Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The two most effective means of preventing disease, disability, and death from infectious diseases have been sanitation and immunization. Both approaches antedated understanding of the germ theory of disease. Artificial induction of immunity began centuries ago with variolation, the practice of inoculating fluid from smallpox lesions into skin of susceptible persons. Although this technique usually produced mild illness without complications, spread of disease did occur, with occasional complications. In 1796, Jenner demonstrated that milkmaids who had contracted cowpox (vaccinia) were immune to smallpox. He inoculated the vesicular fluid from cowpox lesions into the skin of susceptible people and induced protection against smallpox, thus beginning the era of immunization.
Immunization, the act of artificially inducing immunity or providing protection from disease, can be active or passive. Active immunization consists of inducing the body to develop defenses against disease. This usually is accomplished by means of administration of vaccines or toxoids that stimulate the body's immune system to produce antibodies or cell-mediated immunity, or both, which protects against the infectious agent. Passive immunization consists of providing temporary protection through administration of exogenously produced antibody. Two situations in which passive immunization commonly occurs are through transplacental transfer of antibodies to the fetus, which may provide protection against certain diseases for the first 3 to 6 months of life, and injection of immunoglobulins for specific preventive purposes. A more detailed description of the immune mechanisms involved follows.
Immunizing agents include vaccines, toxoids, and antibody-containing preparations from human or animal donors. Several important definitions are provided here.
Vaccine: a suspension of attenuated, live, or killed microorganisms (bacteria, viruses, or rickettsiae), or fractions thereof, that is administered to induce immunity and thereby prevent infectious disease.
Toxoid: a modified bacterial toxin that has been rendered nontoxic but retains the ability to stimulate formation of antitoxin.
Immunoglobulin products include standard immune globulin (IG) for intramuscular (IM) use, hyperimmune globulins that are available for IM and/or intravenous (IV) use, standard immune globulin intravenous (IGIV), immune globulin subcutaneous (IGSC), antibodies of animal origin, and monoclonal antibodies. IG is a sterile, concentrated protein solution containing antibodies from human blood that reflect the infectious and immunization experience of the population from whose plasma the IG was prepared. IG contains 15% to 18% protein, consisting primarily of the immunoglobulin G (IgG) fraction (90%) with trace amounts of immunoglobulin A (IgA) and immunoglobulin M (IgM). IG primarily is indicated for routine protection of certain immunodeficient persons and for passive immunization against measles and hepatitis A. IGIV is indicated primarily for replacement therapy in immunoglobulin G (IgG) deficiency and pediatric human immunodeficiency virus (HIV) infection, treatment of Kawasaki disease, and idiopathic thrombocytopenic purpura. IGSC is indicated primarily for treatment of antibody deficiency.
Specific immunoglobulin: special preparations are obtained from donor pools preselected for high antibody content against a specific disease—for example, hepatitis B immune globulin (HBIG), varicella-zoster immune globulin (VariZIG), rabies immune globulin (RIG), tetanus immune globulin (TIG), and botulism IGIV used to treat infant botulism.
The constituents of immunizing agents include the following:
Suspending fluid: This frequently is as simple as sterile water or saline, but it may be a complex fluid containing small amounts of proteins or other constituents derived from the medium or biologic system in which the immunizing agent is produced (serum proteins, egg antigens, cell culture–derived antigens).
Preservatives, stabilizers, antibiotics: These components of vaccines are used (1) to inhibit or prevent bacterial growth in viral culture or the final product (preservatives and antimicrobial agents) or (2) to stabilize the antigen against changes in temperature and/or pH (stabilizers). They include materials such as mercurials (thimerosal), gelatin, and specific antimicrobial agents. Allergic reactions may occur if the recipient is sensitive to any of these additives. Preservatives are required for multidose vaccine formulations or vials to prevent bacterial or fungal growth, should they be introduced on repeated entry into the vial. Thimerosal, an ethylmercury-containing preservative, has been the major preservative used in vaccines around the world. A review of the mercury content of vaccines in the United States in 1999 indicated that some children had received quantities of ethylmercury from thimerosal in excess of some federal guidelines for methyl mercury. As a precautionary measure, thimerosal as a preservative was removed from most vaccines in the immunization schedule to the extent feasible. However, some of these vaccines may contain trace amounts ( www.fda.gov/cber/vaccine/thimerosal.htm ). Subsequent studies of potential adverse consequences of thimerosal have not demonstrated significant harm from its use in vaccines. It is likely had these data been available in 1999, the United States would not have made the decision to remove thimerosal from vaccines for children. Some vaccines for children contain other preservatives (e.g., 2-phenoxyethanol) or do not need a preservative because they are packaged in single-dose vials. Influenza vaccines in multidose vials used in adults and combined adult-type tetanus and diphtheria toxoids (Td) contain thimerosal as a preservative.
Adjuvants: An aluminum salt is used in some vaccines to enhance the immune response to vaccines containing inactivated microorganisms or their products (e.g., toxoids and hepatitis B vaccine). Bivalent human papillomavirus vaccine contains an aluminum salt combined with monophosphoryl lipid A. An oil-in-water adjuvant is used in one influenza vaccine licensed in the United States and in other vaccines licensed outside the United States. Recombinant zoster vaccine (RZV) contains monophosphoryl lipid A combined with saponin. Vaccines with such adjuvants should be injected deeply into muscle masses because subcutaneous or intracutaneous administration can cause local irritation, inflammation, granuloma formation, or necrosis.
Two major approaches to active immunization have been used: use of live (attenuated) infectious agents, and use of inactivated, or detoxified, agents or their extracts. For many diseases (including influenza, poliomyelitis, typhoid, and measles), both approaches have been used. Live-attenuated vaccines are believed to induce an immunologic response more similar to that resulting from natural infection than do killed vaccines. Inactivated or killed vaccines can consist of inactivated whole organisms (e.g., hepatitis A vaccine), detoxified exotoxin (e.g., diphtheria and tetanus toxoids), soluble capsular material either alone (e.g., pneumococcal polysaccharide), or covalently linked to carrier protein (e.g., Haemophilus influenzae type b [Hib] conjugate vaccines), chemically purified components of the organism (e.g., acellular pertussis, inactivated influenza vaccines [IIVs]), or recombinant proteins (e.g., hepatitis B virus [HBV], serogroup B meningococcal vaccine [MenB-FHbp/MenB-4C], virus-like particles [VLPs; e.g., human papillomavirus (HPV)], or RZV).
The immune system is complex, and antigen composition and presentation are critical for stimulation of the desired immune response. Immunogenicity is determined not only by the chemical and physical states of the antigen but also by the genetic characteristics of the responding individual, the physiologic condition of the individual (e.g., age, nutrition, sex, pregnancy status, stress, infections, immune status), and the manner in which the antigen is presented (route of administration, dose or doses and timing of doses, and presence of adjuvants).
Because the organisms in live vaccines multiply in the recipient, antigen production increases logarithmically until controlled by the immune response induced by the antigen. The live-attenuated viruses (e.g., measles, rubella) generally are believed to confer lifelong protection in those who respond. By contrast, killed vaccines (e.g., diphtheria, tetanus, rabies, typhoid) generally do not induce permanent immunity with one dose, requiring repeated vaccination and subsequent boosters for development and maintenance of high levels of antibody. Exceptions to this general rule may include hepatitis B vaccine, for which long-term immunologic memory has been demonstrated for approximately 30 years after vaccination , and inactivated polio vaccine (IPV), for which the duration of immunity is unknown. Although the amount of antigen initially introduced is greater with inactivated vaccines, multiplication of organisms in the host results in a cumulatively greater antigenic input with live vaccines.
Most vaccines include protein antigens, which generate a T-lymphocyte–dependent immune response. This response induces immunologic memory, booster effects with repeat administration, and good immunogenicity in all age groups. However, purified bacterial capsular polysaccharide vaccines induce a T-lymphocyte–independent immune response, which does not lead to immune memory and cannot be boosted with repeated injections. Polysaccharide vaccines have poor immunogenicity in infants and young children. Covalent linkage of the polysaccharide to a carrier protein converts it from a T-lymphocyte–independent to a T-lymphocyte–dependent antigen (e.g., conjugated Hib, pneumococcal, and meningococcal vaccines), which produces a good immune response in infants and children.
The amount of antigen determines the immune response. Presentation of an insufficient amount of antigen may result in no immune responsiveness. There is usually a dose-response curve relationship between antigen dose and peak response obtained beyond a threshold; however, responsiveness may reach a plateau, failing to increase beyond a certain level despite increasing doses of vaccine.
The immune response to some inactivated vaccines or toxoids can be enhanced by addition of adjuvants, such as aluminum salts (either alone or in combination with monophosphoryl lipid A). Adjuvants are particularly useful with inactivated products, such as diphtheria and tetanus toxoids, acellular pertussis vaccines (DTaP), and hepatitis B vaccine. The mechanism of enhancement of antigenicity by adjuvants is not well defined; however, it is increasingly clear that adjuvants activate the innate immune system through pathogen-associated molecular patterns (PAMPs). Licensed adjuvants for use in humans in the United States include aluminum salts alone or with monophosphoryl lipid A, squalene-based oil-in-water emulsion, and synthetic oligodeoxynucleotides.
The route of administration can determine the nature of the immune response to a vaccine or toxoid. IM or subcutaneous delivery results in a predominantly IgG response. Oral (e.g., rotavirus vaccine and typhoid vaccine Ty21a) or nasal (e.g., live-attenuated influenza vaccine [LAIV]) vaccination is more likely to result in production of local IgA compared with IM injection, although systemic IgG also is induced. The immunogenicity of some vaccines is reduced when not given by the recommended route. For example, administration of hepatitis B vaccine subcutaneously into the fatty tissue of the buttock was associated with substantially lower seroconversion rates than injection intramuscularly into the deltoid muscle.
Most vaccines are administered either intramuscularly or subcutaneously.
The immune response to a vaccine varies with age. Although children and young adults usually respond well to all vaccines, differences in response capability exist during early infancy and older age. The presence of high levels of passively acquired maternal antibody in the first few months of life impairs the initial immune response to some killed vaccines (e.g., hepatitis A vaccine, diphtheria toxoid) and many live vaccines (e.g., measles). Prematurely born infants of low birth weight should be immunized at the usual chronologic age in most cases. Infants with birth weights less than 2000 g may require modification of the timing of hepatitis B immunoprophylaxis, depending on maternal hepatitis B surface antigen (HBsAg) status. Some studies have suggested a reduced immune response in very-low-birth-weight infants (<1500 g) immunized according to the usual schedule; however, antibody concentrations achieved usually are protective. In older adults, the response to antigenic stimulation may be diminished (e.g., influenza, hepatitis B vaccines). This has led to the development of higher-potency influenza vaccines for use in the elderly.
The immune response traditionally is divided into two components: the innate immune response, which is rapid, nonspecific, and serves as an immediate first line of defense against an infection, and the adaptive immune response, which develops over a matter of days, is specific for the foreign antigen, and results in long-term immune memory. The latter protects the host against subsequent challenge with the same or immunologically similar pathogens and is the underlying principle of vaccination. The innate immune response is mediated by natural killer (NK) cells, which recognize and kill virally infected cells; by complement, which is activated by components of bacterial cell walls; and by phagocytes, including macrophages and dendritic cells (DCs), which ingest microorganisms and foreign particulates. The adaptive immune response relies on antigen-presenting cells (APCs), such as DCs, for activation and is mediated by T and B lymphocytes. T lymphocytes can be divided into CD4 (helper) and CD8 (cytotoxic) lymphocytes and are responsible for cell-mediated immune responses. CD4 helper T lymphocytes can be further subdivided into Th1 lymphocytes, which predominantly lead to cell-mediated responses, and Th2 lymphocytes, which predominantly lead to humoral responses. B lymphocytes produce antibody specific for the immunizing agent and require CD4–T-lymphocyte help. Interactions between APCs, helper T-lymphocytes, and B-lymphocytes involve class II major histocompatibility complex (MHC) antigens, whereas interactions between cytotoxic T lymphocytes and their target involve MHC class I antigens. Soluble mediators or cytokines are secreted by all cell types and serve as activation and differentiation factors for different cell lineages. These include interleukins, interferons, and others. A further class of CD4 T lymphocytes (Treg) plays an essential role in the regulation of the adaptive immune response.
The innate immune response is able to respond differently to different types of pathogens, and these differential responses help determine the nature of the subsequent adaptive response. Pattern recognition receptors (PRRs), such as Toll-like receptors (TLRs), nucleotide-binding oligomerization domain (NOD)-like receptors (NLRs), and others, encoded in the germline recognize PAMPs and contribute to immune activation by inducing proinflammatory cytokines, which in turn modulate the adaptive immune response. As alluded to earlier, this has significant implications for adjuvant development.
On exposure to an infectious organism or a vaccine, the innate immune system is mobilized through APC recognition of PAMPs that are present either in the organism or in the adjuvant. Activated APCs (macrophages and DCs) secrete proinflammatory cytokines and chemokines, which recruit other leukocytes to the site of infection. When activated, DCs migrate to the draining lymph nodes, where they interact with T lymphocytes through the MHC-peptide complex. Once the organism or antigen is internalized, it is killed and broken down into peptides. These peptides are transported to the cell surface through membrane trafficking and bind to MHC class I or class II molecules. MHC class I molecules are able to bind peptides that are 8 to 10 amino acids in length, whereas MHC class II molecules are more permissive, binding peptides of 13 amino acids and greater.
