Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Gliomas and glioneuronal tumors constitute the largest and most heterogeneous group of primary central nervous system (CNS) tumors. Normal glia include astrocytes, oligodendrocytes, and ependyma; gliomas are analogously designated as astrocytomas, oligodendrogliomas, and ependymomas to reflect the nonneoplastic cell types that they most closely resemble. Each has a distinct localization, histology, and natural history. Astrocytomas may generally be separated into those that are circumscribed and prognostically favorable tumors, such as pilocytic astrocytoma and subependymal giant cell astrocytoma (SEGA), and those that are infiltrating and challenging to completely resect. Astrocytoma grades 2 through 4 form a malignancy continuum for the diffusely infiltrating astrocytomas. Molecular classification subdivides gliomas into isocitrate dehydrogenase (IDH) mutant and IDH wildtype and histone (H3) altered and H3 wildtype, with glioblastoma reserved for infiltrating astrocytomas that are grade 4 and IDH wildtype/H3 wildtype in adults. The term diffuse glioma is also commonly used to encompass some pediatric astrocytomas with overlapping clinical, radiographic, and histologic features; however, recent molecular studies have prompted the separation of pediatric diffuse gliomas not only from adult gliomas but from morphologically similar appearing gliomas with different prognoses. Glioneuronal tumors also contain neuronal elements—a growing list of rare entities that are being recognized.
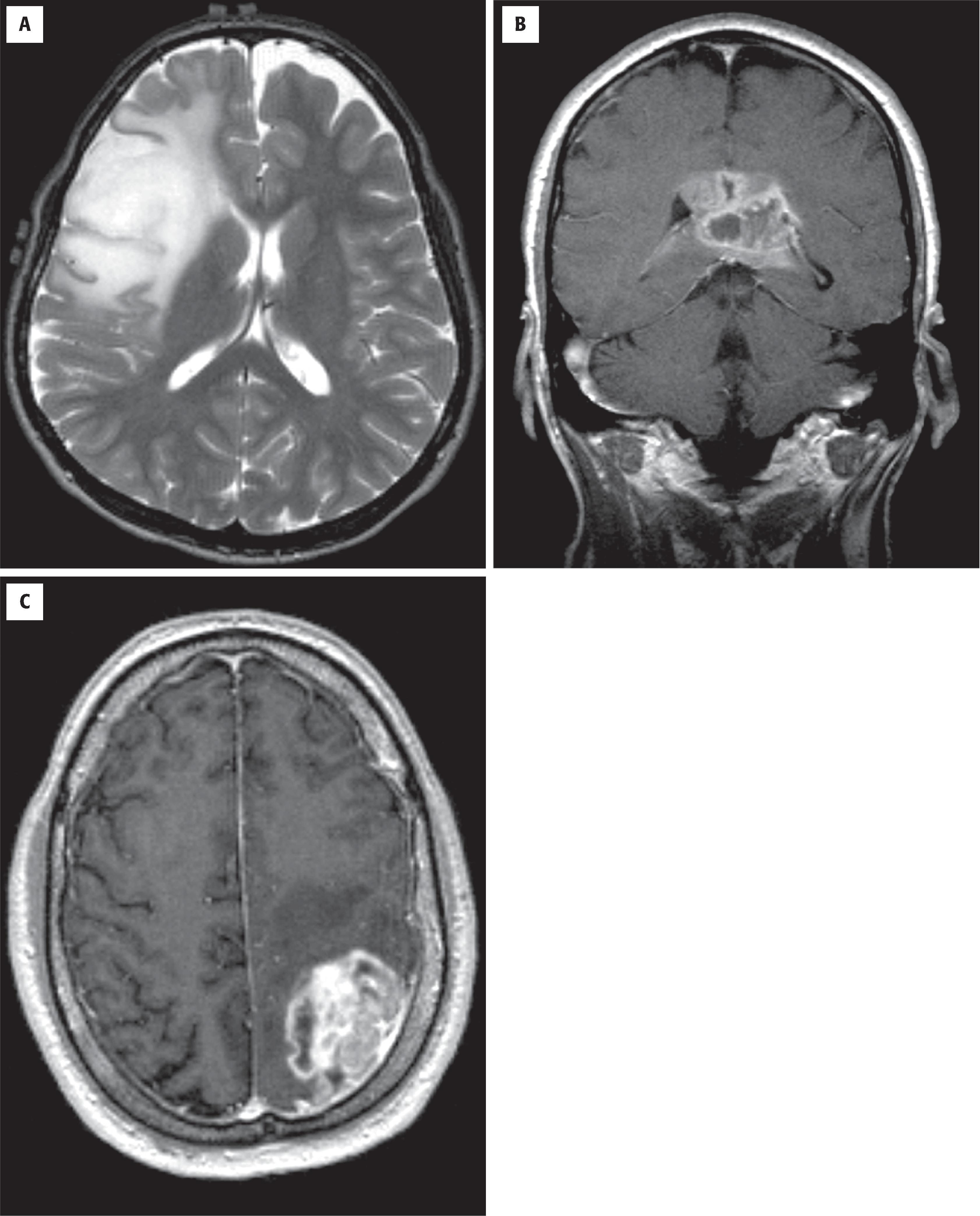
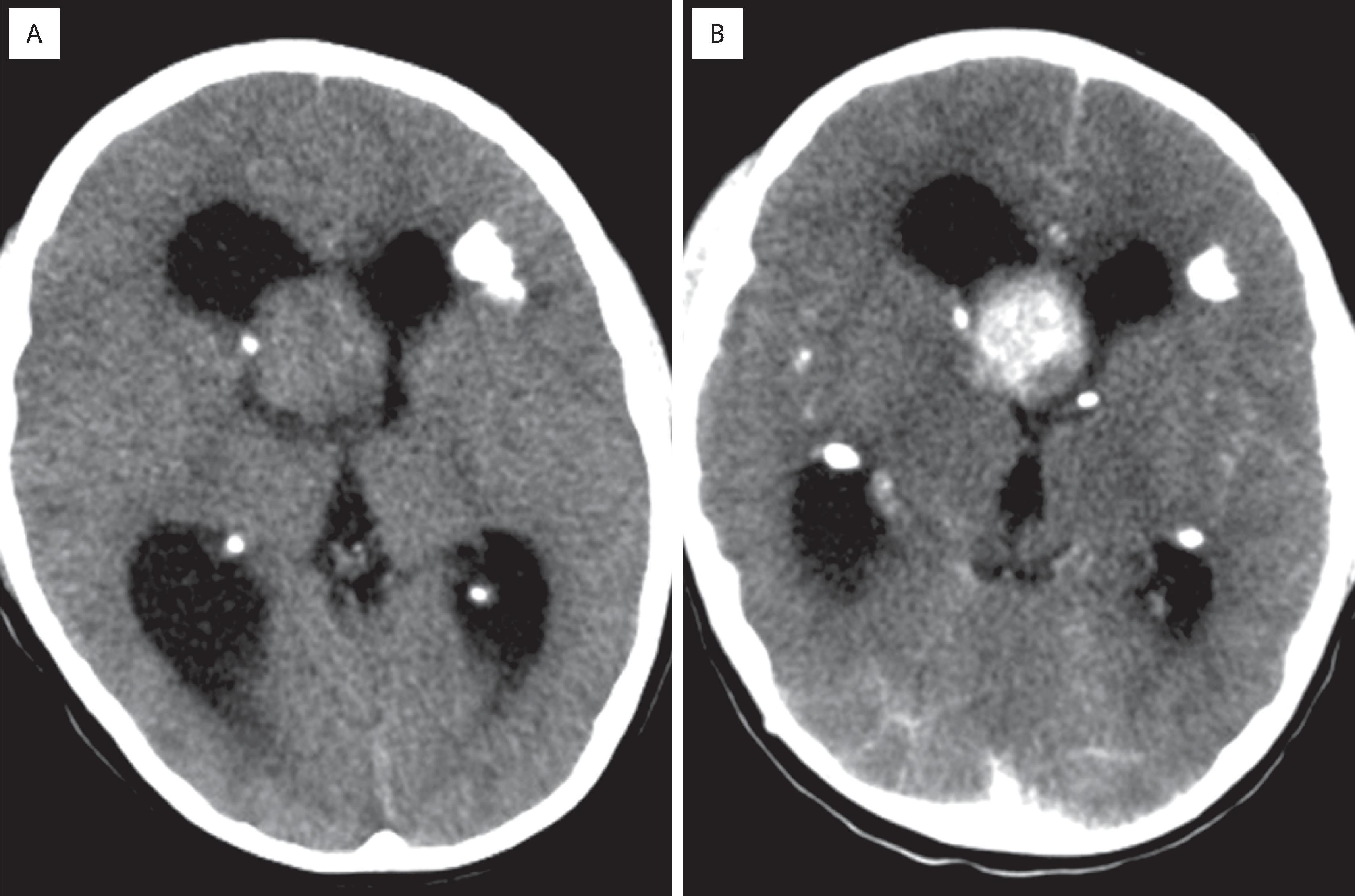
Diffuse astrocytomas (grades 2–4) account for roughly 40 % of primary intracranial tumors. Prior to molecular subtyping, the median age was 30 to 40 years for astrocytoma (grade 2) and 50 to 60 years for glioblastoma (grade 4). Glioblastomas are the most frequent. The clinical manifestations vary according to the site of involvement, but most common are new-onset seizures, motor deficits, and signs/symptoms of increased intracranial pressure (e.g., headaches, nausea/vomiting, papilledema). High-grade (3 and 4) astrocytomas tend to have short histories with rapid progression; low-grade (2) examples are more indolent, often with an insidious onset and a long, protracted clinical course. In adults, IDH-mutant low-grade astrocytomas are associated with longer overall survival than IDH-wildtype astrocytomas that appear histologically low grade and bland. Radiographically, low-grade astrocytomas are most commonly nonenhancing, ill-defined cerebral hemispheric masses best appreciated on T2-weighted or fluid-attenuated inversion recovery (FLAIR) magnetic resonance imaging (MRI) sequences ( Fig. 9.1A ). Because of their infiltrative nature, there are often foci of microscopic disease beyond the suspected tumor margins. Secondary signs of mass effect include midline shift, ventricular compression, and sulcal effacement. Glioblastomas typically have central low-density regions of necrosis surrounded by an irregular rim of contrast enhancement (see Fig. 9.1B ). Those that cross the corpus callosum are often referred to as butterfly lesions because of involvement of the white matter in the centrum semiovale bilaterally. Other diagnostic considerations with similar radiographic appearances include CNS lymphoma, metastasis, and demyelinating processes. In treated gliomas, foci of radiation necrosis are radiographically and grossly very similar to glioblastoma and may produce considerable neurologic worsening as a result of a mass effect ( Fig. 9.2B ; see Fig. 9.1C ). Therefore the differential diagnosis of tumor recurrence/progression versus radiation necrosis is a common conundrum, and biopsy is sometimes necessary.
