Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Pathogenic involvement of joints and other synovium-lined structures, including tendon sheaths and bursae, is a relatively uncommon but important form of infection. Its significance is related to the severe destruction of musculoskeletal structures it can cause, which often results in permanent disability and, in some instances, even death.
Synovial tissue is vital to the normal functioning of the musculoskeletal system. It helps maintain joints, tendons, bursae, and ligaments; is the source of nourishment for articular cartilage; and participates in the immune surveillance and activity of these tissues. The synoviocytes secrete the fluid that provides lubrication, protection, and nourishment to the adjacent structures. Articular cartilage, which lacks its own vascular supply, is wholly dependent on synovial fluid for the provision of oxygen and other essential metabolites. The fluid is also rich in glycoproteins, hyaluronic acid, and lysosomal enzymes that are important in the homeostasis of chondrocytes, fibroblasts, and the structural matrix. Histologically, synoviocytes are cuboidal to elongate and are arranged in a layer, two to four cells thick, which is supported by loose fibrofatty connective tissue containing abundant blood vessels, lymphatics, and nerves. The synovial lining lacks a protective basement membrane, and this, in conjunction with its rich vascular network, makes it a prime target for invasion by a wide variety of microorganisms ( Table 15.1 ).
| Gram-Positive Bacteria (60–70%) | |
| Staphylococcus aureus | 44% |
| Streptococcus pyogenes | 7% |
| Streptococcus pneumoniae | 7% |
| Coagulase-negative staphylococci | 4% |
| Streptococcus agalactiae | 3% |
| Other β-hemolytic streptococci | 4% |
| Gram-Negative Bacteria (20–30%) | |
| Mycobacterium tuberculosis | 4% |
| Escherichia coli | 4% |
| Haemophilus influenzae | 4% |
| Neisseria gonorrhoeae | 3% |
| Pseudomonas aeruginosa | 2% |
| Neisseria meningitidis | 1% |
| Salmonella spp. | 1% |
| Other gram-negatives | 5% |
| Polymicrobial/Anaerobic Bacteria (2–5%) | |
| Bacteroides | |
| Fusobacterium | |
| Peptostreptococcus | |
| Clostridium | |
| Fungal (4%) | |
| Candida spp. | |
| Sporothrix schenckii | |
| Coccidioides immitis | |
| Blastomyces dermatitidis | |
| Cryptococcus neoformans | |
| Histoplasma capsulatum | |
| Miscellaneous (1%) | |
Mechanistically the initial entry of an organism into a joint or synovial structure occurs by the same pathways involved in osteomyelitis; namely, hematogenous seeding, extension from a contiguous site, and direct implantation. In many situations, hematogenous seeding is the most common etiology, followed in frequency by spread from a neighboring infection in bone or soft tissue. Trauma with direct implantation is a common cause of tenosynovitis and bursitis, which often affects the digits of the hands and feet and may less frequently involve larger joints. Because tendon sheaths may communicate with bursae, infection of these structures can produce complicated patterns of extension.
After an organism infects the synovium, there is usually an acute inflammatory response with vascular dilatation and increased permeability, resulting in tissue edema and hyperemia. The inflamed synovium undergoes architectural changes to accommodate its increased girth by forming papillary structures lined by hypertrophied and hyperplasic synoviocytes. The synoviocytes increase their secretion of fluid, and this, in conjunction with serum leakage from reactive vessels and fibrin production, results in effusion of the affected joint, bursa, or tendon sheath. Both pathogenic infiltration and the reactive host inflammatory reaction can trigger necrosis of synoviocytes, chondrocytes, and tendinocytes and digestion of crucial structural components, with subsequent destruction of the tissues. The closed physical nature of joints, tendon sheaths, and bursae limits their expansion, so, after they are inundated by the infectious process, secondary ischemia and its complications (e.g., compartment syndromes) may occur. Over time, chronic inflammatory cells appear and are accompanied by reactive fibrosis and scarring. If located near bone, the infection can initiate osteoclastic activity and erosion of the bone; this frequently manifests as juxtaarticular erosions in joint infections. Eventually, there can be severe and permanent destruction of the joint and periarticular structures, resulting in significant dysfunction. In some instances the infectious agent, because of molecular mimicry between the host and the pathogen, initiates an autoimmune reaction that may be short lived or chronic and may cause further significant damage and disability.
Bacteria are the most common cause of infection of the synovium. Large weight-bearing joints, such as the knee, are particularly prone to hematogenous seeding. Small joints of the hands and feet are more likely to develop septic arthritis secondary to penetrating trauma. Iatrogenic causes of infection include arthroscopy, corticosteroid injection, surgery, and implantation of prosthetic joints.
The primary risk factor for septic arthritis is preexisting joint disease, and up to 47% of patients have prior joint problems or rheumatologic disorders, including rheumatoid arthritis, systemic lupus erythematosus (SLE), osteoarthritis, gout, pseudogout, trauma, or prior joint surgery. Rheumatoid arthritis is most frequently associated with septic arthritis and tends to have worse outcomes. Chronic breach in skin integrity (e.g., psoriasis, chronic skin ulcers, intravenous drug use) is also a risk factor. An immunocompromised state (e.g., malignancy, corticosteroid use, chronic disease, acquired immune deficiency syndrome [AIDS]) is strongly linked to increased risk for infectious arthritis, in particular with atypical microorganisms, such as Listeria , Salmonella , and Actinobacillus , as well as fungal and mycobacterial arthritides.
