Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Dedicated magnetic resonance imaging (MRI) of peripheral nerves is referred to as magnetic resonance neurography (MRN). From the time Howe and Filler et al. originally described MRN, this technique has evolved with constant addition of newer and optimized MRI techniques, and it is being increasingly used in clinical practice.
Basic nerve anatomy, pathophysiology, and injury grading should be understood to detect deviations from the normal morphology and accurately identify injury and entrapment related to nerve pathology. Peripheral nerves are covered by an outer epineurium, which contains multiple fascicles that are covered by perineurium. The fascicles contain endoneurium-covered axons. The peripheral nerves may be host to a large variety of diseases, which can be classified based on the functional involvement (motor, sensory, mixed, or autonomic) or the anatomic site of involvement (neuromuscular junction or nerve-cell body, axon, and myelin sheath). Nerve diseases can be categorized into three large groups:
Group 1: systemic diseases such as vasculitis and ischemia; toxic, endocrine, and metabolic disorders such as diabetes mellitus, thyroid disorders, hyperlipidemia, and amyloidosis; acute and chronic demyelinating inflammatory neuropathies, hereditary neuropathies, and others. MRN is not expected to make these diagnoses, but it can be used to confirm a diagnosis by demonstrating the primary signs of neuropathy and secondary signs of regional muscle denervation changes while excluding any structural cause for mononeuropathy.
Group 2: local conditions such as nerve injury, plexopathy, peripheral compressive lesions, and adhesive neuropathy in failed tunnel release cases, nerve sheath tumors, and so forth. MRN has a vital role in this group of diseases and provides supplementary information gained from clinical examination and electrodiagnostic tests by allowing direct visualization and interrogation of the nerves and muscles.
Group 3: neuropathies related to functional anatomic changes, including habitual leg crossing, repeated typing, or repetitive exercise, which may cause traction as well as compressive neuropathy via functional compartment syndromes. MRN is rarely used in this group because the diagnosis is based on clinical examination and pressure catheter studies. However, MRN can play a role in complicated cases, with imaging before and after the instigating exercise/effort in question to detect muscle signal alterations.
Nerve injuries can be related to blunt or penetrating trauma. Iatrogenic causes also play a role, such as nerve injuries after surgical plating, local surgery, or cryotherapy/radiofrequency ablation. Nerve injuries can also occur with fractures or fracture-dislocations, such as with hip dislocation (sciatic nerve), shoulder dislocation (axillary nerve), humeral fracture (radial nerve), and elbow fracture/dislocation (ulnar nerve). Nerve injuries are traditionally classified based on the Seddon and Sunderland classification system ( Table 65-1 ). Seddon classification describes three different types of nerve injuries: neurapraxia, axonotmesis, and neurotmesis. Neurapraxia is the least severe type of nerve injury and is related to a temporary block of nerve conduction. Neurapraxia usually results from blunt trauma with predominant myelin sheath injury and usually resolves completely in less than 6 to 8 weeks. MRN may show abnormal increased signal on T2-weighted images without muscle denervation changes; no wallerian degenerative changes occur in this process, and electromyography (EMG) studies are normal. Nerve conduction velocity (NCV) studies may demonstrate temporary slowing or conduction block. In the authors' experience, neural fascicular changes (disruption, effacement, or enlargement) are generally not seen in this type of mild nerve injury.
