Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The contributions to this chapter by Dr. Neil D. Theise, Department of Pathology at NYU Grossman School of Medicine, New York, New York, and the late Dr. Nelson Fausto, Department of Pathology, University of Washington, Seattle, Washington, in previous editions of this book are gratefully acknowledged.
The healthy adult liver weighs 1400 to 1600 gm. It has a dual blood supply, with the portal vein providing 60% to 70% of hepatic blood flow and the hepatic artery supplying the remainder. The portal vein and the hepatic artery enter the inferior aspect of the liver through the hilum, or porta hepatis . Within the liver, the branches of the portal veins, hepatic arteries, and bile ducts travel in parallel within portal tracts, ramifying through 10 to 12 orders of branches.
The most common terminology used to describe the hepatic microarchitecture is based on the lobular model ( Fig. 14.1 ). This model divides the liver into lobules 1 to 2 mm in diameter that are centered on a terminal tributary of the hepatic vein and demarcated by portal tracts at their periphery. These lobules are often drawn as hexagonal structures, though the shapes are variable; nonetheless, it is a useful simplification. A second model divides the liver into triangular acini (see Fig. 14.1 ) based on the position of hepatocytes relative to their blood supply. The hepatocytes in the vicinity of the terminal hepatic vein are called centrilobular ; those near the portal tract are periportal. Division of the lobular parenchyma into zones is an important concept because each zone differs with respect to its metabolic activities and susceptibility to various forms of hepatic injury.
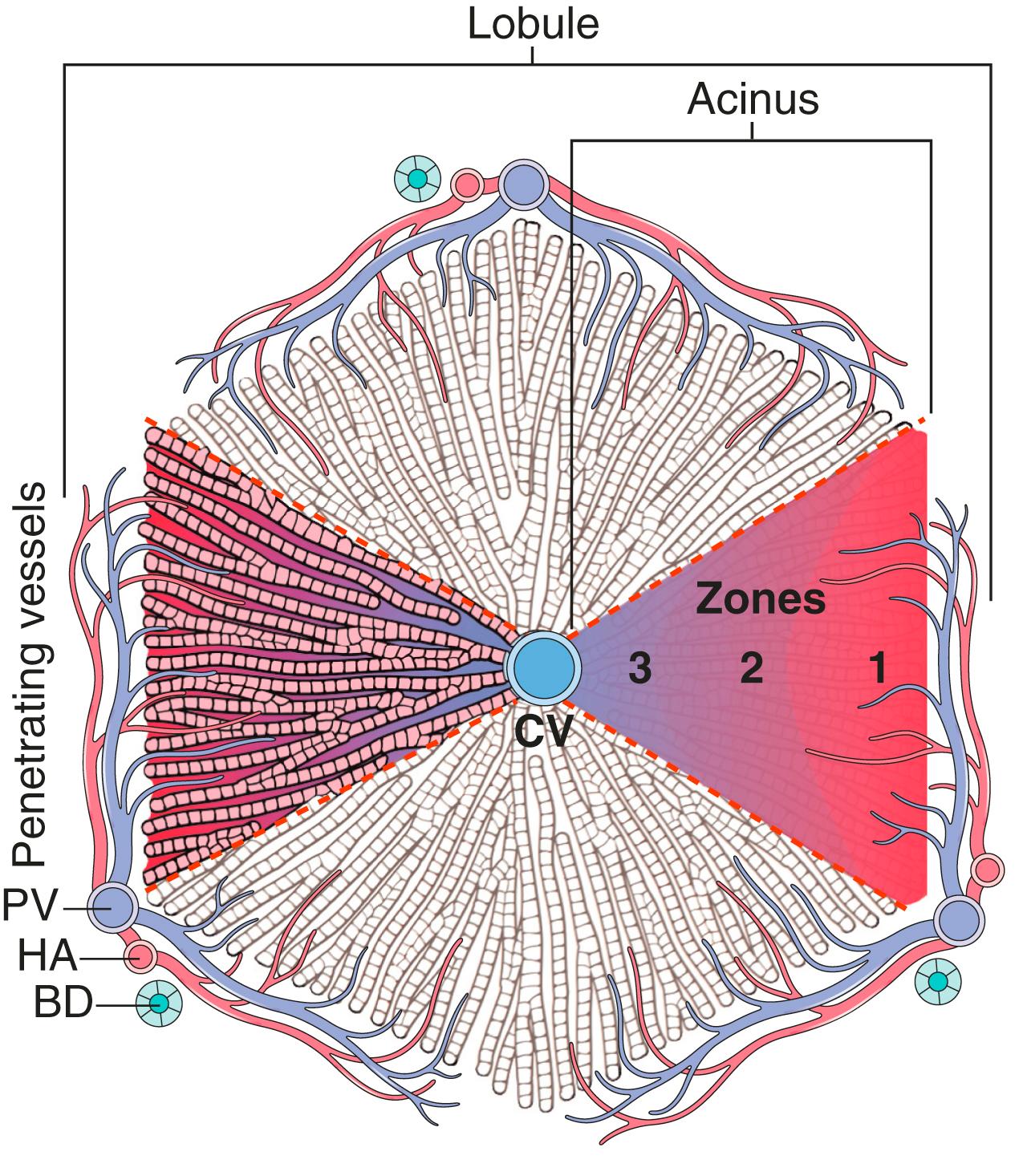
Within the lobule, hepatocytes are organized into anastomosing sheets or “plates” extending from the portal tracts to the terminal hepatic veins. Between the trabecular plates of hepatocytes are vascular sinusoids . Blood flows through the sinusoids and exits into the terminal hepatic veins through numerous orifices in the vein wall. Hepatocytes are thus bathed by well-mixed portal venous blood on one side and hepatic arterial blood on the other. The sinusoids are lined by a fenestrated endothelium that overlies a perisinusoidal space (the space of Disse ) into which abundant hepatocyte microvilli protrude. Attached to the luminal face of the sinusoids are scattered Kupffer cells, specialized long-lived tissue macrophages that arise early in embryogenesis. Another specialized cell type, the hepatic stellate cell, is found in the space of Disse and has a role in the storage of vitamin A. Between abutting hepatocytes are bile canaliculi, channels 1 to 2 μm in diameter that are formed by grooves in the plasma membranes of adjacent hepatocytes and are separated from the vascular space by tight junctions. These channels drain successively into the intralobular canals of Hering, periportal bile ductules, and finally into the terminal bile ducts within the portal tracts.
The most important primary diseases of the liver are viral hepatitis, alcohol-related liver disease, nonalcoholic fatty liver disease, and hepatocellular carcinoma. The liver is also frequently damaged secondarily in a variety of common disorders, such as heart failure, disseminated cancer, and extrahepatic infections. The large functional reserve of the liver reduces the clinical impact of mild liver damage, but severe diffuse liver disease can be life-threatening.
With the rare exception of fulminant hepatic failure, liver disease is an insidious process in which the signs and symptoms of hepatic decompensation appear weeks, months, or even years after the onset of injury. The hepatic injury may be imperceptible to the patient and manifest only as laboratory test abnormalities ( Table 14.1 ); liver injury and healing may also be subclinical. Hence, individuals with hepatic abnormalities who are referred to hepatologists most frequently have chronic liver disease.
| Test Category | Blood Measurement a |
|---|---|
| Hepatocyte integrity |
|
| Biliary excretory function | |
| Hepatocyte synthetic function |
a Most commonly used tests are in italics.
Injured hepatocytes may show several potentially reversible changes, such as accumulation of fat (steatosis) and bilirubin (cholestasis); when injury is not reversible, hepatocytes die by necrosis or apoptosis. Necrosis ( Fig. 14.2 ) is commonly seen following hepatic injury caused by hypoxia and ischemia. Apoptotic cell death ( Fig. 14.3 ) predominates in viral, autoimmune, and drug- and toxin-induced hepatitides.
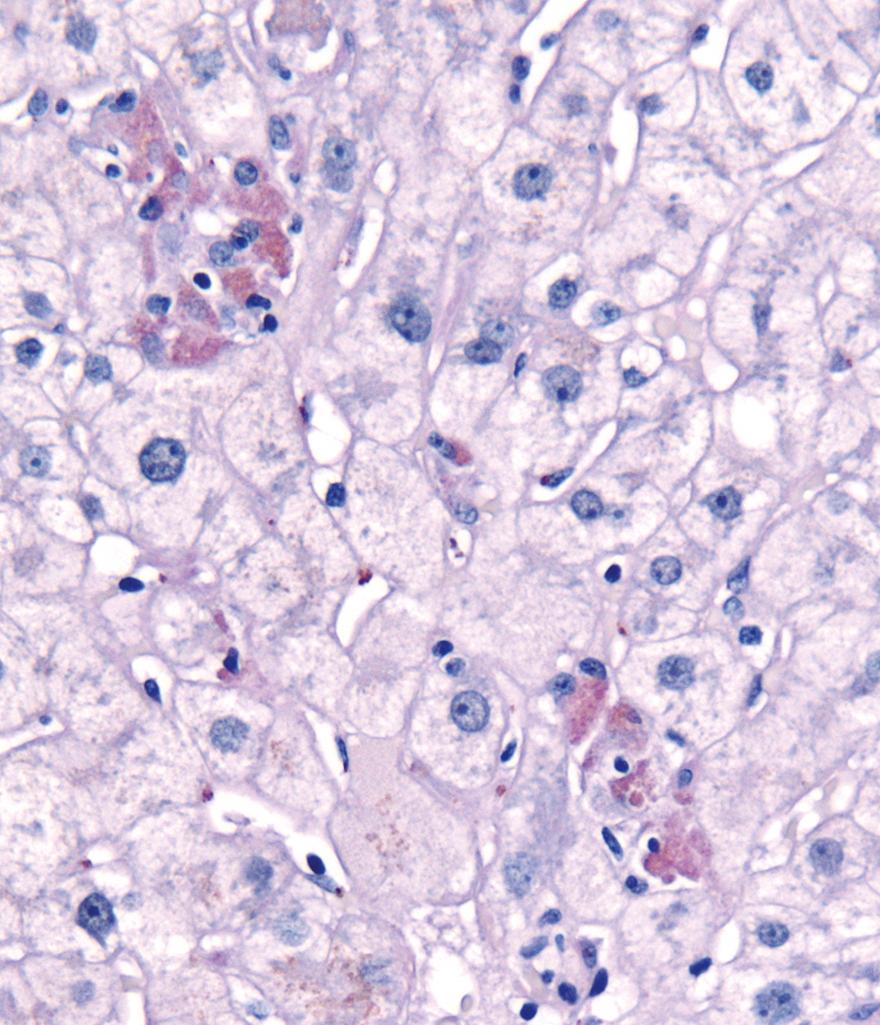
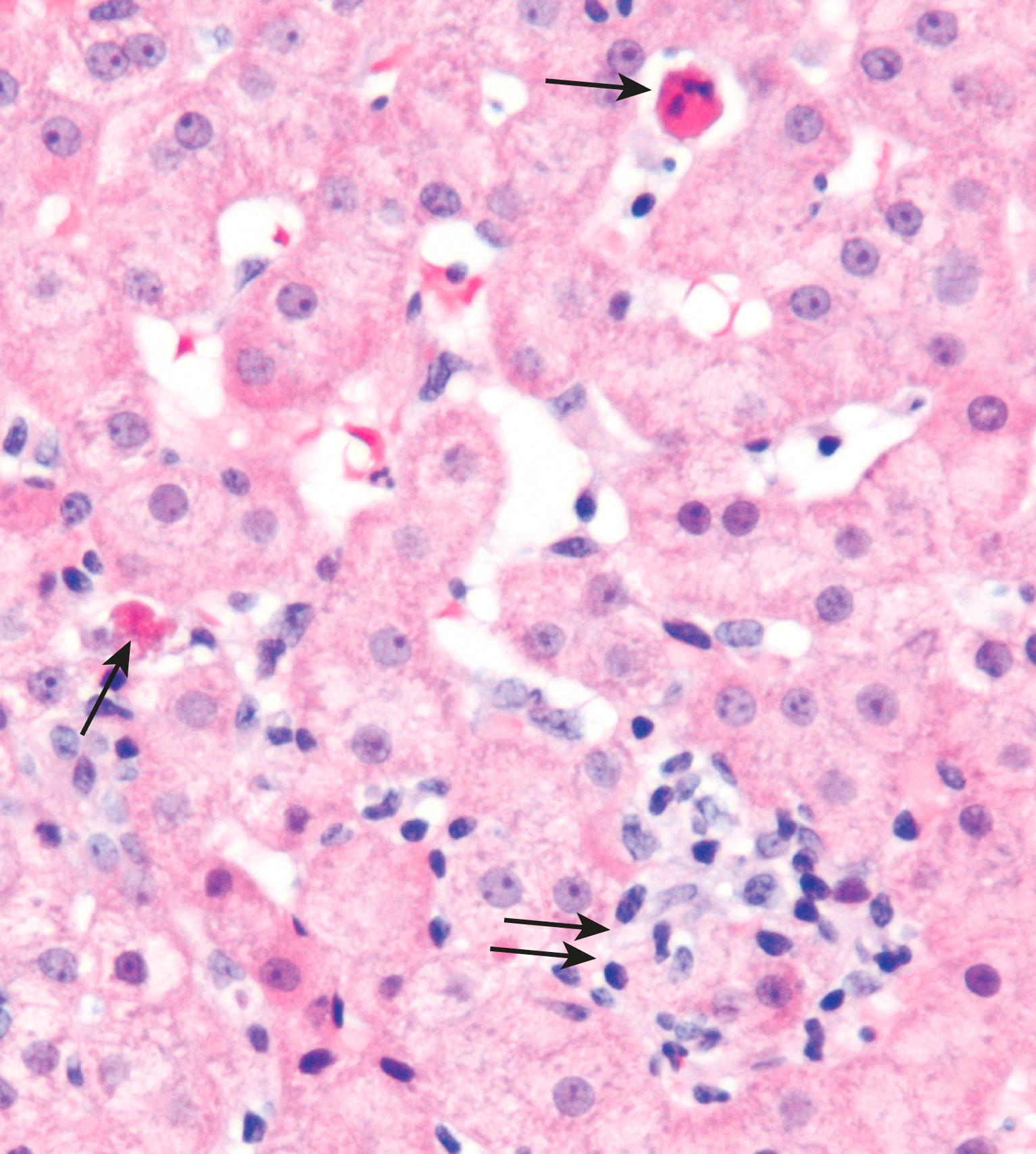
Widespread death of hepatocytes may produce confluent necrosis. This may be seen in acute toxic or ischemic injuries or in severe chronic viral or autoimmune hepatitis. Confluent necrosis begins as a zone of hepatocyte dropout around the central vein. With increasing severity, necrosis “bridges” central veins and portal tracts or adjacent portal tracts.
Regeneration to replace lost hepatocytes takes place primarily by mitotic replication of hepatocytes adjacent to those that have died. In more severe forms of acute liver injury, hepatic stem cells located in a niche near the canal of Hering may also begin to divide. The differentiating progeny of these tissue stem cells produce ductlike structures, called ductular reactions, a morphologic marker of stem cell–mediated liver regeneration.
Scar formation may follow severe acute injury but occurs more often as a reaction to chronic injury. When there is severe injury that causes death of large numbers of hepatocytes and the dropout of liver cells, the underlying reticulin may collapse, precluding orderly regeneration of hepatocytes and laying down of collagen. The principal cell type involved in scar formation is the perisinusoidal hepatic stellate cell. These cells become activated and are converted into highly fibrogenic myofibroblasts, which lay down fibrous septa. Eventually, these fibrous septa encircle surviving, regenerating hepatocytes in late-stage chronic liver disease giving rise to cirrhosis .
Inflammation and immunologic reactions are involved in many forms of liver disease. Systemic inflammation alters the metabolic and biosynthetic activities of the liver, leading to increased secretion of acute-phase reactants such as C-reactive protein, serum amyloid A protein (a precursor of some forms of amyloid), and hepcidin, a key regulator of iron metabolism ( Chapter 10 ). As we will discuss, adaptive immune cells play a critical role in viral hepatitis, with CD4+ and CD8+ T cells being particularly important in the eradication of virus-infected hepatocytes and in causing liver injury in chronic disease.
The most severe clinical consequence of liver disease is liver failure. It occurs primarily in three clinical scenarios: acute, chronic, and acute-on-chronic liver failure.
Acute liver failure is defined as an acute liver illness that produces hepatic encephalopathy within 6 months of the initial diagnosis. In the United States, accidental or deliberate ingestion of acetaminophen accounts for almost 50% of cases of acute liver failure, while autoimmune hepatitis, other drugs and toxins, and acute hepatitis A and B infections account for the remainder of cases. In Asia, acute hepatitis B and E predominate as causes of acute liver failure.
The clinical syndrome of acute liver failure is reflected anatomically and histologically as massive hepatic necrosis. The liver is small and shrunken due to loss of parenchyma ( Fig. 14.4 A ). Microscopically, there are large zones of destruction surrounding occasional islands of regenerating hepatocytes ( Fig. 14.4 B). Scarring is mostly absent because of the acute nature of the process.