The first step in the induction of a T-lymphocyte–dependent antibody response is the activation of naïve CD4 helper T lymphocytes by presentation of an antigen by phagocytes or DCs. The T-lymphocyte receptor recognizes the MHC-peptide complex, and this recognition triggers secretion of cytokines, which stimulate maturation of naïve helper T lymphocytes. In the presence of interleukin-12 (IL-12), Th1 lymphocytes will differentiate, and these in turn will secrete IL-2 and interferon-γ. In the presence of IL-4, Th2 lymphocytes will differentiate and secrete IL-4 and IL-5. These two cytokines are essential for the differentiation and maturation of B lymphocytes into antibody-secreting plasma cells.
Naïve B lymphocytes recognize a specific antigenic epitope on native antigen through the immunoglobulin receptor on their surface but are unable to differentiate into antibody-secreting lymphocytes without T-lymphocyte help. A given B lymphocyte can be activated only by a T lymphocyte responding to the same antigen, though not necessarily to the same epitope. A helper T lymphocyte will recognize the MHC class II complex on the surface of the B lymphocyte and deliver a signal for B-lymphocyte differentiation. This leads to B-lymphocyte proliferation and maturation in a clonal manner. Class switching (from IgM to IgG and IgA) and affinity maturation occur, and antigen-specific plasma cells develop. However, not all B lymphocytes become plasma cells. Some mature into memory B cells, which are long-lived and form the basis of the rapid secondary response on the next encounter with the pathogen. Although the mechanism of maintenance of these cells is not clear, the ability to mount a strong secondary response after many years argues for a homeostatic mechanism that regulates these cells. The antibodies formed after vaccination express a variety of antigen-binding specificities (i.e., recognize different structures on a complex multideterminant antigen), reflecting the sum of the large number of individual clonal B-lymphocyte responses that make up an antibody response.
Antibodies mediate protection through a variety of mechanisms. They may inactivate soluble toxic protein products of bacteria (antitoxins), facilitate intracellular digestion of bacteria by phagocytes (opsonization), interact with components of serum complement to damage the bacterial membrane with resultant bacteriolysis (lysins), prevent infectious virus from infecting cells (neutralizing antibodies), or interact with components of the bacterial surface to prevent adhesion to mucosal surfaces (antiadhesins). Antibodies cannot readily reach intracellular sites of infection, the sites of viral and some bacterial replication. However, antibodies are effective against many viral diseases through interaction with viruses before initial intracellular penetration occurs and through prevention of locally replicating viruses from disseminating from the site of entry to an important target organ, as in the spread of poliovirus from the intestine to the central nervous system or rabies from a puncture wound to peripheral neural tissue.
Virally infected cells can be killed by cytotoxic CD8 T lymphocytes. As the virus replicates in a cell, viral proteins are processed and presented on the cell surface as an MHC class I–peptide complex, which is then recognized by cytotoxic T lymphocytes. Cells infected with intracellular bacteria, such as Mycobacterium leprae, are recognized and killed in the same way.
Independent of antibody production, stimulation of the immune system by vaccination may, on occasion, elicit a hypersensitivity response. Killed measles vaccine, used in the United States between 1963 and 1967, induced incomplete humoral immunity and cell-mediated hypersensitivity, resulting in development of a syndrome of atypical measles in some children on subsequent exposure to measles. In addition, some antibodies produced may not be protective but block the reaction of protective antibodies with antigens, inhibiting the body's defenses. Some vaccines may induce immunologic tolerance that results in blunting of the immune response on subsequent exposure to the antigen (e.g., meningococcal polysaccharide vaccine [MPSV]). Concerns have been raised that immunizations might induce autoimmune disorders. However, careful reviews of both the possible biologic mechanisms and epidemiologic evidence generally have failed to confirm vaccines as causes of these disorders. The evidence was insufficient to accept or reject a causal relationship between vaccines and allergic disorders, particularly asthma. A subsequent epidemiologic study failed to show an association between vaccines and asthma. Concerns also have been raised that the number of antigens in the current vaccine schedule might overwhelm an infant's immune system, leading to chronic diseases and predisposing to other serious infections. As a result of removal of whole-cell pertussis vaccine and smallpox vaccine from the current immunization schedule, the number of immunogenic proteins and polysaccharides a child is exposed to today is actually smaller than in the past. Estimates suggest that an infant is capable of responding to 10,000 vaccine antigens simultaneously. The Institute of Medicine (IOM) concluded that available evidence favored rejection of a causal relationship between vaccines and increased risk for infections. IOM also concluded that available evidence favored rejection of a causal relationship between vaccines and type 1 diabetes mellitus.
On first exposure to a vaccine, a primary response is induced, and a protective immune response will develop in about 2 weeks. Circulating antibodies do not usually appear for 7 to 10 days, and the immunoglobulin class of the response changes over this period of time. Early-appearing antibodies are usually of the IgM class and of low affinity; late-appearing antibodies are usually of the IgG class and display a high affinity. IgM antibodies may fix complement, making lysis and phagocytosis possible. As the titer of IgG rises during the second week (or later) after immunogenic stimulation, the IgM titer falls. IgG antibodies are produced in large amounts and function in the neutralization, precipitation, and fixation of complement. The antibody titer frequently reaches a peak in about 2 to 6 weeks and then falls gradually. The switch from IgM synthesis to predominantly IgG synthesis in B lymphocytes is mediated by T-lymphocyte help. Uncommonly, people may not respond to a vaccine, experiencing a primary vaccine failure. This may be due to a genetic inability to respond to vaccine, but other factors are involved. For example, almost all children who do not respond immunologically to the first dose of measles-mumps-rubella (MMR) vaccine will acquire measles immunity after a second dose.
After a second exposure to the same antigen, a heightened humoral or cell-mediated response, an anamnestic response, is observed. These secondary responses occur sooner than the primary response, usually within 4 to 5 days, and depend on a marked proliferation of antibody-producing cells or effector T lymphocytes. Effector T lymphocytes, also known as memory T cells, are T lymphocytes that have a memory of a previous immune response. The secondary response depends on immunologic memory after the first exposure mediated by both T and B lymphocytes. Infection with measles or varicella vaccine strains has been shown to evoke a cell-mediated in addition to a humoral response.
Many pathogens replicate at mucosal surfaces before host invasion and may induce secretory IgA along the respiratory and gastrointestinal mucous membranes and at other localized sites (e.g., polio, rubella, influenza, rotavirus). IgA antibodies are efficient at virus neutralization (e.g., polio), fix complement through the alternative pathway (e.g., cholera), prevent adsorption of organisms to the intestinal wall (e.g., Escherichia coli, cholera), and can lyse gram-negative bacteria (with the aid of both complement and lysozyme). Current parenteral, especially inactivated, vaccines rarely induce high levels of secretory IgA antibodies.
Response to vaccines is often gauged by measuring the appearance and concentration of specific serum antibodies. For some viral vaccines, such as those for measles and rubella, the presence of circulating antibodies correlates with clinical protection. Although this has served as a dependable indicator of immunity, seroconversion measures only the humoral parameter of the immune response. Secondary vaccine failure occurs when an individual who previously had developed an adequate immune response loses protection over time. This waning immunity can be attributed to a loss of long-lived memory B or T cells in the absence of repeated exposure to the pathogen. Evaluating persistence of antibody has been used to determine the duration of vaccine-induced immunity for those diseases for which antibody is judged to be a good correlate of protection. However, the absence of measurable antibody may not mean that the individual is unprotected. Although a fall in titer occurs, on revaccination or challenge a rapid secondary response is observed in IgG antibodies, with little or no detectable IgM response, suggesting persistent protection. With some vaccines and toxoids, the mere presence of antibodies is not sufficient to ensure clinical protection, but rather a minimal circulating level of antibody is required (e.g., 0.01 IU/mL of tetanus antitoxin). Functional antibody is important in assessing immunity to bacterial polysaccharide vaccines. Opsonophagocytic activity is considered the assay of choice for monitoring vaccine response because the vaccines also induce nonfunctional antibodies that are detected in standard enzyme immunosorbent assay (EIA), although the EIA can be used as a proxy. Some immune responses may not in themselves confer immunity but may be sufficiently associated with protection that they remain useful proxy measures of protective immunity (e.g., vibriocidal serum antibodies in cholera). The measurement of cell-mediated immunity, which would be helpful in assessing the degree of ongoing protection in many circumstances, usually is limited to research laboratories and to only a few vaccines.
Most vaccines in use today have been developed by empirical techniques. For live-attenuated viral vaccines, organisms are repeatedly passaged in various tissue culture cell lines to reduce virulent properties while maintaining immunogenicity. Inactivated vaccines usually have been developed by growing microorganisms, followed by concentration, purification, and inactivation, not necessarily in that order. Component vaccines usually are derived from chemical separation of the needed component from the parent organism.
Future vaccines are likely to be derived from new methods of biotechnology, especially recombinant techniques. Currently available hepatitis B vaccines were developed by cloning the HBsAg gene into yeast, leading to synthesis of HBsAg within the yeast cell. Other new approaches for producing vaccines include live vectors, in which one or more genes encoding critical determinants of immunity from pathogenic microorganisms are inserted into the genome of the vector, followed by the administration of the vector as a component of the vaccine. These vectors may include viruses, such as poxviruses (vaccinia or canarypox), or bacteria, such as Salmonella or bacillus Calmette-Guérin (BCG). Additional newer techniques include microencapsulation of critical antigens in polymers, which can lead to sustained release or pulse release over prolonged periods, mimicking the effect of multiple injections of an antigen over a several-month interval. New technologies also include use of nucleic acids, which encode critical antigens. Injection of the DNA, combined with administration of a protein at a later point in time, leads to production of antigen without risk for producing whole infectious organisms. LAIV was developed using genetic reassortment of the genes encoding two of the surface glycoproteins from wild virus isolates with six other genes contributed from a cold-adapted, temperature-sensitive influenza strain. Similar techniques were used to develop bovine rotavirus vaccines. Last, newer technologies focus on the development of adjuvants to help stimulate the immune response.
Introduction and widespread use of vaccines resulted in global eradication of smallpox, elimination of poliomyelitis caused by wild viruses in the United States and most of the countries of the world, and dramatic reductions in the incidence rates of other diseases ( Tables 316.1 and 316.2 ). Measles and rubella are no longer considered endemic in the Americas. Measles and rubella have been reduced by greater than 90% in developed countries and, if global vaccination efforts can be sustained, may eventually be eliminated from many countries. The World Health Assembly had established a goal to eradicate polio from the world by the end of 2000. Although that goal was not achieved, by the end of 2016 only three countries in the world had never interrupted wild poliovirus transmission ( www.polioeradication.org ). The last case of polio caused by wild virus in the Western Hemisphere was in 1991; four of the six regions of the World Health Organization (WHO)—American, European, Southeast Asian, and Western Pacific—have been certified free of wild poliovirus. Global use of hepatitis B vaccine in infants may have an impact comparable to that of other vaccines in childhood. Hib vaccines have only recently come into widespread use, but disease incidence has been reduced markedly in many developed countries. Reductions based on historical estimates have been achieved for congenital rubella syndrome and Hib invasive disease. Despite these successes, cases of measles and pertussis continue to occur in the United States (see Table 316.1 ). All measles cases are the result of international importations, some of which spread within the US population, whereas pertussis remains endemic.
| DISEASE | 20th CENTURY ANNUAL MORBIDITY a | 2017 REPORTED CASES b | PERCENT DECREASE |
|---|---|---|---|
| Smallpox | 29,005 | 0 | 100% |
| Diphtheria | 21,053 | 0 | 100% |
| Measles | 530,217 | 120 | >99% |
| Mumps | 162,344 | 6109 | 96% |
| Pertussis | 200,752 | 18,975 | 91% |
| Polio (paralytic) | 16,316 | 0 | 100% |
| Rubella | 47,745 | 7 | >99% |
| Congenital rubella syndrome | 152 | 5 | 99% |
| Tetanus | 580 | 33 | 94% |
| Haemophilus influenzae | 20,000 | 33 c | >99% |
a From Roush SW, Murphy TV; Vaccine-Preventable Disease Table Working Group. Historical comparisons of morbidity and mortality for vaccine-preventable diseases in the United States. JAMA . 2007;298;2155–2163.
b Centers for Diease Control and Prevention. National Notifiable Diseases Surveillance System, 2017 Annual Tables of Infectious Disease Data. Atlanta: CDC Division of Health Informatics and Surveillance; 2018.
c H. influenzae type b (Hib) at <5 years of age. An additional 10 cases of Hib are estimated to have occurred among the 203 notifications of H. influenzae (<5 years of age) with unknown serotype.
| DISEASE | PREVACCINE ERA ANNUAL ESTIMATE | 2016 ESTIMATE (UNLESS OTHERWISE SPECIFIED) | PERCENT DECREASE |
|---|---|---|---|
| Hepatitis A | 117,333 a | 4000 b | 97% |
| Hepatitis B (acute) | 66,232 a | 20,900 b | 68% |
| Pneumococcus (invasive) | |||
| All ages | 63,067 a | 30,400 c | 52% |
| <5 yr of age | 16,069 a | 1700 c | 89% |
| Rotavirus (hospitalizations, <3 yr of age) Varicella |
62,500 d 4,085,120 a |
30,625 e 102,128 f |
51% 98% |
a Rousch SW, Murphy TV; Vaccine-Prevenatable Disease Table Working Group. Historical comparisons of morbidity and mortality for vaccine-preventable diseases in the United States. JAMA. 2007;298:2155–2163.
b Centers for Disease Control and Prevention. Viral hepatitis surveillance—United States, 2016.
c Centers for Disease Control and Prevention. Active bacterial core surveillance 2016 (unpublished).
d Cortese MM, Parashar UD; Centers for Disease Control and Prevention. Prevention of rotavirus gastroenteritis among infants and children: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2009;58(RR-2):1-25.
e New Vaccine Surveillance Network 2017 data (unpublished); US rotavirus disease now has a biennial pattern.
f Centers for Disease Control and Prevention. Varicella Program 2017 data (unpublished).
