Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The nondepolarizing neuromuscular blocking agent (NMBA) d-tubocurarine has been used for 500 years as a paralyzing poison. In the 16th century, Sir Walter Raleigh reported that hunters in South America were using darts and arrows dipped in curare to paralyze their living targets. Curare, the poison of the plant Strychnos toxifera , and its active component, d-tubocurarine, were isolated in the 1930s. d-Tubocurarine was introduced into clinical practice in 1942 to induce neuromuscular block. It was not widely used until the second half of the 20th century, when maintenance of neuromuscular blockade during surgery became widely accepted. Clinical use of these agents facilitates endotracheal intubation and mechanical ventilation, improving surgical conditions, and recent data suggest that neuromuscular blocking agents have the potential to improve the outcome in patients with respiratory distress syndrome. Since the introduction of d-tubocurarine into clinical practice, the use of NMBAs has become common. In 2010, more than 100 million patients received NMBAs throughout the world, 80% of them nondepolarizing NMBAs.
While the use of NMBAs has allowed the development of modern anesthesia and surgery, their use is not without risk. Shortly after the introduction of NMBAs into clinical practice, Beecher and Todd reported an increased mortality in patients who had received NMBAs as part of their anesthetic. While their conclusions on cause and effect have been criticized, it has now become clear that residual effects of NMBAs can adversely impact patient outcome. As reported by Beecher, unrecognized residual paralysis negatively affects ability to breathe and protect the airway. While the introduction of the intermediate-acting NMBAs rocuronium, vecuronium, and cisatracurium into clinical practice in the early 1990s initially decreased the incidence of residual neuromuscular blockade, more recent data indicate that as surgical and anesthetic practices and the definition of inadequate recovery of neuromuscular function have evolved, the incidence of residual neuromuscular block, even with these shorter-acting compounds, remains high.
Administration of NMBAs for tracheal intubation decreases the incidence of postoperative upper airway trauma-related symptoms by decreasing the likelihood of tissue trauma, including vocal cord injury and resulting postoperative hoarseness. Administering NMBAs to mechanically ventilated patients with acute respiratory distress syndrome (ARDS) in the intensive care unit for a short period may improve their outcome. Neuromuscular blockade decreases the need to overdose anesthetics in order to decrease reflex movement. It also facilitates surgical exposure and minimizes potentially deleterious complications of intraoperative patient movement. It is not, however, a substitute for an adequate depth of anesthesia. While neuromuscular blockade decreases the likelihood of patient movement, it does not guarantee the absence of pain, recall, and patient movement throughout a surgical procedure; patients can move even when their response to neuromuscular stimulation is significantly reduced (1 or 2 responses to train-of-four [TOF] stimulation).
Since spontaneous recovery of neuromuscular function after administration of a neuromuscular blocking agent does not happen quickly and is not instantaneous even with the administration of an appropriate dose of an anticholinesterase, maintaining a deep level of relaxation throughout a surgical procedure, especially ocular or laparoscopic surgery, might not allow enough time during closure for complete recovery of neuromuscular function. There are a number of possible solutions to ensure adequate recovery of muscle strength at the end of a surgical procedure. First, NMBAs are not always required to optimize surgical conditions. Alternatively, depth of block can be allowed to decrease as the surgical procedure nears completion, allowing for a greater degree of spontaneous recovery before administration of the anticholinesterase. Most recently, it has been possible to use a selective relaxant binding agent rather than an anticholinesterase to facilitate recovery from either rocuronium- or vecuronium-induced neuromuscular block. The “optimal” depth of neuromuscular block to provide the best surgical conditions depends on both the surgical procedure and the anesthetics that are being administered. Administration of volatile anesthetics potentiates NMBAs. Whether profound paralysis (post-tetanic count of 1–3) always improves surgical conditions is currently a matter of debate ; additional work is necessary to determine whether increased use of deep levels of neuromuscular blockade improves or worsens patient outcomes, such as an increase in the odds of hospital readmission within 30 days after surgery. An association between the intraoperative dose of NMBA and 30-day readmission after abdominal surgery has been demonstrated.