Acute liver failure manifests with nausea, vomiting, jaundice, and fatigue, which are followed by the onset of life-threatening encephalopathy, coagulation defects, and portal hypertension associated with ascites. Typically, transaminase levels in the serum are elevated into the thousands. The liver is initially enlarged by swelling and edema related to inflammation, but then as parenchyma is destroyed, the liver shrinks dramatically. Eventually, as hepatocytes are lost, serum transaminase values level off and then decline rapidly as their source disappears. Worsening jaundice, coagulopathy, and encephalopathy develop; with unabated progression, multiorgan failure ensues, potentially resulting in death. Manifestations of acute liver failure include the following:
Jaundice and icterus (yellow discoloration of the skin and sclera, respectively) due to retention of bilirubin, and cholestasis due to systemic retention of bilirubin as well as other solutes eliminated in bile.
Hepatic encephalopathy , with symptoms ranging from subtle behavioral abnormalities to confusion, stupor, coma, and death. Hepatic encephalopathy is believed to be caused by elevated ammonia levels, which correlate with impaired neuronal function and cerebral edema. The principal source of the ammonia is the gastrointestinal tract, where it is produced by microorganisms and by enterocytes during glutamine metabolism. Normally, ammonia is transported in the portal vein to the liver, where it is metabolized in the urea cycle; in severe liver disease, this detoxification mechanism fails. Thus, ammonia enters the systemic circulation. In the CNS, the accumulated ammonia impairs neuronal function and causes cerebral edema. A typical neurologic sign is asterixis, a nonrhythmic, rapid, extension-flexion movement of the head and extremities, best seen as “flapping” of the hands when the arms are held in extension with dorsiflexed wrists.
Coagulopathy. The liver produces a number of coagulation factors whose levels decline in liver failure, leading to easy bruising and bleeding. Paradoxically, disseminated intravascular coagulation ( Chapter 10 ) may also occur due to failure of the damaged liver to remove activated coagulation factors.
Portal hypertension arises when there is diminished flow through the portal venous system, which may occur because of obstruction at the prehepatic, intrahepatic, or posthepatic level. While it can occur in acute liver failure, portal hypertension is more commonly seen with chronic liver failure and is discussed later. In acute liver failure, the obstruction is usually intrahepatic, and its major clinical consequences are ascites and hepatic encephalopathy. In chronic liver disease, portal hypertension develops over months to years, and its effects are more complex and widespread (see later).
Hepatorenal syndrome is a form of renal failure occurring in individuals with acute or chronic liver failure in whom there is no intrinsic renal pathology to account for renal dysfunction. Liver failure results in the production of vasodilators such as nitric oxide that increase blood flow in the abdominal viscera with consequent decreased renal perfusion pressure and reduced glomerular filtration rate. In response to renal hypotension, the renal sympathetic nervous system is activated, as is the renin-angiotensin axis, both of which cause vasoconstriction of the afferent renal arterioles, further decreasing renal perfusion. The syndrome’s onset begins with a decrease in urine output and rising blood urea nitrogen and creatinine levels (azotemia).
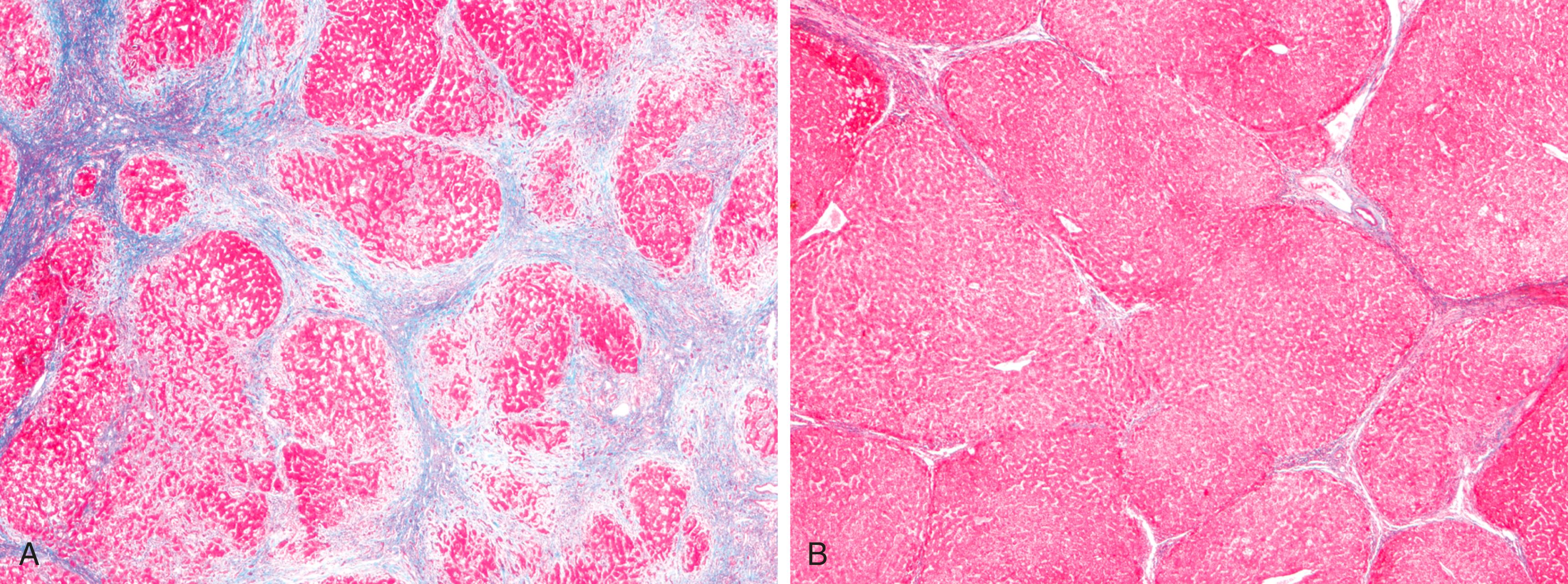
Cirrhosis refers to the diffuse transformation of the liver into regenerative parenchymal nodules surrounded by fibrous bands ( Fig. 14.5 ). It is the morphologic change most often associated with chronic liver disease. The leading causes of chronic liver failure worldwide include chronic hepatitis B, chronic hepatitis C, nonalcoholic fatty liver disease (NAFLD), and alcohol-related liver disease. While cirrhosis is a common feature of a number of chronic liver diseases, it is not a specific entity, and it is important to recognize that (1) not all chronic liver disease terminates in cirrhosis and (2) not all cirrhosis leads to end-stage liver disease. For example, chronic biliary tract diseases often do not lead to cirrhosis even at the end stage, whereas patients with treated autoimmune hepatitis or cured hepatitis C may have adequate liver function despite the presence of cirrhosis. Even in diseases that are likely to give rise to cirrhosis, the morphology and pathophysiology of cirrhosis in each may differ. Thus, while the term cirrhosis implies the presence of severe chronic disease, it is not a specific diagnosis and has variable prognostic implications. There are also some instances in which cirrhosis arises without any clear cause; the term cryptogenic cirrhosis is sometimes applied to such cases.
Cirrhosis is characterized by transformation of the entire liver into regenerative parenchymal nodules surrounded by fibrous bands. The nodular nature of the process is readily evident grossly ( Fig. 14.5 ) and microscopically ( Fig. 14.6 A ). The size of the nodules, the pattern of scarring (linking of portal tracts to each other vs. linking of portal tracts to central veins), the degree of parenchymal loss, and the frequency of vascular thrombosis (particularly of the portal vein) vary between diseases and even, in some cases, between individuals with the same disease.
As mentioned earlier, stem cell activation and differentiation give rise to ductlike structures, the so-called ductular reactions. In chronic liver disease, ductular reactions increase with disease progression and are usually most prominent in cirrhosis.
Regression of fibrosis and even of fully established cirrhosis may follow disease remission or cure. Scars become thinner and more densely compacted, and eventually start to fragment (see Fig. 14.6 B). As fibrous septa break apart, adjacent nodules of regenerating parenchyma coalesce into larger islands. All cirrhotic livers show elements of both progression and regression, with the balance being dictated by the severity and persistence of the underlying disease.
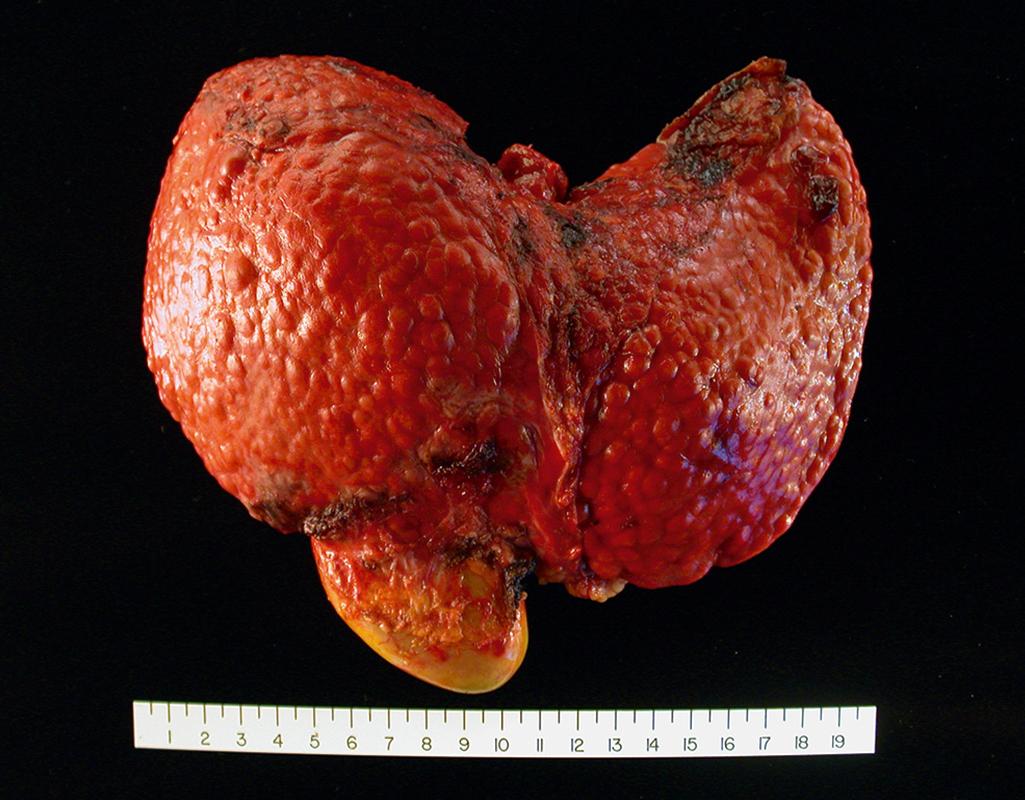
About 40% of individuals with cirrhosis are asymptomatic until the most advanced stages of the disease. Even at late stages, they present with nonspecific clinical manifestations, such as anorexia, weight loss, weakness, and eventually signs and symptoms of liver failure discussed earlier. Jaundice, encephalopathy, and coagulopathy may result from chronic liver disease, much the same as in acute liver failure. However, there are some significant additional features:
Chronic severe jaundice can lead to pruritus (itching), which may be so severe that patients scratch their skin raw and risk repeated bouts of potentially life-threatening infection. Pruritus is also seen in other disorders associated with cholestasis, suggesting that it is related to the accumulation of bile salts in the body; its precise pathogenesis is unknown.
Portal hypertension is more frequent and manifests in more complex ways in chronic liver failure than in acute liver failure ( Fig. 14.7 ). It stems from increased vascular resistance coupled with increased portal blood flow. The increased resistance to portal flow is at the level of the sinusoids and is caused by contraction of vascular smooth muscle cells and myofibroblasts, and disruption of blood flow by scarring and the formation of parenchymal nodules. Increase in portal venous blood flow is due to arterial vasodilation. The increased splanchnic arterial blood flow in turn leads to increased venous efflux into the portal venous system.
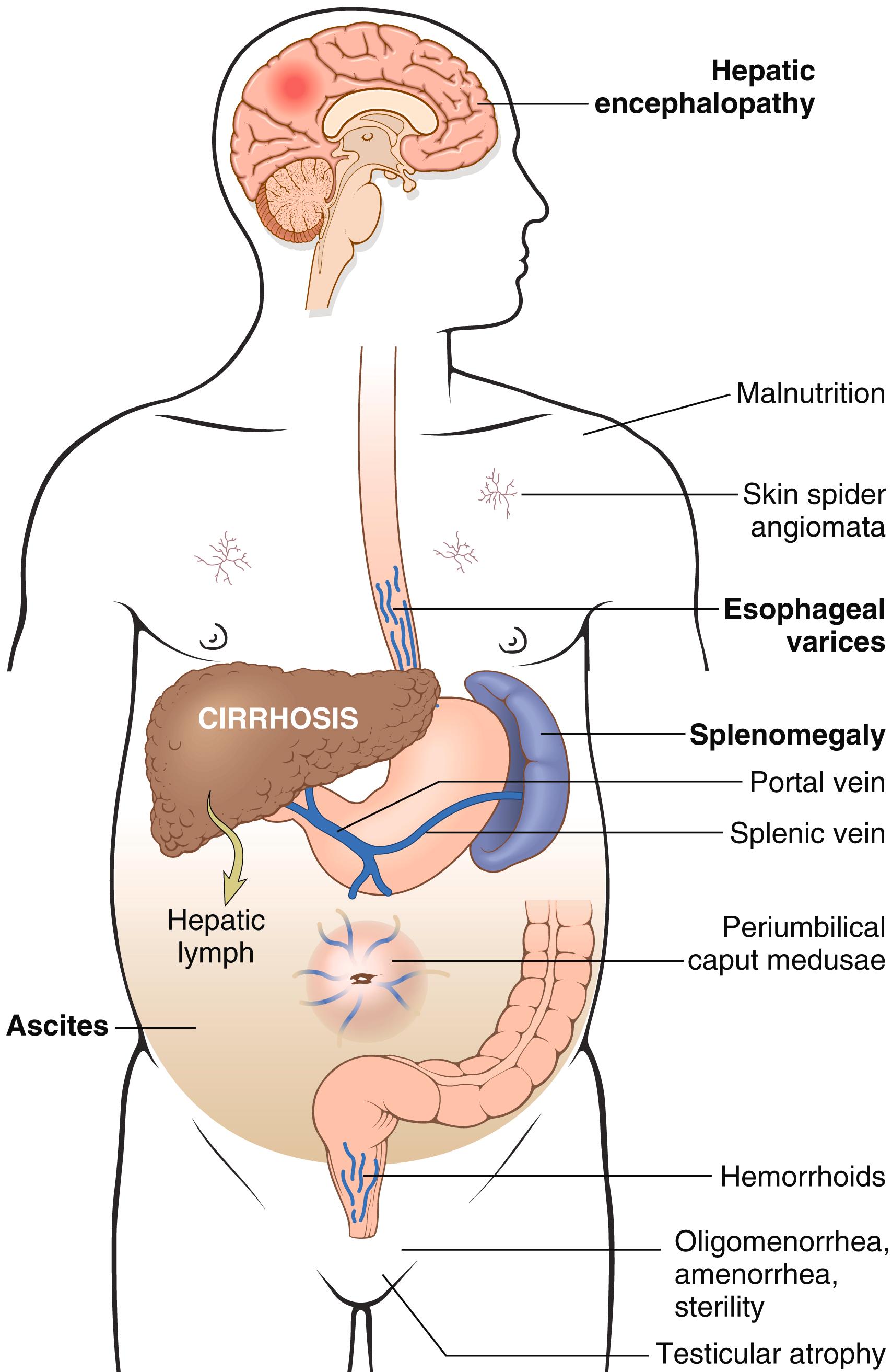
Portosystemic shunts develop due to sustained portal hypertension. These shunts are produced principally by dilation of collateral vessels. Most notably, venous bypasses develop wherever the systemic and the portal circulations share common capillary beds; the most clinically important of these are esophagogastric varices ( Chapter 13 ), which arise in about 40% of individuals with advanced-stage liver disease and may be the source of massive, frequently fatal hematemesis, particularly when there is associated coagulopathy.
Ascites is the accumulation of fluid in the peritoneal cavity. About 85% of cases of ascites are caused by portal hypertension due to cirrhosis. The fluid is a transudate, having less than 3 gm/dL of protein (largely albumin), and a serum-to-ascites albumin gradient of ≥1.1 gm/dL.
Long-standing portal hypertension may cause congestive splenomegaly. The degree of splenic enlargement varies widely, and the splenic weight may reach as much as 1000 gm (five to six times normal), but it is not necessarily correlated with other features of portal hypertension. Splenomegaly may secondarily induce hematologic abnormalities such as thrombocytopenia or even pancytopenia, due to destruction of blood elements by the increased numbers of splenic macrophages. This condition is sometimes referred to as hypersplenism.