Pneumococcal conjugate vaccines (PCVs) have had a marked impact on invasive pneumococcal disease in countries where they have been used widely in children. Decreases in disease were observed not only in children but also in adults, who presumably are not being exposed to infectious children because the latter have had vaccine-type pneumococcal carriage eliminated by vaccination.
Modern vaccines are safe and generally effective. Each vaccine is associated with some adverse effects, which are usually mild, and only rarely life-threatening. No vaccine is 100% effective. Consequently, some persons who have received a complete vaccine or toxoid series may acquire disease after exposure. The effectiveness of vaccines recommended for universal use in children is well defined, with most vaccines protecting more than 80% of recipients after a primary series.
In most studies, acellular pertussis vaccines range in efficacy from 63% to 99% during the first few years after vaccination. One dose of varicella vaccine is 95% or more effective against severe varicella but is less effective against varicella of any severity. With some vaccines, antibody may wane, but immunologic memory is sufficient to prevent disease if the individual is exposed (e.g., hepatitis B). However, for some diseases with short incubation periods (e.g., meningococcal disease), waning antibody after vaccination is associated with waning protection. This waning has occurred with meningococcal conjugate vaccines, resulting in the need for modification of the originally recommended vaccine schedule with the addition of a second dose. Another example of loss of durability has occurred with the Tdap (tetanus, diphtheria, and acellular pertussis) and DTaP (diphtheria and tetanus toxoids and acellular pertussis) vaccines, in which protection begins to wane a few years after administration.
Although high efficacy of each of these vaccines is apparent, there has been substantial controversy about reported adverse events temporally associated with vaccination. Because of these controversies, the IOM reviewed available information, and between 1991 and 2013 published multiple reports. In the 1991 and 1994 studies, the IOM found insufficient evidence to indicate a causal relationship between DTaP and permanent neurologic damage, and the IOM favored rejection of a causal relationship between combined diphtheria and tetanus toxoids (DT) and encephalopathy and between conjugate Hib vaccines and early-onset Hib disease. The IOM also concluded that the evidence establishes a causal relationship between MMR and thrombocytopenia, between rubella vaccine and acute arthritis, between DT and brachial neuritis, and between a variety of vaccines and anaphylaxis. In 2004 the IOM reported the relationships between a variety of disorders and vaccines ( www.iom.edu/Activities/PublicHealth/ImmunizationSafety.aspx ). The IOM panel concluded that evidence did not support a relationship between MMR or thimerosal and autism, between multiple immunizations and heterologous infections, between multiple immunizations and type 1 diabetes, or between hepatitis B vaccine and incident or relapsed multiple sclerosis. In 2011 the IOM looked at the relationship of vaccines with many conditions that are reported after vaccination and, in most cases, found no evidence to support such associations. The IOM specifically found evidence to support rejection of an association between MMR vaccine and autism. Likewise, it found evidence to reject an association between IIV and asthma. In 2013 the IOM studied the impact of giving multiple vaccines to an individual in accordance with Advisory Committee on Immunization Practices (ACIP) of the Centers for Disease Control and Prevention (CDC), American Academy of Pediatrics (AAP), and American Academy of Family Physicians (AAFP) recommendations and found no evidence of a safety concern with adhering to the childhood schedule.
Development of vaccines consists of four phases. Initial studies typically are conducted in animal models to demonstrate protection (or at least production of antibodies) and relative safety. These are called preclinical studies. Then limited numbers of doses are administered to humans to demonstrate antibody production and safety (phase I). After this phase, clinical trials in humans are conducted in a limited number of people to select optimal vaccine schedules and to demonstrate further safety (phase II). Larger trials are conducted to demonstrate efficacy (phase III). Because of their limited size, these field trials can be expected to detect adverse events that occur only relatively frequently (1 per 1000 doses or higher). After clinical trials, licensure may be sought. In the United States, vaccine production is regulated by the Center for Biologics Evaluation and Research (CBER) of the US Food and Drug Administration (FDA). Only after a vaccine has been found to be safe and effective is it licensed for use. Postmarketing surveillance (phase IV) is necessary to detect rare adverse events associated with vaccination and to monitor safety of vaccination practices, such as simultaneous immunization.
There is no direct evidence of risk to the fetus when pregnant women are given vaccines routinely recommended during pregnancy by ACIP. The benefit of IIV to the pregnant mother and the fetus outweighs any risk of vaccination to the mother or the fetus. Some vaccines are recommended for pregnant women in order to provide passive immunity to their fetuses so that when the child is born, the child is protected before active immunity can be induced through direct vaccination of the infant. Thus, Tdap and nonlive influenza vaccine is recommended during each pregnancy. Most live-virus vaccines induce viremia, which at least theoretically could result in infection of the fetus, so live-virus vaccines are not administered to pregnant women except in unusual circumstances, when potential benefit clearly outweighs the risk.
The decision to administer a vaccine involves assessment of risks of disease, benefits of vaccination, and risks associated with vaccination. The relative balance of risks and benefits may change over time; consequently, continuing assessment of vaccines is essential. Recommendations for vaccine use are developed by several different bodies: ACIP develops recommendations for vaccines for children, adolescents, and adults in the civilian population in conjunction with professional societies. These recommendations are updated annually and are available at www.cdc.gov/vaccines/schedules/hcp/index.html . Since 2011, the ACIP process for making vaccine recommendations has included a careful evaluation of the strength of the evidence supporting recommendations, which is known as GRADE (Grading of Recommendations, Assessment, Development and Evaluation; www.cdc.gov/vaccines/acip/recs/GRADE/table-refs.html ). The Committee on Infectious Diseases (COID) of the AAP (the “Red Book “ committee) develops recommendations for vaccine use in infants, children, and adolescents. Since 1995, ACIP, the AAP, and the AAFP have collaborated to issue a harmonized childhood immunization schedule, which is updated annually. The childhood immunization schedule consists of three parts: one based on age, a second that is a catch-up schedule for children who are behind on their immunizations, and a third that is based on underlying medical conditions ( Fig. 316.1 ). ACIP also annually issues an adult immunization schedule in two parts: (1) recommendations based on age group and (2) recommendations based on underlying medical conditions ( Figs. 316.2 to 316.4 ), which can be found at www.cdc.gov/vaccines/schedules/hcp/adult.html . The Adult Immunization Schedule for 2018 was harmonized with the AAFP, the American College of Obstetricians and Gynecologists, the American College of Physicians, and the American College of Nurse-Midwives.