Diffusely infiltrative glioma displaying astrocytic features
Account for roughly 70% of all gliomas, most of which are glioblastoma, and 20% of all primary brain tumors
Most arise in the cerebral hemispheres with a subcortical epicenter, but they may be seen anywhere along the neural axis, including the brainstem, cerebellum, and spinal cord
Slight male preponderance (M/F ratio of 3:2)
Can occur at any age, although the incidence increases with advancing age
Older adults (age >55 years) are more likely to have glioblastoma
Manifestations dependent on location of tumor
Focal neural deficits (e.g., motor, cranial nerve, sensory, visual), increased intracranial pressure, and seizures are most common
Generalized neurologic signs and symptoms of increased intracranial pressure include headaches, nausea/vomiting, and papilledema
Rapid onset and progression suggest high-grade (3 or 4); whereas, a protracted history is more consistent with low grade (2)
Low-grade diffuse glioma is typically a nonenhancing expansile mass with increased signal on T2-weighted (see Fig. 9.1A ) and FLAIR MRI sequences
Diffuse midline glioma, H3K27-altered (World Health Organization [WHO] grade 4) tends to involve thalamus, brainstem, or spinal cord and show infiltrative growth, sometimes with dissemination to leptomeninges
Glioblastoma (i.e., glioblastoma multiforme; WHO grade 4) shows irregular rim enhancement and may cross the corpus callosum (aka butterfly lesion) (see Fig. 9.1B )
Multifocal gliomas usually show separate foci of enhancement, with or without discernible nonenhancing signal abnormalities in between
Foci of radiation necrosis may appear identical to tumor recurrence or progression (see Fig. 9.1C )
Patient age: powerful predictor with survival inversely proportional to patient age
Integrated histologic and molecular features: IDH1 and IDH2 mutations are associated with better outcomes in adults
Preoperative performance status: measure of neurologic function with greater impairment associated with a poorer prognosis
Extent of resection: gross total resection/debulking may be beneficial
Radiotherapy often used to treat subtotally resected and high-grade glioma
Chemotherapy often used in high-grade (3 or 4) or oligodendroglial tumors, the latter being more chemosensitive than their astrocytic counterparts
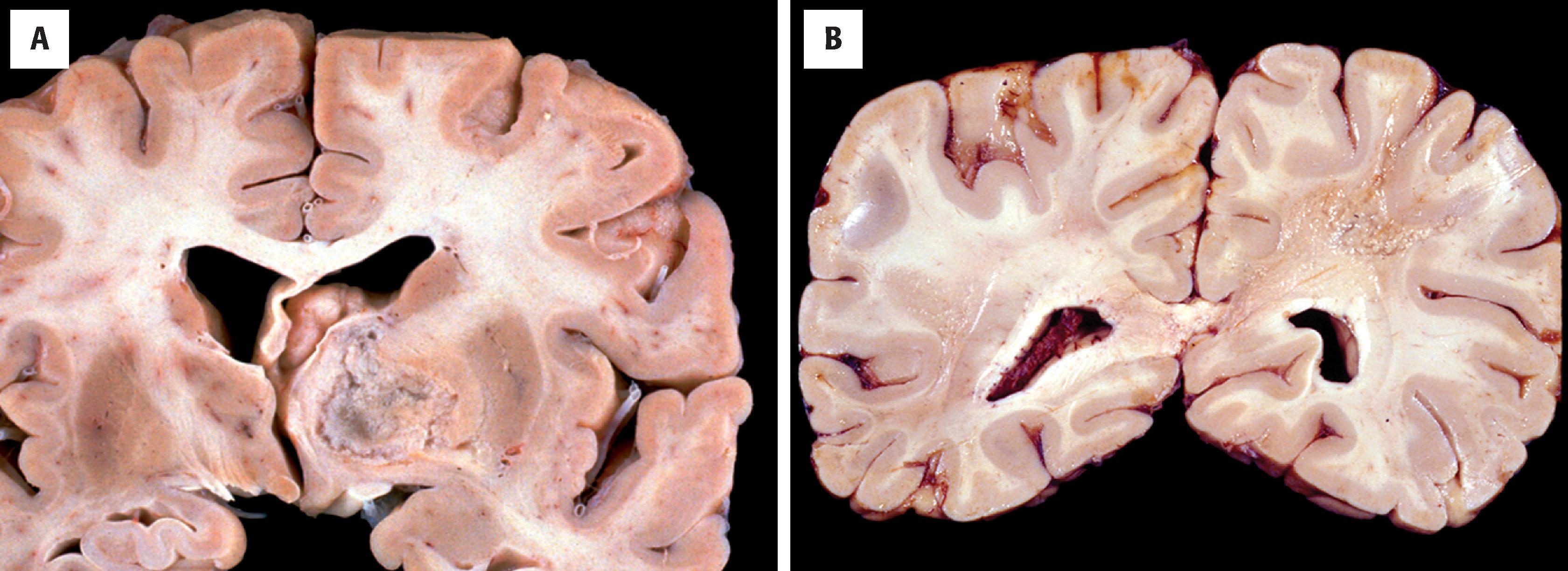
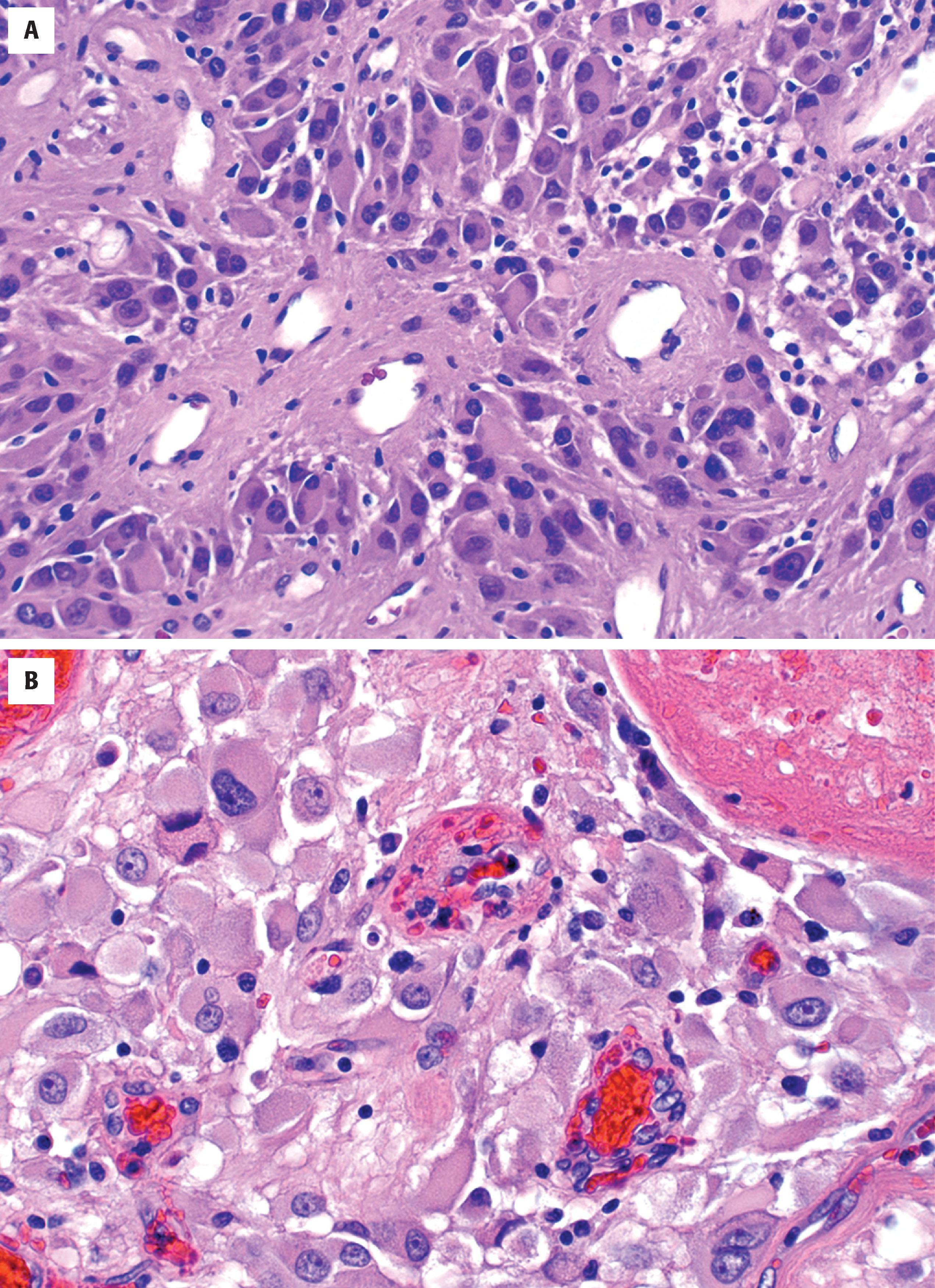
Grossly, low-grade and anaplastic tumors are ill defined and subtly discolored with secondary mass effects identical to those seen radiographically. As described earlier, there are typically foci of microscopic disease beyond the grossly suspected borders that are invisible to the naked eye. Glioblastomas are classically variegated with foci of necrosis and hemorrhage ( Fig. 9.2A ).
