Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Lymph nodes play an important role in reaction to infectious processes. In many cases the histologic changes are nonspecific and a particular etiology cannot be assigned; in other cases the findings are characteristic of specific entities. This chapter focuses on histologically distinctive types of infectious lymphadenitides and on their differential diagnosis.
Infectious mononucleosis is a symptomatic primary infection by Epstein-Barr virus (EBV), characterized by the classic triad of fever, pharyngitis, and cervical lymphadenopathy. Infectious mononucleosis was first described as a distinct entity in 1920, when six college students with pharyngitis, fever, and lymphadenopathy (a syndrome referred to previously as glandular fever) were all found to have an absolute lymphocytosis in the peripheral blood with mononuclear cells with unusually abundant cytoplasm. Subsequently, Paul and Bunnell found that the serum of patients with infectious mononucleosis caused sheep red blood cells to agglutinate due to the presence of what they called a heterophile antibody, providing a laboratory test for the diagnosis of infectious mononucleosis. The definition of infectious mononucleosis was further refined when a laboratory worker exposed to EBV, which had only recently been discovered and identified as being associated with endemic Burkitt lymphoma, developed heterophile-positive infectious mononucleosis, establishing EBV as the causative agent.
Currently the diagnosis of infectious mononucleosis is usually established clinically. When enlarged lymph nodes or tonsils are biopsied, it is often because the presentation is not typical for infectious mononucleosis and the possibility of infectious mononucleosis was not considered. On pathologic evaluation, problems in differential diagnosis are not uncommon.
Infectious mononucleosis is a fairly common disease of adolescents and young adults. Cases of infectious mononucleosis in young children have been reported, although EBV infection in this age group is usually subclinical. Rare cases are also described in older adults. Males and females are affected equally. Development of symptomatic infectious mononucleosis is most likely related to a combination of socioeconomic and genetic factors. Infectious mononucleosis is more commonly encountered among individuals in higher socioeconomic groups, who are less likely to be exposed to EBV during early childhood. Transmission is through close contact with another individual who is EBV infected, with an incubation period of 4 to 7 weeks.
The classic findings at presentation include fever, pharyngitis, and cervical lymphadenopathy, accompanied by an atypical peripheral blood lymphocytosis and a positive heterophile antibody (Monospot) test. Lymphadenopathy is usually symmetrical and tender. Both anterior and posterior triangle lymphadenopathy may be prominent, in contrast to bacterial tonsillitis, in which lymphadenopathy typically involves only the upper anterior cervical lymph nodes. Splenomegaly is also common, occurring in approximately 50% of cases. A maculopapular or urticarial rash may develop, mainly among patients who are treated with a β-lactam antibiotic.
Supportive therapy is usually sufficient, and spontaneous recovery after an illness of up to 4 weeks is typical, except for the small minority of patients with serious complications. In more severe cases, there may be generalized lymphadenopathy, hepatomegaly with hepatic dysfunction, splenic rupture, peripheral cytopenias, and development of a hemophagocytic lymphohistiocytosis. In most cases the illness is self-limited, but, rarely, intercurrent infection, Guillain-Barré syndrome, or splenic rupture with hemorrhage results in death. Rare patients develop chronic active EBV infection (CAEBV).
Problems in establishing a diagnosis on clinical and laboratory grounds sometimes arise because the heterophile antibody test may not be positive, especially early in the illness and among children, sometimes requiring repeated Monospot tests. In some individuals, particularly those at the extremes of age, the heterophile antibody is never detected. In such cases, serologic studies for EBV-associated antigens help to establish a diagnosis. Another scenario creating diagnostic difficulties is when patients have clinical features diverging from the classic presenting symptoms (e.g., presentation with diffuse lymphadenopathy without prominent pharyngitis). Patients with unusual clinical or laboratory features are more likely to undergo lymph node excision to establish a diagnosis than those patients with classic infectious mononucleosis.