Hyperestrogenemia due to impaired estrogen metabolism in male patients with chronic liver failure can give rise to palmar erythema (a reflection of local vasodilatation) and spider angiomas of the skin. Such male hyperestrogenemia also leads to hypogonadism and gynecomastia.
Most chronic liver diseases predispose to development of hepatocellular carcinoma (discussed later).
The course and severity of chronic liver disease with cirrhosis vary widely from patient to patient. Even in those uncommon instances in which cirrhosis regresses following disease remission, portal hypertension may persist due to the presence of irreversible shunts. The most common causes of death are liver failure (as in acute liver disease) and hepatocellular carcinoma. Clinical and laboratory findings are the main criteria used to gauge prognosis and disease progression.
After years of stable, well-compensated, chronic liver disease, some individuals suddenly develop signs of acute liver failure. Hepatic insults that cause sudden decompensation of patients with chronic liver disease include hepatitis D superinfection in those with chronic hepatitis B; emergence of resistance to medical therapy in those with viral hepatitis; and systemic disorders, such as sepsis, acute cardiac failure, or a superimposed toxic injury that tips a well-compensated patient with cirrhosis into liver failure.
The terminology for acute and chronic viral hepatitis can be confusing, because the same word, hepatitis, is used to describe several different entities; careful attention to context can clarify its meaning in each situation. Firstly, hepatitis is applied to diseases caused by viruses (hepatitis A, B, C, D, and E virus) that are hepatotropic, that is, have a specific tropism for the liver. Secondly, hepatitis is applied to patterns of acute and chronic hepatic injuries produced by other viruses (such as Epstein-Barr virus [EBV], cytomegalovirus [CMV], and yellow fever) as well as autoimmune reactions, drugs, and toxins. In this section, we will focus on the main features of hepatotropic viruses, which are summarized in Table 14.2 , and we will then discuss the clinicopathologic characteristics of acute and chronic viral hepatitis.
| Virus | Hepatitis A (HAV) | Hepatitis B (HBV) | Hepatitis C (HCV) | Hepatitis D (HDV) | Hepatitis E (HEV) |
|---|---|---|---|---|---|
| Viral genome | ssRNA | Partially dsDNA | ssRNA | Circular defective ssRNA | ssRNA |
| Viral family | Hepatovirus; related to picornavirus | Hepadnavirus | Flaviviridae | Subviral particle in Deltaviridae family | Hepeviridae family, Hepevirus genus |
| Route of transmission | Fecal-oral (contaminated food or water) | Parenteral, sexual contact, perinatal | Parenteral; intranasal cocaine use is a risk factor | Parenteral | Fecal-oral |
| Incubation period | 2–6 weeks | 2–26 weeks (mean 8 weeks) | 4–26 weeks (mean 9 weeks) | Same as HBV | 4–5 weeks |
| Frequency of chronic liver disease | Never | 5%–10% | >80% | 10% (coinfection); 90%–100% for superinfection | In immunocompromised hosts only |
| Diagnosis | Detection of serum IgM antibodies | Detection of HBsAg or antibody to HBcAg; PCR for HBV DNA | ELISA for antibody detection; PCR for HCV RNA | Detection of IgM and IgG antibodies, HDV RNA in serum, or HDAg in liver biopsy | Detection of serum IgM and IgG antibodies; PCR for HEV RNA |
HAV infection is usually benign and self-limited, does not cause chronic hepatitis, and rarely (in about 0.1% of cases) produces fulminant hepatitis. HAV has an incubation period of 2 to 6 weeks. It is typically cleared by the host immune response, so it does not establish a carrier state. The infection occurs throughout the world and is endemic in countries with poor healthcare infrastructure. Acute HAV tends to cause a febrile illness associated with jaundice and nonspecific symptoms such as fatigue and loss of appetite. Overall, HAV accounts for about 25% of acute hepatitis worldwide. It does not cause chronic hepatitis.
HAV is a nonenveloped, positive-strand RNA picornavirus that occupies its own genus, Hepatovirus . It is spread by ingestion of contaminated water and food and is shed in the stool for 2 to 3 weeks before and 1 week after the onset of jaundice. Thus, close personal contact with an infected individual or fecal-oral contamination accounts for most cases and explains outbreaks in institutional settings such as schools and nurseries, as well as waterborne epidemics in places where people live in overcrowded, unsanitary conditions. HAV can also be detected in serum and saliva of infected individuals.
In high-income countries, sporadic infections may be contracted by the consumption of raw or steamed shellfish that have concentrated the virus from seawater contaminated with human sewage. Infected workers in the food industry are another source of outbreaks. HAV itself does not seem to be cytopathic. The cellular immune response, particularly that involving cytotoxic CD8+ T cells, plays a key role in HAV-mediated hepatocellular injury.
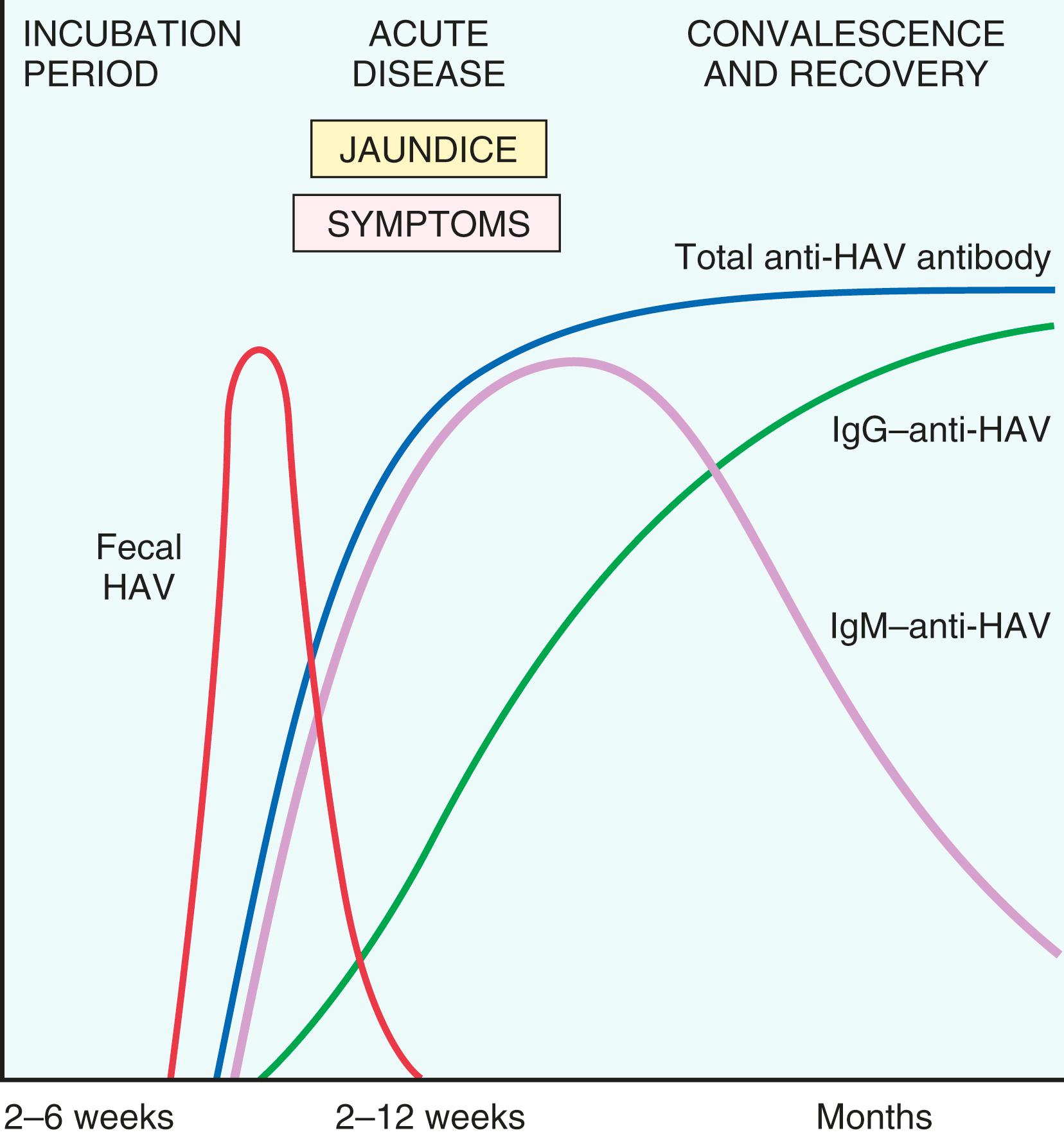
Because HAV viremia is transient, bloodborne transmission is very rare; therefore, donated blood is not specifically screened for this virus. IgM antibody against HAV appears in the blood at the onset of symptoms and is a reliable marker of acute infection ( Fig. 14.8 ). Fecal shedding of the virus ends as the IgM titer rises. The IgM response usually declines in a few months followed by the appearance of IgG anti-HAV that persists for years, often conferring lifelong immunity. The HAV vaccine, available since 1995, is effective in preventing infection. Hepatitis A rates have declined by more than 95% since the introduction of the vaccine; there are now about 2800 cases annually in the United States.
The outcome of HBV infection varies widely and includes: (1) acute hepatitis with recovery and clearance of the virus; (2) nonprogressive chronic hepatitis; (3) progressive chronic disease ending in cirrhosis; (4) fulminant hepatitis with massive liver necrosis; or (5) an asymptomatic “healthy” carrier state. HBV-induced chronic liver disease is also an important precursor for the development of hepatocellular carcinoma.
Liver disease due to HBV infection is an enormous global health problem. One-third of the world’s population (2 billion individuals) has been infected with HBV, and 250 million individuals have chronic infections. Seventy-five percent of chronic carriers live in Asia and the Western Pacific rim. The global prevalence of chronic hepatitis B infection varies from greater than 8% in parts of Africa to less than 2% in Western Europe, North America, and Australia. In Africa the prevalence is highest in West Africa.
The mode of transmission of HBV also varies with the geographic locale. In high-prevalence regions of the world, perinatal transmission during childbirth accounts for 90% of cases. In areas with intermediate prevalence, horizontal transmission, especially in early childhood, dominates. Spread among children usually occurs through minor breaks in the skin or mucous membranes following physical contact with infected individuals. In low-prevalence areas, unprotected sex and intravenous substance use are the chief modes of spread. Transfusion-related spread has been reduced greatly by screening of donated blood for HBsAg and by stopping the practice of paying blood donors. Vaccination induces a protective antibody response in 95% of individuals.
HBV is a member of Hepadnaviridae, a family of DNA viruses that cause hepatitis in multiple animal species. The HBV genome is a partially double-stranded, 3200-nucleotide, circular DNA with four open reading frames, which encode the following proteins:
Nucleocapsid “core” protein (HBcAg, hepatitis B core antigen) and a longer polypeptide with a precore and core region, designated HBeAg (hepatitis B e antigen). The precore region directs the secretion of the HBeAg polypeptide into blood, whereas HBcAg remains in hepatocytes, where it participates in the assembly of virions.
Envelope glycoproteins (HBsAg, hepatitis B surface antigen). Infected hepatocytes synthesize and secrete massive quantities of noninfective envelope glycoproteins (mainly small HBsAg).
A polymerase (Pol) with both DNA polymerase activity and reverse transcriptase activity, which enables genomic replication to occur through a unique DNA → RNA → DNA cycle via an intermediate RNA template. This unusual polymerase is the target of drugs used to treat hepatitis B infection (described later).
HBx protein, which is required for virus replication and may act as a transcriptional transactivator for viral genes and a wide variety of host genes. It has been implicated in the pathogenesis of HBV-associated liver cancer.
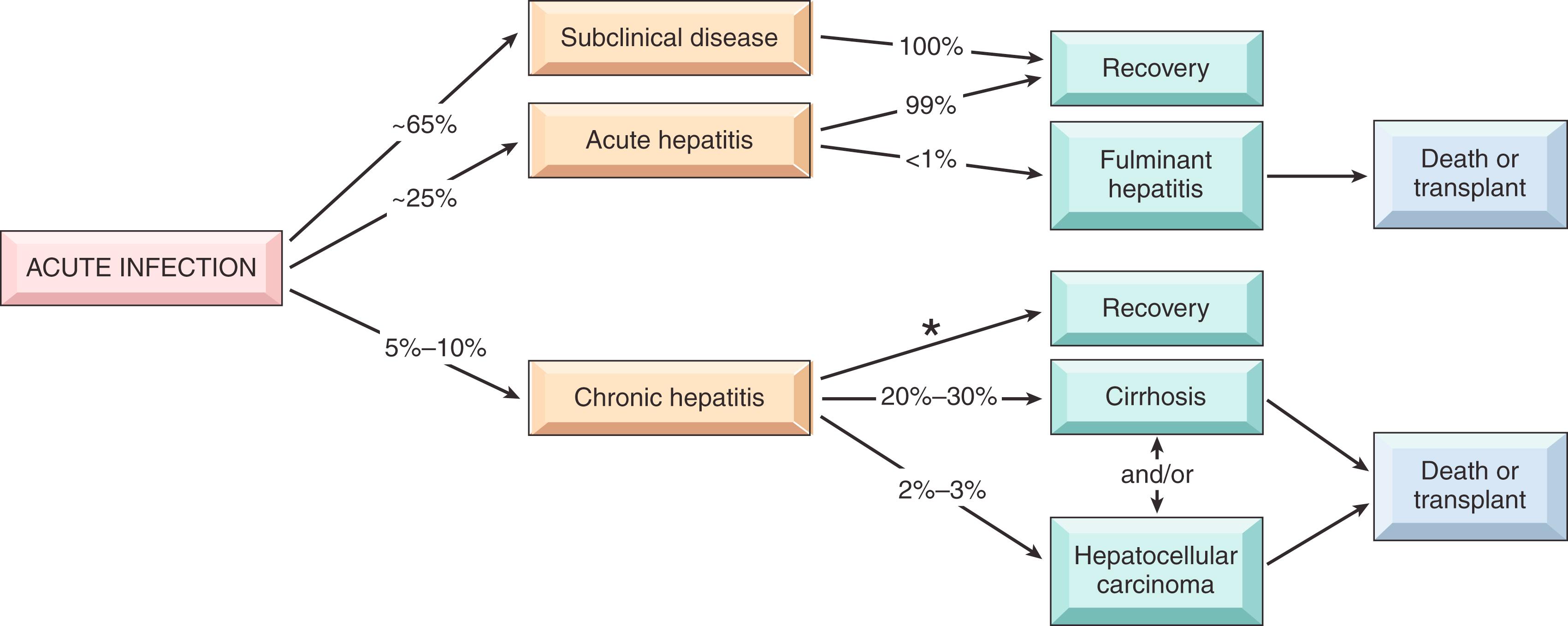
HBV has a long incubation period (2–26 weeks). Unlike HAV, HBV remains in the blood during active episodes of acute and chronic hepatitis. Approximately 65% of adults with newly acquired HBV have mild or no symptoms and do not develop jaundice (acute anicteric hepatitis). The remaining 25% have nonspecific constitutional symptoms such as anorexia, fever, jaundice, and right upper quadrant pain (acute icteric hepatitis). Fulminant hepatitis and chronic hepatitis are uncommon, occurring in approximately 0.1% to 0.5% and 5% to 10%, respectively, of individuals who are acutely infected.
In most cases, the infection is self-limited and resolves without treatment, but chronic disease develops in 5% to 10% of individuals who are infected. The risk of chronic infection is inversely related to age and is greatest (approximately 90%) in infants who are exposed to the virus through transmission from their mothers at birth. Chronic hepatitis may progress to cirrhosis, and a subset develop hepatocellular carcinoma. The approximate frequencies of various clinical outcomes of HBV infection are depicted in Fig. 14.9 .
The host immune response is the main determinant of the outcome of the infection. Innate immune mechanisms, particularly the production of interferon (IFN)-α, protect the host during initial phases of the infection, and a strong response by virus-specific CD4+ and CD8+ interferon γ–producing cells is associated with the resolution of acute infection. Like HAV, HBV is generally not directly hepatotoxic, and most hepatocyte injury is caused by CD8+ cytotoxic T cells attacking infected cells.
The course of the disease can be followed clinically by monitoring certain serum markers ( Fig. 14.10 ).
HBsAg appears before the onset of symptoms, peaks during symptomatic disease, and then usually declines to undetectable levels in 12 weeks (though it may occasionally persist for as long as 24 weeks). By contrast, HBsAg persists in cases that progress to chronicity.
Anti-HBs antibody appears after the acute disease is over and is usually not detected until a few weeks to several months after HBsAg disappears. Anti-HBs antibodies may persist for life and confer protection, which is the rationale for HBsAg-containing vaccines. By contrast, anti-HBs antibodies are not produced in cases that progress to chronic liver disease
HBeAg and HBV DNA appear in serum soon after HBsAg and signify ongoing viral replication. Persistence of HBeAg is an indicator of progression to chronic hepatitis. The appearance of anti-HBe antibodies implies that an acute infection has peaked and is waning.