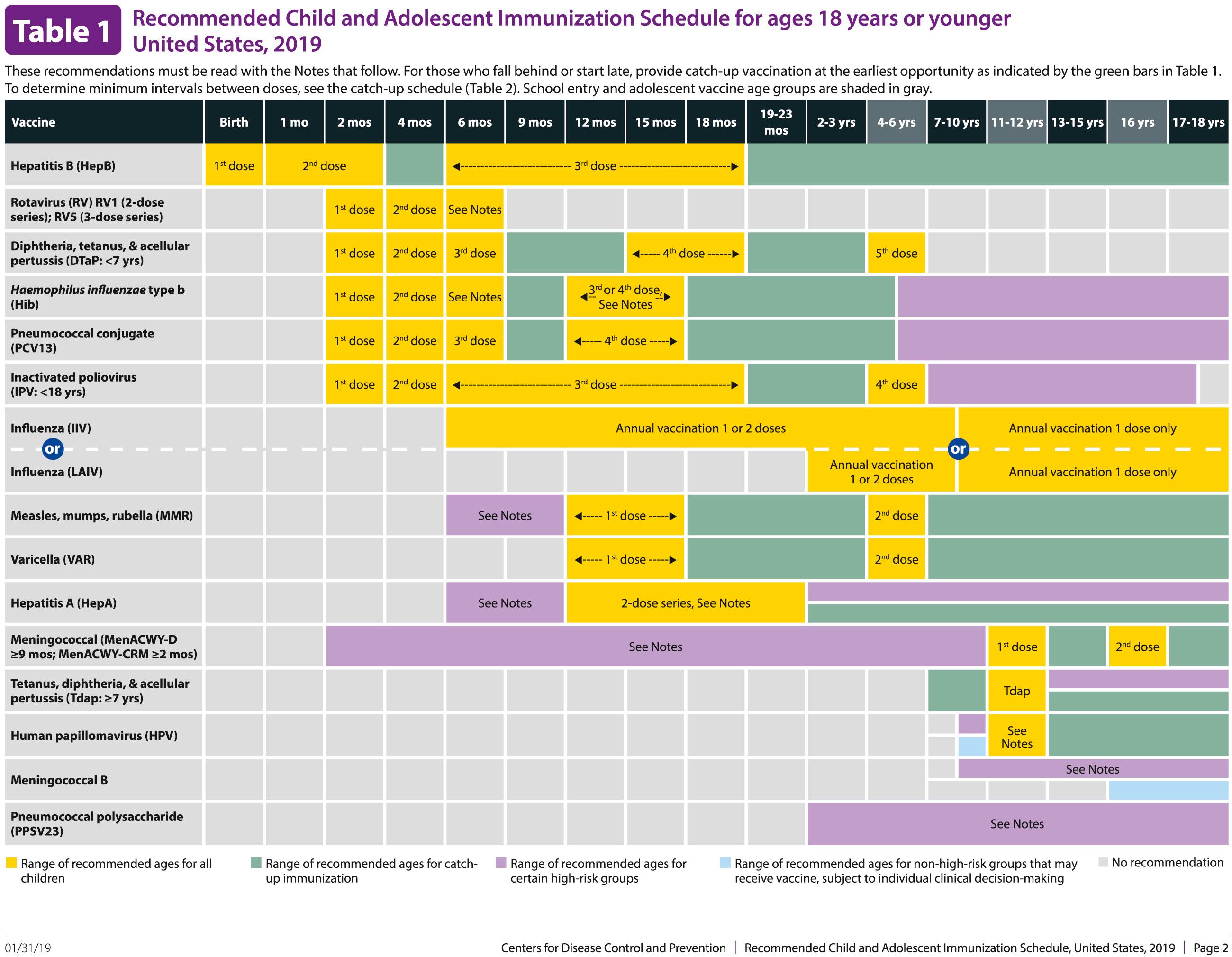
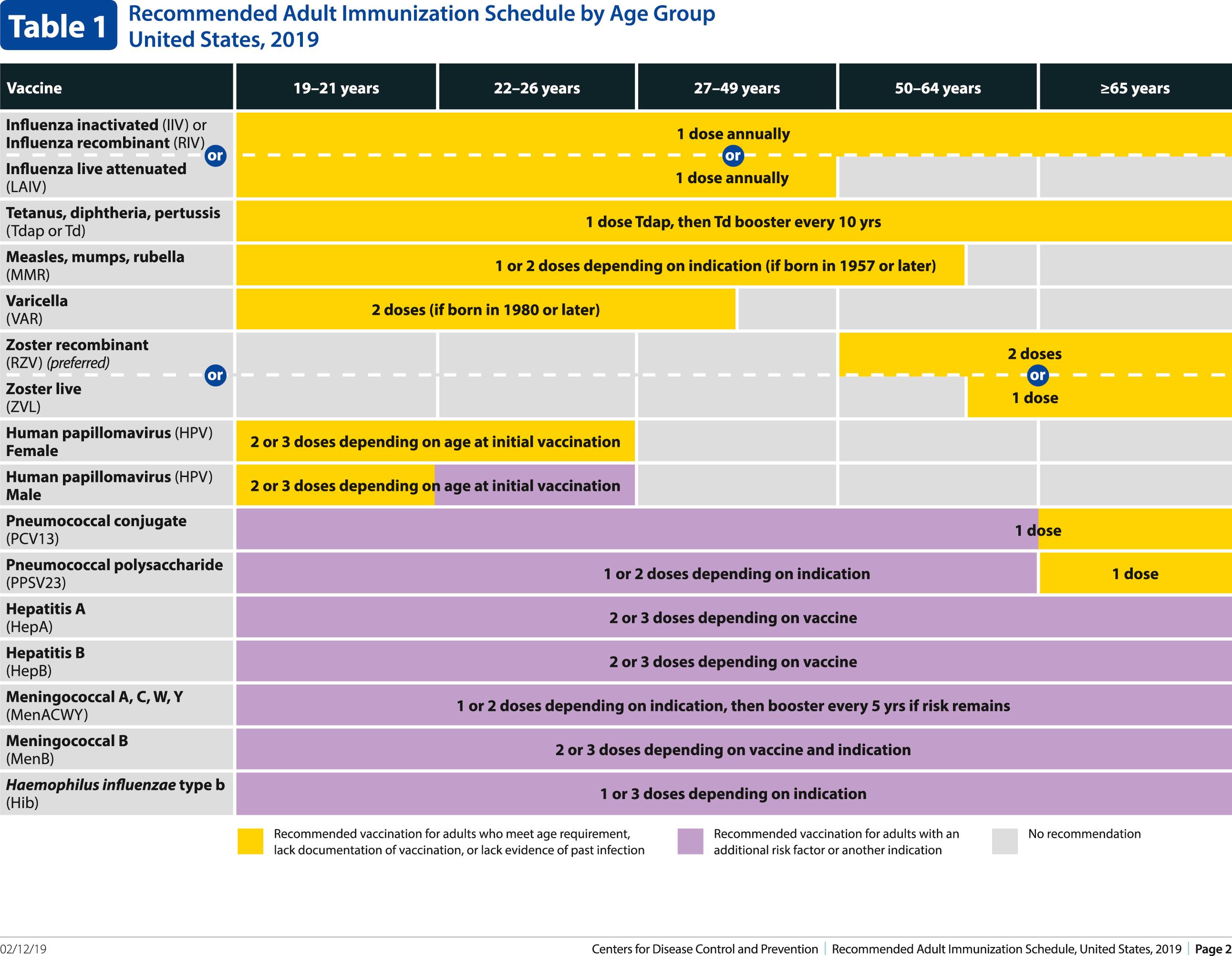
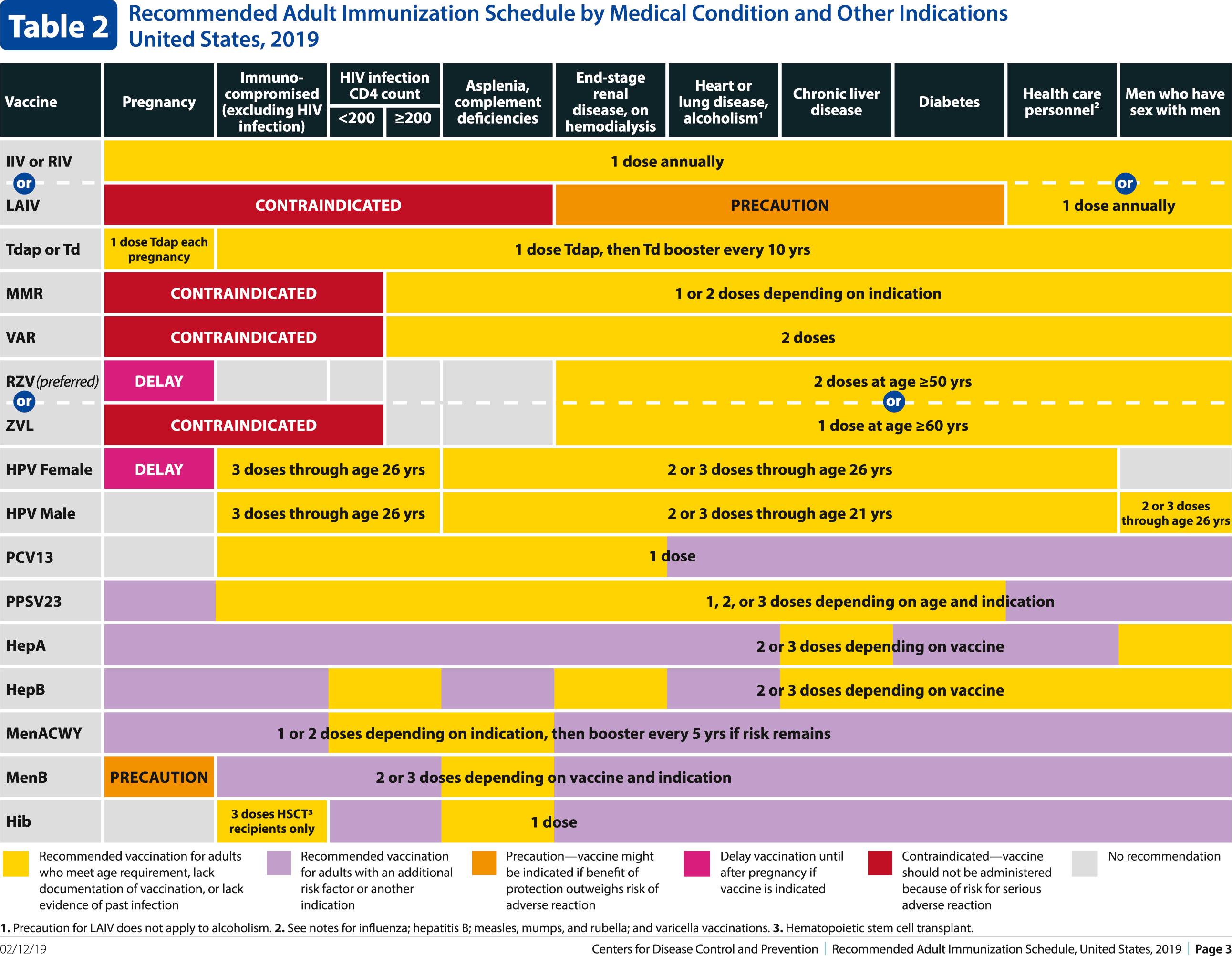
Update: Changes in ACIP Recommendations in Adult Immunization Schedule for 2020
Tables 316.3 and 316.4 list currently licensed immunizing agents and immunoglobulins. This section presents brief information about most immunizing agents, primary indications for use, relative efficacy, number and spacing of doses required, known adverse effects, and precautions and contraindications for use. Package inserts and specific references and recommendations should be consulted for more detailed information. In addition to these licensed products, several other vaccines are under development and may become available.
| PRODUCT | YEAR LICENSED |
|---|---|
| Adenovirus vaccine, live, attenuated | 2014 |
| Anthrax vaccine adsorbed | 1972 |
| Calmette-Guérin bacillus vaccine; live, attenuated | 1950 |
| Cholera vaccine, live, attenuated | 2016 |
| Dengue tetravalent vaccine, live | 2019 |
| Diphtheria and tetanus toxoids and acellular pertussis vaccine | 1991 |
| Diphtheria and tetanus toxoids adsorbed (pediatric use, DT) | 1949 |
| Diphtheria and tetanus toxoids and acellular pertussis vaccine absorbed, Haemophilus B conjugate vaccine, and inactivated polio vaccine combined | 2008 |
| Diphtheria and tetanus toxoids and acellular pertussis vaccine absorbed and inactivated polio vaccine combined | 2008 |
| Diphtheria and tetanus toxoids and acellular pertussis adsorbed, hepatitis B (recombinant), and inactivated poliovirus vaccine combined | 2002 |
| Haemophilus influenzae type b conjugate vaccine | 1987 |
| Hepatitis A vaccine | 1995 |
| Hepatitis A inactivated and hepatitis B (recombinant) vaccine | 2001 |
| Hepatitis B recombinant vaccine | 1987 |
| Human papillomavirus vaccine | 2006 |
| Influenza virus vaccine (cell culture) | 2013 |
| Influenza virus vaccine (inactivated) | 1945 |
| Influenza virus vaccine; live, attenuated, intranasal | 2003 |
| Influenza virus vaccine; recombinant hemagglutinin | 2014 |
| Japanese encephalitis vaccine | 2009 |
| Measles virus vaccine; live, attenuated | 1963 |
| Measles, mumps, rubella, varicella; live, attenuated | 2005 |
| Measles, mumps, and rubella virus vaccine; live, attenuated | 1971 |
| Meningococcal polysaccharide (serogroups A, C, Y, and W) conjugated to diphtheria toxoid | 2005 |
| Pneumococcal conjugate vaccine (13-valent) | 2010 |
| Pneumococcal polysaccharide vaccine (23-valent) | 1983 |
| Poliomyelitis vaccine (inactivated, enhanced potency) | 1987 |
| Rabies vaccine (human diploid) | 1980 |
| Recombinant zoster vaccine | 2017 |
| Rotavirus vaccine, live, attenuated | 2006 |
| Rubella virus vaccine, live, attenuated | 1969 |
| Serogroup B meningococcal vaccine | 2014 |
| Smallpox vaccine, live, attenuated | 2007 |
| Tetanus and diphtheria toxoids, adsorbed (adult use, Td) | 1955 |
| Tetanus toxoid adsorbed | 1949 |
| Tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis vaccine, absorbed | 2005 |
| Typhoid vaccine (polysaccharide) | 1994 |
| Typhoid vaccine; live, attenuated (oral) | 1990 |
| Varicella vaccine; live, attenuated | 1995 |
| Yellow fever vaccine; live, attenuated | 1953 |
| Zoster vaccine; live, attenuated | 2006 |
| NAME | ABBREVIATION | ROUTE OF ADMINISTRATION | YEAR LICENSED |
|---|---|---|---|
| Anthrax immune globulin | Intravenous | 2015 | |
| Botulism intravenous immune globulin | BabyBIG | Intravenous | 2003 |
| Cytomegalovirus immune globulin intravenous | CMV IGIV | Intravenous | 1990 |
| Hepatitis B immune globulin | HBIG | Intramuscular | 1977 |
| Immune globulin | IG | Intramuscular | 1943 |
| Immune globulin intravenous | IGIV | Intravenous | 1981 |
| Immune globulin subcutaneous | IGSC | Subcutaneous | 2006 |
| Rabies immune globulin | RIG | Intramuscular | 1974 |
| Tetanus immune globulin | TIG | Intramuscular | 1957 |
| Vaccinia immune globulin intravenous | VIG-IGIV | Intravenous | 2005 |
| Varicella-zoster immune globulin | VariZIG | Intramuscular | 2012 c |
a Antitoxin preparations from animal sera other than humans are available for botulism and diphtheria.
c A previous preparation of varicella-zoster IG (VZIG) was licensed in 1980.
Update: Revised Recommendations for Immunization with Pneumococcal Vaccines
Adenovirus vaccine contains live adenovirus types 4 and 7. It is recommended only for military personnel who are 17 through 50 years of age. It is taken as two oral tablets (one dose). Serious adverse events possibly associated with receipt of vaccine included hematuria, gastroenteritis, gastritis, pneumonia, and hematochezia.
Anthrax vaccine (AVA) is prepared from microaerophilic cultures of an avirulent nonencapsulated strain of Bacillus anthracis. The vaccine is a cell-free filtrate that contains a mixture of components, including protective antigen (the antigen that is thought to confer immunity) and other bacterial products adsorbed to aluminum hydroxide. Because of concerns about potential use of B. anthracis as a biologic warfare agent, vaccination of selected members of the US Armed Forces was begun in 1998. After the intentional release of anthrax in the United States in 2001, anthrax vaccine was recommended for civilians at risk for repeated exposure to B. anthracis spores, including laboratory personnel handling environmental specimens and performing confirmatory testing for B. anthracis in selected laboratories and workers making repeated entries into sites known to be contaminated with B. anthracis spores. Anthrax vaccine also was used after exposure, in conjunction with antimicrobial prophylaxis, under an investigational protocol. Groups for whom preexposure vaccination is recommended include persons working with production quantities of B. anthracis cultures or in activities with a high potential for aerosol production and selected other workers at high risk for exposure to B. anthracis spores. Efficacy has been demonstrated in protection against cutaneous disease. Data on clinical efficacy against inhaled anthrax in humans are limited, but available human and animal data are consistent with protection. The vaccine induces antibodies in greater than 90% of adults who received the currently recommended primary course of three IM injections given at time zero, 4 weeks, and 6 months, with boosters at 12 months and 18 months, followed by annual boosters. A controlled study of a vaccine similar to the currently available vaccine demonstrated protective efficacy against cutaneous disease of 93% among mill workers. Experience suggests that two doses of vaccine confer some protection. Mild local reactions at the site of injection occur in about 30% of recipients. Studies of adverse events after injection of the alum-precipitated vaccine, which is the precursor to the AVA vaccine, demonstrate that more severe local reactions occur infrequently (<4%) and systemic reactions are rare (0.2%). Surveillance for adverse events in the military program revealed no pattern of serious adverse events. Adverse events, including injection site reaction incidence and duration, were less often seen after IM injection compared with subcutaneous injection. The IM route of administration is indicated for preexposure use. Vaccines containing only recombinant protective antigen are under active development and may be less reactogenic than the current vaccine. In the event of exposure to anthrax spores, the recommended postexposure prophylaxis (PEP) regimen is three doses of AVA administered at 0, 2, and 4 weeks, combined with 60 days of antibiotics.