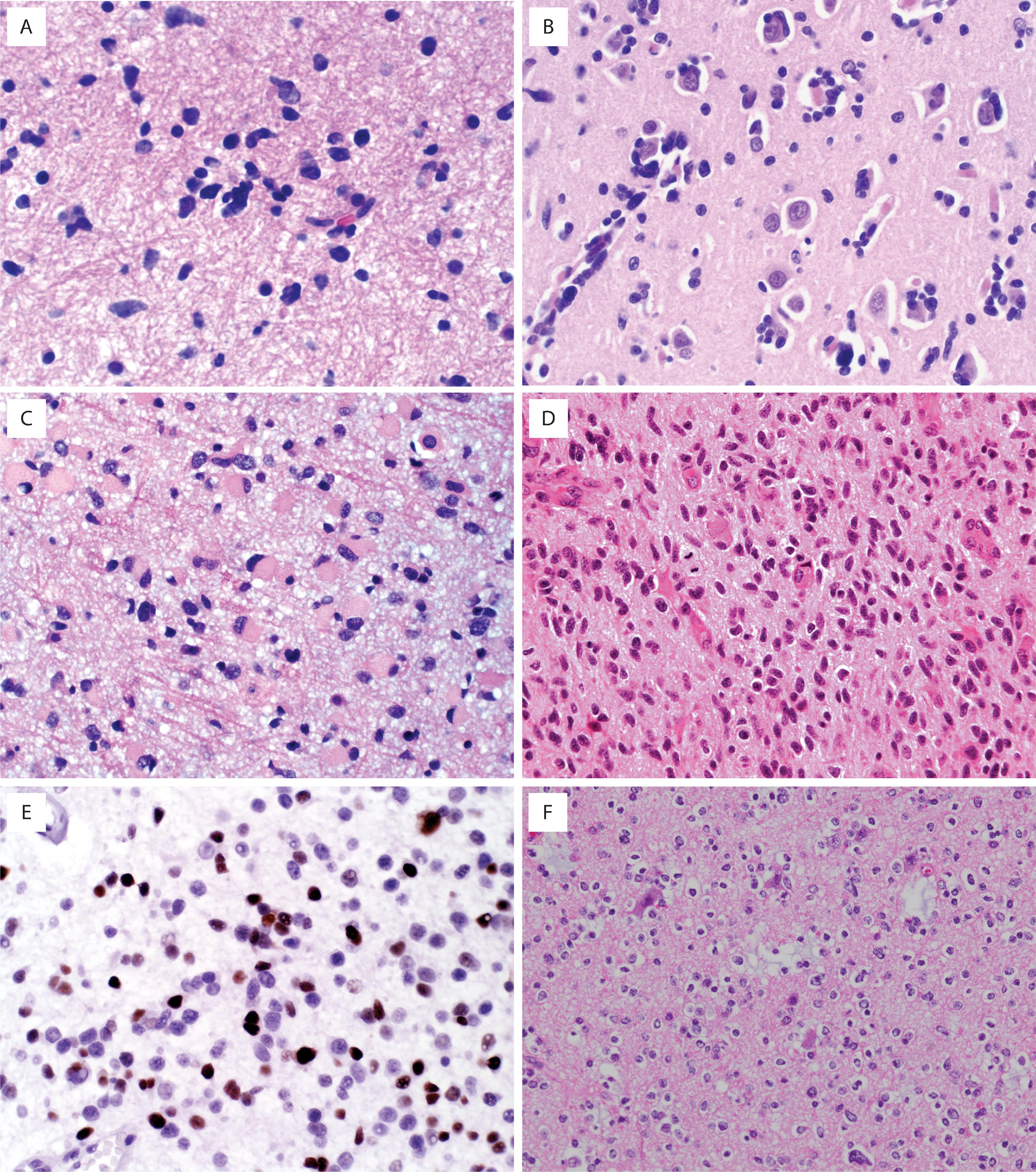
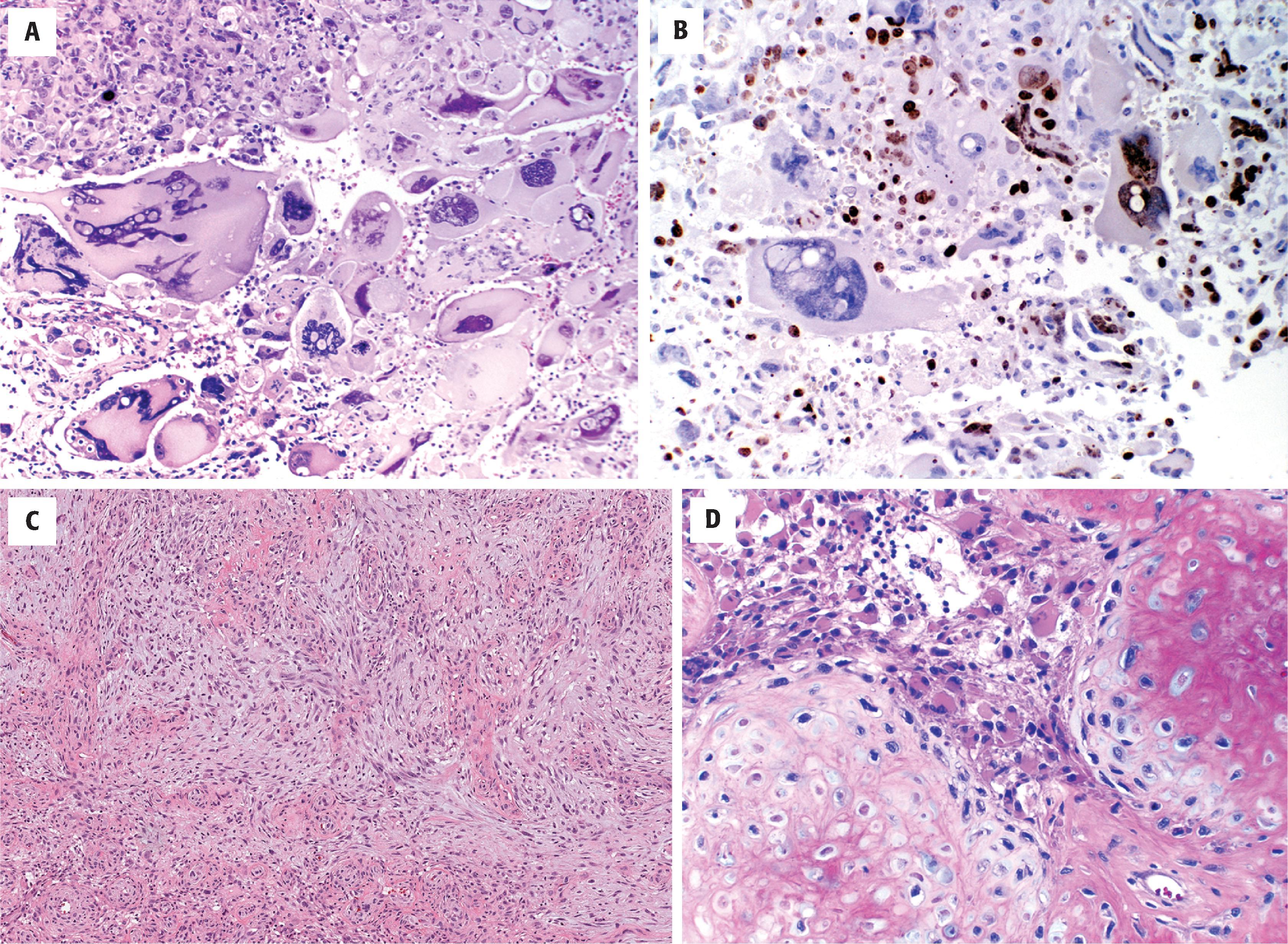
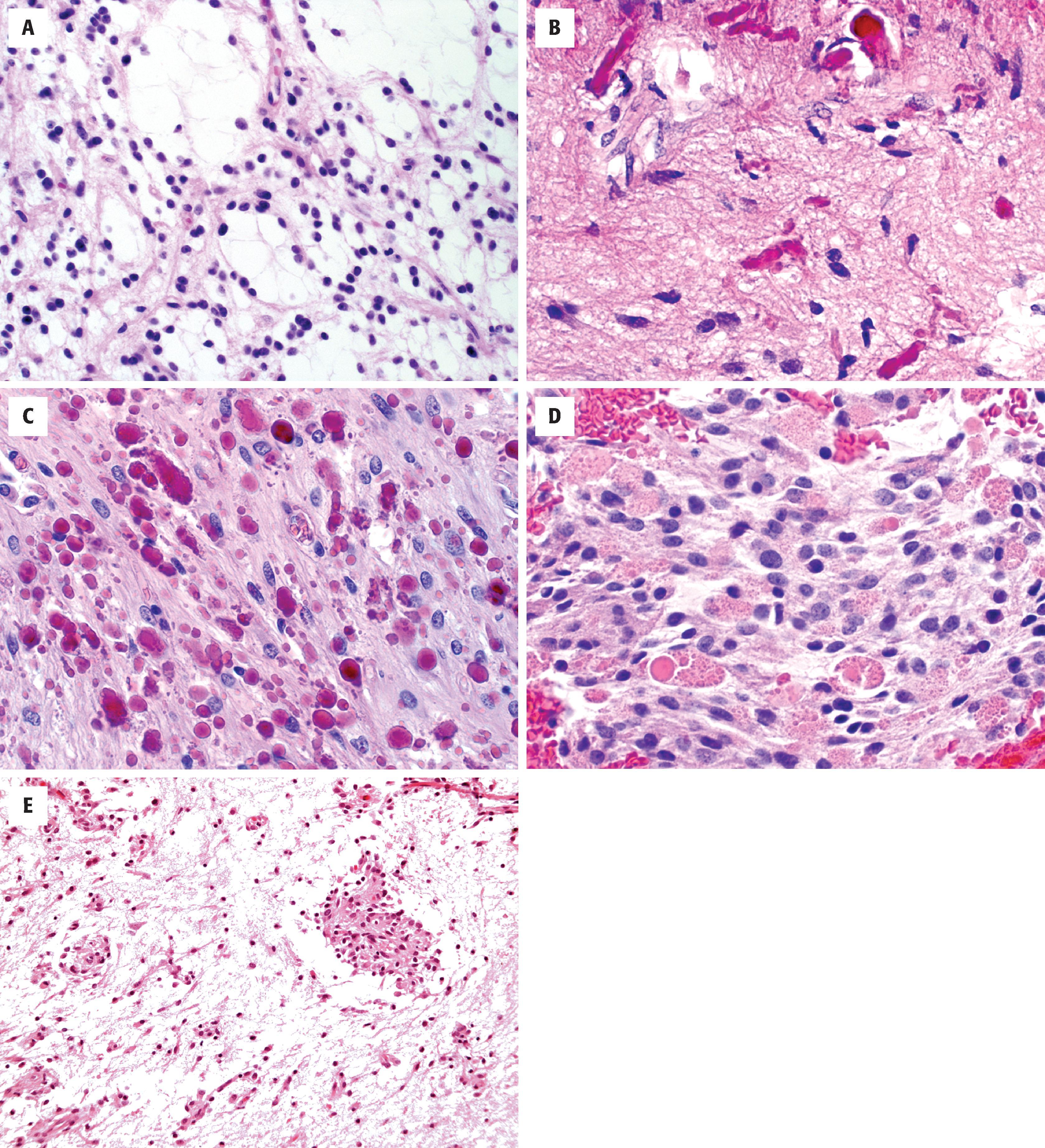
Diffuse astrocytomas may have a wide morphologic spectrum ( Table 9.1 ), although the fibrillary pattern is the prototype—composed of elongated, irregular hyperchromatic nuclei, often with no discernible cytoplasm embedded in a dense fibrillary matrix ( Fig. 9.3A ) with thickened eosinophilic cytoplasmic processes. Other morphologic patterns include gemistocytic (eccentric bellies of cytoplasm) (see Fig. 9.3C ), protoplasmic (wispy cobweb-like processes), giant cell (large mononucleated and multinucleated cells with abundant cytoplasm) ( Fig. 9.4A ), small cell (monomorphic oval nuclei with minimal cytoplasm) (see Fig. 9.4E ), epithelioid (cells with discrete cell membranes) (see Fig. 9.4G ), and granular (large cells with granular, periodic acid-Schiff [PAS]–positive cytoplasm) (see Fig. 9.4H ). Diffuse astrocytomas are characterized by invasive growth such that nonneoplastic cells are often intermixed and may even predominate in some areas. Secondary structures of Scherer include subpial condensation, perineuronal satellitosis, and perivascular aggregation (see Fig. 9.3B ). At their extreme, they may involve multiple lobes of the brain and extend into the brainstem and/or cerebellum (formerly called gliomatosis cerebri). A number of grading schemes have been applied, although the WHO scheme is the standard. Grade 2 astrocytomas have atypia only. Grade 3 astrocytomas additionally have significant mitotic activity, and grade 4 astrocytomas have microvascular proliferation or necrosis, often both. The number of mitotic figures necessary for grade 3 astrocytoma has been debated, but one may be enough in a small biopsy (e.g., stereotactic needle) specimen, whereas more are needed in resection specimens. Microvascular proliferation is defined by multilayering of endothelium/myofibroblasts/pericytes or glomeruloid vessels (i.e., multiple lumina) (see Fig. 9.3I ). The necrosis may be bland and infarct-like, but it is most commonly serpentine with associated nuclear pseudopalisading (i.e., lining up of tumor nuclei at the periphery of the acellular necrotic area) (see Fig. 9.3J ).
