue their medication. However, 35 patients chose to return to SCS. While most of the published studies regarding SCS for CRPS studies are retrospective in nature, a review of 10 studies by found an overall success rate of 82% (148/180) for patients with CRPS Type I and 79% (23/29) for CRPS Type II.
Recent case reports and studies reflect the extraordinary advances in technology and clinical applications that are currently being realized with SCS. A 65-year-old woman with CRPS of the left upper extremity, whose response after the initial success of SCS over a 2-year period became attenuated, with a numerical rating score (NRS) for pain that climbed to 8, underwent burst stimulation which reestablished the previous efficacy and resulted in the NRS returning to 2 ( ). Burst stimulation is a unique method of SCS consisting of delivering spikes of energy, and is discussed in the next section.
Along similar lines, completed an RCT that compared tonic SCS and high-frequency and burst stimulation in patients with CRPS. Five SCS modes (40, 500, 1200 Hz, burst, and placebo) were compared in a blinded manner after a successful trial and implantation using 40 Hz stimulation. After a 3-month follow-up assessment (T1), the remaining modes were tested in a cross-over fashion over 10 weeks. Each patient then chose the stimulation they preferred. This was assessed at the end of 3 months (T2). The answers from this trial will provide important information regarding the most appropriate type of stimulation that can provide optimal pain control and perhaps also improvement of function for patients with CRPS.
The combination of SCS and motor cortex stimulation (MCS) is one example of combining a single neuromodulation modality with another to regain a loss of clinical efficacy. Lopez et al. described the gradual loss of pain relief to SCS over 2 years in a patient with severe CRPS Type II after brachial plexus injury ( ). Using MCS in a cycling fashion with SCS, they were able to regain efficacy by 2 years, with pain reduction and improvement in QOL noted at follow-up. Similar improvements have been achieved by using the combination of SCS and peripheral nerve stimulation (PNS). Sanders et al. described the outcomes of a retrospective case series of 199 patients with failed back surgery syndrome (FBSS) or CRPS who were treated with SCS at an academic medical center over a 10-year period. The oral morphine equivalents and NRS scores decreased significantly from preimplantation levels at 6 months and 1 year ( P = <.02 and P = <.01, respectively) ( ).
Early implementation of SCS was emphasized by Prager and Chang to facilitate interdisciplinary management of CRPS. The externalized SCS lead remained in place for 4 weeks, but if a patient still required SCS for pain relief and promotion of functional restoration, a permanent SCS system would be implanted. This emphasizes the fact that not only may early use of SCS be an important adjunct to exercise therapy, but an externalized SCS (extended trial) may be both therapeutic and cost effective. A second set of 16 patients received a permanent implant if they failed to improve after 4 weeks of comprehensive therapy (exercise therapy with or without a behavioral component). Two patients of this latter group had their SCS system explanted because they were essentially pain free and no longer used their systems at 5 and 18 months after implant ( ). A recent paper by Goff et al. exemplifies the early application of SCS in a patient who developed spontaneous CRPS and was completely refractory to medical management, and whose rehabilitation dramatically responded after SCS, with unassisted ambulation, functional improvement, pain relief, and improved QOL ( ) in the paper by , these investigators also exempified early application of spinal cord stimulation for CRPS.
Singular advances in our understanding of the neural framework and possible sites within which SCS may achieve its clinical response on pain have taken place during the past 10 years. The traditional/conventional model of tonic (paresthesia-based) stimulation has finally been challenged by new waveforms and paradigms. With software and technological advances, tolerance as a byproduct of tonic SCS may be relegated to history. Without exploring the field of SCS mechanisms, which is more than adequately dealt with elsewhere (see Guan et al.), one recent study has suggested a mechanistic basis for clinical phenomena that respond to SCS. Dystonia, a frequent and frustrating phenomenon that occurs in CRPS, is difficult to treat and can prevent the adequate return of function. A case report by in which a 31-year-old female with severe refractory dystonia responded successfully to a combination of SCS, multidisciplinary care, and behavioral measures is an example. The authors make a strong argument that dystonia, in this case, is a consequence of multilevel (peripheral and central) pathological modifications in the nervous system brought about by CRPS ( ). As pointed out by , a number of CRPS pathophysiologies respond not only because of their effects on central autonomic and spinal inhibitory systems, but also because of the beneficial effects on the microvasculature via calcitonin gene-related peptide and nitric oxide, and on small-diameter nerve endings through the expression of transient receptor potential V1. In addition, the α-1a receptor, which is an integral component of CRPS pathophysiology (expression on nerve endings, blood vessels, and sclerocytes in the periphery), responds to SCS with the amelioration of this activity ( ).
In an animal model of a unilateral spinal nerve injury designed to look at the manner in which SCS modulates the descending antinociceptive system (DAS), found that while serotonergic and noradrenergic pathways in the DAS may be important, the dorsal raphe nucleus and, to a lesser extent, the locus coeruleus may be primarily responsible. They also noted that SCS does not potentiate the synthetic enzymes of 5HT and norepinephrine in the neuropathic spinal cord. The authors used methysergide, a 5HT 1 and 5HT 2 antagonist, and idazoxan, an α-2 adrenoceptor antagonist, both of which antagonized the antinociceptive effect of SCS ( ). A disturbance in the locus coeruleus of hemilateral pain processing, due to the disruption of pain-control mechanisms because of a sensitized nociceptive network in CRPS, might be a likely target for SCS. The spread of increased sensitivity and allodynia from an affected limb to the ipsilateral forehead as a reflection of disrupted pain mechanisms was commented on by .
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here