| Degree of Nerve Injury | Myelin | Axon | Endoneurium | Perineurium | Epineurium | MRN Findings | Recovery |
|---|---|---|---|---|---|---|---|
| I: Neurapraxia | +/− | No | No | No | No | Nerve: increased T2 SI Muscle: normal |
Full |
| II: Axonotmesis | Yes | Yes | No | No | No | Nerve: increased T2 SI, abnormal fascicles; mildly enlarges nerves Muscle: denervation |
Full |
| III | Yes | Yes | Yes | No | No | Same as II; worsening abnormalities | Slow/incomplete |
| IV | Yes | Yes | Yes | Yes | No | Nerve: focal enlargement, effaced/disrupted fascicles, neuroma in continuity Muscle: denervation |
Poor |
| V: Neurotmesis | Yes | Yes | Yes | Yes | Yes | Nerve: complete discontinuity Muscle: denervation |
None |
| VI: Dellon-Mackinnon | Combination of above | Nerve: mixed T1/T2 SI; surrounding soft tissue, ligament, and muscle injury changes | Poor to none | ||||
Axonotmesis is a more severe nerve injury that results from severe nerve contusion or crush injury leading to axonal injury and wallerian degeneration distally. Axonotmesis results in loss of axonal continuity and disruption of the endoneurium with preservation of the epineurium and perineurium, and there is both sensory and motor function loss. EMG and NCV are both abnormal. On MRN there are muscle denervation changes with increased T2 signal intensity of the nerve, as well as neural fascicular enlargement, disruption, or effacement. Since the peripheral myelin sheath is intact, functional loss related to axonotmesis eventually recovers, albeit slowly.
Neurotmesis is the most severe type of nerve injury, leading to axonal injury as well as disruption of the surrounding perineurium and epineurium. There is complete loss of motor, sensory, or autonomic function. EMG is usually unable to differentiate between axonotmesis and neurotmesis. On MRN there is complete transection of the nerve with end-bulb neuroma formation or sometimes neuroma in continuity. This type of injury does not have much potential for recovery. MRN plays a significant role in differentiating a reversible injury from irreversible injury by depicting nerve discontinuity and neuroma, and may therefore help guide treatment planning by identifying cases amenable to surgical intervention. Sunderland grading is a further expanded injury grading system based on an inside-out anatomy model (i.e., injuries to endoneurium, perineurium, and epineurium are encompassed in grades 3/4/5 in this system). The respective MRN findings are shown in Table 65-1 . Dellon and Mackinnon added a sixth grade based on outside-in partial circumference injury to the nerve. The worst type of Sunderland (1-V) injury grade dictates the prognosis in such injury.
MRN provides information that may assist in diagnosis, therapeutic follow-up, and surgical planning for peripheral nerve disorders. The following are the most common indications for MRN based on the authors' experience:
To confirm the clinical suspicion of peripheral neuropathy
To grade nerve injury
To assess the extent of the nerve abnormality
To perform preoperative mapping before neurolysis, as well as postoperative evaluation
To differentiate between postradiation changes and recurrent malignancy
To depict the intraneural or extraneural space-occupying lesions causing neuropathy
To help exclude the diagnosis of major neuropathy by depicting normal nerves and regional muscles
To provide imaging guidance for perineural and perimuscular injections
MRN is a tissue-selective MRI technique. This technique may be broadly categorized as conventional and diffusion-based sequences. Conventional imaging encompassing T1- and T2-weighted images provides good anatomic and pathologic imaging. It is also widely available and provides consistent and reproducible imaging quality. Although still being optimized for widespread clinical application, diffusion-based imaging allows nerve signal quantification and the potential for functional imaging. The essentials of good conventional MRN are spin echo–type contrast, thin-section (2-4 mm), two-dimensional (2D), T1-weighted and fat-suppressed T2-weighted MRI with in-plane resolution of 0.3 to 0.5 mm, uniform and excellent fat suppression, high echo times (TE > 60 milliseconds) to minimize magic-angle artifacts, and superior resolution (256 × 392 or higher) employing a combination of 2D nerve perpendicular and three-dimensional (3D) isotropic images.
MRN can be performed using both 1.5 tesla (T) and higher-field 3T scanners. The advantages of higher-field scanners are higher signal-to-noise ratio (SNR), better gradient performance, wider available bandwidth, and improved coil design. Increased SNR helps improve spatial resolution by allowing reduced slice thickness and voxel size. On non–fat-suppressed T2-weighted images, differentiation of abnormal high signal within the nerve from the normal hyperintense T2 signal of the adjacent fat and the fascicular morphology can be difficult to appreciate. Fat suppression can be performed in various ways, including frequency-selective fat saturation, short tau inversion recovery (STIR), spectral adiabatic inversion recovery (SPAIR), chemical shift imaging (CSI), and Dixon-type fat-suppression technique.
