Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
![]() This chapter includes an accompanying lecture presentation that has been prepared by the authors: .
This chapter includes an accompanying lecture presentation that has been prepared by the authors: .
Microelectrode recording (MER) offers the most accurate structure differentiation tool to localize deep nuclei in the brain.
MER requires knowledge of anatomy, disease, and anesthetic management for proper localization.
The relationship between the surgeon and physiologists is critical for proper MER application in the operating room.
Firing rates, morphology, and variability are all critical when determining the structure from which the firing originates.
After recoding, it is important to fit the data to the appropriate structures to fuse the recordings to the anatomy.
Intraoperative neurophysiologic monitoring (IONM) is an outcrop of techniques used originally in both clinical and research laboratories. At the 2000 International Intraoperative Neurophysiologic Monitoring symposium, Dr. Marc Sindou coined the term interventional neurophysiology to describe the area of intraoperative monitoring that includes those techniques that directly guide segments of surgical intervention. The application of many standard IONM techniques can warn the surgical team that something potentially correctable has occurred, whereas other IONM applications are integral elements of the surgical procedure itself such as mapping and localization methods during surgery. Microelectrode recording (MER) represents a specific application for such mapping and localization in deeper brain targets. The technique of recording from single units (cells) in research laboratories dates to 1939 with Renshaw and colleagues’ recordings of pyramidal cells in the cat hippocampus. The inclusion of single-unit recordings in the operating room setting dates to the early 1960s and has now become a common neuromonitoring tool when performing deep brain stimulation (DBS) procedures, the standard surgical treatment for a variety of movement disorders, such as Parkinson disease (PD), essential tremor, and dystonia.
Before the mid-1990s, surgical interventions to treat movement disorders relied on therapeutic lesioning. Since the early 2000s, almost all surgical treatments place stimulating electrodes within deep brain structures to treat these disorders. The deep location of these targets does not allow for safe direct surgical visualization, and surgeons therefore rely on a combination of image-guided stereotactic techniques and intraoperative neurophysiology to place the stimulating electrodes with a high level of accuracy and safety. Neurophysiology is critical because unlike tumors and vascular deformities, which are relatively large and easily identified with CT or MRI, functional neurosurgical targets typically are small (<3 mm) and somewhat poorly visualized with typical current, easily accessible imaging modalities. In addition, even as imaging technology improves, the optimal placement is still a physiologic target, which presently cannot be differentiated using only imaging technology. Together the surgeon and physiologist use MER data and other neurophysiologic data sets to fine-tune their anatomic targeting before completing the therapeutic intervention. Thus employed, intraoperative neurophysiology does not simply monitor surgical activity; it guides it.
The first operation applied to the communication networks of the basal ganglia for movement disorders was performed in 1939 by Meyers, , who demonstrated the potential benefits of surgery for relief of PD symptoms, even given the high mortality rates (10%–12%) of this open procedure. During these and other surgical procedures, Meyers was observing and describing the frequency, phase, and amplitude of what we would today call low-frequency potentials (LFPs) from the striatum, pallidum, corpus callosum, internal capsule, subcallosal bundle, and dorsal thalamus in patients with and without movement disorders. , Meyers quickly realized the potential value of the accumulated data, which he ultimately used to help localize specific deep brain structures during movement disorder surgery.
Early electrophysiologic studies of the human thalamus and basal ganglia were performed with macroelectrode techniques recording LFPs. As electrode manufacturing and electronics were refined over subsequent decades, the inclusion of single-cell MER in the localization process was made possible. Key work done by Albe-Fessard, who refined MER techniques that had been used for experimental purposes, paved the way for their intraoperative use. , She suggested that MER would “provide a powerful tool in improving stereotactic localization and that it would furthermore reduce the risk due to anatomical variability.”
Presently, movement disorder surgery is focused on three “deep brain” structures: the ventral-intermediate (VIM) thalamic nucleus, the internal globus pallidus (GPi), and the subthalamic nucleus (STN) ( Fig. 115.1 ) . Initially, these targets were destroyed using either a radiofrequency ablation or cryoablation technique. The present state of the art is to insert a permanent electrode for chronic electrical stimulation. , , , , The choice of target is based largely on clinical diagnosis and the symptoms to be treated.
There is no single “best” surgical method for performing movement disorder surgery. Currently accepted techniques involve both frame-based and frameless stereotactic anatomic localization methodologies supported by imaging and intraoperative physiologic confirmation of proper targeting. No matter the approach, it is generally accepted that some form of intraoperative physiologic confirmation is needed. This chapter focuses on the techniques of intraoperative single-unit recordings (MER) and macroelectrode stimulation testing: VIM thalamic nucleus for essential tremor, STN or GPi for PD, and GPi for dystonia.
Microelectrodes provide the most detailed physiologic picture of the neural elements encountered during movement disorder surgery. a
aReferences 10, 12, 26, 29, 36, 37, 40–42, 63, 71, 78–80, 83–95.
