Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
In the heart, the main repolarizing currents are carried by potassium ions. A number of potassium currents shape and control the action potential (AP). The inward rectifier K + current is crucial for maintaining the membrane potential near the equilibrium potential for potassium, –90 mV during diastole. The voltage-dependent outward currents regulate the activation and termination of the plateau phase of the AP, and the delayed rectifier currents control the final repolarization phase of the action potential. The molecular basis of potassium currents is diverse, with more than 40 genes encoding potassium channels expressed in the heart. In addition, a number of auxiliary subunits and protein partners contribute to the trafficking, anchoring, and stabilization of potassium channels at the plasma membrane and to their organization in macromolecular complexes. These partners confer important properties to the potassium currents that contribute to the plasticity of cardiac electrical properties both in normal conditions and during cardiac diseases. Not only protein but also lipids of the plasma membrane appear as major partners for potassium channels. This chapter focuses on the description of partners involved in the formation of macromolecular K V potassium channel complexes and their role in cardiac excitability.
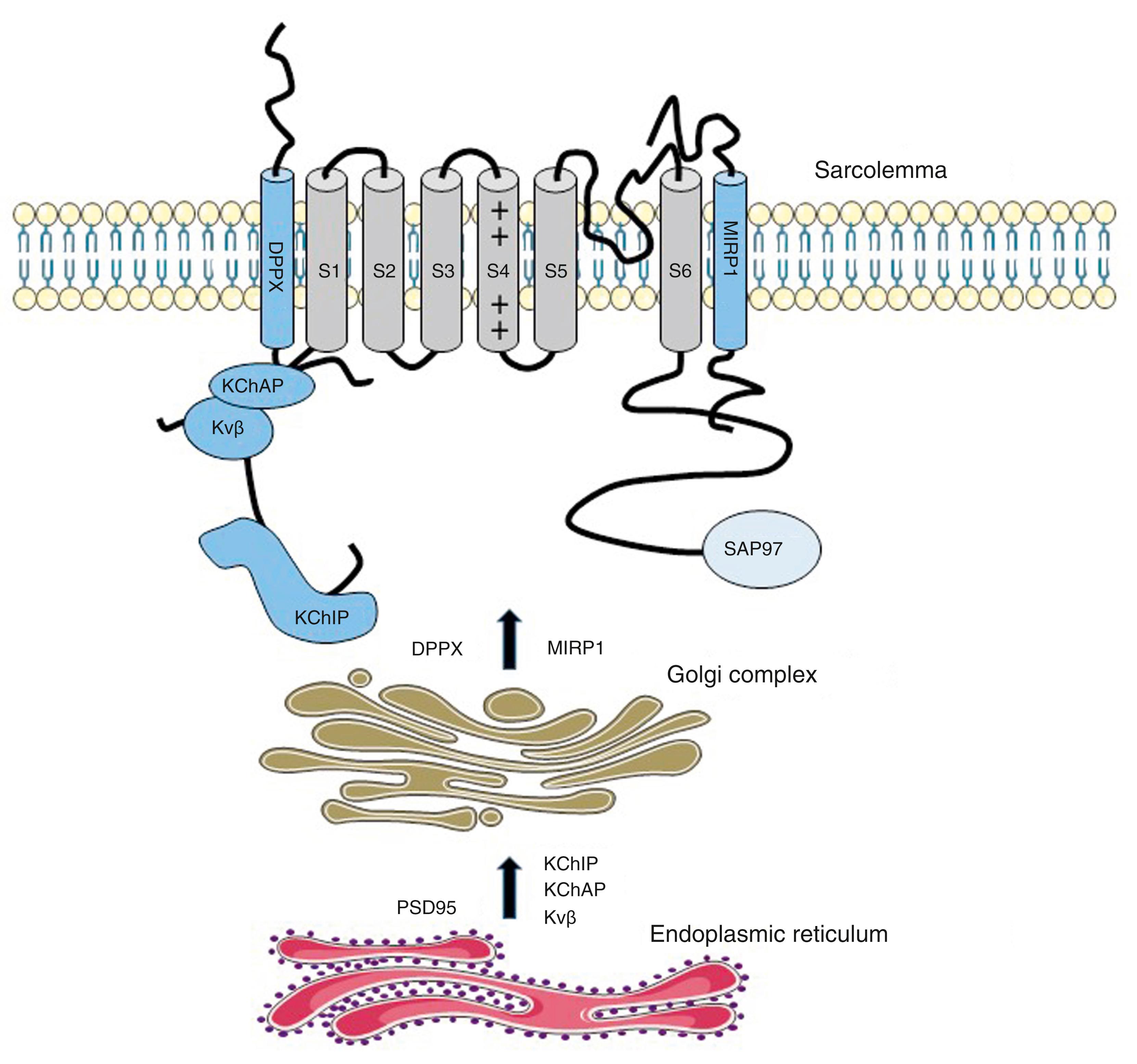
The first identified auxiliary subunits of potassium channels are K V β-subunits that associates with K V channels (referred as α-subunits). Initially purified from bovine brain, , nine β-subunits encoded by four genes have been identified. In mammals, three genes are expressed in the heart (Kβ1.1, K V β1.2, and K V β1.3), and variants from alternative splicing are also found. K V β-subunits are localized in the cytosol with a conserved C-terminal and a variable N-terminal.