| Diffuse (fibrillary) astrocytoma (see Fig. 9.3 ) Prototype of diffuse astrocytomas Cells with inconspicuous cytoplasm embedded in a densely fibrillary matrix (naked nuclei) or with thin glial fibrillary acidic protein (GFAP)–positive cytoplasmic processes Enlarged, elongated hyperchromatic nuclei with irregular nuclear contours Hypocellular examples may be difficult to distinguish from reactive gliosis |
Gliosarcoma (see Fig. 9.4C and D ) Biphasic variant of glioblastoma with astrocytic and mesenchymal differentiation Sarcomatous element lacks GFAP and resembles fibrosarcoma but may show bone, cartilage, or muscle differentiation Often superficial and deceptively circumscribed Prognosis and response to therapy do not differ significantly from IDH-wildtype glioblastomas |
| Gemistocytic astrocytoma (see Fig. 9.3C ) Eccentrically placed eosinophilic, GFAP-positive cytoplasm with short, polar cytoplasmic processes; round, variably hyperchromatic nuclei; and asymmetric distribution of tumor cells (unlike reactive gemistocytes) Associated perivascular inflammation Proliferating astrocytes may have small, difficult to find mitotic figures High rate of malignant progression to glioblastomas Granular cell astrocytoma (see Fig. 9.4H ) Contains large cells with granular, periodic acid-Schiff–positive, CD68-positive cytoplasm Granular cell change related to increased cytoplasmic lysosomes May represent a degenerative phenomenon |
Giant cell glioblastoma (see Fig. 9.4A–C ) Mononucleate and multinucleate giant cells with abundant cytoplasm and bizarre nuclei Often deceptively circumscribed grossly Previously known as monstrocellular glioblastomas Usually IDH wildtype, TP53 mutant Small cell glioblastoma (see Fig. 9.4E )
|
| Epithelioid glioblastoma (see Fig. 9.4G ) Marked by cells with distinct cytoplasmic borders, paucity of fibrillary processes, dyshesion, maybe rhabdoid or gemistocytic Resembles metastatic carcinoma or melanoma GFAP positive SMARB1(INI-1) and SMARCA4 (BRG1) intact Half have BRAF V600E mutations |
By immunohistochemistry, most astrocytomas are positive for glial fibrillary acidic protein (GFAP), although the degree of cytoplasmic positivity is highly variable. It may be difficult to distinguish reactive astrocytes in the background from neoplastic cells. S-100 protein is a less specific glial marker but is positive in most gliomas. Neurofilament may be useful for highlighting entrapped axons, thus calling attention to the infiltrative growth pattern. Diffuse astrocytomas may show p53 overexpression. A subset show evidence of IDH1 (R132H) immunoreactivity, a feature also shared by many oligodendrogliomas but not seen with nonneoplastic lesions. A subset of tumors also demonstrate evidence of an ATRX mutation (loss of immunostaining). Finally, MIB-1 (Ki-67) is useful for estimating the proliferative index, which is roughly proportional to the histologic grade (see Fig. 9.3E ).
Borders are ill defined and there are often foci of tumor involvement that are grossly invisible (see the subsequent section)
Tumor epicenter most common in white matter or deep gray matter (e.g., thalamus or basal ganglia) (see Fig. 9.2A )
Blurring of corticomedullary and other gray-white junctions
Mass effect with a midline shift, blunting of sulci, compression of ventricles, or any combination of these effects
A variegated appearance with foci of necrosis and hemorrhage suggests glioblastoma (see Fig. 9.2A )
Foci of radiation necrosis may appear grossly similar to glioblastoma ( Fig. 9.2B )
Hypercellularity with intermingling of neoplastic and nonneoplastic elements (i.e., infiltrative)
Hyperchromatic, ovoid, elongated nuclei with irregular contours (see Fig. 9.3A )
Mild to moderate nuclear atypia
Either no discernible cytoplasm (naked nuclei) or eosinophilic cytoplasmic processes
Secondary structures of Scherer (e.g., subpial condensation, perineuronal satellitosis, perivascular aggregation) are less common than in oligodendrogliomas but occasionally prominent (see Fig. 9.3B )
See Table 9.1 for histologic variants of diffuse astrocytoma (see Figs. 9.3C and 9.4 )
Mitotic figures/increased proliferation present in grades 3 and 4 (see Fig. 9.3D and E )
Microvascular proliferation or necrosis (or both) present in grade 4 (see Fig. 9.3I and J )
Electron-dense cells with prominent cytoplasmic intermediate filaments corresponding to GFAP
Immunohistochemical Features
GFAP: usually positive but sometimes difficult to distinguish reactive background from tumor processes
S-100 protein: often positive; less specific but more sensitive than GFAP for glial lineage
SOX10: variable
Olig2: variable in most types; loss in diffuse hemispheric glioma with H3 G34-mut (also shows ATRX loss and p53 overexpression)
Cytokeratins: negative but may get false-positive result with AE1/AE3 cocktail
Epithelial membrane antigen (EMA): usually negative
HMB-45, MelanA, leukocyte common antigen (LCA/CD45): negative
Neurofilament highlights entrapped axons, suggesting infiltrative growth pattern
p53 protein overexpression (corresponding to strong nuclear positivity in majority of tumor cells)
IDH1 R132H immunopositivity and ATRX loss in secondary/progressive diffuse astrocytoma (see Fig. 9.3G and H )
MIB-1 (Ki-67) labeling index roughly proportional to grade (see Fig. 9.3E )
Secondary astrocytoma, adult: IDH1 R132H or other IDH1/IDH2 mutation, ATRX mutation, TP53 mutation, CDKN2A/B homozygous deletion associated with grade 4
De novo/primary glioblastoma, adult: IDH wildtype, EGFR amplification, chromosome 7 gain and chromosome 10 loss, TERT promoter mutation, PTEN mutation/deletion
Low grade, pediatric: IDH wildtype, MYB/MYBL1 altered, BRAF V600E or BRAF fusions, FGFR1/2 altered
High grade, pediatric: IDH wildtype, histone H3 variants H3F3A or HIST1H3B K27M (midline), H3F3A G34R/V (cerebral hemispheres), ATRX mutation, TP53 mutation, PIK3CA mutation, PDGFRA amplification
High grade, infantile: receptor tyrosine kinase rearrangements in ALK , ROS1 , NTRK1/2/3 , and MET (more amenable to therapy with better survival outcomes than other pediatric high-grade gliomas)
Low-grade morphology: oligodendroglioma, reactive gliosis
High-grade morphology: metastatic carcinoma or melanoma, CNS lymphoma, anaplastic oligodendroglioma, progressive multifocal leukoencephalopathy (bizarre astrocytes), radiation necrosis ( Fig. 9.6 )
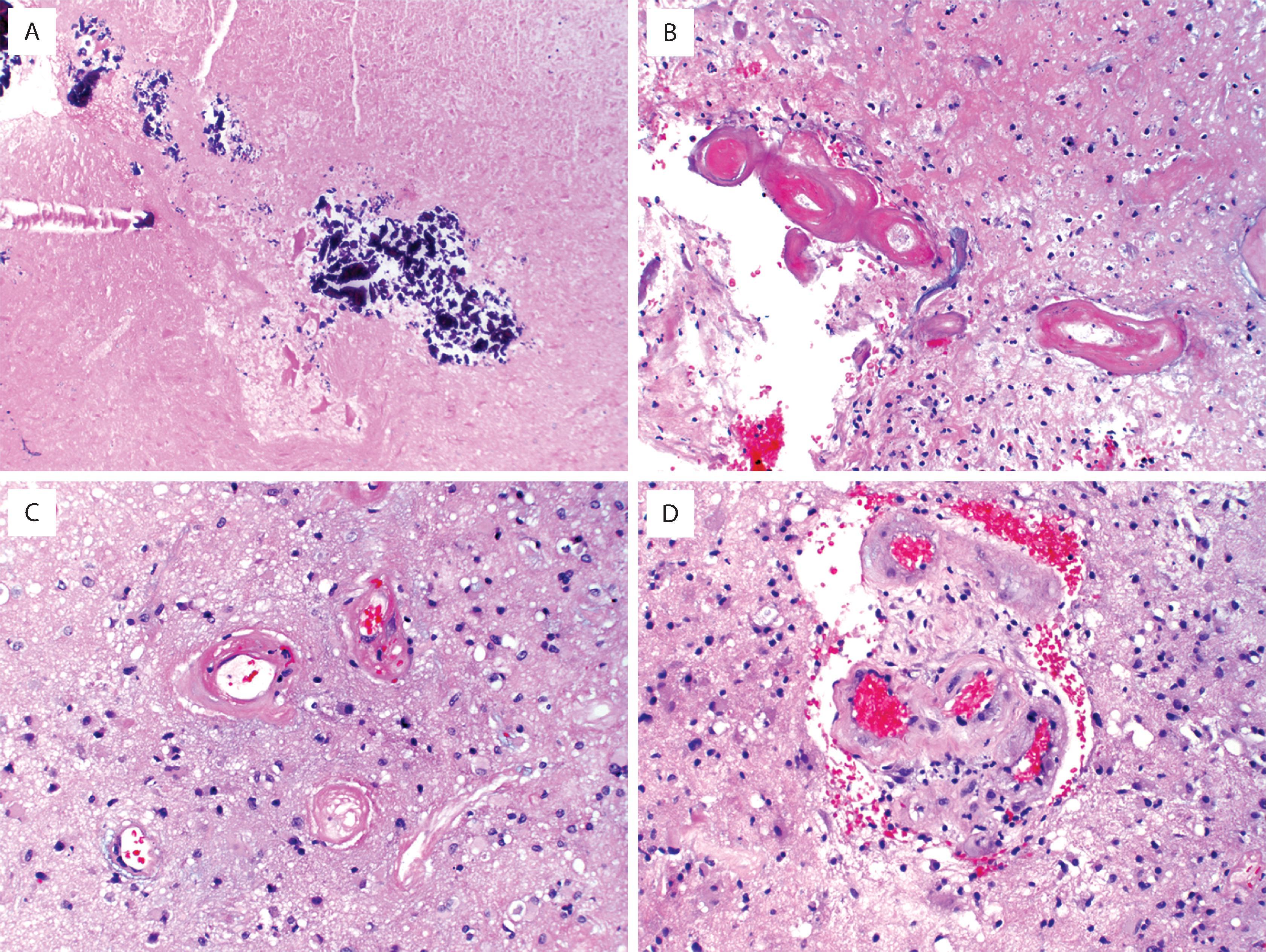
The most common and morphologically challenging diagnostic consideration in diffuse astrocytoma is oligodendroglioma. There is considerable morphologic overlap between low-grade astrocytoma and oligodendroglioma, and perinuclear halos may not be notable in a previously frozen or rapidly fixed specimen. Therefore molecular classification is necessary to look for IDH mutation and ATRX loss (see Figs. 9.3F–H and 9.5 ). In poorly differentiated cases, metastatic carcinoma (GFAP negative, cytokeratin positive) and melanoma (HMB-45, MelanA positive) enter the differential diagnosis. One caveat to keep in mind is that keratin cocktails, such as AE1/AE3, often cross-react with GFAP intermediate filaments and may lead to false-positive results with astrocytomas. In general, low-molecular-weight keratins such as CAM5.2 do not suffer this problem and are thus preferable in this differential. Neurofilament may be helpful for highlighting the infiltrative nature of astrocytomas as opposed to metastases, as the latter tend to push axon-rich parenchyma to the side rather than infiltrate. CNS lymphomas are similarly infiltrative but are characterized by angiocentricity and immunoreactivity for lymphocytic (CD45) and B-cell (CD20, CD79A, PAX5) markers. In post-treated astrocytomas, a common differential diagnosis is that of tumor recurrence or progression versus radiation necrosis. Histologically there is often a combination of each with some suggestion that the prognosis is improved when radiation necrosis predominates. Although it is not always possible to distinguish radiation necrosis from tumor necrosis, the former is typified by large geographic zones of infarct-like necrosis unassociated with nuclear pseudopalisading, with or without dystrophic calcification. Other radiation effects consist of parenchymal rarefaction and vascular changes, including telangiectasia, hyalinization, and fibrinoid necrosis of vessel walls (see Fig. 9.8 ) . In patients with low-grade astrocytomas with low cellularity and minimal atypia, distinction from reactive gliosis may be difficult. Findings supportive of neoplasia include radiographic features of diffuse glioma, nuclear enlargement/hyperchromasia, nuclear clustering, IDH mutation, increased MIB-1 proliferative index, and p53 overexpression.