IgM anti-HBc becomes detectable in serum shortly before the onset of symptoms, concurrent with the onset of elevated serum aminotransferase levels (indicative of hepatocyte destruction). Over a period of months, the IgM anti-HBc antibody is replaced by IgG anti-HBc.

Treatment of chronic hepatitis B with HBV polymerase inhibitors and IFN-α can slow disease progression, reduce liver damage, and prevent liver cirrhosis or liver cancer but does not eliminate the infection.
HCV is a major cause of chronic liver disease, with approximately 170 million individuals affected worldwide. Approximately 2.7 million Americans have chronic HCV infection. HCV is a blood-borne infection. Notably, there has been a decrease in the annual incidence of infection from a mid-1980s peak of over 230,000 new infections per year to 17,000 new infections per year currently, due primarily to a reduction in transfusion-associated cases as a result of effective screening procedures. It is worrisome that these gains made may not be sustained. There is a recent increase in new HCV infections primarily due to the ongoing opioid epidemic and the associated injection drug use. Until recently, the number of patients with chronic infection appeared likely to continue to increase, but new therapies (discussed later) are improving the outlook.
The risk factors for HCV infection are as follows:
Intravenous substance use
Needlestick injury
Perinatal transmission of HCV at the time of birth occurs in about 5% to 6% of infants born to HCV infected women.
Currently, transmission of HCV by blood transfusion is close to zero in the United States; the risk for acquiring HCV by needlestick is about six times higher than that for HIV (1.8 vs. 0.3%). The efficiency of HCV transmission by sexual intercourse is low, as is transmission by household contacts. One-third of individuals have no identifiable risk factors, an enduring mystery.
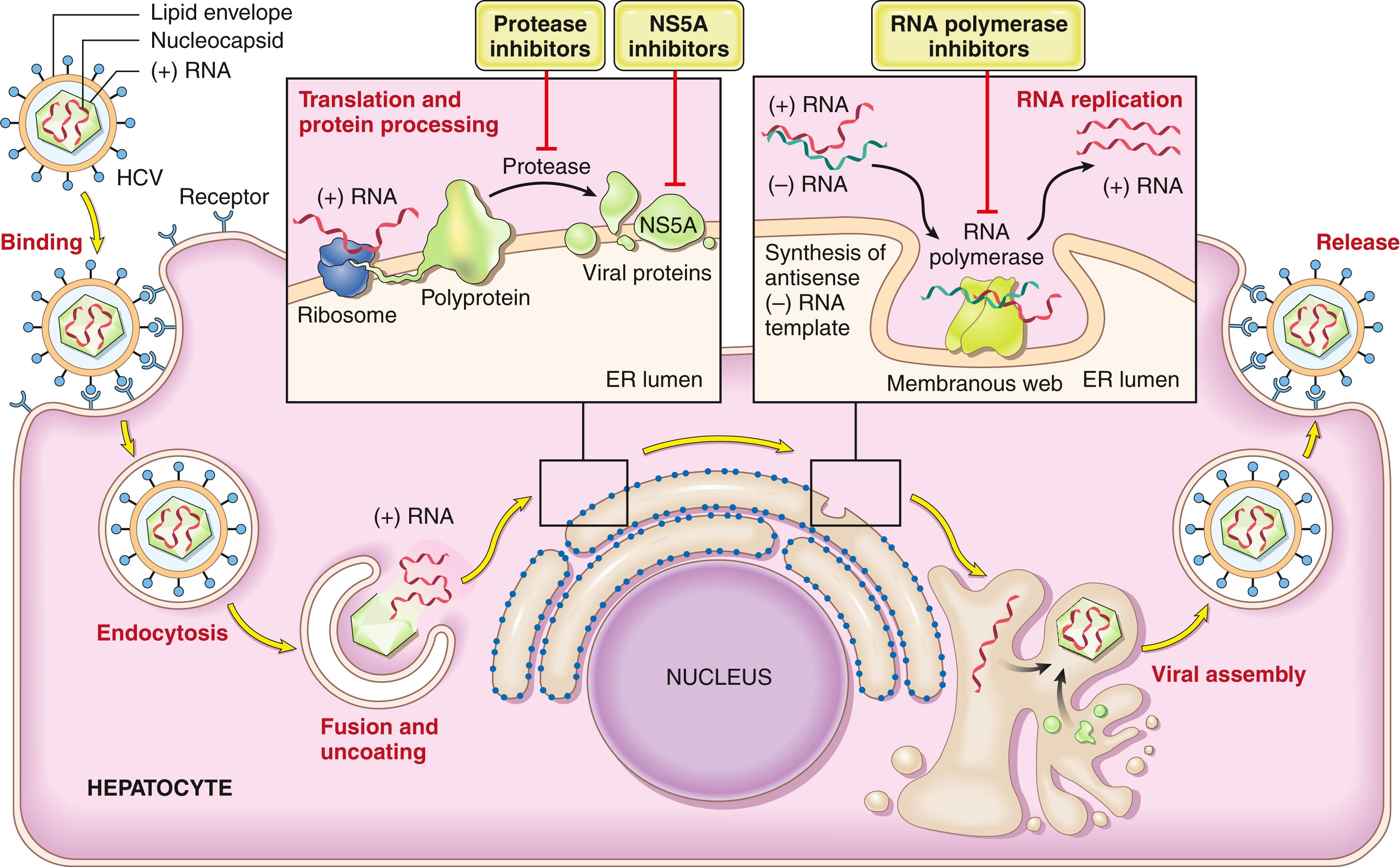
HCV is a member of the Flaviviridae family. Just as with HIV, an understanding of viral replication and assembly has facilitated the development of highly effective anti-HCV drugs (described below). HCV is an enveloped, single-stranded RNA virus with a genome encoding a single polyprotein that is processed by several proteases into 10 functional proteins. Included among these proteins is a protease that is needed for complete processing of the polyprotein; NS5A, which is essential for assembly of HCV into mature virions; and a RNA polymerase that is necessary for replication of the viral genome ( Fig. 14.11 ). Because of the inability of the host immune response to eliminate HCV and the low fidelity of the HCV RNA polymerase, new genetic variants develop at a rapid pace. This has led to the appearance of seven major HCV genotypes worldwide, each with one or more “subspecies.” Infections in most individuals are due to a virus of a single genotype, but new genetic variants are generated in the host while viral replication persists. As a result, each patient usually comes to be infected with a population of divergent but closely related HCV variants known as quasispecies.
The incubation period for HCV ranges from 4 to 26 weeks, with a mean of 9 weeks. In about 85% of individuals, the acute infection is asymptomatic and goes unrecognized. HCV RNA is detectable in blood for 1 to 3 weeks, coincident with elevations in serum transaminases ( Fig. 14.12 ). The clinical course of acute HCV hepatitis is milder than that of HBV; severe acute hepatitis is rare.
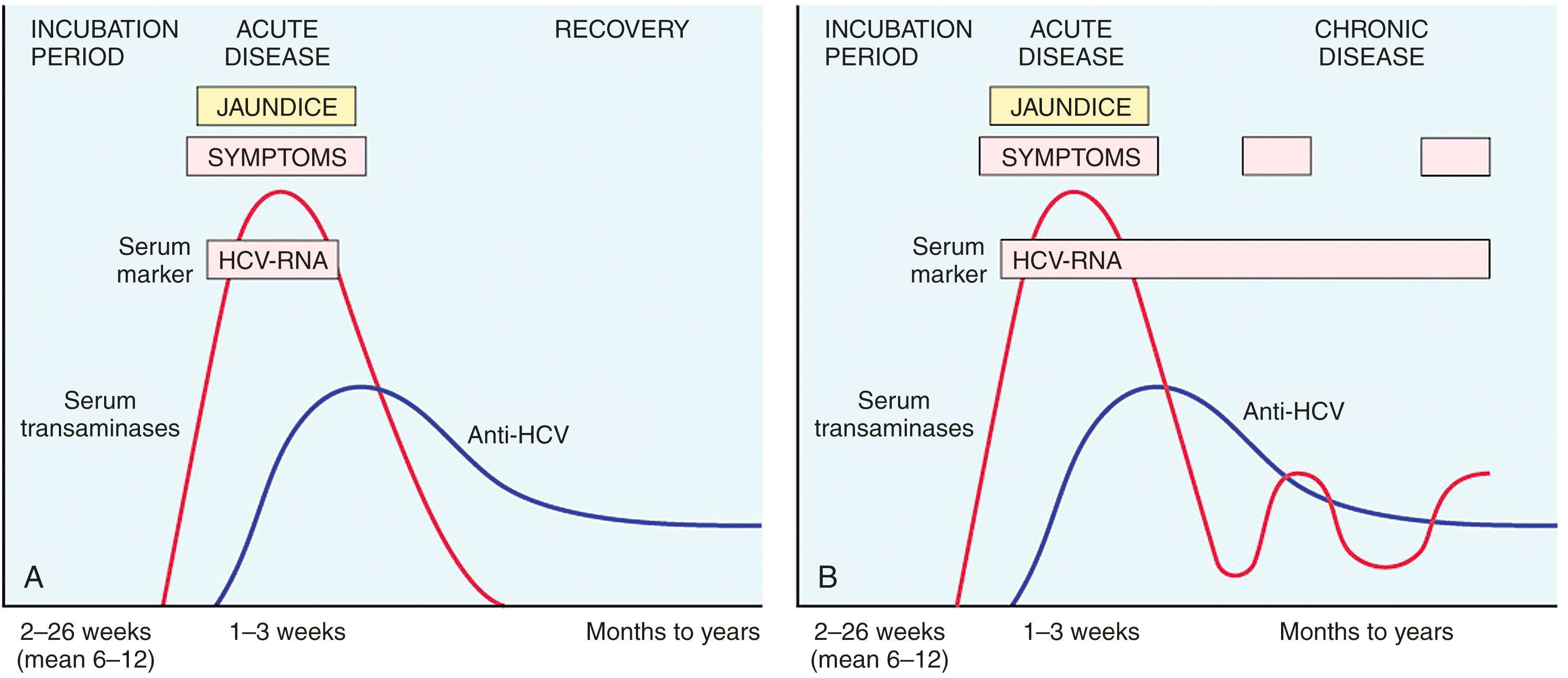
Persistent infection and chronic hepatitis are the hallmarks of HCV infection, despite the generally asymptomatic nature of the acute illness. In contrast to HBV, chronic disease occurs in the majority of individuals infected with HCV (80%–90%), and cirrhosis eventually occurs in approximately 20% over a period of 20 to 30 years. The mechanisms leading to chronicity are not well understood. Older age, male gender, alcohol use, immunosuppressive drugs, hepatitis B/HIV coinfection, and diseases associated with insulin resistance, including obesity, type 2 diabetes, and metabolic syndrome, have been associated with progression. Those who develop cirrhosis are at risk for development of hepatocellular carcinoma. Although the overall risk is small, in the United States, HCV is responsible for about one-third of cases of liver cancer.
In chronic HCV infection, circulating HCV RNA persists in 90% of patients despite the presence of neutralizing antibodies (see Fig. 14.12 B). Hence, testing for HCV RNA is done to confirm the diagnosis of chronic HCV infection. A characteristic clinical feature of chronic HCV infection is episodic elevations in serum aminotransferases separated by periods of normal or near-normal enzyme levels. However, even patients infected by HCV who have normal transaminases are at high risk for developing permanent liver damage, and anyone with detectable serum HCV RNA needs treatment and long-term medical follow-up.
Fortunately, recent years have seen dramatic improvements in treatment of HCV infection that stem from development of drugs that specifically target the viral protease, RNA polymerase, and NS5A protein, all of which are required for production of virus (see Fig. 14.11 ). Combination therapy with these drugs (a strategy akin to triple drug therapy for HIV) is remarkably effective. The goal of current treatment is to eradicate HCV, which is defined by the absence of detectable HCV RNA in the blood after treatment is stopped. Currently, over 95% of HCV infections are curable.
Also called delta agent, HDV is a unique RNA virus that is dependent for its life cycle on HBV. Infection with HDV arises in the following settings:
Coinfection occurs following exposure to serum containing both HDV and HBV. Coinfection can result in a clinical syndrome that is indistinguishable from acute hepatitis B. It is self-limited and is usually followed by clearance of both viruses. However, there is a higher rate of acute hepatic failure in individuals who use intravenous drugs.
Superinfection occurs when a chronic carrier of HBV is exposed to a new inoculum of HDV. This results in disease 30 to 50 days later, presenting either as severe acute hepatitis in a previously asymptomatic HBV carrier, or as an exacerbation of chronic hepatitis B infection. Chronic HDV infection occurs in more than 80% of superinfections and may have two phases: (1) an acute phase with active HDV replication and suppression of HBV associated with high transaminase levels and (2) a chronic phase in which HDV replication decreases, HBV replication increases, and transaminase levels fluctuate.
HDV infection occurs worldwide and affects an estimated 15 million individuals (about 5% of the 300 million individuals infected by HBV). Its prevalence varies, being highest in the Amazon basin, Africa, the Middle East, and Southern Italy, and lowest in Southeast Asia and China. In most higher-income countries, it is largely restricted to individuals who use intravenous drugs and those who have had multiple blood transfusions. Coinfection with HDV and HBV increases the risk of progression to cirrhosis and HCC.
HDV RNA is detectable in the blood and liver at the time of onset of acute symptomatic disease. Anti-HDV IgM is a reliable indicator of recent HDV exposure but is frequently short-lived. Acute coinfection by HDV and HBV is associated with the presence of IgM against HDAg and HBcAg (denoting new infection with hepatitis B). When chronic hepatitis arises from HDV superinfection, HBsAg is present in serum, and anti-HDV antibodies (IgG and IgM) persist for months or longer. Because of its dependency on HBV, HDV infection is prevented by vaccination against HBV.
HEV is an enterically transmitted, waterborne infection that usually produces a self-limiting disease. The virus typically infects young to middle-aged adults. HEV is a zoonotic disease with animal reservoirs that include monkeys, cats, pigs, and dogs. Epidemics have been reported in Asia and the Indian subcontinent, sub-Saharan Africa, and Mexico, and sporadic cases are seen in the United States, Canada, and Europe, particularly where pig farming is common, and in travelers returning from regions of high incidence. Of greater importance, HEV infection accounts for 30% to 60% of cases of sporadic acute hepatitis in India, exceeding the frequency of HAV. A characteristic feature of HEV infection is the high mortality rate among pregnant women, approaching 20%. In most cases, HEV is not associated with chronic liver disease or persistent viremia. The average incubation period following exposure is 4 to 5 weeks.
HEV is an unenveloped, positive-stranded RNA virus in the Hepeviridae family ( Hepevirus genus). Virions are shed in stool during the acute illness. Before the onset of clinical illness, HEV RNA and HEV virions can be detected by PCR in stool and serum. The onset of rising serum aminotransferases, clinical illness, and elevated IgM anti-HEV titers are virtually simultaneous. Symptoms resolve in 2 to 4 weeks, during which time the IgM titers fall and anti-HEV IgG titers rise.
As already discussed, infection with hepatitis viruses produces a wide range of outcomes. Acute infection by each of the hepatotropic viruses may be symptomatic or asymptomatic. HAV and HEV do not cause chronic hepatitis, and only a small number of adults infected with HBV develop chronic hepatitis. By contrast, HCV commonly causes chronic infections. Fulminant hepatitis is unusual and is seen primarily with HAV, HBV, or HDV infections. HEV can cause acute liver failure in pregnant women. Although HBV and HCV are responsible for most cases of chronic hepatitis, there are many other causes of similar clinicopathologic presentations, including autoimmune hepatitis and drug- and toxin-induced hepatitis (discussed later). Therefore, serologic and molecular studies are essential for the diagnosis of viral hepatitis and for distinguishing the various types.
Major features of the main clinicopathologic syndromes associated with hepatitis viruses are as follows:
Acute asymptomatic infection with recovery. Patients in this group are identified incidentally because of elevated serum transaminases or the presence of antiviral antibodies. HAV and HBV infections, particularly in childhood, are frequently subclinical.
Acute symptomatic infection with recovery. Acute disease follows a similar course for all viruses and consists of (1) an incubation period of variable length (see Table 14.2 ); (2) a symptomatic preicteric phase; (3) a symptomatic icteric phase; and (4) convalescence. Peak infectivity occurs during the last asymptomatic days of the incubation period and the early days of acute symptoms.
Acute liver failure. Viral hepatitis is responsible for about 10% of cases of acute hepatic failure. HAV is the most common cause worldwide, but HBV is more common in Asia and the Mediterranean. Survival for more than 1 week may permit recovery to occur via replication of residual hepatocytes.
Chronic hepatitis is defined as symptomatic, biochemical, or serologic evidence of continuing or relapsing hepatic disease for more than 6 months. In some patients, the only signs of chronic disease are elevations of serum transaminases. Laboratory studies may reveal impaired liver functions such as prolongation of the prothrombin time and hyperbilirubinemia. Occasionally, in cases of HBV and HCV, immune complex disease develops that results in vasculitis ( Chapter 8 ) and glomerulonephritis ( Chapter 12 ). Cryoglobulinemia is found in about 35% of individuals with chronic hepatitis C.
