Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Sudden cardiac death (SCD) is a leading cause of death, with an annual mortality of approximately 1 in 1000. SCD in the young is predominantly caused by inherited cardiac disorders that predispose the patient to the development of life-threatening arrhythmias. Such disorders include conditions with structural cardiac abnormalities (cardiomyopathies) and primary electrical disorders (PED) with disturbances in the electric currents underlying the cardiac action potential (AP). The diagnosis of PED can be challenging because the electrophysiologic abnormalities associated with these disorders can be masked unless provoked by specific triggers. Additionally, PED can often remain asymptomatic before sudden death. Genetic testing of individuals with PED can be of great value because the identification of a pathogenic variant creates the opportunity for presymptomatic genetic testing in family members. By identifying at-risk individuals before the development of symptoms, preventative measures can be taken to decrease the risk for sudden death.
To improve genetic testing in the clinic, next-generation sequencing methods have been used to create sequencing panels containing multiple genes associated with PED. Recently, even more extensive sequencing technologies, such as whole exome sequencing, are finding their way to the clinic. Although this increases the rate of molecular diagnosis, clinicians are often confronted with results that are difficult to interpret. Many genetic variants identified with sequencing panels are rare and have never been described in other patients or functionally studied in cell or animal models. It is therefore difficult to assess their pathogenicity.
Although in silico methods for the prediction of variant pathogenicity or the pathogenicity of a variant do exist, the programs often produce contradictory results. Therefore there is an increasing need to functionally model genetic variants identified in the clinic, preferably in a high throughput manner. Although PED variants have traditionally been experimentally validated by heterologous expression and patch-clamp analysis, this method is limited to the study of isolated ion channels and is less suitable for assessing genetic variants in associated proteins. Moreover, these heterologous expression systems lack the patient specific genetic background. Alternatively, induced pluripotent stem cell cardiomyocytes (iPSC-CMs) offer the opportunity to study genetic variants in the patient’s own genomic background. Nevertheless, generating iPSC-CMs is expensive and time consuming. Additionally, this model also is not perfect because iPSC-CMs suffer from immaturity and a high degree of variability.
Zebrafish offer several advantages for high throughput research. Although the zebrafish cardiac electrophysiology differs from human, it is still more compatible than that of mice. Most importantly, zebrafish can be easily combined with in vivo optical mapping techniques, which simplifies their phenotypical characterization. This chapter discusses the advantages and challenges of zebrafish as a PED model and optical mapping techniques in detail. By providing this overview, we aim to demonstrate how, by combining the zebrafish model with optical mapping, it is possible to meet the need for a high throughput in vivo system for the assessment of genetic variants in PED disorders.
Zebrafish ( Danio rerio ) are small freshwater fish originating from South Asia. In the last 25 years, they have become an increasingly popular research model because they offer several advantages. Although zebrafish require a dedicated aquatic facility, their husbandry is considerably less expensive than the care for rodents. Zebrafish reach adulthood and become capable of spawning at 3 months of age. They can easily produce over 100 eggs in a single spawning. Zebrafish larvae are translucent during the first days of life and develop externally. This enables a direct observation of organogenesis and makes this model particularly useful for the implementation of fluorescent techniques in vivo. They also develop rapidly, as evidenced by the observation of cardiac contractions as early as 24 hours after fertilization. Genetically, approximately 70% of the zebrafish genes have a human orthologue with varying degrees of conservation. The zebrafish genome underwent a duplication event in their evolutionary past, resulting in multiple zebrafish orthologues for many of the human genes. This phenomenon complicates genetic modeling because multiple gene copies need to be targeted.
In contrast to human anatomy, the zebrafish heart consists of only one atrium and ventricle, separated by an atrioventricular valve. Another valve, called the sinoatrial valve, is formed between the atrium and the central venous circulation (sinus venosus). The blood exiting the ventricle drains into the bulbus arteriosus, a pear-shaped chamber that functions as a reservoir. Similar to humans, the cardiac tissue is subdivided in endocardial, myocardial, and epicardial cells. The zebrafish cardiomyocytes are considerably smaller and narrower than those of humans. Additionally, they do not contain T-tubules. It is likely that the smaller size of the cells abolishes the need for such structures.