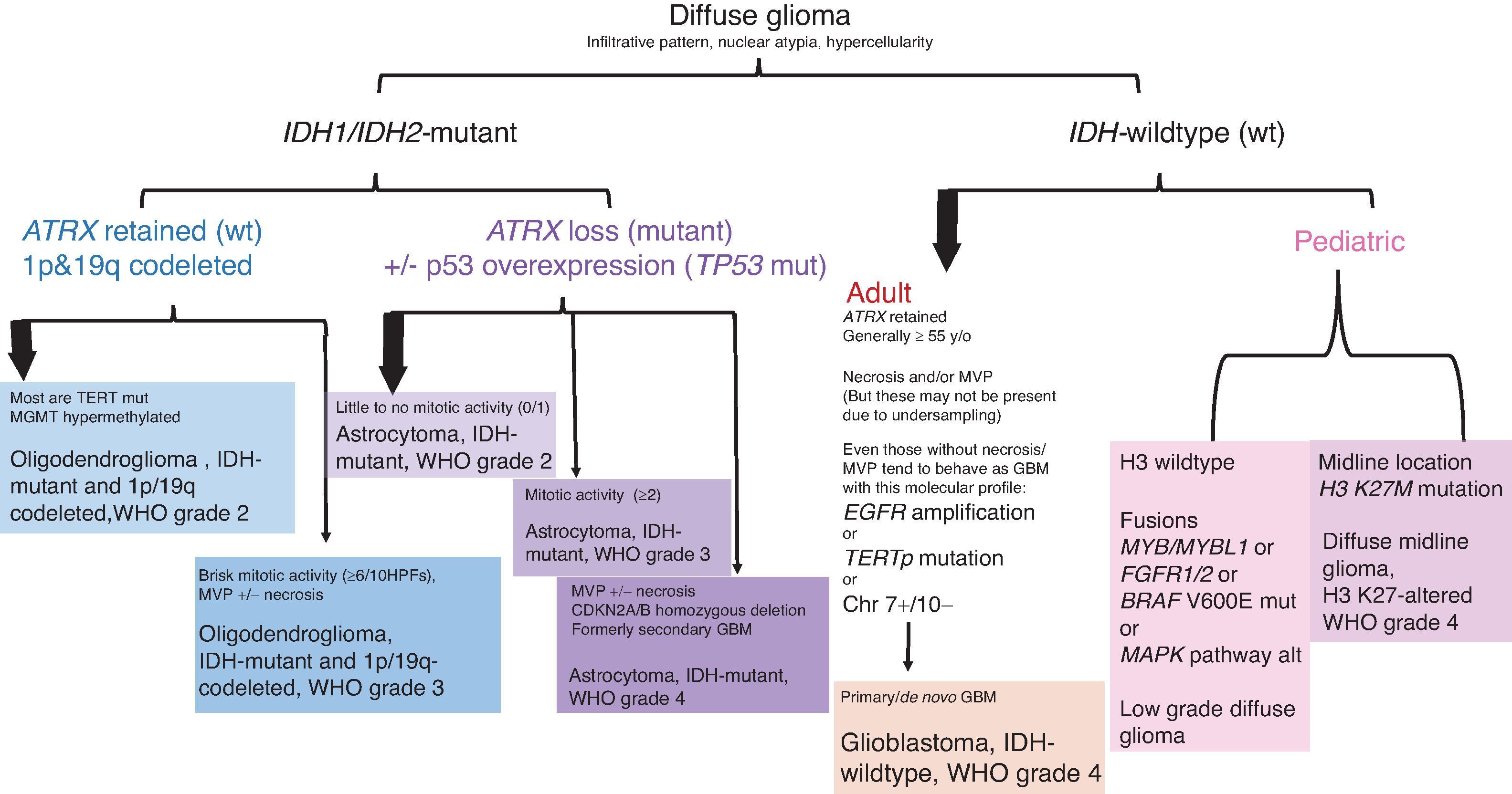
The biologic behavior of diffuse astrocytic neoplasms is highly variable, although all are considered malignant. Pre-IDH era, the two most powerful prognostic variables were patient age and histologic grade, and average survival times by grade were 5 to 10 years, 2 to 3 years, and 1 year for grades 2, 3, and 4, respectively. As more studies emerge, histologic grading criteria may not stratify risk for patients with IDH-mutant astrocytomas in the WHO grade 2 and 3 categories. IDH1/2 mutations are associated with better outcomes than IDH wildtype. Other molecular features can be integrated with morphology for further prognostic classification (see Fig. 9.5 ). IDH-wildtype (de novo) glioblastomas have the worst prognosis with median overall survival (OS) of 9.9 months following surgery and radiation and 15 months following surgery, radiation, and chemotherapy. Astrocytoma, IDH mutant, grade 4 tends to fair better with 24-month OS following surgery and radiation and 31-month OS following surgery, radiation, and chemotherapy. H3.3 G34-mutant high-grade gliomas have better survival than IDH-wildtype glioblastoma and H3 K27M-altered diffuse midline glioma but worse than that of IDH-mutant astrocytoma grade 4. Methylation of the O6-methylguanine DNA methyltransferase (MGMT) promoter is a prognostic factor associated with an improved response to chemotherapy with temozolomide and longer overall survival in glioblastoma. Less powerful prognostic variables include Karnofsky performance status (degree of neurologic impairment) and extent of surgical resection.
Overall, pilocytic astrocytomas account for only about 2 % of primary CNS tumors, but in children they represent the most common glioma. They account for roughly 10 % of cerebral and 85 % of cerebellar astrocytomas overall. Besides the cerebellum, they are particularly common in the optic nerves/chiasm, hypothalamus, dorsal aspect of the brainstem, and spinal cord. Vague clinical terms such as cerebellar astrocytoma, optic pathway glioma, tectal glioma, and dorsal exophytic brainstem or medullary glioma generally refer to pilocytic astrocytomas, although they should be clarified whenever possible because diffuse astrocytomas may also involve these sites. Although generally considered a pediatric tumor, they may also be seen in adults of virtually any age. Some of these tumors have probably been present in the patient in an asymptomatic form for years to decades. Most pilocytic astrocytomas are sporadic, but patients with neurofibromatosis type 1 (NF1) are known to be predisposed to this tumor type, particularly in the optic pathway. On neuroimaging studies the majority of these tumors appear well circumscribed, and they may be cystic with an enhancing mural nodule ( Fig. 9.7 ). In the optic pathway they usually appear solid and expand the optic nerves, the chiasm, or both.
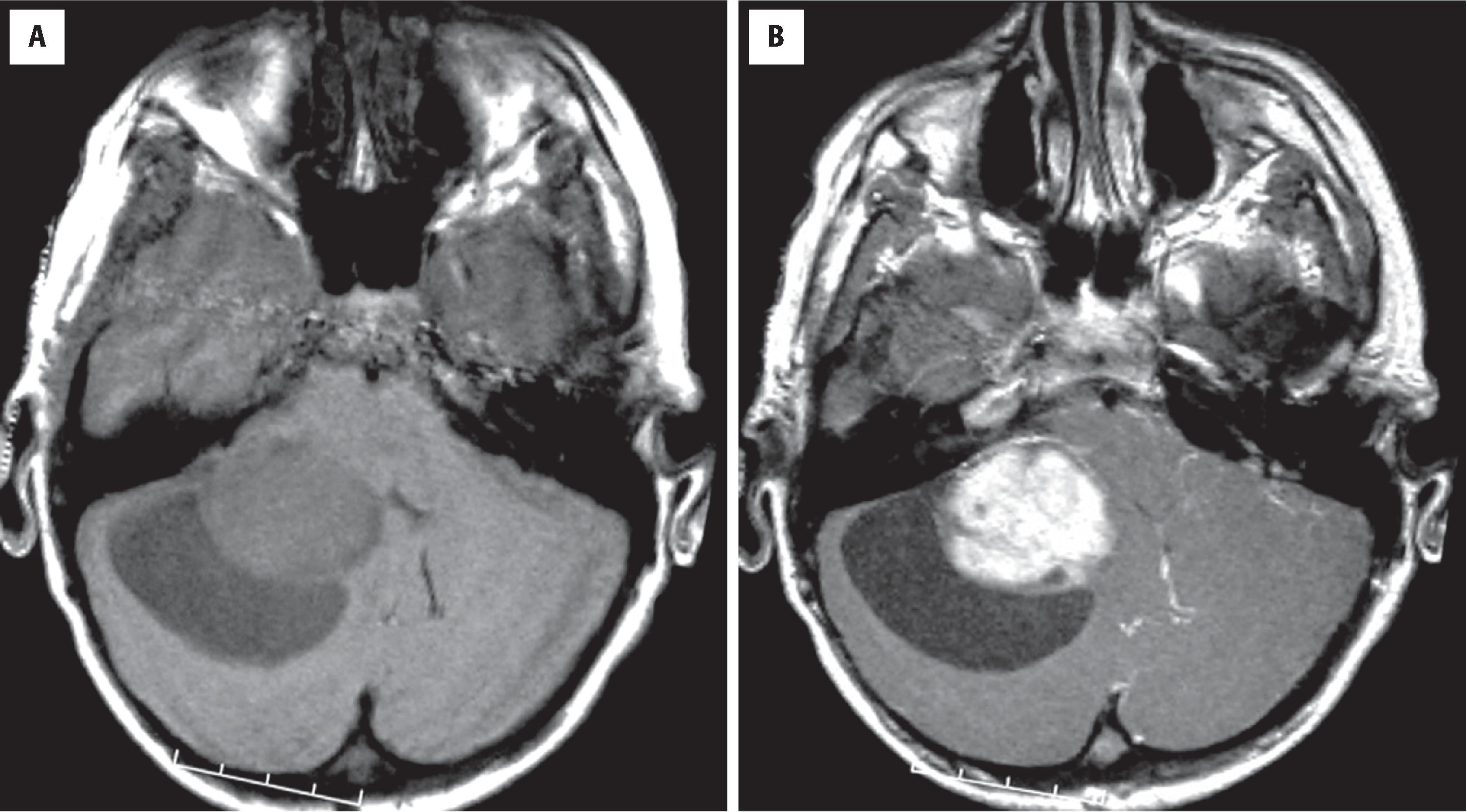
A rare variant of pilocytic astrocytoma—pilomyxoid astrocytoma—has perivascular orientation of tumor cells in a markedly myxoid background, presents in children, most often under 3 years of age; the hypothalamus/chiasmic region is the most common site of origin. These tumors are associated with higher rates of recurrence.