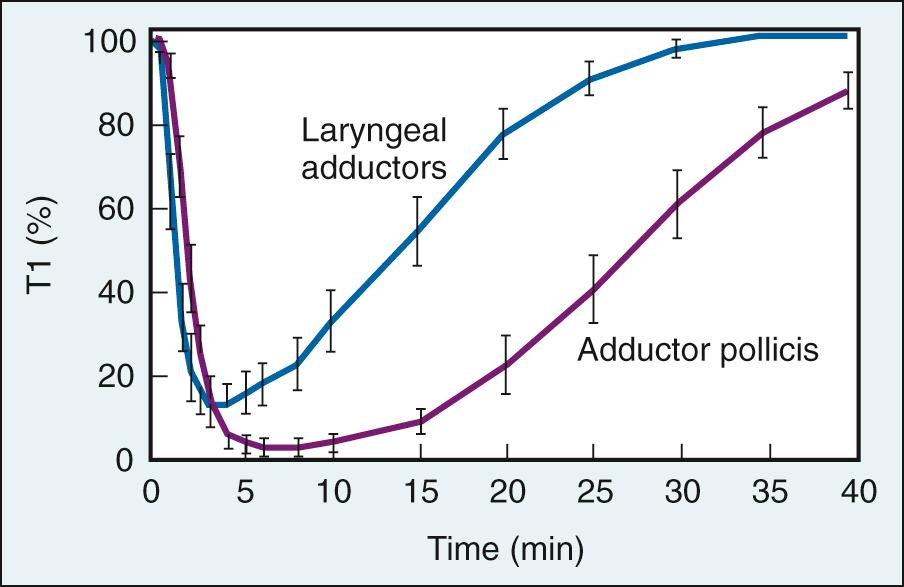
The effects of NMBAs are different in different muscles owing to their physiologic differences ( Chapter 21 ). The diaphragm is less susceptible to the effects of NMBAs than either peripheral muscles or pharyngeal upper airway dilator muscles. Additionally, recovery of diaphragmatic function occurs more rapidly than in muscles of the extremity, such as the adductor pollicis, which is the muscle commonly used in clinical practice for monitoring depth of neuromuscular block following administration of an intubating dose of a nondepolarizing NMBA. The resistance of the diaphragm to neuromuscular blockade can be explained by the release of a greater number of acetylcholine-containing vesicles from presynaptic terminals following neural stimulation and the presence of a greater number of postjunctional nicotinic acetylcholine receptor binding sites than exist in peripheral muscles.
In clinical practice, the response of the adductor pollicis muscle to stimulation of the ulnar nerve is recommended for monitoring, as recommendations for dosing of NMBAs are based on the response of this neuromuscular unit to stimulation. When used, its response is assessed by either visual or tactile evaluation. While other superficially located neuromuscular units can be monitored, their response to neuromuscular stimulation will be different than that of the adductor pollicis in the same patient. This occurs because different neuromuscular units have different sensitivities to NMBAs and different time courses for onset of and recovery from neuromuscular block ( Fig. 22.1 ). This has been attributed to different blood flow to these different muscles. Typically, when a patient's arms are tucked and not available for monitoring, the posterior tibial nerve that innervates the plantar muscles of the foot, the common peroneal nerve that through the deep peroneal nerve innervates the muscles of the anterior compartment of the leg, or the facial nerve that innervates the muscles used for expression, may be used for monitoring depth of neuromuscular blockade. When dosing of NMBAs based on the results of monitoring at these different sites, the differences in their response to neural stimulation must be considered. The mimetic muscles recover more quickly than those of the periphery and the depth of block in response to a dose of an NMBA is less profound than that in the arm or the leg. While different sites can be monitored, dosing recommendations for NMBAs are based on the response of the adductor pollicis to stimulation of the ulnar nerve.
The degree of interpatient variability regarding the effects of NMBAs and the potential adverse consequences of their residual effects at the conclusion of an anesthetic are the reasons for the importance of adequately monitoring their effects in clinical practice. Unfortunately, it is not possible to detect reliably residual neuromuscular block with either clinical tests of muscle strength or with commonly used qualitative monitors of neuromuscular function.