Most cases of bacterial arthritis are due to occult bacteremia with gram-positive organisms, typically Staphylococcus aureus . Gram-negative bacilli account for 10% of community-acquired septic arthritis and are associated with intravenous drug use. Gram-positive bacteria are thought to more efficiently bind extracellular matrix proteins, thereby facilitating adhesion to affected tissues. A number of bacterial adhesion genes that may be involved in this process have been identified, including those encoding fibrinogen-binding proteins (fib, cflA, fbpA) , fibronectin-binding proteins (fnbA, fnbB) , an elastin-binding protein (ebpS) , a collagen receptor (cna) , and a less specific binding protein (map) . Bacterial invasion and proliferation result in direct soft tissue and cartilage damage through the production of bacterial enzymes and toxins (e.g., staphylococcal TSST-1, enterotoxins, α-hemolysin). The host inflammatory response further damages articular cartilage through activation of matrix metalloproteinases (MMPs) and release of reactive oxygen species, lysosomal enzymes, and proinflammatory cytokines, including interleukin (IL)-2, interferon (IFN)-γ, and tumor necrosis factor (TNF)-α. Articular cartilage is also subjected to ischemia as accumulation of pus within the joint space leads to increased joint pressure and tamponade of synovial blood flow. If left untreated, infection may spread to adjacent bone and soft tissues, leading to the formation of sinus tracts, osteomyelitis, and possible destruction of tendons and ligaments.
S. aureus accounts for 40% to 50% of cases of septic arthritis, followed by Streptococcus pyogenes (8%), Streptococcus pneumoniae (7%), Haemophilus influenzae (4% to 7%), Escherichia coli (4%), coagulase-negative staphylococci (4%), Neisseria gonorrhoeae (3%), Streptococcus agalactiae (3%), Pseudomonas aeruginosa (2%), Neisseria meningitidis (1%), and Salmonella spp. (1%). Other gram-negative rods and β-hemolytic streptococci are responsible for 5% and 4% of cases, respectively, and 1% to 2% of cases are polymicrobial. Staphylococcus and Streptococcus are the primary bacterial pathogens in pediatric septic arthritis, whereas vaccination against H. influenzae has significantly reduced the incidence of arthritis associated with that organism.
Kingella kingae , a β-hemolytic aerobic/facultative anaerobic gram-negative bacillus that forms part of the normal pharyngeal flora of infants is now recognized as an important pathogen in children younger than 2 years of age. Median age of presentation is 18.6 months, and infection is rare in patients older than 4 years. Infection typically occurs after a breach in mucosal integrity (often a result of upper respiratory illness) that leads to occult bacteremia. Clinical systemic symptoms are mild, and disease is typically monoarticular or oligoarticular, with large weight-bearing joints being the most common sites of involvement. Osteomyelitis and spinal discitis have also been reported. Since the advent of polymerase chain reaction (PCR)-based diagnostic testing, K. kingae has been reported to account for 48% of proven bacterial joint infections in patients under 2 years, and is now thought to be the single most common bacterium responsible for septic arthritis in children younger than 3 years.
Anaerobic pathogens are fastidious and difficult to culture and therefore are often overlooked as a cause of septic arthritis. The infections are often polymicrobial, and their true incidence is uncertain. Bacteroides , Peptostreptococcus , Fusobacterium , Clostridium , and Propionibacterium acnes are the primary culprits, and pigmented Prevotella , Porphyromonas , and Fusobacterium are all associated with bite infections. Bacteroides fragilis has a predilection for hand and foot infections. Risk factors for anaerobic infection include collagen vascular disease, bites, trauma, prosthetic joints, prior surgery, and peripheral neuropathy. Adults are more frequently affected than children.
Bacterial arthritis is usually monoarticular. The knee is the primary site for bacterial arthritis, with 30% to 50% of cases, followed by hip (15% to 22%), ankle (6% to 9%), elbow (8% to 9%), wrist (4% to 6%), and shoulder (5% to 12%). In the pediatric population the most common sites of septic arthritis are the hip and knee, and 90% of cases are monoarticular. Polyarticular disease occurs in approximately 15% of cases and is associated with immunocompromise. Gonococcal, pneumococcal, group B streptococcal, and gram-negative infections also may result in polyarthritis. Septic polyarthritis is asymmetric, and 72% of cases involve the knee. The cartilaginous joints of the axial skeleton are relatively resistant to infection, although an association with intravenous drug use has been established.
The classic presentation of septic arthritis is fevers and rigor accompanying a swollen, warm, erythematous, and painful joint. High-grade fever is present only in 58% of cases, whereas low-grade fever is present 90% of the time. Fewer than 60% of patients present with a serum leukocytosis, and immunocompromised patients may not report pain. Therefore a high index of suspicion should be maintained, even in the absence of classic symptoms, and arthrocentesis should be performed. Synovial fluid chemistries, including protein, glucose, and lactate dehydrogenase concentrations, as well as synovial white blood cell (WBC) counts, are relatively nonspecific for infection. Infectious etiologies should be considered in all synovial fluids with a WBC count greater than 50,000 cells/µL; however, a low WBC count does not exclude septic arthritis ( Table 15.2 ). Gram staining and culture should be routinely performed whenever infection is suspected, as well as PCR or serologic testing where indicated.