The lymph nodal architecture is typically distorted but not effaced by an expanded paracortex containing a polymorphous population of lymphoid cells, including small lymphocytes, intermediate-sized lymphoid cells, immunoblasts, tingible body macrophages, and mature and immature plasma cells. The immunoblasts may occur in clusters or sheets and may be atypical, with pleomorphic or lobated nuclei. Binucleated cells resembling Reed-Sternberg cells may be identified. Mitotic figures may be numerous. Apoptosis is common, and zonal necrosis may be present. Primary follicles or reactive follicles are often present at the periphery of the lymph node, but follicular hyperplasia is not usually a prominent feature. Sinuses are patent in at least some areas, and frequently they are dilated. Sinuses contain histiocytes and a polymorphous population of lymphoid cells similar to that found in the paracortex, including immunoblasts. Lymphoid cells sometimes infiltrate the capsule and extend into perinodal fat. Similar histologic features are found in the tonsils of patients with infectious mononucleosis ( Fig. 12.1 ). In tonsils, crypts are usually present, although the epithelium lining them may be necrotic. Rare patients with acute infectious mononucleosis or with a recent history of infectious mononucleosis have florid follicular hyperplasia, sometimes accompanied by monocytoid B-cell hyperplasia. EBV-infected cells may be found in follicles and the interfollicular areas in such cases. Thus an uncommon pattern of acute or resolving infectious mononucleosis is florid follicular hyperplasia rather than a paracortical immunoblastic reaction.
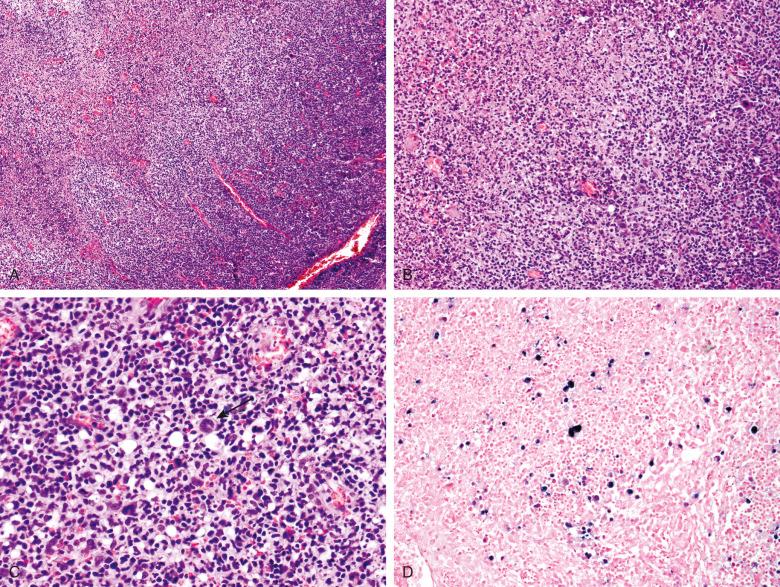
The small and intermediate-sized cells in the paracortex are predominantly T cells, including many that are activated in response to the presence of the virus. The CD4 to CD8 ratio is decreased. The paracortical EBV-infected immunoblasts are B cells that are typically CD20 + , MUM1/IRF4 + , CD10 − , BCL6 −/dim , BCL2 variable, OCT2 + , BOB1 + . Thus the EBV-positive immunoblasts have a postgerminal center immunophenotype. The B-immunoblasts may coexpress CD43. Using immunostains or in situ hybridization for kappa and lambda immunoglobulin light chains, plasma cells are polytypic. Polytypic light chain expression can often also be demonstrated in the immunoblasts. The immunoblasts often show staining for the activation antigen CD30; they are CD15 negative. Using in situ hybridization, EBV-encoded RNA (EBER) can be detected in the paracortical immunoblasts and scattered smaller cells.
Clonal rearrangement of the TCR gamma gene has been described in association with infectious mononucleosis and in an otherwise typical case of infectious mononucleosis should not be considered to support a diagnosis of T-cell lymphoma. The clonal T cells likely represent a focused immune response to EBV.