Fat suppression can be challenging and often inhomogeneous along curvatures of the extremities, particularly on low-field scanners. STIR images frequently provide superior fat saturation, especially on low-field scanners. However, STIR images have poorer spatial resolution, longer acquisition time, and less SNR than fast-spin echo (FSE) imaging overall, and they are prone to pulsation artifacts. 3D imaging is also degraded on low-field scanners; only gradient echo–type 3D sequences are feasible on low-field scanners to reduce the time penalty. Given the above-mentioned consequences, the authors recommend MRN on 3T scanners whenever possible, except when there is expected interference due to susceptibility artifacts, such as from metal prostheses. Susceptibility and chemical shift artifacts are aggravated on 3T images, although these effects can be partially mitigated using SPAIR imaging, employing parallel imaging, and increasing the bandwidth.
To perform 3D imaging, the authors recommend isotropic (sub-1.5 mm), predominantly spin echo–type sequences in most studies. These are single-slab turbo-spin echo (TSE)-type images with variable flip angle evolutions. The parameters can be adjusted, and output can be set to provide T2-weighted, proton density (PD)-, SPAIR-, or STIR-type contrasts. 3D SPAIR TSE provides better images for extremity MRN as compared to STIR TSE, owing to higher SNR. 3D STIR TSE is ideal for brachial and lumbosacral (LS) plexus exams because of its better fat suppression. 3D SHINKEI (3D nerve- SH eath signal increased with INK ed rest-tissue rar E I maging [rapid acquisition with refocused echoes]) (Philips, Best, Netherlands) provides blood signal suppression to provide better nerve identification in the LS plexus. For these 3D images, the authors recommend a repetition time (TR) of 1500 to 2000 milliseconds, echo time (TE) of 70 to 80 effective, and 1.2 to 1.5 mm isotropic voxel. In the future, 3D Dixon sequences will be available with some diffusion weighting (b value, 60-80 sec/mm 2 ) for higher contrast and spatial resolution. In general, these 3D sequences null the arterial signal and allow easier identification of nerves in multiple isotropic planes. In plexus cases, one should add axial and sagittal T2-weighted images of the spine or alternatively 3D non–fat-suppressed T2-weighted TSE sequences, since spine pathology is a major confounder in plexus pathology. These 3D images allow multiplanar isotropic reformats (MPR), curved planar reformats (CPR), and maximum intensity projection (MIP) to demonstrate the longitudinal extent of neural lesions.
The use of intravenous contrast (gadolinium) provides limited value in peripheral neuropathies; in our practice, it is restricted to cases of suspected infections, acute-subacute inflammation, diffuse peripheral nerve lesions, and tumors. Intravenous contrast may better show enhancement of the denervation changes of muscles; however, these changes can already be apparent on fat-suppressed T2-weighted or STIR images.
Diffusion-based MRN is technically demanding and remains in the experimental phase. It increases nerve conspicuity by suppressing the vascular signal, especially on higher b values. For nerve identification, one needs b values more than 60 to 80, whereas for tractography, one needs b values of approximately 600 to 1000 and 12 to 15 directions of interrogation. One may increase the specificity of MRN by parametric analysis of diffusion tensor imaging (DTI) data, including measurement of apparent diffusion coefficient (ADC) and fractional anisotropy (FA). DTI allows generation of quantitative values that can be used for follow-up assessment of nerve regeneration/degeneration. Diffusion-based 3D reversed fast imaging in steady-state free precession (FISP), or 3D DW-PSIF, is a hybrid-type sequence that can be used for effective vascular suppression and selective nerve distribution in extremities. Its parameters include TR, 12 milliseconds; TE, 2.5 milliseconds; b value, 60 to 80; and voxel, 0.8 mm isotropic. It has all the advantages of a 3D isotropic sequence, including MPR, CPR, and MIP.