| Parameter | Normal | Noninflammatory | Inflammatory, Viral, or Reactive | Bacterial | Fungal | Crystal | Trauma |
|---|---|---|---|---|---|---|---|
| Color | Yellow | Yellow | Yellow | Gray-green | Variable | Yellow-white | Red-brown |
| Clarity | Clear | Slightly cloudy | Cloudy | Cloudy | Variable | Cloudy | Bloody |
| Viscosity | High | Reduced | Low | Low | Low | Low | Reduced |
| Clot | No | Rare | Rare | Frequent | — | Rare | Yes |
| WBCs/µL | 0–200 | 0–2000 | 2000–100,000 | 20–200,000 | 10,000–100,000 | 500–200,000 | 50–10,000 |
| % Neutrophils | <25 | <30 | >50 | >75 | >50 | <90 | <50 |
| Serum glucose concentration (mg/dL) | 0–10 | 0–10 | 0–40 | 20–100 | 10–20 | 0–80 | 0–20 |
| Crystals | No | No | ± | ± | ± | Yes | ± |
| Culture | Negative | Negative | Negative | Most organisms | Some organisms | Negative | Negative |
| PCR/Serology | Negative | Negative | Positive | Some organisms | Some organisms | Negative | Negative |
| Stains | Negative | Negative | Negative | Gram stain, AFB, silver stain | Silver stain, PAS, Gram stain | Negative | Negative |
* Synovial WBCs, glucose, protein, and lactate dehydrogenase concentrations are relatively nonspecific indicators of infection. Presence of crystals does not exclude septic arthritis. Gram stain, culture, PCR, or serologic studies remain essential for definitive diagnosis and organism identification.
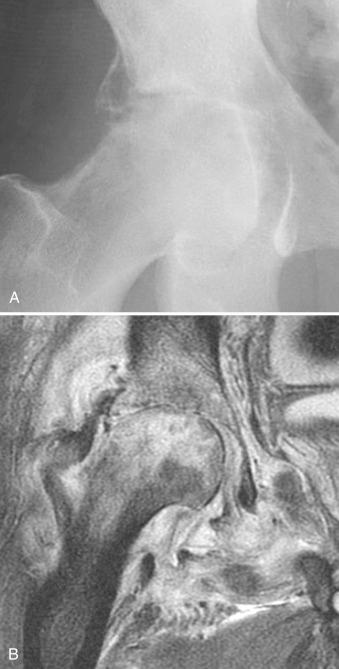
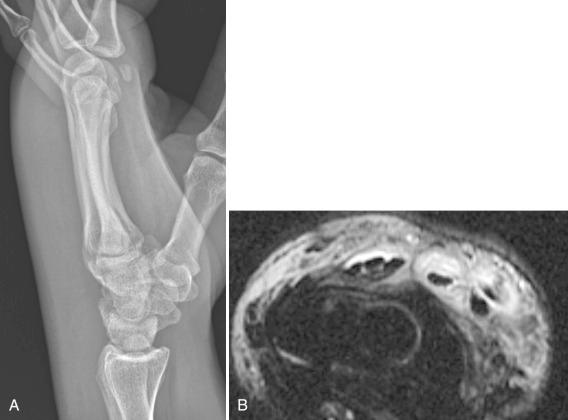
Historically, plain films have been of use only in late-stage disease, demonstrating joint effusions, cartilage loss, or bone destruction ( Fig. 15.1 ). Magnetic resonance imaging (MRI) may be helpful in diagnosing early lesions, including those that involve tendon sheaths, with findings such as effusion, synovial thickening and enhancement, bone marrow edema, and perisynovial edema and enhancement ( Fig. 15.2 ). Reactive bone marrow edema in septic arthritis can be mistaken on imaging for periarticular osteomyelitis. However, in osteomyelitis, the edema demonstrates markedly low signal on T1-weighted images and is more diffuse and robust than that seen in association with septic joints, although these findings are not specific. Ultrasound may also be used to detect early effusion. Echo-free effusions, resulting from clotting hemorrhagic fluid collections, are thought to be characteristic of septic arthritis. Imaging studies can also be used to monitor the response to treatment or to demonstrate underlying conditions such as osteoarthritis.
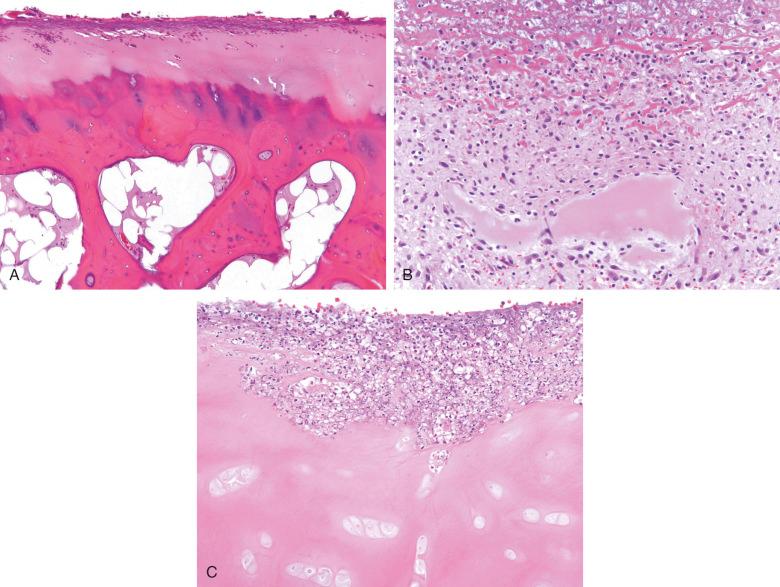
Histologic examination of infected synovial tissue from native joints is rarely performed, because diagnosis relies on clinical findings, arthrocentesis, and analysis and culture of synovial fluid. Animal models have shown that joint injury is rapid, with synovial hypertrophy visible within 24 hours after intravascular bacterial injection, whereas bone erosions and pannus formation may develop within 3 days. Early septic arthritis is dominated by dense neutrophilic infiltration of the synovium, which may coat articular cartilage and result in its necrosis and digestion ( Fig. 15.3 ). Eventually, lymphocytes, plasma cells, and histiocytes appear, and an acute and chronic papillary synovitis with fibrin aggregates develops. These changes resemble those seen in noninfectious inflammatory arthritides, such as rheumatoid arthritis. In long-standing disease the entire articular cartilage may be eroded, resulting in osteomyelitis ( Fig. 15.4 ).