Two main roles have been described for K V β-subunits. Trimmer and coworkers reported that K V β1 and K V β3 early associate with α-subunits during their biosynthesis in the endoplasmic reticulum and exert a chaperone-like effect, facilitating their stable expression at the plasma membrane. , The C-terminal domain of K V β interacts with the α-subunit (stoichiometry of 1α-1β) to regulate channel trafficking. Nevertheless, this chaperone property of the K V β-subunit has not been found for all K V channels , ( Fig. 20.1 ).
The most drastic effect of K V β on the voltage-dependent outward current is to accelerate its rate of inactivation. K V channels inactivate through two main mechanisms: the rapid N-type inactivation called the “ball and chain” model and a slow C-type inactivation. For instance, K V channels such as K V 1.5, which do not have an N-terminus acting as an open channel blocker, are inactivated through a mechanism that involves slow conformational changes of the outer mouth of the pore, referred to as C-type inactivation. The coexpression of K V β1 with K V α-subunits with a C-type inactivation results in a fast inactivating current with increased membrane depolarization. For instance, the K V 1.5-encoded current, one main component of I Kur in atrial myocytes, is transformed by K V β1.3-subunits in a transient outward I to -type current. This effect on the current kinetic is associated with a shift of the voltage-dependent activation and inactivation toward more negative potentials. The effect of K V β1 on channel inactivation is mediated by their N-terminal domain that blocks the inner cavity of the pore of the α-subunit, resembling the ball and chain process. , In addition, by binding to the C-terminus of the channel, K V β can accelerate the C-type inactivation. In heterologous expression systems, coexpression of K V β1.3 with K V 1.5 is necessary for the protein kinase A (PKA, or cyclic adenosine monophosphate–dependent protein kinase)-mediated increase in K + current. In addition, protein kinase C (PKC) has little effect on Kv1.5 channels alone, but, when coexpressed with K V β1.2, the protein kinase reduces the K + current. These observations are consistent with the presence of multiple phosphorylation sites on the β- and α-subunits and could provide an explanation for the effects of the β-adrenergic or PKC stimulation on I Kur in human atrial myocytes. The mode of membrane depolarization (i.e., the duration and the frequency) can persistently modify the rate of inactivation of I Kur in human atrial myocytes. This effect is modulated by the activation of calcium-calmodulin–dependent protein kinase II (CaMKII) activation and could also involve the interaction between K V β and the K V α1.5-subunits.
Another illustration of the important role played by the regulation of K V channels by K V β is provided by the study on pharmacologic properties of K V 1.5 channel. The contribution of I Kur to the shortening of the AP during atrial remodeling, and the fact that K V 1.5 channel is more abundantly expressed in atrial than ventricular myocardium, had opened the possibility of using elective I Kur blockers as potential atria-specific antiarrhythmic agents. Clinical trials using selective K V 1.5 channel, however, have been disappointing. Nevertheless, understanding the action mechanisms of these molecules has provided insights into the gating processes of these channels. In this line, some of these molecules compete with β-subunits to bind in the inner cavity of the K V 1.5 channel pore and are responsible for an apparent open channel block of I Kur . For instance, K V β1.3 decreases the drug affinity of K V 1.5 for local anesthetic and antiarrhythmic drugs (e.g., bupivacaine , ; AVE0118 ; vernakalant ). In atrial myocytes too, bertosamil, the analog of tedisamil, accelerates the rate of inactivation of the outward current with membrane depolarization, which is consistent with a competition between the drug and endogenous K V β at the inner face of the pore.