To perform extremity MRN, one could use a combination of axial T1 weighting, axial T2 SPAIR or T2Dixon, sagittal fat-suppressed proton density–weighting (fsPDW), coronal 3D SPAIR TSE, coronal 3D DW-PSIF, and axial DTI. To perform MRN of the lumbar plexus, one would use a combination of axial T1, axial T2 SPAIR or T2Dixon, coronal 3D STIR TSE or SHINKEI, sagittal 3D T2TSE for spine and axial DTI. Optimal brachial plexus MRN has similar requirements as LS plexus imaging, the principle difference being that axial T2 SPAIR of the LS plexus sequence is replaced by sagittal STIR on both sides.
Accurate MRN interpretation requires detailed knowledge of peripheral nerve anatomy, its common variations, and nerve pathophysiology in various diseases. For 2D evaluation of peripheral nerves, T1- and T2-weighted images should be evaluated in tandem on the reading workstation. T1 images are useful for anatomic evaluation and differentiation of nerves from the adjacent vessels. T1-weighted images nicely demonstrate fascicular anatomy and perineural fibrosis (strandlike T1 and T2 hypointensities). Atrophy and fatty replacement of the regional skeletal muscles are also better appreciated on T1-weighted images. T2-weighted images are better for detection of abnormal signal intensity of the nerve (approaching the signal intensity of the adjacent in-plane vessels). Normal nerves are isointense to regional muscles on T1 and T2 sequences and demonstrate a smooth course with uniformly-sized fascicles, clean surrounding perineural fat planes, and caliber almost similar to adjacent arteries that gradually taper distally. Mild nonspecific T2 hyperintensity due to subclinical neuropathy or magic-angle artifact can be seen. If the nerve is pathologic, one would see constant abnormal signal along the longer segment of the nerve beyond the expected location of magic-angle artifact (e.g., sciatic nerves show magic-angle artifact at the sciatic notch, whereas patients with sciatic neuropathy exhibit asymmetric increased signal of the nerve beyond the expected notch location). Normal nerves do not enhance, owing to a preserved blood-nerve barrier. Fascicle evaluation may demonstrate enlargement, effacement, or disruption due to distal obstruction, axonal degeneration, venous congestion and hyperemia, or direct injury. Postinjury neuroma can be differentiated from peripheral nerve sheath tumor (PNST) based on nonenhancement of the injury-related former lesion.
Symmetric increased signal intensity is particularly common on 3D STIR images and is due to the high fluid sensitivity of these images and extremely good fat suppression. It is seen in all plexus nerves in the retroperitoneal course, whereas isointense signal is seen beyond the inguinal ligament. Therefore one should look for asymmetric signal alteration and other findings suggesting neuropathy, as described earlier. STIR sequences are also very useful for brachial and LS plexus abnormalities. Split variations of nerves (e.g., sciatic, femoral nerves) are common, and these findings by themselves are not pathologic. 3D images should be reconstructed as thick-slab (5-20 mm) MIP images to reduce image blurring and noise and improve conspicuity of the peripheral nerves. 3D images are great for looking at gross abnormal findings such as neuroma formation, focal or diffuse nerve swelling, nerve displacement or abnormal angulation, or nerve discontinuity. Secondary signs of neuropathy are very informative. For instance, muscle denervation changes can give a rough estimate of the acuity of the injury or lesion; acute-phase pathology demonstrates edema-like T2 signal intensity without fatty infiltration or atrophy, subacute phase pathology depicts muscle edema with mild fatty infiltration with or without atrophy, and chronic phase pathology shows frank muscle atrophy and fatty infiltration. As a general rule, diffuse muscle involvement and lack of fascial edema/enhancement can help in differentiating denervation changes from myositis and muscle strains. It is beyond the scope of this chapter to describe evaluation of peripheral nerves based on DWI sequences, given their technically demanding and experimental nature. It suffices to mention that neuropathic nerves are easily seen on tensor images and depict low FA and high ADC values. With nerve regeneration, the FA values increase and ADC values decrease. For nerve sheath tumors, low ADC values (<1.1 × 10 −3 mm 2 /sec) suggest extremely high cellularity and likely malignancy.