Treatment of septic arthritis should be prompt and involves appropriate antimicrobial therapy. Intraarticular antibacterial therapy is not usually indicated. Drainage should be performed by needle aspiration or open drainage and may be repeated. Débridement may be required.
Outcomes in septic arthritis may be poor, especially if the infection accompanies systemic sepsis, with an overall mortality rate as high as 11%. Morbidity is also high, with secondary osteomyelitis occurring in 8% and poor functional outcome in 24% to 40% of cases. Poor outcome is associated with polyarticular arthritis, age greater than 65 years, delay in presentation, existence of medical comorbidities, chronic illness, and rheumatoid arthritis.
N. gonorrhoeae , a gram-negative diplococcus, is the most common sexually transmitted bacterium associated with infective arthritis. In the 1970s through the 1980s it was the most common cause of infective arthritis in the United States. Safer sex practices resulted in a steep decline in disease prevalence through the 1990s. More recently, gonococcal infections have been on the rise, primarily due to high-risk sexual behavior among young men who have sex with men. Of further concern is the appearance of antibiotic-resistant strains. These two trends ensure that gonorrhea will continue to be of clinical importance for the foreseeable future.
Patients with gonococcal arthritis are usually young, healthy, and sexually active. Gonococcal arthritis may take one of two forms: a classic suppurative arthritis similar to the septic arthritis seen with other bacterial infections or a syndrome of disseminated gonococcal infection (DGI) including tenosynovitis, skin lesions, and polyarthralgias without true septic arthritis. Some authors have postulated an immunologic basis for DGI. However, the presence of positive blood cultures in up to 50% of cases and an inability to identify circulating immune complexes argue against this theory.
Gonococcal infection begins with localized mucosal disease. In fewer than 3% of cases, the organism rapidly enters the circulation and produces DGI. Risk factors associated with DGI include delayed diagnosis, SLE, deficiency of the complement system, menstruation, and pregnancy, as well as socioeconomic factors, promiscuity, urban residence, male homosexuality, and educational status. Bacterial strains also play a role, with higher risk associated with piliated N. gonorrhoeae strains that are capable of phase variation. Women have four times the risk of men for development of DGI, most likely because of asymptomatic infection and delayed diagnosis.
Patients with DGI present with migratory arthralgias, fever, chills, dermatitis, and tenosynovitis. Most are found to have asymptomatic mucosal gonococcal infection involving the genitals, anus, or pharynx. Skin lesions are classically limited to the extremities and trunk and begin as small, inconspicuous erythematous papules, which progress to vesicular or pustular lesions. The papules may overlie affected joints. Tenosynovitis causes pain, swelling, and erythema. Polyarthralgias may involve knees, elbows, and distal joints, whereas arthritis and tenosynovitis are polyarticular and preferentially involve the distal joints, including those of the hands and fingers, wrists, elbows, knees, and ankles.
Suppurative gonococcal arthritis is monoarticular or oligoarticular and involves the knees, wrists, ankles, and elbows. Symptoms of bacteremia, skin lesions, or prior polyarthralgias are not present.
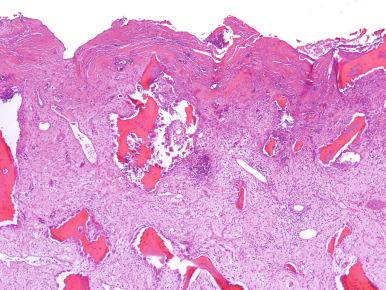
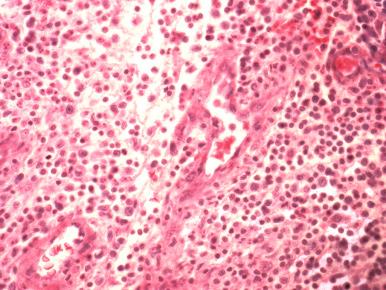
The radiographic and histopathologic findings in gonococcal arthritis are similar to those seen in other septic bacterial arthritides. However, imaging studies and synovial biopsy are rarely performed. In rare biopsied cases the periarticular soft tissues show granulation tissue with mixed polymorphonuclear and mononuclear cell infiltrates ( Fig. 15.5 ).
Synovial fluid analysis and culture are required for diagnosis. Treatment requires appropriate antibacterial therapy in conjunction with aspiration. Surgery is rarely necessary. Patients should be tested for concomitant infection with Chlamydia trachomatis . Appropriate treatment is associated with a good outcome.
The differential diagnosis of DGI includes N. meningitidis infection, which manifests with similar skin lesions and arthralgias. Other bacterial infections also must be ruled out and can usually be distinguished by their clinical features or by culture.
Infection with N. meningitidis is associated with a sterile monoarticular or oligoarticular arthritis that is thought to be secondary to a hypersensitivity reaction with immune complex deposition. Recovery from this form of arthritis is usually complete. Infrequently, meningococcus may lead to a monoarticular septic arthritis.