BCG vaccine contains living Calmette-Guérin bacillus, an attenuated strain of Mycobacterium bovis. In many countries, BCG is used in infants and young children to prevent disseminated tuberculosis infection. In the United States, use of BCG is recommended only in special circumstances because the general risk for infection is low. BCG vaccination can also result in conversion of the purified protein derivative (PPD) or Mantoux tuberculin skin test, thereby removing one of the most important indicators of tuberculosis infection (tuberculin conversion). However, the association of a positive PPD skin test result after immunization with BCG in childhood tends to fade over time, and most individuals will have a PPD reaction of less than 10 mm by 10 years later. BCG does not cross react with the interferon-γ release assay (IGRA), so the IGRA is the preferred test over the PPD for diagnosis of tuberculosis in patients older than 4 years who have received BCG. The IGRA is not as sensitive in children 4 years or younger and requires a blood draw. Although BCG is widely used throughout the world, there has been much controversy regarding its efficacy. Studies have suggested that the vaccine is effective, particularly for preventing complications of disseminated tuberculosis in young children. In the United States, use of BCG should be considered for individuals, such as infants, whose skin test results are negative and who have prolonged, close contact with patients with active tuberculosis who are untreated, are ineffectively treated, or have antibiotic-resistant infection. BCG also may be considered for health care providers in areas in which multidrug-resistant Mycobacterium tuberculosis infection has become a significant problem.
A single dose of vaccine is administered intradermally or by the percutaneous route. (The Tice strain licensed in the United States is approved only for percutaneous administration.) Known adverse reactions include regional adenitis, disseminated BCG infection, and osteitis caused by the BCG organism. Adenitis occurs in about 1% to 10% of vaccinees, whereas disseminated infections and osteitis are quite rare (about 1 case per 1 million vaccinees). The risk for developing osteitis after BCG vaccination varies by country; in one review, this risk ranged from 0.01 cases per million vaccinees in Japan to 32.5 and 43.4 cases per million vaccinees in Sweden and Finland, respectively. Immunocompromised individuals should not receive the vaccine because of increased risk for disseminated BCG infection.
A killed whole-cell cholera vaccine was available in the United States from the 1940s until 2001. Killed whole-cell vaccines are still available in some countries, and improved killed vaccines are licensed in some countries. Two oral whole-cell inactivated vaccines, including one that is combined with the B subunit of cholera toxin, are available in some parts of the world, as is an oral live-attenuated vaccine with a critical moiety of the gene for the cholera toxin deleted. Killed oral cholera vaccines are increasingly being used as important components of cholera prevention in epidemic and endemic settings. A live oral vaccine (CVD 103-HgR or Vaxchora, manufactured by PaxVax) was licensed in the United States in 2016. The vaccine is administered as a single dose with a buffer salt to neutralize stomach acid. It is recommended for travelers 18 to 64 years of age to an area of active cholera transmission. Vaxchora should be administered 8 hours or more after a dose of oral typhoid vaccine.
Diphtheria toxoid is a purified preparation of inactivated diphtheria toxin. It is highly effective in inducing antibodies that will prevent disease, although antibodies may not prevent acquisition or carriage of the organism. In the United States, the toxoid is available in adsorbed form, combined with tetanus toxoid (adult formulation, Td, and pediatric formulation, DT) or with tetanus toxoid and acellular pertussis vaccine (DTaP, childhood formulation; or Tdap, adult formulation). Single-antigen diphtheria toxoid is not distributed in the United States. Two dosage formulations are available: one for use in children through 6 years of age, and one for use in older children and adults. The adult formulation has a lower concentration of diphtheria toxoid (≤2.5 limit of flocculation units [Lf]) than the childhood formulation (6.7–25 Lf) because local reactions are thought to relate to both age and dosage. With all formulations, levels of antitoxin considered protective are induced in more than 90% of recipients who complete the schedule.
Immunization against diphtheria is recommended for all residents in the United States. For children younger than 7 years with no contraindications to pertussis immunization, DTaP is recommended, and the primary series is three doses administered 4 to 8 weeks apart, followed by a first booster dose 6 to 12 months later and a second booster dose at school entry (4–6 years of age). For infants with contraindications to pertussis vaccine, DT is administered in the same schedule as DTaP (see “Pertussis-Containing Vaccine” and Fig. 316.1 ). The primary immunizing series of DT (for children 1–6 years of age) or Td (for older children and adults) consists of at least two doses administered 4 to 8 weeks apart, followed by a third dose 6 to 12 months later. There is no need to restart a series if the schedule is interrupted; the next dose in the series should be given. Booster doses of Td should be given every 10 years. All persons 11 years and older should receive one dose of Tdap, which can serve as one of the recommended booster doses for diphtheria and tetanus. Persons 7 years or older not fully vaccinated with DTaP vaccine should receive one dose of Tdap as part of a catch-up series. If the dose is administered at 7 through 10 years of age, another dose of Tdap should be administered at 11 or 12 years of age. Tdap should be administered to pregnant women during every pregnancy, optimally early between gestational ages 27 weeks and 36 weeks. Tdap administered during pregnancy provides passive immunity to the fetus and should protect newborns and young infants before they have time to make an active immune response to DTaP. Known adverse effects of diphtheria toxoid include local reactions and mild or moderate systemic reactions such as fever; anaphylaxis occurs rarely. Brachial neuritis appears to be a rare consequence of immunization and is most likely due to tetanus toxoid. The only contraindications are in individuals who previously have had severe hypersensitivity reactions after diphtheria or tetanus toxoids or, if combined with pertussis, have had previous similar adverse events to those antigens.
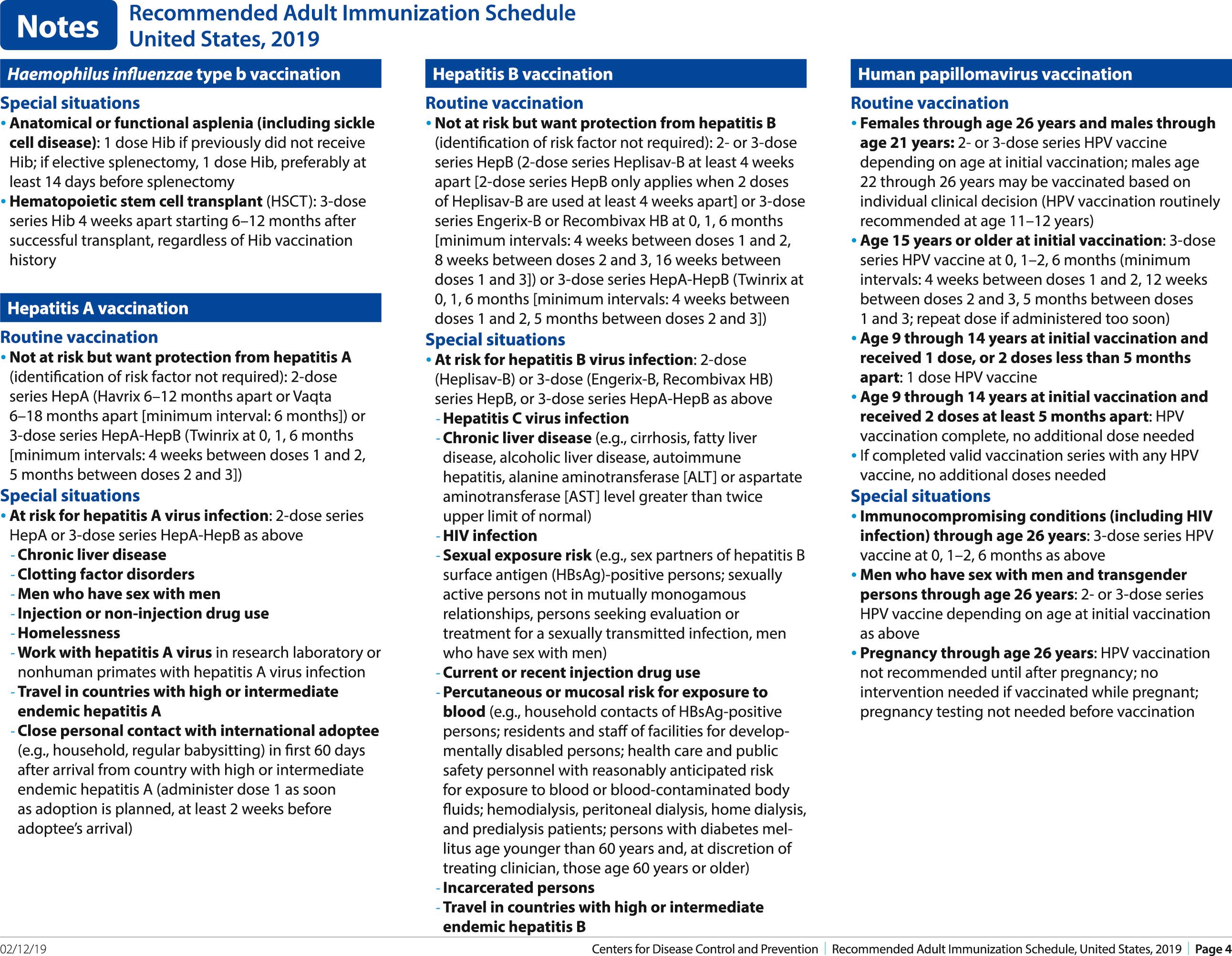
Conjugated vaccines to prevent Hib invasive disease were first licensed at the end of 1987 and have replaced the earlier polysaccharide vaccines because they elicit substantially higher antibody titers and are effective in young infants. The polysaccharide in these vaccines is covalently linked to protein carriers, converting them from T-lymphocyte–independent antigens to T-lymphocyte–dependent antigens. There are four available conjugate vaccines licensed for use in infants. Three are single-component vaccines for prevention of Hib disease. Carrier proteins include a Neisseria meningitidis outer membrane protein complex (PRP-OMP) for PedVaxHib and tetanus toxoid (PRP-T) for ActHIB and Hiberix. PRP-OMP has been demonstrated to be 95% effective in a clinical trial in infants. PRP-T has been licensed for use in infants because it elicits comparable antibody responses to other conjugate vaccines that have been shown to be highly effective. A combination vaccine, DTaP-IPV/Hib, is licensed for any of the recommended first four doses during the first 2 years of life ( www.fda.gov/BiologicsBloodVaccines/Vaccines/ApprovedProducts/ucm174757.htm and www.fda.gov/biologicsbloodvaccines/vaccines/approvedproducts/ucm172502.htm ).
The Hib component of DTaP-IPV contains PRP-T as the conjugate. PRP-OMP behaves differently from PRP-T, inducing high levels of antibody after a single dose. A second dose 2 months later increases those levels; less benefit appears to be derived from a third dose. The basic series for PRP-OMP is two doses given 2 months apart beginning at 2 months of age, followed by a booster dose at 12 to 15 months of age. PRP-OMP is preferred in American Indian/Alaska Native populations because of the younger peak in disease incidence. In contrast, PRP-T does not induce substantial antibody levels until the second dose, and high levels of protection are achieved only after three doses 2 months apart. The basic series for PRP-T starts at 2 months of age with three doses 2 months apart, followed by a booster dose at 12 to 15 months of age. Although use of a single conjugate vaccine for the primary series is recommended, several studies have suggested that mixed sequences of Hib conjugate vaccines induce an adequate immune response. Thus, for infants younger than 6 months, three doses of any licensed Hib vaccine administered at 2-month intervals should confer protection; a booster dose is given at 12 to 15 months of age.
For healthy infants starting immunization at 7to 11 months, two doses of any of the Hib vaccines licensed for infants should be given with at least 4 weeks between the two doses, followed by a booster dose at 12 to 15 months, provided that at least 2 months have elapsed since the second dose. Any of the conjugates can be used for the booster dose.
Healthy children beginning immunization at 12 to 14 months of age can receive two doses of any conjugate, with the second dose given at least 2 months after the first dose. Healthy children who initially are immunized at 15 months or older need only one dose of any of the conjugate vaccines. Unimmunized children aged 60 months or older do not need catch-up vaccination.
High-risk conditions include functional or anatomic asplenia and immunosuppression, particularly IgG2 subclass deficiency, early complement component deficiency, HIV infection, receipt of chemotherapy or radiation therapy for malignant neoplasms, and receipt of a hematopoietic stem cell transplant (HSCT). Children who will be undergoing splenectomy and are age 15 months or older who are unvaccinated or incompletely vaccinated (which means they have received fewer doses than the routine series through 14 months of age and no doses at 14 months or older) should receive a dose of Hib vaccine at least 2 weeks before splenectomy. If they have completed the recommended series, providers may offer an additional dose of Hib vaccine.
Children 12 through 59 months of age who are asplenic and have received fewer than two doses before 12 months of age require two doses of Hib. Persons 5 years of age or older who are asplenic and who are unvaccinated or incompletely vaccinated require one dose of Hib.
Children with HIV infection between 15 months and 18 years of age and who are unvaccinated or incompletely vaccinated require one dose of Hib. Hib vaccination is not recommended for HIV-infected adults.
Patients younger than 59 months undergoing chemotherapy or radiation therapy who receive doses of Hib vaccine within 2 weeks of their therapy should have these doses repeated at least 3 months after completion of therapy. Any recipient of an HSCT should be revaccinated with a three-dose regimen 6 to 12 months after a successful transplant, regardless of vaccination history; at least 4 weeks should separate the doses.
Although vaccine is not indicated for children who had documented invasive Hib infection at 2 years or older, it is indicated for children younger than 2 years who had documented invasive Hib infection because of their potential inadequate antibody response after natural infection.
Hib-containing vaccines have a very good safety record. Local reactions at the injection site and fever have been noted in less than 4% of vaccinees. The vaccines should not be administered if there is a history of anaphylaxis to the specific vaccine or to other vaccine components.