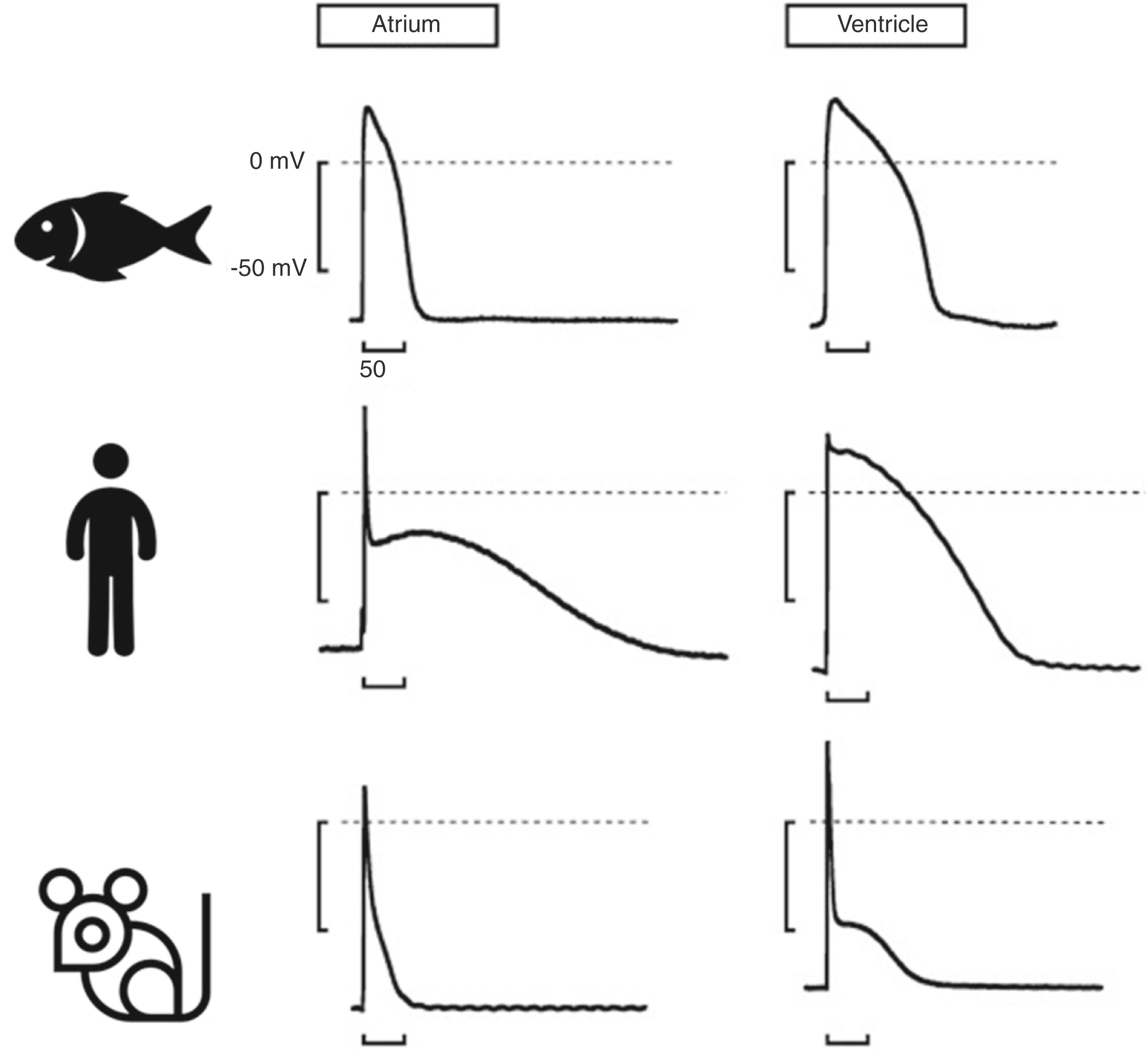
The zebrafish cardiac AP shares most of the characteristics of the human one ( Fig. 26.1 ). The most noticeable difference is the absence of the characteristic “notch” of the early transient repolarization (phase 1) generated by the I to (transient outward) current, which is not observed in zebrafish. The AP starts from a resting membrane potential of approximately –70 mV with a fast depolarization (phase 0), although the maximum AP upstroke velocity is lower in zebrafish (approximately 90 V/s in the zebrafish ventricle compared with 180 V/s in the human ventricle). , There is a distinctive plateau phase (phase 2), which is shorter in atria than in ventricles, and ends in a repolarization phase (phase 3). The AP duration at 90% of repolarization (APD90) in the zebrafish ventricle is more similar to the human (circa 130 ms, compared with 240 ms in humans and 80 ms in mice). The heart rate of adult zebrafish is approximately 100 to 130 beats/min, and therefore also more similar to the human than the much faster heart rates observed in mice (approximately 600 beats/min). The heart rate is slowed, and the APD90 is prolonged by exposure to colder temperatures. Because of deterioration of the sinoatrial node, older fish also have a slower and more variable heart rate.