The differential diagnosis of infectious mononucleosis is broad. A variety of infectious processes can be associated with an illness with clinical features resembling infectious mononucleosis, including cytomegalovirus (CMV), human herpesvirus 6 (HHV-6), herpes simplex virus type 1 (HSV-1), human immunodeficiency virus (HIV), group A β-hemolytic Streptococcus pyogenes (strep throat), and Toxoplasma gondii . Careful clinical evaluation, augmented by laboratory studies, is required to establish a diagnosis.
The differential based on pathologic features includes a variety of reactive and neoplastic conditions. When immunoblasts are numerous, the possibility of diffuse large B-cell lymphoma is often a consideration. In favor of infectious mononucleosis are lack of architectural effacement, presence of areas readily recognizable as reactive hyperplasia, a polymorphous background of lymphoid cells, patent sinuses containing lymphoid cells (including immunoblasts), and lack of monotypic immunoglobulin expression on immunophenotyping. The typical immunophenotype of the immunoblasts (CD10 − , BCL6 −/dim , BCL2 +/− , MUM1 + ) may also be helpful because the majority of diffuse large B-cell lymphomas express BCL6. Diffuse large B-cell lymphomas are usually EBV-negative unless the patient is immunocompromised, so the presence of EBV should raise the possibility of infectious mononucleosis. In EBV-positive diffuse large B-cell lymphoma, EBV-positive cells will virtually all be large, whereas in infectious mononucleosis, they are of a range of sizes.
Classical Hodgkin lymphoma is often included in the differential diagnosis because Reed-Sternberg–like cells are often seen in infectious mononucleosis (see Fig. 12.1C ); however, most large cells in infectious mononucleosis have the appearance of immunoblasts rather than Reed-Sternberg cells or variants. Hodgkin lymphoma is more often associated with obliteration of the lymph nodal architecture. The polymorphous background of lymphoid cells ranging from small to intermediate and large in the paracortex and sinuses seen in infectious mononucleosis is very helpful in excluding Hodgkin lymphoma, in which background lymphocytes are all typically small. Granulocytes, especially eosinophils, and a background of fibrosis are more common in Hodgkin lymphoma. Immunoblasts in infectious mononucleosis are typically CD20 + , CD15 – , in contrast to the CD20 − , CD15 + typically found Reed-Sternberg cells. However, in some cases, CD20 is absent or only weakly expressed and Pax5 may be dim or negative in infectious mononucleosis, overlapping with findings in Hodgkin lymphoma, so that immunophenotype must be relied upon with caution. The CD4 to CD8 ratio is usually normal or elevated in Hodgkin lymphoma, in contrast to the CD8 predominance typically seen in infectious mononucleosis. Reed-Sternberg cells in approximately 40% of cases of classical Hodgkin lymphoma are EBV positive, so the presence of EBV does not exclude Hodgkin lymphoma. Similar to EBV-positive diffuse large B-cell lymphoma, the EBV-positive cells will all, or nearly all, be the large neoplastic cells in Hodgkin lymphoma, in contrast to infectious mononucleosis. Obtaining adequate clinical information is important to make the correct diagnosis. If the patient is known to have clinical or laboratory evidence of infectious mononucleosis, a diagnosis of Hodgkin or non-Hodgkin lymphoma should be made with caution.
Other viral infections, vaccination, and acute reaction to severe necrotizing processes can produce lymphadenopathy with histologic features similar to or indistinguishable from those of infectious mononucleosis. Reaction to certain drugs, including phenytoin and carbamazepine, can mimic the histologic features of infectious mononucleosis. Clinical information can be helpful in investigating the etiology of the lymphadenopathy. In addition, in infectious mononucleosis, EBV-positive immunoblasts are typically present in large numbers, whereas in other conditions, EBV-positive cells are usually absent. The Monospot test and serologic testing for EBV-specific antibodies can be helpful in establishing a diagnosis.
CMV is an uncommon cause of lymph nodal enlargement; lymphadenopathy may be localized or generalized. CMV lymphadenitis may occur in the setting of an infectious mononucleosis-like illness in which patients have sore throat, fatigue, and malaise; it may also be found in patients who are otherwise asymptomatic.
