Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Nerve injuries are often devastating, with associated pain and impaired function.
Motor nerve injuries must be managed expeditiously, because regenerating axons must reach target muscle prior to degeneration and fibrosis – “time is muscle”.
Nerve transfers offer an advantageous method of reconstruction by delivering regenerating nerve fibers to the target end organ more quickly, thus converting a proximal injury to a more distal injury.
Nerve transfers allow for dissection outside the original zone of injury, providing a safer and more technically straightforward procedure.
Unlike tendon transfers, the muscle–tendon biomechanical structure is preserved, thus excursion, origin, insertion, and length–tension relationships are undisturbed.
Nerve transfers require time for the nerve to regenerate and extensive physical therapy for retraining.
Intraneural dissection is technically demanding, and nerve transfers require intimate knowledge of nerve topography.
Nerve transfers are replacing other techniques as the gold standard for brachial plexus and other peripheral nerve injury in the authors’ practice.
Newer applications of nerve transfers to pediatrics, lower extremity injury, brachial neuritis, partial injury and central nervous system disorders (spinal cord injury and transverse myelitis) are exciting.
![]()
![]() Access video and video lecture content for this chapter online at Elsevier eBooks+
Access video and video lecture content for this chapter online at Elsevier eBooks+
Nerve transfers can be performed to restore sensory or motor deficits.
Nerve transfers essentially convert a proximal injury to a distal injury, providing a source of regenerating axons in close proximity to the end target.
Nerve transfers are associated with minimal to no downgrade in donor function.
Nerve transfers preserve the original biomechanical muscle–tendon relationship.
Nerve transfers may provide superior results to tendon transfer, with the ability to restore individual and separate muscle function.
While peripheral nerve reconstruction is an evolving field, nerve transfers have become a more broadly accepted reconstructive option ( ![]() ). As our understanding of internal nerve topography improves, we are able to recognize redundant or non-essential potential donor nerves and fascicles. Recent contributions in basic science are broadening options for nerve transfer with new and improved techniques. Clinical research focusing on outcomes, applications to novel populations, and areas of debate continue to advance the field. This chapter will outline the history and principles of nerve transfers as they pertain to upper extremity nerve reconstruction. The recent literature as it relates to current techniques, debates, and advances in nerve transfers will be reviewed. Current commonly performed nerve transfers are detailed with focus on both motor and sensory nerve transfers, their indications, rehabilitation and postoperative protocols, and a basic overview of selected surgical techniques.
). As our understanding of internal nerve topography improves, we are able to recognize redundant or non-essential potential donor nerves and fascicles. Recent contributions in basic science are broadening options for nerve transfer with new and improved techniques. Clinical research focusing on outcomes, applications to novel populations, and areas of debate continue to advance the field. This chapter will outline the history and principles of nerve transfers as they pertain to upper extremity nerve reconstruction. The recent literature as it relates to current techniques, debates, and advances in nerve transfers will be reviewed. Current commonly performed nerve transfers are detailed with focus on both motor and sensory nerve transfers, their indications, rehabilitation and postoperative protocols, and a basic overview of selected surgical techniques.
The classification of nerve injury is perhaps the single most important factor when determining management ( Table 26.1 ). The six degrees of nerve injury have been well described. First- and second-degree injuries recover spontaneously, as do third-degree, albeit recovery is variable and less than normal, depending on scar tissue. There may be a role for surgery in these patients to augment recovery. Fourth- and fifth-degree injuries are significant and recovery is not expected. These injuries should be managed with prompt surgical intervention to prevent degeneration and fibrosis of the motor endplates. Sixth-degree injuries encompass a variety of nerve injuries within a single nerve, and the need to operate is determined on an individual basis.
| Degree of injury | Tinel's sign present | Recovery | Rate of recovery | Surgical procedure | |
|---|---|---|---|---|---|
| I | Neurapraxia | No | Complete | Up to 12 weeks | None |
| II | Axonotmesis | Yes | Complete | 1 * per month | None |
| III | Yes | Varies * | 1 * per month | None or neurolysis | |
| IV | Neuroma-in-continuity | Yes, but no advancement | None | None | Nerve repair, graft, or transfer |
| V | Neurotmesis | Yes, but no advancement | None | None | Nerve repair, graft, or transfer |
| VI | Mixed injury (I–V) | Some fascicles (II, III) | Some fascicles (II, III) | Depends on degree of injury (I–V) | Neurolysis, nerve repair, graft, or transfer |
* Recovery can vary from excellent to poor depending on the amount of scarring and the sensory versus motor axon misdirection to target receptors.
Once surgical intervention has been deemed necessary, there are a number of surgical options. Traditionally nerve repair or grafting was used with tendon transfers to augment poor recovery or delayed presentation. The difficulty with proximal nerve injuries is that the distance from the injury to the motor endplate is significant, and recovery is prolonged. In addition, delayed intervention may result in denervation and fibrosis prior to muscle reinnervation. Axonal regeneration has been shown to occur at the rate of approximately 1 inch (2.5 cm) per month and reinnervation of denervated muscle is not possible after 12–18 months; therefore, nerve transfers offer a valuable means of providing regenerating axons in closer proximity to the motor endplate. Essentially, a nerve transfer converts a proximal nerve injury to a distal one, enabling faster recovery and quicker return to function.
Nerve transfers were described very early; however they were traditionally reserved for otherwise unsalvageable root avulsions and proximal pan-plexus injuries. Approximately 30 years ago, nerve transfers were revisited and applied to a significantly broader patient population, with applications in partial plexus injuries to recreate specific essential functions in the upper extremity. As our understanding of internal nerve topography has broadened, techniques and equipment for performing microsurgery have improved, and our diagnostic ability for brachial plexus and peripheral nerve injury has progressed, the field of nerve transfers has exploded. Nerve transfers have become the standard of care for treatment of peripheral nerve injuries in the authors' practice and have largely replaced other modalities.
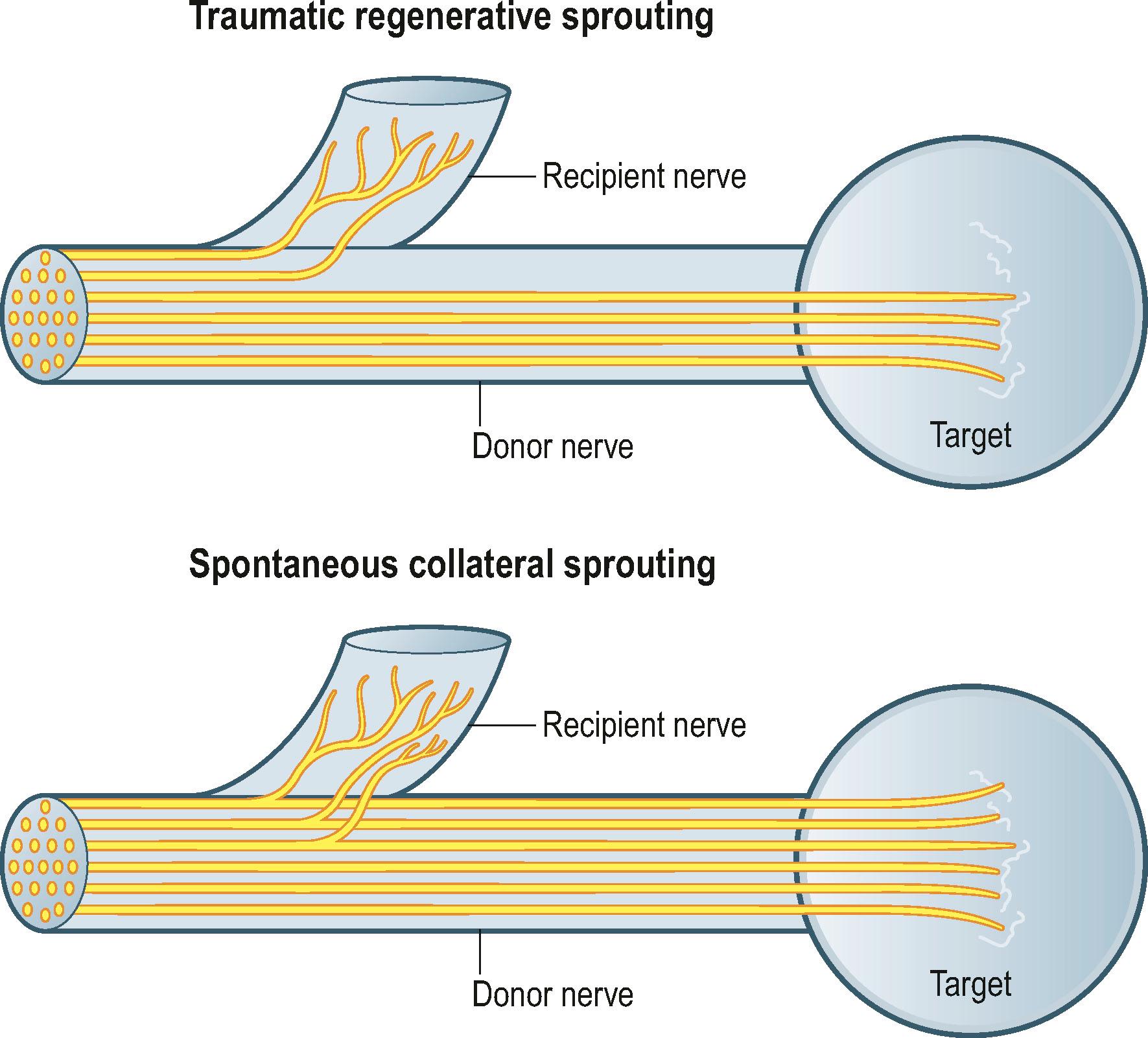
There have been dramatic increases in our knowledge of nerve injury, healing, and regeneration. The most relevant recent basic science advances pertaining to nerve transfers relate to end-to-side transfers ( Fig. 26.1 ). End-to-side neurorrhaphy involves the coaptation of the distal end of an injured recipient nerve into the lateral aspect of an intact nerve, which serves as a proximal source of axons to regenerate into the injured nerve. Reports of end-to-side transfers occurred as early as the late 1800s and then made a resurgence in the 1990s. There has, however, been controversy regarding the success of these transfers, and sensory recovery has typically been more impressive than motor recovery. The sprouting of axons in an end-to-side coaptation, thus eliminating the need for a proximal nerve stump, makes nerve transfers an option for proximal nerve injuries and has been well demonstrated in the literature. While sensory nerves are able to sprout spontaneously (collateral sprouting), proximal donor nerve axonotmetic injury is required for motor neuronal regeneration (regenerative sprouting) across the end-to-side repair ( Fig. 26.2 ). An epineurotomy in the donor nerve is required, and partial axotomy will result in more significant regenerative sprouting into the recipient nerve, albeit at the potential expense of a measurable downgrade in donor nerve function, which may be temporary or permanent.