Reduced strength of contraction during repetitive stimulation of a peripheral nerve is observed with neuromuscular transmission failure, as in myasthenia gravis, and during recovery from NMBAs. Proper muscle function requires different degrees of reserve in terms of neuromuscular transmission depending on the test chosen to assess strength. The ability of a test to measure the effects of an NMBA increases with the force of output required to pass the test. Assessment of fade during a supramaximal 100 Hz tetanic stimulation can detect subtle effects of NMBAs, whereas twitch height after low-frequency stimulation (e.g., 0.1–1 Hz) decreases only after blockade of 90% of acetylcholine receptors. This is clinically important, as clinicians typically assess neuromuscular function using nontetanic stimulation of peripheral skeletal muscles, usually TOF or double-burst stimulation (DBS). While tetanus will detect more subtle degrees of neuromuscular block, it is not a commonly used monitor of residual neuromuscular block. Tetanic stimulation is exceptionally uncomfortable for the patient who is not deeply anesthetized. In addition, interpretation of the significance of fade in the response to tetanic stimulation is difficult and the degree of fade has not been correlated with the TOF response. Therefore, its utility is of relatively limited clinical value.
The technique of train-of-four monitoring was introduced into clinical practice in 1970. For measurement of the TOF response, muscle contraction is induced by stimulation of the corresponding motor nerve 4 times with a frequency of 2 Hz. Any superficial neuromuscular unit can be monitored in this fashion. In response to TOF stimulation, the TOF ratio (TOFR) is the ratio of the amplitude of the fourth response to the first response. If neuromuscular transmission is intact, TOF stimulation causes 4 twitches with essentially identical amplitudes and a resulting TOFR of 0.9 to 1.0. In contrast, after complete relaxation, TOF stimulation does not result in any muscle contraction and the TOF count (number of responses to TOF stimulation) is zero. Return of the first twitch is described as a TOF count of 1. This is followed by consecutive recovery of the second, third, and fourth twitches (TOF counts of 2, 3, and 4, respectively). Once the fourth response to stimulation has returned, the fade between the first and the fourth twitch responses can be measured as the TOFR. For example, if the amplitude of the fourth twitch is 50% of the amplitude of the first twitch, the TOFR is 0.5. The TOF count can also be used to estimate the recovery of the first twitch in the TOF to baseline values. When the first response in the TOF returns, strength of the first response is approximately 10% of baseline values. Similarly, return of the second, third, and fourth responses corresponds to recovery of the first twitch in the TOF to approximately 20, 35, and 45% of baseline values.
While the TOF will measure residual paralysis, ability to accurately detect degree of fade in the TOF response is not reliable once the TOFR has recovered to 40% or more. In other words, it is impossible to reliably detect fade in the TOFR if there is anything less than 60% fade, and a TOFR of 92% looks and feels the same as a TOFR of 52%.
DBS was developed to improve detection of residual neuromuscular block. Fade in the strength of the second response relative to the first response is used to determine whether residual neuromuscular block is present. Fade in the response to stimulation is equivalent to the fade detected with TOF stimulation; however, the reliability of qualitative monitoring with DBS is improved over that of TOF monitoring. DBS allows detection of fade when the second response is 60% of the first response. This occurs because the presence of the second and third responses to TOF stimulation makes the comparison of the strength of the fourth response to that of the first response more difficult. With DBS, either 2 or 3 short bursts of high-frequency tetanic stimuli are administered, followed by a second series of 2 or 3 short bursts of tetanic stimuli, each resulting in a single muscular contraction. With full recovery from neuromuscular block, 2 equal responses occur with DBS.
Typically, NMBA doses of two times the ED 95 (the dose required to cause, on average, 95% suppression of muscle response to stimulation) or greater are administered to facilitate tracheal administration in a reasonable time after induction of anesthesia. However, smaller doses of NMBA might be adequate to optimize intubating conditions during deep anesthesia. While recovery is monitored with either TOF or DBS, onset of block is typically determined with the response to single-twitch stimuli. For this pattern of stimulation, supramaximal stimuli are applied at a frequency of 0.1 Hz, or once every 10 seconds. Onset of neuromuscular blockade is defined as the fade in twitch response with each subsequent stimulus. When larger doses of NMBA are administered, onset of 100% neuromuscular block occurs more quickly and is more likely to develop in all patients. Doubling the dose of rocuronium from 0.6 to 1.2 mg/kg shortens the average onset time from 1.5 minutes to just under 1 minute, and decreasing the dose slows onset time.