CMV lymphadenitis can occur in individuals of any age; males and females are equally affected. The risk of CMV lymphadenitis is increased in the setting of congenital or acquired immunodeficiency, and CMV infection is described in patients who have or have had Hodgkin lymphoma and non-Hodgkin lymphoma, but CMV lymphadenitis also occurs in immunologically normal individuals. CMV infection is common in patients with common variable immunodeficiency. Most patients have a self-limited illness and require no specific therapy. Patients with an associated lymphoproliferative disorder or immunodeficiency may have a more severe course.
On microscopic examination, involved lymph nodes most often show follicular hyperplasia and monocytoid B cell hyperplasia; this may be sufficiently florid to distort the nodal architecture. Paracortical hyperplasia may also be prominent. Infected cells contain a large eosinophilic intranuclear inclusion (mean size: 9 µm) and often also contain multiple tiny eosinophilic to amphophilic cytoplasmic inclusions. Cells with inclusions are usually present focally in relatively small numbers, but occasionally they are numerous. Neutrophils and histiocytes are often scattered around the infected cells. Inclusions are typically found among monocytoid B cells, but the infected cells are most likely histiocytes or endothelial cells rather than lymphocytes; finding inclusions in the paracortex has also been described.
Immunoperoxidase stains for CMV-associated antigens can be used to confirm the diagnosis when inclusions are found on routine sections and can help to identify CMV-infected cells when inclusions are difficult to find. Cells containing inclusions express CD15, usually with a Golgi region or diffuse cytoplasmic pattern of staining, but membrane staining is less common.
The differential diagnosis of CMV lymphadenitis includes reactive hyperplasia due to causes other than CMV and lymphoma, including both Hodgkin and non-Hodgkin lymphoma. If the viral inclusions are overlooked, the changes may be interpreted as nonspecific reactive lymphoid hyperplasia. The histologic features of CMV lymphadenitis overlap with those of toxoplasma lymphadenitis and early cat-scratch disease in that all three are characterized by follicular and monocytoid B-cell hyperplasia. Toxoplasma lymphadenitis is characterized in addition by clusters of epithelioid histiocytes, and cat-scratch disease, even at an early stage, typically also shows small foci of necrosis with fibrin and neutrophils.
Cells with nuclear viral inclusions may resemble Reed-Sternberg cells and variants on routinely stained sections, raising the question of classical Hodgkin lymphoma. CD15 expression by the virally infected cells enhances the resemblance to Hodgkin lymphoma. However, CMV lymphadenitis is much less likely to cause obliteration of the nodal architecture, and Hodgkin lymphoma is unlikely to be found in a lymph node occupied by florid follicular hyperplasia. Cells harboring virus may contain numerous granular cytoplasmic inclusions, in contrast to the agranular cytoplasm of Reed-Sternberg cells. Membrane staining by CD15 is more common in Reed-Sternberg cells than in CMV-infected cells.
Occasionally the follicular and monocytoid B-cell hyperplasia can be so florid as to distort the nodal architecture, potentially suggesting follicular lymphoma or nodal marginal zone lymphoma. Identification of CMV-infected cells, confirmed by immunostaining for CMV in a background of a follicular and monocytoid B-cell proliferation, establishes a diagnosis of CMV lymphadenitis.
HSV-1 or HSV-2 infection is rarely associated with lymphadenitis. In some cases, primary infection with HSV-1 occurs in the setting of an infectious mononucleosis-like syndrome, with pharyngitis, fever, and cervical lymphadenopathy. This type of lymphadenitis has been described in individuals over a wide age range. Males and females are equally affected. The risk of HSV lymphadenitis appears to be increased among immunodeficient individuals; some patients have also had a hematologic malignancy. The most common associated disorder is chronic lymphocytic leukemia, but other B-cell lymphomas and myeloid leukemias are also occasionally associated with HSV lymphadenitis. HSV can cause localized or generalized lymphadenitis or can involve lymph nodes in the setting of widespread visceral infection. When localized, the lymphadenopathy most often affects inguinal nodes, with cervical nodes next most often affected. The enlarged lymph nodes are characteristically tender or even painful. The presence of concurrent ulcerated cutaneous or mucosal lesions provides a clue to the diagnosis, but such lesions are not always present, and, when present, they may be inconspicuous and overlooked. Rarely, HSV-1 is responsible for Parinaud oculoglandular syndrome; manifestations include conjunctivitis, periorbital swelling and tenderness, and enlarged, painful preauricular lymph nodes.