Benign astrocytic neoplasm with piloid (hairlike) processes and a relatively circumscribed growth pattern
Accounts for approximately 2% of primary intracranial neoplasms
Most arise in the cerebellum, optic pathway (optic gliomas), hypothalamus/third ventricle, or dorsal brainstem (tectal glioma, dorsal medullary glioma) but may be seen in the spinal cord and cerebral hemispheres as well
Can occur at any age, although encountered predominantly in children
Median age of 13 years at diagnosis
Manifestations dependent on location of tumor
Typically slow or insidious onset of symptoms
Increased incidence in NF1, particularly in the optic pathway and brainstem
Cyst with an enhancing mural nodule is most common
Well-demarcated (i.e., noninfiltrative) appearance
Diffuse or fusiform enlargement of the optic nerve(s), optic chiasm, or both in the optic gliomas
Excellent prognosis, with a roughly 80% 20-year survival rate
Most are surgically curable
Radiation therapy may be used for subtotally resected or recurrent cases
Pilocytic astrocytomas typically appear well demarcated grossly, although there is nearly always some microscopic evidence of infiltration, usually at the edges. Cystic degeneration is common.
Microscopically, the classic pilocytic astrocytoma has a biphasic appearance with alternating dense and loose/microcystic components ( Fig. 9.8A ). In some cases, only one of the two patterns is encountered. The dense regions often resemble fibrillary astrocytoma, except that the cytoplasmic processes are particularly long and hairlike (i.e., piloid, as indicated by the tumor’s name). The latter is best appreciated on cytologic specimens such as intraoperative smears. They also differ from fibrillary astrocytoma by a typically more solid growth pattern (e.g., lack of neurofilament-positive entrapped axons) and, in most cases, the presence of corkscrew-shaped brightly eosinophilic Rosenthal fibers (see Fig. 9.8B and C ). The loose component sometimes looks remarkably similar to oligodendroglioma, although the long, thin cellular processes are often highlighted with a GFAP immunostain. In addition, this component frequently harbors mulberry-shaped eosinophilic granular bodies (EGBs), which may be numerous or rare (see Fig. 9.8D ). In the latter scenario, staining with PAS and diastase may help draw attention to these structures. The nuclei of pilocytic astrocytomas are typically oval with bland chromatin, but significant atypia (perhaps degenerative in nature) may occasionally be seen and is generally unassociated with increased mitotic/proliferative activity. Multinucleated forms may also be visualized. Glomeruloid vessels with multiple lumina are also typical and should not be mistaken for the multilayered endothelial hyperplasia of diffuse gliomas. Nevertheless, the latter may be encountered in pilocytic astrocytomas as well and does not have the ominous significance that it does in diffuse gliomas. Bland infarctlike necrosis is encountered in roughly 5 % of pilocytic astrocytomas and similarly has no clinical significance. Likewise, extension into the subarachnoid space is quite common and does not alter the prognosis. In contrast, pseudopalisading necrosis and foci of hypercellularity with increased proliferative activity should prompt consideration of an alternative diagnosis or malignant transformation, an exceptionally rare complication in pilocytic astrocytomas with most examples being encountered after radiation therapy.
Cystic to spongy because of microcysts or hypervascularity, variably gelatinous
Often biphasic with dense and loose appearance, relatively circumscribed
Piloid astrocytic cells with long, thin, hair-like processes
Rosenthal fibers and PAS-positive EGBs are characteristic
Round to ovoid (oligodendroglioma-like) nuclei with bland chromatin
Glomeruloid and hyalinized vessels common
Scant mitotic activity
May show degenerative atypia with nuclear pseudoinclusions, multinucleation (pennies-on-a-plate)
Infarct-like necrosis seen in 5% to 10% of cases
The rare pilomyxoid astrocytoma is dominated by a myxoid stroma, monomorphous bipolar cells, and angiocentric arrangement of cells (see Fig. 9.8E ); in general, they do not have Rosenthal fibers or EGBs
Electron-dense cells with prominent cytoplasmic intermediate filaments corresponding to GFAP
GFAP: usually strongly and nearly diffusely positive
S-100 protein, Olig2, SOX10: positive
Neurofilament-positive axons generally lacking, but may be seen at the periphery
KIAA1549 -BRAF fusion is found in 90% of cerebellar and 50% of supratentorial pilocytic astrocytomas
BRAF V600E in a subset, particularly supratentorial pilocytic astrocytomas
KRAS G12R mutation in tectal glioma
About 15% of individuals with NF1 develop PA, especially optic pathway glioma, during the first decade. The NF1 gene, located on chromosome 17, encodes neurofibromin, which acts in the MAPK pathway
High-grade astrocytoma with piloid features: IDH wildtype; deletions of CDKN2A/B , MAPK pathway gene alterations ( NF1 > BRAF , FGFR1 ), ATRX alteration, MGMT promotor methylation (clinical course worse than PA, but better than IDH wildtype glioblastoma)
Diffuse astrocytoma
Oligodendroglioma
Ganglioglioma
Reactive piloid gliosis
The most common differential diagnosis is that of a diffuse astrocytoma or oligodendroglioma. However, the unique clinical, radiographic, and microscopic features help distinguish these tumor types. Given the rare finding of endothelial hyperplasia and necrosis, one may even find oneself in the unsettling differential diagnosis with glioblastoma. A predominantly solid growth pattern with a neurofilament stain and low MIB-1 labeling index help rule out this possibility. Although neither Rosenthal fibers nor EGBs are entirely specific for pilocytic astrocytoma, they generally suggest a benign or slowly evolving process such as pilocytic astrocytoma, pleomorphic xanthoastrocytoma (PXA), and ganglioglioma, all representing more favorable tumor types, often with similar radiographic features. However, PXA has greater pleomorphism, mesenchymal-like spindled elements, vacuolated cells, and reticulin-rich foci not seen in pilocytic astrocytomas, whereas gangliogliomas have a neuronal component characterized by dysmorphic ganglion cells. The rare monomorphous pilomyxoid astrocytoma has many overlapping features; however, this tumor is typically found in the hypothalamic/third ventricular region of infants, produces abundant mucin/myxoid stroma, does not harbor the dense or compact component (i.e., not biphasic), and generally lacks Rosenthal fibers and EGBs. Finally, Rosenthal fibers are also often encountered in a piloid form of reactive gliosis, most often next to craniopharyngiomas, ependymomas, hemangioblastomas, developmental cysts, and syringomyelia. Piloid gliosis is typically less cellular and does not have a microcystic component. Additional sampling and attention to the clinical/radiographic features generally allows one to avoid this pitfall.
Pilocytic astrocytomas are benign (WHO grade 1) neoplasms treated primarily with surgery, with overall 5- and 10-year survival rates of greater than 95 %. However, depending on patient age and location, they occasionally produce significant morbidity and mortality. Therefore subtotally resected cases may undergo radiotherapy and/or chemotherapy for enhanced local control. The pilomyxoid astrocytoma has variable behavior, but most are more aggressive than a pilocytic astrocytoma and more prone to local recurrence and cerebrospinal spread.
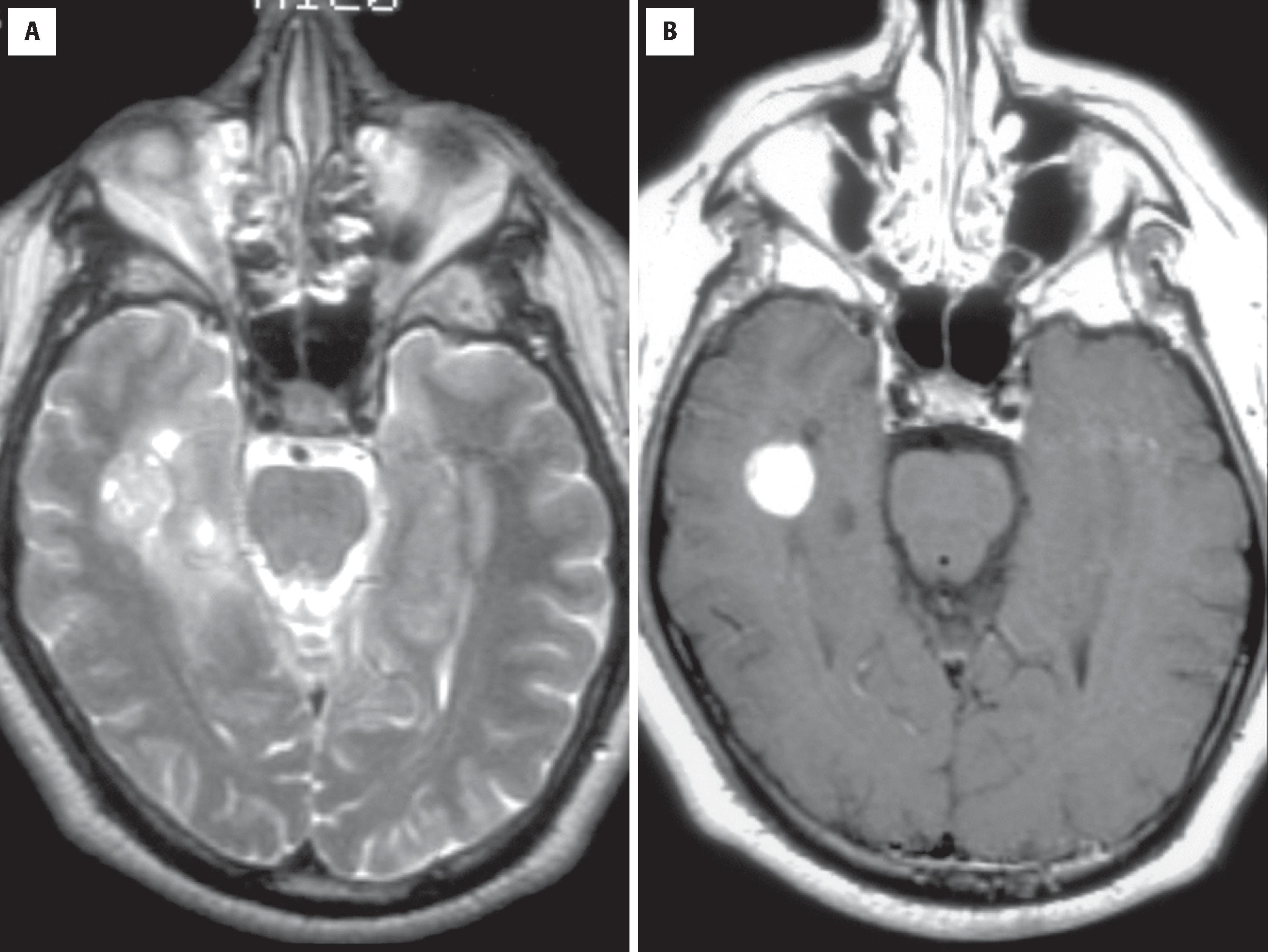
SEGA is a benign intraventricular tumor encountered nearly exclusively in the setting of tuberous sclerosis. It is typically located in the lateral or third ventricle (or both ventricles) near the foramen of Monro and comes to clinical attention because of obstructive hydrocephalus. Radiographically, it appears solid, enhancing, well demarcated, and often calcified ( Fig. 9.9 ). Because it may be the first detected manifestation of this familial disorder, other characteristic features should be sought, including cortical tubers, candle gutterings (smaller masses along the ventricular lining resembling wax drippings), and gray matter heterotopia. Extracranial manifestations include facial cutaneous angiofibromas, renal angiomyolipoma, pulmonary lymphangioleiomyomatosis, subungual fibroma, cardiac rhabdomyoma, intestinal polyps, and visceral cysts. Many think that cases of SEGA without these other manifestations represent a forme fruste of tuberous sclerosis.