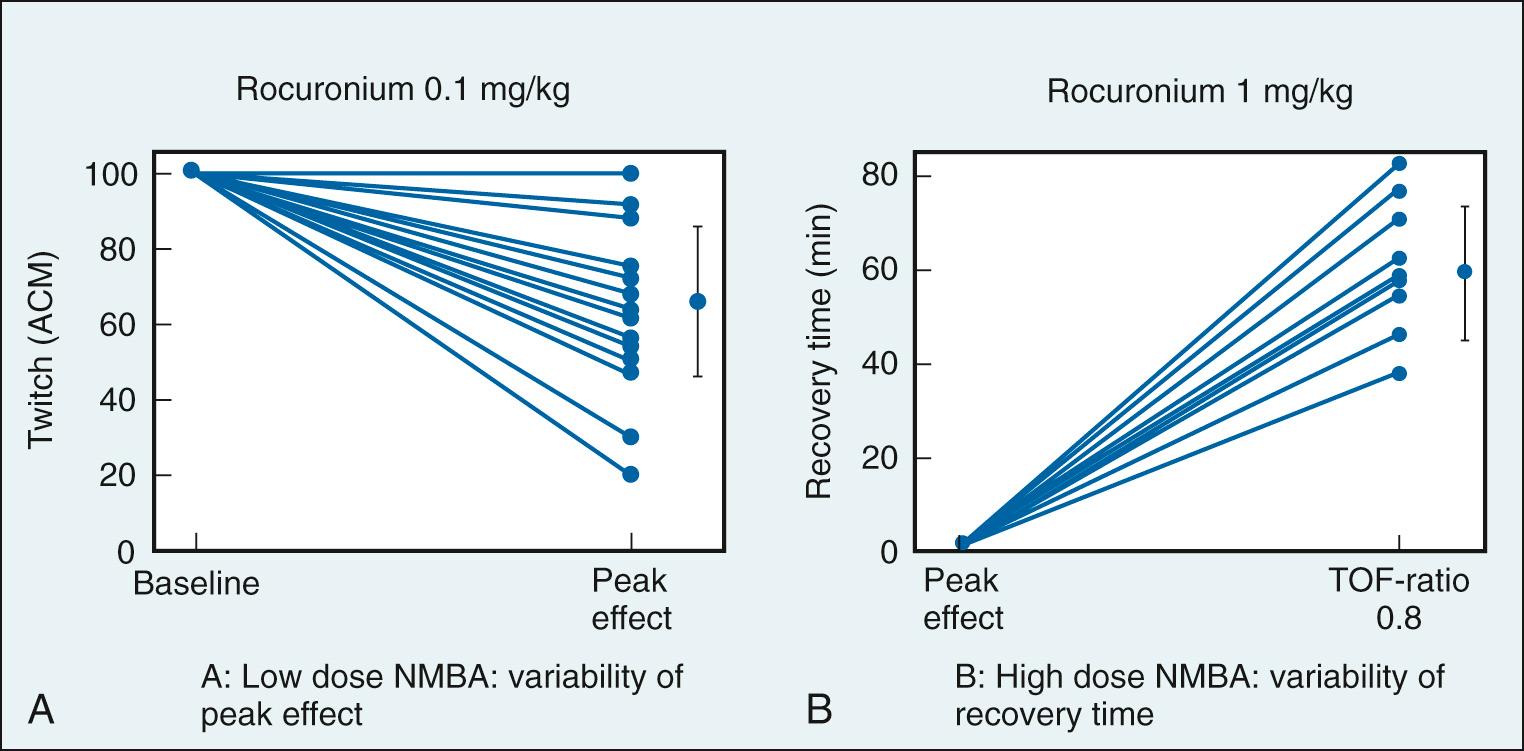
When monitoring the effect of NMBAs administered to facilitate tracheal intubation, monitoring at the muscles of the face more accurately indicates adequacy of neuromuscular block in the upper airway. When lower doses of NMBAs are administered, depth of block at a particular time (60 or 90 seconds after administration) cannot be guaranteed since onset of NMBAs is quite variable. As shown in Fig. 22.2 , patients developed 0% to 80% neuromuscular block following administration of 0.1 mg/kg rocuronium. Just as onset of block is variable with small doses of NMBAs, recovery is quite variable following administration of larger doses (see Fig. 22.2 ). This emphasizes the importance of monitoring depth of neuromuscular block throughout surgery to avoid overdosing.
While the most commonly used monitors of depth of neuromuscular block are qualitative monitors, quantitative monitors of depth of block are also available. Without using these quantitative devices, clinicians can reliably detect only severe residual neuromuscular block (TOFR < 0.4), as twitch height at a TOFR between 0.4 and 1.0 is likely to be perceived as 4 responses that are similar. Optimally, quantitative methods for measurement of the evoked muscular response should be used. Commercially available techniques include mechanomyography, electromyography, kinemyography, and acceleromyography.