Lyme borreliosis was first described in 1975 in the United States, after the perplexing outbreak of juvenile rheumatoid arthritis in the vicinity of Lyme, Connecticut. The causative agent, the spirochete Borrelia burgdorferi , was not identified until 1982. Lyme disease is now recognized as the most common vector-borne disease in North America and Europe, with annual incidence rates as high as 31.6 cases per 100,000 population in endemic areas. In the United States it is localized to three endemic areas: the East Coast (Maine to Maryland), the Midwest (Minnesota and Wisconsin), and the Northwest (Northern California and Oregon). However, these regions continue to enlarge as the geographic range of the vector ticks infected with B. burgdorferi expands.
Three members of the Borrelia genus are known to cause Lyme disease in humans: B. burgdorferi (endemic to North America), Borrelia garinii , and Borrelia afzelii (endemic to Europe and Asia). More recently, Borrelia lonestari has been implicated in a Lyme disease–like illness in the southern United States, and Borrelia miyamotoi is a recently recognized borreliosis transmitted by Ixodes persulcatus , which presents with Lyme disease–like symptoms, including myalgia and arthralgia in 30% to 60% of patients, in the absence of a preceding rash. Borreliosis is a vector-borne disease, with pathogens transmitted by the bite of infected Ixodes ricinus ticks. In the larval and nymph stages of the tick life cycle, ticks feed primarily on small rodents—predominantly the white-footed mouse in the United States and the vole in Europe and Asia. These animals then serve as a disease reservoir for horizontal transmission between nymphs and larval ticks. In contrast, larger mammals, such as deer or humans, preferred as a food source for adult ticks, are dead-end hosts for Borrelia . For Borrelia transmission to occur, the tick must be attached to its host for at least 24 hours ; this allows the bacteria to migrate from the tick midgut into the salivary gland, a process mediated by cell surface adhesion molecules, including OspA and Ospc.
A number of factors have been implicated in disease transmission. Tick saliva is thought to contain vasodilators, anticoagulants, and immunomodulators, which may facilitate Borrelia transmission to the host. Factors on the spirochete membrane function as adhesion molecules, such as p66, which binds to β 3 -chain integrins, and BBK32, which binds to fibronectin. B. burgdorferi then binds to host plasmin and urokinase, resulting in plasmin-mediated MMP activation and proteolysis of extracellular matrix, which allows dissemination of bacteria throughout the host. Additional factors assist in immune evasion, by modulating host response or downregulation of immunogenic cell surface markers.
The pathognomonic lesion of early acute Lyme disease is the erythema migrans rash, which appears at the site of tick bite within 5 to 14 days, then spontaneously resolves after several days to weeks. The rash is erythematous, often with a central clearing, or bull's-eye, and is usually painless and nonpruritic. Erythema migrans has been reported to have an incidence of up to 90% in some studies of Lyme disease patients. Within a few weeks of infection, untreated patients develop symptoms of early disseminated disease, including fever, malaise, aseptic meningitis, facial palsy, radiculoneuropathy, or carditis, as well as migratory arthralgias and myalgias. True arthritis typically develops weeks or months after the initial infection.
Acute Lyme arthritis occurs in up to 50% to 60% of untreated patients infected with B. burgdorferi ; the incidence is lower with B. garinii or B. afzelii infection. Lyme arthritis is usually a monoarticular or oligoarticular arthritis that typically affects the knee or other large joints. Joints of the digits are occasionally involved, and tendonitis and bursitis may also occur. In most patients, arthritis occurs in flares, which resolve over a period of years. In a minority of patients, chronic synovitis may develop despite appropriate antibiotic treatment. Chronic synovitis is accompanied by joint injury and results in permanent joint damage in 2% of patients.
Chronic Lyme disease is considered to be a controversial diagnosis, and theories on its pathogenesis range from autoimmunity, to lingering B. burgdorferi cell bodies or bacterial DNA in tissue provoking an immune response. The crux of this controversy is the persistence of arthritis despite eradication of spirochetes in the affected joint. Evidence for persistent disease has been controversial at best, with the majority of studies finding no serologic or culture evidence of B. burgdorferi within synovial tissues after appropriate antibiotic therapy. The argument for an autoimmune response leading to arthritis is more convincing. Mechanistically this immune response is thought to be caused by focal homology between the B. burgdorferi membrane antigen OspA and hLFA-1α, an adhesion molecule expressed by most lymphoid and myeloid cells that is upregulated during an inflammatory response. An association with major histocompatibility complex (MHC) class II human leukocyte antigens HLA-DRB1*0401 and 0101 has also been identified. T cells are thought to be an important mediator of subsequent inflammatory changes, along with secreted proinflammatory cytokines.
On imaging, acute Lyme arthritis shows effusion of the knee joint. Intraarticular edema can be seen, along with a spectrum of soft tissue changes involving the infrapatellar fat pad, periarticular soft tissues, and entheses. In chronic Lyme arthritis, an inflammatory arthritis is seen, with juxtaarticular osteoporosis, loss of cartilage, and bony erosions. Rarely, degenerative changes, such as subarticular sclerosis and osteophyte formation, may be seen.
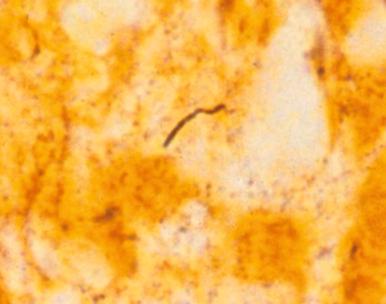
The histologic features of acute Lyme synovitis are similar to those observed in other septic arthritides, with edema and abundant neutrophilic infiltration of synovium and adjacent soft tissues. Organisms have been demonstrated in synovial tissues in the early stages of infection by Warthin-Starry silver stain ( Fig. 15.6 ), and PCR has been shown to improve detection. Histologic examination of synovial tissue from chronic Lyme arthritis demonstrates nonspecific features of chronic inflammatory or rheumatoid arthritis. Fibrin deposition is seen, along with synovial hypertrophy, neovascularization, and infiltration by histiocytes, plasma cells, and a subsynovial mixed lymphocyte population ( Fig. 15.7 ). In this setting the morphologic changes overlap with those of other, noninfectious inflammatory arthropathies.