Benign or hamartomatous astrocytic and partially neuronal intraventricular tumor
Accounts for less than 1% of primary intracranial neoplasms
Involves the lateral or third ventricle (or both ventricles) near the region of the foramen of Monro
Mostly children and young adults
Signs and symptoms of obstructive hydrocephalus with increased intracranial pressure
Almost exclusively seen in the setting of tuberous sclerosis, although this may be the initial manifestation
Some think that sporadic cases represent a forme fruste of tuberous sclerosis
Enhancing intraventricular mass
Well demarcated (i.e., noninfiltrative)
Often calcified
Other features of tuberous sclerosis may be present (e.g., tubers, candle gutterings, gray matter heterotopia)
Excellent prognosis
Most are surgically curable
Grossly, SEGA is a solid, well-demarcated mass, often with zones of dense calcification. Candle gutterings are smaller versions that are more widely distributed along the ventricular surface. They are otherwise identical microscopically.
Histologically, SEGA is a noninfiltrative mass composed of spindled and epithelioid cells arranged in sweeping fascicles or even perivascular pseudorosettes, such as those encountered in ependymomas ( Fig. 9.10A ) . The cells often have a mixed glioneuronal appearance with brightly eosinophilic cytoplasm resembling that of astrocytes, along with large vesicular nuclei and prominent nucleoli similar to those of ganglion cells (see Fig. 9.10B ). Mitoses and necrosis are uncommon and do not have a negative impact on prognosis. Immunostains reveal GFAP positivity in a subset of tumor cells. Neuronal marker immunoreactivity may also be seen in some cells, sometimes in the same cells that are GFAP positive. Other cells are negative for both. Because of the mixed glioneuronal features, some prefer the term subependymal giant cell tumor rather than SEGA.
Solid and well demarcated
Often calcified
Candle gutterings represent smaller, histologically similar lesions that grossly resemble wax drippings throughout the ventricular surface
Epithelioid, gemistocyte-like, or spindled cells (or all three) arranged in sweeping fascicles
Dysmorphic cells with neuronlike nuclei (i.e., vesicular chromatin, prominent nucleoli) and astrocyte-like cytoplasm (i.e., eccentrically placed and eosinophilic)
Overlying ependymal cells sometimes evident
Perivascular pseudorosettes common
Mitotic figures and necrosis uncommon
Electron-dense cells with prominent cytoplasmic intermediate filaments corresponding to GFAP
GFAP: usually positive in a subset of cells, but may be surprisingly weak
S-100 protein: positive
Neuronal markers may be positive in a subset of cells (e.g., synaptophysin, neurofilament, NeuN, chromogranin)
Strong association with tuberous sclerosis, a genetic disease caused by inactivating mutations in TSC1 gene at 9q or TSC2 gene at 16p
Gemistocytic astrocytoma
Ependymoma
SEGA resembles gemistocytic astrocytoma, but it does not grow in an infiltrative pattern, it expresses GFAP less intensely, and it is found within the ventricle rather than the parenchyma. The perivascular pseudorosettes raise the possibility of ependymoma, although in ependymoma they are typically intraparenchymal rather than intraventricular when they are supratentorial. Furthermore, the ganglion-like and gemistocyte-like cytology is not typical of ependymoma. Clinically, other intraventricular tumors in the differential diagnosis include intraventricular meningioma, central neurocytoma, and subependymoma, although these tumors’ histologic characteristics are quite different.
SEGAs are benign tumors (WHO grade 1) and probably represent hamartomatous rather than neoplastic proliferations. Rare examples recur but do not undergo malignant progression. Despite its benign nature, SEGA has significant prognostic implications for patients and their families because of this tumor’s nearly universal association with tuberous sclerosis.
PXA is a specialized variant of astrocytoma with a predilection for the superficial cortex, particularly in the temporal lobe. Patients are typically young and often have a longstanding seizure disorder. Radiographically, some appear as cysts with an enhancing mural nodule ( Fig. 9.11 ) , but they may also be solid or attached to the dura and mimic a meningioma.
Specialized variant of astrocytoma with reticulin deposition and increased pleomorphism but a generally favorable prognosis
Accounts for less than 1% of primary intracranial neoplasms
Predilection for the temporal lobe
Mostly children and young adults
Chronic seizure disorder common
Cyst with an enhancing mural nodule common but may be solid
Superficial cortex; may attach to the overlying dura
Good prognosis in most cases (WHO grade 2), but 15% to 20% undergo malignant transformation (WHO grade 3) and may die of the disease
Many are surgically curable
Treatment is mostly surgical, but adjuvant therapy is often given for subtotally resected, recurrent, or anaplastic (grade 3) cases (or for all three)
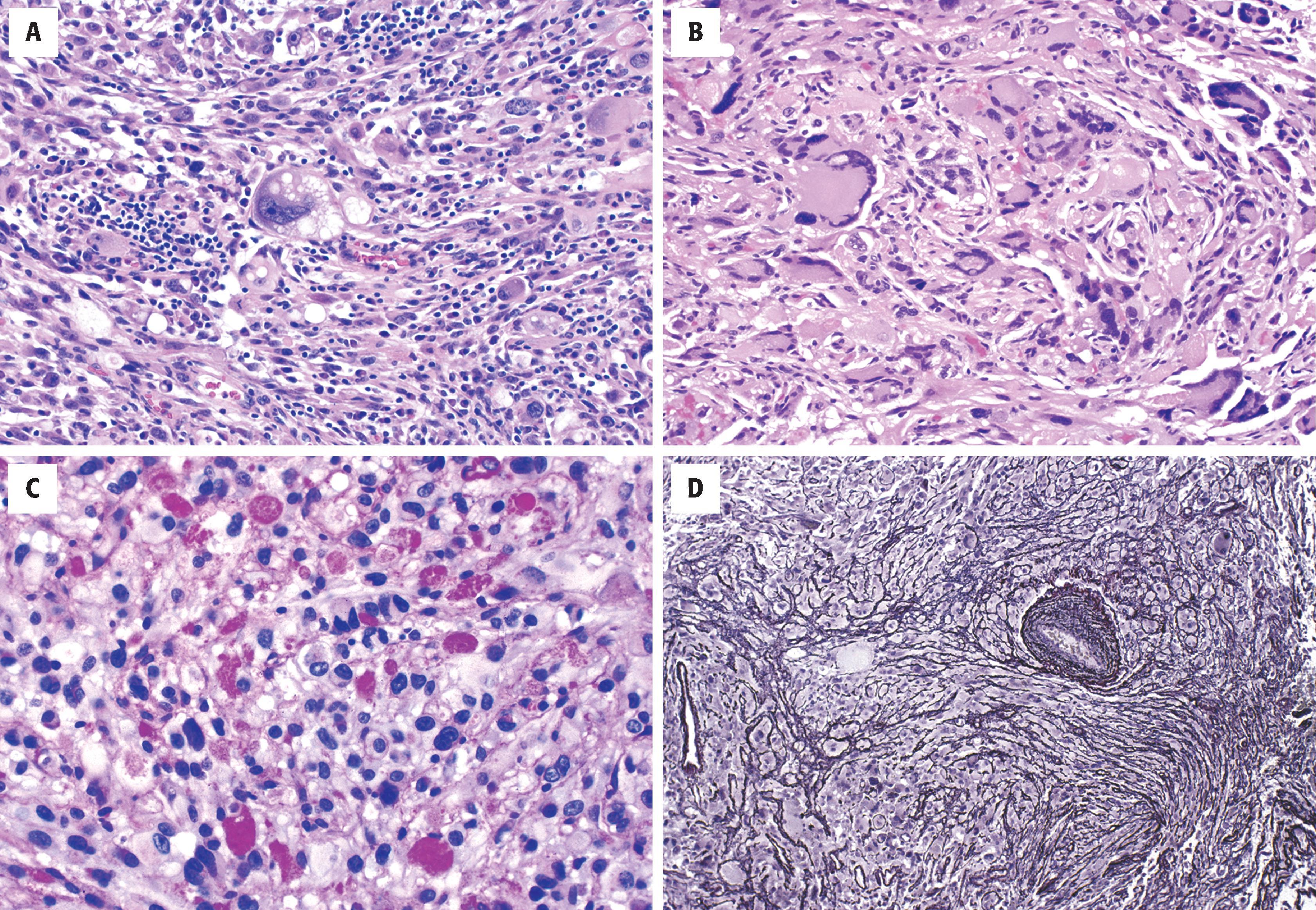
Grossly, PXAs appear well demarcated and firm. Cystic components are seen in some. Histologically they are characterized by foci of pleomorphic astrocytes and spindled mesenchymal-like cells arranged in fascicles or a storiform pattern ( Fig. 9.12 ). Multinucleated cells are common. Lipidized astrocytes (xanthoastrocytes) are seen in roughly one-fourth of cases (see Fig. 9.12A ). Despite the pleomorphism, the mitotic/proliferative index is low in most cases (see Fig. 9.12B ). EGBs are typical (see Fig. 9.12C ), and Rosenthal fibers may also be seen, particularly at the edges of the tumor. Unlike diffuse astrocytomas, there is increased intercellular reticulin deposition, either diffusely or in a patchy fashion (see Fig. 9.12D ). The latter corresponds to basal lamina ultrastructurally, which is why PXA has been speculated to arise from a specialized subpial astrocyte with basal lamina production. Occasional cases are mixed with classic ganglioglioma, intratumoral ganglion cells, or lesser forms of neuronal differentiation, such as synaptophysin or neurofilament positivity, in otherwise astrocytic-looking cells. Subsets of tumor cells are GFAP positive, but the fraction is highly variable. The definition of anaplasia is controversial, although ironically there is less pleomorphism with an overall resemblance to high-grade diffuse astrocytomas, including frequent mitoses, endothelial hyperplasia, pseudopalisading necrosis, or any combination of these findings.
Solid and cystic, well demarcated
Spindled (mesenchymal-like) and atypical gemistocyte-like (pleomorphic) or epithelioid glial cells
PAS-positive EGBs in the vast majority
Increased intercellular reticulin deposition, either focally or diffusely
Rosenthal fibers may be seen, particularly at the periphery of the tumor
Xanthomatous (clear and foamy, lipid laden) astrocytes in roughly one-fourth of cases
Mitotic figures should be infrequent (<5 per 10 high-power fields) and necrosis rare, except in cases with malignant progression (grade 3)
Electron-dense cells with prominent cytoplasmic intermediate filaments corresponding to GFAP
Individual tumor cells surrounded by basement membranes, pericellular basal lamina
GFAP and S-100 protein: positive
The MIB-1 (Ki-67) labeling index is typically low (<1%), except in anaplastic examples
BRAF V600E mutation in 50% to 78%
CDKN2A deletion
Glioblastoma, epithelioid, or gliosarcoma subtype
Ganglioglioma
Because of the prominent nuclear pleomorphism and mesenchymal-like foci, the most common differential diagnoses are glioblastoma or gliosarcoma. However, EGBs are not found in any of these entities, and PXA grade 2 shows very low mitotic activity. PXA has significant overlap with ganglioglioma, and both entities may combine to form a composite neoplasm. In general, however, ganglioglioma has less pleomorphism, a more obvious neuronal component, and no lipidized astrocytes. Similarly, pilocytic astrocytomas may have bizarre, atypical/pleomorphic nuclei, but intercellular deposition of reticulin does not occur.
PXA generally has a favorable prognosis, with many cases curable by surgery alone (5-year overall survival 90 %). Nevertheless, it is recognized that a minority undergo malignant progression, which, along with a somewhat less predictable behavior than that of pilocytic astrocytoma and ganglioglioma, accounts for the WHO grade 2 designation. Anaplastic PXA, WHO grade 3, is associated with aggressive behavior and shortened survival.