Some patients have had no specific therapy and some have received acyclovir or valacyclovir. In those with an associated hematologic disorder, therapy may be directed at that rather than directly at the HSV infection. The prognosis depends on the extent of infection and on the prognosis of any underlying hematologic disorder or immunodeficiency. Disseminated HSV infection has a poor prognosis, but isolated HSV lymphadenitis is self-limited in most cases.
HSV infection results in a necrotizing lymphadenitis. Viable areas show prominent paracortical hyperplasia, often with numerous immunoblasts. Necrosis appears to begin in the paracortex at one pole of the lymph node and often shows prominent extension into perinodal soft tissue. The necrotic areas contain neutrophils, karyorrhectic or amorphous eosinophilic debris, and a variable number of viral inclusions, ranging from rare to abundant. Most infected cells are uninucleate, although a few multinucleated cells are seen ( Fig. 12.2 ). This is in contrast to HSV infection of squamous epithelial cells, in which multinucleated cells with nuclear molding are characteristic. Intact neutrophils are most abundant in early lesions and may be absent in long-standing lymphadenitis. Histiocytes often surround the necrotic areas, but granulomas are not found. Similar changes are found in the tonsils in herpetic tonsillitis. Varicella zoster virus rarely causes lymphadenitis; the histologic features are similar to those of HSV lymphadenitis.
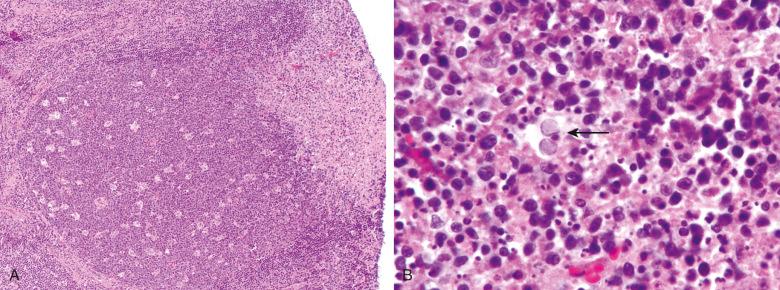
The diagnosis can be confirmed readily using immunohistochemical stains for HSV-1– and HSV-2–associated antigens; polymerase chain reaction (PCR), in situ hybridization, electron microscopy, or viral culture can also be used.
The differential diagnosis includes other types of necrotizing lymphadenitis as well as lymphoma. The lack of granulomatous inflammation with epithelioid or palisading histiocytes provides evidence against infections caused by mycobacteria, fungi, yersinia, cat-scratch bacilli, and lymphogranuloma venereum. Infectious mononucleosis typically shows paracortical expansion with immunoblasts and some necrosis, but EBV does not produce viral inclusions. In those cases with marked paracortical expansion with numerous immunoblasts, a high-grade lymphoma with necrosis may enter the differential diagnosis. In HSV lymphadenitis the nodal architecture may be distorted, although it is not obliterated, in contrast to most nodes involved by lymphoma. Viral inclusions are absent in lymphoma, except in cases in which HSV and lymphoma concurrently involve the same lymph node.
Reactive changes in lymph nodes directly related to HIV infection are discussed below. Lymphadenitis or lymphadenopathy in HIV-positive patients related to infectious agents other than HIV is discussed separately in those sections.
Most patients are young to middle-aged adult males, but occasional women and children of either gender may also be affected. There are a number of different clinical settings in which HIV-associated lymphoid hyperplasia can be found: lymphadenopathy, occurring in the setting of an infectious mononucleosis-like illness, in association with primary infection by HIV; persistent generalized or isolated lymphadenopathy, which may be accompanied by constitutional symptoms; cystic lymphoid hyperplasia, involving periparotid lymph nodes and causing multicystic, often bilateral, masses in the area of the parotid glands; and hyperplasia of extranodal lymphoid tissue, with symptoms related to mass effect.