Upper extremity nerves, especially when they pass through tunnels, are vulnerable to intrinsic or extrinsic abnormalities that lead to compression or impingement syndromes. Tunnel syndromes may results from various etiologies, including acromegaly, pregnancy, myxedema, rheumatoid arthritis, space-occupying lesion (e.g., hematoma), fracture deformity, tenosynovitis, ganglion cyst, anomalous muscle, varicosities, nerve sheath tumors, and other regional benign or malignant tumors. Traditionally EMG and NCV have been the diagnostic modalities of choice for evaluation of upper extremity nerve diseases. However, these studies provide limited information about the nature and severity of the lesion, existence of neuroma, or precise location of the pathology. High-resolution ultrasound (US) has the ability to provide more anatomic information but is very operator dependent, poor in the assessment of deep nerves, and gives limited or no information about regional muscle changes.
The median nerve arises from the medial roots of C8-T1 and lateral roots of C5-C7. It courses along the humerus between the biceps brachii and brachialis muscles and beneath the bicipital aponeurosis. It may travel beneath the ligament of Struthers that connects the supracondylar process to the medial epicondyle. The anterior interosseous nerve (AIN) arises from the radial aspect of the median nerve 5 to 8 cm distal to the medial epicondyle of the humerus. The median nerve may be injured or entrapped at different levels, producing different clinical symptoms.
Radiographs usually demonstrate a supracondylar spur when present. MRN shows high T2 intensity with or without nerve flattening, and sometimes the culprit ligament. Symptoms include paresthesia in the thumb, index and middle fingers, pain in the volar aspect of forearm, and weakness of forearm flexors. Treatment includes rehabilitation or surgical release, based on the severity of symptoms.
Pronator syndrome may be due to a hypertrophied pronator muscle, thickened bicipital aponeurosis, thickened fascial edge of the flexor digitorum superficialis (FDS), aberrant median artery, or any kind of regional space-occupying lesion. Symptoms include pain in the volar forearm and proximal arm, paresthesia of the thumb, index, and middle fingers, and positive Tinel's sign. EMG is positive in about two thirds of patients. MRN demonstrates moderate to severe T2 hyperintensity, with nerve flattening at the entrapment site. Proximal nerve enlargement may be present. Mild hyperintensity can be seen in asymptomatic cases. Treatment includes rehabilitation or surgical release, based on the severity of symptoms.
The AIN is predominantly a motor nerve, and it is the largest branch of the median nerve. It courses between the two heads of the pronator teres muscle and reaches the anterior aspect of the interosseous membrane to travel beside the anterior interosseous artery. The AIN provides motor innervation to the radial belly of the flexor digitorum profundus (FDP), flexor pollicis longus (FPL), and pronator quadratus (PQ) muscles. It also provides sensory innervations to the wrist. Compression of the nerve results in weakness of pinching, even though PQ weakness (pronation) may be compensated for by the concurrent action of the pronator teres. There are many causes of AIN palsy: fibrous bands from the deep (more common) or superficial head of the pronator teres to the brachialis fascia, fractures of the forearm bones or supracondylar fracture, local pressure such as that resulting from prolonged atypical sleeping position or a poorly applied cast, exercise and weightlifting, and viral neuritis.
On MRI of AIN neuropathy, changes in muscle denervation are easily observed in a typical distribution. Although the PQ muscle is always the first one involved in AIN neuropathy, it should be noted that isolated PQ signal change or atrophy is also commonly seen as an asymptomatic finding. On high-resolution MRN, abnormal signal of the AIN itself can easily be differentiated from adjacent vessels, and changes in PQ size are appreciated in cases of neuropathy.