The human SCN5A gene, which encodes the α-subunit of the Na V 1.5 cardiac sodium channel, is responsible for the generation of the sodium current underlying the rapid depolarization in phase 0 of the cardiac action potential (I Na ). This gene is encoded by two orthologues in the zebrafish ( scn5Laa and scn5Lab, Table 26.1 ). Similar to humans, the I Na displays slightly different characteristics in atrial and ventricular cells, with the inactivation shifted toward a more negative potential in atrial cells. The I Na density is lower in zebrafish, which likely contributes to the reduced upstroke velocity.
| Current | Human Gene(s) | Zebrafish Gene(s) | Altered Properties in Zebrafish |
|---|---|---|---|
| I Na | SCN5A | scn5Laa, scn5Lab | Lower density |
| I CaL | CACNA1C | cacna1c | Role in excitation-contraction coupling |
| I CaT | CACNA1H | cacna1g | Nonorthologous Expressed in adult cardiomyocytes Role in excitation-contraction coupling |
| I Ks | KCNQ1 + KCNE2 | kcnq1 | Rapid activation Independent of frequency/β-adrenergic stimulation Altered drug responsiveness |
| I Kr | KCNH2/hERG | kcnh6/zerg2 | Nonorthologous Activation shift toward positive voltages Inactivation shift toward negative voltages |
| I K1 | KCNJ2, KCNJ12, KCNJ4 | kcnj14, kcnj12a | Nonorthologous Altered channel composition |
| I to | KCND2, KCND3, KCNA4 | — | Absent |
As in humans, the plateau phase is maintained by calcium current. In zebrafish, however, two types of calcium current, the transient T-type (I CaT ) and the slow L-type (I CaL ), contribute to the plateau. I CaT is the product of a nonorthologous gene in zebrafish ( cacna1g; see Table 26.1 ). In human adults, I CaT is only expressed in conductive tissues and the sinoatrial node. Both in humans and in zebrafish, the cardiac I CaL is generated by the Ca V 1.2 protein, encoded by the CACNA1C gene. CACNA1C has only one zebrafish orthologue ( cacna1c; see Table 26.1 ). The I CaL density in zebrafish is increased compared with humans. Overall, the kinetics of I CaL in zebrafish appear similar to humans.
It seems that in zebrafish, apart from contributing to the AP plateau phase, I CaL and, in a lesser extent, I CaT also have an important role in cardiac contraction. Presumably, the excitation contraction coupling in zebrafish relies more strongly on calcium influx from outside the cell, mediated by I CaL , I CaT , and the sodium/calcium exchanger. This is in contrast to mammalian cardiomyocytes, where the Ca V 1.2 channel initiates a calcium-induced calcium release from the sarcoplasmic reticulum via the ryanodine channel. This calcium released from the sarcoplasmic reticulum (SR), rather than the calcium originating from the extracellular space, functions as the main activator of contraction.
Apart from the I to current, all major potassium currents are expressed in the zebrafish heart. The presence of the slowly activating potassium current (I Ks ) was challenging to confirm because this current was difficult to measure experimentally and has many differences from its human counterpart. In humans, I Ks is produced by the assembled K V 7.1 α-subunits and β-subunits, encoded by the KCNQ1 and KCNE2 genes, respectively. Although adult zebrafish do express kcnq1 in the heart, the expression of kcne2 is greatly reduced. This dramatically alters the kinetics of the channel (see Table 26.1 ). In mammals, I Ks is characterized by markedly slow activation kinetics. This is compatible with its main function as a repolarization reserve, with the capacity to shorten the AP when the heart rate is increased. In zebrafish, I Ks displays a rapid activation, likely because of the decreased availability of its β-subunit. The β-adrenergic responsiveness and frequency dependence of I Ks are diminished. The absence of the β-subunit also alters the protein conformation of the channel, thereby altering its drug sensitivity.