There are two inactivated single-antigen hepatitis A vaccines available in the United States: Havrix (GlaxoSmithKline Biologicals, Research Triangle Park, NC) and Vaqta (Merck, Whitehouse Station, NJ). Efficacy of one 25-unit dose of Vaqta in children 2 to 16 years of age is 97%.
Preventing hepatitis A at the community level requires widespread vaccination of children and adults. In 1996, ACIP recommended hepatitis A vaccine for children at age 2 years in communities with high rates of disease and children through the teen years in outbreaks. In 1999, the ACIP recommendations were expanded to include children beginning at 2 years or older living in states, counties, or communities with reported annual rates of hepatitis A of 20 per 100,000 or higher between 1987 and 1997, and vaccine was considered in states with rates above the national average of 10 cases per 100,000 population or higher. In 2006, ACIP recommended that all children aged 12 to 23 months be vaccinated. Children who are not vaccinated by age 2 years can be vaccinated at subsequent visits. Hepatitis A vaccine is also recommended for use among populations known to be at increased risk for infection, including persons traveling to hepatitis A–endemic areas, men who have sex with men (MSM), users of injection and noninjection drugs, persons who work with hepatitis A virus–infected primates or who do research with the virus, recipients of clotting factors, and persons who anticipate close personal contact with an international adoptee. Persons with chronic liver disease may be at increased risk for fulminant hepatitis A and should also be vaccinated as well. Homelessness has been associated with hepatitis A cases and outbreaks, and homelessness was approved as an indication for hepatitis A vaccination by ACIP in October 2018.
Havrix is recommended in a two-dose schedule, with doses separated by 6 to 12 months. The dose for children 1 to 18 years of age is 720 enzyme-linked immunosorbent assay (ELISA) units; for adults, it is 1440 ELISA units. Two doses of 25 units of Vaqta 6 to 18 months apart are recommended for persons 1 to 18 years of age, and two doses of 50 units 6 months apart are recommended for persons aged 19 years or older. The second dose is intended to produce lifelong immunity to hepatitis A. Hepatitis A vaccine is not licensed for use in children younger than 12 months. The vaccine is poorly immunogenic in infants born to women who are seropositive for hepatitis A. Simultaneous administration with IG may decrease immunogenicity slightly but should not cause any decrease in protection.
ACIP recommends hepatitis A vaccine for international travelers to countries with high or intermediate hepatitis A endemicity. Hepatitis A vaccine should be administered to infants aged 6 to 11 months traveling outside the United States. The travel-related dose for infants aged 6 to 11 months does not count toward the routine two-dose series; the two-dose series should be initiated at age 12 months according to the routine, age-appropriate vaccine schedule. Healthy travelers 12 months and older who have not received the hepatitis A vaccine should receive a single dose of vaccine as soon as travel is considered. Infants younger than 6 months and travelers who elect not to receive vaccine or for whom vaccine is contraindicated should receive a single dose of IG (0.1 mL/kg). The dose is 0.2 mL/kg if the travel duration is 1 month or longer. Persons with chronic liver disease, older adults (aged >40 years), immunocompromised persons, and persons with other chronic medical conditions planning to depart to a risk area in <2 weeks should receive the initial dose of vaccine, and IG can also be simultaneously administered at a separate anatomic injection site.
Persons who have recently been exposed to hepatitis A virus and who have not received the hepatitis A vaccine previously should receive PEP as soon as possible within 2 weeks of exposure. Persons aged ≥12 months should receive a single dose of vaccine as soon as possible. Infants aged <12 months and persons for whom vaccine is contraindicated should receive IG instead of vaccine for PEP. Immunocompromised persons and persons with chronic liver disease should receive both IG and hepatitis A vaccine simultaneously at a different anatomic site, as soon as possible after exposure. For long-term immunity, the hepatitis A vaccine series should be completed with a second dose at least 6 months after the first dose; the second dose is not necessary for PEP.
The most frequent side effects are local reactions. The only contraindication is for persons with a severe allergic reaction after a previous dose or to a vaccine component.
Hepatitis B vaccine consists of purified HBsAg particles obtained either from plasma of chronic carriers or from yeast through recombinant DNA technology. In the United States, plasma-derived vaccines have been replaced by recombinant vaccines, although the former are still available abroad. There are three single-antigen hepatitis B vaccines available in the United States—Recombivax HB (Merck), Engerix-B (GlaxoSmithKline), and Heplisav-B (Dynavax). Engerix-B is available as a combination product: with hepatitis A vaccine (Twinrix; GlaxoSmithKline), or DTaP and IPV (Pediarix; GlaxoSmithKline). Heplisav-B is a recombinant vaccine that contains an adjuvant, a synthetic oligodeoxynucleotide called CpG, which binds to a molecule on APCs called TLR9, stimulating an immune response to hepatitis B. Because recommended doses vary by age, the package insert should be consulted for the proper dose of each product. When initially licensed, use of vaccine was targeted to individuals at high risk for exposure to hepatitis B, including certain categories of health care personnel (those with risk for exposure to blood or blood products), hemodialysis patients, recipients of certain blood products, MSM, certain institutionalized individuals, parenteral drug abusers, and household or sexual contacts of chronic carriers of HBsAg. Vaccine continues to be indicated for these groups, and federal regulations now mandate that the vaccine be made available at no cost to all health care and public safety workers who anticipate exposure to human blood or body fluids during work. In 2011, adults through 59 years of age with diabetes were added to this list of risk groups, and so providers should offer hepatitis B vaccine to all adults with diabetes younger than 60 years. Providers may offer vaccine to diabetics older than 59 years, particularly if they receive assisted blood glucose screening in a long-term care facility. Failure of vaccination to have substantial impact on disease incidence when targeted only to high-risk groups, along with appreciation that hepatitis B affects larger groups in the general population (such as heterosexuals with multiple partners), has led to development of population-based control strategies. In 2018, ACIP updated recommendations for individuals with chronic liver diseases to whom hepatitis B vaccine should be administered. These included but were not limited to hepatitis C virus infection, cirrhosis, fatty liver disease, alcoholic liver disease, autoimmune hepatitis, and alanine aminotransferase (ALT) or aspartate aminotransferase (AST) levels greater than twice the upper limit of normal ( Fig. 316.4 ).
Currently in the United States, universal hepatitis B vaccine is recommended within 24 hours of birth for medically stable infants weighing ≥2000 g. Primary vaccination generally consists of three IM doses administered on a 0-, 1-, and 6-month schedule. When using combination vaccines, a four-dose schedule, including a birth dose of single-antigen hepatitis B vaccine, is acceptable. Alternative vaccination schedules (e.g., 0, 1, and 4 months or 0, 2, and 4 months) have been demonstrated to elicit dose-specific and final rates of seroprotection similar to those obtained on a 0-, 1-, and 6-month schedule. It is anticipated that those immunized as infants will still be protected when they become adolescents and young adults, the greatest risk period of acute infection in the United States. To protect infants at highest risk for development of chronic hepatitis B infection, all pregnant women should be screened routinely for HBsAg, preferably during an early prenatal visit. The vaccine should be administered within 12 hours of birth, along with hepatitis B IG, to infants born of HBsAg-positive mothers.
For adolescents and adults, the usual schedule is doses at 0, 1, and 6 months. All adolescents who previously have not been vaccinated should receive three doses of vaccine. The final dose of vaccine must be administered at least 8 weeks after the second dose and should follow the first dose by at least 16 weeks; the minimum interval between the first and second doses is 4 weeks. An alternative two-dose regimen of one licensed hepatitis B vaccine (Recombivax) is available for routine vaccination of adolescents, with doses at 0 and 4 to 6 months. For adolescents who have not been vaccinated previously, a good time to begin is at 11 to 12 years of age, when other immunizations also are recommended.
The vaccine should be administered intramuscularly to infants in the anterolateral thigh with a 1-inch 23-gauge needle and to children and adults in the deltoid region. For deltoid vaccination, a ![]() -inch 25-gauge needle may be used in children up to 9 years of age (if the skin is stretched tightly and subcutaneous tissues are not bunched), but generally a 1-inch 23-gauge needle should be used in older children and adults. Gluteal administration is associated with poorer antibody responses and is not recommended. A series of three IM doses produces a protective antibody response (antibody to HBsAg ≥10 mIU/mL) in greater than 95% of infants and children, greater than 90% of adults younger than 40 years, and 75% to 90% of adults older than 40 years. Host factors, such as smoking and obesity, contribute to decreased immunogenicity of the primary vaccine series, but age is the major determinant of vaccine response. Vaccine immunogenicity also may be lower in immunocompromised patients. Follow-up for up to 30 years has shown the virtual absence of clinically significant infections in persons who initially achieved a protective antibody titer. Most persons who lose detectable antibody appear to retain immunologic memory against significant infections. A small study of Alaskan children, vaccinated at birth, suggested that almost half of children lacked anamnestic responses after a booster dose 15 years later. However, none of the children had been infected, as measured by the presence of core antibody. In a study by Middleman and colleagues published 7 years later, 90% of study participants (420 adolescents) immunized against hepatitis B as infants exhibited a seroprotective response to a challenge dose of vaccine. Thus there is no indication at this time for booster doses of vaccine after immunization of immunocompetent children or adults. Additional experience will be necessary to know whether there will be any need for booster doses.
-inch 25-gauge needle may be used in children up to 9 years of age (if the skin is stretched tightly and subcutaneous tissues are not bunched), but generally a 1-inch 23-gauge needle should be used in older children and adults. Gluteal administration is associated with poorer antibody responses and is not recommended. A series of three IM doses produces a protective antibody response (antibody to HBsAg ≥10 mIU/mL) in greater than 95% of infants and children, greater than 90% of adults younger than 40 years, and 75% to 90% of adults older than 40 years. Host factors, such as smoking and obesity, contribute to decreased immunogenicity of the primary vaccine series, but age is the major determinant of vaccine response. Vaccine immunogenicity also may be lower in immunocompromised patients. Follow-up for up to 30 years has shown the virtual absence of clinically significant infections in persons who initially achieved a protective antibody titer. Most persons who lose detectable antibody appear to retain immunologic memory against significant infections. A small study of Alaskan children, vaccinated at birth, suggested that almost half of children lacked anamnestic responses after a booster dose 15 years later. However, none of the children had been infected, as measured by the presence of core antibody. In a study by Middleman and colleagues published 7 years later, 90% of study participants (420 adolescents) immunized against hepatitis B as infants exhibited a seroprotective response to a challenge dose of vaccine. Thus there is no indication at this time for booster doses of vaccine after immunization of immunocompetent children or adults. Additional experience will be necessary to know whether there will be any need for booster doses.
Alopeica has rarely been reported primarily in adults and has been reversible in most cases. A number of case reports have linked hepatitis B vaccine to demyelinating syndromes, including multiple sclerosis. However, data available do not support a causal relationship. The IOM's Immunization Safety Review Committee reviewed available data and concluded that the evidence did not support a relationship between hepatitis B vaccination in adults and multiple sclerosis; the evidence was inadequate to accept or reject a causal relationship with other demyelinating conditions. A more recent review by the IOM reported only anaphylaxis in some individuals that could be linked to vaccine. For most conditions reviewed, the evidence was inadequate to accept or reject a causal relationship. Recombinant hepatitis B vaccine is contraindicated in persons with hypersensitivity to yeast. Immunization is not effective in eliminating the carrier state, but there is no known risk for vaccinating individuals who are carriers or who are already immune.
In February 2018, ACIP recommended use of the new single-antigen recombinant hepatitis B vaccine with a novel cytosine-phosphate-guanine 1018 oligodeoxynucleotide adjuvant (Heplisav-B) for prevention of HBV infection in adults aged ≥18 years. Approved by the FDA in November 2017, Heplisav-B is routinely administered in two doses given ≥4 weeks apart. It can be used as a substitute in a three-dose series with a different hepatitis B vaccine, but a valid two-dose series requires two doses of Heplisav-B with ≥4 weeks between doses. When feasible, a vaccine from the same manufacturer should be used to complete the vaccination series. However, vaccination should not be deferred if the previously administered hepatitis B vaccine is unknown or if a vaccine from the same manufacturer is not available. A pregnant woman with an indication for hepatitis B vaccination should not receive Heplisav-B because no safety data are available on its use during pregnancy.
Three HPV vaccines were developed using L1 capsid proteins, which self-assemble into VLPs that are similar in conformation to the natural virus. All three are produced using recombinant techniques, which incorporate the gene expressing L1 into Saccharomyces cerevisiae or baculovirus-infected insect cells. Only one licensed vaccine is currently available in the United States: nona(nine)valent vaccine (9vHPV) containing types 6, 11, 16, 18, 31, 33, 45, 52, and 58. Types 16 through 58 in the vaccine cause about 80% of cervical cancers worldwide; types 6 and 11 cause about 90% of genital warts. Quadrivalent (types 6, 11, 16, and 18) HPV vaccine (4vHPV) and bivalent HPV vaccine (2vHPV), which contains types 16 and 18, are no longer available in the United States. The nonavalent vaccine is produced in yeast and contains an aluminum hydroxide adjuvant.