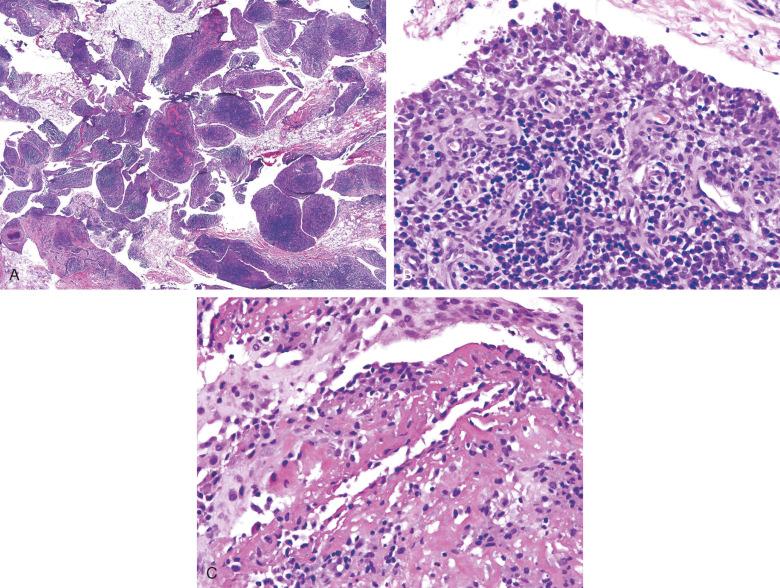
Diagnosis of Lyme arthritis should be considered in patients who have recently been in areas endemic for B. burgdorferi and present with acute or subacute monoarthritis. A history of erythema migrans is helpful, but not required, to proceed to serologic or synovial fluid testing. Treatment of acute Lyme arthritis involves oral or intravenous antibiotic therapy. If arthritis does not resolve with two courses of an appropriate antibiotic regimen and PCR testing of synovial fluid is negative for B. burgdorferi , chronic Lyme arthritis should be considered, and it should be treated with immunosuppressive therapy.
Joint involvement follows infection of the spine as the most common manifestation of musculoskeletal tuberculosis. Peripheral large and medium-sized joints, especially the hip and knee, are most frequently affected. Risk factors for development of tuberculous arthritis include female gender and older age; children rarely develop this form of infection. Seeding of the joint space is thought to occur as a complication of bone infection or through direct inoculation. Hematogenous spread can also result in multifocal joint involvement, especially in immunocompromised patients.
Pain and local swelling are common presenting complaints. Fever and weight loss are rarely present. Tuberculous arthritis has an indolent course, with the gradual development of a nonspecific synovitis and joint effusion.
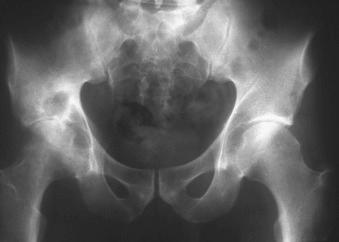
Radiographically the Phemister triad is characteristic. It consists of juxtaarticular osteoporosis, osseous erosions, and slight narrowing of the joint space ( Fig. 15.8 ). In contrast, the MRI findings of synovitis with pannus formation, effusions, and peripheral and central erosions are relatively nonspecific. On T2-weighted images, tuberculous synovitis demonstrates low signal in areas of granuloma formation.
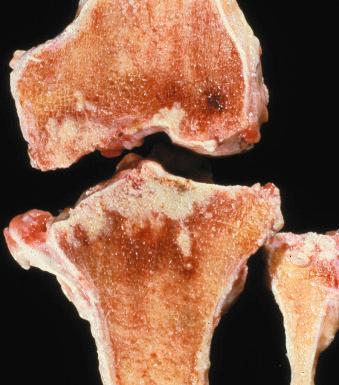
Grossly, the inflamed synovial tissue is tan-yellow, with destruction of the articular cartilage and possible erosion into bone ( Fig. 15.9 ). Histologically the synovium shows acute and chronic inflammation with necrotizing granulomas. The infection frequently extends into bursae and synovial tendon sheaths. Treatment consists of local débridement with multidrug antituberculosis chemotherapy.
Nontuberculous mycobacterial infections rarely affect the joints and are usually associated with underlying immunocompromise or rheumatologic disorders. Direct inoculation or trauma is occasionally the route of infection Hematogenous spread from a primary pulmonary lesion may also occur. Nontuberculous mycobacterial arthritis has also been seen in association with foreign bodies, including prosthetic joints. Among the nontuberculous mycobacteria, Mycobacterium kansasii is the most common cause of monoarthritis, whereas Mycobacterium marinum and Mycobacterium avium complex are the most frequent causes of tenosynovitis. Mycobacterium chelonae , Mycobacterium fortuitum , Mycobacterium abscessus , and Mycobacterium terrae , among others, have also been implicated.