Structure–activity relationships of NMBAs can affect neuromuscular blocking activity, pharmacokinetic properties, and side effect profiles. Since the early classification of NMBAs as rigid bulky molecules with amine functions incorporated into ring structures, much has changed in our understanding of the relationships between their structures and function as neuromuscular blockers.
Postjunctional nicotinic acetylcholine receptors are pentameric members of the superfamily of ligand-gated ion channels. The mature form consists of 5 subunits: 2-alpha (α), 1-delta (δ), 1-beta (β) and 1-epsilon (ε); ( Fig. 22.3 ). In the immature (fetal) form of the receptor, the ε subunit is replaced by a gamma (γ) subunit. The N- and C-terminal ends of each subunit are extracellular, with the protein traversing the lipid bilayer membrane 4 times—creating four transmembrane domains (M1, M2, M3, and M4). The M2 domain of each subunit creates the central ion pore (see Fig. 22.3 ).
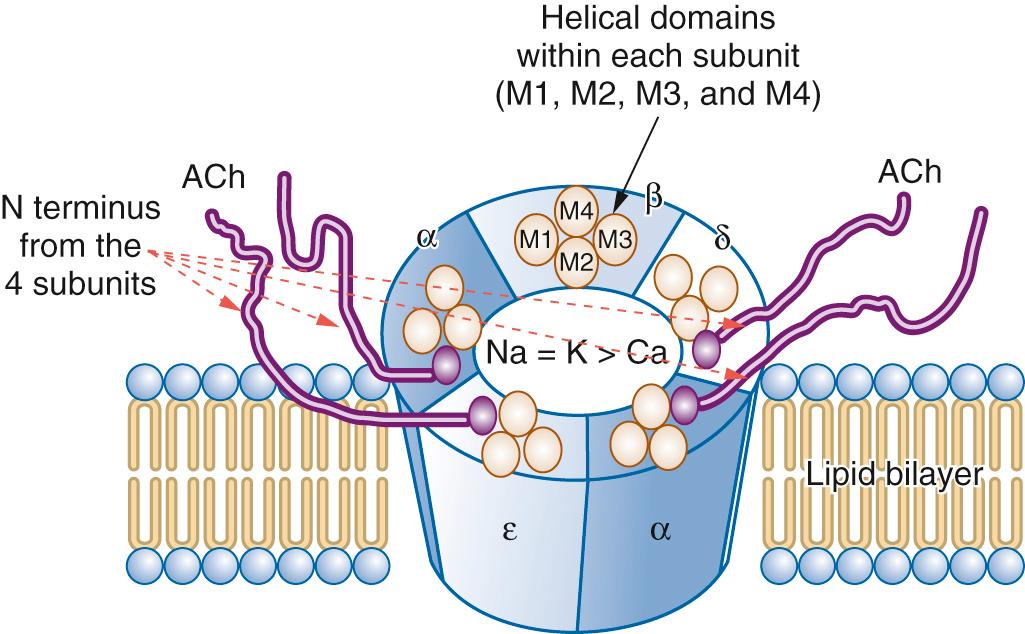
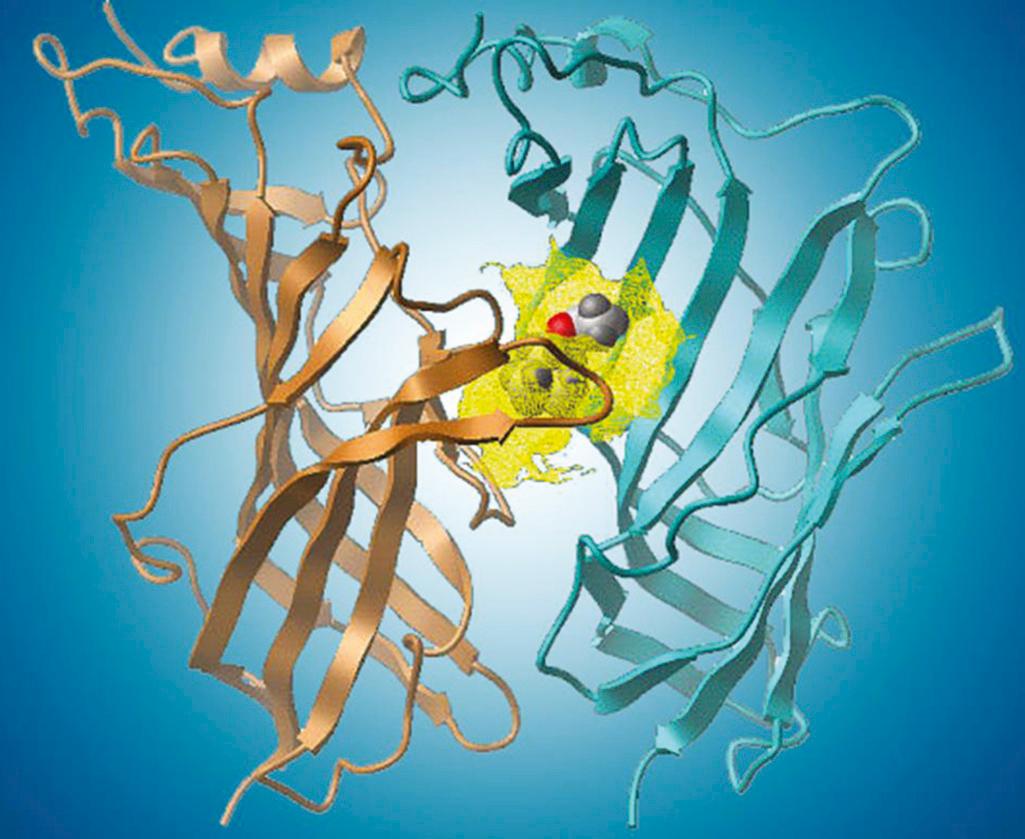
The agonist binding sites of the acetylcholine receptor are located at the interface of the α-δ and α-ε subunits, where the N-terminus of each subunit works with that of the other to form the acetylcholine binding site. In order for the central pore of the receptor to open, allowing for influx of Na + and Ca 2+ and efflux of K + , two agonist molecules must be bound to the receptor. The two binding sites are not identical (the δ-subunit contributes to one receptor and the ε-subunit contributes to the other). These differences lead to varying affinity at each of the sites for agonists and competitive antagonists. The fetal α-γ binding site is generally more sensitive than the mature α-ε one. The α-γ binding site has up to a 500-fold greater affinity for d-tubocurarine than does the α-δ binding site. In mature receptors, the α-δ binding site appears to be more important than the α-ε site in determining receptor affinity for pancuronium, vecuronium, and cisatracurium.