For efficacy studies for 4vHPV, a combined analysis of four clinical trials evaluating high-grade lesions (cervical intraepithelial neoplasia grade 2 or 3 [CIN 2/3] or adenocarcinoma in situ [AIS]) associated with types 16 and 18 revealed an efficacy of 100% with a lower bound of the 95% confidence limit of 92.9%. Effectiveness against genital warts related to any of the four types was 98.9% (95% confidence interval [CI], 93.7%–100%). The duration of protection is unknown, but with over 10 years of data, there is no evidence of waning protection. Efficacy of 4vHPV in males has been demonstrated for prevention of genital warts, anal intraepithelial neoplasia types 2/3 and anal intraepithelial neoplasia types 1/2/3 (88%–89%, 78%, and 75%, respectively).
9vHPV has been shown to have similar immunogenicity to 4vHPV for the four shared types, and is approximately 95% effective against the five additional HPV types in the vaccine.
Local reactions were more common in vaccine recipients. After licensure, concerns were raised about serious adverse events temporally related to HPV vaccine, such as seizures and autoimmune disorders, but the postlicensure studies have not found an elevated risk.
Within 4 years of use of these vaccines, vaccine type prevalence of HPVs decreased from 11.5% to 5.1% among females 14 to 19 years of age. ACIP recommends routine HPV vaccination at age 11 or 12 years. The vaccination schedule can be started at age 9 years. ACIP also recommends vaccination for females aged 13 to 26 years, for males aged 13 to 21 years who were not vaccinated previously, and for males to 26 years of age if they are immunosuppressed, have HIV infection, or are MSM. Vaccine also may be administered to all men 22 years to 26 years of age.
In December 2016, ACIP recommended that a two-dose schedule would be sufficient for girls and boys who initiate the vaccination series at ages 9 through 14 years. The two doses should be administered with 6 to 12 months between the doses. Three doses at 0, 1 to 2 months, and 6 months remain recommended for persons who initiate vaccination at ages 15 through 26 years, for immunocompromised persons, and for people with sickle cell disease.
Most inactivated influenza virus vaccines are manufactured in chicken eggs and are composed of inactivated disrupted (“split”) influenza viruses or of purified surface antigens. Inactivated influenza vaccine (trivalent) or IIV3 contains antigens for two influenza A viruses, H1N1 and H3N2, and one influenza B virus. Most IIV3 is administered intramuscularly; a preparation that is administered intradermally and approved for persons 18 years to 64 years of age was licensed in 2011. The intradermal IIV was changed from a trivalent to a quadrivalent vaccine a few seasons before it was discontinued (it was not marketed in 2018). One formulation of IIV3 contains four times the antigenic content of the others and is considered “high dose,” and is an option for persons aged 65 years or older. Also an option for persons aged 65 years or older is an adjuvanted vaccine, which is an IIV3 vaccine that contains a squalene-based oil-in-water emulsion.
Starting in the 2013–14 influenza season, some vaccines included antigens from two influenza A virus subtypes and two influenza B virus lineages, Yamagata and Victoria, making them quadrivalent vaccines (IIV4). Quadrivalent vaccine is an option, but there is no preference for its use in any group.
There are two forms of IIVs that are not manufactured in eggs. Cell-cultured–based influenza vaccine (ccIIV4) is manufactured in Madin-Darby canine kidney cells, is quadrivalent, is intramuscularly administered, and has been approved for use in persons 4 years of age or older. Quadrivalent recombinant hemagglutinin influenza vaccine (RIV4) is manufactured through reverse genetics in an insect cell line to produce influenza antigen and never uses the entire influenza virus. RIV4 and ccIIV4 avoid use of eggs for manufacture, which would make their production sustainable even if there were a shortage of eggs, as could occur in a pandemic. ccIIV4 uses seed virus that is isolated in eggs and therefore is not considered egg free, although the remaining quantity of egg protein is extremely low. RIV4 is considered egg free.
Because of the frequent antigenic changes in influenza viruses, the antigenic content of influenza virus vaccines may be changed annually to reflect the influenza A and B virus strains in circulation. In most years, at least one of the strains is different from the preceding year's vaccine. The efficacy of the vaccine in protecting against influenza is related to the age of the person immunized and to the degree of concordance between the virus strains included in the vaccine and the strains that are circulating in the community. When periodic changes in the antigenic structure of circulating influenza viruses occur, vaccine that contains antigens representative of prior viruses has decreased or no effectiveness. In recent years, influenza vaccine effectiveness has been approximately 40% to 60% when there is a good match between strains in the vaccine and circulating strains (across all age groups). Influenza vaccine has been estimated to be about 60% effective in preventing influenza in healthy adults younger than 65 years, when there is a good match.
In nursing home settings, effectiveness has often been substantially lower, approximately 20% to 40%. Some studies show higher effectiveness for preventing complications of influenza in such settings—for instance, 50% to 60% in preventing hospitalization or pneumonia and 80% in preventing death; however, such studies may be biased if healthier persons are more likely to be vaccinated than those who are less healthy. Influenza vaccination might reduce the frequency of secondary complications and might reduce the risk for influenza-related hospitalization and death among community-dwelling adults aged 65 years or older with and without high-risk medical conditions. Preliminary estimates of effectiveness of the A/H3N2 component of the 2017–18 vaccine showed about 17% effectiveness in the elderly, compared with 10% to 37% in younger adults. In contrast, effectiveness against influenza B strains was substantially higher in all age groups in that season: 29% to 57% effective in all age groups. Efficacy data among young children are limited. A meta-analysis of five studies showed efficacy of 59% in children 6 months to 15 years of age. In 2010, ACIP recommended that all persons aged 6 months or older be vaccinated annually. This should provide individual benefits to those who are vaccinated but also has the potential to reduce community transmission of the virus and provide indirect benefit to others.
Although routine annual influenza vaccination is recommended for all persons 6 months or older, when vaccine supply is limited, vaccination efforts should focus on delivering vaccination to the following persons (no hierarchy is implied by order of listing): all children aged 6 months to 59 months; all persons aged 50 years or older; adults and children who have chronic pulmonary (including asthma) or cardiovascular (except isolated hypertension), renal, hepatic, neurologic, hematologic, or metabolic disorders (including diabetes mellitus); persons who have immunosuppression (including immunosuppression caused by medications or by HIV infection); women who are or will be pregnant during the influenza season; children and adolescents (aged 6 months to 18 years) who are receiving aspirin or salicylate-containing medications and who might be at risk for experiencing Reye syndrome after influenza virus infection; residents of nursing homes and other long-term care facilities; American Indians/Alaska Natives; and persons who are extremely obese (body mass index ≥40). Influenza vaccination should also be emphasized for health care personnel; household contacts and caregivers of children aged 5 years or younger and adults aged 50 years or older, with particular emphasis on vaccinating contacts of children younger than 6 months; and household contacts and caregivers of persons with medical conditions that put them at higher risk for severe complications from influenza.
For the 2018–19 season, it was recommended that children 6 months to 8 years of age being vaccinated for the first time receive two doses of vaccine with an interval of at least 4 weeks between them. Children who had received two total doses or more of trivalent or quadrivalent vaccine before July 1, 2018 required only one dose of the 2018–19 recommended vaccine. Influenza seasons can peak anywhere from November to May, although the peak most often occurs in January or later, February being the most common month. Thus, although October and November have been the traditional months for influenza vaccination, during many influenza seasons vaccination through February, and even March, will provide benefit.
Adverse events associated with current influenza vaccines are infrequent. During the swine influenza immunization program of 1976, an elevated incidence rate of Guillain-Barré syndrome (GBS) was noted in recipients of the swine influenza vaccine. However, studies during the 1992–93 and 1993–94 influenza seasons suggested that influenza vaccines may have been associated with GBS at an attributable risk of about one additional case per 1 million doses in those years. No cases of GBS within 6 weeks of vaccination were detected in persons 18 to 44 years of age, despite administration of about 4 million doses of vaccine over the two influenza seasons studied.
If GBS is ever caused by current influenza vaccines, this is a rare occurrence. In contrast, the risk for hospitalization from influenza disease and its complications is orders of magnitude higher in most populations in which vaccine is recommended. Given the substantial benefits of influenza vaccine among the targeted populations, risk for GBS, if any, is exceeded by benefits. Several studies have shown an increased risk, but results were variable within and across studies and subject to methodologic challenges due to narcolepsy epidemiology and increased awareness about the association.
An increased incidence of narcolepsy has been reported in those younger than 30 to 40 years who received adjuvanted (AS03) monovalent 2009 pandemic H1N1 vaccines used in Europe in 2009 and 2010, but this vaccine was not licensed in the United States.
A recent Vaccine Safety Datalink (VSD) study found that women vaccinated early in pregnancy with an influenza vaccine containing the A(H1N1) 2009 strain and who also had been vaccinated the prior season with an A(H1N1)pdm09-containing influenza vaccine had an increased risk of spontaneous abortion (miscarriage) in the 28 days after vaccination. Earlier studies did not find a link between influenza vaccination and miscarriage. This study examined data from a small number of women in a subgroup who received H1N1-containing vaccines in consecutive years. The small numbers in the study could have led to imprecise results.There is an ongoing investigation to study this issue further among women who were pregnant and eligible to receive influenza vaccine during the 2012–13 through 2014–15 influenza seasons. Results are anticipated in late 2018 or 2019.
Because pregnant women are at high risk of serious influenza complications, it is recommended that they receive influenza vaccination during any trimester of their pregnancy. Providers should consult current guidelines for more detailed and updated recommendations.
Data demonstrating the safety of IIV for HIV-infected persons are limited, but no evidence exists that vaccination has a clinically important impact on HIV infection or immunocompetence. It is especially important to vaccinate HIV-infected persons because of their increased risk of influenza complications.
In 2003 the FDA licensed LAIV vaccine to be administered intranasally. Each viral strain in the vaccine consists of six internal genes from a cold-adapted, temperature-sensitive, attenuated mutant. The hemagglutinin and neuraminidase are derived from circulating wild strains. The cold adaptation is supportive of growth of the vaccine viruses in the upper airways, and temperature sensitivity decreases their growth in the lower airways. The vaccine had been trivalent, with reassortants for each of the major circulating influenza viruses: A(H3N2), A(H1N1), and B. Since the 2013–14 season, the only LAIV preparation has been quadrivalent, including representative strains of the two influenza B lineages, Yamagata and Victoria.
A meta-analysis of five studies showed a pooled efficacy of 83% for LAIV in children 6 months to 7 years old prior to the 2012–13 season. In a study of healthy children, vaccine was 94% effective after two doses in children 60 to 71 months of age in 1996–97, with a good match between vaccine and circulating wild virus, and 86% in 60- to 84-month-old children in 1997–98, when vaccine and circulating strains substantially diverged. In addition, vaccine reduced influenza A–associated febrile otitis media (vaccine efficacy, 94%). Estimated efficacy of LAIV against laboratory-confirmed influenza in randomized, placebo-controlled studies among 18- to 49-year-old adults was 36% in the 2007–08 season but was not significantly different from zero in either the 2004–05 or the 2005–06 season.
For the 2016–17 seasons, as well as for the 2017–18 seasons, ACIP recommended that LAIV4 not be used, because of concerns regarding low effectiveness against influenza A(H1N1)2009 in the United States during the 2013–14 and 2015–16 seasons. In the 2014–15 season, the effectiveness of LAIV4 among 2- to 8-year-olds was found to be 3% against the H3N2 strain. In the 2015–16 season the effectiveness of LAIV among 2- to 17-year-olds was found to be 5%, and against the H1N1 strain was found to be −19%. This recommendation to not use LAIV4 continued through the 2017–18 seasons. Previous data and recommendations regarding the use of LAIV are further discussed in the following text and in Chapter 165 .
In adults 18 through 49 years of age, solicited adverse reactions occurring in at least 1% of LAIV4 recipients and at a higher rate (≥1% rate difference after rounding) compared with placebo include runny nose (44% LAIV4 vs. 27% placebo), headache (40% LAIV4 vs. 38% placebo), sore throat (28% LAIV4 vs. 17% placebo), tiredness or weakness (26% LAIV4 vs. 22% placebo), muscle aches (17% LAIV4 vs. 15% placebo), cough (14% LAIV4 vs. 11% placebo), and chills (9% LAIV4 vs. 6% placebo).
Contraindications to LAIV include a history of severe allergic reaction to any component of the vaccine or after a previous dose of any influenza vaccine; concomitant aspirin or salicylate-containing therapy in children and adolescents; children aged 2 through 4 years who have received a diagnosis of asthma or whose parents or caregivers report that a health care provider has told them that during the preceding 12 months their child had wheezing or asthma or whose medical record indicates a wheezing episode has occurred during the previous 12 months; children and adults who are immunocompromised from any cause (including immunosuppression caused by medication or by HIV infection); close contacts and caregivers of severely immunosuppressed persons who require a protected environment; pregnancy; and receipt of an influenza antiviral medication within the previous 48 hours. Precautions regarding use of LAIV include moderate or severe acute illness or fever; history of GBS within 6 weeks of a previous dose of influenza vaccine; asthma in persons aged 5 years and older; and other underlying medical conditions that might predispose to complications after wild-type influenza infection (e.g., chronic pulmonary, cardiovascular [except isolated hypertension], renal, hepatic, neurologic, hematologic, or metabolic [including diabetes mellitus] disorders).