Patients with nontuberculous mycobacterial arthritis typically present with an insidious onset of localized swelling and pain over the affected joint. A joint effusion may be present, and erosive changes may be seen on imaging. Nontuberculous mycobacterial tenosynovitis and arthritis most frequently involve the hands, although other sites, including the feet, shoulder, and knee, have been reported. Histologically the findings range from chronic synovitis with scattered macrophages to florid necrotizing granulomas ( Fig. 15.10 ).
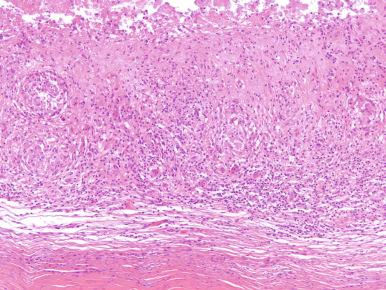
The differential diagnosis for nontuberculous mycobacterial arthritis includes sarcoidosis, especially if the small joints are involved; however, the histopathologic changes, special stains for microorganisms, and culture can establish the correct diagnosis.
Treatment of nontuberculous mycobacterial arthritis usually involves a prolonged course of multidrug antimycobacterial therapy with or without local débridement.
Fungal (mycotic) arthritis is a rare disease that arises mainly from hematogenous dissemination, although direct inoculation or extension from an adjacent region of infection may also occur. As with bacterial arthritis, predisposing factors include chronic disease, immunosuppression, and prolonged use of intravenous antibiotics, as well as the presence of indwelling catheters and joint prostheses. Since the advent of the human immunodeficiency virus (HIV) epidemic, the incidence of fungal arthritis has markedly increased. Although the true incidence is unknown, candidal infection is thought to account for most cases. However, other species, including Sporothrix schenckii , Coccidioides immitis , Blastomyces dermatitidis , Cryptococcus neoformans , and Histoplasma capsulatum , are also known to cause articular disease.
Mycotic arthritis tends to manifest indolently, with an extended course between symptom onset and diagnosis. Although any joint may be affected, the knee and other large joints are the most common sites. Clinical symptoms are nonspecific, and common complaints are decreased range of motion, pain, erythema, and joint effusion.
Radiographic findings demonstrate soft tissue swelling, periarticular bone erosions, effusions, and narrowing of the joint space.
Synovial fluid analysis may be suggestive of an inflammatory aseptic arthritis, although Candida and Cryptococcus are associated with purulent synovial fluid. Routine cultures are frequently negative. Histologically, there is an acute and chronic synovitis, and in some cases necrotizing granulomas are present. Special histochemical stains are useful in identifying the offending organism.
Treatment invariably involves intravenous antifungal therapy, and surgical drainage or débridement is often required.
Nine species of Candida are known to be pathogenic in humans. Candida albicans , Candida parapsilosis , Candida tropicalis , Candida glabrata , Candida guilliermondii , Candida krusei , and Candida zeylanoides have been identified in cases of candidal arthritis. Joint infections involving Candida spp. occur in the setting of disseminated disease in most cases. Other factors predisposing to Candida infection are indwelling catheters, intravenous drug use, parenteral hyperalimentation, malignancy, multiple surgeries, use of broad-spectrum antibiotics, and prosthetic joints. Candidal arthritis may also occur secondary to intraarticular corticosteroid injection.
Candidal arthritis most frequently involves the knee (67% of cases), although hip, shoulder, ankle, and other joints have been reported to be affected. Candidal infection from hematogenous spread usually causes a monoarticular or oligoarticular arthritis. Patients are febrile and may have leukopenia or leukocytosis. There may be a long latency between fungemia or joint surgery and onset of arthritis, and in rare cases symptoms have been reported for up to 4 years before diagnosis. Patients may present with a normal WBC count and no fever. Clinical symptoms include pain, inability to bear weight, and restricted range of motion. The affected joints are swollen, painful, and warm.
Radiologic examination reveals nonspecific soft tissue swelling, joint effusion, and destructive joint changes. If a prosthesis is present, there may evidence of loosening.
Candidal arthritis begins as a synovitis and spreads to adjacent bone. Grossly the synovium may be thickened and hyperemic, with scarring and erosion of articular cartilage. Histologic examination of infected synovium reveals histiocytic and mononuclear cell infiltration and fibrosis. Granulomas are rarely present. Silver stain or tissue Gram stain may demonstrate fungal organisms, but culture of synovial fluid or tissue is more sensitive. Blood cultures are often negative.
Treatment usually involves intravenous antifungal chemotherapy, with or without local débridement and synovectomy. Infected prostheses must be removed. Use of intraarticular antifungal chemotherapy is controversial. The mortality rate in cases involving a systemic fungal infection may be as high as 50%.
Candida is the prototypical fungal pathogen involved in pediatric arthritis. Affected patients are primarily neonates and infants younger than 6 months of age. Joint infections are typically a result of hematogenous spread in the setting of systemic invasive candidiasis. Risk factors include prematurity, low birth weight, respiratory distress syndrome, aspiration pneumonia, gastrointestinal defects, surgery, malnutrition, and use of broad-spectrum antibiotics. Maternal vaginal candidiasis may also be a factor. Patients present with fever and a swollen, tender joint, with abnormal positioning of the infected limb. Thirty percent of cases are polyarticular, and the knee is most frequently involved. There may be associated metaphyseal osteomyelitis, either by direct extension from synovium or by concurrent hematogenous spread. Radiographic findings include joint effusion, joint dislocation, or metaphyseal abnormalities. Intravenous antifungal therapy is the treatment of choice.