The complexity of fitting large molecules, such as NMBAs, into acetylcholine receptor agonist binding sites ( Fig. 22.4 ) implies that conformational changes in the NMBA are required. While these compounds are large, they can bend and fold and will seek a conformation requiring minimal energy. Interaction of the γTyr117 with the 2-N and 13′ positions of d-tubocurarine suggests that allosteric changes in either the antagonist or receptor occur with binding. Several different sites of interaction in the binding site are involved in binding the agonist or antagonist. Different affinities at each of these sites might account for some of the synergism observed when different NMBAs are administered to the same patient.
In addition to opening the ion channel of postjunctional acetylcholine receptors, neuromuscular transmission is modulated by a population of prejunctional cholinergic receptors. These prejunctional nicotinic and muscarinic receptors on the motor nerve endings are involved in modulating the release of acetylcholine into the neuromuscular junction. Prejunctional nicotinic receptors are activated by acetylcholine and function in a positive feedback control system that serves to maintain the availability of acetylcholine when demand is high. Their activation mobilizes acetylcholine-containing synaptic vesicles toward the release sites in the presynaptic membrane of the motor nerve terminal but not the actual process of acetylcholine release. These presynaptic receptors are morphologically different than those at the postjunctional membrane and consist of 3 α subunits and 2 β subunits. All NMBAs tested—including mivacurium, atracurium, cisatracurium, d-tubocurarine, pancuronium, rocuronium, and vecuronium—inhibit presynaptic nicotinic acetylcholine receptors in a concentration-dependent fashion, with concentrations causing 50% inhibition of response in the micromolar range. Vecuronium and d-tubocurarine are the most potent inhibitors of this receptor subtype; mivacurium is the least potent. Inhibition of this presynaptic receptor by NMBAs is primarily competitive, but d-tubocurarine and vecuronium also produce noncompetitive inhibition. The effect of blockade of these presynaptic receptors during periods of stress, such as TOF or tetanic stimulation, likely accounts for the fade observed in the TOF response with small doses of NMBA, such as those administered prior to succinylcholine to decrease the incidence and severity of fasciculations of NMBAs.