Astroblastoma is a rare, cerebral glioma of unknown histogenesis and is not universally accepted as a distinct entity, as astroblastoma-like histology can be found in other tumors. Most occur in children and young adults. Radiologically, they are well-demarcated, noncalcified, enhancing masses that may be lobulated and/or cystic.
Rare form of glioma with combined astrocytic and ependymal features (controversial entity)
Accounts for less than 1% of primary intracranial neoplasms
Most involve the cerebral hemispheres
Female greater than male
Mostly children and young adults
Signs and symptoms depend on location and are similar to those of diffuse astrocytomas
Supratentorial lobulated, cystic, enhancing parenchymal mass
Well demarcated (i.e., noninfiltrative)
Variable prognosis
Insufficient clinical experience
Grossly, astroblastomas appear well demarcated and may be cystic. Histologically, they have mixed astrocytoma-like and ependymoma-like features (see Fig. 9.13 ). Like ependymomas, they grow in a relatively circumscribed fashion and have perivascular pseudorosettes. However, the cells are more epithelioid or cuboidal with broader perivascular processes (see Fig. 9.13B–D ). Characteristically, abundant vascular hyalinization is evident (see Fig. 9.13A ). Papillary foci are common, and the tumor cells are usually strongly GFAP positive (see Fig. 9.13C and D ).
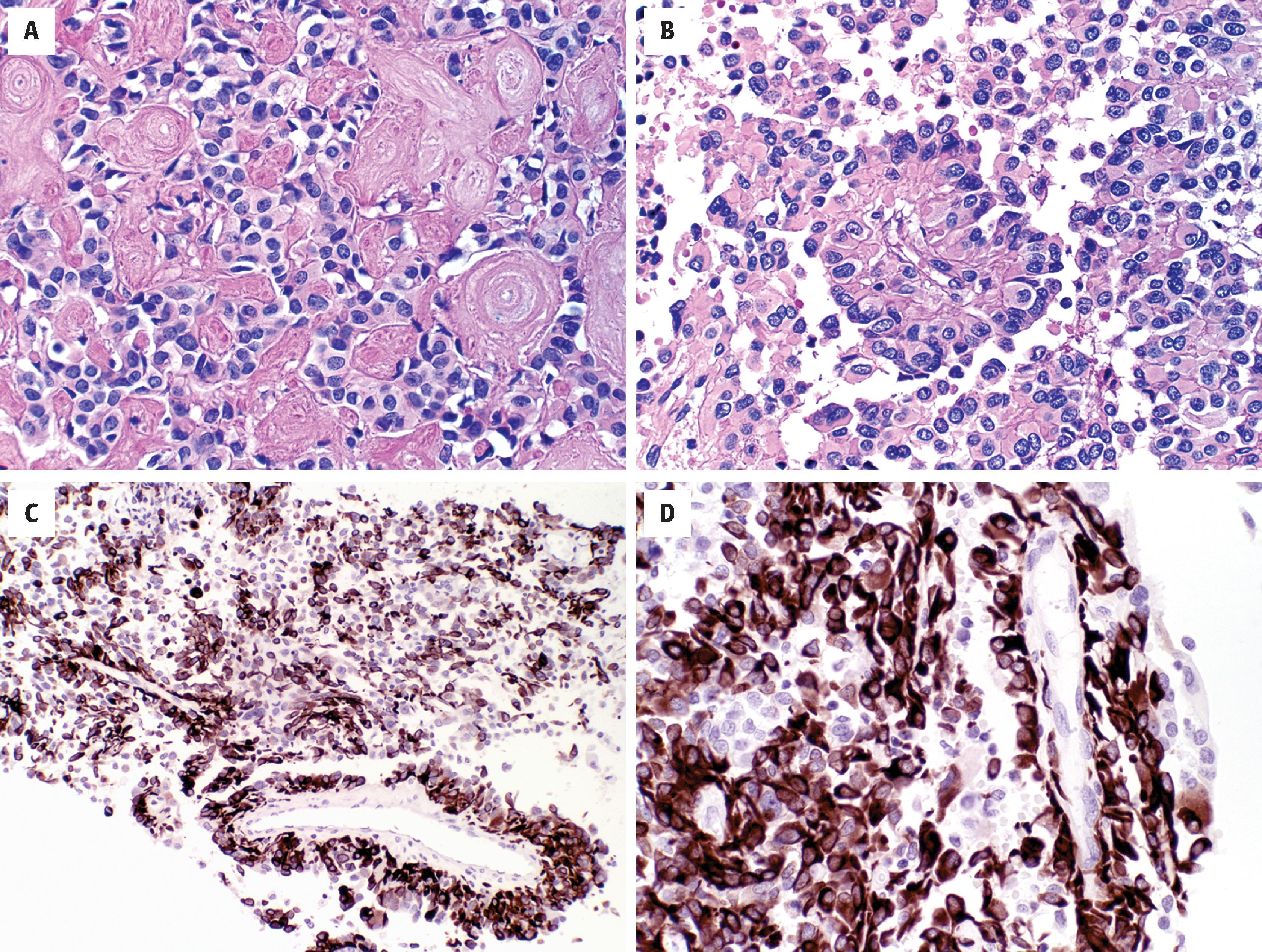
Solid and relatively well demarcated
Perivascular, astroblastic pseudorosettes with broader and shorter processes than those of ependymoma
Round to oval nuclei
Focal to extensive vascular hyalinization
Pseudopapillary pattern with pushing, noninfiltrative border
Mitotic figures, endothelial hyperplasia, and necrosis are uncommon except in the anaplastic variant and thus no definite WHO grade has been established
Electron-dense astrocyte-like cells with prominent cytoplasmic intermediate filaments
Limited ependymal or tanycyte-like features (intercellular junctions, interdigitations/pleatings, cilia, microvilli)
GFAP: usually positive, highlighting short broad perivascular processes
S-100 protein, Olig2: positive
Neuronal markers (e.g., synaptophysin, neurofilament, NeuN, chromogranin) negative
Variable EMA positivity
MN1 alteration required for diagnosis
Ependymoma, papillary variant
PXA
Other papillary tumors
Metastatic disease
The main differential diagnosis is ependymoma, and distinction between the two is made primarily by the presence of broader processes associated with the perivascular pseudorosettes. Diffuse astrocytomas may also look similar, but they are more infiltrative. Those with a prominent papillary growth pattern may resemble another rare entity known as papillary glioneuronal tumor (PGNT), but the latter has a discernible neuronal element. In recent studies, some tumors with astroblastic morphology were reclassified as PXA based on BRAF mutation and concomitant CDKN2A loss. Since astroblastoma is primarily a pediatric neoplasm, astroblastic morphology in an adult’s tumor should prompt further investigation clinically or molecularly to rule out high-grade glioma or metastasis. Sharp circumscription may be seen in metastatic disease, but metastases are not GFAP positive.
Because of the rarity of this tumor type, little clinical experience has accrued. Surgery is the treatment of choice. Currently they are divided into well-differentiated and anaplastic (proliferative index >5 %) types, the latter more likely to recur or lead to patient death.
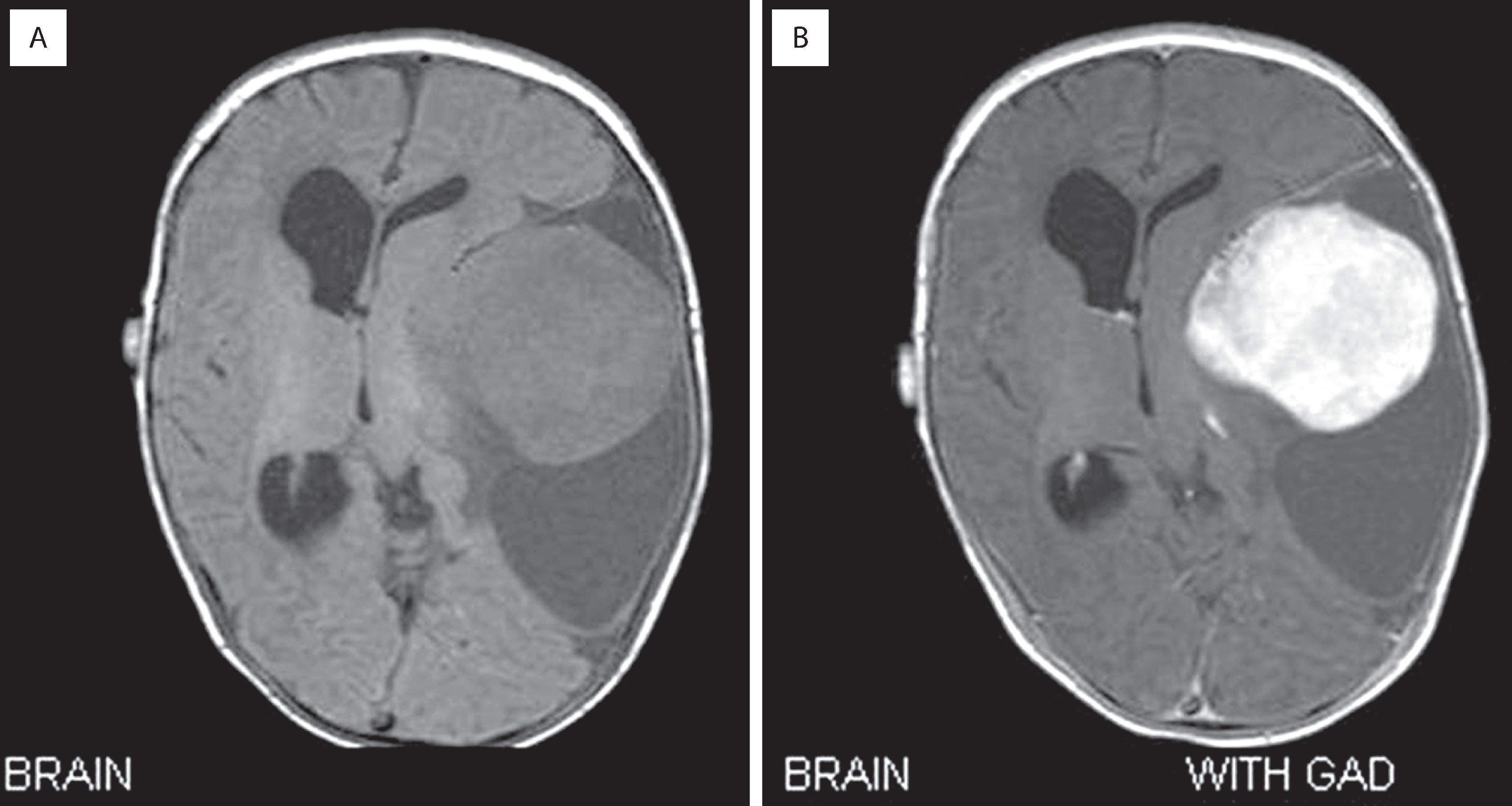
Desmoplastic infantile ganglioglioma (DIG) and its related ganglion cell–deficient entity, desmoplastic infantile astrocytoma (DIA), are benign CNS tumors that develop virtually exclusively in infants with a mean age of 6 months, often as a remarkably large cystic hemispheric mass that may replace much of the brain on one side ( Fig. 9.14 ). Increased head circumference and bulging fontanelles are the most common initial clinical signs, although seizures, hyperreflexia, and cranial nerve palsies may also be encountered. Because of this dramatic manifestation, a malignancy is often expected.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here