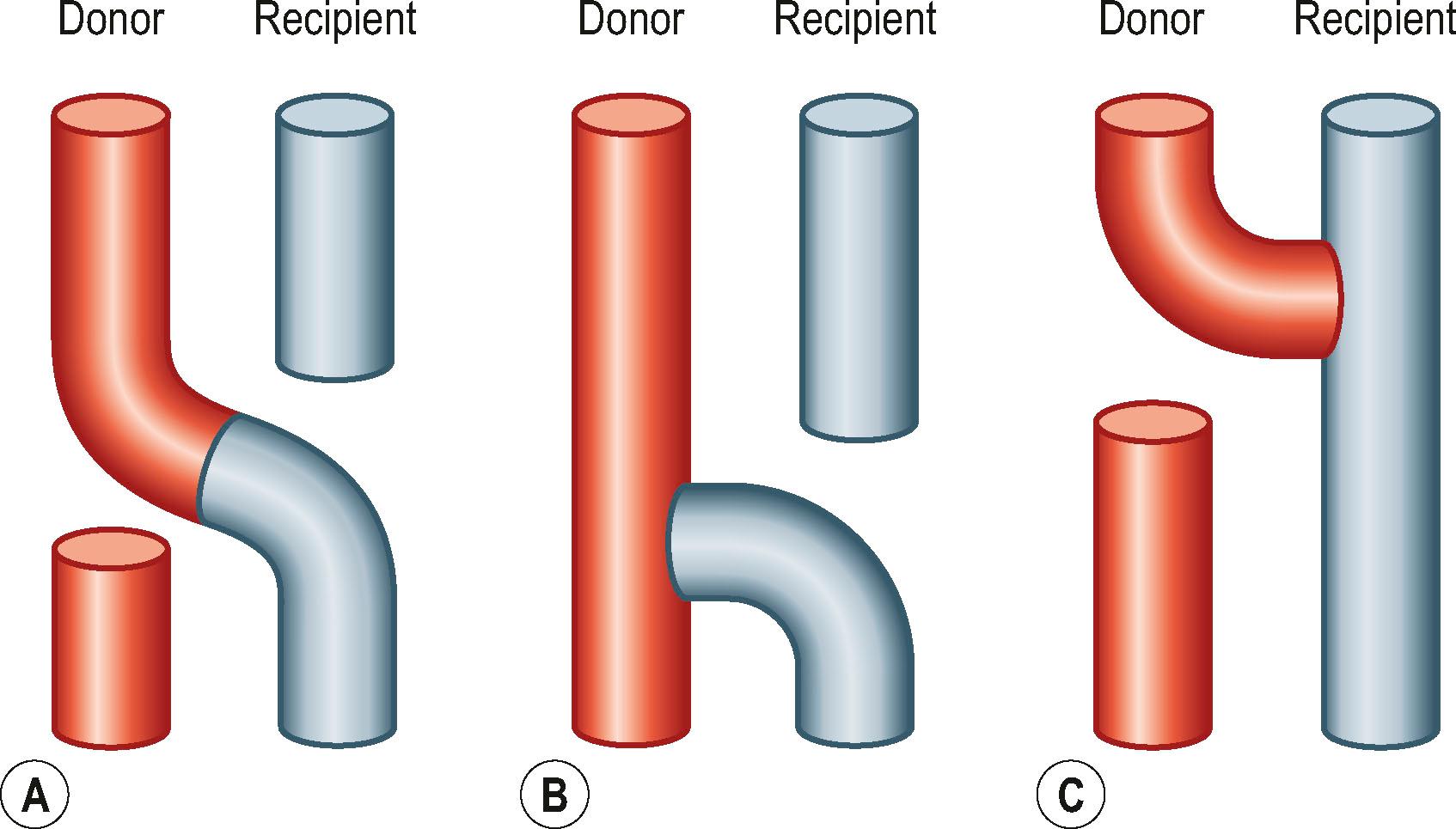
Supercharged end-to-side nerve transfers involve the complete transection of the donor nerve, which is then coapted into the side of the intact recipient nerve. This maximizes the potential number of available motor axons from the donor nerve. This procedure does not interrupt any recovery in the injured recipient nerve because the nerve remains in continuity; however, the additional axons recruited from the donor nerve improve distal target reinnervation, a concept known as “supercharging”. In a proximal partial nerve injury, with a long distance required for reinnervation, this technique can protect target muscles from denervation atrophy and fibrosis. Clinically, the most relevant place to consider this strategy is for preservation of intrinsic hand function in proximal severe ulnar neuropathy with use of the end-to-side distal anterior interosseous (branch to pronator quadratus) to deep motor branch of ulnar nerve end-to-side coaptation. The following algorithm provides some guidance on the indications for utilizing the supercharge-end-to-side transfer in clinical practice ( Algorithm 26.1 ).
The end-to-side transfer can be considered as the recipient nerve “pulling” regenerating axons out of the intact donor, whereas the supercharge end-to-side “SETS” transfer (formally referred to as “reverse end-to-side”) can be considered as the donor nerve “pushing” regenerating axons out into the intact recipient.