Carpal tunnel syndrome (CTS) is the most common neuropathy of the upper extremity. The carpal tunnel is a fibroosseous canal formed by carpal bones and a rigid fibrous transverse carpal ligament. The median nerve branches into a recurrent motor branch that supplies the thenar muscles and multiple sensory branches that provide innervation to the three radial digits and the radial half of the fourth digit.
The pathophysiology of CTS involves a combination of mechanical trauma, increased intratunnel pressure, and ischemic injury to the median nerve, leading to perineurial and epineurial edema and hyperemia, followed by demyelination and axonal loss. Clinically these patients complain of nocturnal pain, hand clumsiness, tingling, numbness, and paresthesia in the distribution of the median nerve. Sensory loss typically precedes the motor deficit. Patients with long-standing disease may have wasting of the soft tissues of the thenar eminence. Clinically there is a positive Phalen test (increased paresthesia after 1 minute of passive wrist flexion) and Tinel's sign (paresthesia in nerve territory after gentle tapping over carpal tunnel).
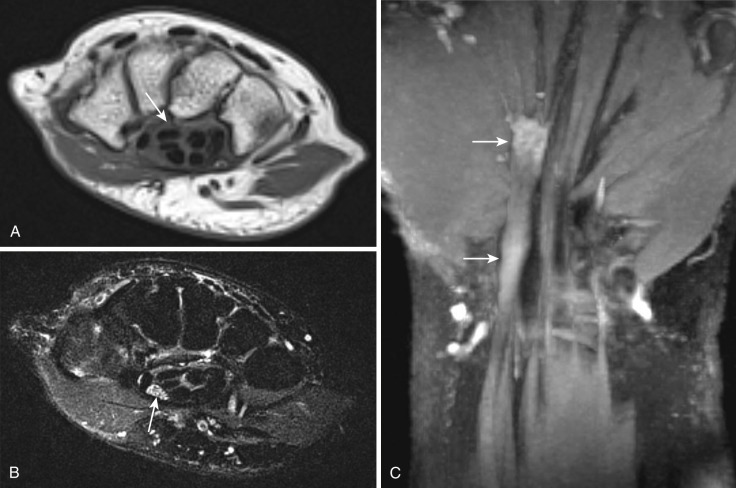
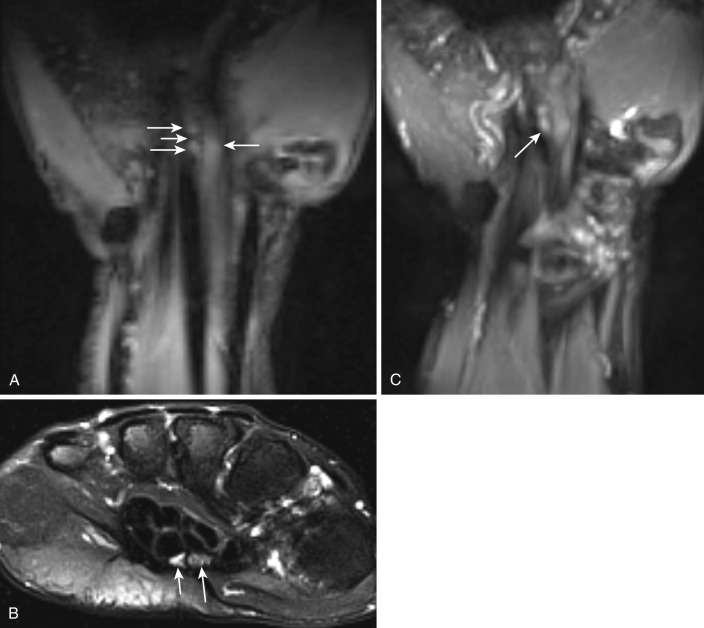
Although electrophysiology studies are frequently performed for wrist neuropathies, false-negative rates can be as high as 10% to 30% and positive predictive values can be as low as 33%, which clearly indicate a need for improved diagnosis. Isolated MRI signs of abnormal nerve T2 hyperintensity have low reported specificities of less than 40%. Other signs such as flattening of the median nerve in the carpal tunnel, proximal median nerve enlargement, volar bowing of the retinaculum, extension of T2 hyperintensity distally in the median nerve branches, and effacement of the deep fat pad of the carpal tunnel increase the specificity of the diagnosis of CTS. MR neurography also shows tenosynovitis, internal wrist derangement, and changes in regional muscle denervation in a noninvasive fashion. 3T MR neurography depicts the individual bundle abnormality of a bifid nerve, and nerve abnormalities can also be shown in the longitudinal plane of the nerve ( Fig. 65-1 ). Failure to recognize the bifid components of the median nerve ( Fig. 65-2 ) may lead to accidental injury to the median nerve during surgical intervention.