Finally, an oxidoreductase activity has been reported for the K V β-subunit as indicated by the observation that K V β2.1 confers sensitivity to oxygen on K V 4.2. This enzymatic activity is likely because of the presence of a binding site for the cofactor NADP + and catalytic domains. In vivo data have supported the idea that K V β2 is crucial for the redox regulation of ventricular cardiac action potential duration. Although native ventricular myocytes exhibit increased inactivation of I K, slow1 and I K, slow2 carried by the K V 1-subunit and prolonged APD when intracellularly dialyzed with a hypoxia-mimicking solution (low NADP + /NADPH ratio), ablation of K V β2 prevents the redox sensitivity of the cardiac AP. Interestingly, K V β2 ablation is also accompanied by a reduction of the left ventricle size, a thinning of the left ventricular wall thickness, and a reduced fractional shortening and ejection fraction. These observations support a role for K V β2 in ventricular remodeling and the metabolic regulation of K V channels during stress conditions, such as ischemia and hypoxia.
Originally thought to be the α-subunit of the delayed-rectifier potassium current I Ks , minK (short for minimal K channel protein) is a small transmembrane protein (14–20 kDa) encoded by KCNE1. The first interaction described for minK is with the K V 7.1 channel (K V LQT1). Expressed alone, K V 7.1 underlies a voltage-dependent outward current of very small amplitude. In contrast, when coexpressed with minK, this channel is responsible for a large slow delayed-type current called I Ks . , MinK can interact with other channels, such as the hERG channel (or K V 11.1), which is responsible for the activation of the rapid delayed rectifier I Kr . This observation, however, was only reported in heterologous expression systems. In mice, deletion of KCNE1 is associated with an impaired QT-RR adaptability on the electrocardiogram (ECG), prolongation of epicardial AP duration, and frequent episodes of atrial tachycardia, all of which point to the physiologic importance of this ancillary subunit for cardiac electrophysiology.
Four other peptides belonging to the minK family have been identified and are known as MinK-related peptides (MiRP; KCNE2-5). In the heart, MiRP1 (KCNE2) is expressed mainly in the nodal tissue and Purkinje cells. In vitro MiRP1 can interact with several potassium channels, including the K V 7.1, K V 4 , and hERG channels. The hERG channel is regulated by PKA, which increases the current amplitude through the shift of the voltage-dependent activation of the channels. This regulation is an important target for the sympathetic regulation and subsequent adaptation of cardiac repolarization to the increased heart rate. It has been reported that KCNE2 facilitates the PKA regulation of I Kr by stabilizing the hERG channel in its phosphorylated form. Transgenic models have helped to establish the physiologic role of MiRP1 in the heart. Mice with a specific deletion of KCNE2 show prolonged ventricular AP and reduced density of fast and slow components of the outward potassium current, which is consistent with the ability of KCNE2 to interact with various potassium channels. , It has also been reported that K V 7.1-KCNE1 association shifts from the conventional secretory pathway (i.e., through Golgi apparatus) to taking a nonconventional route that targets the channel complex to endoplasmic reticulum (ER)-plasma membrane junction in which the two partners oligomerize before reaching the sarcolemma. This new trafficking process explains the location of the channel complex in T-tubules of cardiomyocytes.
Genetic studies of patients with inherited or drug-induced long QT (LQT) syndrome have also provided important information on the physiologic role of this family of potassium partners. Mutations of genes encoding MinK and MiRP1 have been found during the two familial forms of LQT syndrome, LQT5 and LQT6. Some mutations of KCNE2 are responsible for the decrease in I Kr because of the acceleration of hERG inactivation. This results in less repolarizing currents during the termination of the AP, causing the prolongation of phase 3 and a risk for triggered activities. Other mutations have been shown to modify the pharmacologic profile of hERG, providing a molecular explanation for iatrogenic LQT syndrome and torsades de pointes , , (see Fig. 20.1 ). One study sheds light on the connection between sympathetic nervous system hyperactivity and I hERG /I Kr reduction, a well-known cause of torsades de pointes and ventricular fibrillation. Both in expression systems and feline myocytes, α1 adrenergic receptor activation leads to I Kr reduction through direct (and either MinK or MiRP-independent) PKC-mediated phosphorylation of the hERG channel N-terminus. On the other hand, posttranslational modifications of minK are directly responsible for the V 1/2 inherent to native cardiac I Ks currents. For instance, the voltage-dependence of I Ks results from, and is modified by, KCNE1-dependent SUMOylation of KCNQ1.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here