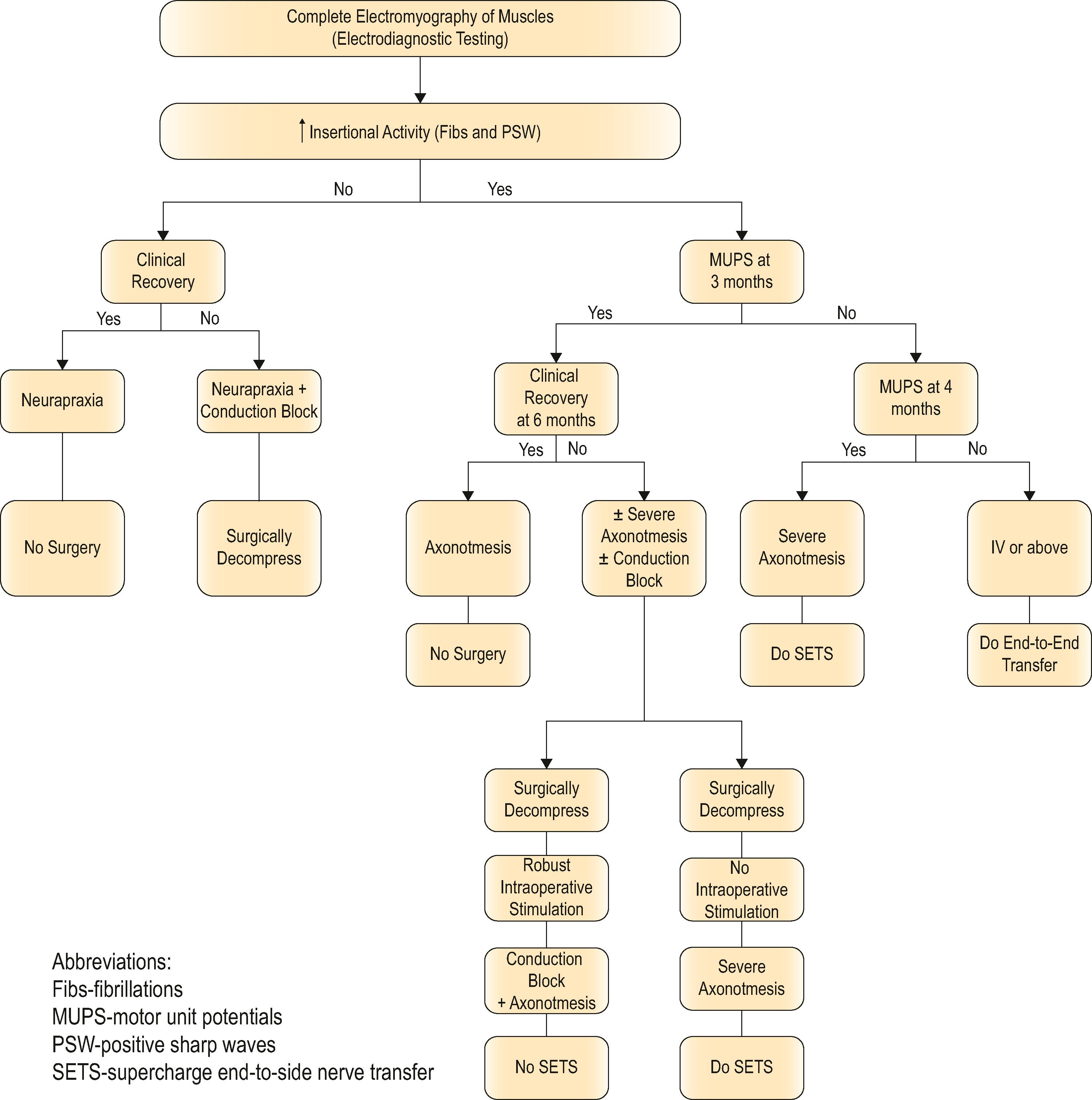
This algorithm provides guidance on use of the supercharge end-to-side (“SETS”) nerve transfer to augment motor recovery. Use of the SETS is not indicated for nerve injuries that will recover completely such as a neurapraxia. It can be used in select axonotometic injuries, where complete motor recovery is not expected. Electodiagnostic studies, specifically electromyography (EMG) of clinically relevant muscles, are performed. Depending on the findings of the EMG and serial clinical exam, surgery or repeat EMG may be indicated. The SETS transfer should not be performed in cases where clinical recovery is seen or where simple nerve decompression alone may lead to complete recovery of function. The SETS transfer is performed in clinical scenarios where less then complete motor recovery is expected. (© nervesurgery.wustl.edu, Washington University in St. Louis.)
The value of a complete history and physical examination in the patient with a potential brachial plexus or proximal nerve injury of the upper extremity cannot be overstated. Patients should be evaluated promptly after injury, both to provide a baseline for future serial examinations and to facilitate timing of surgical intervention where necessary. Documentation of a complete plexus examination helps to prevent missing neurologic deficits.
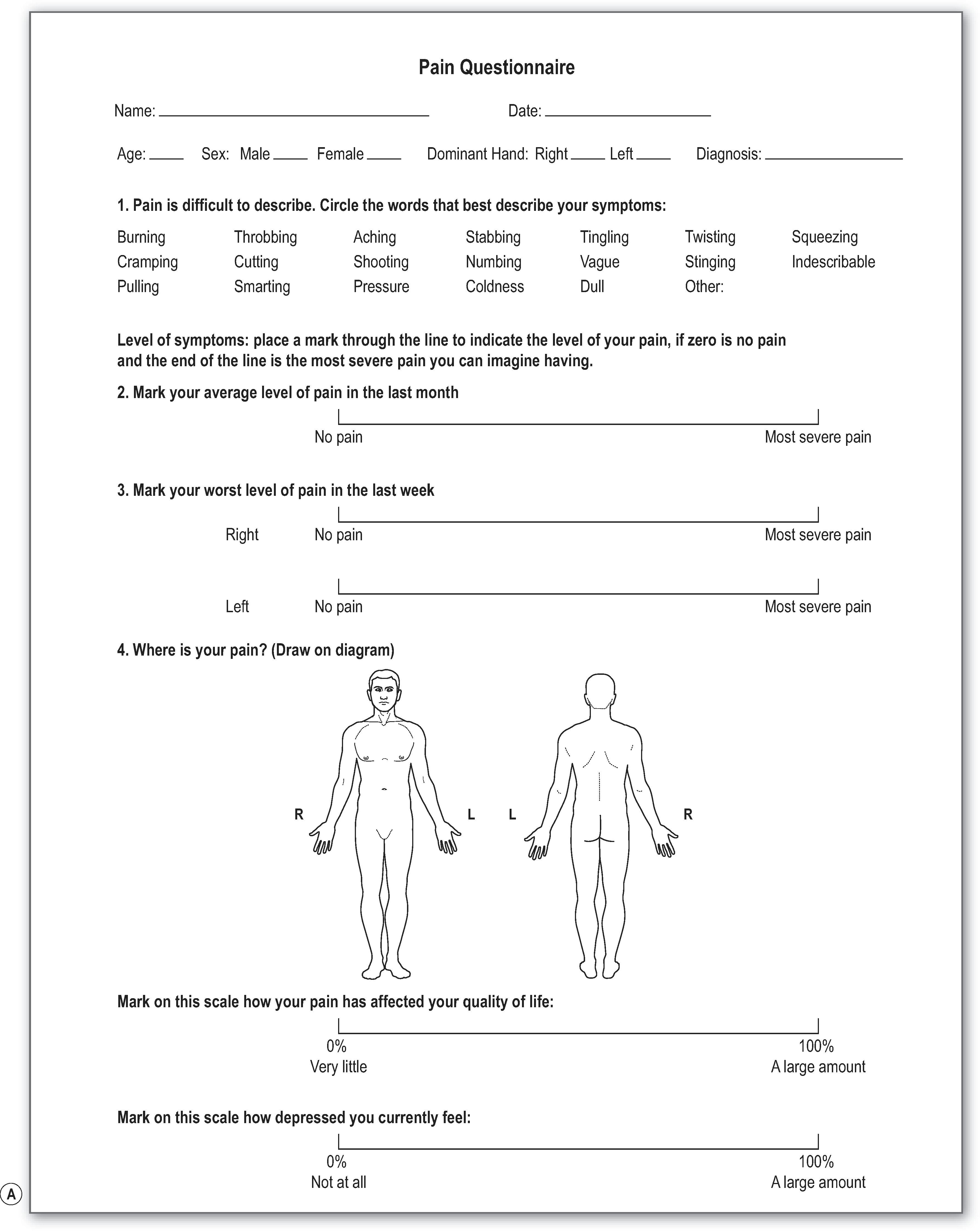
On history, details of the mechanism and timing of injury are crucial. When seeing a patient in a delayed fashion, it is also useful to ascertain any interval recovery of function. The mechanism of injury will determine timing of intervention, with prompt exploration of penetrating sharp trauma, and more expectant management of closed injuries and gunshot wounds. Specific patient symptoms such as loss of function, both sensory and motor, and pain should be precisely elicited, as this will help focus the subsequent physical examination. The pain diagram and questionnaire are particularly useful for eliciting this information ( Fig. 26.3 ). Other factors, such as patient age, handedness, occupation, and comorbidities, will factor into management decisions and foster shared decision-making. Attention to patient goals and expectations allows for individually tailored and nuanced approaches that may or may not involve nerve transfer surgery techniques, which form “a part of the armamentarium” when caring for patients with complex upper extremity injury. Further information on steadily improving quality of life and other gradual but continuing changes over time after nerve transfer in brachial plexus injury patients may further influence treatment choices and patient preference-specific choices.