Intraoperative microelectrodes are metal, as opposed to glass pipets, and have diameters of 10 to 40 μm and impedances of about 0.5 to 1.5 MΩ. By recording individual neuronal activity ( Fig. 115.2C ), microelectrodes provide real-time information concerning the temporal electrophysiologic characteristics of a single neural unit and indirectly about the nucleus within which the cell is located. One drawback to the technique is that interpreting single-cell recordings is a skill that is mastered only with experience and patience. Yet our experience has shown that, when mastered, MER can be performed both quickly and efficiently and yields invaluable data concerning electrode position.
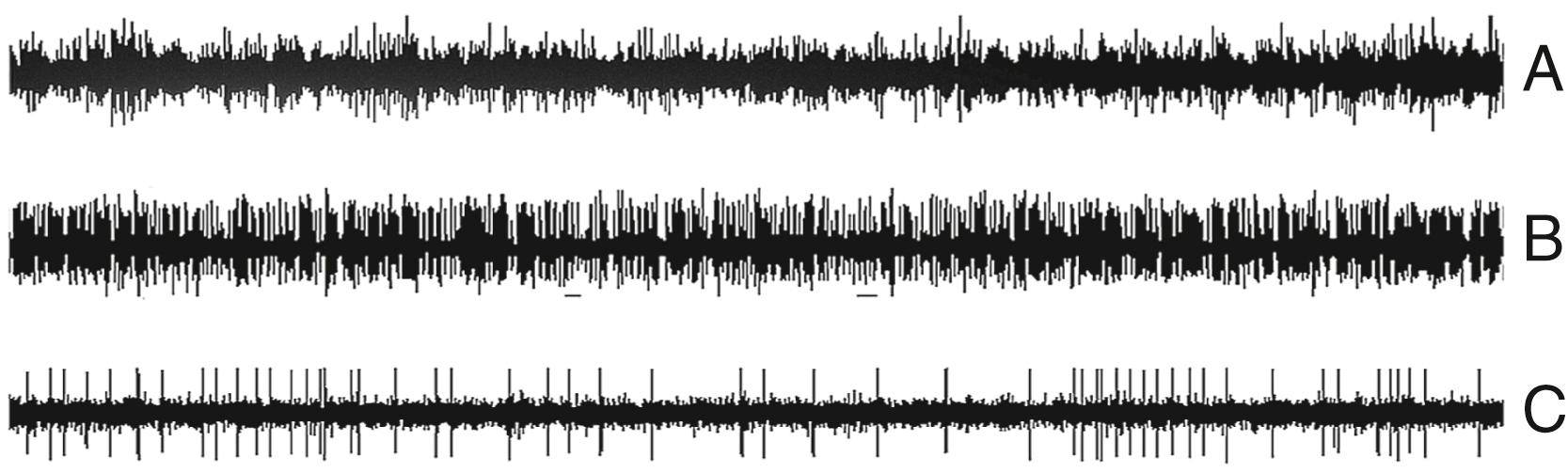
In addition to MER data, some electrodes include additional recording surfaces that record LFPs. The recordings from these non-microelectrodes are similar to those recorded by depth electrodes used, for example, in seizure focus localization in epilepsy patients. Even though the obvious differentiation firing patterns, available through MER, are not apparent, decomposing this signal by using spectral or other statistical time series methods can extract information relating to the complex oscillatory nature of the specific circuits that may be involved in the sensorimotor aspects of basal ganglia processing and the disorder itself. By looking at both the amplitude variations and the variations in the power spectra of these LFPs, a more optimal localization may be found and greater insight into the possible mechanisms behind the disorder may be gained, but more important, understanding the analysis of these data may open a door for closed-loop systems, given the stability of these oscillations over time.
LFPs are the averaged postsynaptic activity of a few hundred to a few thousand cells. When the system activity is composed of correlated events, the analysis of this averaged activity can give much greater insight into the complete network compared with that of a single unit. One analogy is that of a complex orchestral piece wherein sampling of specific instruments gives only one element of the complete piece and in many instances does not allow for a complete understanding of the piece, yet through listening to the complete orchestra the underlying structure is not obvious. On the other hand, if the network is not coherent, then very little information can be added by looking at an averaged ensemble. Work by Brown and colleagues in patients with PD showed that there are changes in power spectral energies in medication “on” versus medication “off” states. , Additional work has shown that placing the electrode in the region of specific spectral band peaks can be somewhat predictive of patient outcome. Brown has hypothesized that movement initiation is reduced because of the high energy of the beta oscillations inhibiting the passage of the normal movement information. The beta frequencies are in the range of 12 (10 in some cases) to 30 Hz. Another important energy range in movement disorders is the gamma band, which is in the range of 60 to 90 Hz. Zaidel and colleagues showed that both beta- and gamma-oscillation changes are present during movement-related activities, yet the beta activity seems to be higher in the “dorsolateral oscillatory region” of the STN compared with the ventrolateral (VL) region, which had less movement-related cell activity, whereas the gamma activity was higher in the VL region. In addition, they demonstrated that placing the final electrodes in the dorsolateral oscillatory region, defined as areas with high beta power, appears to be a good indicator of patient outcome. Presently the role of LFPs as a real-time analysis tool for optimizing electrodes is still under investigation, but many commercial systems now include tools to analyze these data.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here