Carrier state. A “carrier” is an individual who harbors and can transmit an organism but has no symptoms. Carriers include (1) individuals who harbor the virus but have no liver disease and (2) individuals who harbor the virus and have asymptomatic nonprogressive liver damage. In both cases, particularly the latter, affected individuals constitute reservoirs of infection. HBV infection acquired early in life in endemic areas (such as Southeast Asia, China, and sub-Saharan Africa) gives rise to a carrier state in more than 90% of cases, whereas in nonendemic regions the carrier state is rare.
Because of their similar transmission modes and overlapping risk factors, coinfection of HIV and hepatitis viruses is a common clinical problem. In the United States, 10% of individuals who are infected with HIV are coinfected with HBV and 25% with HCV, and, when untreated, chronic HBV and HCV infection are important causes of morbidity and mortality in these individuals. However, in adequately treated immunocompetent patients with HIV, the severity and progression of HBV and HCV infection and response to anti–hepatitis virus therapy resembles that seen in individuals who are not infected with HIV.
The morphologic changes in acute and chronic viral hepatitis are shared among the hepatotropic viruses and can be mimicked by drug reactions or autoimmune hepatitis. They are depicted schematically in Fig. 14.13 .
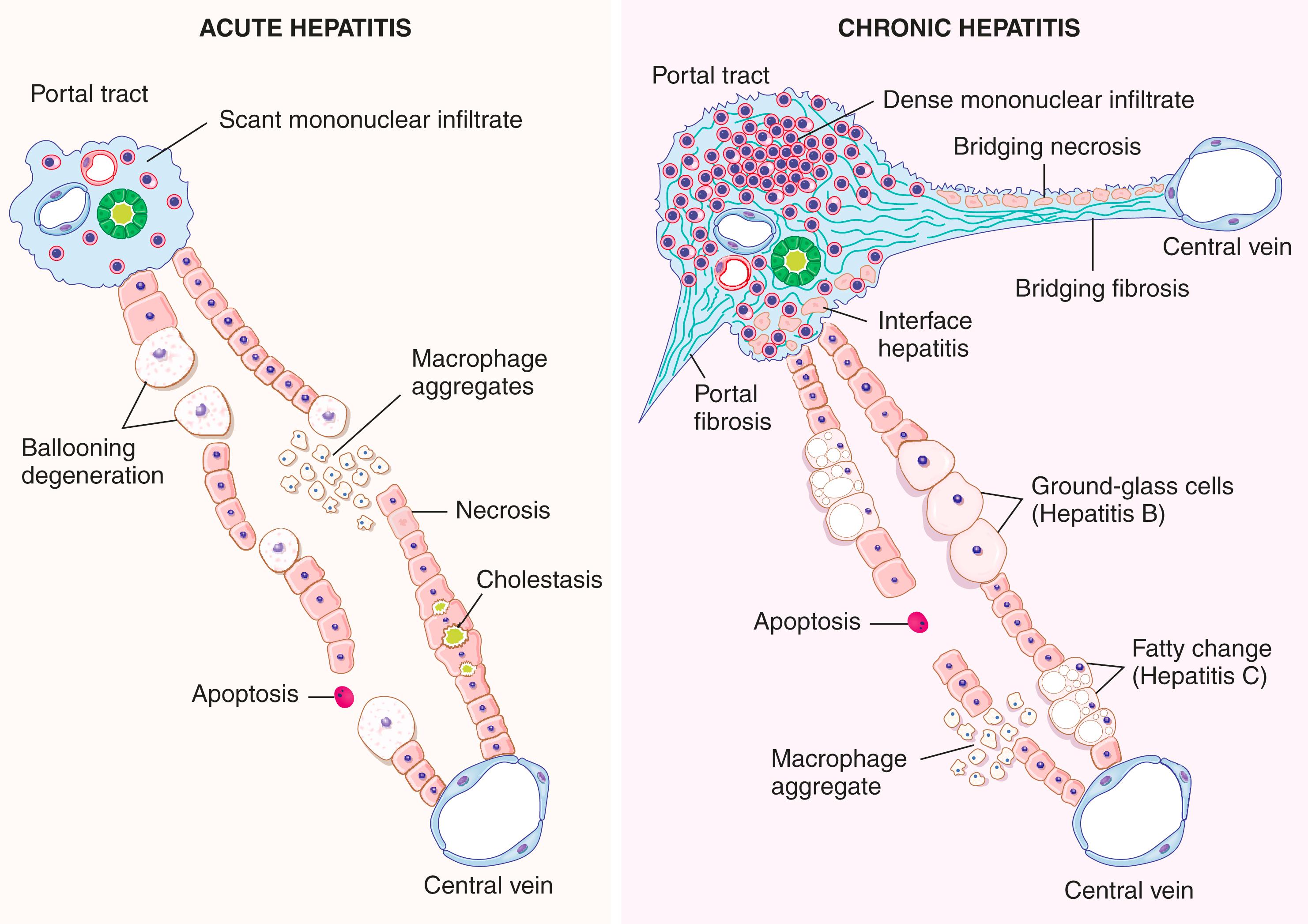
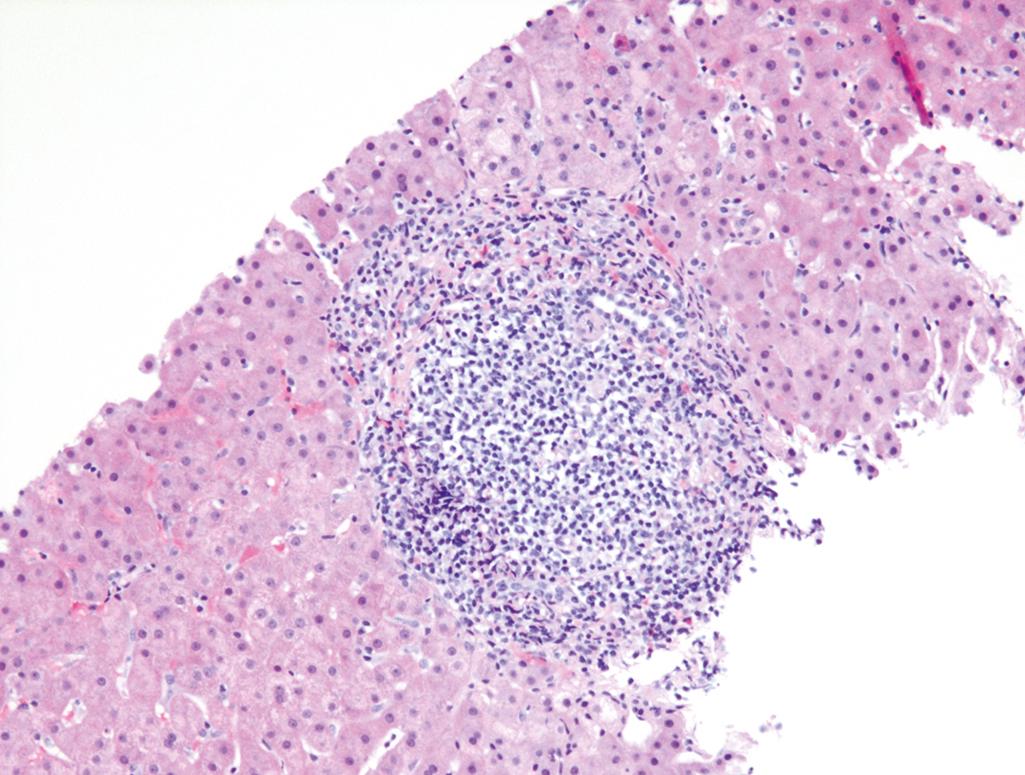
In acute viral hepatitis, the liver may be normal in size, enlarged (due to inflammation), or shrunken (massive liver necrosis due to acute liver failure; see Fig. 14.4 ). Microscopically, there is a portal and lobular inflammatory infiltrate comprising predominantly lymphocytes variably admixed with plasma cells and eosinophils. The hepatocyte injury may result in necrosis or apoptosis (see Figs. 14.2 and 14.3 ). Necrosis of groups of hepatocytes (i.e., confluent necrosis) may be seen in severe cases and can progress to necrosis of the entire lobule (i.e., panlobular or panacinar necrosis) or to connect vascular structures (i.e., bridging necrosis). Liver failure can develop with massive hepatic necrosis.
In chronic viral hepatitis, the defining histologic feature is portal lymphocytic or lymphoplasmacytic inflammation with fibrosis. The inflammatory cells often cross the limiting plate and injure periportal hepatocytes (interface activity). This may be accompanied by a variable degree of lobular inflammation. Fibrosis develops with increasing liver damage, manifesting initially as portal and periportal fibrosis. Fibrous septa develop and lead to portoportal bridging fibrosis, and eventually cirrhosis.
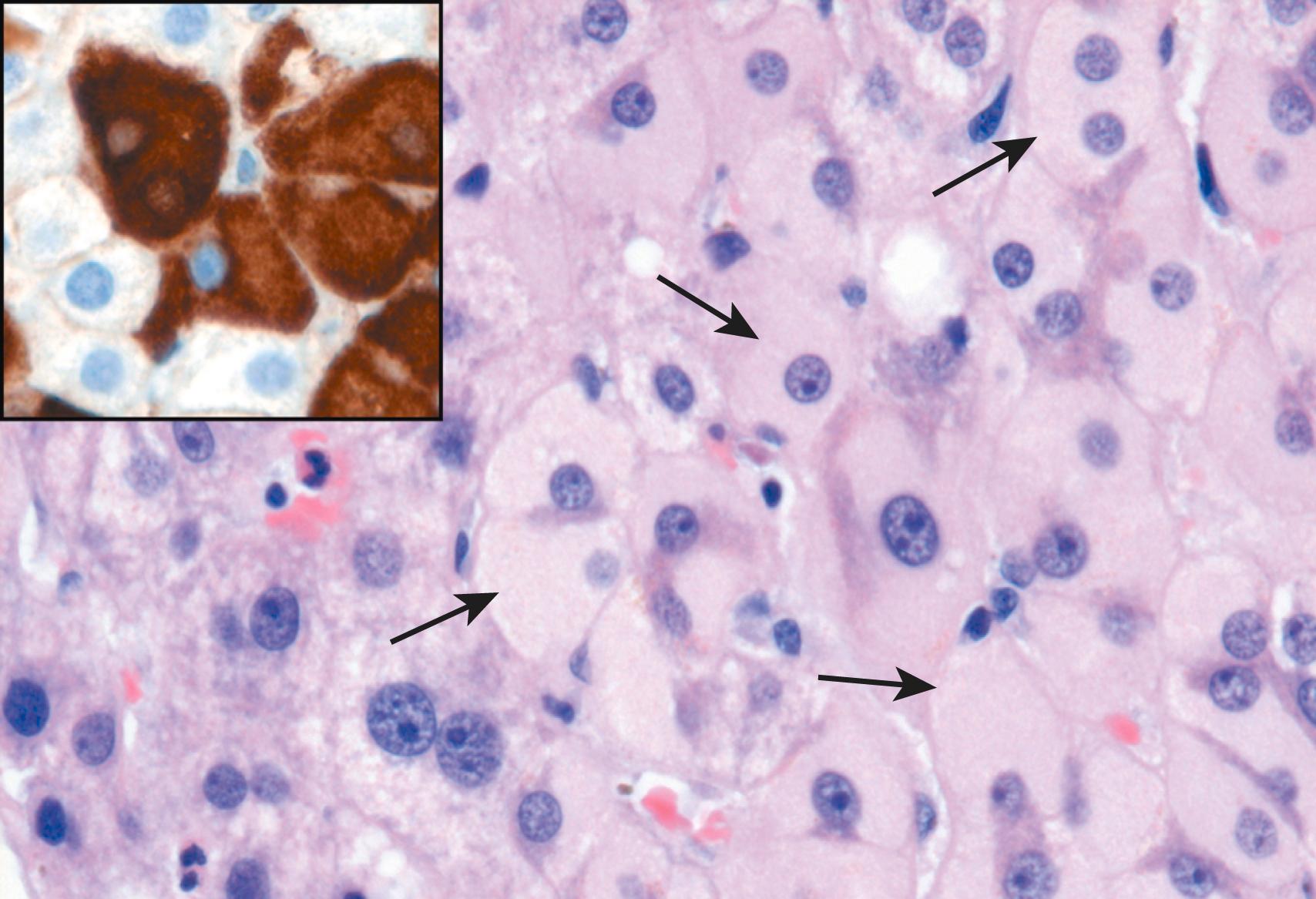
Certain histologic features point to specific viral etiologies in chronic hepatitis. In chronic hepatitis B, “ground-glass” hepatocytes (cells with endoplasmic reticulum swollen by HBsAg) are a diagnostic hallmark, and the presence of viral antigen in these cells can be confirmed by immunostaining ( Fig. 14.14 ). Liver biopsies involved by chronic hepatitis C commonly show large lymphoid aggregates ( Fig. 14.15 ). Often, hepatitis C is associated with fatty change in scattered hepatocytes. Bile duct injury is also prominent in some cases of hepatitis C and may mimic the histologic changes seen in primary biliary cholangitis (see later); clinical parameters easily distinguish these two diseases, however.
A multitude of organisms can infect the liver and biliary tree, including bacteria, fungi, helminths and other parasites, and protozoa. Infectious organisms can reach the liver through several pathways:
Ascending infection, via the gut and biliary tract (ascending cholangitis)
Vascular seeding, most often through the portal system via the gastrointestinal tract
Direct invasion, from an adjacent source (e.g., bacterial cholecystitis)
Penetrating injury
Bacteria that may establish an infection in the liver via the blood include Staphylococcus aureus in toxic shock syndrome, Salmonella typhi in typhoid fever, and Treponema pallidum in secondary or tertiary syphilis. Ascending infections are most common in the setting of partial or complete biliary tract obstruction and are typically caused by gut flora, which can grow in the ducts. Whatever the source of the bacteria, pyogenic organisms can cause intrahepatic abscesses, producing fever, right upper quadrant pain, and tender hepatomegaly. Although antibiotic therapy may sterilize small abscesses, surgical drainage is often necessary for larger lesions. More commonly, extrahepatic bacterial infections, particularly sepsis, induce mild hepatic inflammation and varying degrees of hepatocellular cholestasis indirectly, without establishing an infectious nidus in the liver.
Other nonbacterial infectious agents cause liver disease with important or unusual pathogenic features that merit specific comment. These include the following:
Schistosomiasis, most commonly found in Asia, Africa, and South America. Adult worms in the gut produce numerous eggs, some of which enter the portal circulation, where they lodge and induce a granulomatous reaction associated with marked fibrosis.
Entamoeba histolytica, an important cause of dysentery ( Chapter 13 ), sometimes ascends to the liver through the portal circulation and produces secondary foci of infection that can progress to large necrotic areas called amebic liver abscesses. Amebic abscesses are more common in the right lobe of the liver. The abscess cavity contains necrotic liver cells, but unlike pyogenic abscesses, neutrophils are absent.
Liver fluke infection, most common in Southeast Asia, is associated with a high rate of cholangiocarcinoma. Responsible organisms include Fasciola hepatica , Opisthorchis species, and Clonorchis sinensis.
Echinococcal infections may cause the formation of intrahepatic hydatid cysts that produce symptoms due to pressure on surrounding structures or following rupture.
Autoimmune hepatitis is a chronic, progressive disorder with features that include a genetic predisposition, an association with other autoimmune diseases, the presence of autoantibodies, and therapeutic responsiveness to immunosuppression. Autoimmune hepatitis has a wide range of presentations ranging from asymptomatic disease detected by elevated transaminases to acute and chronic hepatitis. There is a female predominance (78%). It is classified into two types, based on the patterns of circulating antibodies:
Type 1 is characterized by the presence of antinuclear antibodies (ANAs), which are most common but not specific; antismooth muscle antibodies (ASMAs), which are present in 65% of cases; and anti–soluble liver antigen/liver-pancreas antigen (anti-SLA/LP) antibodies, present in 25% to 35% of cases. The latter are also present in type 2 autoimmune hepatitis.
Type 2, usually seen in children and teenagers, is characterized by anti–liver kidney microsome-1 antibodies (anti-LKM-1); anti–liver cytosol-1 (anti-LC1) antibodies; and anti-SLA/LP.
In a small subset of patients, there may be features that overlap with those of primary biliary cholangitis or primary sclerosing cholangitis. Cirrhosis is common in patients with autoimmune hepatitis; up to 30% of patients with adult-onset disease have cirrhosis at the time of diagnosis.