A complete physical examination of the upper extremity includes assessment of sensory and motor function, joint suppleness, range of motion, and a functional assessment with attention paid to adaptive strategies and use patterns. Particularly in patients with late presentation, the presence of fixed joint contractures may preclude functional recovery. Perfusion, bony pathology, presence of edema, scar, previous incisions, and other soft-tissue trauma are factors that influence treatment choices.
Function should be graded at each joint. Manual muscle testing and examination by direct palpation is imperative for assessing the presence of redundant function when checking for expendable donor muscles. Shoulder function should be assessed by examining deltoid, supraspinatus, infraspinatus, trapezius, latissimus dorsi, serratus anterior, and pectoralis major muscles. The patient's ability to abduct/adduct, flex/extend, and internally/externally rotate must be assessed. Caution to avoid confusing limited shoulder abduction due to upper trapezius function and missing a spinal accessory or axillary nerve injury should be taken. This demonstrates the importance of directly palpating the muscle during assessment. The elbow should also be assessed for flexion and extension. Here it is important to differentiate between flexion secondary to biceps brachii and brachialis (musculocutaneous nerve) and brachioradialis (radial nerve). Testing triceps by palpation and resisting overhead elbow extension ensures that adaptive strategies such as active shoulder abduction and passive elbow extension using gravity are not being used to produce motion. The forearm and wrist should be assessed for flexion/extension and pronation/supination. Palpation of the individual tendons with wrist extension and flexion is essential to determine which nerves are involved, as well as the level and degree of injury and the availability of branches (extensor carpi radialis brevis and longus, extensor carpi ulnaris and flexor carpi ulnaris, flexor carpi palmaris, palmaris longus) for use as donor nerves. In the hand, a complete examination of extrinsic and intrinsic function again helps to delineate the level of injury. Special tests can be very useful in examination of the intrinsic muscle function including Froment’s, Wartenberg sign, index-finger cross-over, and examination for a claw deformity (Duchenne’s). Assessment of muscles and grouping the exam by peripheral nerve branch and/or by cervical root level pattern may be helpful, depending on the appropriate clinical scenario.
Sensation should be examined by both dermatome and peripheral nerve distribution and can be helpful in distinguishing these injuries. The authors advocate the use of both two-point discrimination and the ten-test to evaluate sensory loss in the hand. A Tinel's sign, the tingling sensation elicited with percussion over a regenerating nerve, will help to localize the level of nerve injury, and may also be followed on serial clinical examination to check for signs of advancement, indicating spontaneous recovery. An advancing Tinel's sign distal to the site of injury quite often precedes clinical motor recovery.
For completeness, the presence of Horner's syndrome and dysfunction of nerve branches that come off the proximal brachial plexus (dorsal scapular nerve to rhomboids, long thoracic nerve to serratus anterior) indicate a very proximal level of injury.
One of the most important components of the physical examination is the simple determination of what is functioning, and what function has been lost. Not only does this help to determine the level of injury, it can guide future surgical planning. In the presence of an injury requiring surgical intervention, it is important to examine the patient for putative nerve donors, both intraplexal and extraplexal.
Remember to examine the patient for both what muscles have lost function and also for potential nerve transfer donors. Ideally donor nerves have 4.5/5 muscle strength; but in situations with a paucity of donors a recovering muscle’s nerve may need to be used.
Imaging is primarily useful for confirming level of injury in more complex closed-mechanism nerve injury patients. For example, imaging such as computed tomography myelogram and magnetic resonance imaging provides additional confirmation of a nerve root avulsion pattern. Additional X-rays, such as shoulder films that demonstrate scapular notch-level trauma, may provide information of localized trauma that would make proximal transfer to the suprascapular nerve ineffective due to the extensive zone of injury. Other plain films such as chest X-ray might be used to evaluate pathology such as rib fractures or diaphragm dysfunction that might make the use of intercostal and phrenic nerves as donor nerves less palatable, although the former is debatable. The role of direct imaging in nerve injuries is controversial and at the very least requires an experienced radiologist or neuromuscular neurologist familiar with MRI and/or ultrasonography in this setting.
Electrodiagnostic testing, performed by an experienced person, can be a useful adjunct to physical examination for serial assessment of reinnervation in closed nerve injuries. Electromyography records the electrical activity of muscle fibers, tested both at rest and activity, by the insertion of needles into the muscle. Denervated muscles will demonstrated fibrillations and positive sharp waves on needle insertion; however, the findings are not reliable until approximately 4 weeks after injury. Initial electrodiagnostic testing should be deferred at least 4 weeks post-injury to assess for signs of both axonal injury and root-level avulsion. Serial testing will reveal signs of reinnervation with nascent potential and motor unit potentials in the affected muscle groups, often prior to clinical evidence of recovery.
Detailed testing of both putative functioning donors and non-functioning recipient muscles is important to confirm physical exam findings. This is particularly important because subtle abnormalities in donor muscles correlate with subsequent clinical results. In fact, abnormalities seen in the putative donor muscles may dictate a change to a different donor, if available, or an understanding that results may be detrimentally affected.
Nerve conduction studies are used to measure the integrity of a peripheral nerve (sensory nerve action potential [SNAP] or compound motor action potential [CMAP]). A severed nerve will lose the ability to conduct a signal as Wallerian degeneration occurs; however, if measured too early, the distal aspect of the nerve will still conduct, leading to an inaccurate assessment. This is another reason for delaying initial electrodiagnostic studies. Preganglionic injuries will be associated with a loss of sensation but an intact SNAP and an absent CMAP. Thus the level of injury can be confirmed with electrodiagnostic studies.
In patients with no evidence of recovery 3 months after a closed nerve injury, the balance should tilt towards consideration of surgical intervention.
Order baseline electrodiagnostic studies at least 4 weeks after injury and subsequent testing as indicated by the injury:
Electromyography (EMG) showing fibrillations (fibs) and positive sharp waves (PSWs) suggest denervation. Motor unit potentials (MUPs) suggest some retained function, and nascent units suggest reinnervation.
Do not be confused by automated EMG reporting errors – “normal” may appear in some reports by default. If a muscle has fibs and PSWs but the MUP column says “normal” this may be a machine printout error – consult with your electrodiagnostician as it more likely that there are absent MUPs (not “normal” MUPs).
When assessing for recovery over time, ask to check proximal muscles first (example flexor carpi ulnaris not first dorsal interosseous, when evaluating ulnar nerve recovery).
Remember, the EMG provides qualitative information only (not quantitative).
Above all, careful communication with the electrodiagnostician and correlation to clinical exam findings are imperative when interpreting these test results.
Where possible, a combined clinic with both surgeon and electrodiagnostician is preferred.
Overall, the complete picture, including history, physical examination, and adjunct testing, should facilitate surgical decision-making. Appropriate treatment for open injuries with nerve dysfunction begs for timely management with surgery on an urgent or semi-urgent basis. For these injuries, direct exploration and repair have historically been the treatment of choice, but in very proximal injuries, distal nerve transfer can allow for more timely and successful reinnervation. In closed nerve injuries or in gunshot wounds, appropriate management demands a more measured approach and surgery should be delayed to allow for spontaneous recovery, which is often superior to that seen in patients treated with hasty surgery. These patients in particular may benefit from “supercharging” with reverse end-to-side nerve transfer procedures, which can more quickly deliver regenerating axons to the appropriate end organ, while still allowing slower spontaneous recovery.
When considering nerve transfers, be aware of the various advantages, disadvantages, and limitations:
Nerve transfers may allow for an unprecedented level of timely and biomechanically appropriate return of function.
A single donor nerve (from one muscle) can provide individual and isolated muscle function to more than one recipient through motor re-education and cortical adaptation.
It will take months to years for maximal function to be achieved after nerve transfer (time for regeneration, relearning, and strengthening).
A distal nerve transfer will not directly address pain from a proximal neuroma or neuroma-in-continuity.
No transfer, no matter how close to the target, can restore function after terminal muscle denervation has occurred (that deadline typically occurs somewhere between 12–18 months after nerve injury).
