Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Malformations of cortical development (MCDs) may be generalized throughout the cerebral cortex or may be focal dysplasias.
The etiologies of MCD may be genetic as either germline or postzygotic somatic mutations or both or may be epigenetic due to fetal exposure to teratogenic influences of toxins, infections, or trauma.
Mechanisms of cerebral dysgenesis are disorders or arrests of normal developmental processes; the most frequent principal processes are neuroepithelial cell proliferation, neuroblast migration, and organization/lamination of the cortical plate after migration is complete.
Focal cortical dysplasias (FCDs) are a frequent cause of epilepsy in infants and children and may be classified neuropathologically: FCD type I has abnormal lamination but normal neurons; FCD type II has abnormal lamination plus megalocytic dysplastic neurons; FCD type III is type I or II associated with another principal lesion such as hippocampal sclerosis, cortical tumor, vascular malformation, or congenital infarct.
Type II FCD includes hemimegalencephaly (HME), epidermal nevus syndromes, and tuberous sclerosis complex (TSC), all of which share identical or similar neuropathologic and genetic features.
Epileptogenesis of each MCD depends in large part on the ratio of excitatory and inhibitory synaptic input to individual neurons.
Treatment of epileptogenic FCD involves surgical resection as well as antiseizure medications.
Major advances in understanding the pathogenesis and precise diagnosis of MCD involves recent genetic discoveries, neuroimaging studies, and neuropathologic application of immunocytochemistry and biochemistry to tissue sections of brain lesions.
Malformations of cortical development (MCDs) are a heterogenous group of disorders that are characterized by abnormal cerebral cortical cytoarchitecture. MCDs represent a spectrum of disorders from subtle microdysgenesis affecting a small area of the cortex to devastating lissencephaly in which the regional, gyral, and laminar patterning of the cortex is lost. A unifying feature of MCDs is the high association with intractable epilepsy and in many cases with cognitive disabilities, autism, or pervasive developmental disorder. With the routine use of MRI, many subtle MCDs are being identified with greater frequency and with higher resolution, although so-called minimal MCDs often remain radiographically undetectable.
There is considerable variability in the molecular mechanisms and pathologic features of each MCD; those affecting broad areas of the cortex tend to have greater neurological manifestations, whereas minor malformations often manifest with pharmacoresistant epilepsy. Infantile spasms can be associated with virtually any type of malformation. MCDs can occur as autosomal recessive, autosomal dominant, or X-linked disorders; however, many occur sporadically without a clear family pedigree or as discordant findings in monozygotic twins. By contrast, some MCDs result from intrauterine toxins (e.g., alcohol, drugs), congenital infections such as cytomegalovirus, or perinatal hypoxic-ischemic injury. Over the past 15 years, progress has been made in the identification of single gene defects associated with individual MCDs. The generation of animal models for some of these MCDs has generated significant insights into the molecular mechanisms of epilepsy and cognitive disabilities seen in patients.
A growing number of MCDs have been associated with single pathogenic mutations and may in fact be part of a known specific genetic syndrome. The development of the normal six-layered human cortex results from a complex series of events, in large part controlled by known and unknown genes. Disruptions in these developmentally regulated genes can result in cortical malformations. A number of new genes have been discovered that cause certain forms of MCD ( Table 79.1 ). The specific malformation resulting from each mutation is related to the normal temporal and anatomic expression of the gene product that has been lost. Epigenetic factors also may generate MCD, due to embryonic or fetal exposure to teratogenic toxins including drugs, alcohol, pesticides, heavy metals (e.g., lead, mercury), and environmental pollutants. Abnormal DNA methylation without altering the nucleic acid sequence is one epigenetic mechanism, exemplified in fetal alcohol syndrome.