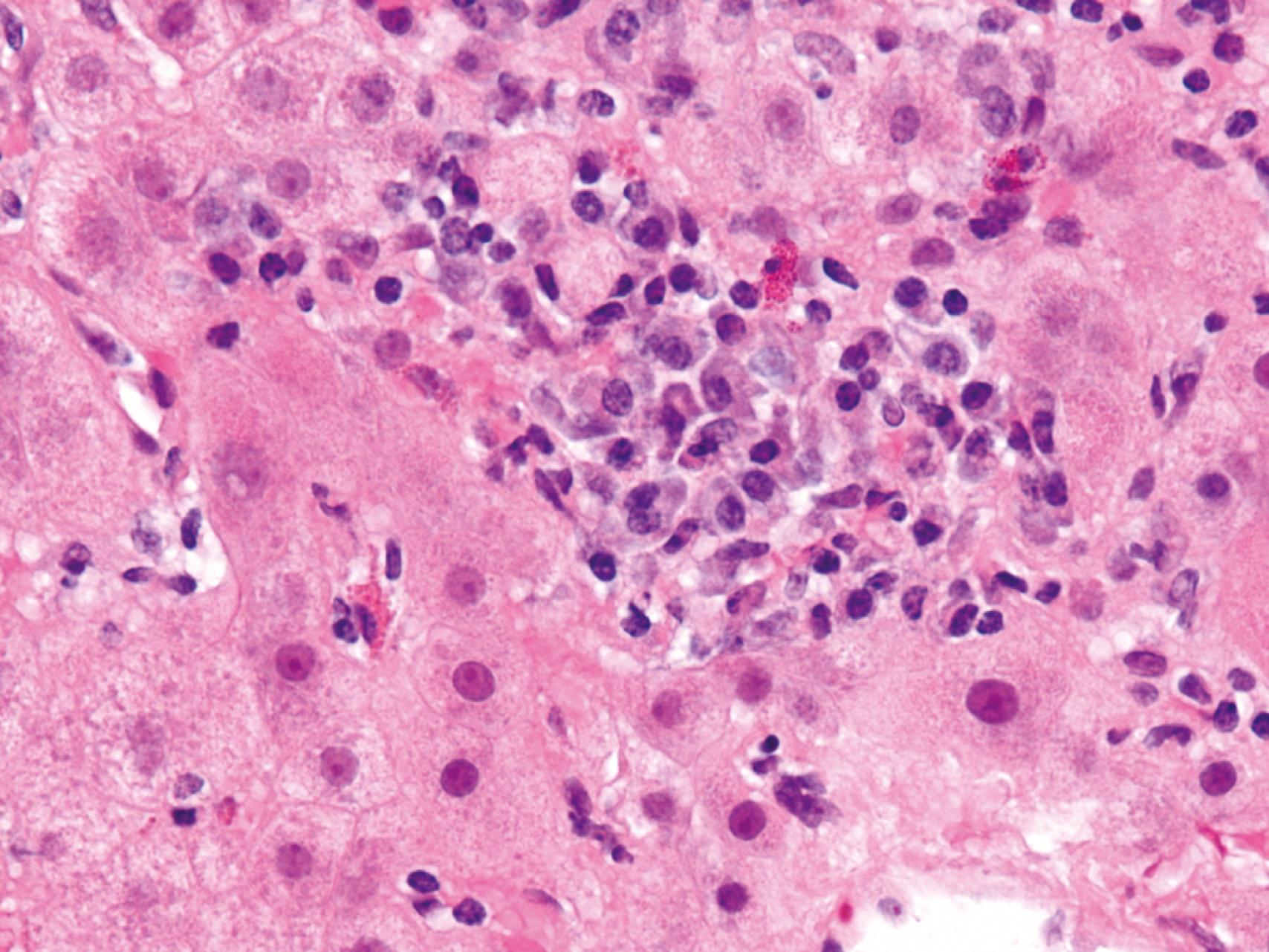
Autoimmune hepatitis shares patterns of injury with acute or chronic viral hepatitis. The following features are typical of autoimmune hepatitis ( eFig. 14.1 ):
Necrosis and inflammation, indicated by extensive interface hepatitis or foci of confluent (perivenular or bridging) necrosis or parenchymal collapse
Plasma cell predominance in the mononuclear inflammatory infiltrates
Hepatocyte “rosettes” comprising a circular arrangement of regenerating hepatocytes around a dilated canaliculus
Immunosuppressive therapy is usually effective, leading to remission in 90% of patients including those who have cirrhosis at diagnosis. End-stage disease is an indication for liver transplantation. The 10-year survival rate after liver transplant is 75%, but recurrence in the transplanted organ occurs in 20% of cases.
As the major drug metabolizing and detoxifying organ in the body, the liver is subject to injury from an enormous array of therapeutic and environmental chemicals. Injury may result from direct toxicity, occur through hepatic conversion of a xenobiotic compound to an active toxin, or be produced by immune mechanisms in which the drug or its metabolite chemically bond with a cellular protein and convert it into an immunogen. A diagnosis of drug- or toxin-induced liver injury may be made on the basis of a temporal association of liver damage with drug or toxin exposure, recovery (usually) upon removal of the inciting agent, and exclusion of other potential causes. Exposure to a toxin or therapeutic agent should always be included in the differential diagnosis of any form of liver disease.
Principles of drug and toxic injury are discussed in Chapter 7 . Here it suffices to note that drug reactions may be predictable (intrinsic) or unpredictable (idiosyncratic). Predictable drug or toxin reactions affect all individuals in a dose-dependent fashion. Unpredictable reactions depend on host-specific factors, such as a propensity to mount an immune response to the drug or toxin or to metabolize the responsible agent in an unusual fashion. Both classes of injury may be immediate or take weeks to months to develop ( Table 14.3 ).
A classic, predictable hepatotoxin is acetaminophen, now the most common cause of acute liver failure necessitating transplantation in the United States. The toxic agent is not acetaminophen itself but rather toxic metabolites produced by the cytochrome P-450 system. Since these enzymes are more active in the central zone of the lobule, necrosis of perivenular hepatocytes is a typical feature of drug-induced liver injury. Eventually necrosis can involve the entire lobule.
Examples of drugs that can cause idiosyncratic reactions include chlorpromazine, an agent that causes cholestasis in patients who are slow to metabolize it, and halothane and its derivatives, which can cause a fatal immune-mediated hepatitis after repeated exposure.
| Pattern of Injury | Morphologic Findings | Examples of Associated Agents |
|---|---|---|
| Cholestatic | Bland hepatocellular cholestasis, without inflammation | Contraceptive and anabolic steroids, antibiotics, antiretroviral therapy |
| Cholestatic hepatitis | Cholestasis with lobular necroinflammatory activity; may show bile duct destruction | Antibiotics, phenothiazines, statins |
| Hepatocellular necrosis | Spotty hepatocyte necrosis | Methyldopa, phenytoin |
| Massive necrosis | Acetaminophen, halothane | |
| Chronic hepatitis | Isoniazid | |
| Fatty liver disease | Large and small droplet fat | Ethanol, corticosteroids, methotrexate, total parenteral nutrition |
| “Microvesicular steatosis” (diffuse small droplet fat) | Valproate, tetracycline, aspirin (Reye syndrome), antiretroviral therapy | |
| Steatohepatitis with Mallory hyaline | Ethanol, amiodarone, irinotecan | |
| Fibrosis and cirrhosis | Periportal and pericellular fibrosis | Alcohol, methotrexate, enalapril, vitamin A and other retinoids |
| Granulomas | Noncaseating epithelioid granulomas | Sulfonamides, amiodarone, isoniazid |
| Fibrin ring granulomas: granulomas with fibrin surrounding a central lipid vacuole | Allopurinol | |
| Vascular lesions | Sinusoidal obstruction syndrome (veno-occlusive disease): obliteration of central veins | High-dose chemotherapy, bush teas |
| Budd-Chiari syndrome | Oral contraceptives | |
| Peliosis hepatis: blood-filled cavities, not lined by endothelial cells | Anabolic steroids, tamoxifen | |
| Neoplasms | Hepatocellular adenoma | Oral contraceptives, anabolic steroids |
| Hepatocellular carcinoma | Alcohol, Thorotrast | |
| Cholangiocarcinoma | Thorotrast | |
| Angiosarcoma | Thorotrast, vinyl chloride |
Alcohol is a well-known cause of fatty liver disease in adults and can manifest histologically as steatosis, steatohepatitis, and cirrhosis. In recent years, it has become evident that another entity, so-called nonalcoholic fatty liver disease (NAFLD), which is associated with insulin resistance and the metabolic syndrome, can mimic the entire spectrum of hepatic changes associated with excessive alcohol use. Since the morphologic changes of alcohol-related liver disease and NAFLD are indistinguishable, they are discussed together, followed by the pathogenesis and distinctive clinical features of each entity.
Three types of liver alterations are observed in fatty liver disease: steatosis (fatty change), steatohepatitis, and fibrosis.
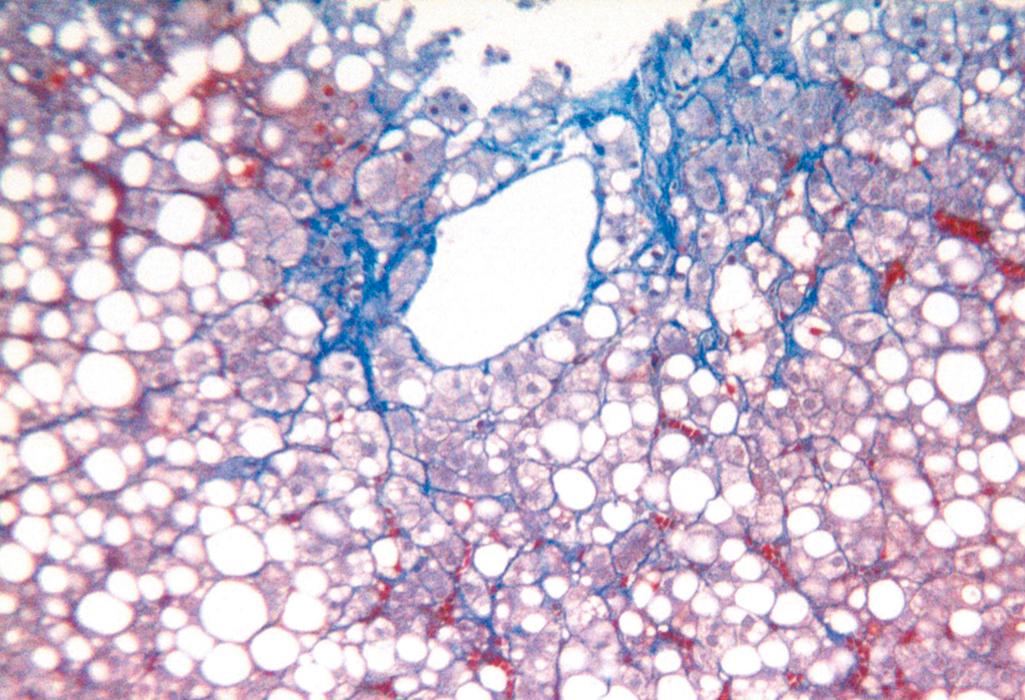
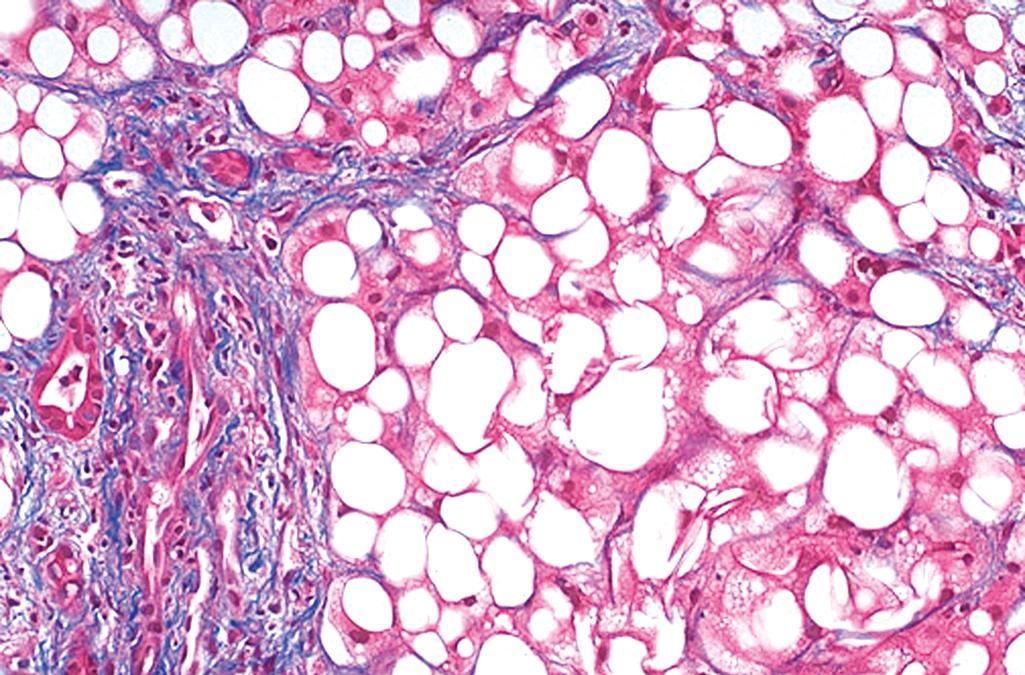
Hepatocellular steatosis. Fat accumulation typically begins in centrilobular hepatocytes. The lipid droplets range from small (microvesicular) to large (macrovesicular); the largest fill and expand the cell and displace the nucleus. As steatosis becomes more extensive, the lipid accumulation spreads outward from the central vein to hepatocytes in the midlobule and then the periportal regions ( Fig. 14.16 ). Macroscopically, fatty livers with widespread steatosis are large (weighing 4–6 kg or more), soft, yellow, and greasy. In general, fatty change is completely reversible if there is abstention from further intake of alcohol.
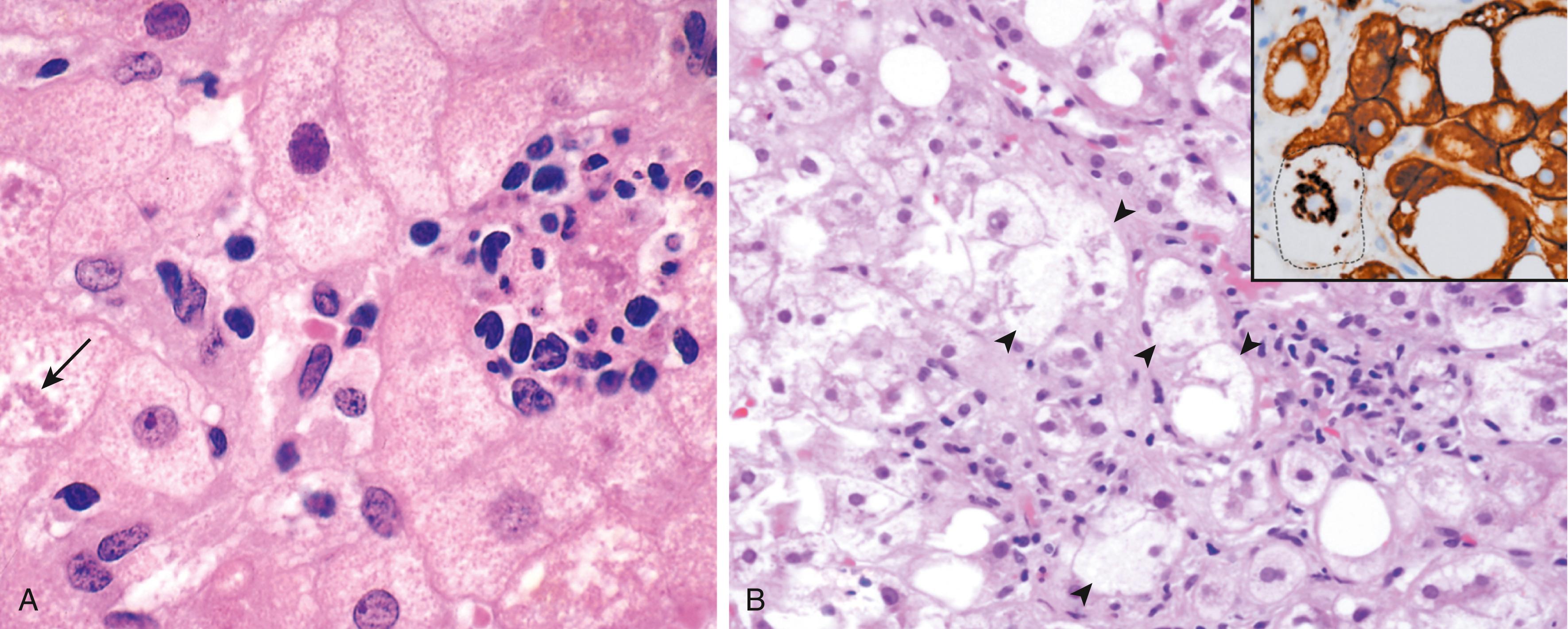
Steatohepatitis. These changes typically are more pronounced with alcohol use than in NAFLD but can be seen in either ( Fig. 14.17 ):
Hepatocyte ballooning. Single or scattered foci of cells undergo swelling and necrosis; as with steatosis, these features are most prominent in the centrilobular regions.