In humans, the rapidly activating outward rectifying potassium current (I Kr ) is the main repolarizing current in the heart. This is also the case for zebrafish, although the I Kr kinetics are slightly different. In humans, this current is produced by a channel encoded by the human ether-a-gogo related gene ( hERG or KCNH2 ). Zebrafish express several zebrafish ether-a-gogo related genes ( zerg genes) in the heart ( kcnh6, kcnh2a, kcnh2b, and kcnh7 ). The kcnh2a gene shows the highest sequence similarity to the human KCNH2. Kcnh2a knockdown experiments demonstrated a dose-dependent reduction of the heart rate.
It appears, however, that the main zerg gene expressed in the zebrafish heart is kcnh6 rather than kcnh2a, suggesting that I Kr might be produced by a nonorthologous gene in zebrafish (see Table 26.1 ). The human KCNH6 or HERG2 gene is mainly expressed in the cerebral cortex. Kcnh6 knockdown resulted in a reduced or irregular heart rate and atrioventricular block, whereas heterozygous missense mutants had a prolonged QTc interval. , Nonetheless, kcnh2a may still play a role, as evidenced by the experiments in kcnh2a knockdown zebrafish.
Several potassium channels contribute to the human inward rectifier potassium current (I K1 ), which is responsible for the stabilization of the cardiac resting membrane potential (see Table 26.1 ). Although I K1 is present in zebrafish, its channel composition is different compared with human. Kir2.4, encoded by the kcnj14 gene, forms the dominant isoform of the I K1 channel in zebrafish. This is in contrast to humans, where KCNJ14 is primarily expressed in the brain. ,
The cardiac ionic currents have mainly been studied in cardiomyocytes extracted from adult zebrafish. A few studies have focused on the electrophysiologic properties of zebrafish embryonic cardiomyocytes. , Although the cardiac AP of the embryo has a similar morphology to the adult, there are a few differences. The duration of the AP is longer (action potential duration at 90% of repolarization of approximately 230 ms in the ventricle in embryos, approximately 130 ms in ventricles of adult fish). The AP upstroke velocity depends on both the I Na and the I CaT currents and appears to be much slower (approximately 5 to 6 V/s in embryo ventricles versus 90 V/s in adult ventricles). As in the adult fish, the I CaT and I CaL calcium currents strongly influence the plateau phase. I Kr is also present in embryos. Apart from its role in repolarization, it appears to also contribute to the diastolic potential, which is not the case in adult fish. Both I Ks and I to have not been demonstrated in zebrafish embryos.
Transposon-based methods are popular genetic modification techniques that can be used to stably insert large genetic sequences, such as transgenes, into the genome of a living organism. These techniques are based on endogenous genetic mechanisms in which mobile DNA sequences (transposon elements) are capable of “jumping” to another location in the genome. This displacement is mediated by the action of enzymes that either transcribe the DNA sequence to RNA or cut the DNA sequence from its original location and insert it elsewhere.
The Tol2 transposon sequence was first identified in the genome of medaka fish, a species closely related to zebrafish. In contrast to most transposon elements identified in vertebrate genomes, Tol2 is autonomous (i.e., it is able to alter its genomic location on its own without the requirement of additional external components). The transposase protein encoded by the Tol2 element is capable of catalyzing a transposition of a transgene flanked by specific DNA sequences ( Tol2 ends). The Tol2 method is capable of inserting transgenes, even up to 11 kilo base pairs in size, into the host genome. The site of genomic integration of the transgene is random. To use the Tol2 method in zebrafish, a plasmid containing the transgene flanked by the Tol2 ends needs to be injected into fertilized zebrafish eggs together with Tol2 mRNA. Overall, approximately 50% to 70% of the injected fish will produce transgenic offspring.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here