| Syndrome | Locus | Gene | Protein |
|---|---|---|---|
| Autosomal recessive periventricular heterotopia and microcephaly , , , | 8p23 | MCPH1 | Microcephalin |
| 1q31 | ASPM | Abnormal spindle-like microcephaly | |
| 17q25.3 | ARFGEF2 | ARFGEF2 | |
| Autosomal recessive microcephaly | 9q32 | CDK5RAP2 | Cyclin-dependent kinase 5 regulatory-associated protein 2 |
| 13q12.2 | CENPJ | Centromere-associated protein J | |
| Amish lethal microcephaly | 17q25.3 | SLC25A19 | Nuclear mitochondrial deoxynucleotide carrier |
| Seckel syndrome | 3q22-q24 | ATR | Ataxia telangiectasia and Rad3-related protein |
| Isolated lissencephaly sequence , , , , | Xq22.3–q23 | DCX, XLIS | DCX, doublecortin |
| 17p13.3 | LIS1 | PAFAH1B1 | |
| Subcortical band heterotopia , , | Xq22.3–q23 | DCX, XLIS | DCX, doublecortin |
| 17p13.3 | LIS1 | PAFAH1B1 | |
| Miller-Dieker syndrome , , | 17p13.3 | Several genes | PAFAH1B1 and others |
| Lissencephaly with cerebellar hypoplasia | 7q22 | RELN | Reelin |
| X-linked lissencephaly with abnormal genitalia , | Xp22.13 | ARX | Aristaless homeobox protein |
| Fukuyama congenital muscular dystrophy , , | 9q31 | FCMD | FCMD, fukutin |
| Muscle-eye-brain disease , , | 1p33–34 | POMGnT1 | POMGnT1 |
| 19q13.3 | FKRP | Fukutin-related protein | |
| Congenital muscular dystrophy , | 19q13.3 | FKRP | Fukutin-related protein |
| 22q12.3–q13.1 | LARGE | ||
| Walker-Warburg syndrome , , | 9q34.1 | POMT1 | O -mannosyl-transferase 1 |
| 19q13.3 | FKRP | Fukutin-related protein | |
| 9q31 | FCMD | FCMD | |
| Bilateral periventricular nodular heterotopia , | Xq28 | FLNA | Filamin-A |
| 20q13.3 | ARFGEF2 | BIG2 | |
| 5p15 | Unknown | Unknown | |
| Tuberous sclerosis , , , | 9q32 | TSC1 | Hamartin |
| 16p13.3 | TSC2 | Tuberin | |
| Pretzel syndrome | 17q23.3 | LYK5 | Strad |
| Bilateral frontoparietal polymicrogyria | 16q13 | GPR56 | Unknown |
| Warburg microsyndrome-1 | 2q21.3 | RAB3GAO | |
| Bilateral perisylvian polymicrogyria | Xq28 | Unknown | Unknown |
The dynamic process of cortical development can be disrupted at many time points by gene mutation or an environmental event. The formation of the normal cerebral cortex extends from weeks 8 to 26 of human gestation and is orchestrated by a complex array of genes that ultimately results in the correct cortical laminar organization. The cortex forms from neuroepithelial progenitor (stem) cells in the ventricular zone that undergo successive cycles of mitosis until the ventricular ependyma differentiates and arrests mitotic activity. After exiting the cell cycle, neurons migrate via both radial and tangential pathways in response to various trophic and repulsive cues to form the cortical plate (the nascent cortex). Excitatory neurons follow a radial inside-out migratory gradient along radial glial cells from layers VI to II. Layer I is an early embryonic layer that predates the deeper layers. This process also requires extracellular adhesion molecules to attach premigratory neuroblasts to radial glial fibers and disadhesion molecules to detach them at the cortical plate so that neuroblasts behind them on the same radial glial fiber can bypass. Radially migratory neuroblasts become excitatory glutamatergic cortical neurons. Inhibitory GABAergic interneurons are generated in the ganglionic eminence (a specialized portion of the germinal matrix) and migrate by tangential pathways into the cortical plate. They comprise about 20% of total mature cortical neurons distributed in all layers, but more in laminae 2 and 4. Radially migrating neuroblasts begin extending axons before reaching the cortical plate, but dendritic growth and ramification followed by synaptogenesis occurs only after arrival at their appropriate cortical destination. Conceptually, normal corticogenesis can be broadly divided into three stages: proliferation, migration, and organization/lamination.