Mallory hyaline bodies. These consist of tangled skeins of intermediate filaments (including ubiquitinylated keratins 8 and 18) and are visible as eosinophilic cytoplasmic inclusions in degenerating hepatocytes ( Fig. 14.17 B).
Neutrophil infiltration. Neutrophilic infiltration may permeate the lobule and accumulate around degenerating hepatocytes, particularly those containing Mallory hyaline bodies. Lymphocytes and macrophages also may be seen in portal tracts or parenchyma.
Steatofibrosis. Fatty liver disease of all kinds has a distinctive pattern of scarring. Like other changes, fibrosis appears first in the centrilobular region as central vein sclerosis. Perisinusoidal scarring appears next in the space of Disse of the centrilobular region and then spreads outward, encircling individual or small clusters of hepatocytes in a chicken-wire fence pattern (see Fig. 14.16 ). Tendrils of fibrosis eventually link to portal tracts and then condense to create central portal fibrous septa. As these become more prominent, the liver takes on a nodular, cirrhotic appearance. Because the underlying cause persists in most cases, the continual subdivision of established nodules by new perisinusoidal scarring leads to a classic micronodular cirrhosis . Early in the course, the liver is yellow-tan, fatty, and enlarged, but with persistent damage over the course of years, the liver is transformed into a brown, shrunken organ composed of cirrhotic nodules that are usually less than 0.3 cm in diameter—smaller than is typical for most forms of chronic viral hepatitis. The end-stage cirrhotic liver may enter a “burned-out” phase devoid of fatty change and other typical features. A majority of cases of cryptogenic cirrhosis, without clear etiology, are now recognized as “burned-out” NAFLD.
Excessive ethanol consumption causes more than 60% of cases of chronic liver disease in Western countries and accounts for 40% to 50% of deaths resulting from cirrhosis. Among the most important adverse effects of chronic alcohol consumption are the overlapping forms of alcohol-related fatty liver disease already discussed: (1) hepatic steatosis, (2) steatohepatitis, and (3) fibrosis and cirrhosis, collectively referred to as alcohol-related liver disease ( Fig. 14.18 ).
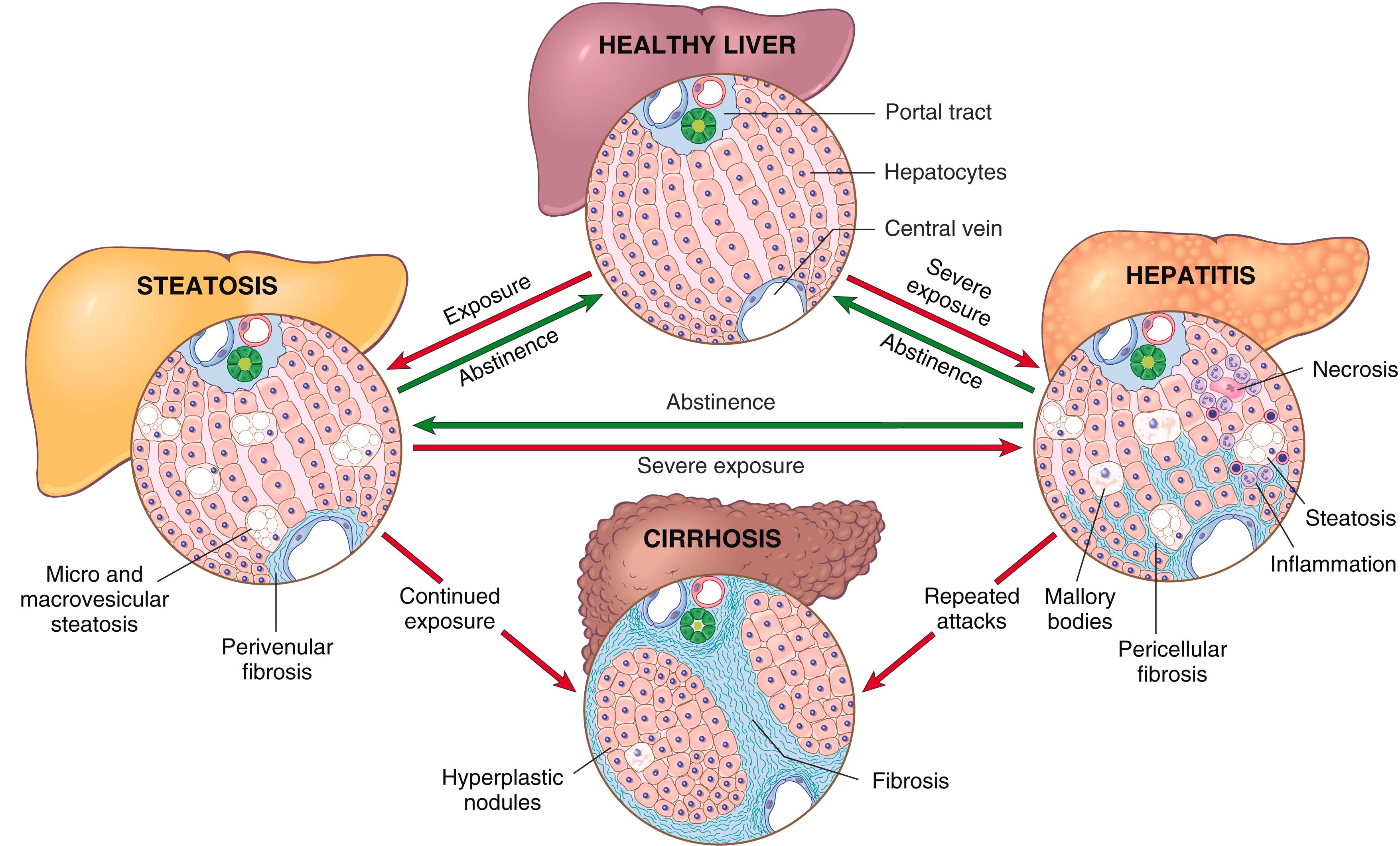
Between 90% and 100% of individuals who chronically consume excess alcohol develop fatty liver (i.e., hepatic steatosis), and of those, 10% to 35% develop steatohepatitis, whereas only 8% to 20% of this population develop cirrhosis. Steatosis, steatohepatitis, and fibrosis may develop sequentially or independently, so they do not necessarily represent a sequential continuum of changes. Hepatocellular carcinoma arises in 10% to 20% of patients with cirrhosis secondary to excess alcohol use.
Short-term ingestion of as much as 80 gm of ethanol per day (5–6 beers or 8–9 ounces of 80-proof liquor) generally produces mild reversible hepatic changes, such as fatty liver. Chronic intake of 40 to 80 gm/day is considered a borderline risk factor for severe injury. The risk of severe hepatic injury becomes significant with intake of 80 gm or more of ethanol per day. However, only 10% to 15% of individuals who chronically consume excess alcohol develop cirrhosis. In the absence of a clear understanding of the factors that influence liver damage, it is difficult to state what constitutes a safe level of alcohol consumption. For reasons that may relate to decreased gastric metabolism of ethanol and differences in body composition, women are more susceptible than men to hepatic injury. It seems that how often one drinks may affect the risk for liver disease development. For example, binge drinking causes more liver injury than that associated with steady, lower-level consumption.
Hepatocellular steatosis is caused by alcohol through several mechanisms. First, metabolism of ethanol by alcohol dehydrogenase and acetaldehyde dehydrogenase generates large amounts of nicotinamide-adenine dinucleotide (NADH), which shunts fatty acid precursors away from catabolism and toward lipid biosynthesis. Second, ethanol impairs the assembly and secretion of lipoproteins. The net effect is to cause the accumulation of intracellular lipids.
The cause of steatohepatitis secondary to alcohol is uncertain, but it may stem from one or more of the following toxic byproducts of ethanol and its metabolites:
Acetaldehyde (a major metabolite of ethanol) induces lipid peroxidation and acetaldehyde-protein adduct formation, which may disrupt cytoskeleton and membrane function.
Alcohol directly affects mitochondrial function and membrane fluidity.
Reactive oxygen species generated during oxidation of ethanol by the microsomal ethanol oxidizing system react with and damage membranes and proteins. Reactive oxygen species are also produced by neutrophils, which infiltrate areas of hepatocyte necrosis.
Because generation of acetaldehyde and free radicals is maximal in the centrilobular region, this region is most susceptible to alcohol-induced injury. Pericellular and sinusoidal fibrosis develop first in this area of the lobule. Concurrent viral hepatitis, particularly hepatitis C, is a major accelerator of alcohol-related liver disease.
For unknown reasons, cirrhosis develops in only a small fraction of people who chronically drink excess alcohol. With complete abstinence, at least partial regression of scarring occurs, and by parenchymal regeneration the micronodular liver transforms into a macronodular cirrhotic organ (see Fig. 14.6 ); rarely, there is complete regression of cirrhosis.
Steatosis may be innocuous or give rise to hepatomegaly with mild elevations of serum bilirubin and alkaline phosphatase. Severe hepatic compromise is unusual. Alcohol cessation and an adequate diet are sufficient treatment.
It is estimated that 15 to 20 years of excessive drinking are necessary to develop cirrhosis, but steatohepatitis can occur after just weeks or months of heavy alcohol use. The onset of steatohepatitis is typically acute and often follows an episode of particularly heavy drinking. Symptoms and laboratory abnormalities range from minimal to severe. Most patients present with malaise, anorexia, weight loss, upper abdominal discomfort, tender hepatomegaly, and fever. Typical laboratory findings include hyperbilirubinemia, elevated serum alkaline phosphatase levels, and neutrophilic leukocytosis. Serum alanine and aspartate aminotransferases are elevated but usually remain below 500 U/mL. In contrast to other chronic liver diseases, in which serum ALT tends to be higher than serum AST, in alcohol-related liver disease serum AST tends to be higher than serum ALT levels by a ratio of 2 : 1 or greater. The outlook is unpredictable; each bout of alcohol-related hepatitis carries a 10% to 20% risk for death. With repeated bouts, cirrhosis appears in about one-third of patients within a few years.
The manifestations of alcohol-related cirrhosis are similar to those of other forms of cirrhosis. In addition, when ethanol becomes the major source of calories in the diet, other nutrients are displaced, leading to malnutrition and vitamin deficiencies (e.g., thiamine, folate). Compounding these effects is impaired digestive function, primarily related to chronic gastric and intestinal mucosal damage and pancreatitis.
The long-term outlook for alcohol-related liver disease is variable. The most important part of treatment is abstinence from alcohol. The 5-year survival rate approaches 90% in those who abstain and who are free of jaundice, ascites, and hematemesis but drops to 50% to 60% in individuals who continue to imbibe. Among those with end-stage alcohol-related liver disease, the immediate causes of death are as follows:
Hepatic failure
Massive gastrointestinal hemorrhage
Intercurrent infection (to which affected individuals are predisposed)
Hepatorenal syndrome
Hepatocellular carcinoma (3%–6% of cases)
Nonalcoholic fatty liver disease (NAFLD) is a common condition in which fatty liver disease develops in association with insulin resistance and the metabolic syndrome ( Chapter 18 ). The liver can show any of the three types of changes that occur in alcohol-related liver disease (steatosis, steatohepatitis, and cirrhosis), though on average inflammation is less prominent ( eFig. 14.2 ). The term nonalcoholic steatohepatitis (NASH) is used to describe overt clinical features of liver injury, such as elevated transaminases, and the histologic features of hepatitis already discussed. Since systemic metabolic dysfunction underlies NAFLD, the term metabolic-associated fatty liver disease (MAFLD) has been proposed. In addition to insulin resistance and the metabolic syndrome, NAFLD is characterized by the following:
Type 2 diabetes (or family history of the condition)
Obesity, primarily central obesity
Dyslipidemia (hypertriglyceridemia, low high-density lipoprotein cholesterol, high low-density lipoprotein cholesterol)
Hypertension
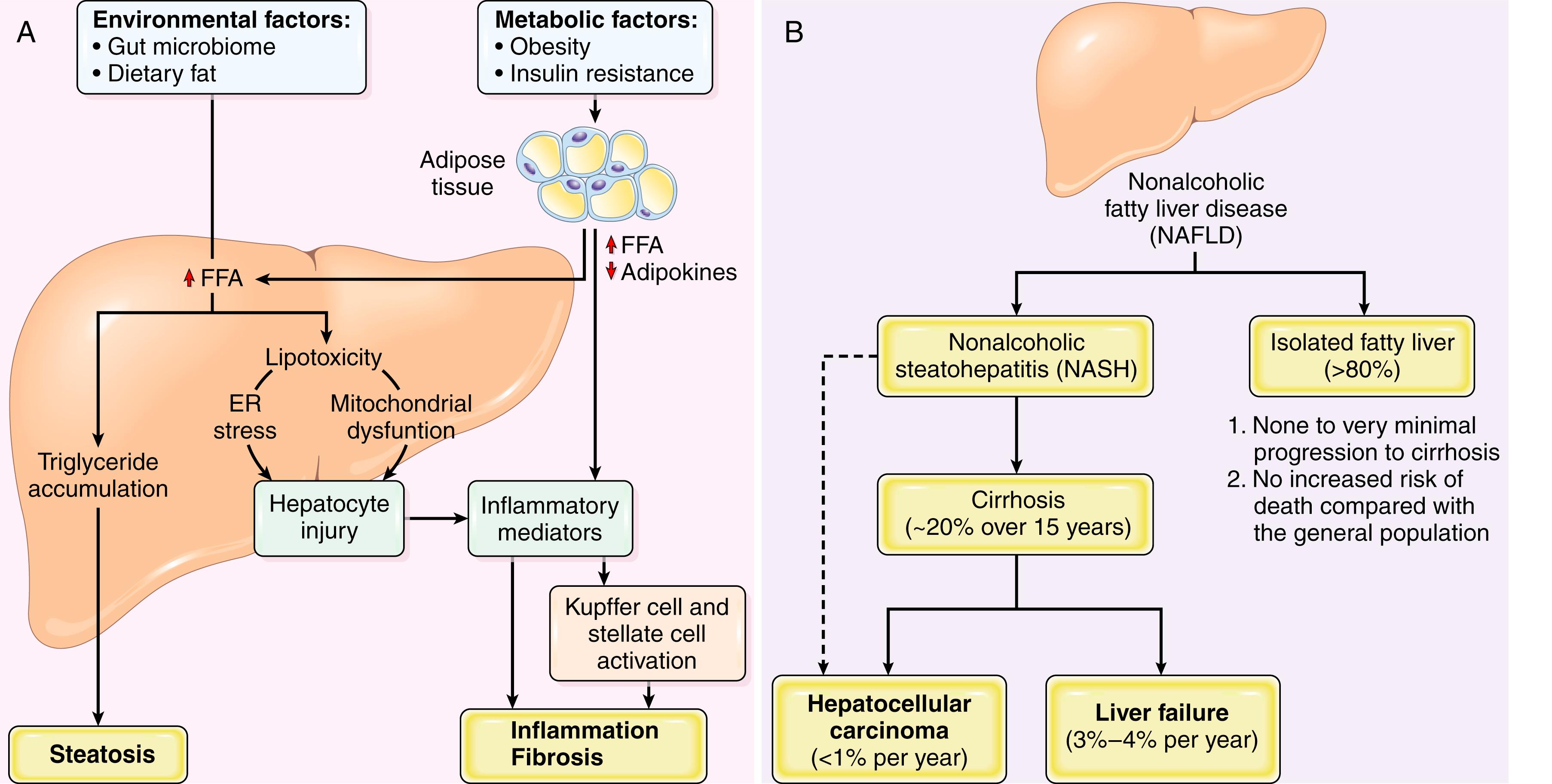
The key initiating events in NAFLD appear to be the development of obesity and insulin resistance ( Fig. 14.19 ). The latter leads to increased release of free fatty acids from adipocytes due to overactivity of lipoprotein lipase. This is associated with reduced production of the hormone adiponectin from adipocytes, which decreases oxidation of free fatty acids by skeletal muscle and increases free fatty acid uptake into hepatocytes, where the fatty acids are stored as triglycerides. In addition, hepatocytes in patients with NASH show evidence of inflammasome activation, possibly due to direct or indirect effects of particular lipids, leading to local release of the proinflammatory cytokine IL-1. Other products of lipid metabolism appear to be directly toxic to hepatocytes; proposed mechanisms include increased production of reactive oxygen species, induction of ER stress, and disruption of mitochondrial function. Alterations in the gut microbiome and increased gut-derived endotoxin production may also play a role in liver inflammation and injury. Hepatocyte injury resulting from these various insults causes stellate cell activation, collagen deposition, and hepatic fibrosis, which along with ongoing hepatocyte damage lead to full-blown NASH.