Upon acute infection by HIV, some patients develop an infectious mononucleosis-like illness with fever, pharyngitis, and cervical lymphadenopathy. This has been referred to as the acute retroviral syndrome. Persistent generalized lymphadenopathy, defined as extrainguinal lymphadenopathy persisting for at least 3 months, involving at least two noncontiguous node groups, is common among HIV-positive patients. Persistent generalized lymphadenopathy mainly affects adult males and is often accompanied by fever, weight loss, headaches, and malaise. When periparotid nodes are affected, the hyperplastic lymphoid tissue can obstruct intranodal ductal structures, resulting in the formation of cysts within the nodes. This results in multicystic, often bilateral lesions in the area of the parotid. The process has been called cystic lymphoid hyperplasia and also lymphoepithelial cysts. When the lymphoid tissue of Waldeyer ring is affected, patients may present with a large, obstructive nasopharyngeal mass, nasal stuffiness, or nasal bleeding. The hyperplastic lymphoid tissue may obstruct the eustachian tubes and cause hearing loss.
Therapy is directed against the HIV infection itself. Outcome is related to the underlying HIV infection, but with antiretroviral therapy (ART) the prognosis has significantly improved.
A range of changes is found in lymphadenopathy associated with HIV infection. Similar changes may be found in organized extranodal lymphoid tissue. In early stages of HIV infection, lymph nodes show florid follicular hyperplasia. Follicle centers are often large and irregularly shaped and have an expanded dark zone with numerous blast cells, numerous mitoses, many tingible body macrophages, and attenuated or absent follicle mantles. Follicle lysis, in which the follicle center appears disrupted and fragmented by irregularly infiltrating small lymphocytes, sometimes accompanied by tiny foci of hemorrhage, is common. The interfollicular region contains a mixture of immunoblasts, plasma cells, lymphocytes, and histiocytes. Monocytoid B cells are often prominent. Sinuses are patent and may show sinus histiocytosis, sometimes with erythrophagocytosis. Scattered epithelioid histiocytes and polykaryocytes/Warthin-Finkeldey–type giant cells may be seen.
In advanced stages of immunodeficiency, there is lymphoid depletion. Follicles are decreased in number. Those follicles present are small and may be ill-defined and difficult to identify. Follicle centers are “burnt out” or regressively transformed and contain a decreased number of B cells. The paracortex appears hypocellular. It is occupied by scattered lymphocytes and immunoblasts, often with prominent vascularity. As in early stages of infection, plasma cells are often abundant. Amorphous eosinophilic interstitial material may be present, or there may be fibrosis, and the node may have a pale, depleted appearance. In some cases, lymph nodes show changes intermediate between florid follicular hyperplasia and lymphoid depletion.
Cutaneous rashes are common among HIV-positive patients; these individuals often have a component of dermatopathic lymphadenopathy.
In both early and late stages, B cells in follicles are polytypic. T cells show a decreased, usually reversed CD4 to CD8 ratio. Follicles with follicle lysis show a fragmented follicular dendritic cell (FDC) network; this may be seen with antibodies to CD21 or CD23. Few to many scattered cells infected by EBV may be found using in situ hybridization for EBER, reflecting poor immunologic control of EBV-infected B cells.
The pathologic changes are not completely specific, but if an individual has lymphadenopathy with the features described previously and the patient is not known to be HIV positive, performing a test for HIV is warranted. HIV-positive patients, particularly those with histologic changes consistent with advanced immunodeficiency, are at increased risk for opportunistic infections, Kaposi sarcoma, or lymphoma, and lymph nodes should be examined carefully to be certain one of these serious complications of HIV infection is not present. If follicles show changes of the type that may be seen in Castleman disease, a stain for HHV-8/Kaposi sarcoma–associated herpesvirus (KSHV) should be performed to investigate the possibility of HHV-8–associated multicentric Castleman disease (MCD) (see Castleman disease , later).