A classification scheme for abnormal cortical development (i.e., MCD) was defined in 1996 and later modified in 2001 and 2004 on the basis of proposed steps for normal cortical development and the recent identification of single genes responsible for many MCDs. A task force commissioned by the International League Against Epilepsy (ILAE) has updated the classification scheme for focal cortical dysplasia (FCD). , The MCD taxonomy is best organized when viewed according to putative cellular mechanisms disrupted in development. These groups included (1) disorders of cellular proliferation, (2) disorders of neuroblast migration, (3) disorders of cortical architectural organization and lamination, and (4) disorders with unknown mechanisms ( Table 79.2 ).
| Group | Subgroup | Condition |
|---|---|---|
| Disorders of cellular proliferation | Abnormal proliferation | Microcephaly |
| Macrocephaly | ||
| Cortical tubers (tuberous sclerosis complex) | ||
| Focal cortical dysplasia with balloon cells | ||
| Hemimegalencephaly | ||
| Abnormal proliferation | Dysembryoplastic neuroepithelial tumor | |
| Neoplastic process | Gangliogliomas | |
| Gangliocytoma | ||
| Disorders of neuroblast migration | Lissencephaly | |
| Heterotopia | ||
| Muscular dystrophy | Congenital muscular dystrophy | |
| Muscle-eye-brain disease | ||
| Walker-Warburg syndrome | ||
| Disorders of cortical organization | Polymicrogyria | |
| Schizencephaly | ||
Maturation of individual neuroblasts and synapse formation in fetal brain at autopsy can be much better defined by modern immunocytochemical markers than by traditional histologic stains such as hematoxylin-eosin (H&E) or cresyl violet (Nissl) stain. Some of these label early and others late stages of neuroblast maturation. Synaptogenesis is a good example of the value of immunocytochemistry. The normal spatial and temporal sequence of synapse formation in the cerebral cortex begins at midgestation. In alobar and semilobar holoprosencephaly (HPE), not only is the cortical architecture disrupted, but there is precocious synaptogenesis beginning much earlier than in control fetal brains, which may enable the early formation of epileptic networks postnatally. In many other cerebral dysgeneses, by contrast, synaptogenesis is delayed. Myelin stains similarly can demonstrate early or late myelination in white matter, which has an advantage of demonstration by MRI in living infants as well as in tissue sections. Hypermetabolic neurons with increased numbers of mitochondria and mitochondrial enzymatic activity are found in epileptic foci as demonstrated by histochemistry and electron microscopy. The hypermetabolism of these neurons results from repetitive discharges that deplete their store of neurotransmitter and an increased energy requirement for increased synthesis of replacement transmitters.