Nerve transfers have become increasingly popular for a number of reasons, the most compelling of which is the “time is muscle” issue. The biggest challenge facing peripheral nerve surgeons is that with increasing time since injury, the ability to achieve good motor function becomes increasingly limited. In fact, for any nerve injury where there is complete discontinuity with the motor end organ, no reinnervation procedure will be able to restore muscle once denervation and fibrosis have occurred, a process that occurs as early as 1 year. Nerve transfers, by bringing the regenerating motor fibers closer to the target end organ more rapidly, essentially convert a more proximal-level injury to a more distal-level injury, thus increasing the chance of achieving meaningful muscle function.
Another advantage of nerve transfers is that they enable surgical reconstruction outside the zone of the original injury, avoiding complex dissections and limiting injury to critical neurovascular structures. In patients with brachial plexus trauma, the proximity of vital structures (great vessels, thoracic duct, lung) makes the occurrence of potentially life-threatening complications a real possibility. In other cases, such as concomitant vascular injury, previous blast injury, or soft-tissue deficits requiring flap reconstruction, the ability to avoid doing a complex nerve exploration in the setting of dense fibrotic scar is particularly advantageous.
Nerve transfers allow for a very targeted intervention in cases of partial nerve injury such as in treatment of a sixth-degree or neuroma-in-continuity injury, where transfer can be done distal to the site of injury specifically to the non-functioning nerve branch. This allows for preservation of all intact function and restoration of only missing function. In addition, the ability to “supercharge” a recovering nerve is invaluable when it is unclear whether the nerve transfer or intrinsic recovery will be more beneficial. Another example that is somewhat controversial is the setting of brachial neuritis (neuralgic amyotrophy). Previously thought to have excellent prognosis, recovery following brachial neuritis is not always reliable and therefore augmenting with a “supercharge” procedure offers additional motor axons without impeding the change for spontaneous recovery, even in a delayed fashion.
When operating in the setting of brachial neuritis or a partial nerve injury, consider a supercharge end-to-side nerve transfer to allow for the event of spontaneous recovery.
Unlike tendon transfers, nerve transfers typically do not require immobilization, which is especially valuable in patients presenting with significant baseline stiffness. Nerve transfers also preserve the biomechanical properties of the musculotendinous unit, such as end organ origin, insertion, excursion, and length–tension relationships. Finally, nerve transfers can restore unique function such as pronation, which is incredibly difficult to restore by traditional surgical techniques.
Thus nerve transfers are indicated in a number of situations ( Box 26.1 ), for example, in proximal brachial plexus injury where grafting is not possible or the required distance for reinnervation will not allow motor axons to reach the target motor before permanent atrophy and fibrosis due to denervation have occurred. In situations of extreme scarring, or major upper extremity trauma, a nerve transfer is preferable to risking damage to critical structures within the scarred region. Nerve transfers are also indicated in segmental nerve loss, and in partial nerve injury patterns with functional loss. Patients presenting in a delayed manner with inadequate time to reinnervate the distal targets are good candidates for distal nerve transfers. Also, patients with sensory nerve deficits in critical regions should be considered for nerve transfer to restore sensation.
Proximal brachial plexus injuries where grafting is not possible
Proximal peripheral nerve injuries requiring long distance for reinnervation of distal targets
Severely scarred areas with risk of damage to critical structures
Segmental nerve loss
Major upper extremity trauma
Partial nerve injuries with functional loss
Delayed presentation with inadequate time for reinnervation of distal targets with grafting
Sensory nerve deficits in critical regions
The main reason a nerve transfer should not be performed is similar to the contraindication for any other traditional nerve repair/graft intervention, namely end organ unresponsiveness. An old peripheral nerve injury will not respond to the new “magic” of nerve transfers. Muscle that is in complete discontinuity with the nerve for greater than 1 year will not be reinnervated, no matter how elaborate the reinnervation strategy employed. The exception to this is in children, where the potential window for reinnervation appears to be longer.
More relative contraindications for nerve transfer include issues such as the time required for regeneration, challenges of the surgery, the anatomic knowledge required, and problems of postoperative retraining and therapy, as these techniques are less familiar to hand therapists. There are some patients, such as the young manual laborer with a radial nerve injury, who may prefer the more rapid recovery associated with tendon transfer at the expense of the independent fine motor control that could be achieved through the use of nerve transfers. The surgery itself is challenging because of the detailed knowledge of internal nerve topography required. Most surgeons are not formally trained in the intraneural dissection techniques required to perform nerve transfers and the results are not apparent until months down the line. This requires a significant leap of faith for surgeons who are more used to immediate gratification and a clear result in weeks, not months or years!
Direct nerve repair and nerve grafting also remain valuable tools for the peripheral nerve surgeon and should continue to be the treatment of choice in a variety of scenarios. These include cases of multiple nerve injuries where there is a paucity of nerve donor material for nerve transfer. Also, in distal single-function nerve injuries, direct or graft repair is preferable to nerve transfer because one-to-one function is preserved, no retraining is necessary, no donor function is sacrificed, and the distance to the end target is short.
Patient general health, comorbidities, and associated injuries factor into the decision to perform nerve transfers. These procedures can be lengthy, and are not without significant risk from anesthesia in the fragile patient. In addition, patient compliance is an integral part of the recovery process, and patients require education preoperatively about the prolonged recovery and therapy times associated with nerve transfer.
Avoid paralytics, including short-term paralytics such as succinylcholine with anesthesia induction to allow for nerve stimulation as injured nerves do not always repolarize within the normal time frame, which could lead to inaccurate assessment.
Minimize or avoid tourniquet time to avoid interference with nerve stimulation.
Use plain epinephrine in proximal incisions to minimize blood loss without lidocaine paralysis.
Obtain wide surgical exposure to identify nerves and appropriate branches.
Choice of optimal nerve donor is based on quantity of motor axons, proximity to target muscles, synergy of muscle function, and donor expendability.
Conduct neurolysis with your “eyes” by following the course of the nerve, longitudinal vessels within the nerve, and/or by using microforceps to “fall” into the planes between fascicles, except at the site of actual transfer, to avoid prolonged dissection and increased trauma to nerve branches.
Confirm no intraoperative stimulation in putative recipient before dividing donor.
Divide donor nerve distally and recipient nerve proximally.
Use 8-0 or 9-0 nylon and the operating microscope to perform tension-free epineurial repair.
Use bupivacaine block at end of case for postoperative pain control.
Patients with upper plexus injuries present with glenohumeral joint subluxation, loss of shoulder abduction and external rotation, and absent elbow flexion. When C7 is also involved, additional deficits in function may include loss of elbow extension and weakness of wrist and digit extension. Numbness over the corresponding dermatomes is noted.
Priorities for upper plexus injuries focus first on restoring elbow flexion and second on stabilizing the shoulder. Restoration of shoulder abduction and external rotation is desired. Standard nerve transfers include: (1) double fascicular nerve transfer; (2) medial triceps to axillary; and (3) spinal accessory to suprascapular nerve.