Most patients who have been treated with ART show improvement in lymphoid architecture, as well as an increase in CD4 + T cells and a decrease in CD8 + T cells within lymphoid tissue. However, HIV often is detectable in FDCs in lymphoid tissue, even after a prolonged course of ART, and even in the absence of detectable virus in the peripheral blood.
Measles is an infectious disease caused by a paramyxovirus of the genus Morbillivirus . Measles is uncommon in the United States because of widespread inoculation of children; however, it continues to be a significant health problem in multiple countries. Patients typically present with fever, rash, malaise, cough, sore throat, and headache. Lymphadenitis in the setting of measles occurs in a minority of cases. Lymph nodes show follicular hyperplasia; the most distinctive feature is the presence of Warthin-Finkeldey giant cells within reactive germinal centers. Warthin-Finkeldey giant cells are found in the prodromal stage of infection and generally disappear as a rash develops.
HHV-6 is the etiologic agent of a common febrile illness among young children that is known as exanthem subitum, roseola infantum, or sixth disease. Adults infected by HHV-6 may develop a heterophile-negative mononucleosis-like illness with cervical lymphadenopathy. Rare cases of lymphadenitis in the setting of a severe acute febrile illness due to HHV-6 in adults have been described. Lymph nodes in these systemically ill patients showed marked paracortical expansion by a polymorphous population of cells of varying sizes, including many atypical-appearing cells containing large eosinophilic nuclear and/or cytoplasmic viral inclusions. Virally infected cells appear to be CD4 + T cells. The differential diagnosis in such cases may include cytomegaloviral lymphadenitis because the inclusions bear some resemblance to those of CMV. The paracortical expansion in conjunction with many immunoblasts or virally infected cells resembling immunoblasts could raise the possibility of lymphoma. A small proportion of cases of lymphadenitis with the morphology of Kikuchi disease may be due to HHV-6 infection (see Kikuchi disease , later). Immunostains for HHV-6–associated antigens, electron microscopy, PCR for HHV-6, and serologic studies may help to establish a diagnosis.
Kawasaki disease, also known as mucocutaneous lymph node syndrome and infantile polyarteritis, is a systemic vasculitis of uncertain etiology, but evidence strongly suggests that infection triggers the development of Kawasaki disease in genetically susceptible children. For this reason, it is included in this chapter.
Kawasaki disease is an acute febrile disease of young children, with 85% of patients under the age of 5 years. Boys are more often affected than girls, with a male to female ratio of approximately 2 : 1. In the United States the incidence is approximately 17 cases per 100,000 children younger than 5 years per year. The incidence is up to 20-fold higher among Northeast Asians than among whites. There is also a higher risk among family members of patients with Kawasaki disease.
The Centers for Disease Control and Prevention requirements for the diagnosis of Kawasaki disease include finding fever of 5 or more days, not responding to antibiotics, and a minimum of four of the following five findings:
bilateral conjunctival congestion,
abnormalities of lips and oral cavity, including diffuse erythema, dry fissured lips, and prominent lingual papillae (strawberry tongue),
abnormalities of the skin of the distal extremities, including erythema of palms and soles with edema early on and desquamation of fingertips later in the course of the disease,
polymorphous, nonvesicular, primarily truncal, rash, and
acute, nonsuppurative cervical lymphadenopathy, not due to any other identifiable cause. The lymphadenopathy is typically in the anterior cervical triangle. Although cervical lymphadenopathy is most characteristic, lymphadenopathy in a variety of other sites can develop.
In addition to the diagnostic criteria noted previously, patients may have cardiac abnormalities (electrocardiogram changes, cardiomegaly, murmurs), diarrhea, arthritis or arthralgia, peripheral blood abnormalities (neutrophilic leukocytosis with a leftward shift, anemia and/or thrombocytosis), elevated sedimentation rate, aseptic meningitis, mild jaundice, proteinuria, sterile pyuria, elevated transaminases, or a combination of these. Most patients recover after an illness of 3 to 4 weeks' duration, but without therapy 20% to 25% develop complications of coronary arteritis (aneurysm, thrombosis), which may be fatal, sometimes years later. If coronary artery dilatation is demonstrated on echocardiography, a diagnosis of Kawasaki disease is confirmed, even if four of the five previously mentioned criteria are not met. A subset of patients present initially with fever and cervical lymphadenopathy alone; these patients are slightly older on average than patients with more classical presentation of Kawasaki disease. They may be thought to have some form of infectious lymphadenitis, potentially leading to a delay in diagnosis and delay in instituting appropriate therapy.