Malformations in this group include disorders that result in either increased or decreased growth or in increased or decreased apoptosis. These disorders often manifest as abnormal brain size or localized areas of enlargement. Abnormalities in brain size can manifest as microcephaly with a normal to thin cortex, microlissencephaly, microcephaly with polymicrogyria, or macrocephaly. , , ,
Microcephaly is defined as a head circumference that is greater than 2 standard deviations smaller than normal. Micrencephaly refers to a small brain. Patients with this condition have a brain that may be up to 70% smaller than normal, but the brain has essentially normal cortical laminar architecture. In some cases of micrencephaly, there is a simplified gyral folding pattern observed on MR images. Because any process that interferes with brain growth can lead to reduced head and brain size, microcephalies are a highly heterogeneous group of disorders. Microcephaly can result from a number of prenatal insults including hypoxia-ischemia and fetal alcohol exposure. In addition, a number of genes ( ASPM, MCPH1, CENPJ, and CDK5RAP2 ) have been shown to result in autosomal recessive forms of microcephaly. , , , Rare syndromic forms include Amish lethal microcephaly and Seckel syndrome. , The ASPM (abnormal spindle-like microcephaly-associated) gene is of particular interest because it is the single most common genetic mutation associated with microcephaly, and it has provided new insights into evolutionary mechanisms governing brain size across species. ASPM is an ortholog of a Drosophila gene that has been shown to be essential for normal mitotic spindle function in embryonic neuroblasts. When the gene is absent, the neuroblasts are arrested in the cell cycle and fail to proliferate, resulting in reduction in the number of cells in the brain and consequently a smaller brain size. When the human ASPM gene sequence was compared with that in primates, there was a correlation between sequence and brain size. Thus ASPM appears to have played a central role in the genetic component in the evolutionary expansion in brain size from primates to Homo sapiens.
Macrocephaly, defined as measurable (>2 standard deviations) enlargement of the head, can occur in association with a large number of syndromes and conditions, some of which, such as hydrocephalus, autism, leukodystrophies, and organic acidurias, are not associated with MCD. Megalencephaly (ME) is the condition in which both brain and head size are increased. In recent years a number of new gene mutations in AKT, PI3KCA, PTEN, and TBC1D7 have been identified to account for ME. Hemimegalencephaly (HME) is a malformation characterized by the abnormal enlargement of one entire hemisphere ( Fig. 79.1 ); a variant of HME is lobar dysplasia in which an entire brain lobe is enlarged. HME may be seen in isolation or in the setting of known syndromes, such as linear