NAFLD is the most common cause of incidental elevation of serum transaminases. The AST to ALT ratio is typically less than one (unlike alcohol-related fatty liver disease, which usually has a ratio greater than two). Most individuals with steatosis are asymptomatic; patients with active steatohepatitis or fibrosis may also be asymptomatic, while others have fatigue, malaise, right upper quadrant discomfort, or more severe symptoms of chronic liver disease. Liver biopsy is required to identify NASH and distinguish it from uncomplicated NAFLD. Fortunately, the frequency of progression from steatosis to active steatohepatitis and then from active steatohepatitis to cirrhosis is low. Nevertheless, NAFLD is considered to be a significant contributor to the pathogenesis of “cryptogenic” cirrhosis. Because they share common risk factors, the incidence of coronary artery disease is also increased in patients with NAFLD. One of the worrisome complications of NASH is development of hepatocellular carcinoma (see Fig. 14.19 ). With successful treatment of hepatitis C, the proportion of liver cancers arising in the setting of NASH is increasing and is likely to overtake HCV as a risk factor for HCC in the United States.
Current therapy is directed toward weight reduction and reversal of insulin resistance. Lifestyle modifications such as diet and exercise appear to be the most effective form of treatment. In selected cases bariatric surgery can help.
Pediatric NAFLD is becoming an increasing problem as obesity and metabolic syndrome approach epidemic proportions. In children, the appearance of the histologic lesions is somewhat different: inflammation and scarring tend to be more prominent in the portal tracts and periportal regions, and mononuclear infiltrates rather than neutrophilic infiltrates predominate.
Although there are many inherited metabolic liver diseases, only some relatively common, pathogenically interesting entities are discussed here: hereditary hemochromatosis, Wilson disease, and α 1 -antitrypsin (α 1 AT) deficiency.
Hemochromatosis is caused by excessive absorption of iron, which is deposited in organs such as the liver and pancreas, as well as in the heart, joints, and endocrine organs. It results most commonly from an inherited disorder, hereditary hemochromatosis .
As discussed in Chapter 10 , the total body iron pool ranges from 3 to 4 gm in healthy adults; about 0.5 gm is stored in hepatocytes. In severe hemochromatosis, total iron may exceed 50 gm, one-third of which accumulates in the liver. Fully developed cases exhibit (1) micronodular cirrhosis; (2) diabetes (up to 80% of patients); and (3) abnormal skin pigmentation (up to 80% of patients).
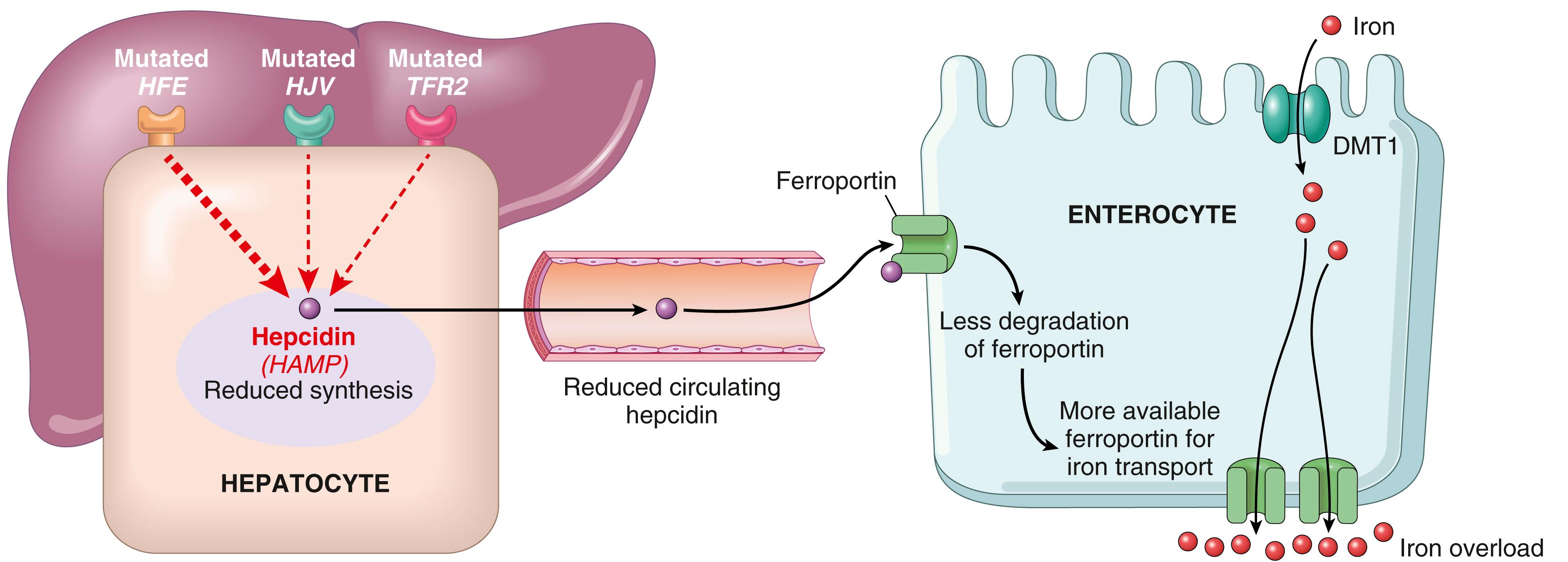
Because there is no regulated iron excretion from the body, the total body content of iron is tightly regulated by intestinal absorption. As discussed in Chapter 10 , hepcidin, encoded by the HAMP gene, is a circulating peptide hormone that acts as a key negative regulator of intestinal iron uptake. HFE, HJV, and TFR2 are membrane proteins expressed on hepatocytes. In a manner that is still poorly understood, HFE, HJV, and TFR2 (a transferrin receptor) function together as a sensor for iron, such that when iron is plentiful, signals are transmitted that stimulate the expression of HAMP transcripts and the secretion of hepcidin. Hepcidin in turn circulates to the gut and binds to ferroportin on enterocytes, leading to its internalization and degradation, thereby reducing the efflux of iron from enterocytes. Diverse loss-of-function mutations in the components of this negative feedback loop lead to increased iron absorption and hemochromatosis ( Fig. 14.20 ). The most common genetic alterations underlying hereditary hemochromatosis are as follows:
HFE (for H ereditary Fe [iron]) is the most frequently mutated gene in patients with hereditary hemochromatosis. It encodes an HLA class I–like molecule that regulates the synthesis of hepcidin in hepatocytes. Loss-of-function mutations of the HFE gene reduce hepcidin levels and are present in over 70% of patients diagnosed with hereditary hemochromatosis.
Less commonly, hereditary hemochromatosis is caused by mutations in genes encoding proteins that are directly involved in iron trafficking, such as the receptor for transferrin (the plasma transport molecule for iron) or ferroportin (a transmembrane iron transporter). With these mutations, the associated clinical condition is milder in some cases and more severe in others, sometimes resulting in disease that manifests in young adults or even during childhood.
An acquired form of hemochromatosis (secondary hemochromatosis) may develop in patients who receive multiple blood transfusions or have chronic ineffective erythropoiesis, as occurs in β-thalassemia and certain myeloid neoplasms. Ineffective erythropoiesis is marked by the premature death of red cell progenitors in the bone marrow, causing anemia that triggers increased production of erythropoietin from the kidney. This leads to an expansion of early red cell progenitors, which release a hormone called erythroferrone that suppresses hepcidin production. If uncorrected, the inevitable result is hemochromatosis.
Whatever the underlying defect, the net result is an increase in intestinal absorption of dietary iron, leading to an accumulation of 0.5 to 1 gm of iron per year, with disease developing after iron stores reach about 20 gm. Excessive iron appears to be directly toxic to host tissues. Mechanisms of liver injury include the following:
Lipid peroxidation via iron-catalyzed free radical reactions
Stimulation of collagen formation by activation of hepatic stellate cells
DNA damage by reactive oxygen species, leading to lethal cell injury or predisposition to hepatocellular cancer
The deleterious effects of iron on cells that are not fatally injured are reversible, and removal of excess iron with therapy promotes recovery of tissue function.
The morphologic changes in severe hemochromatosis are characterized principally by (1) tissue deposition of hemosiderin in the following organs (in decreasing order of severity): liver, pancreas, myocardium, pituitary gland, adrenal gland, thyroid and parathyroid glands, joints, and skin; (2) cirrhosis; and (3) pancreatic fibrosis. In the liver, iron becomes evident first as golden-yellow hemosiderin granules in the cytoplasm of periportal hepatocytes, which can be histochemically stained with Prussian blue ( Fig. 14.21 ). With increasing iron load, there is progressive deposition in the rest of the lobule, the bile duct epithelium, and Kupffer cells. At this stage, the liver is typically slightly enlarged and chocolate brown. Fibrous septa develop slowly, linking portal tracts to each other and leading ultimately to cirrhosis in an intensely pigmented (very dark brown to black) liver.

The pancreas also becomes pigmented, acquires diffuse interstitial fibrosis, and may show parenchymal atrophy. Hemosiderin is found in the acinar and the islet cells and sometimes in the interstitial fibrous stroma. The heart is often enlarged, with hemosiderin granules within the myocardial fibers. The pigmentation may induce a striking brown coloration to the myocardium. A delicate interstitial fibrosis may appear. Although darkening of the natural skin color is partially attributable to hemosiderin deposition in dermal macrophages and fibroblasts, most of the coloration results from increased epidermal melanin production. The combination of these pigments gives the skin a grayish tinge. With hemosiderin deposition in the joint synovial linings, an acute synovitis may develop. There is also excessive deposition of calcium pyrophosphate, which damages the articular cartilage and sometimes produces disabling polyarthritis, referred to as pseudogout. With the onset of cirrhosis, the testes may become atrophic.
Symptoms usually appear earlier in men than in women since menstrual bleeding limits the accumulation of iron until menopause. This results in a male-to-female ratio of clinically significant iron overload of approximately 5 : 1 to 7 : 1. In the most common form (due to HFE mutations), symptoms usually appear in the fifth and sixth decades of life in men and later in women.
The principal manifestations include hepatomegaly, abdominal pain, changes in skin pigmentation (particularly in sun-exposed areas of light skinned individuals), glucose intolerance or diabetes due to destruction of pancreatic islets, cardiac dysfunction (e.g., arrhythmias, cardiomyopathy), and atypical arthritis. In some patients, the presenting complaint is hypogonadism (e.g., amenorrhea in the female, impotence and loss of libido in the male). As noted, clinically apparent disease is more common in males and rarely becomes evident before 40 years of age. Death may result from cirrhosis or cardiac disease. In those with untreated disease, the risk for hepatocellular carcinoma is increased 200-fold, presumably because of ongoing liver damage and the genotoxic effects of oxidants generated by iron.
Fortunately, hemochromatosis can be diagnosed long before irreversible tissue damage has occurred. Screening of family members of probands is important. Heterozygotes also accumulate excessive iron, but not to a level that causes significant tissue damage. Currently, most patients with hemochromatosis are diagnosed in the subclinical, precirrhotic stage due to routine serum iron measurements (as part of another diagnostic workup). The diagnosis can be confirmed by sequencing the HFE gene. Regular phlebotomy results in steady removal of excess tissue iron, and, with this simple treatment, life expectancy is normal.
Wilson disease is an autosomal recessive disorder caused by loss-of-function mutations of the ATP7B gene, which results in impaired copper excretion into bile and a failure to incorporate copper into ceruloplasmin ( Fig. 14.22 ). This disorder is marked by the accumulation of toxic levels of copper in many tissues and organs, principally the liver, brain, and eye. Normally, 40% to 60% of ingested copper (2–5 mg/day) is absorbed in the duodenum and proximal small intestine, where it is transported in a complex with albumin and histidine to the liver. Within hepatocytes copper binds to ATP7B, a copper-transporting transmembrane protein that is found predominantly in the trans-Golgi network and in lysosomes. In the trans-Golgi network, ATP7B mediates the transport of copper into apoceruloplasmin to form ceruloplasmin, which is then secreted into the bloodstream. In the lysosomes, ATP7B transports nonceruloplasmin-bound hepatic copper to bile canaliculi for excretion through bile, the major route of copper excretion from the body.
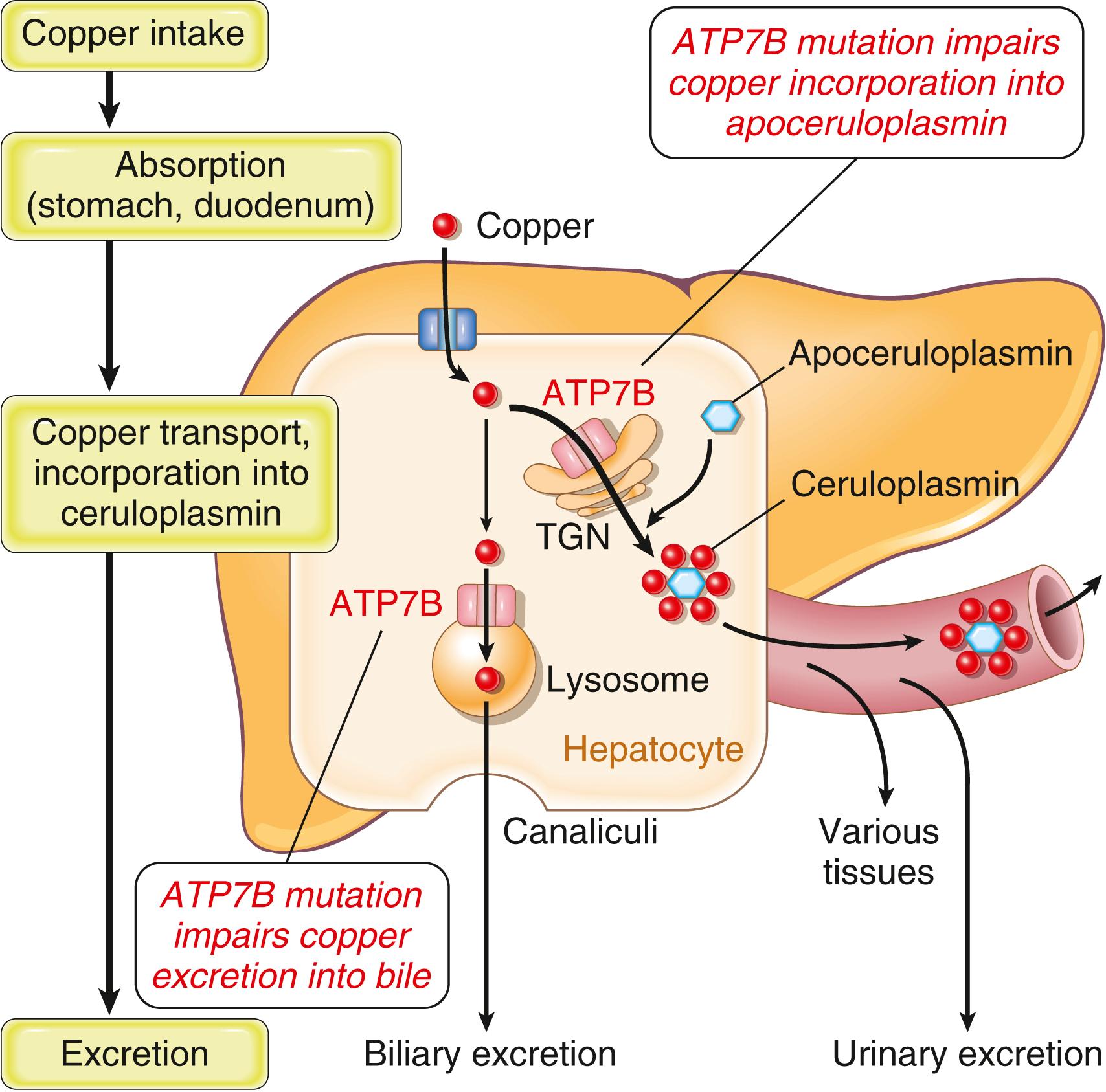
In Wilson disease ATP7B-dependent copper transport out of hepatocytes into the blood and bile is impaired. Hence it accumulates in the cytoplasm and in lysosomes, which increases ROS production, damaging hepatocytes. Although low serum ceruloplasmin levels are a hallmark of Wilson disease, the reduction in ceruloplasmin plays no role in the pathogenesis of this disorder. With progressive accumulation of copper in the liver, nonceruloplasmin-bound copper is released from injured hepatocytes into the circulation, causing red cell hemolysis and allowing copper to deposit in other tissues, such as the brain, corneas, kidneys, bones, joints, and parathyroid glands. Concomitantly, urinary excretion of copper increases markedly from its normal minuscule levels.
The liver often bears the brunt of injury. The hepatic changes are variable, ranging from relatively minor to severe, and mimic many other disease processes. There may be mild to moderate fatty change (steatosis) associated with focal hepatocyte necrosis. Acute, fulminant hepatitis can mimic acute viral hepatitis. Chronic hepatitis in Wilson disease exhibits moderate to severe inflammation and hepatocyte necrosis, areas of fatty change, and features of steatohepatitis (hepatocyte ballooning with prominent Mallory hyaline bodies). In advanced cases, cirrhosis may be seen. Copper deposition in hepatocytes can be demonstrated by special stains ( eFig. 14.3 ).
Toxic injury to the brain primarily affects the basal ganglia. Nearly all patients with neurologic involvement develop eye lesions called Kayser-Fleischer rings, green to brown deposits of copper in the Desçemet membrane in the limbus of the cornea.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here