Onset of neuromuscular block is proportional to the dose of NMBA administered and is typically described in terms of multiples of the ED 95 (the dose causing 95% suppression of twitch response; Table 22.1 ). The use of larger doses of NMBAs is limited for a number of reasons, including an increase in the duration of action (the time required from administration to recovery of twitch height to 25% of baseline, which increases with increasing dose), more frequent and severe side effects, and the limited benefit of increasing the dose beyond a certain point.
| Neuromuscular Blocking Agent | Approximate ED 95 (mg/kg) | Intubating dose (× ED 95 ) |
|---|---|---|
| Pancuronium | 0.07 | 1–1.5 |
| Rocuronium | 0.30 | 2–4 |
| Vecuronium | 0.05 | 2–4 |
| Atracurium | 0.25 | 2 |
| Cisatracurium | 0.05 | 3–5 |
Potency is inversely related to onset of neuromuscular block; the more potent a compound, the slower its onset of effect. Larger doses of NMBAs with a lower potency are administered, increasing the driving force for diffusion of these agents down their concentration gradient to the acetylcholine receptors of the neuromuscular junction. This has been found for the aminosteroid compounds, a series of tetrahydroisoquinolinium chlorofumarates, 3 structurally unrelated compounds with long durations of action and for compounds of different durations of action and structure. Pharmacokinetic modeling with a fixed number of acetylcholine receptors shows that there is a set requirement for the number of antagonist molecules needed to establish block; an ED 95 greater than 0.1 mg/kg is necessary for a rapid onset of effect.
In order to exert its effect, an NMBA must be able to enter the neuromuscular junction, which is facilitated by its lipophilicity. The speed of onset of neuromuscular block after administration of an NMBA is also related to the speed of recovery of neuromuscular function. This appears to be due to the more rapid equilibration between the plasma and effect compartment with drugs that are metabolized or redistributed more quickly ( Fig. 22.5 ). Because of this, equipotent doses of mivacurium or succinylcholine have a slower onset of effect in patients who are homozygous for atypical butyrylcholinesterase.
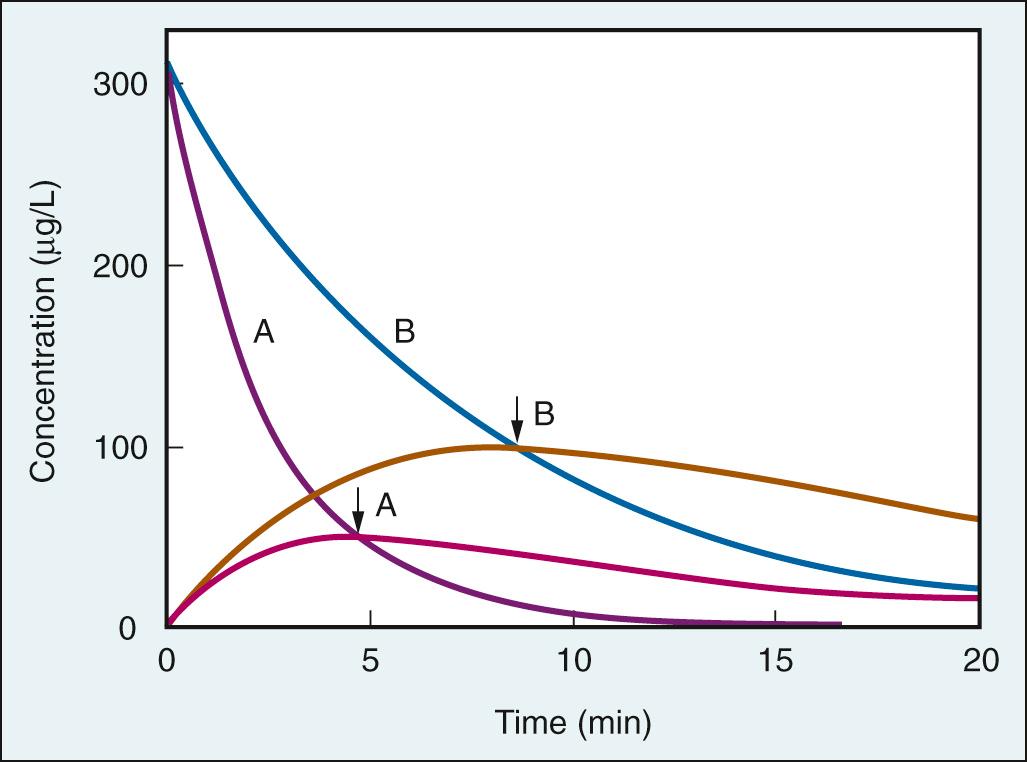
Understanding some of the factors impacting onset of neuromuscular block has led to the development of NMBAs with a faster onset of effect. Structural changes in the steroidal NMBAs have yielded compounds with a rapid onset of effect ( Fig. 22.6 ). In clinical practice, these structural changes provide a real alternative to succinylcholine when intubation within 60 seconds is required. Rocuronium, 1 to 1.2 mg/kg, provides rapid onset of neuromuscular block and can be used effectively in the setting of a rapid sequence induction and intubation.