sebaceous nevus, hypomelanosis of Ito, tuberous sclerosis complex (TSC), or Proteus syndrome. HME is highly associated with intractable seizures and, often, infantile spasms. Pathologic analyses of HME brains demonstrate loss of cortical lamination dysmorphic neurons and cytomegalic cells similar to those seen in FCD and TSC (discussed later). Over the past several years, somatic or germline mutations in a number of genes including AKT, PI3KCA, PTEN, and DEPDC5 have been linked to HME.
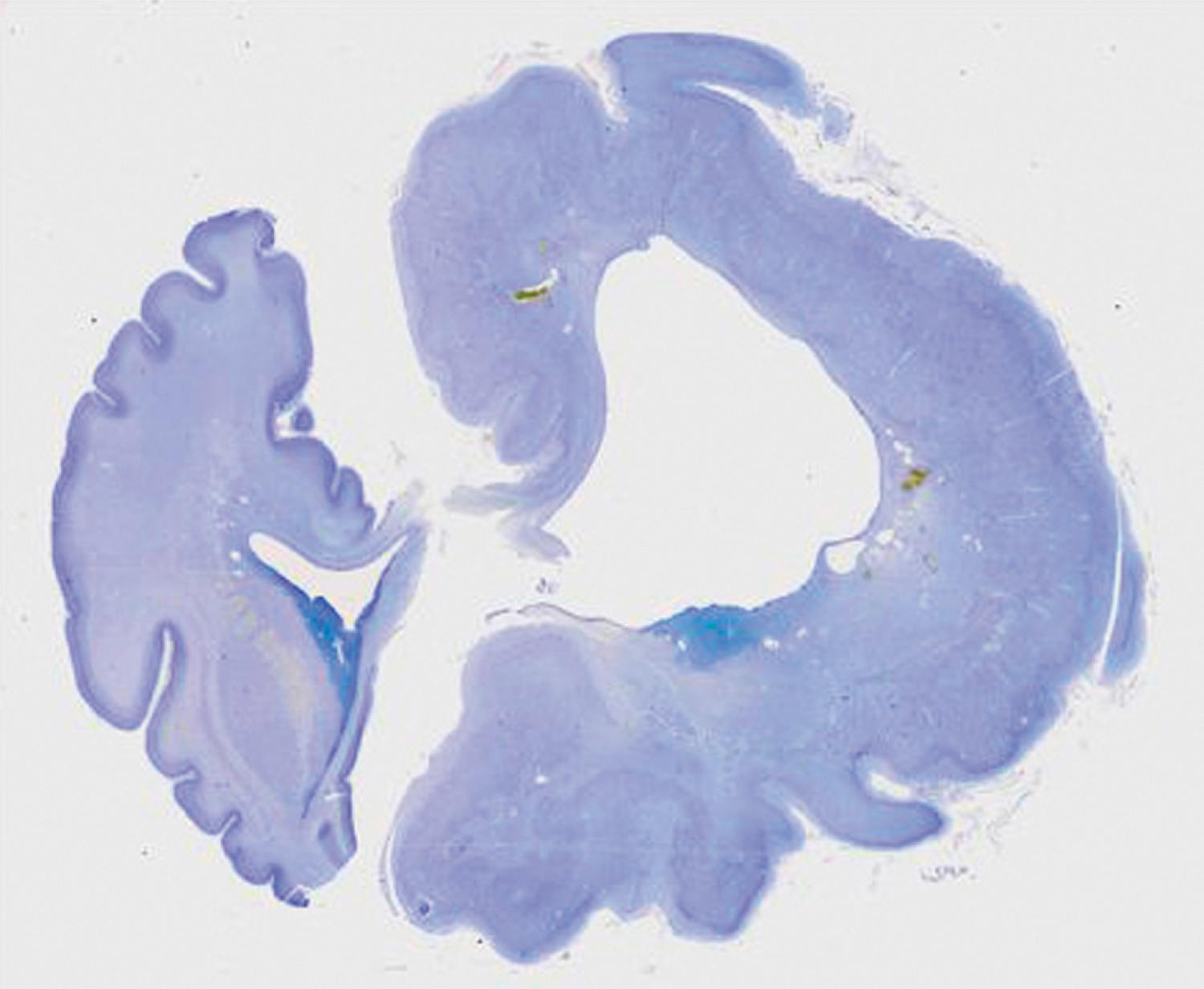
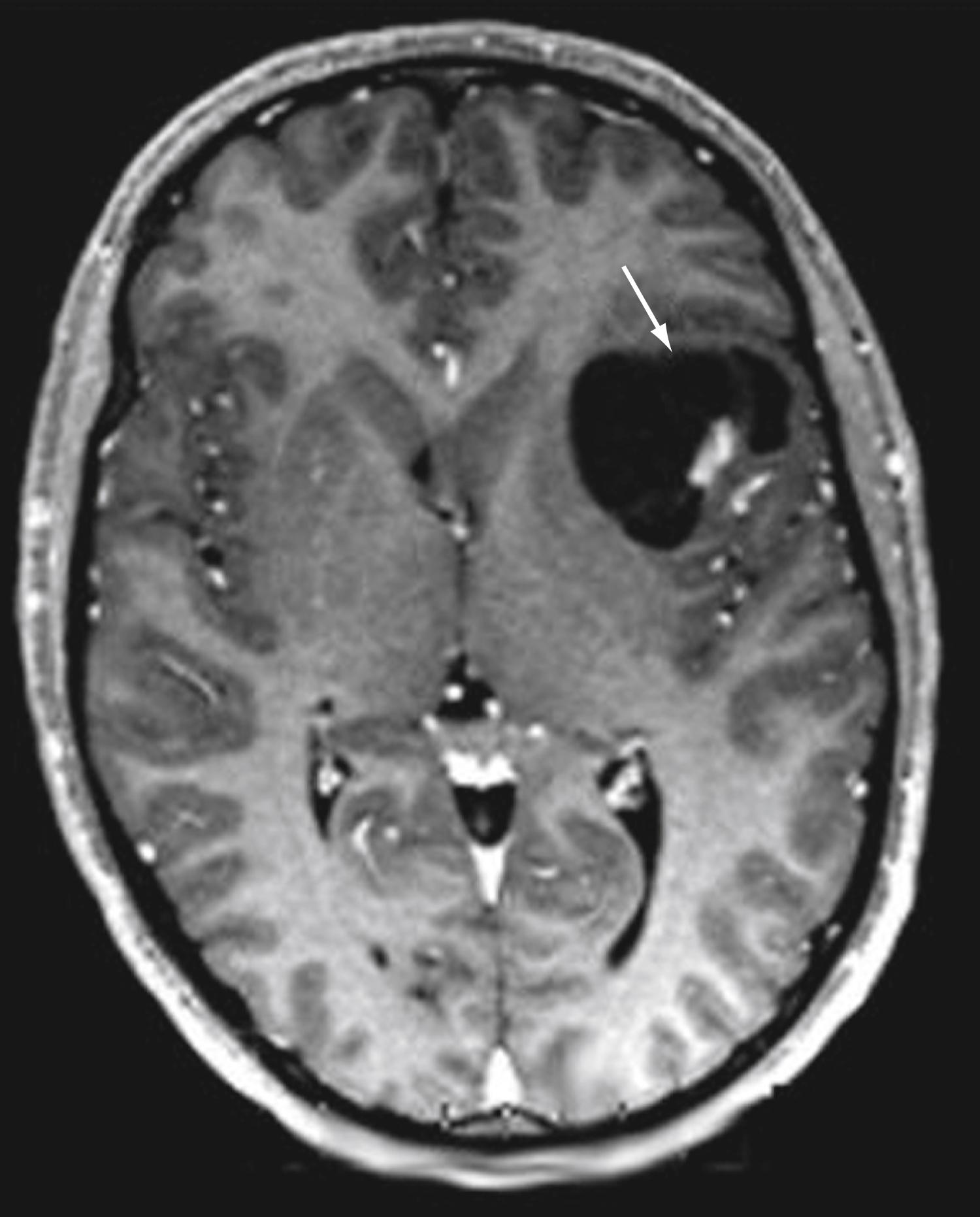
In addition to changes across broad cortical areas, disorders of cellular proliferation can result in focal malformations or dysplasias. These lesions include FCDs, which have been divided into types Ia, Ib, Ic, IIa, IIb, and III (see Crino ). In some schemas, low-grade neoplastic lesions such as dysembryoplastic neuroepithelial tumor, ganglioglioma ( Fig. 79.2 ), and gangliocytoma are linked to MCD. This is not irrational, because they share some genetic mutations and other features such as upregulation of abnormal tau, one of several microtubular proteins important in cellular growth and differentiation. ,
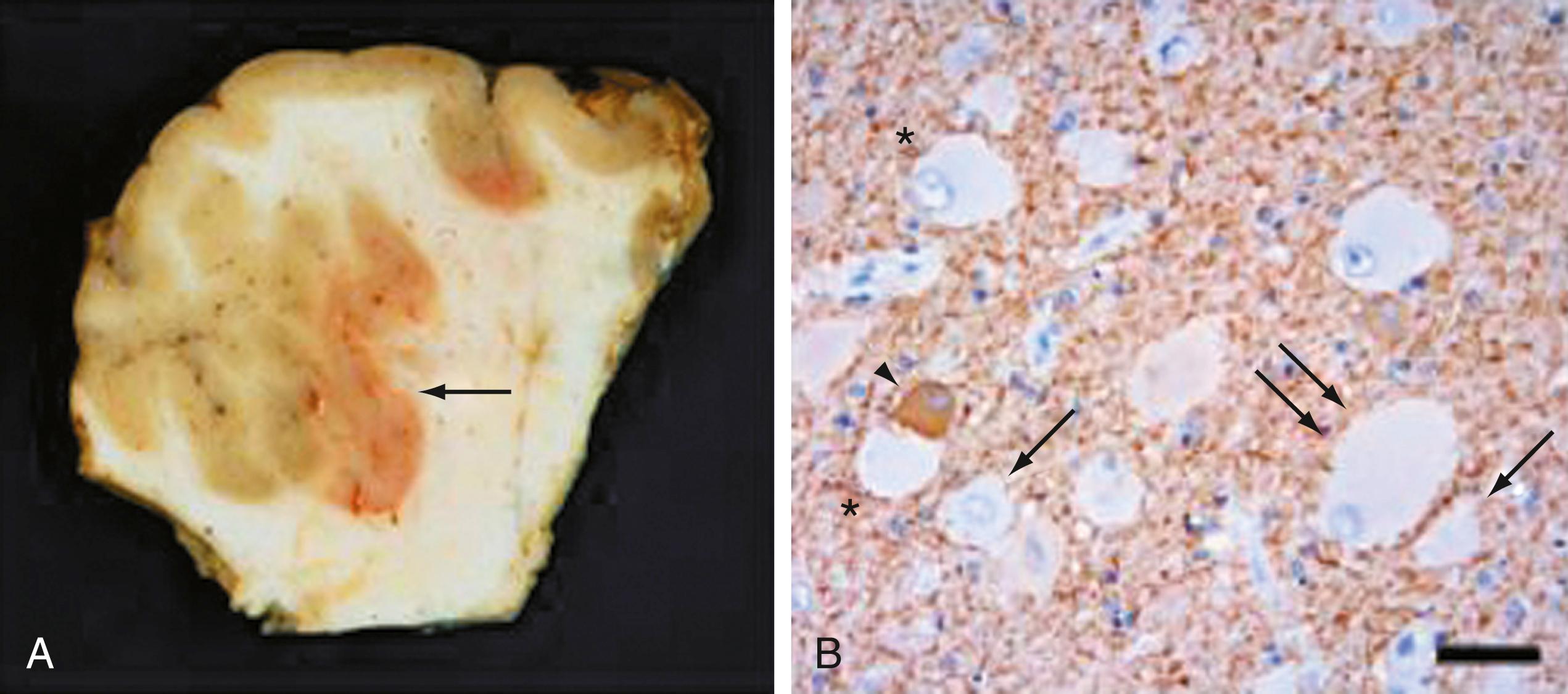
TSC results from mutations in TSC1 , encoding TSC1 or hamartin, or TSC2 , encoding TSC2 or tuberin. , TSC1 and TSC2 are known modulators of the mammalian target of rapamycin (mTOR) pathway, which governs cell size and proliferation. Neurological manifestations of TSC include epilepsy, infantile spasms, cognitive disabilities, and autism. Cortical tubers are focal areas of disorganized cortical lamination characterized by the presence of cytomegalic cells known as giant cells, which are morphologically similar to balloon cells in FCD type IIb (see later). The number of tubers across patients is variable and does not seem to vary with mutation type, patient age, or, necessarily, neurological phenotype.
FCD is an often sporadic condition for which specific gene mutations have been recently identified, especially among regulatory components of the mTOR pathway. , The existing classification scheme for FCD delineates three broad categories that reflect a spectrum of pathologic severities. FCD types Ia and Ib are characterized by often subtle alterations in, respectively, columnar and laminar architecture of restricted areas of cortex; type Ic is a combination of Ia and Ib. Gene mutations have not been clearly associated with these subtypes. Types IIa and IIb exhibit more dramatic histopathologic changes, including large dysmorphic neurons in type IIa and the presence of balloon cells in type IIb that are similar to giant cells found in TSC ( Fig. 79.3 ). Both germline and somatic mutations in a number of genes associated with the mTOR pathway including PI3K, AKT3, DEPDC5, and MTOR itself have been associated with types IIa and IIb FCD.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here