Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The authors would like to thank Ms. Kelsey Lansdale for her assistance with the illustrations.
Intracranial hemorrhage and subsequent seizures, headaches, and focal neurological deficits are common presentations in patients with symptomatic iAVMs.
Multidisciplinary care of patients with iAVMs in the neurointensive care unit is warranted, particularly for those with hemorrhage and for patients undergoing surgical and/or endovascular interventions.
The preoperative management in cases of ruptured iAVMs centers around limiting hematoma expansion and mass effect, preventing/treating seizures, and preventing rerupture of the AVM.
Loss of cerebral autoregulation and changes in flow dynamics after iAVM treatment can result in postoperative intraparenchymal hemorrhage.
Strict blood pressure control (systolic blood pressure < 140 mm Hg) should be implemented for at least the first 24 hours after iAVM treatment, with close monitoring for neurological deterioration.
Critical care management of intracranial arteriovenous malformations (iAVMs) incorporates principles common to the management of intracranial hemorrhage (ICH) that focus on mitigating primary and secondary injury from the hematoma. The emphasis, particularly in the acute period after AVM rupture, and the immediate postoperative period following definitive AVM treatment, regardless of previous rupture status, essentially involves strict blood pressure regulation. ICH from AVM rupture is the most dreaded complication that can develop in the pre- or posttreatment setting. Understanding the pathophysiology and flow dynamics of these lesions, adapting principles of treatments of other acute brain injuries, and close monitoring and prompt intervention in a dedicated neurointensive care unit (NICU) are crucial and should be an essential part of treating critically ill patients with iAVMs. This chapter first delves into the details of intensive care management of patients with ruptured iAVMs and subsequently discusses the complexity of postoperative care of iAVM patients as the brain acclimates to reorganization of blood flow dynamics.
Although unruptured iAVMs are more common than ruptured iAVMs, patients with unruptured iAVMs often present with progressive neurological deficits in an outpatient setting and therefore generally do not require intensive care monitoring prior to the first planned elective treatment. Therefore this section will focus on the preoperative management of patients with ruptured iAVMs.
Rupture of an iAVM warrants formal admission to the NICU. The most common cause of symptoms from iAVMs is rupture (ICH) and, in particular, intraparenchymal hemorrhage (IPH). Though less common, intraventricular, subdural, and subarachnoid hemorrhage may also be seen ( Fig. 27.1 ). Recent observational data suggest that patients with IPH from a ruptured iAVM enjoy a more favorable prognosis than those with primary IPH (i.e., due to hypertension or amyloid angiopathy). Factors that likely contribute to lower mortality and disability with AVM-associated IPH include younger age, hematoma within the AVM nidus with relative sparing of the surrounding brain parenchyma, and smaller hematoma volumes on presentation.
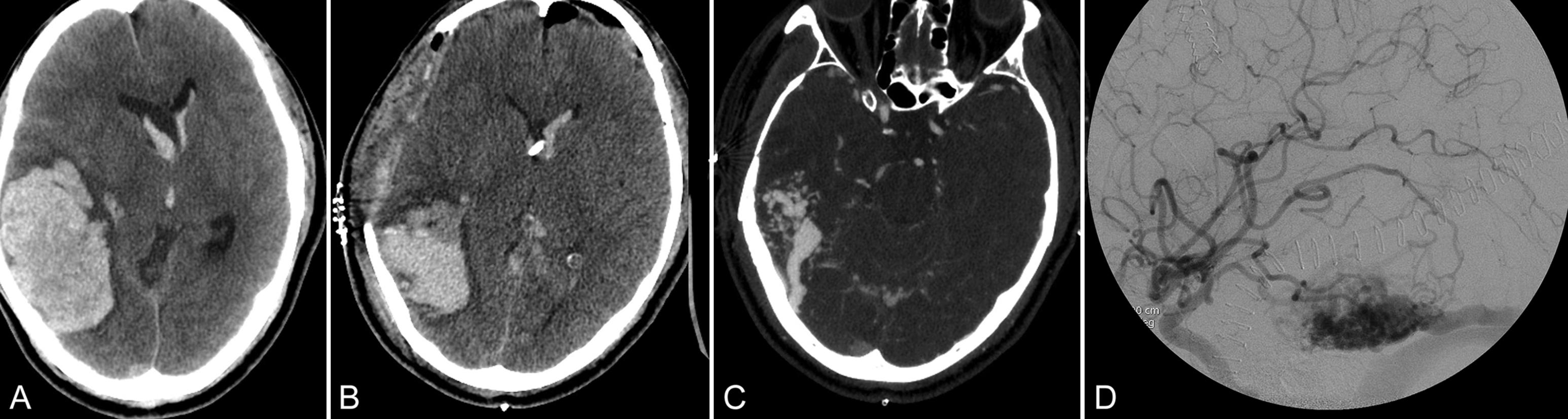
In patients with ruptured iAVMs, basal subarachnoid cisternal hemorrhages carry the worst outcomes compared to hemorrhages in other locations. This hemorrhage distribution may reflect an aneurysmal source with the attendant poorer overall outcomes. Moreover, the risk of recurrent rupture is nearly 2–3 times higher—between 4% and 7% per year—than the risk of a first-time rupture, which is usually about 1%–2% per year. Finally, the risk of recurrence is highest in the first year after an AVM rupture (up to 15%), and presumably much higher in the first few days after the initial bleed than later in that first year. Early aggressive care in an ICU setting should therefore be initiated soon after IPH diagnosis.
The critical care principles applicable to the management of ICH from other causes may be extrapolated for managing AVM-associated ICHs. Endovascular interventional neuroradiology and neurosurgery departments should be consulted, preferably at the time of admission, for treatment planning. Timely and effective patient assessment, following the ABCs of emergent evaluation/intervention, is essential as this can change patient outcomes. If a patient scores 8 or less on the Glasgow Coma Scale (GCS), endotracheal intubation should be strongly considered to protect the airway when necessary. Ventilation and oxygenation should be optimized to prevent secondary brain injury.
Hyperacute management applies to the first 6 hours after symptom onset, when the majority of patients experience hematoma expansion, an early devastating factor independently associated with mortality and severe disability after ICH. Thus serial CT scans at 2 and 12 hours after presentation are typically obtained in stable patients. Several interventions have been proposed to limit hematoma expansion and include blood pressure control, correction of coagulopathy, and treating seizures.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here