The ulnar nerve arises from the medial cord of the brachial plexus (C8-T1 and sometimes C7) and courses along the medial border of the medial head of the triceps muscle. The nerve travels beneath the medial intermuscular septum and descends posteriorly through the cubital tunnel. The nerve courses distally between the two heads of flexor carpi ulnaris (FCU) muscle (Osborne band) and reaches the Guyon's canal (ulnar tunnel). It provides a palmar cutaneous branch and a dorsal cutaneous branch to the medial 1.5 digits. The ulnar nerve gives off motor branches to the hypothenar, third palmar and fourth dorsal interossei, third and fourth lumbricals, adductor pollicis, and flexor pollicis brevis muscles. Like other major nerves, the ulnar nerve may be injured or entrapped at different levels, producing different clinical syndromes.
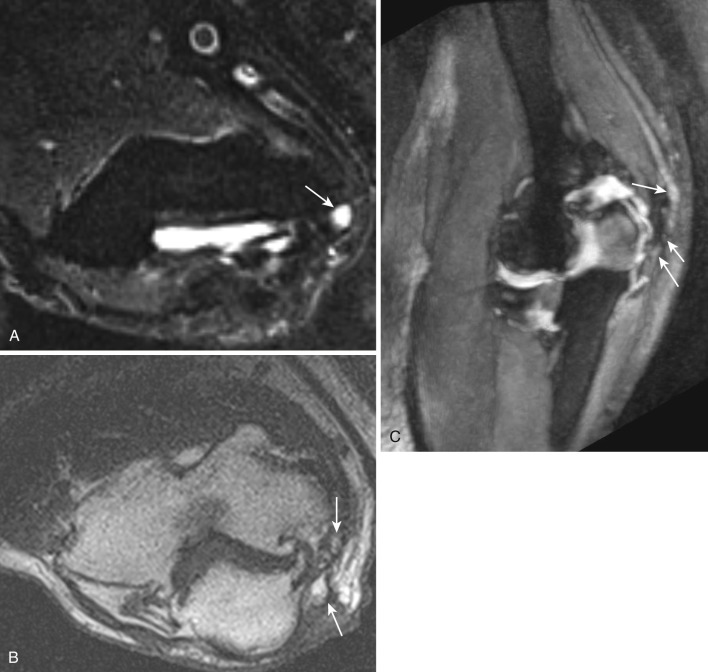
Also known as sleep palsy or cell phone neuritis, cubital tunnel syndrome is usually due to repetitive flexion of the elbow joint. Sometimes prolonged external pressure (driver's elbow) can cause this syndrome. It can also be secondary to other intraneural or extraneural compressive lesions described earlier. Although clinical examination, EMG, NCV, and US can be diagnostic, MRN can clearly depict the underlying problem in most cases ( Fig. 65-3 ). Mild increased T2 signal intensity can be seen in asymptomatic individuals. The T2 signal intensity and its longitudinal extent increase with worsening neuropathy. Enlarged fascicles, nerve enlargement and flattening, angulation or displacement, and regional muscle changes can be visualized using MRN. Treatment includes rehabilitation or surgical release. Reentrapment of the anteriorly transposed nerve may occur secondarily to overzealous dissection and development of perineural fibrosis, leading to abnormal nerve flattening and angulation. MRN is quite useful in these cases as well.