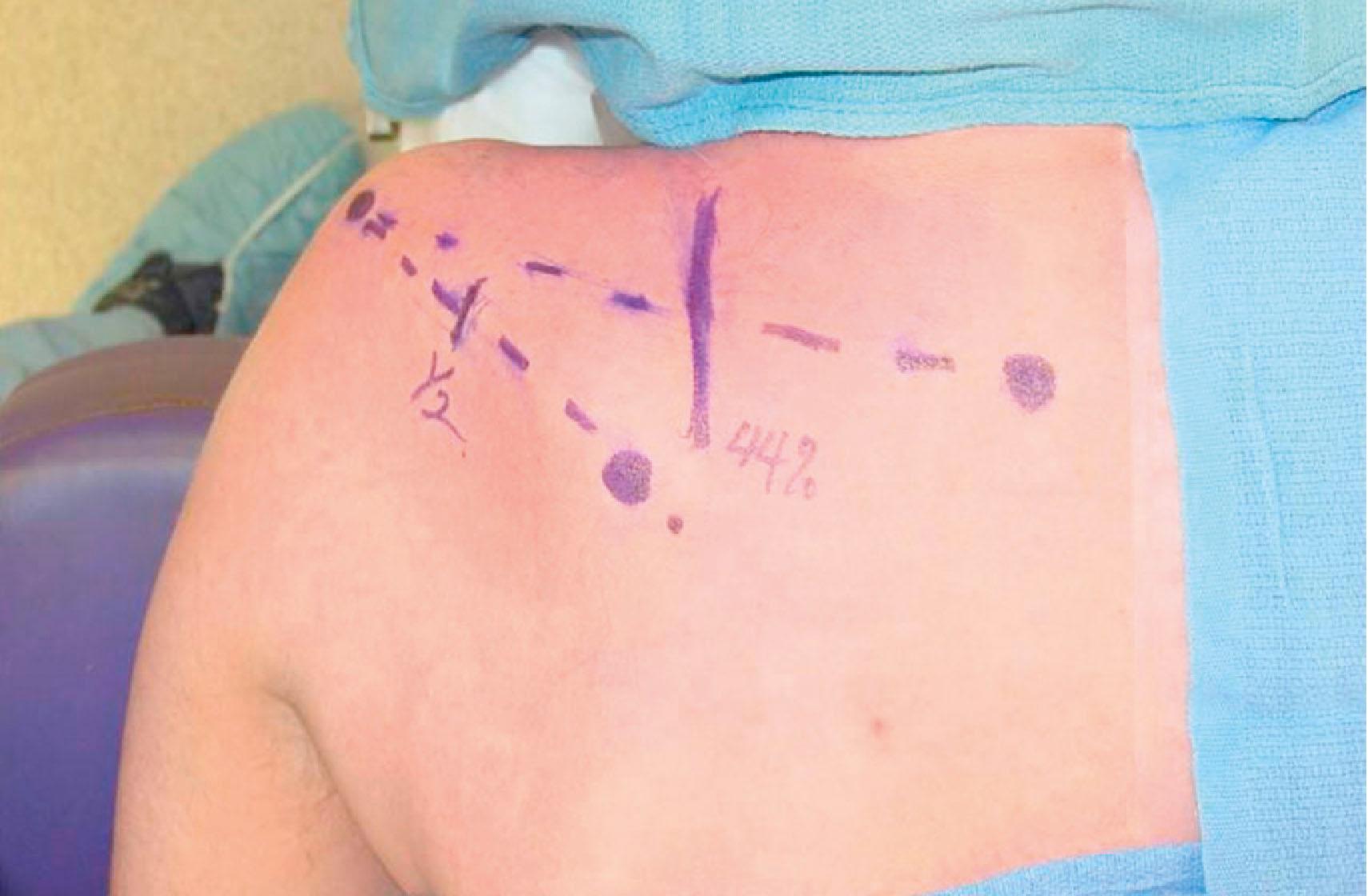
Restoration of shoulder stability and external rotation are facilitated by transferring the spinal accessory nerve (cranial nerve XI) to the suprascapular nerve. This transfer can be conducted by either an anterior or a posterior approach. The posterior approach is used only if scarring precludes the anterior approach. In the posterior approach, the patient is positioned prone and surface landmarks are used to approximate the position of the nerves ( Fig. 26.4 ). The spinal accessory nerve runs parallel to the border of the trapezius and is localized 44% of the way along a line connecting the acromion to the dorsal midline at the level of the superior border of the scapula ( Fig. 26.5 ). The suprascapular nerve is located midway between the medial border of the scapula and the acromion as it runs through the suprascapular notch. Dissection is carried through the trapezius in a muscle-splitting fashion and an end-to-end coaptation, sparing the upper trapezius nerve branches, is conducted.
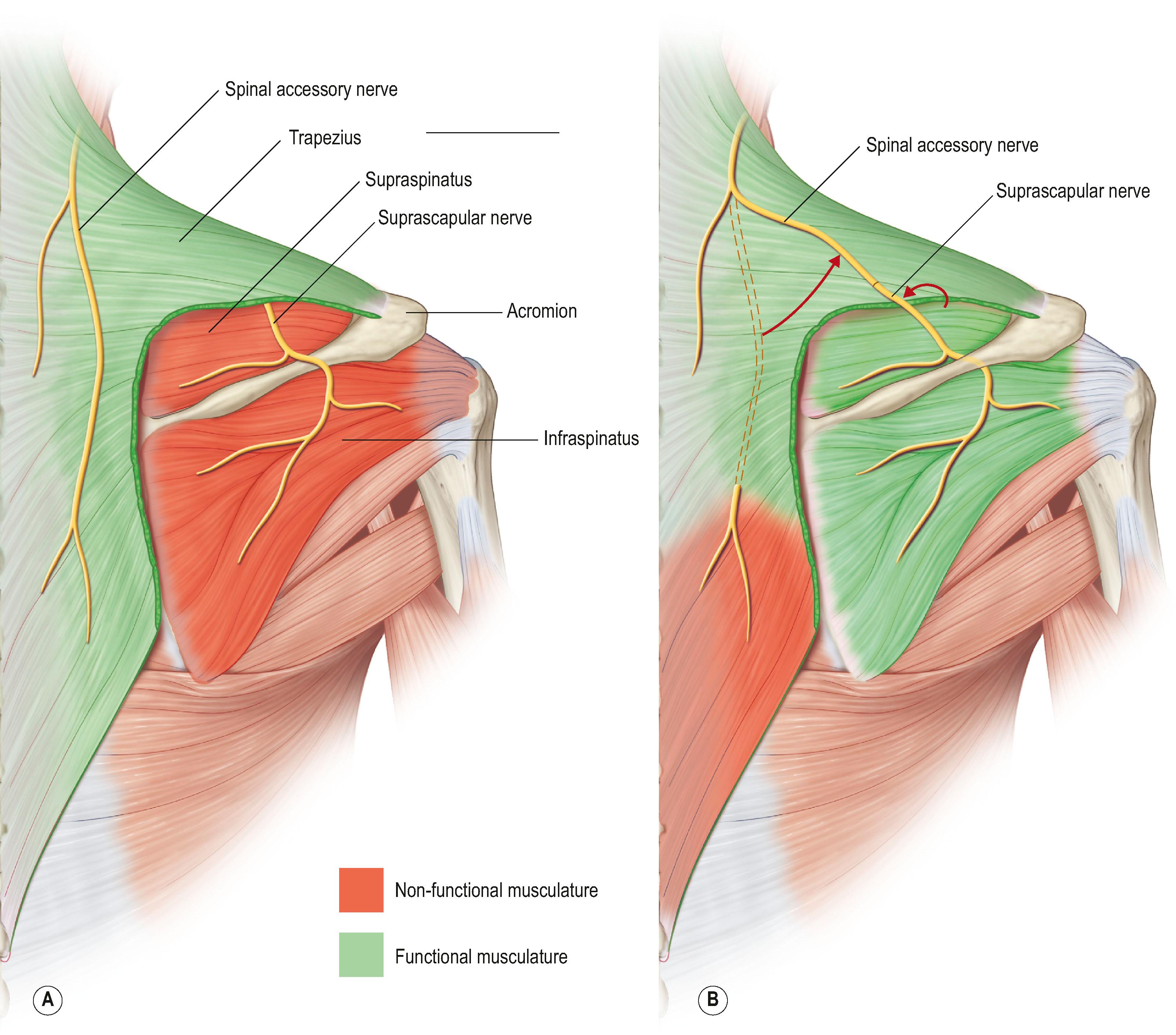
For the anterior approach, a supraclavicular approach to the brachial plexus is made; the lateral aspect of the trapezius is released off of the clavicle and the spinal accessory nerve is identified on its anterior surface. The suprascapular nerve sits at the superolateral aspect of the upper trunk, which is identified between the anterior and middle scalene muscles. Coaptation can be performed in an end-to-side fashion. In some cases, an interpositional nerve graft may be required. A proximal crush to the spinal accessory donor nerve is needed for this motor donor, which remains otherwise in-continuity to its recipient musculature.
Additional abduction of the shoulder is provided by transferring a branch of the triceps, usually from the medial head, to the axillary nerve ( Fig. 26.6 ). Better results in upper plexus injury patients are achieved by reinnervating both the suprascapular and axillary nerves. A longitudinal incision is made on the posterior surface of the arm ( Fig. 26.7 ). The axillary nerve is identified in the quadrangular space and dissected proximally to include the branch to teres minor, then divided proximally. The natural cleavage plane between the lateral and long heads of the triceps muscles is identified and blunt dissection is conducted to expose the donor radial nerve running along the humerus.
The branch to the medial triceps sits superficially and medially on the surface of the radial nerve as a distinct branch. This makes dissection of this branch quick and minimizes risk of creating a donor deficit.