C. neoformans infection primarily involves the lungs and central nervous system. Disseminated disease is thought to occur by hematogenous spread from infected lungs. Cryptococcal joint infection is an extremely rare event that usually occurs by direct spread from adjacent infected bone. Cryptococcal arthritis may affect one or several joints, and the knee is the most common site of involvement. In one case, cryptococcal infection was documented in tendonitis and tenosynovitis of the forearm. Patients are usually immunocompromised as a result of organ transplantation, malignancy, corticosteroid use, or AIDS.
Patients present with warmth, swelling, tenderness, and limited range of motion in the affected joint. Symptoms of systemic disease may also be present. Radiographs show narrowing of the joint space, along with bony changes of osteomyelitis. MRI demonstrates synovial thickening and enhancement if tenosynovitis is present.
Histologic examination reveals epithelioid granulomas with multinucleated giant cells, moderate lymphocytic infiltration, and areas of necrosis. Intracytoplasmic and extracytoplasmic spheroidal budding organisms ranging in diameter from 5 to 20 µm can be seen on silver or periodic acid–Schiff (PAS) stains ( Fig. 15.11 ). Organisms may be cultured from synovial tissue or fluid.
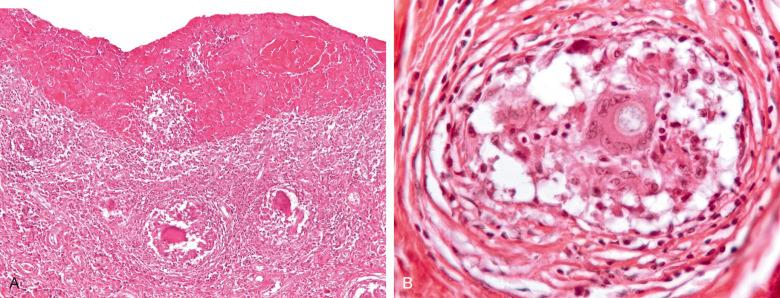
Treatment requires débridement and intravenous antifungal chemotherapy. Despite treatment, patients may develop postinfectious degenerative arthritis.
C. immitis infection begins with a pulmonary infection in immunocompetent individuals. In fewer than 1% of cases, infection disseminates, with involvement of meninges, skin and subcutaneous tissues, lymph nodes, and bones and joints. Acute polyarticular arthritis with fever, rash, eosinophilia, and bilateral hilar lymphadenopathy may develop as part of a hypersensitivity syndrome in 30% of cases during primary infection. The arthritis is migratory, and joints are tender and painful on motion. Effusions are generally not present. The ankle and knee are the primary joints affected, and the arthritis resolves within 1 month without permanent sequelae. In disseminated disease, arthritis arises from hematogenous seeding or direct extension from areas of osteomyelitis. Symptoms begin as intermittent, painful swelling of one joint and progress to large effusions, swelling, and development of draining sinus tracts. The knee is affected in 50% to 70% of cases, but any joint may be involved. Tenosynovitis may also occur.
Imaging findings include effusion, joint space narrowing, and bone destruction. Histology demonstrates villonodular synovitis, or pannus formation with nonnecrotizing granulomas, and spherules containing coccidioidal endospores. Treatment is with antifungal chemotherapy. Drainage and débridement may be necessary.
B. dermatitidis arthritis is a monoarticular arthritis that usually affects men from the third to fifth decade of life. The knee is the main site of involvement, followed by ankle, elbow, wrist, and hand. Joint infection may occur by hematogenous seeding or by spread from a focus of osteomyelitis. If left untreated, infection may spread to additional joints. The clinical onset may be abrupt, and pain and swelling are associated with fever and weight loss. Synovial fluid can be purulent. Diagnosis requires evidence of fungus, in synovial fluid or in any other site, and can be made on wet mount microcopy. Treatment with antifungal agents results in resolution of arthritis. Drainage of infected fluid may be necessary.
Joint manifestations of H. capsulatum infection occur as part of a hypersensitivity reaction during primary infection. This self-limited arthritis is migratory and polyarticular and may coexist with other hypersensitivity reactions, such as erythema nodosum, pleuritis, or pericarditis. Disseminated disease rarely results in more serious articular involvement, which is associated with immunocompromise. Histoplasma arthritis manifests as a monoarthritis, typically of the knee. Tenosynovitis involving the flexor tendon sheaths of the hand has been reported. Radiologically, destructive joint lesions, erosions, sclerosis, and joint space narrowing may be present. Diagnosis depends on detection of fungal organisms or on serology. Treatment is with antifungals, with or without débridement.
Infection with S. schenckii occurs after cutaneous inoculation or, less commonly, after inhalation of spores. Dissemination develops infrequently but may involve any organ system. The arthritis is chronic and can be monoarticular or polyarticular; it primarily involves the knee or, less often, the wrist or joints of the hands and feet. Symptoms are chronic, with an insidious onset. The diagnosis is often delayed, sometimes up to several years from symptom onset, and requires culture of synovial fluid or tissue. Radiographic findings include joint space narrowing, effusions, periarticular erosions, soft tissue swelling, and periarticular osteopenia. Treatment is with intravenous or intraarticular antifungal agents. Synovectomy may also be effective.
Aspergillus fumigatus and Aspergillus flavus are the most frequently identified Aspergillus spp. in human infection. Aspergillus infection in an immunosuppressed host very rarely causes arthritis and is usually associated with adjacent osteomyelitis. Aspergillus arthritis manifests with fever, chills, or malaise, as well as pain, swelling, and tenderness of the affected joint. A synovial effusion is present, and there is a restricted range of motion. On imaging, soft tissue swelling and osteomyelitis may be present. Diagnosis requires isolation of fungus from tissue or synovial fluid. Treatment requires both débridement and intravenous antifungal agents.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here