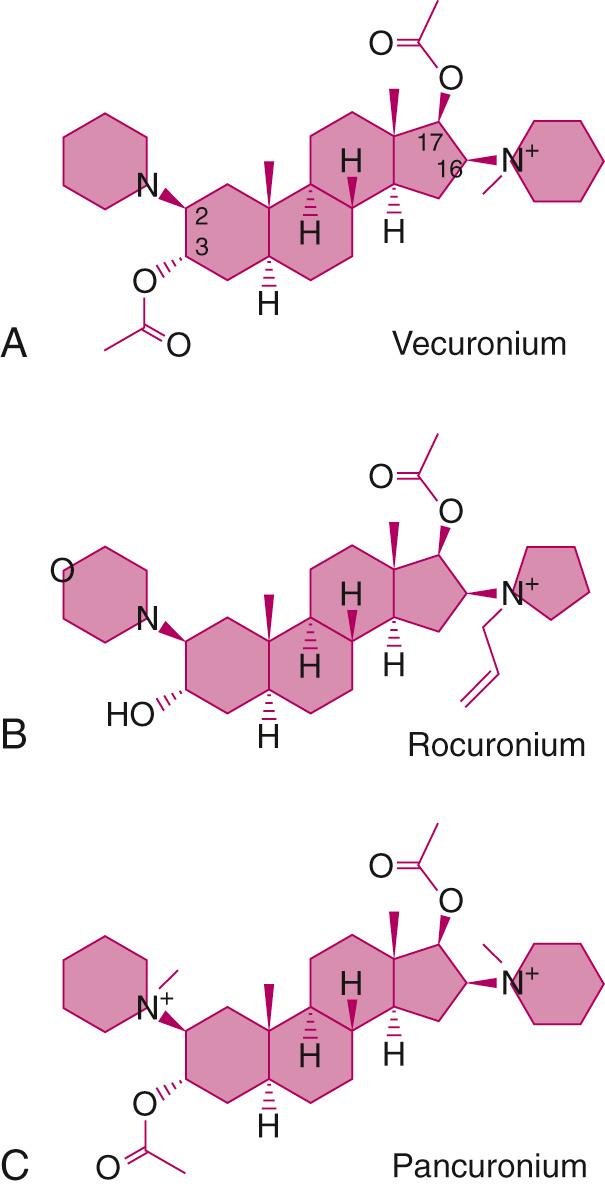
As the understanding of the adverse effects of residual neuromuscular blockade is increasingly appreciated, development of NMBAs with a shorter duration of action has become a priority. The two ways to shorten recovery from neuromuscular block involve decreasing the concentration of NMBA at the acetylcholine receptor relative to acetylcholine ( Fig. 22.7 ). This can be done through increasing the metabolism of the NMBA to rapidly remove it from the neuromuscular junction or inhibiting the activity of acetylcholinesterase so that the availability of acetylcholine at the neuromuscular junction is increased. Both mivacurium and succinylcholine have short durations of action and are metabolized by butyrylcholinesterase. Two more recently studied compounds, gantacurium and CW002, are inactivated through adduction of the amino acid cysteine at the fumarate double bond. Administration of exogenous cysteine shortens the duration of action of CW002 through its rapid inactivation so that acetylcholine available at the neuromuscular junction is more effective. Sugammadex, a selective relaxant binding agent, is a cyclodextrin that encapsulates steroidal NMBAs so that they can no longer bind to the acetylcholine receptor (see the section Antagonism of Residual Neuromuscular Block , to follow). Calabadions provide a broader (increased binding selectivity) and expanded (reversal of benzylisoquinolines) spectrum of indications.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here