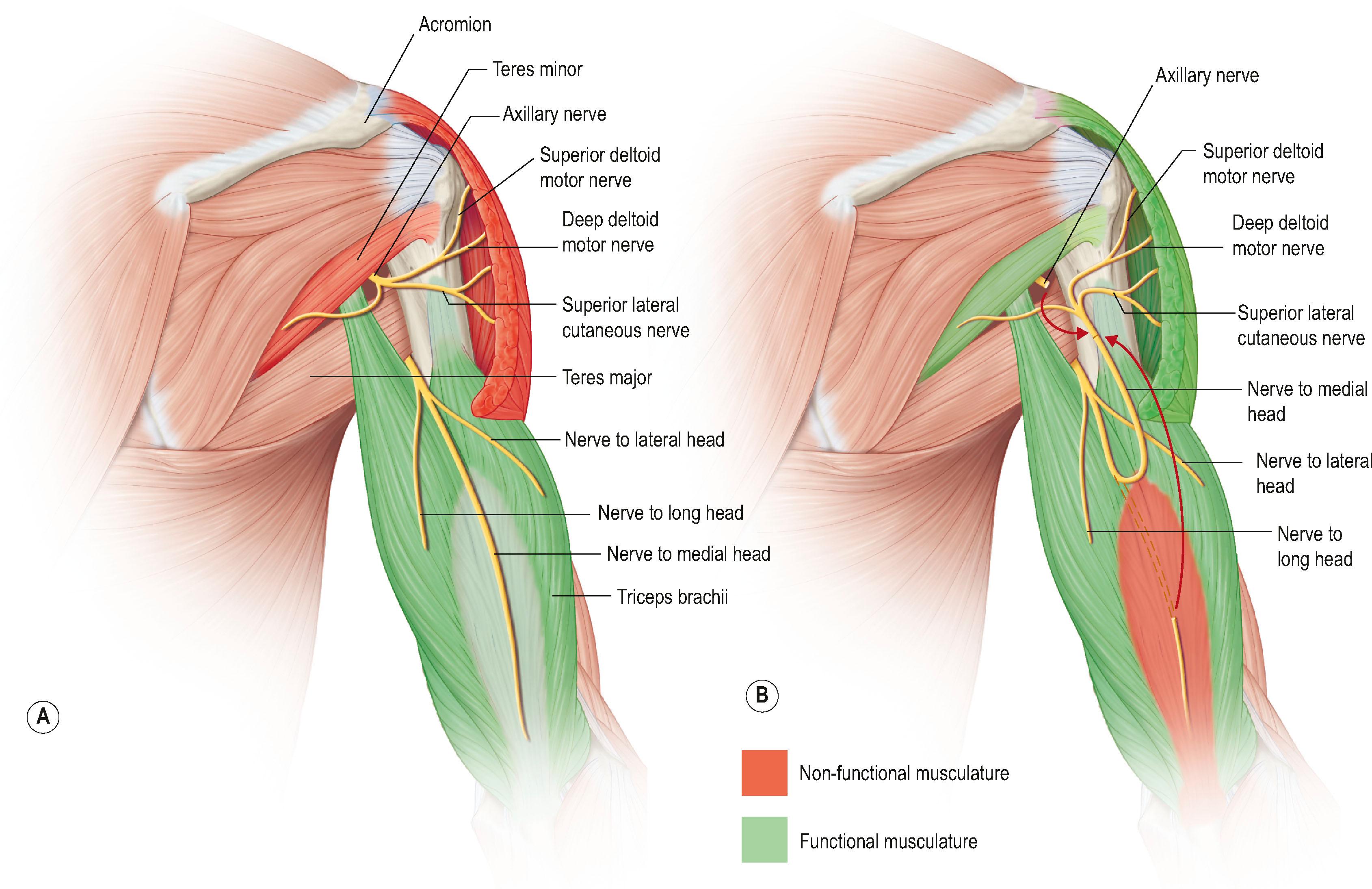
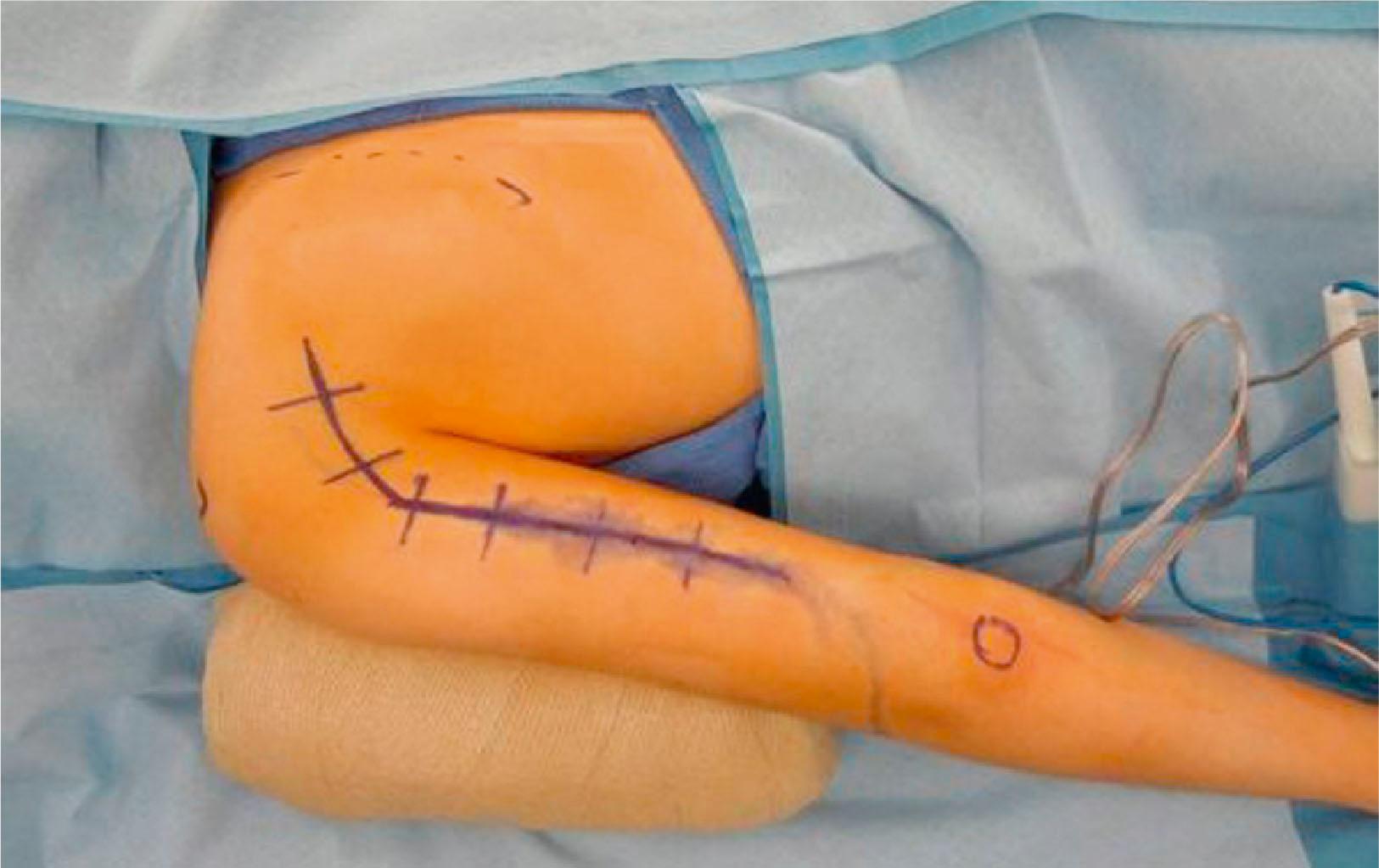
The donor triceps nerve is dissected as far distally as possible and then coapted to the axillary nerve ( Fig. 26.8 ).