Kawasaki disease can often be treated successfully with intravenous immunoglobulin (IVIg) and aspirin; steroids and other antiinflammatory agents can be used if symptoms persist. Prompt administration of IVIg reduces the risk of coronary arterial changes, and thus of serious or fatal complications of this disease.
Only a limited amount of information is available on histologic findings in lymph nodes in Kawasaki disease. Lymph nodes are reported to show paracortical expansion by lymphocytes, plasma cells, and monocytes/macrophages, as well as small to large areas of necrosis containing karyorrhectic debris, sometimes with admixed neutrophils. Some reports also describe an admixture of immunoblasts. Increased numbers of blood vessels lined by swollen endothelial cells and containing fibrin thrombi may surround the necrotic areas. The capsule is inflamed, and in some instances there is inflammation and vasculitis in perinodal soft tissue. Some lymph nodes show nonspecific changes without necrosis. Activation of the immune response is believed to underlie the pathogenesis of the disease; important components are proposed to be immunoglobulin A (IgA), cytotoxic T cells, and monocytes/macrophages.
Kikuchi disease, also known as histiocytic necrotizing lymphadenitis, subacute necrotizing lymphadenitis, Kikuchi lymphadenitis, and Kikuchi-Fujimoto disease, is an uncommon cause of lymphadenopathy. This disease is of unknown etiology, but an infectious etiology has been implicated in the pathogenesis of at least a subset of cases of Kikuchi disease.
Most patients with Kikuchi disease are between adolescence and middle age, but it is mainly a disease of young adults, with a mean and median age in the third decade. There is a female preponderance that ranges from modest to pronounced in different series. Kikuchi disease appears to be more prevalent among Asians than in Western populations. Most patients present with unilateral, often painful, cervical lymphadenopathy. Other lymph nodes are affected much less often. Infrequently, there is generalized lymphadenopathy. Approximately one-half of patients have fever at presentation. Some patients have anemia or neutropenia; one-quarter of patients have an atypical lymphocytosis. Leukocytosis is uncommon. Occasionally, patients have a rash.
The minority of patients who are in the pediatric age range show a slight male preponderance, in contrast to adolescents and adults. Children are also more likely to have fever and to have a rash than older patients.
Kikuchi disease is typically self-limited, and most patients recover without therapy. A small number develop recurrent lymphadenopathy. Rarely, Kikuchi disease occurring in immunocompromised patients results in a severe illness. A few patients thought to have Kikuchi disease later develop systemic lupus erythematosus; this may be coincidental or the patient may have had systemic lupus erythematosus mimicking Kikuchi disease from the outset.
A variety of viral and other types of infectious etiologies have been suggested as potential causes of Kikuchi disease, including EBV, parvovirus B19, HTLV-1, HHV-6, and HIV. However, no one infectious agent stands out as the principle cause of Kikuchi disease, and when large series of Kikuchi disease are studied, the majority of cases have no associated specific, identifiable infectious agent. The apparent evolution to systemic lupus erythematosus in a small number of cases raises the question of Kikuchi disease being an autoimmune disease that is self-limited in the vast majority of cases. Alternatively, it may be that these cases represented unrecognized cases of systemic lupus erythematosus that were misdiagnosed as Kikuchi disease. Some patients have developed lymphadenitis with features of Kikuchi disease as a reaction to foreign material or other types of noninfectious conditions. Kikuchi disease appears to be more common among individuals with certain human leukocyte antigen (HLA) class II genes. It is possible that the histologic and immunohistologic features of Kikuchi disease can be produced by a variety of stimuli, depending on the susceptibility of the individual affected.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here