The ulnar nerve can be affected at this level by external compression, space-occupying lesions, or chronic traction neuritis. Symptoms vary based on three zonal locations of involvement:
Zone 1: proximal edge of the volar carpal ligament to bifurcation of the ulnar nerve, where patients present with combined motor and sensory deficits
Zone 2: from the nerve bifurcation to just distal to the fibrous arch of the hypothenar muscles, where patients may present with pure motor deficit
Zone 3: distal end of the canal containing the superficial sensory branch, where patients may present with pure sensory deficit
Lesions in zones 1 and 3 are more common. Symptoms include sensory loss of the volar aspect of the medial fourth and fifth digits, with sparing of the dorsal aspect of the medial hand and digits and palmar aspect of the proximal hand because the respective nerves arise 5 cm above the wrist joint. The value of MRI in Guyon's canal syndrome is in the detection of space-occupying lesions within the canal and/or signal intensity and size changes of the nerve itself. Secondary signs of neuropathy include denervation atrophy/edema of the hypothenar muscles, third/fourth lumbricals, and interossei muscles. Isolated deep-bundle neuropathy may also be diagnosed on high-resolution 3T MRN, which is a difficult diagnosis on electrodiagnostic testing. In the authors' experience, neuropathy in Guyon's canal without perineural fibrosis or mass lesion is very uncommon.
The radial nerve arises from the posterior cord of the brachial plexus (C6-T1). It courses posterior to the axillary and brachial arteries and anterior to the latissimus dorsi and teres major tendons, and it enters the spiral groove distally. It pierces the lateral intermuscular septum and arrives in the front of the lateral epicondyle. It enters the radial tunnel at the level of the capitellohumeral joint and divides into superficial and deep branches. The deep motor branch is also called the posterior interosseous nerve (PIN). The radial nerve innervates the triceps and anconeus above the spiral groove and the brachioradialis below the spiral groove. The PIN supplies all the other muscles of the dorsolateral aspect of the forearm. Anatomic sites where the radial nerve is prone to injury and entrapment are associated with several syndromes.
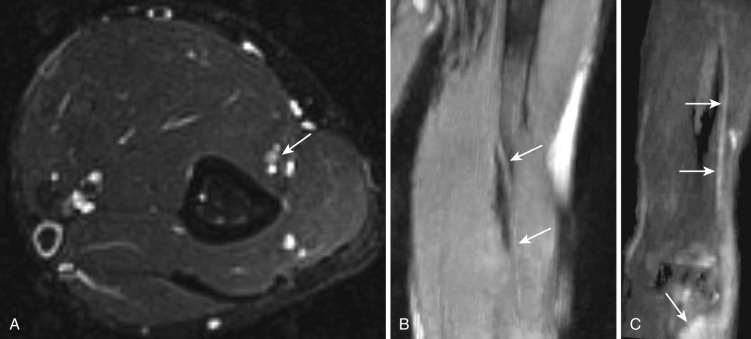
Spiral groove syndrome represents the most common type of radial nerve injury and mostly occurs after trauma/fracture. Less commonly it is due to a thickened lateral intermuscular septum or a prominent myotendinous edge of the lateral head of the triceps muscle, leading to “Saturday night palsy” due to improper positioning of the upper extremity during sleep, or it is secondary to other common pathologies affecting nerves in different locations as previously described. Symptoms include weak elbow flexion and supination, wrist extension, and finger extension. EMG is useful for diagnosis; however, MRN might be needed to demonstrate the underlying cause, such as thickened fascia or PNST. MRN shows abnormally increased T2 signal intensity at or above the spiral groove. Certain conditions, such as hereditary neuropathy with liability to pressure palsies, may be associated with multiple nerve entrapments ( Fig. 65-4 ), including radial nerve entrapment in the spiral groove.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here