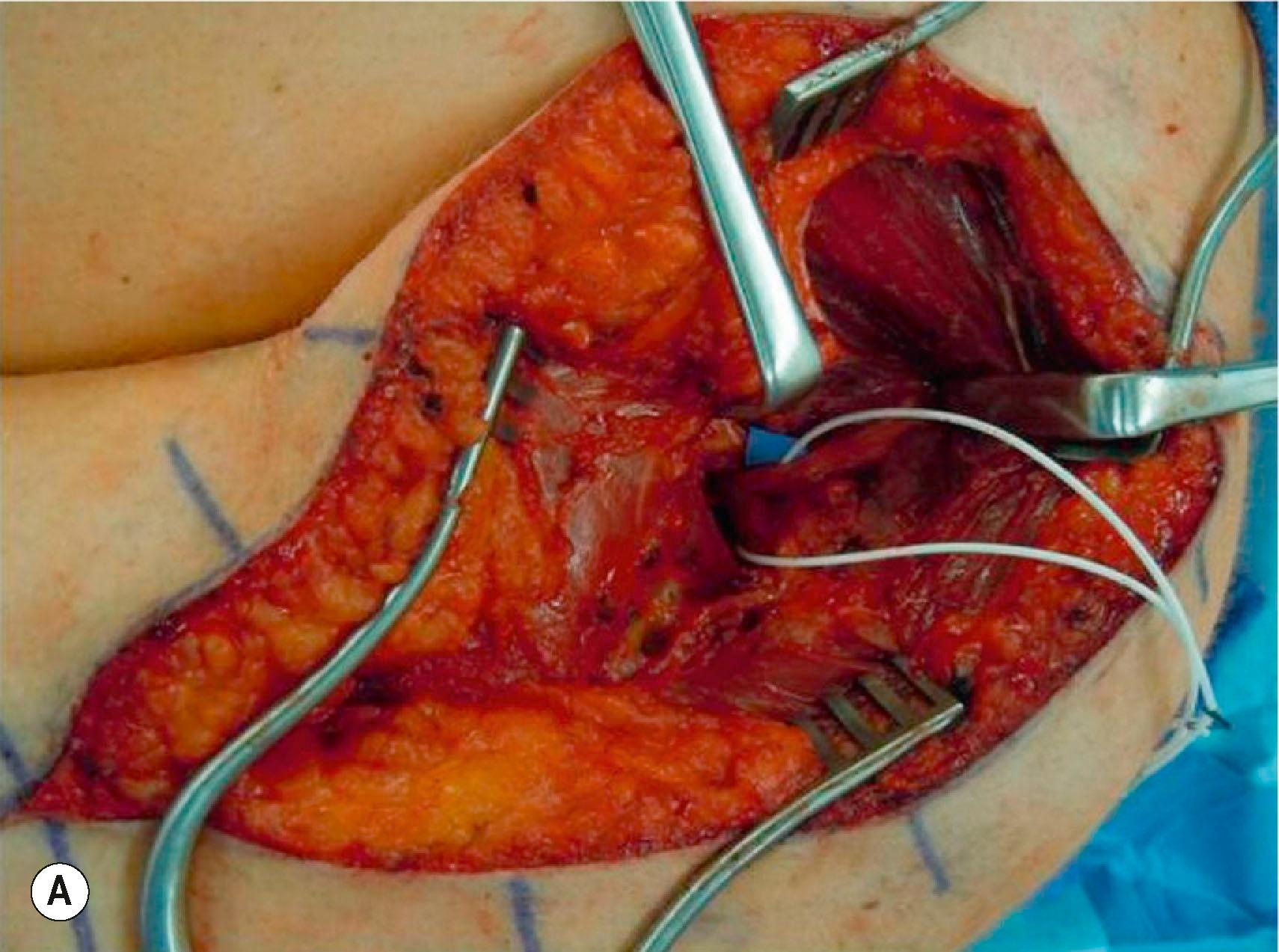
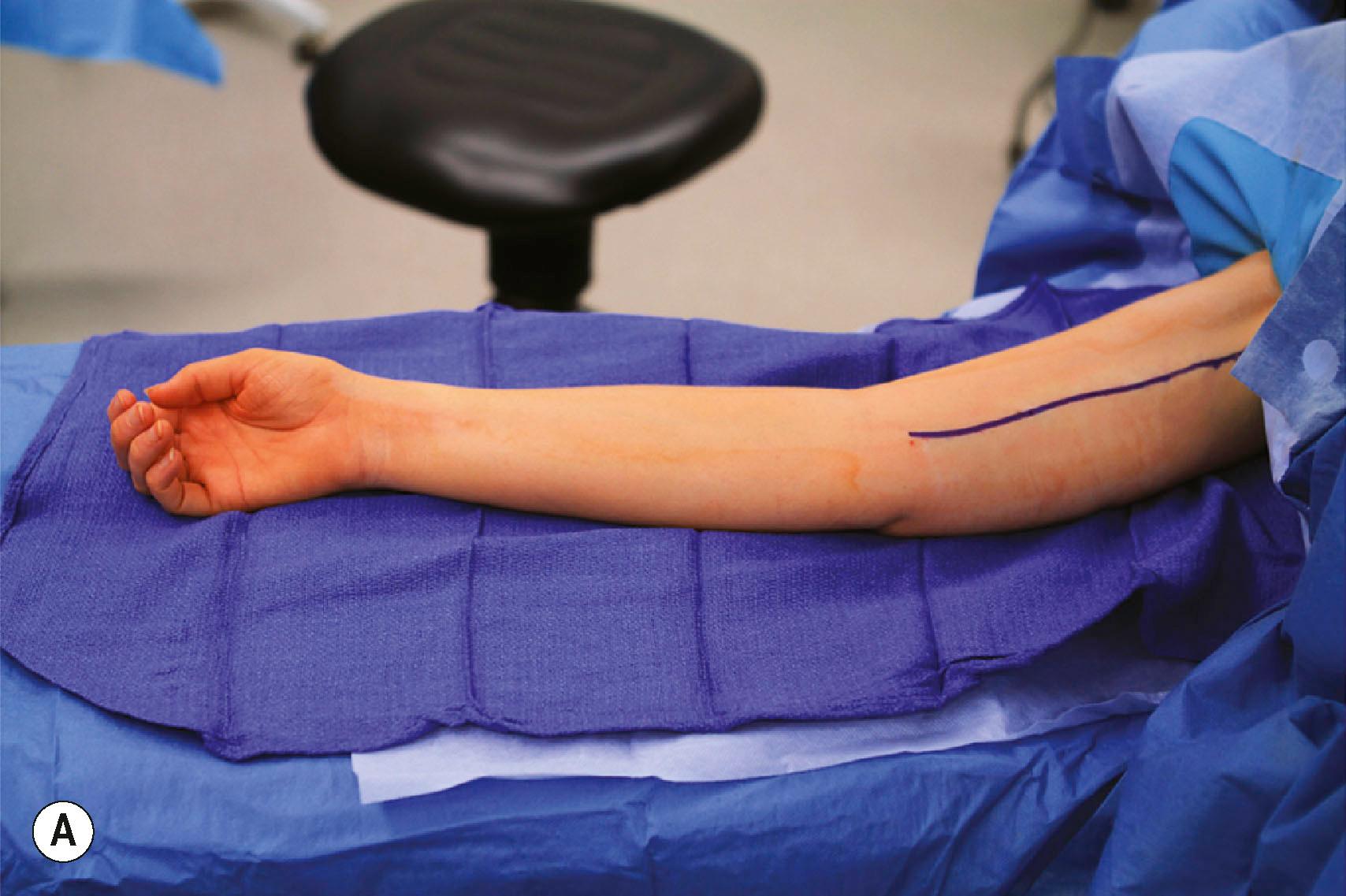
Restoration of elbow flexion is achieved with the double fascicular (ulnar/median redundant branches to biceps brachii and brachialis branches of the musculocutaneous) nerve transfer ( Fig. 26.9 ). This transfer reinnervates both the biceps brachii and brachialis muscles using redundant fascicles from the ulnar and median nerves, if available. However, in cases of limited donor availability, reinnervation of biceps alone may provide adequate antigravity elbow flexion. A medial arm incision in the bicipital groove allows exposure of the musculocutaneous, median, and ulnar nerves. Elevating the biceps brachii muscle laterally allows exposure of the proximal biceps and distal brachialis nerve branches. These are divided and draped over the median and ulnar nerves to determine best donor–recipient pairings. Most frequently, the biceps will be paired with a fascicle from the median nerve and the brachialis will be paired with a redundant fascicle from the ulnar nerve. The ulnar and median nerves are then neurolyzed at the appropriate level, and expendable fascicles to the flexor carpi ulnaris (FCU) (ulnar), flexor carpi radialis (FCR), or flexor digitorum superficialis (FDS) (median) are divided distally and coapted end to end. Reinnervation occurs at approximately 4–6 months postoperatively.
Avoid harvesting both the FCU and FCR branches when doing a double fascicular nerve transfer to preserve strong wrist flexion.
This transfer can also be conducted in a SETS fashion, with meticulous dissection of the donor nerves in order to facilitate reaching the recipients without an interpositional nerve graft.
Other potential donors that can be used to restore elbow flexion include the medial pectoral nerves, the thoracodorsal nerve, and the intercostal nerves.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here