Although there had been promise that LAIV would be highly effective based on prelicensure trials and early experience, the consistent poor effectiveness documented in the United States starting in the 2013–14 influenza season, particularly against H1N1 viruses, led ACIP to recommend the vaccine not be used through 2017–18. Recent data led to the recommendation that LAIV is an option for influenza vaccination of those in whom it is appropriate to use LAIV in 2018–19.
Persons with a history of egg allergy who have experienced only hives after exposure to eggs should receive influenza vaccine. Any licensed and recommended influenza vaccine (i.e., any age-appropriate IIV, RIV4, or LAIV4) that is otherwise appropriate for the recipient's age and health status may be used.
Persons who report having had reactions to egg involving symptoms other than hives, such as angioedema, respiratory distress, lightheadedness, or recurrent emesis, or who required epinephrine or another emergency medical intervention, may similarly receive any licensed and recommended influenza vaccine (i.e., any age-appropriate IIV, RIV4, or LAIV4) that is otherwise appropriate for the recipient's age and health status. The selected vaccine should be administered in an inpatient or outpatient medical setting (including but not necessarily limited to hospitals, clinics, health departments, and physician offices). Vaccine administration should be supervised by a health care provider who is able to recognize and manage severe allergic conditions.
In 2009, inactivated Vero (green monkey kidney) cell culture–derived Japanese encephalitis (JE) vaccine (JE-VC; Ixiaro [Intercell Biomedical, Livingston, United Kingdom]) was licensed for use in persons aged 17 years or older and subsequently was recommended for travelers in this age group at high risk of JE. This is the only JE vaccine that is licensed and available in the United States. In May 2013 the FDA extended the indication for use of JE-VC to include children 2 months to 16 years of age, and subsequently ACIP extended recommendations for use in this age group. The vaccine was licensed in the United States based on a noninferiority immunogenicity study comparing neutralizing antibodies elicited by the new vaccine with the previously available JE vaccine grown in mouse brains ([JE-MB]-[JE-VAX]). The JE-MB vaccine was associated with hypersensitivity and neurologic adverse reactions. Fewer vaccine-associated hypersensitivity or neurologic adverse events are expected to occur after use of JE-VC compared with the previously used JE-MB vaccine. JE-VC vaccine consists of purified, inactivated JE proteins derived from attenuated virus propagated in Vero cells. Immunogenicity studies have demonstrated noninferiority to the JE-MB vaccine, which was proved to be 91% effective in a large-scale trial in Thailand. JE-VC vaccine is recommended for travelers who plan to spend a month or longer in endemic areas during the Japanese encephalitis virus (JEV) transmission season. This includes long-term travelers, recurrent travelers, or expatriates who will be based in urban areas but are likely to visit endemic rural or agricultural areas during a high-risk period of JEV transmission. Vaccine should also be considered for the following: short-term (less than 1 month) travelers to endemic areas during the JEV transmission season, if they plan to travel outside an urban area and their activities will increase the risk of JEV exposure; travelers to an area with an ongoing JEV outbreak; and travelers to endemic areas who are uncertain of specific destinations, activities, or duration of travel. The immunization schedule is two doses administered 28 days apart.
Measles vaccine is a live-attenuated virus vaccine recommended for use in all children 12 months and older who do not have contraindications. When administered to a child 12 to 15 months or older, the median one-dose efficacy is 93% and the median two-dose efficacy is 97%. Only a single dose is needed to provide long-lasting, probably lifelong, immunity in those who respond to the vaccine. However, evidence indicates that measles transmission can be sustained among the 2% to 5% of vaccinated persons who fail to be protected after an initial dose of vaccine. Therefore, beginning in 1989 a two-dose schedule of measles-containing vaccine was recommended in the United States. The first dose should be administered at 12 to 15 months of age. Lower levels of maternal antibody from currently vaccinated mothers allow higher rates of seroconversion at 12 months than in the past, when most maternal antibody came from mothers with naturally acquired disease.
The second dose should be administered 1 month or more after the first dose, typically at entry to school (4–6 years of age). Both doses should routinely be given as combined MMR vaccine or MMR and varicella (MMRV). Both MMR and MMRV vaccines are associated with an elevated febrile seizure risk, but data suggest that MMRV, because it is associated with a higher risk for fever than the separate administration of MMR and varicella, also may be associated with an increased risk for febrile seizures compared with simultaneous separate MMR and varicella vaccines after the first dose of the two-dose series. In June 2009, after consideration of the postlicensure data and other evidence, ACIP adopted new recommendations regarding use of MMRV vaccine for the first and second doses and identified a personal or family (i.e., sibling or parent) history of seizure as a precaution for use of MMRV vaccine. For the first dose of measles, mumps, rubella, and varicella vaccines at age 12 to 47 months, either MMR vaccine and varicella vaccine or MMRV vaccine may be used. Providers who are considering administering MMRV vaccine should discuss the benefits and risks of both vaccination options with the parents or caregivers. Unless the parent or caregiver expresses a preference for MMRV vaccine, ACIP recommends that MMR vaccine and varicella vaccine should be administered separately for the first dose in this age group. For the second dose of measles, mumps, rubella, and varicella vaccines at any age (15 months through 12 years—the age for which the vaccine is approved) and for the first dose at age 48 months or older, use of MMRV vaccine generally is preferred over separate injections of its equivalent component vaccines (i.e., MMR vaccine and varicella vaccine). Recent data from Australia suggest that MMRV is not associated with an increased risk of febrile seizures when it is the second dose of an MMR-containing vaccine.
All college entrants who have not received two doses of MMR vaccine on or after their first birthday should receive two doses. The two doses can be given separated by 1 month. Immunization is recommended for all people not known to be immune. Because people born before 1957 are likely to have been infected naturally, they usually are considered immune. Other acceptable evidence of measles immunity is documentation of adequate vaccination or laboratory evidence of immunity to measles. Health care facilities should consider recommending a dose of MMR to unvaccinated providers born before 1957 who do not have laboratory evidence of immunity to both measles and rubella or other acceptable evidence of measles immunity.
Because measles is much more prevalent outside the United States, adequate vaccination is recommended for all travelers born after 1956. These travelers should have evidence of having received two doses. Infants 6 months to 11 months of age should receive a dose of MMR vaccine if they travel internationally. One dose is recommended for travel in this age group, but this dose is not considered part of the routine two-dose childhood series (beginning at 12 months of age), so two additional doses should be administered at the appropriate age.
Adverse reactions associated with measles vaccine include fever of 39.4°C or greater in 5% of recipients and transient rashes in about 5% of vaccinees. Because measles vaccine can cause fever, it can be associated with febrile seizures. Children with prior personal histories of seizures or histories of seizures in the immediate family may be at increased risk for febrile seizures after MMR vaccination. Anaphylaxis and thrombocytopenic purpura also appear to be caused rarely by MMR. Encephalopathy with onset about 10 days after vaccination has been reported in vaccine recipients, with a frequency of approximately 1 in 2 million vaccinations; although a causal role for measles vaccine has not been established. There is no association between MMR vaccine and autism.
Measles vaccine is contraindicated for pregnant women and in persons who are immunocompromised because of either congenital or acquired disorders (e.g., leukemia or immunosuppressive drugs), with the exception of persons infected with HIV. Because measles may cause severe disease in HIV-infected people, MMR vaccine is recommended for persons who do not have evidence of severe immunosuppression. Absence of severe immunosuppression is defined as CD4 percentages greater than or equal to 15% for 6 months, or longer for persons 5 years old or younger, and CD4 percentages greater than or equal to 15% and CD4 count greater than or equal to 200 lymphocytes/mm 3 for 6 months, or longer for persons older than 5 years. When only CD4 counts or CD4 percentages are available for those older than 5 years, the assessment of severe immunosuppression can be on the basis of CD4 values that are available. When CD4 percentage is not available for children 5 years old or younger, the assessment of severe immunosuppression can be on the basis of age-specific CD4 counts at the time the CD4 counts were measured (i.e., absence of severe immunosuppression is defined as 6 months’ duration above age-specific CD4 count criteria: CD4 count greater than 750 lymphocytes/mm 3 in those 12 months old or younger and CD4 count greater than or equal to 500 lymphocytes/mm 3 in those 1–5 years of age).
Four meningococcal containing vaccines are available in the United States. Two vaccines contain purified meningococcal capsular polysaccharides of groups A, C, Y, and W, conjugated to protein (MenACWY), which results in a vaccine that is immunogenic in infants and young children. Immunization involves induction of T-lymphocyte cell-dependent responses, and induces immunologic memory to meningococcal polysaccharide. Two vaccines are serogroup B meningococcal (MenB) recombinant protein vaccines. MPSV is no longer available in the United States.
Conjugate meningococcal vaccines reduce carriage and induce herd protection.
One conjugate vaccine, MenACWY-D (Menactra), is licensed for persons 9 months to 55 years of age, and the other conjugate vaccine, MenACWY-CRM (Menveo), is licensed for persons 2 months to 55 years of age. The antibody responses to each of the four conjugated polysaccharides included in each of the quadrivalent vaccines are serogroup specific, independent, and comparable for the two vaccines. Meningococcal conjugate vaccines routinely are indicated for immunization of adolescents, for control of outbreaks attributable to a vaccine serogroup, and for use among certain high-risk groups, such as persons with persistent complement component deficiencies, eculizumab use, HIV infection, or anatomic or functional asplenia, and laboratory personnel who routinely are exposed to isolates of N. meningitidis. Meningococcal conjugate vaccine (MenACWY) routinely is recommended for all adolescents beginning at 11 to 12 years of age, with a booster dose at 16 years of age. Adolescents who receive their first dose of MenACWY at 11 to 12 years of age routinely are recommended for a booster at 16 years of age. Adolescents who receive their first dose of vaccine at 13 to 15 years of age are recommended to receive a booster at 16 to 18 years of age. First-year college students 19 years of age or older living in residence halls should receive a dose if they have not been vaccinated after the 16th birthday. Regardless of attendance in a college, if a high-risk scenario develops (e.g., travel to a region in the “the meningitis belt” of sub-Saharan Africa [which stretches from Senegal to Ethiopia], entering the military, routine exposure to N. meningitidis through microbiology laboratory work), a dose should be provided if it has been 5 years since the most recent dose. Children traveling to the meningitis belt (or to the Hajj in Saudi Arabia) should receive a quadrivalent meningococcal conjugate vaccine (MenACWY-CRM or MenACWY-D).
When initiated at 2 months of age, a four-dose schedule is recommended for MenACWY-CRM, with doses at 2, 4, 6, and 12 months of age; when initiated at 9 to 23 months of age, a two-dose schedule is recommended. Children aged 9 months and older can receive MenACWY-CRM or MenACWY-D. MenACWY-D is recommended as a two-dose primary series, with 3 months separating the doses (8 weeks minimum). Children 2 to 23 months of age with functional or anatomic asplenia or HIV infection should receive MenACWY-CRM vaccine. To avoid interference with the immunologic response to the infant series of PCV13, children younger than 24 months with functional or anatomic asplenia or HIV infection should not receive MenACWY-D vaccine. In contrast, MenACWY-CRM does not demonstrate immune interference with PCV7 (and, by extrapolation, PCV13) after the 12-month dose, and can therefore be administered concomitantly with PCV13. Because a potential for immunologic interference with MenACWY-D response has been demonstrated when MenACWY-D is administered 30 days after DTaP vaccine, it is recommended that MenACWY-D be given either before or concomitantly with DTaP in children at increased risk for meningococcal disease.
Adults at increased risk for meningococcal disease (functional or anatomic asplenia, complement component deficiency, travel or residence in the meningococcal belt, exposed to an outbreak of vaccine serogroup) also should receive either MenACWY-D or MenACWY-CRM. A two-dose primary series is recommended for adults with functional or anatomic asplenia, HIV infection, and complement deficiency.
For children first vaccinated before 7 years of age with MenACWY, revaccination should be considered after 3 years if they remain at high risk, and then every 5 years thereafter for subsequent booster doses, as long as they remain at high risk.
The development of vaccines against meningococcus serogroup B has been hampered because the serogroup B polysaccharide is very poorly immunogenic in humans. Through the use of reverse genetics, recombinant serogroup B antigens that can provide protection against serogroup B have been identified, and two vaccines were licensed in the United States in 2014 and 2015, respectively: MenB-FHbp (Trumenba) and MenB-4C (Bexsero). ACIP recommended that the vaccines be used to immunize individuals aged 10 years or older who are at increased risk for serogroup B disease (persistent complement component deficiencies, eculizumab use, anatomic or functional asplenia, or at risk because of an outbreak of serogroup B disease). While ACIP does not routinely recommend a serogroup B meningococcal vaccine for all teens and young adults without risk factors for serogroup B disease, all teens and young adults may get vaccinated, preferably at 16 through 18 years old; this decision is left to individual consideration of health care providers, parents, and patients. For adults at increased risk for meningococcal disease and for use during serogroup B meningococcal disease outbreaks, three doses of MenB-FHbp should be administered at 0, 1 to 2, and 6 months. When given to healthy adolescents and young adults who are not at increased risk for meningococcal disease, two doses of MenB-FHbp should be administered at 0 and 6 months. The other MenB vaccine, Men-4C, is a two-dose series, with the two doses administered at least a month apart. (see Fig. 316.4 ).
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here